Abstract
Objective
To study the relationship among brain natriuretic peptide (BNP), cholesterol and lipoprotein.
Design
A retrospective, cross-sectional study.
Setting
Tokushima University Hospital area.
Patients
A retrospective study of 46 patients (nine inpatients and 37 outpatients) with angina pectoris or arrhythmias who were seen at Tokushima University Hospital Cardiovascular Division and had measurements of their BNP, fatty acid and lipid profile. The average age of patients was 57±17 years, and 39% were male subjects.
Main outcome measures
BNP, dihomo-γ-linolenic acid, arachidonic acid, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), apolipoproteinA1, apolipoprotein A2 (ApoA2), apolipoprotein B (ApoB), apolipoprotein C2, apolipoprotein C3, apolipoprotein E, total cholesterol (TC), triglyceride, high density lipoprotein cholesterol and low density lipoprotein cholesterol.
Results
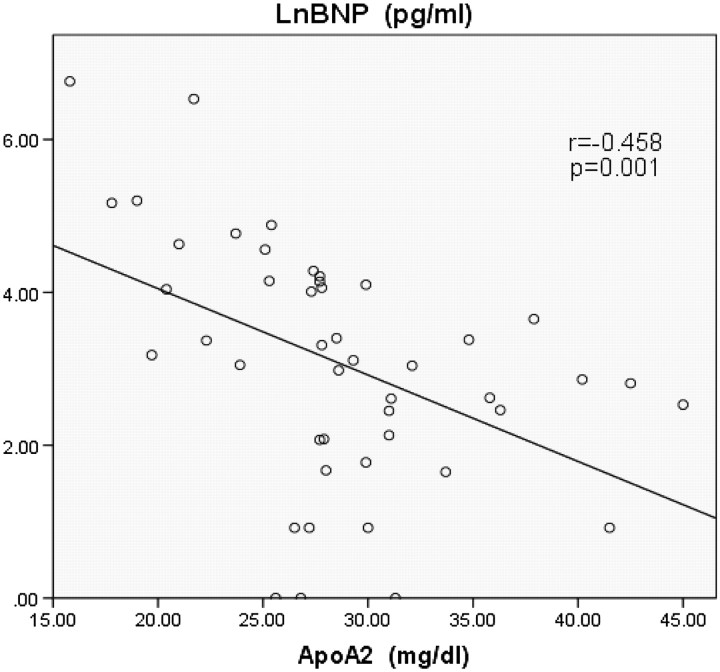
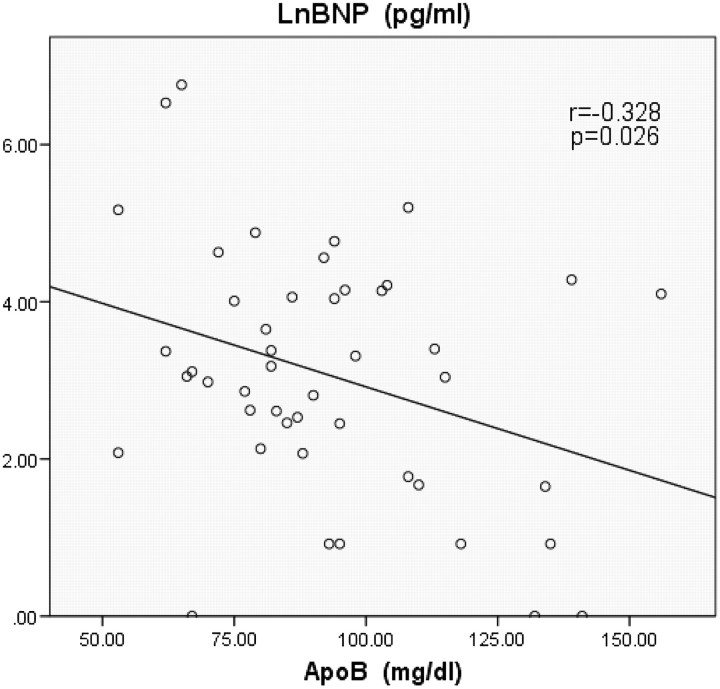
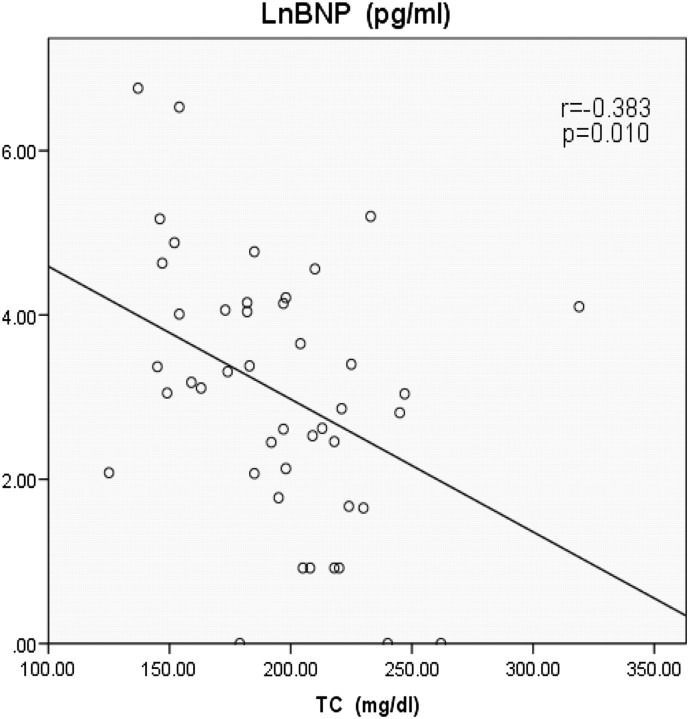
The baseline characteristics of the patients were shown in table 1 and the data of lipoprotein were shown in table 2. Table 3 shows the relationship among BNP, cholesterol and lipoprotein. The authors found significant negative correlation between serum levels of BNP and ApoA2 (figure 1; r=−0.458, p=0.001), serum levels of BNP and ApoB (figure 2; r=−0.328, p=0.026) and serum levels of BNP and TC (figure 3; r=-0.383, p=0.010). There is a possibility that dietary EPA and DHA may modulate cardiac mitochondrial and autonomic nervous system dysfunction via fatty-acids-PPARs-PTEN-PI3K/Akt-SREBPs system and affect serum BNP levels indirectly.
Conclusion
