Abstract
Rho family GTPases, and the proteins that regulate them, have important roles in many cellular processes, including cell division, survival, migration and adhesion. Although most of this understanding has come from studies using cell lines, more recent gene targeting studies in mice are providing insights into the in vivo function of these proteins. Here we review recent progress revealing crucial roles for these proteins in lymphocyte development, activation, differentiation and migration. The emerging picture shows that Rho family GTPases transduce signals from receptors for antigens, chemokines and cytokines, as well as adhesion molecules and pattern recognition receptors, and that they act as focal points for crosstalk between different signalling pathways.
Introduction
Rho family GTPases have been recognized as crucial signal-transducing proteins for almost 20 years. Whereas initial analysis of the prototypical family members RHOA (RAS homologue gene family member A), RAC1 and CDC42 (cell division cycle 42) identified important roles for these proteins in the regulation of the cytoskeleton, Rho GTPases also have crucial functions in cell division, survival, migration and adhesion. Most of our insight into these cellular functions has come from cell-based studies using dominant negative or constitutively active proteins. More recently, however, gene targeting in mice has allowed selective inactivation of different Rho GTPases and their regulators, and subsequent analysis of in vivo function1. In this review we discuss how this approach has led to a large increase in our understanding of the physiological roles of these proteins in lymphocyte development and activation.
General features of Rho GTPases and their regulators
Mammalian Rho GTPases constitute a family of 23 proteins that are related in primary sequence. Phylogenetic analysis shows that these proteins cluster into several subfamilies based on sequence similarity (Figure 1). The prototypical members of the family, RAC1, CDC42 and RHOA, can bind both GTP and GDP. When bound to GTP they are active and able to transduce signals by binding to effector proteins. By contrast, the GDP-bound forms do not bind effector proteins and are generally assumed to be inactive. However the RND, RHOBTB, RHOH, RHOU and RHOV GTPases are atypical and do not conform to this stereotypical mechanism of regulation as they are mainly GTP bound. They might instead be regulated by expression, phosphorylation or stability.
Figure 1.
Mouse Rho GTPases. Phylogenetic tree of 23 mouse Rho GTPases, showing how they cluster into different subfamilies, for example the Rac, RhoA/RhoB/RhoC and Rnd subfamilies. We note that it has been proposed that MIRO1, MIRO2 and RHOBTB3 do not belong to the Rho GTPase family120.
The switch between the GDP- and GTP-bound forms of typical Rho GTPases is regulated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) (Figure 2). Most Rho GTPases are modified at the C-terminus by the attachment of a prenyl group2. This contributes to membrane localization of the GTPases and therefore to their signalling function, as some effectors of the Rho GTPases are membrane-associated proteins and others are recruited to membranes by binding to the GTPases. Guanine nucleotide dissociation inhibitors (GDIs) bind to the C-terminal lipid groups on GTPases, thereby inhibiting membrane binding and maintaining them in a state where they cannot be activated and are unable to interact with effector proteins.
Figure 2.
Regulation of Rho GTPases. This figure shows a generic Rho GTPase anchored to the membrane with a prenyl group near the C-terminus (zigzag line). The GTPase binds either GDP or GTP. Guanine nucleotide-exchange factors (GEFs) catalyse the release of GDP from the GTPase, allowing GTP to bind. GTPase-activating proteins (GAPs) increase the intrinsic GTPase activity of the Rho proteins, causing GTP to be hydrolysed to GDP and phosphate (Pi). GEFs and GAPs are often constitutively or inducibly associated with membranes. GDP-bound Rho proteins can be sequestered by Rho guanine nucleotide dissociation inhibitors (GDIs), which bind to the lipid modification and thereby inhibit membrane binding of the GTPase. GTP-bound Rho proteins transduce signals by binding to effector proteins.
When in the GTP-bound active form, Rho family GTPases transduce signals by binding to effector proteins (Box 1). These in turn are involved in many cellular processes including regulation of the actin cytoskeleton, microtubule dynamics, cell division, migration and adhesion (see recent reviews1,3 for more detail regarding effector proteins).
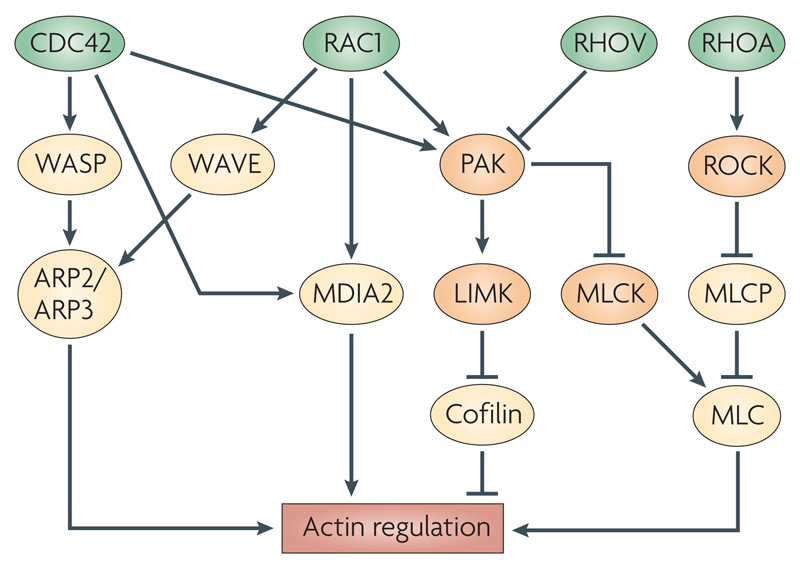
Box 1. Effector proteins of Rho GTPases that regulate the actin cytoskeleton.
Regulation of the actin cytoskeleton downstream of the Rho GTPases CDC42 (cell division cycle 42), RAC1, RHOV and RHOA is mediated by several effector proteins (see figure). CDC42 activates WASP (Wiskott–Aldrich syndrome protein), which, via ARP2 (actin-related protein 2 homologue)-ARP3, leads to actin polymerization from the sides of existing filaments, creating branched structures. RAC1 activates the same pathway through the WASP-related WAVE (WASP-family verprolin homologous protein) family of proteins. Both CDC42 and RAC1 activate mDIA2, leading to the nucleation of unbranched actin filaments. The same two GTPases also activate the PAK family kinases, which phosphorylate and activate LIMK (LIM domain kinase), a kinase which in turn phosphorylates and inhibits cofilin. Cofilin promotes severing of actin filaments and depolymerization, hence its inhibition leads to increased stability of polymerized actin. RHOV inhibits PAK activation117. LIMK is also activated by ROCK (RHO-associated coiled-coil-containing protein kinase), a kinase effector that is downstream of RHOA. ROCK increases phosphorylation of myosin light chain (MLC) by phosphorylating and inhibiting myosin light chain phosphatase (MLCP), and might also directly phosphorylate MLC. Phosphorylation of MLC leads to its increased association with actin filaments. Finally, PAK counteracts ROCK function by inhibiting myosin light chain kinase (MLCK), thereby reducing phosphorylation of MLC. Interestingly, in migrating T cells, there is spatial segregation of these pathways, with MLCK activated predominantly at the leading edge and ROCK activated in the trailing edge118. Many of the pathways illustrated here have been identified in non-lymphoid cells and it is not known if they exist in B or T cells.
There are 79 identified GEFs for Rho-family GTPases in the mammalian genome4. The largest subgroup of these, containing 66 members, is the Dbl family, all of which contain a Dbl homology (DH) domain, which has the catalytic GEF activity. The Dock family of GEFs is distinguished from the Dbl family by not having a DH domain. Instead, catalytic activity resides in a Dock homology region 2 (DHR2). Finally, the related SWAP70 and IBP proteins are GEFs for Rac GTPases but they contain neither DH nor DHR2 domains.
There are currently 65 Rho family GAPs known in mice. All of these are typified by a conserved RhoGAP domain, which contains the catalytic activity of the enzymes5. Both the GEFs and the RhoGAPs usually contain other domains, including protein-protein interaction domains and catalytically active domains. The reasons for the large number of GEFs and GAPs relative to Rho family GTPases are not known. Whereas some GEFs are specific for only one or a few GTPases (such as ITSN1 for CDC42), others have a broader specificity (for example, VAV1 acts on RAC1, RHOA, RHOG and CDC42). The same is true for GAPs. It is probable that this diversity of regulators serves to transduce signals from many different receptors to the GTPases, suggesting that these GTPases act as focal points for cross-talk between different signalling pathways.
Finally, there are three GDIs for Rho family GTPases: GDIα, GDIβ and GDIγ. Readers are referred to recent reviews4–6 for a more comprehensive discussion of the GEFs, GAPs and GDIs that regulate Rho family GTPases.
Analysis of mRNA levels for Rho family GTPases and their regulators shows that approximately half of the members of these large families are expressed by lymphoid cells (Table 1 and Supplementary Table 1). In particular, 14 out of 23 GTPases, 36 out of 79 RhoGEFs, 38 out of 65 RhoGAPs and 2 out of 3 RhoGDIs are expressed in one or more lymphoid lineages, suggesting that this large group of signal transducers has important roles in lymphocyte biology.
Table 1.
Expression of some Rho GTPases and their regulators in lymphocytes. Expression data is compiled from NCBI Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) under accession numbers GSE6259 and GSE6506 119. Only those genes that have been targeted for deletion in mice are listed; a full list of all Rho GTPases and their regulators is shown in Supplementary Table 1. +++, high level expression; ++, intermediate level expression; + low level expression; -, not expressed.
| Gene name | B cell | CD4+ T cell | CD8+ T cell | NK cell |
|---|---|---|---|---|
| Rho GTPases | ||||
| Rac1, Rac2, Rhog, Cdc42 | +++ | +++ | +++ | +++ |
| Rac3 | – | – | – | – |
| Rhoh | +++ | +++ | +++ | ++ |
| Rhob | +++ | ++ | ++ | ++ |
| Rhoc | – | + | – | ++ |
| RhoGEFs | ||||
| Abr | – | ++ | ++ | ++ |
| Als2 | ++ | + | + | ++ |
| Arhgef1 | +++ | +++ | +++ | ++ |
| Arhgef6, Arhgef18, Sos1, Sos2, Dock2, Def6, Vav1 | +++ | +++ | +++ | +++ |
| Arhgef9 | – | – | + | – |
| Arhgef12 | ++ | ++ | ++ | + |
| Arhgef15, Kalrn, Mcf2, Ngef, Prex2, Rasgrf1, Rasgrf2, Tiam1, Dock3, Dock7 | – | – | – | – |
| Bcr | + | – | – | – |
| Ect2 | + | – | – | ++ |
| Fgd2 | +++ | – | – | – |
| Itsn1 | + | + | + | + |
| Trio | + | + | + | – |
| Vav2 | +++ | ++ | ++ | |
| Vav3 | ++ | ++ | ++ | +++ |
| Dock1 | – | + | + | + |
| Dock5 | ++ | ++ | +++ | |
| Swap70 | +++ | ++ | – | ++ |
| RhoGAPs | ||||
| Abr, Chn2 | – | ++ | + | ++ |
| Arhgap1, Inpp5b, Pik3r1, Tagap1 | +++ | +++ | +++ | +++ |
| Arhgap5 | – | + | + | – |
| Arhgap6 | ++ | + | – | ++ |
| Arhgap12, Srgap2 | ++ | ++ | ++ | +++ |
| Bcr | + | – | – | – |
| Chn1, Dlc1, Grit, Ocrl, Ophn1 | – | – | – | – |
| Pik3r2 | + | + | – | – |
| Ptpdc1 | + | + | + | + |
| Racgap1 | – | – | ++ | ++ |
| Ralbp1 | ++ | ++ | ++ | ++ |
| RhoGDIs | ||||
| Arhgdia, Arhgdib | +++ | +++ | +++ | +++ |
| Arhgdig | – | – | – | – |
GTPases and their regulators in B cells
B cell development starts in the bone marrow, and proceeds through an ordered series of maturation steps resulting in the release of IgM-expressing immature B cells, which migrate to the spleen, where they undergo further maturation to give rise to follicular B cells and marginal zone (MZ) B cells (Figure 3). Follicular B cells recirculate between secondary lymphoid organs through the blood and lymphatic systems. Upon engagement with antigen through the B cell receptor (BCR), they are activated, move to germinal centres, and, with T cell help, differentiate into plasma cells secreting high affinity antibodies and into long-lived memory B cells.
