ABSTRACT
In many parts of the world forest disturbance regimes have intensified recently, and future climatic changes are expected to amplify this development further in the coming decades. These changes are increasingly challenging the main objectives of forest ecosystem management, which are to provide ecosystem services sustainably to society and maintain the biological diversity of forests. Yet a comprehensive understanding of how disturbances affect these primary goals of ecosystem management is still lacking. We conducted a global literature review on the impact of three of the most important disturbance agents (fire, wind, and bark beetles) on 13 different ecosystem services and three indicators of biodiversity in forests of the boreal, cool‐ and warm‐temperate biomes. Our objectives were to (i) synthesize the effect of natural disturbances on a wide range of possible objectives of forest management, and (ii) investigate standardized effect sizes of disturbance for selected indicators via a quantitative meta‐analysis. We screened a total of 1958 disturbance studies published between 1981 and 2013, and reviewed 478 in detail. We first investigated the overall effect of disturbances on individual ecosystem services and indicators of biodiversity by means of independence tests, and subsequently examined the effect size of disturbances on indicators of carbon storage and biodiversity by means of regression analysis. Additionally, we investigated the effect of commonly used approaches of disturbance management, i.e. salvage logging and prescribed burning. We found that disturbance impacts on ecosystem services are generally negative, an effect that was supported for all categories of ecosystem services, i.e. supporting, provisioning, regulating, and cultural services (P < 0.001). Indicators of biodiversity, i.e. species richness, habitat quality and diversity indices, on the other hand were found to be influenced positively by disturbance (P < 0.001). Our analyses thus reveal a ‘disturbance paradox’, documenting that disturbances can put ecosystem services at risk while simultaneously facilitating biodiversity. A detailed investigation of disturbance effect sizes on carbon storage and biodiversity further underlined these divergent effects of disturbance. While a disturbance event on average causes a decrease in total ecosystem carbon by 38.5% (standardized coefficient for stand‐replacing disturbance), it on average increases overall species richness by 35.6%. Disturbance‐management approaches such as salvage logging and prescribed burning were neither found significantly to mitigate negative effects on ecosystem services nor to enhance positive effects on biodiversity, and thus were not found to alleviate the disturbance paradox. Considering that climate change is expected to intensify natural disturbance regimes, our results indicate that biodiversity will generally benefit from such changes while a sustainable provisioning of ecosystem services might come increasingly under pressure. This underlines that disturbance risk and resilience require increased attention in ecosystem management in the future, and that new approaches to addressing the disturbance paradox in management are needed.
Keywords: fire, wind, bark beetles, disturbance effect, biodiversity, ecosystem services, forest management, salvage logging, prescribed burning, disturbance paradox
I. INTRODUCTION
In recent decades, forest disturbance regimes have intensified in many parts of the world (Chapin et al., 2000; Schelhaas, Nabuurs & Schuck, 2003; Balshi et al., 2007; Gardiner et al., 2010). The frequency of large wildfires in western North America has, for instance, increased by nearly four times in the period 1987–2003 compared to the average for 1970–1986 (Westerling et al., 2006), while at the same time bark beetle damage has reached unprecedented levels (Meddens, Hicke & Ferguson, 2012). A similar trend is evident for wildfire, windthrow, and bark beetles in Europe (Schelhaas et al., 2003; Seidl et al., 2014). This trend is likely to continue in the future as a result of the climatic changes expected for the coming decades (Seidl, Schelhaas & Lexer, 2011b; Li et al., 2013; Reichstein et al., 2013; Temperli, Bugmann & Elkin, 2013; Seidl et al., 2014). In many areas, changes in the disturbance regime (i.e. in the distinctive type, size, severity, and frequency of disturbance over extended spatio‐temporal scales) are expected to be among the most severe climate change impacts on forest ecosystems (Lindner et al., 2010; Turner, 2010). Disturbances are important natural drivers of forest ecosystem dynamics (Franklin et al., 2002; Kuuluvainen & Aakala, 2011), and strongly modulate the structure and functioning of forest ecosystems (Weber & Flannigan, 1997; Turner, 2010). Changing disturbance regimes might thus considerably alter forest ecosystems, with potentially far‐reaching impacts on their biological diversity and capacity to provide ecosystem services to society.
With the aim to provide ecosystem services to society while fostering biodiversity, ecosystem management requires a comprehensive understanding of the impacts of natural disturbances. Notwithstanding this high relevance, natural disturbances have hitherto been discussed inconclusively in the context of ecosystem management, with views and recommendations ranging from strict avoidance of disturbance (due to negative effects on selected ecosystem services) to emulating disturbance in management (to utilize their beneficial effects on biodiversity). On the one hand, substantial efforts are undertaken in research and management to quantify disturbance risk, with the aim to minimize their negative impacts through increasing the resistance of forests to disturbances (e.g. Jactel et al., 2009; Overbeck & Schmidt, 2012). Measures such as fostering individual‐tree stability through thinning (Schelhaas, 2008), adapting landscape‐scale harvesting patterns to disturbance risk [e.g. stand edges versus the main wind direction (Byrne & Mitchell, 2013)], and choosing a rotation period that balances disturbance risk with economic considerations (Loisel, 2014) have long been practiced in forestry in order to avoid disturbance‐related losses particularly with regard to timber production. On the other hand, with the advent of science‐based ecosystem management and a growing understanding of the integral role of disturbances in natural forest ecosystem dynamics, mimicking natural disturbance regimes to foster elemental processes of ecosystem dynamics is increasingly advocated (e.g. Toivanen & Kotiaho, 2007; Newton et al., 2011). Hypothesizing a positive effect of disturbances on biodiversity and acknowledging their role in creating keystone habitats within forested landscapes, these ideas view disturbances as inherently positive. In human‐altered boreal forest ecosystems, for instance, where fire is the major natural disturbance agent, there are suggestions for the application of prescribed burning as a measure to restore natural forest conditions (Bergeron et al., 2002; Toivanen & Kotiaho, 2007; Olsson & Jonsson, 2010). In wind‐ and bark beetle‐dominated disturbance regimes the creation of gaps of various sizes and shapes is recommended to mimic natural disturbance regimes and stimulate biodiversity (Franklin et al., 2002; Seymour, White & DeMaynadier, 2002; Kern et al., 2014).
The valuation of disturbances and their role in management thus seems to vary strongly with the particular objective considered (e.g. biodiversity conservation versus timber production). However, only a small proportion of forests serve a sole objective: only about 5% of the world's forests are strict reserves for the conservation of biodiversity (Hoekstra et al., 2005), while a similar fraction are designated plantations for the production of wood and biomass (Carnus et al., 2006). The large majority of forest landscapes need to fulfill a multitude of functions and services simultaneously, including but not limited to serving as habitat, protecting the soil from erosion, producing timber and biomass, storing carbon, etc. In such situations where multiple objectives need to be met within a forest landscape, disturbances can be expected to have both positive and negative impacts on possible objectives of ecosystem management (see e.g. Huston & Marland, 2003), a hypothesis that we here refer to as the ‘disturbance paradox’. Considering that not only disturbances have increased recently but also the range and demand for societally relevant ecosystem services has been growing steadily in recent decades, we estimate that addressing this paradox will be a key challenge for future forest ecosystem management.
Here we attempt to describe and quantify the various effects of natural disturbances in a literature review and meta‐analysis of disturbance impacts at the global scale. In particular, we examine the effects of three of the most detrimental disturbance agents globally [i.e. fire, wind, and bark beetles (FAO, 2010)], focusing on forest ecosystems of the boreal and temperate biomes, a forest area of approximately 13.5 million km2 (Hansen, Stehman & Potapov, 2010). Acknowledging the growing societal importance of a variety of different ecosystem services we not only survey disturbance impacts on traditionally important forest goods (such as timber production) but also include a total of 13 different ecosystem services from all four categories distinguished by the Millennium Ecosystem Assessment in our analysis: provisioning, supporting, regulating, and cultural services (MEA, 2005). Furthermore, we also investigated disturbance impact on three important indicators of biodiversity. Our overall objectives were (i) to synthesize the effect of natural disturbances on a wide range of possible objectives of forest ecosystem management, and (ii) to investigate standardized effect sizes of disturbance impacts for selected indicators via a quantitative meta‐analysis. Based on these analyses we discuss pathways to addressing disturbances in ecosystem management in the particular context of changing disturbance regimes.
II. MATERIALS AND METHODS
(1). Literature review
We searched the literature for studies on disturbance by fire, wind and bark beetles, and their impacts on ecosystem services as defined by the Millennium Ecosystem Assessment (MEA, 2005), as well as their effects on biodiversity, focusing on species richness and habitat quality as well as on indices of diversity (e.g. Shannon‐Index, Simpson‐Index, etc.). We restricted our literature review to boreal and temperate forest ecosystems as subtropical and tropical forests differ considerably in ecological processes and anthropogenic impacts. In particular, extratropical forests are generally less diverse than tropical forests, and share a common set of genera as well as drivers of forest dynamics (e.g. temperature) (Thomas & MacLellan, 2002). Furthermore, land‐use history and recent management differ strongly between tropical and extratropical regions, with a long history of intensive human use and several decades of sustainable management in the temperate and boreal zone (Siry, Cubbage & Ahmed, 2005; Canadell & Raupach, 2008). Focusing solely on the boreal and temperate subset of the literature controlled for these broad differences in our analysis, and thus increased the inferential potential with regard to disturbance effects. The literature search was performed using the Scopus database (SciVerse Scopus, 2013), and the cutoff date for the inclusion of publications was June 6th, 2013. The search terms and synonyms used are listed as supporting online information in Appendix S1. In total, 1958 papers were identified for screening. From this overall body of literature, reviews and syntheses were excluded in order to avoid double counting and the potential transfer of artifacts or errors from one review to the next (Whittaker, 2010). Furthermore, we excluded articles which did not compare disturbed forests with long‐lasting undisturbed ‘control’ sites. Depending on the study scale and context, either the state before a disturbance, an undisturbed reference, or an assumption about an equilibrium condition was assumed as a reference to determine the disturbance effect. From the 1958 papers screened initially 478 were selected for further analysis. For each of these studies we collected information on geographical location, spatial and temporal scales, assessment methodologies and management treatments (Tables 1 and 2, see online Appendix S2). We furthermore recorded whether the reported disturbance effect is related to single or multiple disturbance events (i.e. disturbance regime). If studies included expert opinions on certain disturbance effects they were initially included in our database, but were subsequently omitted from quantitative analyses. We allowed multiple entries per study, for instance if a study examined more than one disturbance agent, ecosystem service or biodiversity indicator. Furthermore, considering that ecological effects can change over time, we also recorded the temporal time frame for every study. In order to alleviate potential autocorrelation issues, effects were grouped into four different time horizons (i.e. short term: 1–5 years, mid term: 6–25 years, long term: 26–100 years, very long term: >100 years). The final database for analysis contained 887 entries of disturbance effects on ecosystem services and biodiversity.
Table 1.
Geographic distribution of observations (N = 887) of disturbance impacts on ecosystem services and biodiversity reported in 478 peer‐reviewed publications included in the analysis
| Disturbance agent | ||||
|---|---|---|---|---|
| Biome | Continent | Fire | Wind | Bark beetles |
| Boreal | Africa | 0 | 0 | 0 |
| Asia | 11 | 1 | 0 | |
| Europe | 28 | 23 | 3 | |
| North America | 221 | 24 | 30 | |
| South America | 0 | 0 | 0 | |
| Australasia | 0 | 0 | 0 | |
| Cool temperate | Africa | 0 | 0 | 0 |
| Asia | 2 | 10 | 0 | |
| Europe | 54 | 38 | 11 | |
| North America | 198 | 25 | 18 | |
| South America | 9 | 0 | 0 | |
| Australasia | 28 | 6 | 0 | |
| Warm temperate | Africa | 2 | 0 | 0 |
| Asia | 10 | 0 | 0 | |
| Europe | 33 | 0 | 0 | |
| North America | 55 | 18 | 0 | |
| South America | 2 | 0 | 0 | |
| Australasia | 24 | 1 | 0 | |
| Total | 677 | 146 | 62 | |
Note that two observations addressing fire and wind impact, respectively, at the global scale, are not included.
Table 2.
