Abstract
Objectives
Peripheral arterial disease can be regarded as a systemic inflammatory disorder affecting the entire vascular system. In the early clinical stages, it is characterised by the deterioration of endothelial function, which does not progress with the development of the disease. This study analyses the pleiotropic effects upon the plasma nitrite and C-reactive protein (CRP) levels in claudicating patients after 12 months of treatment with statins.
Study design
A prospective randomised controlled translational study was made in patients with Fontaine grade II ischaemia, treated with the best medical treatment with or without statins for 12 months from the time of diagnosis for assessing the pleiotropic effects of those statins.
Methods
Measurements of plasma high-sensitivity CRP (hsCRP), lipid profile and nitrites were made at baseline and after 1 month and 1 year of treatment with atorvastatin 40 mg/day.
Results
A significant reduction in nitrite levels was observed after 1 month of treatment (11.8±7.8 μM vs 5.7±1.8 μM, p=0.0001), but this effect did not persist after 1 year (11.8±7.8 μM vs 9.4±8.9 μM, p=0.27). HsCRP underwent a significant reduction after both 1 month (7 (2.2–12) vs 3.4 (1.6–5.5), p<0.01) and 1 year of treatment with atorvastatin (7 (2.2–12) vs 2.25 (1.67–6.7), p=0.02). Statin treatment reduced hsCRP levels in 9.64 (95% CI (1.60 to 17.68)) after 1 month and in 9.14 (95% CI (0.18 to 18.47)) after 1 year.
Conclusions
The long-term biological pleiotropic effects of statins provide information on the role of endothelial function and systemic inflammation in the aetiopathogenesis of peripheral arterial disease. Statins slow endothelial degradation at the start of the disease, with no effects over the long term. These drug substances reduce progressive inflammation throughout the treatment period. This supports the novel hypothesis that endothelial dysfunction is only a disease-triggering phenomenon, while systemic inflammation would be responsible for both the origin and the maintenance of peripheral arterial disease.
Keywords: Endothelium, endothelial function, gene therapy, peripheral vascular disease, aorta, great vessels and trauma
Introduction
Peripheral arterial disease (PAD), the clinical manifestation of atherosclerosis in the lower limbs, is caused by a systemic chronic inflammatory state that affects the entire vascular bed.1 2 Endothelial dysfunction and inflammation play a crucial role in the etiopathogenia of this disease.3
Endothelial dysfunction is considered to be an early marker for atherosclerosis, preceding evidence of atherosclerotic plaques on angiography or ultrasound scan. Such endothelial dysfunction has been attributed to deterioration in nitric oxide (NO) bioactivity, and an increase in the formation of reactive oxygen species.4 Patients with PAD have been shown to have increased plasma nitrite levels from the early stages of the disease, though this condition is unrelated to the severity of PAD.3
C-reactive protein (CRP) is a systemic inflammation marker, and its concentration is correlated with the future development of atherothrombotic events, both in patients with established cardiovascular disease and in apparently healthy subjects.5 It has been shown that the clinical severity of PAD is linearly correlated to increased plasma high-sensitivity CRP (hsCRP) levels.2 Meanwhile, CRP participates in the modulation of the deleterious effects of oxidised low-density lipoprotein (LDL) on endothelial function, favouring oxidative stress and free-radical production (superoxide anion), which are able to inactivate NO, producing peroxynitrite. The latter in turn is a cytotoxic, proinflammatory and potent oxidant; as a result, it can contribute to damage and endothelial dysfunction, and to oxidation of the lipoproteins in atherosclerotic lesions.6 7
In vitro studies have shown that statins are capable of increasing endothelial nitrous oxide synthase (eNOS) expression, the NO/peroxynitrite ratio and levels of tetrahydrobiopterin (BH4) in the endothelial cells, preventing the formation of atherosclerotic plaques and reducing CRP levels.8–12 In a previous study of patients with recently diagnosed PAD, 1 month of statin therapy was shown to reduce in vivo plasma nitrite and CRP levels.13
This study analyses the effect of statins upon plasma nitrite and CRP levels in this same group of patients after 1 year of treatment, with a view to collecting further information on the aetiopathogenesis of PAD.