Figure 3.
Role of Rho GTPases and their regulators in B cell development and function. The earliest B cell progenitors in the bone marrow, pro-B cells, rearrange immunoglobulin heavy chain genes. If they successfully generate a heavy chain, this assembles into the pre-B cell receptor (pre-BCR). Signals from the pre-BCR allow cells to differentiate into pre-B cells and proliferate. Pre-B cells rearrange light chain genes, and if this process is successful, the light chain pairs with the heavy chain to generate cell surface-bound BCR in the form of IgM. These cells, known as immature B cells, exit the bone marrow and migrate through the circulation to the red pulp of the spleen, where we have recently described them as transitional type 0 (T0) cells (R. Henderson and V. Tybulewicz, unpublished observations). Chemokine-induced migration of T0 cells into the white pulp is accompanied by maturation into transitional type 1 (T1) and then type 2 (T2) cells, and finally into either follicular or marginal zone (MZ) B cells. Follicular B cells recirculate between secondary lymphoid organs, including spleen, lymph node, Peyer’s patches and bone marrow. B1 cells, a distinct lineage of B cells, which predominate in the peritoneal and pleural cavities, are derived from foetal progenitors (not shown). Activation of naïve follicular B cells (thick arrow) results in their movement into germinal centres (GCs) where, as GC B cells, with T cell help, they undergo somatic hypermutation, affinity maturation and class switching. Eventually the cells mature into antibody-secreting plasma cells and memory B cells. Points where roles for Rho GTPases or their regulators have been identified are indicated, and discussed more fully in the main text.
B cell development
The roles of RAC1 and RAC2 in B cell development have been investigated using mice deficient in either or both GTPases. Whereas loss of RAC1 had no apparent effect, a deficiency in RAC2 results in loss of MZ and B1 cells, without affecting follicular B cells7,8. By contrast, loss of both RAC1 and RAC2 led to a complete block in the development of all B cell lineages, with arrest at the transitional B cell stage in the spleen8. Analysis showed that RAC1 and RAC2 were required for these earliest transitional B cells to migrate from the red pulp to the white pulp of the spleen, most probably because of a crucial role for RAC GTPases in transducing chemokine receptor signals (R. Henderson and V. Tybulewicz, unpublished observations). This migration is essential for the survival of transitional B cells and their subsequent maturation into follicular and MZ B cells. The redundancy between RAC1 and RAC2 revealed by these studies is an emerging theme of Rho GTPases and their regulators.
B cell development is also perturbed in the absence of the Vav GEFs. A deficiency of VAV1 results in a decreased number of B1 cells, but has little or no effect on the development of either follicular or MZ B cells, despite impaired BCR signalling (see later)9. By contrast, deficiency of VAV2 alone had no effect on B cell development, whereas mice lacking both VAV1 and VAV2 had a strong developmental block which resulted in decreased numbers of follicular and MZ B cells, which was further enhanced in the absence of all three VAV proteins (VAV1, VAV2 and VAV3), once again revealing redundancy of function10–13. The developmental block in these mice occurs between T1 and T2 transitional splenic stages, indicating that VAV proteins have a crucial role in this late step of B cell maturation. This is at least partly due to a function for VAV proteins in transducing nuclear factor-κB (NF-κB)-dependent survival signals from the BCR13. Interestingly, the developmental block seen in VAV-deficient B cells is distinct from that seen in the absence of RAC1 and RAC2. Whereas deficiency of RAC proteins results in a block in the earliest T0 transitional subset, before migration into the splenic white pulp, lack of all three VAV proteins arrests development at the later T1 transitional stage and has no effect on migration of these cells into the white pulp, or on chemokine responsiveness in vitro, implying that a non-VAV GEF, such as a DOCK family member, is required for chemokine-induced RAC activation in transitional B cells (R. Henderson and V. Tybulewicz, unpublished observations).
Deletion in pro-B cells of RACGAP1, a RhoGAP with broad specificity, results in defective interleukin-7 (IL-7)-induced cell proliferation and survival14. Interestingly, RACGAP1 has been shown recently to act as a nuclear chaperone that is required for the nuclear import of phosphorylated signal transducers and activators of transcription (STATs), which have essential roles in transducing signals from cytokine receptors15.
B cell activation and function
Inhibition of RHOA in both primary B cells and a B cell line showed that this GTPase is required for BCR-induced synthesis of phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2), the substrate for phospholipase Cγ2 (PLCγ2), which hydrolyses it to generate inositol-1,4,5-trisphosphate (InsP3) and diacylglycerol (DAG)16 (Figure 4). Thus inhibition of RHOA leads to less InsP3 production, less calcium flux and decreased B cell proliferation. RHOA might mediate this function by either directly activating or controlling the localization of phosphatidylinositol 4-kinase and phosphatidylinositol-4-phosphate 5-kinase, lipid kinases that are required for the synthesis of PtdIns(4,5)P2 in response to BCR signalling.
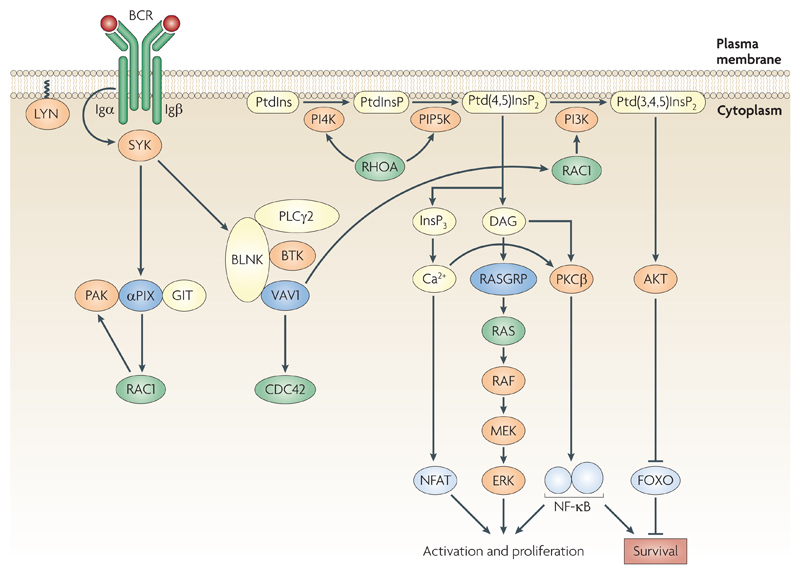
Figure 4.
Signal transduction from the B cell receptor. Binding of antigen to the B cell receptor (BCR) leads to activation of the tyrosine kinase LYN, phosphorylation of the immunoreceptor tyrosine-based activation motifs (ITAMs) in the cytoplasmic domains of Igα and Igβ, recruitment and activation of the tyrosine kinase SYK (spleen tyrosine kinase), phosphorylation of the adaptor protein BLNK (B cell linker) and assembly of a complex including BTK (Bruton’s tyrosine kinase), VAV1 and PLCγ2 (phospholipase Cγ2). Activation of PLCγ2 leads to hydrolysis of the lipid phosphatidylinositol-4,5-bisphosphate (Ptd(4,5)InsP2) to the second messengers inositol-3,4,5-trisphosphate (InsP3) and diacylglycerol (DAG). Ptd(4,5)InsP2 is synthesized by sequential phosphorylation of phosphatidylinositol (PtdIns) to phosphatidylinositol-4-phosphate (Ptd(4)InsP) and then to Ptd(4,5)InsP2 by phosphatidylinositol-4-kinase (PI4K) and phosphatidylinositol-4-phosphate-5-kinase (PIP5K) respectively. Both of these latter kinases are activated by RHOA. VAV1 may contribute to the activation of PLCγ2 through an adaptor function, and, via RAC1 lead to increased synthesis of PtdIns(3,4,5)P3, potentially by activating phosphatidylinositide 3-kinase (PI3K). Increased levels of InsP3 lead to an increase in intracellular Ca2+ and eventually to activation of nuclear factor of activated T cells (NFAT). DAG activates the RAS exchange factor RASGRP, leading to activation of RAS and the RAF, MEK, ERK kinase cascade. Ca2+ and DAG may also contribute to activation of nuclear factor-kB (NF-κB) via PKCβ. PtdIns(3,4,5)P3 leads to activation of the kinase AKT which signals cell survival through inhibition of the FOXO transcription factors. Signalling from the BCR also activates αPIX, and hence RAC1 and PAK.
In B cells that lack RAC2 alone, or RAC2 and one allele of RAC1 (Rac1+/-Rac2-/-), BCR-induced proliferation of B cells was decreased, as was BCR-induced calcium flux, AKT activation and induction of expression of BCL-XL and cyclin D28. RAC2-deficient B cells have defects in the formation of an immunological synapse, owing to defective activation of lymphocyte function-associated antigen 1 (LFA1)-mediated adhesion17. This might be due to a role for RAC2 in transducing BCR signals to activate RAP1 and to induce actin polymerization, both of which have been proposed to regulate LFA1 avidity18. Finally, a deficiency of RAC2 results in defective T cell-independent B cell responses7.
RHOG-deficient mice do not have a severe phenotype, showing a mild increase in the humoral immune response to T-cell-dependent antigens and a subtle increase in the proliferation of B and T cells following antigen receptor cross-linking19. As RHOG is most closely related to the Rac group of GTPases (Figure 1), there may be functional redundancy between RHOG and the Rac proteins.
BCR-induced cell proliferation is partially compromised in the absence of VAV1, a defect that is enhanced in the absence of the other VAV isoforms9–12,20. This is due at least in part to a role for VAV proteins in transducing BCR signals, leading to intracellular calcium flux and activation of phosphatidylinositide 3-kinase (PI3K), AKT and NF-κB10–13,21,22 (Figure 4). Interestingly, BCR-induced activation of extracellular signal-regulated kinase (ERK) is normal, even in the absence of all three VAV proteins12. This is in contrast to a key role for VAV proteins in transducing signals from the T cell receptor (TCR) to ERK activation (see later), illustrating differences in signalling pathways between B and T cells. Microscopy studies have shown that B cells deficient in both VAV1 and VAV2 have defective BCR-induced cell adhesion and spreading on intercellular adhesion molecule 1 (ICAM1)-coated surfaces, decreased actin polymerization, and defective formation of signalling microclusters and the immunological synapse17,23.