BNP had significant negative correlation with ApoA2, ApoB and TC. The findings suggest that increasing serum levels of ApoA2, ApoB and TC may have an effect on improving heart function. But the mechanism is presently unclear.
Keywords: Heart failure treatment, hypertension, CT scanning, gene expression, coronary artery disease
Background
Cholesterol and lipoprotein are important substances for body function and brain natriuretic peptide (BNP) is a marker of heart function. Increased serum BNP levels represent decreasing cardiac ventricular function.
BNP is produced mainly from the ventricle in response to volume expansion, pressure overload and elevated diastolic pressure,1 2 and modified by many factors including angiotensin 2, endothelin 1,3 neuron restrictive silencer element,4 hypoxia5 and cytokines.6 The serum level of BNP is correlated with the extent of ventricular dysfunction7–9 and coronary artery disease.10 11 And even in community-dwelling asymptomatic subjects, the serum level of BNP is correlated with a first cardiovascular event, heart failure and death.12
Cholesterol is an essential substance required for many functions, such as to maintain integrity of cell membranes, production of vitamin D on the surface of skin, and production of bile acid and steroidal hormones.13 14 While elevated plasma low density lipoprotein cholesterol (LDL-C) levels can result in plaque formation in the arterial wall, low plasma high density lipoprotein cholesterol (HDL-C) level is associated with progression of coronary atherosclerosis in the general population.15–19
Cholesterol is transported in the blood bound to apoproteins and phospholipids. Homeostasis of cholesterol is maintained by the metabolism of lipoproteins such as apoproteins and fatty acids, which mediate transport of the lipid to and from liver and tissues.