Assessment methodology and focal scale of observations (N = 887) regarding disturbance impacts on ecosystem services and biodiversity reported in 478 peer‐reviewed publications included in the analysis
| Assessment methodology | |||||||
|---|---|---|---|---|---|---|---|
| Temporal scale | Spatial scale | Empirical | Remote sensing | Simulation | Questionnaire | Expert opinion | Mixed |
| Short term (1–5 years) | Stand | 237 | 1 | 12 | 0 | 14 | 1 |
| Patch | 23 | 0 | 2 | 0 | 0 | 0 | |
| Landscape | 28 | 0 | 5 | 2 | 14 | 3 | |
| Region | 6 | 2 | 24 | 0 | 4 | 2 | |
| Global | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mid term (6–25 years) | Stand | 117 | 0 | 16 | 0 | 7 | 0 |
| Patch | 16 | 0 | 2 | 0 | 3 | 0 | |
| Landscape | 12 | 0 | 9 | 0 | 8 | 2 | |
| Region | 5 | 10 | 23 | 1 | 3 | 1 | |
| Global | 0 | 0 | 0 | 0 | 0 | 0 | |
| Long term (26–100 years) | Stand | 50 | 0 | 12 | 0 | 6 | 0 |
| Patch | 5 | 0 | 2 | 0 | 3 | 0 | |
| Landscape | 4 | 0 | 11 | 0 | 4 | 0 | |
| Region | 1 | 1 | 24 | 1 | 10 | 0 | |
| Global | 0 | 0 | 2 | 0 | 0 | 0 | |
| Very long term (>100 years) | Stand | 22 | 0 | 6 | 0 | 8 | 0 |
| Patch | 1 | 0 | 2 | 0 | 2 | 0 | |
| Landscape | 4 | 0 | 11 | 0 | 16 | 0 | |
| Region | 4 | 0 | 14 | 0 | 17 | 0 | |
| Global | 0 | 0 | 0 | 0 | 0 | 0 | |
| NA | Stand | 0 | 0 | 0 | 0 | 2 | 0 |
| Patch | 0 | 0 | 0 | 0 | 1 | 0 | |
| Landscape | 3 | 0 | 0 | 0 | 10 | 0 | |
| Region | 0 | 0 | 3 | 4 | 5 | 0 | |
| Global | 0 | 0 | 0 | 0 | 0 | 0 | |
| NA | 0 | 0 | 0 | 0 | 1 | 0 | |
| Total | 538 | 14 | 180 | 8 | 138 | 9 | |
Stand: 1–10 ha, patch: 11–100 ha, landscape: 101–100000 ha, region: >100000 ha. NA: undefined temporal or spatial scale.
(2). Analysis
We analysed our literature‐derived database of disturbance effects in two steps. First, we assessed the disturbance effect on indicators of ecosystem services and biodiversity. To that end, a descriptive classification of the disturbance impact was made based on the findings reported in the literature (i.e. negative, neutral, mixed, or positive impact of disturbance on the respective indicator). This classification allowed us to synthesize results consistently from different methodological approaches. It furthermore enabled a comparison of disturbance impacts between ecosystem services measured on different scales (e.g. recreational value versus carbon storage in a forest landscape), as well as between the impacts on ecosystem services and biodiversity. Initially, we tested whether the observed distribution of studies over response categories differed significantly from a random distribution, i.e. we assessed whether a significant disturbance effect can be established from the literature. Subsequently, we tested for differences in disturbance impact among agents, biomes, and study approaches, evaluating the variation of disturbance impacts with these categories. In an attempt to confirm or reject the hypothesized diverging impacts of disturbance on criteria of relevance for ecosystem management (disturbance paradox hypothesis) we also tested whether disturbance impacts differ between indicators of ecosystem services and biodiversity. Another controversial issue in the context of disturbance management is the effect of salvage harvesting after disturbance, i.e. partial or complete removal of disturbance‐killed trees from a site (Donato et al., 2006; Lindenmayer, Burton & Franklin, 2008; Thorn et al., 2014). We thus also tested the hypothesis that disturbance effects after salvage differ significantly from unsalvaged conditions. Finally, we also compared impacts of prescribed burning to those of wildfires, in order to test for differences in disturbance impacts from intended and unintended fires. All these tests were conducted using independence tests, a powerful, permutation‐based approach to test the null hypothesis that two variables (measured on arbitrary scales) are independent of each other (Hothorn et al., 2008), using the package coin (Hothorn et al., 2013) within the R language and environment for statistical computing (R Development Core Team, 2014).
In a second step, in order to determine effect size, we conducted a meta‐analysis based on quantitative information on disturbance impact for two particularly well‐researched criteria: biodiversity and carbon storage. For biodiversity, we analysed disturbance‐induced changes in species richness (S′, N = 57) and species entropy (H′, N = 28), the latter represented by the Shannon‐Index of diversity. Due to the limited sample size further subdivision into the effects of disturbance on specific taxonomic groups was not possible. With regard to carbon storage, we distinguished between disturbance effects on total ecosystem carbon (TEC, N = 27), aboveground live carbon (ALC, N = 38), dead aboveground carbon (DAC, N = 25), and soil organic carbon (SOC, N = 39) in our meta‐analysis. For all variables the effect size was calculated as the per cent change induced by disturbance relative to the reference condition (control). Only entries from single disturbance events without subsequent salvage logging were considered in this second analysis step. We used multiple linear regression analysis to examine the size and statistical significance of disturbance effects on indicators of carbon storage and biodiversity. To generalize the disturbance regime and allow a comparison across studies we used time since disturbance (in years) and disturbance severity (i.e. proportion of timber volume, basal area, or forest area affected by disturbance, using a scale of 0–1) as covariates in the analysis. These parameters were recently used by Miller, Roxburgh & Shea (2011) in an attempt to generalize disturbance effects on diversity. We analysed the residuals of our regression models for trends as well as for temporal autocorrelation (using a Durbin–Watson test), and found support for the assumptions of homoscedasticity and independence. From these regression models we analysed both the intercepts (i.e. the standardized effect at fixed severity and time since disturbance) and slopes (i.e. how the disturbance effect changes with time and severity). To aid the interpretation of the former we transformed severity to 1–severity in our analysis, making the intercept a standardized effect of 100% severity. Additionally, we fitted multiple linear regression models with disturbance agents and biomes as covariates in order to test for the generality of our findings across agents and geographical locations.
III. RESULTS
(1). Disturbance effects on ecosystem services and biodiversity
Overall, 478 studies from the boreal (34.9%), cool (47.1%) and warm temperate (18.0%) biomes addressing effects of disturbances on forest ecosystems were reviewed. The overwhelming majority of articles originated from North America (63.8%), followed by Europe (21.3%) and Australasia (8.8%) (Fig. 1, Table 1). With regard to disturbance agents the effects of forest fires were addressed most frequently (78.0%), while only 15.4% of studies investigated impacts of wind and 6.6% of bark beetles. 60.9% of the research results compiled in our database were empirical, while 19.3% were based on expert opinion, 16.0% derived from simulation studies, and the remaining 3.8% either investigations based on remote sensing, public questionnaires or a combination of different approaches (Table 2). Studies from recent years were overrepresented in our database, with publications on disturbance impact increasing at a rate of approximately 3.1 papers per year between 1996 and 2012 (before 1996 the number of studies was sparse and irregular). This rate of increase of +11.9% year−1 is considerably higher than that of the general literature on, e.g. ecosystem management, which was +7.0% over the same period (Seidl, 2014).
Figure 1.
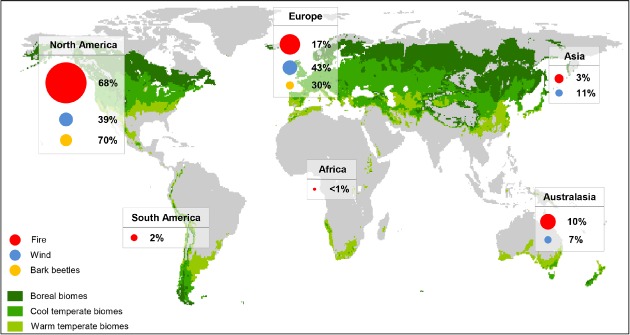
Geographical distribution of papers addressing the impacts of fire (red, comprising wildfire and prescribed burning), wind (blue) and bark beetles (orange) on ecosystem services and biodiversity. The size of the circles represents the number of peer‐reviewed papers per agent and region, while percentages indicate the relative share of disturbance agents per continent. The focal areas of our analysis were the boreal, cool‐ and warm‐temperate biomes as defined by Holdridge (1947, modified using World Clim data), illustrated here in different shades of green.
Overall, there is strong evidence for a distinct impact of disturbances on criteria relevant to ecosystem management, with only 19.3% of entries in our database showing no or mixed effects of disturbance. The fact that in our sample of the literature negative impacts (45.1%) and positive effects (35.6%) were nearly equally distributed confirms the hypothesized disturbance paradox in ecosystem management. These divergent impacts are primarily driven by the disparity of disturbance effects on biodiversity and ecosystem services (Fig. 2). We found that all ecosystem service categories [i.e. supporting, provisioning, regulating and cultural services (see online Fig. S1)] were affected predominately negatively by disturbance (P < 0.001). At the level of individual ecosystem service indicators, the only investigated aspect that was positively influenced was albedo (Fig. 3), as related to the climate change mitigation function of forest ecosystems (Jin et al., 2012). Timber and primary production, fresh‐water provisioning as well as protection against gravitational natural hazards were found to be predominately negatively affected by disturbances. Moreover, the large majority of studies reported a negative disturbance impact on carbon storage, mainly due to a reduction of live biomass in the ecosystem. However, there were also some examples of a positive disturbance effect on carbon storage: in a boreal forest ecosystem in Ontario, ALC peaked 92 years after disturbance then declined to a significantly lower level during the following decades, stabilizing 140 years after disturbance (Seedre & Chen, 2010). For the same forest, SOC peaked between 29 and 140 years after disturbance, before decreasing by approximately one‐third over the next 63 years (Chen & Shrestha, 2012). This suggests that not only direct disturbance‐related C losses in ALC but also the enhanced growth of a regenerating forest as well as the rate of decomposition of dead organic matter need to be considered for a comprehensive assessment of disturbance effects on forest C budgets. Overall, however, 96.3% of 27 observations on C cycle impacts indicated a negative effect of disturbances on TEC.
Figure 2.
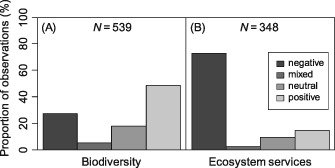
Disturbance effects on (A) biodiversity and (B) ecosystem services. N indicates the number of observations in our database of disturbance effects synthesized from 478 peer‐reviewed articles.
Figure 3.
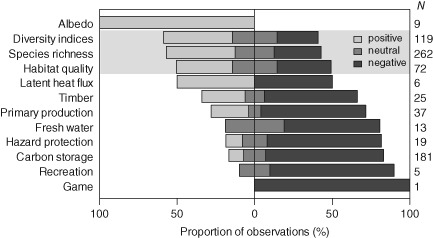
Disturbance effects on indicators of ecosystem services and biodiversity (shaded). Bars show the distribution of positive, neutral and negative disturbance effects per indicator; N denotes the total number of observations. Note that neutral and mixed effects were subsumed under the neutral category here, and that findings based on expert opinions were excluded.
By contrast, we found an overall positive effect of disturbances on biodiversity (P < 0.001). Species richness, habitat quality, and diversity indices were equally positively affected by disturbances. However, the disturbance effect is less consistent for biodiversity than for many ecosystem service indicators, and a number of studies also report negative impacts of disturbances on the indicators of biodiversity investigated here. Hingston & Grove (2010), for example, reported reduced bird species richness in Tasmanian lowland wet eucalypt forests during the first 50 years after wildfire. By contrast, Klaus et al. (2010) found a positive effect of fire on the number of bird species in southern Appalachian upland forests. This illustrates that some species groups might react differently to disturbances depending on the context and specific ecosystem investigated. Also belowground diversity is affected by disturbances, yet dedicated studies are still rare to date. Negative impacts on earthworm biomass and diversity at sites with uprooted trees were reported from areas as different as Belgium and northern Iran (Nachtergale et al., 2002; Kooch & Hosseini, 2010). Another belowground species group that was reported to be negatively affected by windthrow (salvaged) and fire disturbance was Oribatida in the Slovakian High Tatra Mountains (Lóšková et al., 2013). However, a positive impact of fire was reported on soil collembolan diversity in a northern hardwood forest (Huebner, Lindo & Lechowicz, 2012) as well as on soil microbial communities in Spain (Fontúrbel et al., 2012), indicating that disturbances can have both positive and negative impacts on soil diversity. Overall, however, 73.1, 69.8 and 65.3% of studies reported either a positive or neutral response of diversity, species richness and habitat quality, respectively, to disturbance.