The similarity of the BCR signalling defects between B cells deficient for VAV1 and VAV2 and B cells deficient for RAC1 and RAC2 indicates that these proteins lie in the same pathway, with VAV proteins transducing BCR signals to RAC activation, which in turn leads to calcium flux, activation of PI3K and increases in adhesion and actin polymerization.
In VAV1-deficient mice, both T-cell-dependent and T-cell-independent antibody responses were reduced9,20. This was further exacerbated in the absence of VAV2 and VAV310–12. The decreased T-cell-dependent response of VAV1-deficient mice was most probably due to defective help from VAV1-deficient T cells9.
Follicular B cells deficient in VAV1 and VAV2 were reported to have reduced lipopolysaccharide (LPS)-induced proliferation and activation of AKT and NF-κB, suggesting that VAV proteins transduce signals from the receptor for LPS, Toll-like receptor 4 (TLR4)11,24. By contrast, MZ B cells that lack all three VAV proteins had normal LPS-induced proliferation25.
αPIX and βPIX are GEFs for RAC1 and CDC42. Mice deficient in αPIX have reduced numbers of mature B cells in lymph nodes and defects in BCR-induced proliferation26. Deficiency of αPIX does not affect BCR-induced calcium flux, but results in decreased phosphorylation, and therefore activation, of PAK1 and PAK2, which are kinases that transduce signals to the regulation of the actin cytoskeleton (Figure 4 and Box 1).
B cell migration
RAC2-deficient follicular B cells have partially decreased migration in response to the chemokines CXC-chemokine ligand 12 (CXCL12) and CXCL13 in vitro, and reduced homing to lymph nodes in vivo7. A more severe phenotype is seen in transitional B cells deficient in both RAC1 and RAC2, which have a severe defect in migration from the blood through the red pulp of the spleen into the white pulp, and are very defective in chemotaxis in response to CXCL12, CXCL13 and CCL21, chemokines which direct the migration of B cells into follicles of secondary lymphoid tissue (R. Henderson and V. Tybulewicz, unpublished observations).
LSC is a haematopoietic-cell-restricted GEF for RHOA, which interacts with G-protein-coupled receptor (GPCR)-associated Gα12 and Gα13 proteins (Figure 5). In addition to its DH domain with GEF activity, LSC has an RGS domain, which binds to Gα proteins and increases their intrinsic GTPase activity, thereby turning off GPCR signalling. So LSC seems to carry out two functions. First, it transduces GPCR signals from Gα12/13 to the activation of RHOA and second, it attenuates other Gα12/13-coupled GPCR signals. In LSC-deficient mice, the number of B cells is reduced as are T-cell-dependent antibody responses to protein antigens, due to a cell-intrinsic requirement for LSC in B cells27,28. Further analysis has shown that LSC-deficient MZ B cells migrate more effectively to sphingosine 1-phosphate (S1P) in vitro, which might be due to the loss of LSC-mediated inhibition of signalling from Gα12/13-coupled S1P receptors. By contrast, LSC-deficient MZ B cells have a defect in their ability to detach from ICAM1, and in vivo, they do not migrate to follicles following LPS stimulation. These latter defects might reflect a requirement for LSC-mediated RHOA activation in the disengagement of integrin binding at the trailing edge of the migrating B cell28. Further understanding of LSC function would benefit from analysis of mutants that selectively perturb either the GEF or the RGS activity of LSC.
Figure 5.
Signal transduction from G-protein-coupled receptors and integrins. Receptors for sphingosine 1-phosphate (S1P) and thromboxane A2 (TXA2) are members of the G-protein-coupled receptor (GPCR) family, and signal through Gα12 and Gα13 proteins to LSC and RHOA activation. LSC inhibits Gα12/13 function through its RGS domain. Chemokine receptors signal through Gαi2 to DOCK2 and other guanine nucleotide-exchange factors (GEFs) leading to the activation of RAC1 and RHOA. These GTPases transduce signals to integrin activation through phospholipase D (PLD) and PIP5K1C. Integrin signalling is inhibited by RHOH through an unknown mechanism. Integrin signalling leads to the activation of the αPIX GEF and thus the activation of RAC1 and PAK. In myeloid cells, integrin signalling also leads to activation of SRC family kinases which phosphorylate immunoreceptor tyrosine-based activation motif (ITAM)-bearing receptor subunits, such as Fc receptor γ-chain (FcRγ), leading to recruitment and activation of spleen tyrosine kinase (SYK). SYK in turn phosphorylates SH2 domain-containing leukocyte protein of 76 kDa (SLP76) leading to assembly of a complex including PLCγ1, NCK and VAV1, and hence to activation of RAC1 and CDC42. It is not known if this latter pathway operates in lymphocytes.
DOCK2 is a RAC-specific GEF with crucial roles in chemokine receptor signalling (Figure 5). DOCK2-deficient B cells have greatly reduced chemotaxis in vitro in response to CXCL12 and CXCL13, and defective homing to lymph nodes and splenic white pulp in vivo29. The cells also show reduced chemokine-induced activation of RAC1 and RAP1, actin polymerization and integrin-mediated adhesion29,30. Intravital microscopy shows that DOCK2-deficient B cells have a reduced ability to adhere strongly to high endothelial venules (HEVs) of lymph nodes and show reduced interstitial mobility within lymph node follicles30,31. Finally, DOCK2 is also required for S1P-mediated egress of B cells from lymph nodes31.
B cells deficient in the RAC-specific GEF SWAP70 are defective in constitutive trafficking to lymph nodes, as well as in homing to inflamed lymph nodes32. Unlike DOCK2, SWAP70 is not required for chemokine-induced chemotaxis or adhesion to integrin ligands. Instead, SWAP70-deficient B cells have perturbed morphology, with unstable lamellipodia and fewer uropods. Intravital microscopy shows that SWAP70-deficient B cells roll and adhere normally to the HEVs of lymph nodes, but they are defective in transmigration32. The physiological effect of these defects is to impair the ability of B cells to participate in the germinal centre and memory responses to T-cell-dependent antigens33. Surprisingly, however, loss of SWAP70 results in increased numbers of high-affinity plasma cells in the bone marrow and splenic red pulp.
In summary, these studies show that in B cells, Rho GTPases lie at points of crosstalk between signalling pathways from multiple receptors. As a result, they have crucial roles in B cell development, migration and activation.
GTPases and their Regulators In T Cells
T cell development begins in the thymus where CD4-CD8- double negative (DN) thymocytes differentiate into CD4+CD8+ double positive (DP) cells and then undergo positive selection into either CD4+CD8- or CD4-CD8+ single positive (SP) thymocytes (Figure 6). These cells exit the thymus, becoming naïve CD4+ or CD8+ T cells, which continuously recirculate between secondary lymphoid organs. Activation of T cells through the TCR leads to their differentiation into effector and memory T cells.
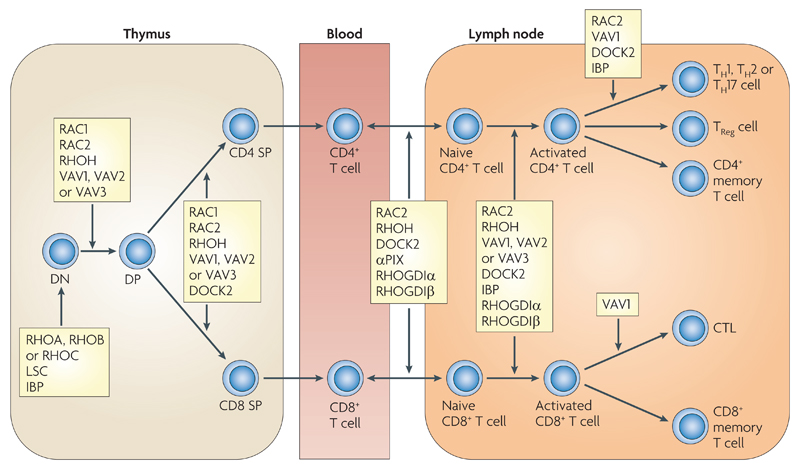
Figure 6.
Role of Rho GTPases and their regulators in T cell development and function. The earliest T cell progenitors, CD4-CD8- double negative (DN) thymocytes, rearrange T cell receptor β (TCRβ) chain genes. If they successfully generate a TCRβ chain, this assembles into the pre-TCR. Signals from the pre-TCR allow cells to differentiate into CD4+CD8+ double positive (DP) cells and proliferate. DP thymocytes rearrange TCRα chain genes, and if this process is successful, the TCRα chain pairs with TCRβto generate cell-surface-bound TCR. Signalling from the TCR on DP cells results in positive or negative selection depending on avidity of binding to self-peptide MHC complexes. DP cells with a MHC class I-restricted TCR differentiate into CD4-CD8+ single positive (CD8 SP) cells, whereas MHC class II-restricted cells are directed into CD4+CD8- single positive (CD4 SP) cells. SP thymocytes exit the thymus, migrate through the blood to secondary lymphoid organs, and become either CD4+ or CD8+ T cells. Activation of naïve CD4+ or CD8+ T cells (thick arrow) results in their proliferation and differentiation. CD4+ T cells can become T helper 1 (Th1), Th2 or Th17 cells, T regulatory (Treg) cells or memory T cells. CD8+ T cells differentiate into cytotoxic T lymphocytes (CTL) and memory T cells. Points where roles for Rho GTPases or their regulators have been identified are indicated and discussed more fully in the main text.
T cell development
Mice deficient in RHOC have no alterations in the development, activation or migration of B or T cells34. RHOB-deficient mice have been generated, but no analysis of the lymphoid compartment has been carried out35. Most information about the role of this subfamily of Rho GTPases has come from transgenic mice expressing in the T cell lineage either a constitutively active form of RHOA (V14RHOA) or Clostridium botulinum C3 transferase. C3 is a toxin that ADP-ribosylates RHOA, RHOB and RHOC in the effector-binding domains, thereby inactivating their function. Transgenic expression of V14RHOA results in more efficient positive selection of CD8+ SP thymocytes and T cells36. C3-transgenic mice have a marked inhibition of thymopoiesis, with a large decrease in the size of the DN type 2 (DN2) and DN3 subsets because of increased apoptosis, leading in turn to reduced numbers of DP and SP thymocytes and T cells37,38. Thymocytes expressing C3 have specific reductions in the expression of transcription factors from the EGR1 and AP1 families39. This may account for the developmental arrest in these mice, as these factors are important for the proliferation and differentiation of early thymocyte subsets.