Our aim is to describe the effect of statins on the endothelial dysfunction and inflammation that surrounds PAD in order to provide new insights in the field of the aetiological pathophysiology of atherosclerosis. For this purpose, we have carried out a randomised translational study (not a clinical trial), in which internal validity precedes external validity. The design of these types of studies is based on the best-possible control of the experimental variables in order to achieve results, as reliable as possible, for the pathogenic mechanisms of the disease, using human models within the highest ethical corrections, in order to obtain information on the studied mechanisms, as accurate as it is obtained in ‘in vitro’, ‘ex vivo’ and in animal-model studies. On the other hand, this study does not claim to obtain information that could be efficiently applied to the daily clinical practice; therefore, the external validity parameters that are evaluated in clinical trials are not applicable for this type of translational study.
Material and methods
A prospective, experimental, randomised controlled, translational study was carried out, involving the sequential randomised inclusion of 60 patients at the time of diagnosis of PAD in grade II of Fontaine. All the patients included met the inclusion criteria of the study: those older than 18 years having been diagnosed as having PAD confirmed by a haemodynamic study (Doppler ultrasound) and assessment of claudication during a previous visit no earlier than 1 month have given informed consent. The patients had not previously undergone revascularisation and were not receiving treatment with statins or contraindications for their use. We also excluded all patients with coexistence of chronic inflammatory diseases or steroidal medication.
This is a translational phase IV study to determine the long-term pleiotropic effects of statins in patients with PAD. The target patients who could meet the inclusion criteria were assessed in a screening visit at the outpatient clinic of the Angiology and Vascular Surgery Department of the Getafe University Hospital. When the criteria were met, and after signing the informed consent, the patients were randomly allocated to the intervention arm or control in a 1:1 ratio (figure 1). A computer-generated random number series was used for randomisation. The sequence was concealed until the study arm was assigned. There were three independent vascular surgeons investigators: one who evaluated the potential target in the screening visit and recorded cardiovascular risk factors, treatment and general condition after 1 month and 1 year of the inclusion; one who generated the allocation sequence; and another who enrolled participants and assigned them to their groups.
Figure 1.
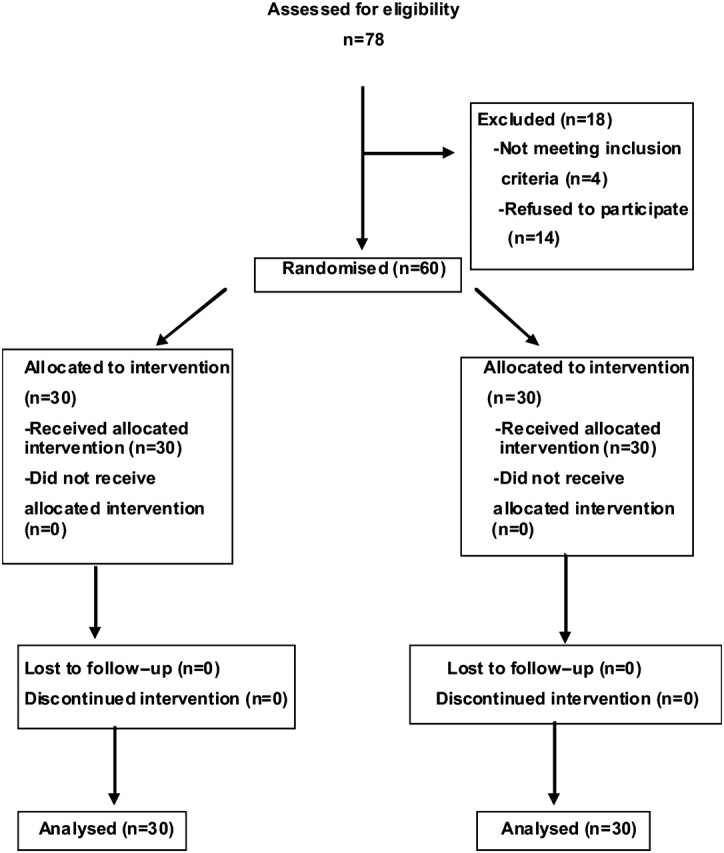
Method of randomisation and allocation concealment.