At the level of different disturbance agents we found no support for significant differences between the effects of fire, wind, and bark beetles on indicators of biodiversity. With regard to ecosystem services, however, the impacts of fire differed significantly from those of wind and bark beetles (P < 0.001 and P = 0.006, respectively), with the latter agents being more frequently reported to have no influence on ecosystem services. This indicates that bottom‐up disturbances such as fire (i.e. susceptibility decreasing with tree size and/or age) might have different impacts than top‐down disturbances such as wind and bark beetles (where susceptibility increases with tree size and/or age). Differences in disturbance impacts between biomes were evident in our data: the effect of disturbances on ecosystem services differed among the boreal and temperate biomes (P < 0.001 and P = 0.005 for cool‐ and warm‐temperate biomes, respectively), while boreal and cool‐temperate biomes differed with regard to disturbance impacts on biodiversity (P = 0.022). Generally, disturbance effects were least distinctive in the boreal biome, with negative disturbance impacts on ecosystem services more pronounced in the temperate biomes compared to boreal ecosystems. However, disturbances also had a stronger positive effect on biodiversity in the cool‐temperate biome than in the boreal biome.
By comparing results across different types of methodologies, e.g. simulation studies versus empirical approaches, we found some noteworthy deviations from the null hypothesis of consistent disturbance impacts across study methods. Concerning the impacts of disturbances on ecosystem services we found a significant difference between empirical studies and simulation studies (P = 0.030) as well as an indication for differences between empirical studies and expert opinions (P = 0.057), with simulation studies and experts reporting a stronger negative effect than empirical analyses. With regard to the effects of biodiversity, we found that both simulation studies (P = 0.007) and expert opinions (P < 0.001) differed significantly from empirical studies. Here, our data indicate that simulation studies underestimate the positive effects of disturbance on biodiversity compared to empirical analyses, while experts overestimate this positive effect. It is also interesting to note that neutral effects (i.e. no disturbance impact on biodiversity) were more commonly reported in empirical studies than in any other methodological approach.
(2). The effect of salvage logging and prescribed burning
We tested whether the reported disturbance impacts of prescribed burning differed relative to those of wildfires, hypothesizing that controlled burns will have fewer negative effects on ecosystem service provisioning. We found no support for this hypothesis: prescribed burns were more frequently reported to have a negative impact on ecosystem services than wildfires (P < 0.001). Yet, this result must be interpreted with caution as it is based only on a small sample of studies for the effect of prescribed burning (N = 13). With regard to the predominately positive effects of fire on indicators of biodiversity, prescribed burns did not differ significantly from wildfires (P = 0.413).
Another frequently discussed management intervention in the context of disturbance management is salvage logging. Based on previous findings, we hypothesized a negative impact of salvage logging on biodiversity (Lindenmayer et al., 2008). Although a slight trend was evident in our data (i.e. the positive disturbance effect on biodiversity indicators was more pronounced for non‐salvaged forests), it was not significant in our comparison of 38 observations on salvage logging with 145 observations of unsalvaged disturbance effects (P = 0.205). Moreover, with regard to the impact on ecosystem services no significant differences between salvaged and unsalvaged studies were found (P = 0.168), however the data reveal a negative trend for salvaged forests.
(3). The size of disturbance effects on biodiversity and forest carbon storage
Disturbance effects on forest ecosystems differ greatly with disturbance severity and time since disturbance, which is why we studied effect sizes using these two variables as covariates. Time since disturbance significantly explained disturbance effects for all investigated carbon compartments (Table 3). Effects on ALC and DAC were particularly strongly related to this variable, and differences to undisturbed conditions (−91.3 and +155.5% in the first year after disturbance for ALC and DAC, respectively) decreased by +0.6% (ALC) and −1.4% (DAC) on average with every passing year following disturbance. Disturbance severity was not significant in any model, but was retained in the analysis due to its ecological relevance (see also Miller et al., 2011). While the analysis of disturbance impacts on indicators of C storage yielded acceptable coefficients of determination (R 2 from 0.736 to 0.124), the explanatory value of disturbance regime covariates was poor with regard to species richness and entropy. Neither species richness nor entropy was found to differ significantly with time since disturbance and disturbance severity. Tests for differences between agents and biomes overall supported a common global meta‐analysis under consideration of disturbance regime covariates for both response variables (data not shown).
Table 3.
Meta‐analysis (multiple linear regression) of disturbance effects on indicators of carbon and biodiversity (response variables) and their relation to covariates describing the disturbance regime
| Time since disturbance | 1–severity | ||||
|---|---|---|---|---|---|
| Indicator | Coefficient | P‐value | Coefficient | P‐value | R 2 |
| ALC | 0.606 | <0.001 | 33.461 | 0.064 | 0.736 |
| TEC | 0.192 | 0.006 | 12.860 | 0.361 | 0.280 |
| DAC | −1.435 | 0.014 | −477.129 | 0.200 | 0.258 |
| SOC | 0.260 | 0.042 | −9.075 | 0.792 | 0.124 |
| S′ | −0.307 | 0.291 | −19.400 | 0.576 | 0.022 |
| H′ | −2.608 | 0.589 | −175.386 | 0.555 | 0.020 |
ALC, aboveground live carbon; TEC, total ecosystem carbon; DAC, dead aboveground carbon; SOC, soil organic carbon; S′, species richness; H′, species entropy (Shannon‐Index).
The analysis of the standardized disturbance effect (i.e. the calculated impact for a year of an event with 100% severity) showed that indicators of biodiversity as well as deadwood C stocks increased with disturbance, while aboveground and soil carbon stocks decreased (Fig. 4). The mean ± 95% C.I. standardized effect of disturbance on total ecosystem carbon was −38.5 ± 8.3% (P < 0.001), while species richness was significantly increased by +35.6 ± 32.3% (P = 0.035).
Figure 4.
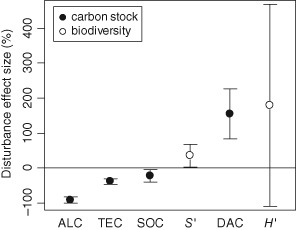
Standardized disturbance effect size (i.e. per cent disturbance‐induced change relative to reference condition) for indicators of carbon stock (filled symbols) and biodiversity (open symbols). Values are standardized coefficients for a disturbance severity level of 100%, and whiskers denote the 95% confidence interval. ALC, aboveground live carbon; TEC, total ecosystem carbon; SOC, soil organic carbon; DAC, dead aboveground carbon; S′: species richness; H′, species entropy (Shannon‐Index).
IV. Discussion
(1). What we know about disturbance impacts on forest ecosystems
We investigated disturbance effects of fire, wind, and bark beetles in a search for general differences in disturbance impacts on ecosystem services and biodiversity. The large number of studies available for analysis not only indicates the importance of disturbance impacts to forest ecosystems, but also provides a suitable basis for a global synthesis on disturbance effects. The increasing number of publications over time may represent a response of the scientific community to the increase in disturbance frequency observed in recent decades (Westerling et al., 2006; Seidl et al., 2014), and should imply a growing understanding of disturbance processes. However, while disturbance impacts on biodiversity are increasingly well researched, we found more variability in information on different ecosystem services. While the main focus of the reviewed papers was on regulating services (predominately on C storage as an important mechanism of climate regulation), supporting and provisioning services are less well studied. The disturbance impact on cultural services has barely been assessed to date (see online Fig. S1).
In addition, the information available on disturbance impacts also differs with disturbance agent and region. The impact of fire on biodiversity and ecosystem services is the most intensively studied disturbance agent, reflecting the dominant role of wildfire in disturbance regimes around the globe (e.g. Conard et al., 2002; Schelhaas et al., 2003; Littell et al., 2009; Newton et al., 2011; Knox & Clarke, 2012). Regional differences were apparent in our database of published studies on disturbance impacts: Asia, for instance, is underrepresented in our analysis; we found only 10 unique studies on disturbance impacts on biodiversity and 11 on ecosystem services for that continent. However, it has to be noted that not the entire geographic imbalance in disturbance studies is likely to be related to regional differences in scientific understanding of disturbance processes. The main cause of such variation in peer‐reviewed information available from different regions is likely to be the language barrier (Powell, 2012). Differences in local research agendas are also likely to play a role (see e.g. Kajala & Watson, 1997). Nonetheless, we advocate research programs that facilitate a broader study of disturbance effects (geographically as well as in terms of the indicators studied), in order to close some of the remaining gaps in our understanding of the role of disturbances in forest ecosystems.
(2). Challenges for synthesizing disturbance impacts
One challenge for a global synthesis lies in a comparison of the different methodological approaches used to study disturbance impacts. Simulation approaches appear to underestimate the effect of disturbances on biodiversity perhaps because current disturbance models are rarely able to assess effects on diversity over a broad variety of guilds. Future improvements in simulation modelling should thus aim to capture the multiple impacts of disturbances better on ecosystems and their diversity (see also Seidl et al., 2011a). Another interesting finding was that expert knowledge differed significantly from the results of empirical studies. Part of this difference could be explained by expert knowledge being reported for different systems and contexts, i.e. systems and indicators that are less well represented by empirical studies. However, the finding that disturbance impacts estimated by experts are more negative on ecosystem services and more positive on biodiversity than those estimated empirically strongly suggests that expert opinions should be omitted from further quantitative analysis (Whittaker, 2010). It should also be noted that our data – like most published literature reviews – are likely to incorporate a degree of publication bias (Møller & Jennions, 2001), i.e. neutral or mixed effects are likely to be underrepresented.
A second challenge relates to the general ability to synthesize the published literature. Although we found a large number of papers dealing with disturbance impacts on biodiversity and carbon storage, only a limited number (18.4 and 22.4%, respectively) could be used in a quantitative meta‐analysis. In most instances we had to exclude studies due to inconclusive reporting of disturbance severity, or the absence of a proper control, consequently making it impossible to quantify the disturbance effect. We thus call for better reporting, especially the inclusion of summary statistics in publications, and advocate a BACI (before – after, control – impact) design (Stewart‐Oaten, Murdoch & Parker, 1986) to facilitate future syntheses on this topic. The increasing requirement to make the results of studies available upon publication, either as an electronic supplement or in archiving services such as Dryad (http://datadryad.org/) should benefit such syntheses in the future. However, some variation in the choice of an appropriate control to disturbed systems is likely to persist, as, for example, the definition of ‘old‐growth’ conditions often differs regionally. Note also that historic land‐use and management practices may influence reference conditions as well as disturbance drivers and impact (e.g. Carcaillet et al., 2009), an aspect that cannot be rectified in a global review and meta‐analysis such as that presented here.
Another difficulty for synthesis and generalization arises from the inherent complexity of disturbance regimes in temperate and boreal forests (see also White & Jentsch, 2001). While we studied three of the most influential disturbance agents globally, other agents of high regional significance were not considered. For example, ash dieback, a disease affecting common ash (Fraxinus excelsior L.) trees of all age‐classes, is currently strongly impacting forest ecosystems in many European countries (Halmschlager & Kirisits, 2008; Ogris, Hauptman & Jurc, 2009), but was not included in this analysis. Our first analysis step revealed significant differences in impact among disturbance agents, documenting that the unique ecology of every agent is important for understanding its effects (e.g. which trees are affected and how). In the second step of our analysis we included severity and time since disturbance as covariates in order to generalize across agents in our meta‐analysis. Tests of this generalization assumption show that differences among agents could be explained satisfactorily with these two covariates (data not shown), enabling a statistical analysis across agents and scales. This underlines the potential for a process‐based analysis of disturbance regimes in synthesizing knowledge from individual observations to reach general patterns and principles (Turner et al., 1993; White & Jentsch, 2001; Miller et al., 2011; Seidl et al., 2011a).
However, this ability to generalize might to some degree be attributed to the inclusion of only temperate and boreal forest ecosystems in our data set. Whether the general patterns deduced for these biomes also hold for tropical forests remains to be tested. Martin, Newton & Bullock (2013), conducted a review on the effects of anthropogenic disturbance on carbon stocks and plant diversity for more than 600 secondary forest sites in the tropics. They show that both biodiversity and carbon storage were negatively affected by clearing (a high‐severity disturbance), and took several decades to recover. Assuming that salvage logging after natural disturbance results in an impact comparable to anthropogenic clearing we here find contrasting results for biodiversity effects in temperate and boreal forests: our data suggest a weak positive effect of disturbance on biodiversity (not significantly affected by salvage logging, P = 0.205). This indicates that further studies are needed to establish whether the disturbance paradox described here also applies to tropical forests.