Expression of a constitutively active form of RAC1 (L61RAC1) in the thymus can partially overcome the developmental block in recombination-activating gene 1 (RAG1)-deficient mice. Whereas in the absence of RAG1 T-cell development is arrested at the DN stage due to lack of a pre-TCR, expression of L61RAC1 caused the appearance of some DP thymocytes, although in smaller numbers than normally found in a wild-type thymus40. Furthermore, expression of L61RAC1 diverts DP thymocytes from positive to negative selection41. These results imply that RAC1 might normally have a role in these developmental transitions. By contrast, transgenic mice expressing a constitutively active form of RAC2 in the thymus had increased apoptosis of both DP and SP thymocytes 42. This could reflect a different function for RAC2 compared with RAC1, or might be due to different levels of expression of the transgenes. More recently, however, mice lacking RAC1 or RAC2 or both in the T cell lineage have been analysed. Whereas elimination of either GTPase alone has no effect on T cell development, in mice lacking both GTPases there is a marked block in development at the pre-TCR checkpoint, and also a defect in the positive selection of DP thymocytes, demonstrating functional redundancy between these highly related GTPases43,44. Unexpectedly, loss of both RAC1 and RAC2 leads to the aberrant appearance of a population of thymocytes that lack expression of TCRβ, with hallmarks of hyperactive signalling from Notch receptors, which have important roles in controlling differentiation of DN thymocytes45. These results suggest that RAC1 and RAC2 may negatively regulate Notch function during early thymic development.
T cell development in mice deficient in VAV1 is partially blocked at the pre-TCR checkpoint, resulting in decreased numbers of DP thymocytes46–49. Furthermore, there is also a marked defect in both positive and negative selection of DP thymocytes46,48,50. These defects are most probably due to a crucial role for VAV1 in transducing pre-TCR and TCR signals. In agreement with this, VAV1-deficient DP thymocytes are defective in TCR-induced calcium flux and TCR-induced activation of ERK MAP kinases, protein kinase D and PI3K46,51,52. The defects in calcium flux and ERK activation most probably result from defective activation of PLCγ1. Interestingly, the block at the pre-TCR checkpoint in VAV1-deficient mice can be rescued by transgenic expression of constitutively active RAC1, suggesting that VAV1 transduces pre-TCR signals to the activation of RAC140. These blocks in thymic development are more pronounced in the absence of all three VAV proteins, resulting in an almost complete block in development at the pre-TCR and TCR checkpoints and arguing strongly for overlapping redundant functions between these three related GEFs12. In contrast to its role in the development of T cells expressing αβTCRs, VAV1 is not required for the development of γδTCR-expressing T cells53. Nonetheless, VAV1-deficient γδ T cells are unable to proliferate or secrete cytokines normally in response to TCR stimulation.
In the absence of the RhoGEF LSC, thymocyte numbers are increased, most probably because of defective apoptosis54. This might be due to the reduced apoptosis of LSC-deficient thymocytes in response to thromboxane A2 (TXA2), a prostanoid which signals through the Gα12- and Gα13-coupled thromboxane prostanoid receptor (TPR) leading to cell death (Figure 5). LSC selectively couples to only a subset of TPR signalling pathways. So whereas TXA2-induced RHOA activation, actin polymerization and cofilin phosphorylation were all reduced in the absence of LSC, there was no effect on TXA2-induced activation of ERK or p38 MAP kinase pathways54. By contrast, lack of LSC had no effect on TCR-induced apoptosis, demonstrating selective coupling of GEFs to specific receptors.
DBS is a GEF for RHOA and CDC42, which is expressed throughout thymocyte development. Transgenic mice expressing an activated form of DBS had larger numbers of CD4-CD8- DN thymocytes, but increased apoptosis in later DP and SP thymocyte subsets55.
DOCK2-deficient mice have impaired positive and negative selection of DP thymocytes56. In particular, there is a large reduction in the number of CD1d-restricted NKT cells expressing a Vα14+ TCR. It is unclear why this subset is affected disproportionately57.
Finally, in mice deficient in IBP (also known as SLAT and DEF6), a SWAP70-related GEF that acts on both RAC1 and CDC4258–60, one study found that T cell development was normal61, whereas a second report showed that lack of IBP results in a smaller thymus due to reduced numbers of the earliest DN1 cells62.
T cell activation
RAC2-deficient T cells have a partial defect in TCR-induced activation and proliferation63. This is accompanied by a small decrease in TCR-induced calcium flux and TCR-induced activation of ERK and p38 MAP kinase pathways. It is notable that this phenotype is much milder than that seen in the absence of the RacGEF VAV1, implying that in the absence of RAC2, other GTPases might have an important role. In view of the marked redundancy between RAC1 and RAC2 in T cell development43,44, it is probable that the same is true for T cell activation.
The RHOH GTPase is expressed predominantly in the haematopoietic system and is atypical in that it mainly binds GTP. RHOH-deficient T cells are defective in TCR-induced activation and proliferation64,65. In the absence of RHOH, TCR-induced calcium flux and ERK activation are reduced, as is the recruitment of the tyrosine kinase ZAP70 (ζ-chain-associated protein kinase of 70 kDa) to the plasma membrane, most probably because RHOH acts as an adaptor (Figure 7). RHOH contains two tyrosines, which are phosphorylated following TCR engagement and function as binding sites for the SH2 domains of ZAP7065. Thus, in the absence of RHOH, there is less ZAP70 recruitment to the plasma membrane and hence less signal transduction to downstream components such as ERK. This may account for the marked developmental block in the thymus of RHOH-deficient mice at both the pre-TCR and positive selection checkpoints, resembling that seen in ZAP70-deficient mice64,65. These studies exemplify another emerging theme where Rho GTPases or their regulators have functions that are unrelated to their canonical GTPase roles.
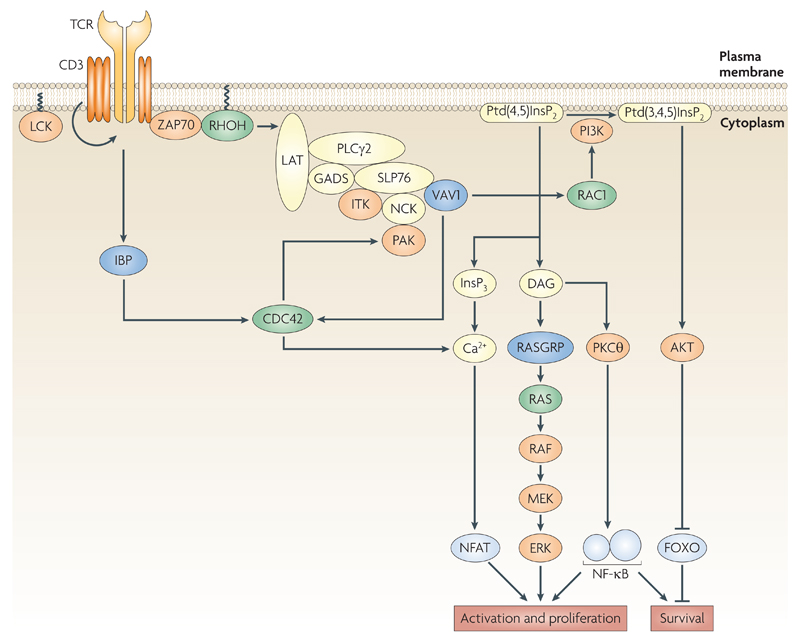
Figure 7.
Signal transduction from the T cell receptor. Binding of peptide:MHC complex to the T cell receptor (TCR) leads to activation of the tyrosine kinases LCK and ZAP70 (ζ-chain-associated protein kinase of 70 kDa), phosphorylation of the adaptor protein LAT (linker for activation of T cells) and assembly of a complex including the adaptors GADS, SLP76 and NCK, the ITK and PAK kinases, VAV1 and PLCγ1. Activation of PLCγ1 leads to hydrolysis of PIP2 to the second messengers InsP3 and DAG. VAV1 may contribute to the activation of PLCγ2 through an adaptor function, and, via RAC1 to increased synthesis of PIP3, potentially by activating phosphatidylinositol-3-kinase (PI3K). Increased levels of InsP3 lead to a rise in intracellular Ca2+ and eventually to activation of the NFAT transcription factor. DAG activates the Ras exchange factor RASGRP, leading to activation of RAS and the RAF, MEK, ERK kinase cascade. DAG may also contribute to activation of NFκB via PKCθ. PIP3 leads to activation of the AKT kinase which signals cell survival via inhibition of the FOXO1 transcription factor. TCR signalling via IBP leads to CDC42 activation and increased intracellular Ca2+.
Stimulation of T cells through the TCR results in rapid tyrosine phosphorylation of VAV1, which activates its GEF activity towards RAC1, CDC42 and RHOA66–69. VAV1-deficient T cells proliferate less well and secrete less IL-2 in response to stimulation through the TCR47,49,70–72. In the absence of VAV1, there is a reduction of TCR-induced calcium flux, and less activation of ERK and AKT and the transcription factors nuclear factor of activated T cells (NFAT), NF-κB and serum response factor (SRF)70,71,73–75 (Figure 7). Similar changes were also seen in a VAV1-deficient Jurkat T cell line76. Defects in both calcium flux and ERK activation in the absence of VAV1 seem to be secondary to a failure to activate PLCγ1, which results in decreased production of InsP3 and DAG52. Whereas InsP3 is required for calcium flux, DAG activates RASGRP (a RasGEF) and therefore RAS, B-RAF, MEK and ERK51. The defective TCR-induced activation of AKT in VAV1-deficient T cells is probably due to reduced PI3K activation, and in turn results in reduced phosphorylation and nuclear translocation of the transcription factor FOXO1, and thus less induction of the cell cycle inhibitor p27Kip1 (REFS 52,75). However one study has failed to find a role for VAV1 in TCR-induced activation of AKT77. VAV1 may control the activation of NF-κB by associating with PKCθ, and recruiting it to the plasma membrane78,79. Interestingly, caspase 3-mediated cleavage of VAV1 has been proposed to contribute to unresponsiveness to TCR stimulation in anergic T cells80.
VAV1 is required to transduce inside-out signals leading to the activation of LFA1, and hence to adhesion between T cells and antigen-presenting cells (APCs)81,82. In the absence of VAV1 there is less actin polymerization, less clustering of TCR and lipid rafts at the immunological synapse and reduced movement of the microtubule organizing centre towards the T cell interface with the APC71,81–84. VAV1 may control the reorganization of the actin cytoskeleton through its GEF activity on RAC1, or through its association with dynamin 2 or the EZH2 methyltransferase85,86. Finally, VAV1 and RAC1 have been shown to transduce TCR signals leading to the dephosphorylation and inactivation of ezrin, radixin and moesin (ERM) proteins, which act as linkers between the actin cytoskeleton and the plasma membrane, thereby leading to a transient increase in cell deformability and more efficient conjugate formation87.
A recent study using T cells from mice with a knock-in mutation that eliminates the GEF activity of VAV1, but leaves its other domains intact, has shown that the GEF activity is required for TCR-induced activation of AKT, but not for calcium flux or activation of the ERK MAP kinase pathway, demonstrating that VAV1 must have GEF-independent functions (A. Saveliev and V. Tybulewicz, unpublished observations). VAV1 could act as an adaptor protein that controls the formation of a signalling complex that is required to activate PLCγ152,88,89. Some evidence for this has come from studies showing that overexpression of a GEF-inactive VAV1 in Jurkat T cells results in an increase in NFAT-dependent gene transcription90. Furthermore, mutation of the calponin homology domain interferes with the ability of VAV1 to activate calcium flux in Jurkat T cells, suggesting that this domain may be involved in the adaptor function of VAV176,91. This work provides another example of a GTPase regulator whose function is in part unrelated to its canonical role as a GEF.