Patients in the control group were prescribed treatment with aspirin 100 mg, or clopidogrel 75 mg daily if aspirin-intolerant, ACE inhibitors and cardiovascular-risk-factor control. All patients in the experimental group received treatment in the form of atorvastatin 40 mg daily for 1 year.
Cardiovascular risk factors, treatment and general condition were recorded on inclusion and after 1 month and 1 year by a vascular surgeon blinded for the study group in which the patients were included. The ankle–brachial index was recorded under resting conditions following the standard technique, applied to the pedal and posterior tibial arteries of both lower limbs.14 Basic biochemical parameters were recorded (blood glucose, renal function, ions), together with lipid profile, at baseline and after 1 month and 1 year of inclusion. Hypertension was taken to correspond to any patient diagnosed as having high blood pressure (systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg) and/or receiving antihypertensive treatment for at least 1 year before inclusion in the study.15 Dyslipidaemia in turn was defined as total cholesterol >250 mg/dl, LDL cholesterol >160 mg/dl or triglycerides >200 mg/dl, or as any patient currently receiving lipid-lowering therapy.16 Diabetes was defined by a baseline blood glucose of >120 mg/dl or the need for glucose-lowering treatment.17 The chronic renal failure in turn was taken to correspond to a serum creatinine of >1.5 mg/dl.18
A venous blood sample was collected to determine plasma nitrites and high-sensitivity CRP (hsCRP), at baseline, after 1 month and after 1 year of inclusion. Plasma nitrite measurement was carried out after a fasting period of at least 12 h, during which the usual medication of the patient was also suspended. Venous blood was drawn from the antecubital vein and centrifuged for 10 min at 800 g. The resulting plasma fraction was then stored at 4°C. Colorimetric analysis based on the Griess reaction was used for the measurement of plasma nitrites.19 This technique is able to detect nitrites (NO2−) in a range of biological and experimental fluids, the limit of detection being 2.5 μM (125 pmol). Each sample was analysed in triplicate, and the mean of the three determinations was calculated. In a control group of 10 patients, we repeated blood sampling to evaluate the reproducibility of the test, the coefficient of variation being <5%.
Likewise, a venous blood sample was collected for measuring plasma CRP based on an automatised, ultrasensitive immunoassay (Roche Diagnostics, Meylan, France) with a lower limit of detection of 0.2 mg/l and a coefficient of variation of 4.2% in 4 mg/l and 6.3% in 1 mg/l.20
The lab data were determined anonymously, so that the results would not be biased.
This study was approved by the Ethical Committee of Getafe University Hospital.
Statistical analysis
The sample size to obtain significant differences with 80% degree of statistical power was calculated on the basis of previous studies analysing plasma NO and hsCRP2 3 was 30 patients in each group with a predetermined α error of 0.5. This study has been designed to obtain information about the disease pathogenic mechanisms, not to serve as a basis for clinical practice decision-making. With these objectives in mind, we have proposed strict inclusion/exclusion criteria in order to achieve a highly selective sample that ensures a high internal validity. Our design allows us to adjust the sample size to what we found in our calculation without unnecessarily oversizing the sample to achieve valid results.