The existence and strength of simultaneous positive and negative impacts of disturbances on objectives of ecosystem management, described here as the disturbance paradox, might not only vary geographically but is likely also strongly dependent on the indicators selected for analysis, and hence the local relevance of specific ecosystem services and aspects of biodiversity. Generalist species might, for instance, benefit strongly from disturbance events while specialists and late‐seral species – which are often a priority for conservation – could be negatively affected (Devictor & Robert, 2009). Moreover, disturbances might benefit invasive alien species (see e.g. Crawford et al., 2001), widely regarded as negative for biodiversity. Owing to the broad scope of this study such aspects were not explicitly considered in our analysis. They might, however, be of high relevance in local assessments and management decisions, and could thus strongly modify the disturbance paradox, described here based on a global synthesis for boreal and temperate forests. A context‐specific assessment of biodiversity effects at the level of guilds, red‐listed species, and alien/native/endemic species in future studies is thus suggested in order to scrutinize further the generality of the disturbance paradox presented here.
(3). The disturbance paradox and how to address it in ecosystem management
We found strong evidence for the existence of the disturbance paradox in our global analysis of disturbance impact. Disturbance effects on ecosystem services and biodiversity clearly differ in the published literature, with ecosystem services being overall negatively affected while biodiversity is predominately positively influenced by natural disturbances. Our meta‐analysis of the disturbance effect on species richness and total ecosystem carbon storage aptly illustrates this paradox: while species richness increases by 35.6% on average for a high‐severity disturbance event, a simultaneous loss of 38.5% of total ecosystem carbon storage is to be expected. When management goals are to increase carbon storage while at the same time fostering biological diversity, managers are faced with ambiguity with regard to assessing the impact of a disturbance event, and gauging the implications of future disturbance regimes. Are disturbances to be prevented (as far as possible) to reduce negative impacts on ecosystem services, or are they to be welcomed and incorporated into management due to their positive effects on biodiversity?
While our global study cannot resolve this paradox of ecosystem management – which needs to be addressed in the local context of stakeholder preferences, habitat quality, and other constraints – several interesting insights for disturbance management can be deduced from our analysis. Since negative disturbance impacts on carbon storage are strongly reduced with time since disturbance, but positive effects on biodiversity do not vary significantly over time, our global meta‐analysis suggests that managing for a low‐ to medium‐frequency disturbance regime would result in limited impacts on provisioning services while still benefiting biodiversity. In other words, our data indicate that the disturbance event itself matters for biodiversity, while having enough time between these events ensures recovery of ecosystem services. Albeit not significant in our analysis, the same is true with regard to severity, i.e. moderate‐ or mixed‐severity disturbances (see e.g. Perry et al., 2011) are likely to be the best balance between negative effects on ecosystem services and positive effects on biodiversity. Traditional disturbance management approaches such as salvage harvesting and prescribed burning, for instance, are not able to moderate between negative ecosystem service impacts and positive diversity effects according to our analysis. We even found a higher proportion of papers reporting negative effects from prescribed burning on ecosystem services provisioning compared to wildfire. However, due to sample‐size limitations we were not able to analyse these data for differences in effect size, although differences in severity (i.e. mean severity over all studies for prescribed burning = 26.2%, wildfire = 88.1%) suggest a positive effect of prescribed burning (Hurteau & North, 2009; Meigs et al., 2009).
Ongoing climatic changes will likely increase disturbance frequency and severity in many parts of the world (Li et al., 2013; Temperli et al., 2013; Seidl et al., 2014) which – according to our findings – may have negative implications for ecosystem service provisioning. Hence, adaptation of forest ecosystems to such changes in disturbance regime is of great importance in current forest ecosystem management, in order to sustain future ecosystem services provisioning to society. However, as many important drivers of the disturbance regime such as species composition respond to management changes only on time scales of decades to centuries (e.g. Hicke & Jenkins, 2008; Thom et al., 2013), such management considerations need to take long lead‐times into account. On the other hand, our analysis indicates that intensifying disturbance regimes may also represent an opportunity to foster biodiversity in forest ecosystems, and might thus to some degree alleviate the ongoing biodiversity crisis (Stuart et al., 2004; Thomas et al., 2004). In this context it is interesting to note that more diverse ecosystems are often more resistant and resilient to disturbance impacts (Bengtsson et al., 2000), so that in the long term disturbance effects on ecosystem services might be buffered by increasing structural and compositional diversity.
V. CONCLUSIONS
(1) Over the last decades, the number of peer‐reviewed publications on forest disturbances and their effects has increased, mirroring the increasing relevance of disturbance regimes and the changes therein. However, the available literature is heterogeneously distributed over agents and regions, with most studies addressing forests in North America and Europe, and mainly focusing on fire impacts.
(2) Disturbances in forest ecosystems can have both positive and negative impacts on objectives relevant to ecosystem management. We here find that ecosystem services of all four categories defined by the MEA (2005) (provisioning, supporting, regulating, and cultural) are predominately negatively impacted by natural disturbances. Biological diversity, as represented by species richness, habitat quality, and diversity indices is, on the other hand, predominately positively affected by natural disturbances.
(3) In a meta‐analysis we determined that on average a disturbance event decreases total ecosystem carbon by 38.5% (standardized coefficient for a stand‐replacing disturbance event in the year of the disturbance), while species richness increases by on average 35.6%.
(4) For ecosystem management, which aims to provide ecosystem services sustainably to society while preserving and fostering biodiversity, these divergent disturbance impacts present a paradox – they are at the same time risk factors and facilitators of management objectives. Our analysis suggests that measures of disturbance management such as salvage logging and prescribed burning do not significantly moderate these diverging impacts. However, a meta‐analysis of carbon storage (an important regulating service in the context of climate change mitigation) and biodiversity suggests that managing for a disturbance regime of low to medium frequency and severity could limit impacts on ecosystem services while still being beneficial for biodiversity.
(5) Our review suggests that intensifying disturbance regimes under climate change will largely benefit biological diversity of forest ecosystems. Ecosystem services provisioning on the other hand will mostly be negatively impacted by such changes in the disturbance regime. This might require a timely adaptation to changing disturbance regimes in order to provide important ecosystem services sustainably in the future.
Supporting information
Fig. S1. Reported disturbance effects on biodiversity and ecosystem service categories (following the definition of the Millenium Ecosystem Assessment, 2005): (A) biodiversity, (B) supporting services, (C) provisioning services, (D) regulation services and (E) cultural services. N indicates the number of observations.
Appendix S1. Indicators of biodiversity and ecosystem services and their respective synonyms used in the literature search.
Appendix S2. Database of disturbance impacts on ecosystem services and biodiversity derived from the literature.
VI. ACKNOWLEDGEMENTS
This study was supported by the project ‘Climate sensitivity of disturbance regimes and implications for forest ecosystem management’ (DICE), funded by the Austrian Science Fund FWF (grant P 25503‐B16). R. Seidl acknowledges further support by a European Commission's Marie Curie Career Integration Grant (PCIG12‐GA‐2012‐334104). We thank M. Pedro and F. Pasztor as well as two anonymous reviewers for helpful comments on an earlier version of the manuscript.
VII. REFERENCES
References marked with asterisk have been cited within the supporting information.
- *Abbott, I. , Liddelow, G. L. , Vellios, C. V. , Mellican, A. E. & Williams, M. R. (2011). Forestcheck: the response of birds to silviculture in jarrah (Eucalyptus marginata) forest. Australian Forestry 74, 328–335. [Google Scholar]
- *Abrams, M. D. , Sprugel, D. G. & Dickmann, D. I. (1985). Multiple successional pathways on recently disturbed jack pine sites in Michigan. Forest Ecology and Management 10, 31–48. [Google Scholar]
- *Ager, A. A. , Finney, M. A. , Kerns, B. K. & Maffei, H. (2007). Modeling wildfire risk to northern spotted owl (Strix occidentalis caurina) habitat in Central Oregon, USA. Forest Ecology and Management 246, 45–56. [Google Scholar]
- *Amiro, B. D. , Barr, A. G. , Black, T. A. , Iwashita, H. , Kljun, N. , McCaughey, J. H. , Morgenstern, K. , Murayama, S. , Nesic, Z. , Orchansky, A. L. & Saigusa, N. (2006). Carbon, energy and water fluxes at mature and disturbed forest sites, Saskatchewan, Canada. Agricultural and Forest Meteorology 136, 237–251. [Google Scholar]
- *Amiro, B. D. , Chen, J. M. & Liu, J. (2000). Net primary productivity following forest fire for Canadian ecoregions. Canadian Journal of Forest Research 30, 939–947. [Google Scholar]
- *Ammann, M. , Böll, A. , Rickli, C. , Speck, T. & Holdenrieder, O. (2009). Significance of tree root decomposition for shallow landslides. Forest Snow and Landscape Research 82, 79–94. [Google Scholar]
- *Aravena, J. C. , Carmona, M. R. , Pérez, C. A. & Armesto, J. J. (2002). Changes in tree species richness, stand structure and soil properties in a successional chronosequence in northern Chiloé Island, Chile. Revista Chilena de Historia Natural 75, 339–360. [Google Scholar]
- *Arkle, R. S. & Pilliod, D. S. (2010). Prescribed fires as ecological surrogates for wildfires: a stream and riparian perspective. Forest Ecology and Management 259, 893–903. [Google Scholar]
- *Armstrong, G. W. (2004). Sustainability of timber supply considering the risk of wildfire. Forest Science 50, 626–639. [Google Scholar]
- *Arthur, M. A. , Paratley, R. D. & Blankenship, B. A. (1998). Single and repeated fires affect survival and regeneration of woody and herbaceous species in an oak‐pine forest. Journal of the Torrey Botanical Society 125, 225–236. [Google Scholar]
- *Azeria, E. T. , Bouchard, M. , Pothier, D. , Fortin, D. & Hébert, C. (2011). Using biodiversity deconstruction to disentangle assembly and diversity dynamics of understorey plants along post‐fire succession in boreal forest. Global Ecology and Biogeography 20, 119–133. [Google Scholar]