Most VAV1 is localized to the cytoplasm in naive unstimulated T cells, and is recruited to the plasma membrane following TCR stimulation, however VAV1 might also have a role in the nucleus92. In a surprising finding, CD28 stimulation in T cells was shown to result in the methylation of VAV1 on an arginine residue93. The significance of this modification remains unknown, but, interestingly, methylated VAV1 is selectively localized to the nucleus.
Mice deficient in αPIX have reduced numbers of mature T cells, and these show defective TCR-induced proliferation and cytokine secretion26. Analysis showed that many TCR proximal signalling events were not disturbed by the absence of αPIX, including TCR-induced activation of both RAC1 and CDC42, showing that αPIX does not transduce TCR signals to the activation of these GTPases. However αPIX-deficient T cells have defective TCR-induced phosphorylation of PAK1 and PAK2 and less recruitment of these kinases to the immunological synapse, probably through a GIT-αPIX-PAK1 complex26,94. They are also less efficient at forming antigen-specific conjugates with APCs, which may be due to defective inside-out activation of LFA1, and reduced recruitment and clustering of LFA1 at the immunological synapse26.
DOCK2-deficient T cells have defects in TCR-induced proliferation56. Analysis of signalling showed that in the absence of DOCK2, TCR-induced calcium flux and the activation of LCK, ZAP70, JNK, PYK2, AKT and PLCγ1 were all unaffected. However, DOCK2-deficient T cells had reduced TCR-induced activation of RAC1, RAC2 and ERK, as well as impaired immunological synapse formation. Although DOCK2-deficient T cells formed antigen-specific conjugates normally with APCs, and showed the expected polarization of LFA1 and PKCθ to the synapse, there was greatly reduced recruitment of both the TCR and of lipid rafts to the T cell–APC interface56. It remains unknown how DOCK2 contributes to this process. It is curious that two GEFs, VAV1 and DOCK2, are both required for TCR-induced RAC1 activation. It could be that they function together, a possibility suggested by the observation that DOCK2 and VAV1 bind to each other95.
IBP-deficient CD4+ T cells have reduced TCR-induced proliferation, at high doses of TCR stimulation, but increased proliferation at low doses61,62,96. IBP is required for TCR-induced calcium flux, ERK activation and nuclear translocation of the NFAT transcription factor62,97. The defect in NFAT nuclear translocation seen in IBP-deficient T cells can be rescued either by a membrane-tethered domain of IBP containing the GEF activity or by a constitutively active form of CDC42. Constitutively active CDC42 also restores the TCR-induced calcium flux. Taken together these results suggest that the GEF activity of IBP regulates NFAT activation through CDC42 and an increase in intracellular calcium (Figure 7).
In mice deficient for RHOGDIα and RHOGDIβ, there are decreased numbers of T cells and these have reduced TCR-induced proliferation98. These mutant T cells have decreased levels of RHOA, RAC1 and CDC42 proteins, perhaps because of increased proteolysis, although a larger fraction of the remaining GTPases is in the active GTP-bound state. It is likely that the phenotype of these mice is a complex superposition of partial disruptions to the function of the multiple GTPases that bind GDIs.
While most analysis has been carried out on VAV1, it is clear that other GEFs (αPIX, DOCK2 and IBP) also transduce crucial signals during T cell activation and development. The interplay between these GEFs remains unclear.
T cell differentiation and function of effector subsets
RAC2 is expressed at higher levels in T helper 1 (Th1) cells than in Th2 cells99. This expression is important as RAC2-deficient T cells have selective defects in Th1 cell differentiation and interferon-γ (IFNγ) production99. However a subsequent study showed only a minor skewing of the T cell response towards the Th2 cell lineage in RAC2-deficient mice challenged with Leishmania major100.
In vitro culture of VAV1-deficient T cells under neutral or Th2-cell-polarizing conditions resulted in reduced expression of IL-4, suggesting that VAV1 is required for normal Th2 cell differentiation101. Furthermore, VAV1 is required in CD4+ T cells for IL-4 production in vivo, which could account for the defective T-cell-dependent antibody responses seen in VAV1-deficient mice9. However, VAV1 is also involved in Th1 and/or Th17 cell responses, as VAV1-deficient mice are resistant to experimental autoimmune encephalomyelitis, showing inefficient priming and expansion of antigen-specific T cells and reduced production of IL-2, IL-4 and IFNγ102.
VAV1 is important for primary antiviral CD8+ cytotoxic T cell responses, which are reduced in the absence of VAV172. However secondary recall responses are normal, demonstrating that the loss of VAV1 only partially disrupts T cell activation. Finally, mice deficient in either VAV1 alone or VAV1 and VAV2 show long-term survival of MHC-mismatched cardiac transplants, most probably due to defective allogeneic T cell responses103.
DOCK2-deficient mice develop an allergic disease that is characterized by increased numbers of Th2 cells104. In the absence of DOCK2, internalized IL-4Rα is re-routed from lysosomes to recycling endosomes, and is therefore not degraded, but re-expressed on the cell surface, thereby promoting Th2 cell differentiation. DOCK2 may control this trafficking by regulating microtubule dynamics through phosphorylation and inactivation of stathmin104.
Loss of the SWAP70-like GEF IBP results in a reduction of both Th1 and Th2 cell responses62. In vitro culture of IBP-deficient CD4+ T cells in polarizing conditions showed decreased production of both IFNγ and IL-4, which may be due to defects in TCR-induced activation of NFAT transcription factors62. However, another study found no defects in IL-4 production and reported reduced IFNγ secretion in T cells cultured in non-polarizing conditions61. Female IBP-deficient mice over 5 months of age develop autoantibodies and an accumulation of effector/memory T cells and IgG1+ B cells61. Interestingly, when the same genetic mutation was crossed to transgenic mice expressing a monoclonal MHC class II-restricted TCR, the mice spontaneously develop an inflammatory arthritic disease characterized by hyperresponsive T cells producing increased quantities of IL-17 and IL-21, and an apparent skewing of T cell differentiation to the Th17 cell lineage96. This effect seems to be due to the regulation of the transcription factor interferon-regulatory factor 4 (IRF4) by IBP, which binds IRF4 and inhibits its function96. It is unclear whether this inhibitory function requires the GEF activity of IBP. In the absence of IBP, IRF4 activates the expression of IL-17 and IL-21, as well as retinoic acid receptor-related orphan receptor-γt (RORγt), a transcription factor that is required for the development of Th17 cells. Thus IBP contributes to T helper cell differentiation, and at least some of its functions may be GEF independent.
T cell migration
GTPases of the RHOA subfamily have an important role in the regulation of integrin-mediated adhesion. Transgenic expression of constitutively active V14RHOA results in increased adhesion of both thymocytes and T cells through β1 and β2 integrins, whereas expression of C3 transferase, the RHOA/B/C inhibitor causes decreased adhesion105. Thymocytes expressing C3 transferase also show reduced chemotaxis in response to CXCL12 and CCL25 when migrating across membranes coated in fibronectin, a ligand for β1 integrins.
RAC2-deficient T cells show defects in chemotaxis to the lymphoid chemokines CCL19 and CCL21, and a mildly reduced ability to home to lymph nodes100. The partial nature of these phenotypes suggests that there might be redundancy between RAC2 and other GTPases (such as RAC1) in transducing chemokine receptor signals. Studies in human T cells show that both RAC1 and RHOA are required to transduce chemokine receptor signals to the activation of LFA1 via PLD and PIP5K1C106 (Figure 5).
In the Jurkat T cell line, mutagenesis of RHOH showed that it is required for the maintenance of LFA1 in a non-adhesive state107 (Figure 5). A similar result was found using RNA interference to knockdown expression of RHOH in primary human T cells, which resulted in increased LFA1-mediated adhesion. These opposing functions of RHOA and RHOH in integrin regulation illustrate the complexity of Rho GTPase function.
Stimulation of human T cells with CXCL12 causes VAV1 to become phosphorylated and to relocalize to the leading edge of the cell108. Expression of a mutant form of VAV1 in these cells impairs CXCL12-induced cell polarization and migration, implying that VAV1 may be important for transducing signals from CXCR4 (the receptor for CXCL12). However, VAV1-deficient mouse T cells have normal chemotactic responses to CXCL12108. This difference may be due to functional redundancy with VAV2 and VAV3.
DOCK2 is required for the in vitro polarization and migration of T cells in response to CCL21 and CXCL12, and for their efficient homing to lymph nodes and splenic white pulp in vivo29,30. Furthermore, intravital microscopy showed that DOCK2-deficient T cells had reduced interstitial movement within lymph nodes31. In the absence of DOCK2, T cells have normal CXCL12-induced calcium flux and activation of AKT and ERK, but reduced RAC1 activation and actin polymerization. In contrast to the requirement for DOCK2 in B cells for chemokine-induced adhesion to integrin ligands, DOCK2-deficient T cells adhere normally in vitro30. Furthermore, lack of DOCK2 does not affect either the rolling and arrest of T cells in HEVs of lymph nodes, or their subsequent transmigration through the endothelial cell layer30,109. However DOCK2 is required for the lateral mobility of T cells on both the apical and basal endothelial surfaces, and this could explain the inefficient homing of T cells to peripheral lymphoid organs in the absence of DOCK2109. DOCK2 is also required for S1P-dependent egress of T cells from secondary lymphoid organs31.
Finally, αPIX-deficient T cells migrate more efficiently both in the presence and absence of chemokines, and T cells deficient in both RHOGDIα and RHOGDIβ migrate poorly in response to CXCL12, CCL19 and CCL2126,98.
Lymphocyte polarization is crucial for in vivo migration. Polarized T cells are characterized by an actin-dependent leading edge, and a uropod and MTOC at the rear. Rho GTPase signalling is compartmentalized in these distinct regions, with RAC1 and CDC42 acting at the leading edge through WASP and WAVE, and Rho at the rear acting through ROCK110 (Box 1). Lymphocyte polarity has not been analysed in GTPase-deficient mice, however expression of dominant negative and constitutively active GTPases demonstrates that RAC1, RHOA and CDC42 are required to establish polarity in T cell lines111–113. T cells deficient for the RAC1 GEF T-cell lymphoma invasion and metastasis 1 (TIAM) show reduced polarization and chemotaxis to CXCL12. In addition to activating RAC1, TIAM1 may function as a scaffold protein, associating with active RAP1 which activates the PAR polarity complex through CDC42114. Genetic dissection of the proposed associations of TIAM1 with RAP1 and the polarity complex would be informative. In a assay of T cell shape regulation, αPIX and PAK1 were shown to be important for CXCL12-induced migration through restrictive barriers115. Imaging analysis of GTPase activity in distinct subcellular localizations within lymphocytes would greatly improve our understanding of the roles of individual GTPases in T cell polarization.
In summary, a complex picture is emerging of the roles of Rho GTPases and their regulators in T cell biology. The multiplicity of processes perturbed by deficiencies of these proteins support the idea that they are involved in cross-talk between multiple signalling pathways.
Future Directions and Implications
Analysis of the lymphoid system in mice deficient in Rho GTPases or their regulators has revealed important roles for these proteins in signalling through antigen receptors, chemokine receptors and integrins, with critical functions in development, activation, differentiation and migration.