Normality of variables was assessed using the Kolmogorov–Smirnov and Shapiro–Wilk tests. The Student t test for independent samples was used to compare plasma NO data from two groups, and the Mann–Whitney test for the hsCRP in the analysis in the treatment groups. The Student t test for paired samples was used for variables exhibiting a normal distribution. For variables that did not follow normal-distribution non-parametric statistical tests for paired samples, the Wilcoxon test was used. The continuous data were expressed as the mean±SD, while categorical variables were reported as percentages. Data relating to hsCRP were expressed as the median ±25th and 75th percentiles. We analysed the data in a linear regression adjusted model for age, sex, baseline nitrite and hs-CRP levels. Statistical significance was considered for p<0.05.
Results
Seventy-eight patients were consecutively assessed for inclusion in the study. Four out of 18 were excluded for not meeting inclusion criteria. Fourteen refused to participate, after we had explained in detail the conditions of the study and the hard recommendation of meeting the follow-up visits established per protocol.
Thirty patients with Fontain stage II PAD were recruited and randomly assigned in each group, treatment and control (table 1). Thus, there were no patient dropouts during the study. All 60 patients included completed the study protocol and were analysed for the primary outcome. No major adverse reactions to the treatment with statins were recorded.
Table 1.
Demographic data and treatments
| Treatment group | Control group | ||
|---|---|---|---|
| N=30 | N=30 | p Value | |
| Age (years) | 71.37±10.81 | 70.51±9.7 | NS |
| Sex | |||
| Males (%) | 26/30 (86.7) | 25/30 (83.3) | NS |
| Ischaemia grade (%) | |||
| IIA | 20/30 (66.7) | 20/30 (66.7) | NS |
| IIB | 10/30 (33.3) | 10/30 (33.3) | NS |
| Hypertension (%) | 22/30 (73.3) | 20/30 (66.7) | NS |
| Diabetes (%) | 12/30 (40) | 13/30 (43.3) | NS |
| Smoking (%) | |||
| Active | 12/30 (40) | 13/30 (43.3) | NS |
| Ex-smokers | 10/30 (33.3) | 11/30 (36.6) | NS |
| Acute myocardial infarction (%) | 4/30 (13.3) | 3/30 (10) | NS |
| Dyslipidaemia (%) | 8/30 (26.7) | 5/30 (16.6) | NS |
| Stroke (%) | 2/30 (6.7) | 1/30 (3.3) | NS |
| Chronic obstructive pulmonary disease (%) | 9/30 (18) | 10/30 (33.3) | NS |
| Antiplatelet agents (%) | 26/30 (86.7) | 27/30 (90) | NS |
| Angiotensin-converting enzyme inhibitors (%) | 14/30 (46.7) | 15/30 (50) | NS |