Fatty acids include n-6 polyunsaturated fatty acids such as arachidonic acid (AA) and dihomo-γ-linolenic acid (DGLA), and n-3 polyunsaturated fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Although AA and EPA have the opposite effects, hepatic lipogenic gene expression is downregulated by both AA and EPA.20–22 The plasma DHA and EPA+DHA levels have been shown to correlate with the risk of coronary heart disease and fatal ischaemic heart disease.23–25 The Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza cardiaca (GISSI) heart failure study reported that when a low dose of EPA+DHA (0.85 g/day) was administered to heart failure patients for 3.9 years, mortality and hospitalisation were significantly decreased compared with placebo.26 The mechanisms by which EPA and DHA could prevent the development and progression of heart failure are unclear.
Apolipoproteins are the structural components of the lipoproteins. Most apolipoprotein A1 (ApoA1) are included in HDL and are produced within the small intestine and liver. ApoA1 controls activation of lecithin-cholesterol acyltransferase. Apolipoprotein A2 (ApoA2) is the second major protein component of HDL (25%), but its role in HDL metabolism is unclear.27 ApoA2 acts on lecithin-cholesterol acyltransferase and cholesteryl ester transfer protein.
Apolipoprotein B (ApoB) is the most abundant protein constituent of all lipoproteins except HDL. ApoB100 is produced in the liver and is the major component of the very low density lipoprotein (VLDL). ApoB48 is produced in the small intestine and is a major component of the chylomicron. HDL-C is composed of ApoA1, ApoA2, apolipoprotein E (ApoE) and cholesterol, and LDL-C is composed of ApoB100, triglyceride (TG) and cholesterol.
Apolipoprotein C2 (ApoC2) is a normal component of VLDL, where it plays an important physiological role as an activator of lipoprotein lipase, present in chylomicron and one of several members of the plasma apolipoprotein family that form amyloid fibrils. Apolipoprotein C3 (ApoC3) is present in HDL at the time of hunger and, with caloric intake, moves to chylomicron and VLDL. (ApoE) is present in HDL, chylomicron and VLDL mainly. ApoE participates in receptor-mediated uptake of these particles by liver.
There are few studies that have examined the direct relation among serum levels of BNP, cholesterol and lipoprotein in cardiovascular patients.
The aim of this study is to examine the relationship among serum levels of BNP and cholesterol and lipoprotein (apolipoprotein and phospholipid) in patients with cardiovascular disease.
Methods
We obtained blood samples from 46 consecutive patients (nine inpatients and 37 outpatients) with angina pectoris (AP), palpitation, arrhythmia such as atrial fibrillation and premature ventricular contraction of Tokushima University Hospital Cardiovascular Division between April 2010 and May 2011. The average age of patients was 57±17 years and 39% were male subjects. The mean value of BMI was 22.7±3.3 kg/m2 (range; 18.3~32.9 kg/m2).
The diagnostic criteria for AP was >75% stenosis of at least one segment of a major coronary artery found on coronary angiography, which was performed because of clinical angina and exercise stress ECG changes with ST-segment elevation or depression of >0.5 mm or T-wave inversion in two or more leads accompanied by ischaemic chest discomfort.
Of the 46 patients, 25 patients had hypertension, 13 patients had AP, 24 patients had cardiac arrhythmias, two patients had hypertrophic cardiomyopathy and one patient had atrial septal defect. Although there were 19 hypertensive patients with a mix of AP or arrhythmias, there were no patients with a mix of AP and arrhythmias.
Patients who were included in the study had measurement of serum levels of BNP, DGLA, AA, EPA, DHA, ApoA1, ApoA2, ApoB, ApoC2, ApoC3, ApoE, TC, TG, HDL-C and LDL-C. Among 46 patients, nine (20%) were treated with statins. And among 46 patients, 14 (30%) were treated with EPA. Among those treated with EPA, two were treated with EPA (1800 mg), eight were treated with EPA (900 mg) and four were treated with EPA (640 mg)+DHA (432 mg). No patients were co-treated with statins and EPA.
Statistical analysis
Data were presented as means±SD. Correlation was analysed by Pearson's correlation coefficient. A probability value less than 0.05 was accepted as statistically significant. Statistical analyses were done with the statistical package for social sciences software (SPSS). We used the Japanese version of SPSS V.19.0 (SPSS Tokyo, Japan).
Results
The baseline characteristics of the patients were shown in table 1 and the data of lipoproteins were shown in table 2. Table 3 showed the relationship among BNP, cholesterols and lipoproteins.