Several themes have emerged from these studies. First, there is often redundancy between related proteins (for example, RAC1 and RAC2). Second, the GTPases seem to lie at focal points of crosstalk between signalling pathways, which may explain the large number of GEFs and GAPs compared to GTPases. Finally, in many cases proteins in these families have functions unrelated to the regulation of GTPases. For example, VAV1 and IBP may have GEF-independent functions and RHOH acts as an adaptor protein.
Several techniques will be essential to the resolution of these issues. Tissue-specific or inducible gene inactivation will be an essential tool in establishing cell-intrinsic roles for proteins, and for avoiding lethality and/or developmental blocks, both of which can preclude use of conventional knockout mice. The use of gene targeting to “knock-in” point mutations will be an invaluable technique to dissect the role of different signalling inputs and outputs from the same GTPase116. This can also be used to distinguish different functions within the same signaling molecule, for example determining which aspects of a GEF’s function require its enzymatic activity, and which might be due to adaptor function. It is possible that some of the GTPases or their regulators are compartmentalized in several intracellular pools, which would have distinct functions. Resolution of such pools will require careful biochemical or imaging analysis with higher resolution techniques than are currently available.
We note that at least 90 of these proteins are expressed in lymphocytes (Supplementary Table 1) and, for the majority, their roles in lymphocyte development and function have not yet been analysed. In many cases, suitable genetically modified mice have not been generated. Clearly, the analysis of the in vivo function of this key family of signal transducers has only just begun and far more remains to be discovered.
Supplementary Material
Online summary.
In mammals, the Rho family of GTPases has 23 members, which are regulated by 79 guanine nucleotide exchange factors (GEFs), 65 GTPase activating proteins (GAPs) and 3 guanine nucleotide dissociation inhibitors (GDIs). About half of these are expressed in lymphocytes.
RAC1 and RAC2 have overlapping redundant roles in both B and T cell development, activation and migration.
RHOH has an important role in T cell activation as an adaptor protein recruiting ζ-chain-associated protein kinase of 70 kDa (ZAP70) to the plasma membrane.
The VAV1, VAV2 and VAV3 GEFs have overlapping redundant roles in both B and T cell development, and activation but not migration. VAV1 has GEF-independent functions, possibly as an adaptor protein.
The DOCK2 GEF is a crucial transducer of chemokine receptor signals in both B and T cells, and is also required for T cell activation.
The SWAP70 GEF is required for polarization of B cells and transmigration across high endothelial venules. The related IBP GEF regulates T helper cell differentiation, in part by binding the transcription factor interferon-regulatory factor 4, a function that may be GEF independent.
Acknowledgements
We thank S. Saveliev and E. Schweighoffer for critical reading of the manuscript, A. Ridley for helpful discussions, and S. Shah (Bloomsbury Centre for Bioinformatics, UCL) for help in analysis of transcriptome data.
Glossary
- prenyl group
An isoprenoid lipid, either farnesyl or geranylgeranyl. Most Rho proteins are modified by the addition of a prenyl group at their C termini. This modification anchors Rho GTPases in membranes.
- follicular B cell
A recirculating mature B-cell subset that populates the follicles of secondary lymphoid organs such as the spleen, lymph nodes and Peyer’s patches.
- Marginal zone B cell
A non-circulating B-cell subset found predominantly in the marginal zone of the spleen which is located between the non-lymphoid red pulp and the lymphocyte-rich white pulp. These cells are thought to mount early responses to blood-borne antigens.
- secondary lymphoid organs
Organs in which naïve B and T cells reside such as spleen, lymph nodes, Peyer’s patches.
- B1 cell
A cell belonging to the B-cell lineage that populates the peritoneal and pleural cavities and secretes polyspecific, low-affinity IgM.
- transitional B cell
An immature B-cell subset found in the spleen, recently arrived from the bone marrow. These cells are short-lived, with half-lives of 2–4 days, which mature into follicular or marginal zone B cells.
- immunological synapse
A region that can form between two cells of the immune system in close contact. The immunological synapse originally referred to the interaction between a T cell and an antigen-presenting cell. It involves adhesion molecules, as well as antigen receptors and cytokine receptors. The immunological synapse is characterized by polarization of the lymphocyte resulting, for example, in directed secretion towards the antigen presenting cell.
- G-protein-coupled receptor
(GPCR). One of a large group of receptors that bind a diverse set of molecules, including chemokines, complement components, biologically active amines and neurotransmitters. GPCRs are seven-transmembrane-spanning receptors and are coupled to heterotrimeric, GTP-regulated signalling proteins.
- sphingosine 1-phosphate
(S1P). A sphingolipid involved in signalling. In the immune system, S1P induces egress of lymphocytes from lymphoid organs by binding to SIP receptors on the cells.
- Intravital microscopy
This is used for examination of biological processes, such as leukocyte–endothelial-cell interactions, in living tissue. In general, translucent tissues are used, such as the mesentery or cremaster muscle, or lymph nodes, which can be exposed and mounted for microscopic observation.
- high endothelial venules
(HEVs). Specialized venules with a cuboidal endothelial lining. They are found in peripheral lymph nodes and Peyer’s patches. HEVs are the sites of entry of lymphocytes into lymphoid tissues from the bloodstream.
- lamellipodia
Flattened, sheet-like protrusions that are supported by a meshwork of F-actin and extend at the leading edge of crawling cells.
- uropods
Slender protrusions that form at the rear end of migrating leukocytes.
- positive selection
A process that leads to the survival of immature thymocytes that express a T-cell receptor that binds with an appropriate affinity to self MHC.
- negative selection
The process in the thymus that eliminates thymocytes that express T-cell receptors with high affinity for self antigens.
- γδ T cells
T cells that express the γδT-cell receptor. These T cells are present in the skin, vagina and intestinal epithelium as intraepithelial lymphocytes (IELs). Although the exact function of γδT cells is unknown, it has been suggested that mucosal γδT cells are involved in innate immune responses.
- microtubule-organizing centre
A region of the cell from which microtubules grow.
- ERM proteins
Ezrin, radixin and moesin (ERM) proteins act as general cytolinkers between the cortical actin-filament network and the plasma membrane, and are thought to function mainly through their ability to bind both F-actin and the cytoplasmic regions of integral membrane proteins.
- Experimental autoimmune encephalomyelitis
(EAE). An experimental model of the human disease multiple sclerosis. EAE is an autoimmune disease mediated by CD4+ T helper 1 (Th1) cells and interleukin-17-producing Th17 cells reactive to components of the myelin sheath that infiltrate the nervous parenchyma, release pro-inflammatory cytokines and chemokines, promote leukocyte infiltration and contribute to demyelination.
Biographies
Author Biographies
Victor Tybulewicz
Victor Tybulewicz is Head of the Division of Immune Cell Biology at the MRC National Institute for Medical Research in London, UK. He obtained his PhD at the MRC Laboratory of Molecular Biology, Cambridge, UK studying the ATP synthase, and then trained as a postdoctoral fellow at the Whitehead Institute, Cambridge, MA, USA developing mouse gene targeting techniques. His current interests are in lymphocyte signal transduction and the genetics of Down’s syndrome.
Robert B. Henderson
Robert Henderson received his PhD from Leeds University and then trained as a postdoctoral fellow at the CRUK London Research Institute studying integrin regulation of myeloid cell migration. Currently he is a scientific investigator at the MRC National Institute of Medical Research, London UK, studying the role of Rac-dependent migration in B cell development.
References
- 1.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 2.Wennerberg K, Der CJ. Rho-family GTPases: it's not only Rac and Rho (and I like it) J Cell Sci. 2004;117:1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- 3.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 5.Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. 2007;99:67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- 6.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Croker BA, et al. The Rac2 guanosine triphosphatase regulates B lymphocyte antigen receptor responses and chemotaxis and is required for establishment of B-1a and marginal zone B lymphocytes. J Immunol. 2002;168:3376–3386. doi: 10.4049/jimmunol.168.7.3376. [DOI] [PubMed] [Google Scholar]
- 8.Walmsley MJ, et al. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [Reference 8, together with references 43 and 44 examine the effects of removing RAC1 and RAC2 on B and T cell development.] [DOI] [PubMed] [Google Scholar]
- 9.Gulbranson-Judge A, et al. Defective immunoglobulin class switching in Vav-deficient mice is attributable to compromised T cell help. Eur J Immunol. 1999;29:477–487. doi: 10.1002/(SICI)1521-4141(199902)29:02<477::AID-IMMU477>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 10.Doody GM, et al. Signal transduction through Vav-2 participates in humoral immune responses and B cell maturation. Nat Immunol. 2001;2:542–547. doi: 10.1038/88748. [DOI] [PubMed] [Google Scholar]
- 11.Tedford K, et al. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat Immunol. 2001;2:548–555. doi: 10.1038/88756. [DOI] [PubMed] [Google Scholar]
- 12.Fujikawa K, et al. Vav1/2/3-null Mice Define an Essential Role for Vav Family Proteins in Lymphocyte Development and Activation but a Differential Requirement in MAPK Signaling in T and B Cells. J Exp Med. 2003;198:1595–1608. doi: 10.1084/jem.20030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vigorito E, Gambardella L, Colucci F, McAdam S, Turner M. Vav proteins regulate peripheral B-cell survival. Blood. 2005;106:2391–2398. doi: 10.1182/blood-2004-12-4894. [DOI] [PubMed] [Google Scholar]
- 14.Yamada T, Kurosaki T, Hikida M. Essential roles of mgcRacGAP in multilineage differentiation and survival of murine hematopoietic cells. Biochem Biophys Res Commun. 2008;372:941–946. doi: 10.1016/j.bbrc.2008.05.170. [DOI] [PubMed] [Google Scholar]