| Angiotensin II Receptor Antagonists (%) | 4/30 (13.3) | 3/30 (10) | NS |
| Beta-blockers (%) | 4/30 (13.3) | 3/30 (10) | NS |
| Nitrites (%) | 2/30 (6.7) | 5/30 (16.6) | NS |
| Ca antagonists | 6/30 (20) | 8/30 (26.7) | NS |
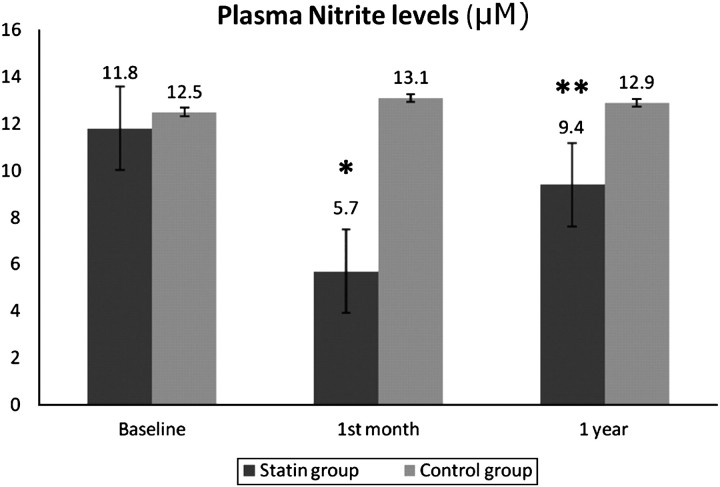
A significant reduction in nitrite levels was observed after 1 month of treatment both in the intragroup comparison (11.8±7.8 μM vs 5.7±1.8 μM, p=0.0001) and in the comparison between groups (p=0.001). This effect did not persist after 1 year (11.8±7.8 μM vs 9.4±8.9 μM, p=0.27) (figure 2). No changes were found in the nitrite levels in the control group over the full course of the study (12.5 ±5.1 μM vs 13.1±9.1 μM vs 12.9±8.7 μ, p=0.83). The difference in the mean nitrite levels in experimental group after 1 month was 6.09 (95% CI (3.22 to 8.95)) vs the control group −0.6 (95% CI (−2.7 to 1.9)) (p<0.05). Otherwise, this reduction was not maintained at 1 year, with a difference in the mean nitrite levels in the experimental group of 3.3 (95% CI (−0.2 to 6.1)) vs −0.4 (95% CI (−3.1 to 1.8)) for the control group.
Figure 2.
Comparison of plasma nitrite levels (μM) at baseline and after 1 month and 1 year of statin therapy (*p<0.05 on comparing baseline vs 1 month of treatment; **p>0.05 on comparing baseline vs 1 year of treatment). There were no statistical differences between the control-group data.
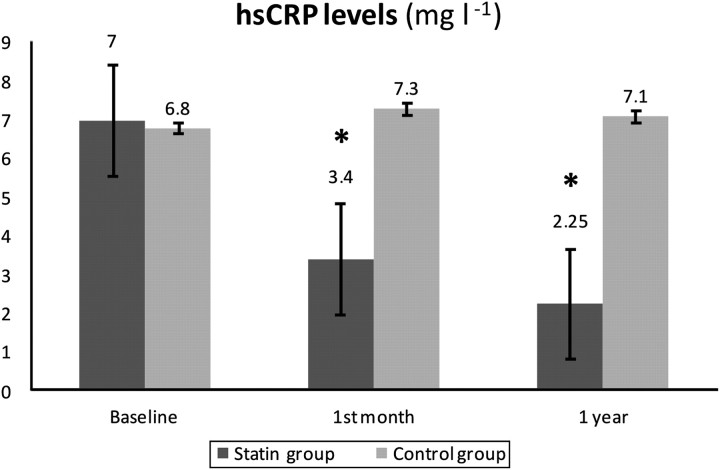
The hsCRP levels likewise underwent a significant reduction after both 1 month (7 (2.2–12) vs 3.4 (1.6–5.5), p<0.01) and 1 year of treatment with atorvastatin (7 (2.2–12) vs 2.25 (1.67–6.7), p=0.02) in both the comparison intragroups and intergroups (p=0.01) (figure 3). There were no changes regarding hsCRP levels in the controls (6.8 (1.9–11.5) vs 7.3 (2.5–12) vs 7.1 (2.4–11.8), p=0.59).
Figure 3.
Comparison of high-sensitivity C-reactive protein levels (mg/l) at baseline and after 1 month and 1 year of statin therapy (*p<0.05). There were no statistical differences between the control-group data.
Statin treatment reduced hsCRP levels in 3.6 (95% CI (2.1 to 5.8)) after 1 month versus the control group −0.5 (95% CI (−1.7 to 0.5)) and in 4.75 (95% CI (2.5 to 6.3)) after 1 year in the experimental group versus −0.3 (95% CI (−1.5 to 0.4)) in the control group.
Lastly, a significant reduction in LDL cholesterol (130.78±43.41 vs 82.50±26.33, p=0.024) and total cholesterol was recorded (207.60±49.24 vs 150.13±33.54, p=0.002), over the full course of the study in the treatment group.