Table 1.
Clinical characteristics of the patients
| Characteristic | n (%) |
|---|---|
| n | 46 |
| Age (years) | 57±17 |
| Gender (F/M) | 18/28 |
| Laboratory results | Means±SD (normal range) |
| TC (mg/dl) | 196±38 (120–220) |
| TG (mg/dl) | 122±88 (35–150) |
| HDL-C (mg/dl) | 72±25 (40–100) |
| LDL-C (mg/dl) | 109±31 (70–140) |
| BNP (pg/dl) | 71±159 (<18.4) |
| FT4 (ng/dl) | 1.08±0.16 (0.93–1.75) |
| FT3 (pg/ml) | 2.98±0.54 (2.5~3.5) |
| TSH (µU/ml) | 2.67±1.91 (0.65–5.55) |
BNP, brain natriuretic peptide; FT3, free 3,3′,5′-triiodo-L-thyronine; FT4, free thyroxine (3,5,3′,5′-tetraiodo-L-thyronine); HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; TSH, thyroxine stimulating hormone.
Table 2.
The data of lipoproteins
| Laboratory results | Means±SD (normal range) |
|---|---|
| ApoA1 (mg/dl) | 151±27 (126–165) |
| ApoA2 (mg/dl) | 28.6±6.4 (24.6–33.3) |
| ApoB (mg/dl) | 92.7±24.3 (66–101) |
| ApoC2 (mg/dl) | 4.74±7.15 (1.5–3.8) |
| ApoC3 (mg/dl) | 9.16±4.20 (5.4–9.0) |
| ApoE (mg/dl) | 4.73±1.67 (2.8–4.6) |
| DGLA (µg/ml) | 35.1±15.7 (10.9–43.5) |
| AA (µg/ml) | 166±40 (85.1–207.8) |
| EPA (µg/ml) | 67±39 (11.6–107.2) |
| DHA (µg/ml) | 129±88 (48.6–152.4) |
AA, arachidonic acid; ApoA1, apolipoprotein A1; ApoA2, apolipoprotein A2; ApoB, apolipoprotein B; ApoC2, apolipoprotein C2; ApoC3, apolipoprotein C3; ApoE, apolipoprotein E; DGLA, dihomo-γ-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Table 3.
The relations among brain natriuretic peptide (BNP), cholesterols and lipoproteins (n=46)
| ApoA1 | ApoA2 | ApoB | ApoC2 | ApoC3 | ApoE | DGLA | ||
| LnBNP | r | −0.277 | −0.458 | −0.328 | −0.075 | −0.121 | −0.234 | −0.247 |
| p | 0.062 | 0.001 | 0.026 | 0.619 | 0.422 | 0.117 | 0.098 | |
| AA | EPA | DHA | TC | TG | HDL-C | LCL-C | ||
| LnBNP | r | −0.275 | −0.241 | −0.280 | −0.383 | −0.165 | −0.218 | −0.235 |
| p | 0.064 | 0.107 | 0.060 | 0.010 | 0.284 | 0.155 | 0.125 | |
AA, arachidonic acid; ApoA1, apolipoprotein A1; ApoA2, apolipoprotein A2; ApoB, apolipoprotein B; ApoC2, apolipoprotein C2; ApoC3, apolipoprotein C3; ApoE, apolipoprotein E; DGLA, dihomo-γ-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; LnBNP, common logarithmic transformed serum levels of BNP; TC, total cholesterol; TG, triglyceride.
We found significant negative correlation between serum levels of BNP and ApoA2 (figure 1; r=−0.458, p=0.001), serum levels of BNP and ApoB (figure 2; r=−0.328, p=0.026) and serum levels of BNP and total cholesterol (TC) (figure 3; r=−0.383, p=0.010). No significant correlation was found between serum levels of BNP and EPA (table 3; r=−0.241, p=0.107), serum levels of BNP and DHA (table 3; r=−0.280, p=0.060), serum levels of BNP and HDL-C (table 3; r=−0.218, p=0.155) and serum levels of BNP and LDL-C (table 3; r=−0.235, p=0.125).
Figure 1.