- 15.Kawashima T, et al. A Rac GTPase-activating protein, MgcRacGAP, is a nuclear localizing signal-containing nuclear chaperone in the activation of STAT transcription factors. Mol Cell Biol. 2009;29:1796–1813. doi: 10.1128/MCB.01423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saci A, Carpenter CL. RhoA GTPase regulates B cell receptor signaling. Mol Cell. 2005;17:205–214. doi: 10.1016/j.molcel.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Arana E, et al. Activation of the small GTPase Rac2 via the B cell receptor regulates B cell adhesion and immunological-synapse formation. Immunity. 2008;28:88–99. doi: 10.1016/j.immuni.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T-cell receptor signaling to integrins. Immunol Rev. 2007;218:65–81. doi: 10.1111/j.1600-065X.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 19.Vigorito E, et al. Immunological function in mice lacking the Rac-related GTPase RhoG. Mol Cell Biol. 2004;24:719–729. doi: 10.1128/MCB.24.2.719-729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachmann MF, et al. The guanine-nucleotide exchange factor Vav is a crucial regulator of B cell receptor activation and B cell responses to nonrepetitive antigens. J Immunol. 1999;163:137–142. [PubMed] [Google Scholar]
- 21.Inabe K, et al. Vav3 modulates B cell receptor responses by regulating phosphoinositide 3-kinase activation. J Exp Med. 2002;195:189–200. doi: 10.1084/jem.20011571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vigorito E, et al. Vav-dependent and vav-independent phosphatidylinositol 3-kinase activation in murine B cells determined by the nature of the stimulus. J Immunol. 2004;173:3209–3214. doi: 10.4049/jimmunol.173.5.3209. [DOI] [PubMed] [Google Scholar]
- 23.Weber M, et al. Phospholipase C-gamma2 and Vav cooperate within signaling microclusters to propagate B cell spreading in response to membrane-bound antigen. J Exp Med. 2008;205:853–868. doi: 10.1084/jem.20072619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hebeis B, Vigorito E, Kovesdi D, Turner M. Vav proteins are required for B-lymphocyte responses to LPS. Blood. 2005;106:635–640. doi: 10.1182/blood-2004-10-3919. [DOI] [PubMed] [Google Scholar]
- 25.Stephenson LM, Miletic AV, Kloeppel T, Kusin S, Swat W. Vav proteins regulate the plasma cell program and secretory Ig production. J Immunol. 2006;177:8620–8625. doi: 10.4049/jimmunol.177.12.8620. [DOI] [PubMed] [Google Scholar]
- 26.Missy K, et al. AlphaPIX Rho GTPase guanine nucleotide exchange factor regulates lymphocyte functions and antigen receptor signaling. Mol Cell Biol. 2008;28:3776–3789. doi: 10.1128/MCB.00507-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girkontaite I, et al. Lsc is required for marginal zone B cells, regulation of lymphocyte motility and immune responses. Nat Immunol. 2001;2:855–862. doi: 10.1038/ni0901-855. [DOI] [PubMed] [Google Scholar]
- 28.Rubtsov A, et al. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity. 2005;23:527–538. doi: 10.1016/j.immuni.2005.09.018. [References 27 and 28 analyse the role of the RHOA GEF LSC in B cell development and provide insights into the regulation of marginal zone B cell migration and retention.] [DOI] [PubMed] [Google Scholar]
- 29.Fukui Y, et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412:826–831. doi: 10.1038/35090591. [First description of the crucial role of DOCK2 in lymphocyte migration, which has since been analysed further in great detail.] [DOI] [PubMed] [Google Scholar]
- 30.Nombela-Arrieta C, et al. Differential requirements for DOCK2 and phosphoinositide-3-kinase γ during T and B lymphocyte homing. Immunity. 2004;21:429–441. doi: 10.1016/j.immuni.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Nombela-Arrieta C, et al. A central role for DOCK2 during interstitial lymphocyte motility and sphingosine-1-phosphate-mediated egress. J Exp Med. 2007;204:497–510. doi: 10.1084/jem.20061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce G, et al. Signaling protein SWAP-70 is required for efficient B cell homing to lymphoid organs. Nat Immunol. 2006;7:827–834. doi: 10.1038/ni1365. [DOI] [PubMed] [Google Scholar]
- 33.Quemeneur L, Angeli V, Chopin M, Jessberger R. SWAP-70 deficiency causes high-affinity plasma cell generation despite impaired germinal center formation. Blood. 2008;111:2714–2724. doi: 10.1182/blood-2007-07-102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hakem A, et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 2005;19:1974–1979. doi: 10.1101/gad.1310805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu AX, Rane N, Liu JP, Prendergast GC. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol Cell Biol. 2001;21:6906–6912. doi: 10.1128/MCB.21.20.6906-6912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corre I, Gomez M, Vielkind S, Cantrell DA. Analysis of thymocyte development reveals that the GTPase RhoA is a positive regulator of T cell receptor responses in vivo. J Exp Med. 2001;194:903–914. doi: 10.1084/jem.194.7.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galandrini R, Henning SW, Cantrell DA. Different functions of the GTPase Rho in prothymocytes and late pre-T cells. Immunity. 1997;7:163–174. doi: 10.1016/s1074-7613(00)80519-1. [DOI] [PubMed] [Google Scholar]
- 38.Henning SW, Galandrini R, Hall A, Cantrell DA. The GTPase Rho has a critical regulatory role in thymus development. EMBO J. 1997;16:2397–2407. doi: 10.1093/emboj/16.9.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullin M, et al. The RhoA transcriptional program in pre-T cells. FEBS Lett. 2007;581:4309–4317. doi: 10.1016/j.febslet.2007.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez M, Tybulewicz V, Cantrell DA. Control of pre-T cell proliferation and differentiation by the GTPase Rac-1. Nat Immunol. 2000;1:348–352. doi: 10.1038/79808. [DOI] [PubMed] [Google Scholar]
- 41.Gomez M, Kioussis D, Cantrell DA. The GTPase Rac-1 controls cell fate in the thymus by diverting thymocytes from positive to negative selection. Immunity. 2001;15:703–713. doi: 10.1016/s1074-7613(01)00235-7. [DOI] [PubMed] [Google Scholar]
- 42.Lores P, Morin L, Luna R, Gacon G. Enhanced apoptosis in the thymus of transgenic mice expressing constitutively activated forms of human Rac2GTPase. Oncogene. 1997;15:601–605. doi: 10.1038/sj.onc.1201378. [DOI] [PubMed] [Google Scholar]
- 43.Dumont C, et al. Rac GTPases play critical roles in early T cell development. Blood. 2009;113:3990–3998. doi: 10.1182/blood-2008-09-181180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo F, Cancelas JA, Hildeman D, Williams DA, Zheng Y. Rac GTPase isoforms Rac1 and Rac2 play a redundant and crucial role in T-cell development. Blood. 2008;112:1767–1775. doi: 10.1182/blood-2008-01-132068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radtke F, Wilson A, Mancini SJ, MacDonald HR. Notch regulation of lymphocyte development and function. Nat Immunol. 2004;5:247–253. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- 46.Fischer KD, et al. Defective T-cell receptor signalling and positive selection of Vav-deficient CD4+ CD8+ thymocytes. Nature. 1995;374:474–477. doi: 10.1038/374474a0. [DOI] [PubMed] [Google Scholar]
- 47.Tarakhovsky A, et al. Defective antigen receptor-mediated proliferation of B and T cells in the absence of Vav. Nature. 1995;374:467–470. doi: 10.1038/374467a0. [DOI] [PubMed] [Google Scholar]
- 48.Turner M, et al. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity. 1997;7:451–460. doi: 10.1016/s1074-7613(00)80367-2. [DOI] [PubMed] [Google Scholar]
- 49.Zhang R, Alt FW, Davidson L, Orkin SH, Swat W. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature. 1995;374:470–473. doi: 10.1038/374470a0. [References 46 – 49 highlight the key role of VAV1 in T cell development and TCR signalling.] [DOI] [PubMed] [Google Scholar]
- 50.Kong YY, et al. Vav regulates peptide-specific apoptosis in thymocytes. J Exp Med. 1998;188:2099–2111. doi: 10.1084/jem.188.11.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reynolds LF, et al. Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos and RasGRP1. J Biol Chem. 2004;279:18239–18246. doi: 10.1074/jbc.M400257200. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds LF, et al. Vav1 transduces T cell receptor signals to the activation of phospholipase C-γ1 via phosphoinositide 3-kinase-dependent and -independent pathways. J Exp Med. 2002;195:1103–1114. doi: 10.1084/jem.20011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swat W, et al. Essential role for Vav1 in activation, but not development, of gd T cells. Int Immunol. 2003;15:215–221. doi: 10.1093/intimm/dxg021. [DOI] [PubMed] [Google Scholar]
- 54.Harenberg A, Girkontaite I, Giehl K, Fischer KD. The Lsc RhoGEF mediates signaling from thromboxane A2 to actin polymerization and apoptosis in thymocytes. Eur J Immunol. 2005;35:1977–1986. doi: 10.1002/eji.200425769. [DOI] [PubMed] [Google Scholar]
- 55.Klinger MB, Guilbault B, Kay RJ. The RhoA- and CDC42-specific exchange factor Dbs promotes expansion of immature thymocytes and deletion of double-positive and single-positive thymocytes. Eur J Immunol. 2004;34:806–816. doi: 10.1002/eji.200324400. [DOI] [PubMed] [Google Scholar]
- 56.Sanui T, et al. DOCK2 is essential for antigen-induced translocation of TCR and lipid rafts, but not PKC-theta and LFA-1, in T cells. Immunity. 2003;19:119–129. doi: 10.1016/s1074-7613(03)00169-9. [DOI] [PubMed] [Google Scholar]
- 57.Kunisaki Y, et al. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J Cell Biol. 2006;174:647–652. doi: 10.1083/jcb.200602142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta S, et al. T cell receptor engagement leads to the recruitment of IBP, a novel guanine nucleotide exchange factor, to the immunological synapse. J Biol Chem. 2003 doi: 10.1074/jbc.M308960200. [DOI] [PubMed] [Google Scholar]
- 59.Gupta S, et al. Molecular cloning of IBP, a SWAP-70 homologous GEF, which is highly expressed in the immune system. Hum Immunol. 2003;64:389–401. doi: 10.1016/s0198-8859(03)00024-7. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka Y, et al. SWAP-70-like adapter of T cells, an adapter protein that regulates early TCR-initiated signaling in Th2 lineage cells. Immunity. 2003;18:403–414. doi: 10.1016/s1074-7613(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 61.Fanzo JC, et al. Loss of IRF-4-binding protein leads to the spontaneous development of systemic autoimmunity. J Clin Invest. 2006;116:703–714. doi: 10.1172/JCI24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Becart S, et al. SLAT regulates Th1 and Th2 inflammatory responses by controlling Ca2+/NFAT signaling. J Clin Invest. 2007;117:2164–2175. doi: 10.1172/JCI31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu H, Leitenberg D, Li B, Flavell RA. Deficiency of Small GTPase Rac2 Affects T Cell Activation. J Exp Med. 2001;194:915–926. doi: 10.1084/jem.194.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dorn T, et al. RhoH is important for positive thymocyte selection and T-cell receptor signaling. Blood. 2007;109:2346–2355. doi: 10.1182/blood-2006-04-019034. [DOI] [PubMed] [Google Scholar]
- 65.Gu Y, et al. RhoH GTPase recruits and activates Zap70 required for T cell receptor signaling and thymocyte development. Nat Immunol. 2006;7:1182–1190. doi: 10.1038/ni1396. [References 64 and 65 reveal an unexpected role for RHOH as an adaptor for ZAP70.] [DOI] [PubMed] [Google Scholar]
- 66.Bustelo XR, Ledbetter JA, Barbacid M. Product of vav proto-oncogene defines a new class of tyrosine protein kinase substrates. Nature. 1992;356:68–71. doi: 10.1038/356068a0. [DOI] [PubMed] [Google Scholar]
- 67.Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 68.Han J, et al. Lck regulates Vav activation of members of the Rho family of GTPases. Mol Cell Biol. 1997;17:1346–1353. doi: 10.1128/mcb.17.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Margolis B, et al. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature. 1992;356:71–74. doi: 10.1038/356071a0. [DOI] [PubMed] [Google Scholar]