Discussion
The results of this study evaluate and analyse in vivo the pleiotropic effects of long-term of the HMG-coenzyme A reductase inhibitors upon NO bioavailability and the plasma levels of the inflammatory marker CRP, in patients with PAD, and offer an aetiopathogenic interpretation of the results obtained.
We know of these effects on the inflammatory cascade and on the metabolic pathway of NO thanks to in vitro laboratory studies involving experimental models with endothelial cells and animal models using cholesterol-rich diets. It has been seen that statins increase the bioavailability of NO and reduce levels of peroxynitrite (the main component of oxidative stress), increasing the NO/peroxynitrite ratio, which is fundamental for the maintenance of endothelial function. The statin-mediated effect upon NO release by the endothelial cells is the result of increased expression of the eNOS enzyme and a reduction in eNOS decoupling. When the tetrahydrobiopterin (BH4) levels are low and/or the production of superoxide anion increases, eNOS ‘uncoples’ and behaves like an NADPH oxidase, increasing the production of superoxide anion and hydrogen peroxide more than that of NO, whereby the net balance is a reduction in NO activity.7 21 The statins are capable of reducing superoxide anion levels through the inhibition of NADPH oxidase.22 They increase GTP cyclohydrolase-I (GTPCH), which is the rate-limiting enzyme in BH4 synthesis in the endothelial cells. Thus, the statins increase levels of BH4, an essential cofactor involved in the functioning of eNOS, both via GTPCH and by preventing the oxidation of BH4 to radical BH3, thereby interrupting perpetuation of the vascular oxidative stress cycle.8 9 23
Our results offer a new insight into the effects on this pathway over the long term in real patients with PAD. A significant reduction in plasma nitrite levels was observed after 1 month of treatment with atorvastatin 40 mg, though such a result did not persist after 1 year. These observations support the hypothesis that endothelial dysfunction only plays a role in triggering the initial development of PAD, but would not be responsible for perpetuation of the disease over time.2 3 In addition, it is seen that statin treatment restores endothelial function during initial action of the drug, though over the long term the biological mechanisms by which statins are able to increase NO bioavailability become depleted. As a result, they exert no sustained long-term effect.
At the other extreme, there is increasing evidence that inflammation plays a predominant role in the processes that trigger and develop PAD. In fact, and considering our results, the metabolic pathway of NO could be defined as being collateral to inflammation. In this context, CRP is an independent predictor of cardiovascular events in patients with PAD,24 and a linear correlation has been established between rising plasma CRP levels and the severity of the disease in these individuals.2 25 These findings point to the existence of an inflammatory substrate in the aetiopathogenesis of PAD. CRP participates in the modulation of the deleterious effects of LDL oxidases upon endothelial function, favouring oxidative stress and free-radical production (superoxide anions), by increasing the expression of NADPH oxidase.26 In addition, CRP directly inhibits GTPCH enzyme activity, lowering the BH4 concentrations and thus favouring eNOS decoupling and a reduction in the bioavailability of NO.27 It has been seen that when inducible nitric oxide synthase (iNOS) is expressed, the peroxynitrite levels increase.7 28 Thus, CRP stimulates NO production via iNOS, increasing its oxidation and nitrosylation.
On the other hand, in vitro studies have found that CRP is able to stimulate NO production independently of iNOS stimulation,28 and has intrinsic and independent deleterious action as a pathogenic agent in the aetiopathogenesis of PAD. A decrease in CRP levels has been reported in atherosclerotic patients treated with statins, independently of the baseline lipid concentrations.12 In this study, we likewise observed a significant reduction in hsCRP in the PAD patients during both short- and long-term treatment with statins. By inverse deduction, this confirms inflammation as the process giving rise to the disease, and which continues to intervene over the course of the disorder, thereby inducing the advance of the processes that trigger and develop PAD.