Significant negative correlation between serum levels of brain natriuretic peptide (BNP) and apolipoprotein A2 (ApoA2) (r=−0.458, p=0.001). LnBNP, common logarithmic transformed serum levels of BNP.
Figure 2.
Significant negative correlation between serum levels of brain natriuretic peptide (BNP) and apolipoprotein B (ApoB) (r=−0.328, p=0.026). LnBNP, common logarithmic transformed serum levels of BNP.
Figure 3.
Significant negative correlation between serum levels of brain natriuretic peptide (BNP) and total cholesterol (TC) (r=−0.383, p=0.010). LnBNP, common logarithmic transformed serum levels of BNP.
Discussion
As far as we know, this is the first study to show that heart function, as represented by the biomarker BNP, was associated with plasma levels of cholesterol and apolipoprotein. The results of this study showed serum ApoA2 levels had the strongest negative relation to serum BNP levels. In addition, serum ApoB and TC levels had a significant negative relation to serum BNP levels.
There were a few papers that have reported a direct relationship between serum levels of BNP and HDL-C. Lupattelli et al 28 reported that there was a ‘positive’ correlation between serum levels of BNP and HDL-C in the hyperlipidemic patients with serum BNP levels under 20 pg/ml (r=0.23, p=0.06).
But our results showed that there was no positive correlation but instead there was a trend towards a ‘negative’ correlation between serum levels of BNP and HDL-C (r=−0.218, p=0.155) even though it was not statistically significant. Although the range of serum levels of BNP was quite different, it was interesting that the relationship showed a trend that was opposite to previous studies.
In the GISSI heart failure trial, a low dose of EPA+DHA (0.85 g/day) administered to heart failure patients was correlated with a significant decrease in mortality and hospitalisation.26 The mechanisms of these effects of EPA+DHA on the heart were unclear. According to our results of the negative relations between the serum level of BNP and the serum level of ApoA2, ApoB and TC, a possible mediator may be alteration in serum apolipoprotein composition and TC levels; however, the administration effects of EPA+DHA were reviewed to make no definitive effects on the serum level of ApoA2, ApoB and TC in another paper.29
Our results showed serum levels of EPA and DHA had no significant negative relation to serum BNP levels, which might mean the effect of serum EPA and DHA level was weaker than ApoA2, ApoB and TC directly. In the GISSI heart failure trial, there was a possibility that dietary EPA and DHA may have good effects on heart via affecting mitochondria and autonomic nervous system directly. In previous studies, McMillin et al 30 demonstrated that dietary fish oil favourably affected mitochondrial function. Sjoberg et al 31 showed that dietary DHA, dose dependently, improved heart rate variability. However, the signalling mechanism remains elusive.
We will demonstrate one possible explanation of the signalling mechanism. First, fatty acids such as EPA, DHA and AA were well known to be identified as ligands of peroxisome proliferator-activated receptors (PPARs) by many studies.32–37
Second, Patel et al 38 demonstrated PPARγ played a role in regulating phosphatidylinositol 3′-kinase (PI3K) and Akt (protein kinase B), which were signalling kinases involved in cell survival and proliferation, by modulating the phosphatase and tensin homologue (PTEN) tumour suppressor gene expression in inflammatory and tumour-derived cells. Lee et al 39 showed that the administration of PPARγ agonists increased PTEN expression, which was correlated with decreased PI3K activity. PI3K pathway is downstream target pathway of PTEN,40 which plays a critical role in transmitting signals from growth factors to cell death and cell cycle machineries.41
Third, Fleischmann and Iynedjian 42 demonstrated that an activation of Akt causes the sterol-regulatory element-binding protein 1 (SREBP-1), master transcriptional regulators of lipid metabolism and mRNA accumulation in primary hepatocytes. In addition, the relation between the PI3K/Akt pathway and the sterol-regulatory element-binding proteins (SREBPs) has been studied extensively in many researches,42–46 in which PI3K seemingly activates SREBP function. Krycer et al 47 reviewed the role of PI3K/Akt on SREBPs. They reviewed that recent evidence suggested PI3K/Akt activated SREBPs, but the precise molecular mechanisms were controversial and differed between SREBP isoforms.