- 70.Fischer K-D, et al. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol. 1998;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 71.Holsinger LJ, et al. Defects in actin cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr Biol. 1998;8:563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- 72.Penninger JM, et al. The oncogene product Vav is a crucial regulator of primary cytotoxic T cell responses but has no apparent role in CD28-mediated co-stimulation. Eur J Immunol. 1999;29:1709–1718. doi: 10.1002/(SICI)1521-4141(199905)29:05<1709::AID-IMMU1709>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 73.Costello PS, et al. The Rho-family GTP exchange factor Vav is a critical transducer of TCR signals to the calcium, ERK and NF-kB pathways. Proc Natl Acad Sci USA. 1999;96:3035–3040. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Charvet C, Auberger P, Tartare-Deckert S, Bernard A, Deckert M. Vav1 couples T cell receptor to serum response factor-dependent transcription via a MEK-dependent pathway. J Biol Chem. 2002;277:15376–15384. doi: 10.1074/jbc.M111627200. [DOI] [PubMed] [Google Scholar]
- 75.Charvet C, et al. Vav1 promotes T cell cycle progression by linking TCR/CD28 costimulation to FOXO1 and p27kip1 expression. J Immunol. 2006;177:5024–5031. doi: 10.4049/jimmunol.177.8.5024. [DOI] [PubMed] [Google Scholar]
- 76.Cao Y, et al. Pleiotropic defects in TCR signaling in a Vav-1-null Jurkat T-cell line. EMBO J. 2002;21:4809–4819. doi: 10.1093/emboj/cdf499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wood JE, Schneider H, Rudd CE. TCR and TCR-CD28 engagement of protein-kinase (BPKB/akt) and gylcogen synthase kinase-3 (GSK-3) operates independently of guanine nucleotide exchange factor VAV-1. J Biol Chem. 2006 doi: 10.1074/jbc.M604878200. [DOI] [PubMed] [Google Scholar]
- 78.Villalba M, et al. Translocation of PKCq in T cells is mediated by a nonconventional, PI3-K- and Vav-dependent pathway, but does not absolutely require phospholipase C. J Cell Biol. 2002;157:253–263. doi: 10.1083/jcb.200201097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Villalba M, et al. A novel functional interaction between Vav and PKCq is required for TCR-induced T cell activation. Immunity. 2000;12:151–160. doi: 10.1016/s1074-7613(00)80168-5. [DOI] [PubMed] [Google Scholar]
- 80.Puga I, Rao A, Macian F. Targeted cleavage of signaling proteins by caspase 3 inhibits T cell receptor signaling in anergic T cells. Immunity. 2008;29:193–204. doi: 10.1016/j.immuni.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ardouin L, et al. Vav1 transduces TCR signals required for LFA-1 function and cell polarization at the immunological synapse. Eur J Immunol. 2003;33:790–797. doi: 10.1002/eji.200323858. [DOI] [PubMed] [Google Scholar]
- 82.Krawczyk C, et al. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity. 2002;16:331–343. doi: 10.1016/s1074-7613(02)00291-1. [DOI] [PubMed] [Google Scholar]
- 83.Villalba M, et al. Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J Cell Biol. 2001;155:331–338. doi: 10.1083/jcb.200107080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wülfing C, Bauch A, Crabtree GR, Davis MM. The vav exchange factor is an essential regulator in actin-dependent receptor translocation to the lymphocyte-antigen-presenting cell interface. Proc Natl Acad Sci USA. 2000;97:10150–10155. doi: 10.1073/pnas.97.18.10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gomez TS, et al. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat Immunol. 2005;6:261–270. doi: 10.1038/ni1168. [DOI] [PubMed] [Google Scholar]
- 86.Su I-h, et al. Polycomb Group Protein Ezh2 Controls Actin Polymerization and Cell Signaling. Cell. 2005;121:425–436. doi: 10.1016/j.cell.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 87.Faure S, et al. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat Immunol. 2004;5:272–279. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- 88.Tybulewicz V, Ardouin L, Prisco A, Reynolds LF. Vav1: a key signal transducer downstream of the TCR. Immunol Rev. 2003;192:42–52. doi: 10.1034/j.1600-065x.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 89.Tybulewicz VLJ. Vav-family proteins in T-cell signalling. Curr Opin Immunol. 2005;17:267–274. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 90.Kuhne MR, Ku G, Weiss A. A guanine nucleotide exchange factor-independent function of Vav1 in transcriptional activation. J Biol Chem. 2000;275:2185–2190. doi: 10.1074/jbc.275.3.2185. [DOI] [PubMed] [Google Scholar]
- 91.Billadeau DD, Mackie SM, Schoon RA, Leibson PJ. Specific subdomains of Vav differentially affect T cell and NK cell activation. J Immunol. 2000;164:3971–3981. doi: 10.4049/jimmunol.164.8.3971. [DOI] [PubMed] [Google Scholar]
- 92.Houlard M, et al. Vav1 is a component of transcriptionally active complexes. J Exp Med. 2002;195:1115–1127. doi: 10.1084/jem.20011701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blanchet F, Cardona A, Letimier FA, Hershfield MS, Acuto O. CD28 costimulatory signal induces protein arginine methylation in T cells. J Exp Med. 2005;202:371–377. doi: 10.1084/jem.20050176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Phee H, Abraham RT, Weiss A. Dynamic recruitment of PAK1 to the immunological synapse is mediated by PIX independently of SLP-76 and Vav1. Nat Immunol. 2005;6:608–617. doi: 10.1038/ni1199. [DOI] [PubMed] [Google Scholar]
- 95.Nishihara H, et al. DOCK2 associates with CrkL and regulates Rac1 in human leukemia cell lines. Blood. 2002;100:3968–3974. doi: 10.1182/blood-2001-11-0032. [DOI] [PubMed] [Google Scholar]
- 96.Chen Q, et al. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29:899–911. doi: 10.1016/j.immuni.2008.10.011. [The IBP GEF regulates Th cell development by inhibiting IRF4.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Becart S, et al. Tyrosine-Phosphorylation-Dependent Translocation of the SLAT Protein to the Immunological Synapse Is Required for NFAT Transcription Factor Activation. Immunity. 2008;29:704–719. doi: 10.1016/j.immuni.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ishizaki H, et al. Defective chemokine-directed lymphocyte migration and development in the absence of Rho guanosine diphosphate-dissociation inhibitors alpha and beta. J Immunol. 2006;177:8512–8521. doi: 10.4049/jimmunol.177.12.8512. [DOI] [PubMed] [Google Scholar]
- 99.Li B, et al. Role of the guanosine triphosphatase Rac2 in T helper 1 cell differentiation. Science. 2000;288:2219–2222. doi: 10.1126/science.288.5474.2219. [DOI] [PubMed] [Google Scholar]
- 100.Croker BA, et al. Rac2-deficient mice display perturbed T-cell distribution and chemotaxis, but only minor abnormalities in T(H)1 responses. Immunol Cell Biol. 2002;80:231–240. doi: 10.1046/j.1440-1711.2002.01077.x. [DOI] [PubMed] [Google Scholar]
- 101.Tanaka Y, So T, Lebedeva S, Croft M, Altman A. Impaired IL-4 and c-Maf expression and enhanced Th1-cell development in Vav1-deficient mice. Blood. 2005;106:1286–1295. doi: 10.1182/blood-2004-10-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Korn T, et al. Vav1-deficient mice are resistant to MOG-induced experimental autoimmune encephalomyelitis due to impaired antigen priming. J Neuroimmunol. 2003;139:17–26. doi: 10.1016/s0165-5728(03)00128-0. [DOI] [PubMed] [Google Scholar]
- 103.Weckbecker G, et al. Strongly reduced alloreactivity and long-term survival times of cardiac allografts in Vav1- and Vav1/Vav2-knockout mice. Transpl Int. 2007;20:353–364. doi: 10.1111/j.1432-2277.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 104.Tanaka Y, et al. T helper type 2 differentiation and intracellular trafficking of the interleukin 4 receptor-alpha subunit controlled by the Rac activator Dock2. Nat Immunol. 2007;8:1067–1075. doi: 10.1038/ni1506. [DOCK2 regulates intracellular trafficking of the IL-4 receptor α subunit.] [DOI] [PubMed] [Google Scholar]
- 105.Vielkind S, Gallagher-Gambarelli M, Gomez M, Hinton HJ, Cantrell DA. Integrin regulation by RhoA in thymocytes. J Immunol. 2005;175:350–357. doi: 10.4049/jimmunol.175.1.350. [DOI] [PubMed] [Google Scholar]
- 106.Bolomini-Vittori M, et al. Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nat Immunol. 2009;10:185–194. doi: 10.1038/ni.1691. [DOI] [PubMed] [Google Scholar]
- 107.Cherry LK, Li X, Schwab P, Lim B, Klickstein LB. RhoH is required to maintain the integrin LFA-1 in a nonadhesive state on lymphocytes. Nat Immunol. 2004;5:961–967. doi: 10.1038/ni1103. [DOI] [PubMed] [Google Scholar]
- 108.Vicente-Manzanares M, et al. Control of lymphocyte shape and the chemotactic response by the GTP exchange factor Vav. Blood. 2005;105:3026–3034. doi: 10.1182/blood-2004-07-2925. [DOI] [PubMed] [Google Scholar]
- 109.Shulman Z, et al. DOCK2 regulates chemokine-triggered lateral lymphocyte motility but not transendothelial migration. Blood. 2006;108:2150–2158. doi: 10.1182/blood-2006-04-017608. [DOI] [PubMed] [Google Scholar]
- 110.Krummel MF, Macara I. Maintenance and modulation of T cell polarity. Nat Immunol. 2006;7:1143–1149. doi: 10.1038/ni1404. [DOI] [PubMed] [Google Scholar]
- 111.Stowers L, Yelon D, Berg LJ, Chant J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc Natl Acad Sci USA. 1995;92:5027–5031. doi: 10.1073/pnas.92.11.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.D'Souza-Schorey C, Boettner B, Van Aelst L. Rac regulates integrin-mediated spreading and increased adhesion of T lymphocytes. Mol Cell Biol. 1998;18:3936–3946. doi: 10.1128/mcb.18.7.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.del Pozo MA, Vicente-Manzanares M, Tejedor R, Serrador JM, Sanchez-Madrid F. Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur J Immunol. 1999;29:3609–3620. doi: 10.1002/(SICI)1521-4141(199911)29:11<3609::AID-IMMU3609>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 114.Gerard A, Mertens AE, van der Kammen RA, Collard JG. The Par polarity complex regulates Rap1- and chemokine-induced T cell polarization. J Cell Biol. 2007;176:863–875. doi: 10.1083/jcb.200608161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Volinsky N, Gantman A, Yablonski D. A Pak- and Pix-dependent branch of the SDF-1alpha signalling pathway mediates T cell chemotaxis across restrictive barriers. Biochem J. 2006;397:213–222. doi: 10.1042/BJ20051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saveliev A, Tybulewicz VL. Lymphocyte signaling: beyond knockouts. Nat Immunol. 2009;10:361–364. doi: 10.1038/ni.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weisz Hubsman M, Volinsky N, Manser E, Yablonski D. Aronheim, A. Autophosphorylation-dependent degradation of Pak1, triggered by the Rho-family GTPase, Chp. Biochem J. 2007;404:487–497. doi: 10.1042/BJ20061696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smith A, Bracke M, Leitinger B, Porter JC, Hogg N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J Cell Sci. 2003;116:3123–3133. doi: 10.1242/jcs.00606. [DOI] [PubMed] [Google Scholar]
- 119.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 120.Boureux A, Vignal E, Faure S, Fort P. Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol Biol Evol. 2007;24:203–216. doi: 10.1093/molbev/msl145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.