The limitations of this study include, in the first place, the fact that nitrite levels are influenced by endogenous and exogenous factors such as dietary nitrates, the inhalation of atmospheric NO, its formation in the salivary glands and renal function. Although it is not possible to exclude these factors, these data are in line with observations from earlier studies. While dealing with plasma nitrites and not other nitrates may seem to be a limitation, it is known that over 70–90% of all plasma nitrites originate from eNOS activity.29
The observational translational design of this study allows us to obtain valid results with the sample size used, which could be applicable in the atherosclerosis aetiopathogenesis knowledge field (internal validity).
Long-term statin therapy reduces the CRP levels, while in contrast the nitrite concentrations initially decrease during the first month of treatment, only to increase again afterwards. In vitro endothelial studies have demonstrated that the statins reduce plasma nitrite levels. It is important to mention that the differences in these results are explained by the fact that our study is a long-term in vivo study, while the rest of the studies have been carried out in vitro and/or over the short-term only.
PAD can be regarded as a systemic, chronic inflammatory disorder, with a direct relationship between the levels of inflammation and its severity.25 On the other hand, previous studies have shown the initial stages of PAD to be characterised by functional endothelial alterations that could trigger the occurrence of a range of events leading to clinical development of the disease.2 3
Conclusion
Statin therapy reduces the plasma nitrite and CRP levels in patients with PAD from the first month of treatment. The initial effect upon NO bioavailability is not maintained over time, in contrast to what is seen in the case of the inflammatory process. This would support the hypothesis that endothelial dysfunction only exerts a PAD-triggering effect, and that the homeostatic mechanisms available in the body for regulating NO bioavailability are not depleted.
Footnotes
Competing interests: None.
Ethics approval: Ethics approval was provided by the Ethics Committee of Hospital Universitario de Getafe.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- 2.De Haro J, Acín F, López-Quintana A, et al. Direct association between C-reactive protein serum levels and endothelial dysfunction in patients with claudication. Eur J Vasc Endovasc Surg 2008;35:480–6. [DOI] [PubMed] [Google Scholar]
- 3.De Haro J, Martinez-Aguilar E, Flórez A, et al. Nitric oxide: link between endothelial dysfunction and inflammation in patients with peripheral arterial disease of lower limbs. Interact Cardiovasc Thorac Surg 2009;9:107–12. [DOI] [PubMed] [Google Scholar]
- 4.Ignarro LJ, Cirino G, Casini A, et al. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol 1999;34:879–86. [DOI] [PubMed] [Google Scholar]
- 5.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003;111:1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbato JE, Tzeng E. Nitric oxide and arterial disease. J Vasc Surg 2004;40:187–93. [DOI] [PubMed] [Google Scholar]
- 7.Miyoshi T, Li Y, Shih DM, et al. Deficiency of inducible NO synthase reduces advanced but not early atherosclerosis in apolipoprotein E-deficient mice. Life Sci 2006;79:525–31. [DOI] [PubMed] [Google Scholar]
- 8.Heeba G, Hassan MK, Khalifa M, et al. Adverse balance of nitric oxide/peroxitrite in the dysfunctional endothelium can be reversed by statins. J Cardiovasc Pharmacol 2007;50:391–8. [DOI] [PubMed] [Google Scholar]
- 9.Hattori Y, Nakanishi N, Akimoto K, et al. HMG-CoA reductase inhibitor increases GTP cyclohydrolase I mRNA and tetrahydrobiopterin in vascular endothelial cells. Arterioscler Thromb Vasc Biol 2003;23:176–82. [DOI] [PubMed] [Google Scholar]