- *Azevedo, J. C. , Possacos, A. , Aguiar, C. F. , Amado, A. , Miguel, L. , Dias, R. , Loureiro, C. & Fernandes, P. M. (2013). The role of holm oak edges in the control of disturbance and conservation of plant diversity in fire‐prone landscapes. Forest Ecology and Management 297, 37–48. [Google Scholar]
- *Bachelet, D. , Neilson, R. P. , Lenihan, J. M. & Drapek, R. J. (2004). Regional differences in the carbon source‐sink potential of natural vegetation in the U.S.A. Environmental Management 33, S23–S43. [Google Scholar]
- *Balshi, M. S. , McGuire, A. D. , Duffy, P. , Flannigan, M. , Kicklighter, D. W. & Melillo, J. (2009). Vulnerability of carbon storage in North American boreal forests to wildfires during the 21st century. Global Change Biology 15, 1491–1509. [Google Scholar]
- Balshi, M. S. , McGuire, A. D. , Zhuang, Q. , Melillo, J. , Kicklighter, D. W. , Kasischke, E. , Wirth, C. , Flannigan, M. , Harden, J. , Clein, J. S. , Burnside, T. J. , McAllister, J. , Kurz, W. A. , Apps, M. & Shvidenko, A. (2007). The role of historical fire disturbance in the carbon dynamics of the pan‐boreal region: a process‐based analysis. Journal of Geophysical Research 112, 1–18. [Google Scholar]
- *Banfield, G. E. , Bhatti, J. S. , Jiang, H. & Apps, M. J. (2002). Variability in regional scale estimates of carbon stocks in boreal forest ecosystems: results from West‐Central Alberta. Forest Ecology and Management 169, 15–27. [Google Scholar]
- *Banks, S. C. , Blyton, M. D. J. , Blair, D. , McBurney, L. & Lindenmayer, D. B. (2012). Adaptive responses and disruptive effects: how major wildfire influences kinship‐based social interactions in a forest marsupial. Molecular Ecology 21, 673–684. [DOI] [PubMed] [Google Scholar]
- *Banks, S. C. , Knight, E. J. , McBurney, L. , Blair, D. & Lindenmayer, D. B. (2011). The effects of wildfire on mortality and resources for an arboreal marsupial: resilience to fire events but susceptibility to fire regime change. PLoS One 6, e22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Bansal, S. , Nilsson, M.‐C. & Wardle, D. A. (2012). Response of photosynthetic carbon gain to ecosystem retrogression of vascular plants and mosses in the boreal forest. Oecologia 169, 661–672. [DOI] [PubMed] [Google Scholar]
- *Barton, A. M. , Swetnam, T. W. & Baisan, C. H. (2001). Arizona pine (Pinus arizonica) stand dynamics: local and regional factors in a fire‐prone madrean gallery forest of Southeast Arizona, USA. Landscape Ecology 16, 351–369. [Google Scholar]
- *Bässler, C. , Müller, J. , Svoboda, M. , Lepšová, A. , Hahn, C. , Holzer, H. & Pouska, V. (2012). Diversity of wood‐decaying fungi under different disturbance regimes‐A case study from spruce mountain forests. Biodiversity and Conservation 21, 33–49. [Google Scholar]
- *Bebi, P. , Teich, M. , Hagedorn, F. , Zurbriggen, N. , Brunner, S. H. & Grêt‐Regamey, A. (2012). Changes in forest cover and ecosystem services in Davos under climate change [Veränderung von Wald und Waldleistungen in der Landschaft Davos im Zuge des Klimawandels]. Schweizerische Zeitschrift für Forstwesen 163, 493–501. [Google Scholar]
- *Bekessy, S. A. , Wintle, B. A. , Gordon, A. , Fox, J. C. , Chisholm, R. , Brown, B. , Regan, T. , Mooney, N. , Read, S. M. & Burgman, M. A. (2009). Modelling human impacts on the Tasmanian wedge‐tailed eagle (Aquila audax fleayi). Biological Conservation 142, 2438–2448. [Google Scholar]
- *Bendix, J. & Cowell, C. M. (2010). Impacts of wildfire on the composition and structure of riparian forests in Southern California. Ecosystems 13, 99–107. [Google Scholar]
- Bengtsson, J. , Nilsson, S. G. , Franc, A. & Menozzi, P. (2000). Biodiversity, disturbances, ecosystem function and management of European forests. Forest Ecology and Management 132, 39–50. [Google Scholar]
- Bergeron, Y. , Leduc, A. , Harvey, B. D. & Gauthier, S. (2002). Natural fire regime: a guide for sustainable management of the Canadian boreal forest. Silva Fennica 36, 81–95. [Google Scholar]
- *Berglund, H. , Jönsson, M. T. , Penttilä, R. & Vanha‐Majamaa, I. (2011). The effects of burning and dead‐wood creation on the diversity of pioneer wood‐inhabiting fungi in managed boreal spruce forests. Forest Ecology and Management 261, 1293–1305. [Google Scholar]
- *Bess, E. C. , Parmenter, R. R. , McCoy, S. & Molles, M. C. Jr. (2002). Responses of a riparian forest‐floor arthropod community to wildfire in the middle Rio Grande Valley, New Mexico. Environmental Entomology 31, 774–784. [Google Scholar]
- *Bhardwaj, M. , Uniyal, V. P. , Sanyal, A. K. & Singh, A. P. (2012). Butterfly communities along an elevational gradient in the Tons valley, Western Himalayas: implications of rapid assessment for insect conservation. Journal of Asia‐Pacific Entomology 15, 207–217. [Google Scholar]
- *Bjune, A. E. , Ohlson, M. , Birks, H. J. B. & Bradshaw, R. H. W. (2009). The development and local stand‐scale dynamics of a Picea abies forest in southeastern Norway. Holocene 19, 1073–1082. [Google Scholar]
- *Blais, J. M. , France, R. L. , Kimpe, L. E. & Cornett, R. J. (1998). Climatic changes in northwestern Ontario have had a greater effect on erosion and sediment accumulation than logging and fire: evidence from 210Pb chronology in lake sediments. Biogeochemistry 43, 235–252. [Google Scholar]
- *Blarquez, O. , Bremond, L. & Carcaillet, C. (2010). Holocene fires and a herb‐dominated understorey track wetter climates in subalpine forests. Journal of Ecology 98, 1358–1368. [Google Scholar]
- *Blarquez, O. , Carcaillet, C. , Bremond, L. , Mourier, B. & Radakovitch, O. (2010. b). Trees in the subalpine belt since 11 700 cal. BP: origin, expansion and alteration of the modern forest. Holocene 20, 139–146. [Google Scholar]
- *Bogle, T. & van Kooten, G. C. (2012). Why mountain pine beetle exacerbates a principal‐agent relationship: exploring strategic policy responses to beetle attack in a mixed species forest. Canadian Journal of Forest Research 42, 621–630. [Google Scholar]
- *Bogle, T. & van Kooten, G. C. (2013). Options for maintaining forest productivity after natural disturbance: a principal‐agent approach. Forest Policy and Economics 26, 138–144. [Google Scholar]
- *Bond‐Lamberty, B. , Peckham, S. D. , Ahl, D. E. & Gower, S. T. (2007). Fire as the dominant driver of central Canadian boreal forest carbon balance. Nature 450, 89–92. [DOI] [PubMed] [Google Scholar]
- *Bork, E. W. , Hudson, R. J. & Bailey, A. W. (1997). Populus forest characterization in Elk Island National Park relative to herbivory, prescribed fire, and topography. Canadian Journal of Botany 75, 1518–1526. [Google Scholar]
- *Boucher, J. , Azeria, E. T. , Ibarzabal, J. & Hébert, C. (2012). Saproxylic beetles in disturbed boreal forests: temporal dynamics, habitat associations, and community structure. Ecoscience 19, 328–343. [Google Scholar]
- *Bouget, C. (2005). Short‐term effect of windstorm disturbance on saproxylic beetles in broadleaved temperate forests – Part I: do environmental changes induce a gap effect? Forest Ecology and Management 216, 1–14. [Google Scholar]
- *Bourg, N. A. , Mcshea, W. J. & Gill, D. E. (2005). Putting a CART before the search: successful habitat prediction for a rare forest herb. Ecology 86, 2793–2804. [Google Scholar]
- *Bradford, J. B. , Fraver, S. , Milo, A. M. , D'Amato, A. W. , Palik, B. & Shinneman, D. J. (2012). Effects of multiple interacting disturbances and salvage logging on forest carbon stocks. Forest Ecology and Management 267, 209–214. [Google Scholar]
- *Braithwaite, N. T. & Mallik, A. U. (2012). Edge effects of wildfire and riparian buffers along boreal forest streams. Journal of Applied Ecology 49, 192–201. [Google Scholar]
- *Brawn, J. D. (2006). Effects of restoring oak savannas on bird communities and populations. Conservation Biology 20, 460–469. [DOI] [PubMed] [Google Scholar]
- *Brewer, J. S. , Bertz, C. A. , Cannon, J. B. , Chesser, J. D. & Maynard, E. E. (2012). Do natural disturbances or the forestry practices that follow them convert forests to early‐successional communities? Ecological Applications 22, 442–458. [DOI] [PubMed] [Google Scholar]
- *Bright, B. C. , Hicke, J. A. & Hudak, A. T. (2012). Estimating aboveground carbon stocks of a forest affected by mountain pine beetle in Idaho using lidar and multispectral imagery. Remote Sensing of Environment 124, 270–281. [Google Scholar]
- *Brown, M. , Black, T. A. , Nesic, Z. , Foord, V. N. , Spittlehouse, D. L. , Fredeen, A. L. , Grant, N. J. , Burton, P. J. & Trofymow, J. A. (2010). Impact of mountain pine beetle on the net ecosystem production of lodgepole pine stands in British Columbia. Agricultural and Forest Meteorology 150, 254–264. [Google Scholar]
- *Brown, C. D. & Johnstone, J. F. (2011). How does increased fire frequency affect carbon loss from fire? A case study in the northern boreal forest. International Journal of Wildland Fire 20, 829–837. [Google Scholar]
- *Brudvig, L. A. , Wagner, S. A. & Damschen, E. I. (2012). Corridors promote fire via connectivity and edge effects. Ecological Applications 22, 937–946. [DOI] [PubMed] [Google Scholar]
- *Buchalski, M. R. , Fontaine, J. B. , Heady, P. A. III , Hayes, J. P. & Frick, W. F. (2013). Bat response to differing fire severity in mixed‐conifer forest California, USA. PLoS One 8, e57884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Buddle, C. M. , Langor, D. W. , Pohl, G. R. & Spence, J. R. (2006). Arthropod responses to harvesting and wildfire: implications for emulation of natural disturbance in forest management. Biological Conservation 128, 346–357. [Google Scholar]
- *Buhk, C. , Götzenberger, L. , Wesche, K. , Gómez, P. S. & Hensen, I. (2006). Post‐fire regeneration in a Mediterranean pine forest with historically low fire frequency. Acta Oecologica 30, 288–298. [Google Scholar]
- *Buscardo, E. , Rodríguez‐Echeverría, S. , Barrico, L. , García, M. Á. , Freitas, H. , Martín, M. P. , De Angelis, P. & Muller, L. A. H. (2012). Is the potential for the formation of common mycorrhizal networks influenced by fire frequency? Soil Biology and Biochemistry 46, 136–144. [Google Scholar]
- *Buscardo, E. , Rodríguez‐Echeverría, S. , Martín, M. P. , De Angelis, P. , Pereira, J. S. & Freitas, H. (2010). Impact of wildfire return interval on the ectomycorrhizal resistant propagules communities of a Mediterranean open forest. Fungal Biology 114, 628–636. [DOI] [PubMed] [Google Scholar]
- *Busing, R. T. , White, R. D. , Harmon, M. E. & White, P. S. (2009). Hurricane disturbance in a temperate deciduous forest: patch dynamics, tree mortality, and coarse woody detritus. Plant Ecology 201, 351–363. [Google Scholar]
- Byrne, K. E. & Mitchell, S. J. (2013). Testing of WindFIRM/ForestGALES_BC: a hybrid‐mechanistic model for predicting windthrow in partially harvested stands. Forestry 86, 185–199. [Google Scholar]
- *Camp, A. E. (1999). Age structure and species composition changes resulting from altered disturbance regimes on the eastern slopes of the cascades range, Washington. Journal of Sustainable Forestry 9, 39–67. [Google Scholar]
- *Campbell, I. D. & Campbell, C. (2000). Late holocene vegetation and fire history at the southern boreal forest margin in Alberta, Canada. Palaeogeography, Palaeoclimatology, Palaeoecology 164, 263–280. [Google Scholar]
- *Campbell, J. W. , Hanula, J. L. & Waldrop, T. A. (2007). Effects of prescribed fire and fire surrogates on floral visiting insects of the blue ridge province in North Carolina. Biological Conservation 134, 393–404. [Google Scholar]