Finally, some groups reported that SREBPs were associated with the gene regulation of mitochondria.48–50 And the relation between SREBPs and autonomic nervous system was recently discovered. Park et al 51 reported a novel relationship between SREBP-1 and regulation of the cardiac parasympathetic response, and in another paper, Park et al 52 demonstrated that SREBP-1 modulated parasympathetic response in type 1 diabetic Akita mice.
Therefore, dietary EPA and DHA might favourably affect the heart by ameliorating mitochondria and autonomic nervous system dysfunction via PPARs-PTEN-PI3K/Akt-SREBPs system, and so they improved mortality and hospitalisation.
Increased ApoA2 is obviously desirable for the reduction of cardiovascular disease including heart failure. It is known that increased LDL-C is associated with deteriorating atherosclerosis; however, not increased LDL-C but increased ApoB, which is main protein component of LDL-C, is associated with improved heart function.
Although it is not difficult to increase serum levels of EPA and DHA by food, it is difficult to increase apolipoprotein such as ApoA2 and ApoB by food directly. By understanding the regulation of ApoA2 and ApoB gene expressions, methods to increase ApoA2 and ApoB can be better understood. These interactions are important to understand the relation between heart function and cholesterol and lipoprotein.
Study limitations
The present study had several potential limitations because of the retrospective study nature and the number of patients was small, and so that it might not be representative of all patients. And the present study was of local patients who lived around Tokushima University Hospital (most patients live in Tokushima Prefecture), so that it might not be representative of all the patients globally. The people living in Tokushima eat more fish than Westerners, which may affect the results. In the future, a worldwide study is desirable.
Conclusion
This is the first reported study to show a direct correlation between serum levels of BNP and ApoA2, ApoB and TC. The findings suggest that increasing serum levels of ApoA2, ApoB and TC may have an effect on improving heart function. But the mechanism is presently unclear. In addition, the serum levels of DGLA, AA, EPA, DHA, ApoA1, ApoC2, ApoC3, ApoE, TG, HDL-C and LDL-C showed a trend towards negative correlation with serum BNP levels.
Footnotes
Contributors: HT collected the whole data and contributed substantially to analysis and interpretation of data. HT wrote the article. All authors approved the final version.
Competing interests: None.
Patient consent: Consent was not obtained but the presented data are anonymised and risk of identification is low.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Baughman KL. B-type natriuretic peptide—a window to the heart. N Engl J Med 2002;347:158–9. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa O, Ogawa Y, Itoh H, et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest 1995;96:1280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piuhola J, Szokodi I, Ruskoaho H. Endothelin-1 and angiotensin II contribute to BNP but not c-fos gene expression response to elevated load in isolated mice hearts. Biochim Biophys Acta 2007;1772:338–44. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa E, Saito Y, Kuwahara K, et al. Fibronectin signaling stimulates BNP gene transcription by inhibiting neuron-restrictive silencer element-dependent repression. Cardiovasc Res 2002;53:451–9. [DOI] [PubMed] [Google Scholar]
- 5.Weidemann A, Klanke B, Wagner M, et al. Hypoxia, via stabilization of the hypoxia-inducible factor HIF-1alpha, is a direct and sufficient stimulus for brain-type natriuretic peptide induction. Biochem J 2008;409:233–42. [DOI] [PubMed] [Google Scholar]
- 6.Ma KK, Ogawa T, de Bold AJ. Selective upregulation of cardiac brain natriuretic peptide at the transcriptional and translational levels by pro-inflammatory cytokines and by conditioned medium derived from mixed lymphocyte reactions via p38 MAP kinase. J Mol Cell Cardiol 2004;36:505–13. [DOI] [PubMed] [Google Scholar]