- 10.Laufs U, La Fata V, Plutzky J, et al. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 1998;97:1129–35. [DOI] [PubMed] [Google Scholar]
- 11.Rikitake Y, Kawashima S, Takeshita S, et al. Anti-oxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis 2001;154:87–96. [DOI] [PubMed] [Google Scholar]
- 12.Ridker P, Rifai N, Pfeffer MA, et al. Long-term effects of pravastatin on plasma concentration of C-reactive protein. Circulation 1999;100:230–5. [DOI] [PubMed] [Google Scholar]
- 13.Martinez Aguilar E, De Haro J, Flórez A, et al. In vivo confirmation of the role of statins in reducing nitric oxide and C-reactive protein levels in peripheral arterial disease. Eur J Vasc Endovasc Surg 2009;37:443–7. [DOI] [PubMed] [Google Scholar]
- 14.Newman AB, Shemanski L, Manolio TA, et al. Ankle–arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol 1999;19:538–45. [DOI] [PubMed] [Google Scholar]
- 15.Verdecchia P, Angeli F. The seventh report of the Joint National Committee on the prevention, detection, evaluation and treatment of high blood pressure: the weapons are ready. Rev Esp Cardiol 2003;56:843–7. [DOI] [PubMed] [Google Scholar]
- 16. National Heart, Lung, and Blood Institute. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III, or ATP III). http://www.nhlbi.nhi.gov/guidelines/cholesterol/atglance.htm (accessed 25 Feb 2006).
- 17. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26:s5–20. [DOI] [PubMed] [Google Scholar]
- 18.Ferrer R, Hérnandez-Jara J. Chronic renal insufficiency. I: definition, clinical course stages, progression mechanisms, etiology and diagnostic criteria. Nefrología 2001;21:18–20. [PubMed] [Google Scholar]
- 19.Kleinbongard P, Rassaf T, Dejam A, et al. Griess method for nitrite measurement of aqueous and protein containing sample. Methods Enzymol 2002;359:158–68. [DOI] [PubMed] [Google Scholar]
- 20.Eda S, Kaufmann J, Roos W, et al. Development of a new microparticle-enhanced turbidimetric assay for C-reactive protein with superior features in analytical sensitivity and dynamic range. J Clin Lab Anal 1998;12:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gokce N, Keaney JF, Jr, Hunter LM, et al. Predictive value of noninvasive determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 2003;41:1769–75. [DOI] [PubMed] [Google Scholar]
- 22.Wagner AH, Kohler T, Ruckschloss, et al. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol 2000;20:61–9. [DOI] [PubMed] [Google Scholar]
- 23.Bevers LM, Braam B, Post JA, et al. Tetrahydrobiopterin, but not l-arginine, decreases NO synthase uncoupling in cells expressing high levels of endothelial NO synthase. Hypertension 2006;47:87–94. [DOI] [PubMed] [Google Scholar]
- 24.Brevetti G, Silvestro A, Di Giacomo S, et al. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripherial circulatory impairment but not to classic risk factors and aterosclerosis burden. J Vasc Surg 2003;38:374–9. [DOI] [PubMed] [Google Scholar]
- 25.De Haro J, Acín F, Medina FJ, et al. Relationship between the plasma concentration of C-reactive protein and severity of peripheral arterial disease. Clin Med Cardiol 2009;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi S, Inoue N, Ohashi Y, et al. Interaction of oxidative stress and inflammatory response in coronary plaque instability: important role of CRP. Arterioscler Thromb Vasc Biol 2003;23:1398–404. [DOI] [PubMed] [Google Scholar]
- 27.Singh U, Devaraj S, Vasquez-Vivar J, et al. C-reactive protein decreases endotelial nitric oxide synthase activity via uncoupling. J Mol Cell Cardiol 2007;43:780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clapp BR, Hirschfield GM, Storry C, et al. Inflammation and endothelial function. Direct vascular effects of human C-reactive protein on nitric oxide bioavailability. Circulation 2005;111:1530–6. [DOI] [PubMed] [Google Scholar]
- 29.Kleinbongard P, Dejam A, Lauer T, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med 2003;35:1551–9. [DOI] [PubMed] [Google Scholar]