- *Campbell, J. W. , Hanula, J. L. & Waldrop, T. A. (2008). Effects of prescribed fire and fire surrogates on saproxylic Coleoptera in the southern Appalachians of North Carolina. Journal of Entomological Science 43, 57–75. [Google Scholar]
- Canadell, J. G. & Raupach, M. R. (2008). Managing forests for climate change mitigation. Science 320, 1456–1457. [DOI] [PubMed] [Google Scholar]
- *Capitanio, R. & Carcaillet, C. (2008). Post‐fire Mediterranean vegetation dynamics and diversity: a discussion of succession models. Forest Ecology and Management 255, 431–439. [Google Scholar]
- Carcaillet, C. , Ali, A. A. , Blarquez, O. , Genries, A. , Mourier, B. & Bremond, L. (2009). Spatial variability of fire history in subalpine forests: from natural to cultural regimes. Ecoscience 16, 1–12. [Google Scholar]
- *Carlson, C. H. , Dobrowski, S. Z. & Safford, H. D. (2012). Variation in tree mortality and regeneration affect forest carbon recovery following fuel treatments and wildfire in the Lake Tahoe Basin, California, USA. Carbon Balance and Management 7, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Carmona, M. R. , Armesto, J. J. , Aravena, J. C. & Pérez, C. A. (2002). Coarse woody debris biomass in successional and primary temperate forests in Chiloé Island, Chile. Forest Ecology and Management 164, 265–275. [Google Scholar]
- Carnus, J. , Parrotta, J. , Brockerhoff, E. , Arbez, M. , Kremer, A. , Lamb, D. , Hara, K. O. & Walters, B. (2006). Planted forests and biodiversity. Journal of Forestry 104, 65–77. [Google Scholar]
- *Catling, P. (2009). Vascular plant diversity in burned and unburned alvar woodland: more evidence of the importance of disturbance to biodiversity and conservation. Canadian Field‐Naturalist 123, 240–245. [Google Scholar]
- Chapin, F. S. , McGuire, A. D. , Randerson, J. , Pielke, R. SR. , Baldocchi, D. , Hobbie, S. E. , Roulet, N. , Eugster, W. , Kasischke, E. , Rastetter, E. B. , Zimov, A. S. & Running, S. W. (2000). Arctic and boreal ecosystems of western North America as components of the climate system. Global Change Biology 6, 211–223. [DOI] [PubMed] [Google Scholar]
- *Chapman, J. I. & McEwan, R. W. (2012). Tree regeneration ecology of an old‐growth central Appalachian forest: diversity, temporal dynamics, and disturbance response. Journal of the Torrey Botanical Society 139, 194–205. [Google Scholar]
- *Chen, W. , Chen, J. & Cihlar, J. (2000). An integrated terrestrial ecosystem carbon‐budget model based on changes in disturbance, climate, and atmospheric chemistry. Ecological Modelling 135, 55–79. [Google Scholar]
- *Chen, J. M. , Ju, W. , Cihlar, J. , Price, D. , Liu, J. , Chen, W. , Pan, J. , Black, A. & Barr, A. (2003). Spatial distribution of carbon sources and sinks in Canada's forests. Tellus 55B, 622–641. [Google Scholar]
- Chen, H. Y. H. & Shrestha, B. M. (2012). Stand age, fire and clearcutting affect soil organic carbon and aggregation of mineral soils in boreal forests. Soil Biology and Biochemistry 50, 149–157. [Google Scholar]
- *Chertov, O. , Bhatti, J. S. , Komarov, A. , Mikhailov, A. & Bykhovets, S. (2009). Influence of climate change, fire and harvest on the carbon dynamics of black spruce in Central Canada. Forest Ecology and Management 257, 941–950. [Google Scholar]
- *Chiang, J.‐M. , McEwan, R. W. , Yaussy, D. A. & Brown, K. J. (2008). The effects of prescribed fire and silvicultural thinning on the aboveground carbon stocks and net primary production of overstory trees in an oak‐hickory ecosystem in southern Ohio. Forest Ecology and Management 255, 1584–1594. [Google Scholar]
- *Chipman, S. J. & Johnson, E. A. (2002). Understory vascular plant species diversity in the mixedwood boreal forest of western Canada. Ecological Applications 12, 588–601. [Google Scholar]
- *Cissel, J. H. , Swanson, F. J. , Grant, G. E. , Olson, D. H. , Gregory, S. V. , Garman, S. L. , Ashkenas, L. R. , Hunter, M. G. , Kertis, J. A. , Mayo, J. H. , McSwain, M. D. , Swetland, S. G. , Swindle, K. A. & Wallin, D. O. (1998). A landscape plan based on historical fire regimes for a managed forest ecosystem: the Augusta Creek study. USDA Forest Service ‐ General Technical Report PNW 422, 1–82. [Google Scholar]
- *Claridge, A. W. , Trappe, J. M. , Mills, D. J. & Claridge, D. L. (2009). Diversity and habitat relationships of hypogeous fungi. III. Factors influencing the occurrence of fire‐adapted species. Mycological Research 113, 792–801. [DOI] [PubMed] [Google Scholar]
- *Clark, D. A. , Anthony, R. G. & Andrews, L. S. (2013). Relationship between wildfire, salvage logging, and occupancy of nesting territories by northern spotted owls. Journal of Wildlife Management 77, 672–688. [Google Scholar]
- *Clark, J. S. , Daniel Royall, P. & Chumbley, C. (1996). The role of fire during climate change in an eastern deciduous forest at Devil's Bathtub, New York. Ecology 77, 2148–2166. [Google Scholar]
- *Clavero, M. , Brotons, L. & Herrando, S. (2011). Bird community specialization, bird conservation and disturbance: the role of wildfires. Journal of Animal Ecology 80, 128–136. [DOI] [PubMed] [Google Scholar]
- *Cobb, T. P. , Langor, D. W. & Spence, J. R. (2007). Biodiversity and multiple disturbances: boreal forest ground beetle (Coleoptera: Carabidae) responses to wildfire, harvesting, and herbicide. Canadian Journal of Forest Research 37, 1310–1323. [Google Scholar]
- *Cobb, T. P. , Morissette, J. L. , Jacobs, J. M. , Koivula, M. J. , Spence, J. R. & Langor, D. W. (2011). Effects of postfire salvage logging on deadwood‐associated beetles [Efectos del Rescate de Madera Después de Incendios sobre Escarabajos Asociados a Madera Muerta]. Conservation Biology 25, 1310–1323. [DOI] [PubMed] [Google Scholar]
- *Coleman, T. W. & Rieske, L. K. (2006). Arthropod response to prescription burning at the soil‐litter interface in oak‐pine forests. Forest Ecology and Management 233, 52–60. [Google Scholar]
- *Colombaroli, D. , Beckmann, M. , van der Knaap, W. O. , Curdy, P. & Tinner, W. (2013). Changes in biodiversity and vegetation composition in the central Swiss Alps during the transition from pristine forest to first farming. Diversity and Distributions 19, 157–170. [Google Scholar]
- *Colombaroli, D. , Tinner, W. , Van Leeuwen, J. , Noti, R. , Vescovi, E. , Vannière, B. , Magny, M. , Schmidt, R. & Bugmann, H. (2009). Response of broadleaved evergreen Mediterranean forest vegetation to fire disturbance during the Holocene: insights from the peri‐Adriatic region. Journal of Biogeography 36, 314–326. [Google Scholar]
- *Colombo, S. J. , Chen, J. , Ter‐Mikaelian, M. T. , McKechnie, J. , Elkie, P. C. , MacLean, H. L. & Heath, L. S. (2012). Forest protection and forest harvest as strategies for ecological sustainability and climate change mitigation. Forest Ecology and Management 281, 140–151. [Google Scholar]
- Conard, S. G. , Sukhinin, A. I. , Stocks, B. J. , Cahoon, D. R. , Davidenko, E. P. & Ivanova, G. A. (2002). Determining effects of area burned and fire severity on carbon cycling and emissions in Siberia. Climatic Change 55, 197–211. [Google Scholar]
- *Concilio, A. , Ma, S. , Ryu, S.‐R. , North, M. & Chen, J. (2006). Soil respiration response to experimental disturbances over 3 years. Forest Ecology and Management 228, 82–90. [Google Scholar]
- *Converse, S. J. , White, G. C. & Block, W. M. (2006). Small mammal responses to thinning and wildfire in ponderosa pine‐dominated forests of the southwestern United States. Journal of Wildlife Management 70, 1711–1722. [Google Scholar]
- *Coomes, D. A. , Holdaway, R. J. , Kobe, R. K. , Lines, E. R. & Allen, R. B. (2012). A general integrative framework for modelling woody biomass production and carbon sequestration rates in forests. Journal of Ecology 100, 42–64. [Google Scholar]
- *Coops, N. C. & Wulder, M. A. (2010). Estimating the reduction in gross primary production due to mountain pine beetle infestation using satellite observations. International Journal of Remote Sensing 31, 2129–2138. [Google Scholar]
- *Cott, P. , Zajdlik, B. , Bourassa, K. J. , Lange, M. & Gordon, A. M. (2010). Effects of forest fire on young‐of‐the‐year Northern Pike, Esox lucius, in the Northwest Territories. Canadian Field‐Naturalist 124, 104–112. [Google Scholar]
- *Coursolle, C. , Margolis, H. A. , Barr, A. G. , Black, T. A. , Amiro, B. D. , McCaughey, J. H. , Flanagan, L. B. , Lafleur, P. M. , Roulet, N. T. , Bourque, C. P.‐A. , Arain, M. A. , Wofsy, S. C. , Dunn, A. , Morgenstern, K. , Orchansky, A. L. , Bernier, P. Y. , Chen, J. M. , Kidston, J. , Saigusa, N. & Hedstrom, N. (2006). Late‐summer carbon fluxes from Canadian forests and peatlands along an east‐west continental transect. Canadian Journal of Forest Research 36, 783–800. [Google Scholar]
- *Cowden, C. C. & Peterson, C. J. (2013). Annual and seasonal dynamics of ectomycorrhizal fungi colonizing white pine (Pinus strobus) seedlings following catastrophic windthrow in northern Georgia, USA. Canadian Journal of Forest Research 43, 215–223. [Google Scholar]
- *Craig, M. D. , Hobbs, R. J. , Grigg, A. H. , Garkaklis, M. J. , Grant, C. D. , Fleming, P. A. & Hardy, G. E. S. J. (2010). Do thinning and burning sites revegetated after bauxite mining improve habitat for terrestrial vertebrates? Restoration Ecology 18, 300–310. [Google Scholar]
- Crawford, J. A. , Wahren, C.‐H. A. , Kyle, S. & Moir, W. H. (2001). Responses of exotic plant species to fires in Pinus ponderosa forests in northern Arizona. Journal of Vegetation Science 12, 261–268. [Google Scholar]
- *Cruise, G. M. , Macphail, R. I. , Linderholm, J. , Maggi, R. & Marshall, P. D. (2009). Lago di Bargone, Liguria, N Italy: a reconstruction of Holocene environmental and land‐use history. Holocene 19, 987–1003. [Google Scholar]
- *Cuchta, P. , Miklisová, D. & Kovác, L. (2012. a). Changes within collembolan communities in windthrown European montane spruce forests 2 years after disturbance by fire. Annals of Forest Science 69, 81–92. [Google Scholar]
- *Cuchta, P. , Miklisová, D. & Kovác, L. (2012. b). The impact of disturbance and ensuing forestry practices on Collembola in monitored stands of windthrown forest in the Tatra National Park (Slovakia). Environmental Monitoring and Assessment 185, 5085–5098. [DOI] [PubMed] [Google Scholar]
- *Czimczik, C. I. , Preston, C. M. , Schmidt, M. W. I. & Schulze, E.‐D. (2003). How surface fire in Siberian Scots pine forests affects soil organic carbon in the forest floor: stocks, molecular structure, and conversion to black carbon (charcoal). Global Biogeochemical Cycles 17, 1–13. [Google Scholar]
- *Daly, C. , Bachelet, D. , Lenihan, J. M. , Neilson, R. P. , Parton, W. & Ojima, D. (2000). Dynamic simulation of tree‐grass interactions for global change studies. Ecological Applications 10, 449–469. [Google Scholar]
- *D'Amato, A. W. , Fraver, S. , Palik, B. J. , Bradford, J. B. & Patty, L. (2011). Singular and interactive effects of blowdown, salvage logging, and wildfire in sub‐boreal pine systems. Forest Ecology and Management 262, 2070–2078. [Google Scholar]
- *Davis, M. R. , Allen, R. B. & Clinton, P. W. (2003). Carbon storage along a stand development sequence in a New Zealand Nothofagus forest. Forest Ecology and Management 177, 313–321. [Google Scholar]
- *Dawson, R. D. & Bortolotti, G. R. (2006). Fire in the boreal forest: proximate effects on reproduction and long‐term consequences for territory occupancy of American kestrels. Ecoscience 13, 75–81. [Google Scholar]
- *Death, R. G. , Baillie, B. & Fransen, P. (2003). Effect of Pinus radiata logging on stream invertebrate communities in Hawke's Bay, New Zealand. New Zealand Journal of Marine and Freshwater Research 37, 507–520. [Google Scholar]
- *DeGayner, E. J. , Kramer, M. G. , Doerr, J. G. & Robertsen, M. J. (2005). Windstorm disturbance effects on forest structure and black bear dens in southeast Alaska. Ecological Applications 15, 1306–1316. [Google Scholar]