- 7.Tsutamoto T, Wada A, Maeda K, et al. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation 1997;96:509–16. [DOI] [PubMed] [Google Scholar]
- 8.Niizeki T, Takeishi Y, Arimoto T, et al. Combination of heart-type fatty acid binding protein and brain natriuretic peptide can reliably risk stratify patients hospitalized for chronic heart failure. Circ J 2005;69:922–7. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa C, Tsutamoto T, Fujii M, et al. Prediction of mortality by high-sensitivity C-reactive protein and brain natriuretic peptide in patients with dilated cardiomyopathy. Circ J 2006;70:857–63. [DOI] [PubMed] [Google Scholar]
- 10.Richards AM, Nicholls MG, Espiner EA, et al. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation 2003;107:2786–92. [DOI] [PubMed] [Google Scholar]
- 11.Katayama T, Nakashima H, Takagi C, et al. Predictors of mortality in patients with acute myocardial infarction and cardiogenic shock. Circ J 2005;69:83–8. [DOI] [PubMed] [Google Scholar]
- 12.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004;350:655–63. [DOI] [PubMed] [Google Scholar]
- 13.Cannon B, Lewis A, Metze J, et al. Cholesterol supports headgroup superlattice domain formation in fluid phospholipid/cholesterol bilayers. J Phys Chem B 2006;110:6339–50. [DOI] [PubMed] [Google Scholar]
- 14.Pandit SA, Bostick D, Berkowitz ML. Complexation of phosphatidylcholine lipids with cholesterol. Biophys J 2004;86:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genest JJ, Jr, Martin-Munley SS, McNamara JR, et al. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation 1992;85:2025–33. [DOI] [PubMed] [Google Scholar]
- 16.Ballantyne CM, Herd JA, Ferlic LL, et al. Influence of low HDL on progression of coronary artery disease and response to fluvastatin therapy. Circulation 1999;99:736–43. [DOI] [PubMed] [Google Scholar]
- 17.Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 1987;317:1237–45. [DOI] [PubMed] [Google Scholar]
- 18.Ballantyne CM, Olsson AG, Cook TJ, et al. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation 2001;104:3046–51. [DOI] [PubMed] [Google Scholar]
- 19.Robins SJ, Collins D, Wittes JT, et al. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA 2001;285:1585–91. [DOI] [PubMed] [Google Scholar]
- 20.Mater MK, Thelen AP, Jump DB. Arachidonic acid and PGE2 regulation of hepatic lipogenic gene expression. J Lipid Res 1999;40:1045–52. [PubMed] [Google Scholar]
- 21.Kim HK, Choi S, Choi H. Suppression of hepatic fatty acid synthase by feeding alpha-linolenic acid rich perilla oil lowers plasma triacylglycerol level in rats. J Nutr Biochem 2004;15:485–92. [DOI] [PubMed] [Google Scholar]
- 22.Dentin R, Benhamed F, Pegorier JP, et al. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J Clin Invest 2005;115:2843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon JA, Hodgkins ML, Browner WS, et al. Serum fatty acids and the risk of coronary heart disease. Am J Epidemiol 1995;142:469–76. [DOI] [PubMed] [Google Scholar]
- 24.Holub DJ, Holub BJ. Omega-3 fatty acids from fish oils and cardiovascular disease. Mol Cell Biochem 2004;263:217–25. [DOI] [PubMed] [Google Scholar]
- 25.Lemaitre RN, King IB, Mozaffarian D, et al. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr 2003;77:319–25. [DOI] [PubMed] [Google Scholar]
- 26.Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1223–30. [DOI] [PubMed] [Google Scholar]
- 27.Blanco-Vaca F, Escola-Gil JC, Martin-Campos JM, et al. Role of apoA-II in lipid metabolism and atherosclerosis: advances in the study of an enigmatic protein. J Lipid Res 2001;42:1727–39. [PubMed] [Google Scholar]
- 28.Lupattelli G, Marchesi S, Siepi D, et al. Natriuretic peptides levels are related to HDL-cholesterol with no influence on endothelium dependent vasodilatation. Vasa 2006;35:215–20. [DOI] [PubMed] [Google Scholar]
- 29.Harris WS. Fish oils and plasma lipid and lipoprotein metabolism in humans: a critical review. J Lipid Res 1989;30:785–807. [PubMed] [Google Scholar]
- 30.McMillin JB, Bick RJ, Benedict CR. Influence of dietary fish oil on mitochondrial function and response to ischemia. Am J Physiol 1992;263:H1479–85. [DOI] [PubMed] [Google Scholar]
- 31.Sjoberg NJ, Milte CM, Buckley JD, et al. Dose-dependent increases in heart rate variability and arterial compliance in overweight and obese adults with DHA-rich fish oil supplementation. Br J Nutr 2010;103:243–8. [DOI] [PubMed] [Google Scholar]
- 32.Kliewer SA, Sundseth SS, Jones SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A 1997;94:4318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krey G, Braissant O, L'Horset F, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol 1997;11:779–91. [DOI] [PubMed] [Google Scholar]
- 34.Michaud SE, Renier G. Direct regulatory effect of fatty acids on macrophage lipoprotein lipase: potential role of PPARs. Diabetes 2001;50:660–6. [DOI] [PubMed] [Google Scholar]
- 35.Sethi S, Ziouzenkova O, Ni H, et al. Oxidized omega-3 fatty acids in fish oil inhibit leukocyte-endothelial interactions through activation of PPAR alpha. Blood 2002;100:1340–6. [DOI] [PubMed] [Google Scholar]
- 36.Lin Q, Ruuska SE, Shaw NS, et al. Ligand selectivity of the peroxisome proliferator-activated receptor alpha. Biochemistry 1999;38:185–90. [DOI] [PubMed] [Google Scholar]
- 37.Diep QN, Amiri F, Touyz RM, et al. PPARalpha activator effects on Ang II-induced vascular oxidative stress and inflammation. Hypertension 2002;40:866–71. [DOI] [PubMed] [Google Scholar]
- 38.Patel L, Pass I, Coxon P, et al. Tumor suppressor and anti-inflammatory actions of PPARgamma agonists are mediated via upregulation of PTEN. Curr Biol 2001;11:764–8. [DOI] [PubMed] [Google Scholar]
- 39.Lee KS, Park SJ, Hwang PH, et al. PPAR-gamma modulates allergic inflammation through up-regulation of PTEN. Faseb J 2005;19:1033–5. [DOI] [PubMed] [Google Scholar]
- 40.Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell 2000;100:387–90. [DOI] [PubMed] [Google Scholar]
- 41.Carpenter CL, Cantley LC. Phosphoinositide kinases. Curr Opin Cell Biol 1996;8:153–8. [DOI] [PubMed] [Google Scholar]
- 42.Fleischmann M, Iynedjian PB. Regulation of sterol regulatory-element binding protein 1 gene expression in liver: role of insulin and protein kinase B/cAkt. Biochem J 2000;349:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol 2008;128:1286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou RH, Yao M, Lee TS, et al. Vascular endothelial growth factor activation of sterol regulatory element binding protein: a potential role in angiogenesis. Circ Res 2004;95:471–8. [DOI] [PubMed] [Google Scholar]
- 45.Porstmann T, Griffiths B, Chung YL, et al. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene 2005;24:6465–81. [DOI] [PubMed] [Google Scholar]
- 46.Du X, Kristiana I, Wong J, et al. Involvement of Akt in ER-to-Golgi transport of SCAP/SREBP: a link between a key cell proliferative pathway and membrane synthesis. Mol Biol Cell 2006;17:2735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krycer JR, Sharpe LJ, Luu W, et al. The Akt-SREBP nexus: cell signaling meets lipid metabolism. Trends Endocrinol Metab 2010;21:268–76. [DOI] [PubMed] [Google Scholar]
- 48.Guha P, Aneja KK, Shilpi RY, et al. Transcriptional regulation of mitochondrial glycerophosphate acyltransferase is mediated by distal promoter via ChREBP and SREBP-1. Arch Biochem Biophys 2009;490:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura MT, Cheon Y, Li Y, et al. Mechanisms of regulation of gene expression by fatty acids. Lipids 2004;39:1077–83. [DOI] [PubMed] [Google Scholar]
- 50.Lu RH, Ji H, Chang ZG, et al. Mitochondrial development and the influence of its dysfunction during rat adipocyte differentiation. Mol Biol Rep 2010;37:2173–82. [DOI] [PubMed] [Google Scholar]
- 51.Park HJ, Georgescu SP, Du C, et al. Parasympathetic response in chick myocytes and mouse heart is controlled by SREBP. J Clin Invest 2008;118:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park HJ, Zhang Y, Du C, et al. Role of SREBP-1 in the development of parasympathetic dysfunction in the hearts of type 1 diabetic Akita mice. Circ Res 2009;105:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]