- *Degen, T. , Devillez, F. & Jacquemart, A.‐L. (2005). Gaps promote plant diversity in beech forests (Luzulo‐Fagetum), North Vosges, France. Annals of Forest Science 62, 429–440. [Google Scholar]
- *Delong, S. C. & Kessler, W. B. (2000). Ecological characteristics of mature forest remnants left by wildfire. Forest Ecology and Management 131, 93–106. [Google Scholar]
- *Delong, S. C. & Tanner, D. (1996). Managing the pattern of forest harvest: lessons from wildfire. Biodiversity and Conservation 5, 1191–1205. [Google Scholar]
- *DeSantis, R. D. , Hallgren, S. W. , Lynch, T. B. , Burton, J. A. & Palmer, M. W. (2010). Long‐term directional changes in upland Quercus forests throughout Oklahoma, USA. Journal of Vegetation Science 21, 606–618. [Google Scholar]
- Devictor, V. & Robert, A. (2009). Measuring community responses to large‐scale disturbance in conservation biogeography. Diversity and Distributions 15, 122–130. [Google Scholar]
- *Diadema, K. , Médail, F. & Bretagnolle, F. (2007). Fire as a control agent of demographic structure and plant performance of a rare Mediterranean endemic geophyte. Comptes Rendus ‐ Biologies 330, 691–700. [DOI] [PubMed] [Google Scholar]
- *Dickson, B. G. , Noon, B. R. , Flather, C. H. , Jentsch, S. & Block, W. M. (2009). Quantifying the multi‐scale response of avifauna to prescribed fire experiments in the southwest United States. Ecological Applications 19, 608–621. [DOI] [PubMed] [Google Scholar]
- *Dodge, R. S. , Fulé, P. Z. & Hull Sieg, C. (2008). Dalmatian toadflax (Linaria dalmatica) response to wildfire in a southwestern USA forest. Ecoscience 15, 213–222. [Google Scholar]
- *Dodson, E. K. , Metlen, K. L. & Fiedler, C. E. (2007). Common and uncommon understory species differentially respond to restoration treatments in ponderosa pine/Douglas‐fir forests, Montana. Restoration Ecology 15, 696–708. [Google Scholar]
- *Dodson, E. K. & Peterson, D. W. (2009). Seeding and fertilization effects on plant cover and community recovery following wildfire in the Eastern Cascade Mountains, USA. Forest Ecology and Management 258, 1586–1593. [Google Scholar]
- *Dodson, E. K. , Peterson, D. W. & Harrod, R. J. (2008). Understory vegetation response to thinning and burning restoration treatments in dry conifer forests of the eastern Cascades, USA. Forest Ecology and Management 255, 3130–3140. [Google Scholar]
- *Don, A. , Bärwolff, M. , Kalbitz, K. , Andruschkewitsch, R. , Jungkunst, H. F. & Schulze, E.‐D. (2012). No rapid soil carbon loss after a windthrow event in the High Tatra. Forest Ecology and Management 276, 239–246. [Google Scholar]
- Donato, D. C. , Fontaine, J. B. , Campbell, J. L. , Robinson, W. D. , Kauffman, J. B. & Law, B. E. (2006). Post‐wildfire logging hinders regeneration and increases fire risk. Science (New York, N.Y.) 311, 352. [DOI] [PubMed] [Google Scholar]
- *Donato, D. C. , Fontaine, J. B. , Robinson, W. D. , Kauffman, J. B. & Law, B. E. (2009). Vegetation response to a short interval between high‐severity wildfires in a mixed‐evergreen forest. Journal of Ecology 97, 142–154. [Google Scholar]
- *Donner, D. M. , Probst, J. R. & Ribic, C. A. (2008). Influence of habitat amount, arrangement, and use on population trend estimates of male Kirtland's warblers. Landscape Ecology 23, 467–480. [Google Scholar]
- *Dore, S. , Kolb, T. E. , Montes‐Helu, M. , Eckert, S. E. , Sullivan, B. W. , Hungate, B. A. , Kaye, J. P. , Hart, S. C. , Koch, G. W. & Finkral, A. (2010). Carbon and water fluxes from ponderosa pine forests disturbed by wildfire and thinning. Ecological Applications 20, 663–683. [DOI] [PubMed] [Google Scholar]
- *Dore, S. , Kolb, T. E. , Montes‐Helu, M. , Sullivan, B. W. , Winslow, W. D. , Hart, S. C. , Kaye, J. P. , Koch, G. W. & Hungate, B. A. (2008). Long‐term impact of a stand‐replacing fire on ecosystem CO2 exchange of a ponderosa pine forest. Global Change Biology 14, 1801–1820. [Google Scholar]
- *Dore, S. , Montes‐Helu, M. , Hart, S. C. , Hungate, B. A. , Koch, G. W. , Moon, J. B. , Finkral, A. J. & Kolb, T. E. (2012). Recovery of ponderosa pine ecosystem carbon and water fluxes from thinning and stand‐replacing fire. Global Change Biology 18, 3171–3185. [DOI] [PubMed] [Google Scholar]
- *Driscoll, D. A. , Kirkpatrick, J. B. , McQuillan, P. B. & Bonham, K. J. (2010). Classic metapopulations are rare among common beetle species from a naturally fragmented landscape. Journal of Animal Ecology 79, 294–303. [DOI] [PubMed] [Google Scholar]
- *Dun, S. , Wu, J. Q. , Elliot, W. J. , Robichaud, P. R. , Flanagan, D. C. , Frankenberger, J. R. , Brown, R. E. & Xu, A. C. (2009). Adapting the Water Erosion Prediction Project (WEPP) model for forest applications. Journal of Hydrology 366, 46–54. [Google Scholar]
- *Dunford, J. S. , McLoughlin, P. D. , Dalerum, F. & Boutin, S. (2006). Lichen abundance in the peatlands of northern Alberta: implications for boreal caribou. Ecoscience 13, 469–474. [Google Scholar]
- *Elliott, K. J. , Hitchcock, S. L. & Krueger, L. (2002). Vegetation response to large scale disturbance in a southern Appalachian forest: hurricane Opal and salvage logging. Journal of the Torrey Botanical Society 129, 48–59. [Google Scholar]
- *Elliott, K. J. & Vose, J. M. (2005). Effects of understory prescribed burning on shortleaf pine (Pinus echinata Mill.)/mixed‐hardwood forests. Journal of the Torrey Botanical Society 132, 236–251. [Google Scholar]
- *Emelko, M. B. , Silins, U. , Bladon, K. D. & Stone, M. (2011). Implications of land disturbance on drinking water treatability in a changing climate: demonstrating the need for “source water supply and protection” strategies. Water Research 45, 461–472. [DOI] [PubMed] [Google Scholar]
- *Euskirchen, E. S. , McGuire, A. D. , Rupp, T. S. , Chapin, F. S. III & Walsh, J. E. (2009). Projected changes in atmospheric heating due to changes in fire disturbance and the snow season in the western Arctic, 2003–2100. Journal of Geophysical Research 114, G04022. [Google Scholar]
- *Everett, R. , Schellhaas, D. , Spurbeck, D. , Ohlson, P. , Keenum, D. & Anderson, T. (1997). Structure of northern spotted owl nest stands and their historical conditions on the eastern slope of the Pacific Northwest Cascades, USA. Forest Ecology and Management 94, 1–14. [Google Scholar]
- FAO (2010). Forest health and vitality In Global Forest Resources Assessment 2010, pp. 65–84, Food and Agriculture Organization of the United Nations (FAO), Rome. [Google Scholar]
- *Farr, J. D. , Wills, A. J. , Van Heurck, P. F. , Mellican, A. E. & Williams, M. R. (2011). Forestcheck: the response of macro‐invertebrates to silviculture in jarrah (Eucalyptus marginata) forest. Australian Forestry 74, 315–327. [Google Scholar]
- *Fenton, N. J. & Bergeron, Y. (2008). Does time or habitat make old‐growth forests species rich? Bryophyte richness in boreal Picea mariana forests. Biological Conservation 141, 1389–1399. [Google Scholar]
- *Ffolliott, P. F. , Stropki, C. L. , Chen, H. & Neary, D. G. (2011). The 2002 Rodeo‐Chediski Wildfire's Impacts on Southwestern Ponderosa Pine Ecosystems, Hydrology, and Fuels. Research Paper RMRS‐RP 85 RP. USDA Forest Service, Fort Collins. [Google Scholar]
- *Flint, C. , Qin, H. & Ganning, J. P. (2012). Linking local perceptions to the biophysical and amenity contexts of forest disturbance in Colorado. Environmental Management 49, 553–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Fontaine, J. B. , Donato, D. C. , Robinson, W. D. , Law, B. E. & Kauffman, J. B. (2009). Bird communities following high‐severity fire: response to single and repeat fires in a mixed‐evergreen forest, Oregon, USA. Forest Ecology and Management 257, 1496–1504. [Google Scholar]
- Fontúrbel, M. T. , Barreiro, A. , Vega, J. A. , Martín, A. , Jiménez, E. , Carballas, T. , Fernández, C. & Díaz‐Raviña, M. (2012). Effects of an experimental fire and post‐fire stabilization treatments on soil microbial communities. Geoderma 191, 51–60. [Google Scholar]
- *Franklin, S. B. , Robertson, P. A. & Fralish, J. S. (2003). Prescribed burning effects on upland Quercus forest structure and function. Forest Ecology and Management 184, 315–335. [Google Scholar]
- Franklin, J. F. , Spies, T. A. , Van Pelt, R. , Carey, A. B. , Thornburgh, D. A. , Berg, D. R. , Lindenmayer, D. B. , Harmon, M. E. , Keeton, W. S. , Shaw, D. C. , Bible, K. & Chen, J. (2002). Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas‐fir forests as an example. Forest Ecology and Management 155, 399–423. [Google Scholar]
- *Fukui, D. , Hirao, T. , Murakami, M. & Hirakawa, H. (2011). Effects of treefall gaps created by windthrow on bat assemblages in a temperate forest. Forest Ecology and Management 261, 1546–1552. [Google Scholar]
- *Fulé, P. Z. , Covington, W. W. , Smith, H. B. , Springer, J. D. , Heinlein, T. A. , Huisinga, K. D. & Moore, M. M. (2002). Comparing ecological restoration alternatives: Grand Canyon, Arizona. Forest Ecology and Management 170, 19–41. [Google Scholar]
- *Gaboury, S. , Boucher, J.‐F. , Villeneuve, C. , Lord, D. & Gagnon, R. (2009). Estimating the net carbon balance of boreal open woodland afforestation: a case‐study in Québec's closed‐crown boreal forest. Forest Ecology and Management 257, 483–494. [Google Scholar]
- *Gagnon, P. R. , Passmore, H. A. & Platt, W. J. (2013). Multi‐year salutary effects of windstorm and fire on river cane. Fire Ecology 9, 55–65. [Google Scholar]
- *Gaines, W. L. , Harrod, R. J. , Dickinson, J. , Lyons, A. L. & Halupka, K. (2010). Integration of Northern spotted owl habitat and fuels treatments in the eastern Cascades, Washington, USA. Forest Ecology and Management 260, 2045–2052. [Google Scholar]
- *Gandhi, K. J. K. , Gilmore, D. W. , Haack, R. A. , Katovich, S. A. , Krauth, S. J. , Mattson, W. J. , Zasada, J. C. & Seybold, S. J. (2010). Application of semiochemicals to assess the biodiversity of subcortical insects following an ecosystem disturbance in a sub‐boreal forest. Journal of Chemical Ecology 35, 1384–1410. [DOI] [PubMed] [Google Scholar]
- *Gandhi, K. J. K. , Gilmore, D. W. , Katovich, S. A. , Mattson, W. J. , Zasada, J. C. & Seybold, S. J. (2008). Catastrophic windstorm and fuel‐reduction treatments alter ground beetle (Coleoptera: Carabidae) assemblages in a North American sub‐boreal forest. Forest Ecology and Management 256, 1104–1123. [Google Scholar]
- *Garbalinska, P. & Sklodowski, J. (2008). Body size differentiation in selected carabid species inhabiting puszcza piska forest stands disturbed by the hurricane. Baltic Journal of Coleopterology 8, 101–114. [Google Scholar]
- Gardiner, B. , Blennow, K. , Carnus, J.‐M. , Fleischer, P. , Ingemarson, F. , Landmann, G. , Lindner, M. , Marzano, M. , Nicoll, B. , Orazio, C. , Peyron, J.‐L. , Reviron, M.‐P. , Schelhaas, M.‐J. , Schuck, A. , Spielmann, M. & Usbeck, T. (2010). Destructive Storms in European Forests: Past and Forthcoming Impacts. EUROPEAN FOREST INSTITUTE: Atlantic European Regional Office – EFIATLANTIC, Cestas. [Google Scholar]
- *Gaulton, R. , Hilker, T. , Wulder, M. A. , Coops, N. C. & Stenhouse, G. (2011). Characterizing stand‐replacing disturbance in western Alberta grizzly bear habitat, using a satellite‐derived high temporal and spatial resolution change sequence. Forest Ecology and Management 261, 865–877. [Google Scholar]
- *George, C. , Rowland, C. , Gerard, F. & Balzter, H. (2006). Retrospective mapping of burnt areas in Central Siberia using a modification of the normalised difference water index. Remote Sensing of Environment 104, 346–359. [Google Scholar]
- *Gerald, G. W. , Bailey, M. A. & Holmes, J. N. (2006). Habitat utilization of Pituophis melanoleucus melanoleucus (Northern Pinesnakes) on Arnold Air Force Base in Middle Tennessee. Southeastern Naturalist 5, 253–264. [Google Scholar]
- Glasgow, L. S. & Matlack, G. R. (2007). Prescribed burning and understory composition in a temperate deciduous forest, Ohio, USA. Forest Ecology and Management 238, 54–64. [Google Scholar]
- *Glaubitz, J. C. , Wu, H. X. & Moran, G. F. (2003). Impacts of silviculture on genetic diversity in the native forest species Eucalyptus sieberi . Conservation Genetics 4, 275–287. [Google Scholar]
- *González‐Tagle, M. A. , Schwendenmann, L. , Pérez, J. J. & Schulz, R. (2008). Forest structure and woody plant species composition along a fire chronosequence in mixed pine‐oak forest in the Sierra Madre Oriental, Northeast Mexico. Forest Ecology and Management 256, 161–167. [Google Scholar]
- *Grant, R. F. , Black, T. A. , Gaumont‐Guay, D. , Klujn, N. , Barr, A. G. , Morgenstern, K. & Nesic, Z. (2006). Net ecosystem productivity of boreal aspen forests under drought and climate change: mathematical modelling with Ecosys. Agricultural and Forest Meteorology 140, 152–170. [Google Scholar]
- *Greenberg, C. H. (2001). Response of reptile and amphibian communities to canopy gaps created by wind disturbance in the southern Appalachians. Forest Ecology and Management 148, 135–144. [Google Scholar]
- *Greenberg, C. H. (2002). Response of white‐footed mice (Peromyscus leucopus) to coarse woody debris and microsite use in southern Appalachian treefall gaps. Forest Ecology and Management 164, 57–66. [Google Scholar]
- *Greenberg, C. H. & Lanham, J. D. (2001). Breeding bird assemblages of hurricane‐created gaps and adjacent closed canopy forest in the southern Appalachians. Forest Ecology and Management 154, 251–260. [Google Scholar]
- *Greenberg, C. H. & Miller, S. (2004). Soricid response to canopy gaps created by wind disturbance in the southern Appalachians. Southeastern Naturalist 3, 715–732. [Google Scholar]
- *Griffis, K. L. , Crawford, J. A. , Wagner, M. R. & Moir, W. H. (2001). Understory response to management treatments in northern Arizona ponderosa pine forests. Forest Ecology and Management 146, 239–245. [Google Scholar]
- *Gustafson, E. J. , Shvidenko, A. Z. , Sturtevant, B. R. & Scheller, R. M. (2010). Predicting global change effects on forest biomass and composition in south‐central Siberia. Ecological Applications 20, 700–715. [DOI] [PubMed] [Google Scholar]
- *Haeussler, S. , Bergeron, Y. , Brais, S. & Harvey, B. D. (2007). Natural dynamics‐based silviculture for maintaining plant biodiversity in Populus tremuloides‐dominated boreal forests of eastern Canada. Canadian Journal of Botany 85, 1158–1170. [Google Scholar]
- *Hagemann, U. , Moroni, M. T. , Shaw, C. H. , Kurz, W. A. & Makeschin, F. (2010). Comparing measured and modelled forest carbon stocks in high‐boreal forests of harvest and natural‐disturbance origin in Labrador, Canada. Ecological Modelling 221, 825–839. [Google Scholar]
- *Haimi, J. , Fritze, H. & Moilanen, P. (2000). Responses of soil decomposer animals to wood‐ash fertilisation and burning in a coniferous forest stand. Forest Ecology and Management 129, 53–61. [Google Scholar]
- Halmschlager, E. & Kirisits, T. (2008). First report of the ash dieback pathogen Chalara fraxinea on Fraxinus excelsior in Austria. Plant Pathology 57, 1177. [Google Scholar]
- *Hamlin, B. T. , Kittredge, W. T. , Lubin, D. P. & Wright, E. B. (2012). Changes in the vascular flora of the middlesex fells reservation, Middlesex County, Massachusetts, from 1895 to 2011. Rhodora 114, 229–308. [Google Scholar]
- *Hancock, M. H. , Amphlett, A. , Proctor, R. , Dugan, D. , Willi, J. , Harvey, P. & Summers, R. W. (2011). Burning and mowing as habitat management for capercaillie Tetrao urogallus: an experimental test. Forest Ecology and Management 262, 509–521. [Google Scholar]
- Hansen, M. C. , Stehman, S. V. & Potapov, P. V. (2010). Quantification of global gross forest cover loss. Proceedings of the National Academy of Sciences of the United States of America 107, 8650–8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hart, S. A. & Chen, H. Y. H. (2008). Fire, logging, and overstory affect understory abundance, diversity, and composition in boreal forest. Ecological Monographs 78, 123–140. [Google Scholar]
- *Harvey, B. J. & Holzman, B. A. (2013). Divergent successional pathways of stand development following fire in a California closed‐cone pine forest. Journal of Vegetation Science 1, 89–99. [Google Scholar]
- *Hayes, D. J. , McGuire, A. D. , Kicklighter, D. W. , Gurney, K. R. , Burnside, T. J. & Melillo, J. M. (2011). Is the northern high‐latitude land‐based CO 2 sink weakening? Global Biogeochemical Cycles 25, GB3018. [Google Scholar]
- *Henderson, M. K. & Keith, D. A. (2002). Correlation of burning and grazing indicators with composition of woody understorey flora of dells in a temperate eucalypt forest. Austral Ecology 27, 121–131. [Google Scholar]
- *Hernández‐Rodríguez, M. , Oria‐de‐Rueda, J. A. & Martín‐Pinto, P. (2013). Post‐fire fungal succession in a Mediterranean ecosystem dominated by Cistus ladanifer L. Forest Ecology and Management 289, 48–57. [Google Scholar]
- *Herrando, S. & Brotons, L. (2002). Forest bird diversity in Mediterranean areas affected by wildfires: a multi‐scale approach. Ecography 25, 161–172. [Google Scholar]
- *Hicke, J. A. , Asner, G. P. , Kasischke, E. S. , French, N. H. F. , Randerson, J. T. , Collatz, G. J. , Stocks, B. J. , Tucker, C. J. , Los, S. O. & Field, C. B. (2003). Postfire response of North American boreal forest net primary productivity analyzed with satellite observations. Global Change Biology 9, 1145–1157. [Google Scholar]
- Hicke, J. A. & Jenkins, J. C. (2008). Mapping lodgepole pine stand structure susceptibility to mountain pine beetle attack across the western United States. Forest Ecology and Management 255, 1536–1547. [Google Scholar]
- *Hindrum, L. , Hovenden, M. J. , Neyland, M. G. & Baker, S. C. (2012). The effects of mechanical disturbance and burn intensity on the floristic composition of two‐year old aggregated retention coupes in Tasmanian wet eucalypt forests. Forest Ecology and Management 279, 55–65. [Google Scholar]
- Hingston, A. B. & Grove, S. (2010). From clearfell coupe to old‐growth forest: succession of bird assemblages in Tasmanian lowland wet eucalypt forests. Forest Ecology and Management 259, 459–468. [Google Scholar]
- *Hirao, T. , Murakami, M. , Iwamoto, J. , Takafumi, H. & Oguma, H. (2008). Scale‐dependent effects of windthrow disturbance on forest arthropod communities. Ecological Research 23, 189–196. [Google Scholar]
- *Hockaday, W. C. , Masiello, C. A. , Randerson, J. T. , Smernik, R. J. , Baldock, J. A. , Chadwick, O. A. & Harden, J. W. (2009). Measurement of soil carbon oxidation state and oxidative ratio by 13C nuclear magnetic resonance. Journal of Geophysical Research 114, G02014. [Google Scholar]
- Hoekstra, J. M. , Boucher, T. M. , Ricketts, T. H. & Roberts, C. (2005). Confronting a biome crisis: global disparities of habitat loss and protection. Ecology Letters 8, 23–29. [Google Scholar]
- *Holden, S. R. , Gutierrez, A. & Treseder, K. K. (2013). Changes in soil fungal communities, extracellular enzyme activities, and litter decomposition across a fire chronosequence in Alaskan boreal forests. Ecosystems 16, 34–46. [Google Scholar]
- Holdridge, L. R. (1947). Determination of world plant formations from simple climatic data. Science 105, 367–368. [DOI] [PubMed] [Google Scholar]
- *Holzmueller, E. J. , Jose, S. & Jenkins, M. A. (2009). The response of understory species composition, diversity, and seedling regeneration to repeated burning in southern Appalachian oak‐hickory forests. Natural Areas Journal 29, 255–262. [Google Scholar]
- *Hopton, M. E. , Cameron, G. N. , Cramer, M. J. , Polak, M. & Uetz, G. W. (2009). Live animal radiography to measure developmental instability in populations of small mammals after a natural disaster. Ecological Indicators 9, 883–891. [Google Scholar]
- *Horrocks, M. , Nichol, S. L. , Gregory, M. R. , Creese, R. & Augustinus, P. C. (2001). A holocene pollen and sediment record of Whangape Harbour, far Northern New Zealand. Journal of the Royal Society of New Zealand 31, 411–424. [Google Scholar]
- *Hossack, B. R. , Eby, L. A. , Guscio, C. G. & Corn, P. S. (2009). Thermal characteristics of amphibian microhabitats in a fire‐disturbed landscape. Forest Ecology and Management 258, 1414–1421. [Google Scholar]
- *Hossack, B. R. , Lowe, W. H. , Honeycutt, R. K. , Parks, S. A. & Corn, P. S. (2013. a). Interactive effects of wildfire, forest management, and isolation on amphibian and parasite abundance. Ecological Applications 23, 479–492. [DOI] [PubMed] [Google Scholar]
- *Hossack, B. R. , Lowe, W. H. , Ware, J. L. & Corn, P. S. (2013. b). Disease in a dynamic landscape: host behavior and wildfire reduce amphibian chytrid infection. Biological Conservation 157, 293–299. [Google Scholar]
- Hothorn, T. , Hornik, K. , van de Wiel, M. A. & Zeileis, A. (2008). Implementing a class of permutation tests: the coin Package. Journal of Statistical Software 28, 1–23. [Google Scholar]
- Hothorn, T. , Hornik, K. , van de Wiel, M. A. & Zeileis, A. (2013). coin: conditional inference procedures in a permutation test framework.
- *Houseman, G. R. & Anderson, R. C. (2002). Effects of jack pine plantation management on barrens flora and potential Kirtland's warbler nest habitat. Restoration Ecology 10, 27–36. [Google Scholar]
- *Huang, S. , Liu, H. , Dahal, D. , Jin, S. , Welp, L. R. , Liu, J. & Liu, S. (2013). Modeling spatially explicit fire impact on gross primary production in interior Alaska using satellite images coupled with eddy covariance. Remote Sensing of Environment 135, 178–188. [Google Scholar]
- *Huber, C. (2005). Long lasting nitrate leaching after bark beetle attack in the highlands of the Bavarian Forest National Park. Journal of Environmental Quality 34, 1772–1779. [DOI] [PubMed] [Google Scholar]
- *Huber, C. , Aherne, J. , Weis, W. , Farrell, E. P. , Göttlein, A. & Cummins, T. (2010). Ion concentrations and fluxes of seepage water before and after clear cutting of Norway spruce stands at Ballyhooly, Ireland, and Höglwald, Germany. Biogeochemistry 101, 7–26. [Google Scholar]
- *Hudiburg, T. , Law, B. , Turner, D. P. , Campbell, J. , Donato, D. & Duane, M. (2009). Carbon dynamics of Oregon and Northern California forests and potential land‐based carbon storage. Ecological Applications 19, 163–180. [DOI] [PubMed] [Google Scholar]
- Huebner, K. , Lindo, Z. & Lechowicz, M. J. (2012). Post‐fire succession of collembolan communities in a northern hardwood forest. European Journal of Soil Biology 48, 59–65. [Google Scholar]
- *Huisinga, K. D. , Laughlin, D. C. , Fulé, P. Z. , Springer, J. D. & McGlone, C. M. (2005). Effects of an intense prescribed fire on understory vegetation in a mixed conifer forest. Journal of the Torrey Botanical Society 132, 590–601. [Google Scholar]
- *Hunt, L. M. & Haider, W. (2004). Aesthetic impacts of disturbances on selected boreal forested shorelines. Forest Science 50, 729–738. [Google Scholar]
- *Hurteau, M. D. , Hungate, B. A. & Koch, G. W. (2009). Accounting for risk in valuing forest carbon offsets. Carbon Balance and Management 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hurteau, M. & North, M. (2008). Mixed‐conifer understory response to climate change, nitrogen, and fire. Global Change Biology 14, 1543–1552. [Google Scholar]
- Hurteau, M. & North, M. (2009). Fuel treatment effects on tree‐based forest carbon storage and emissions under modeled wildfire scenarios. Frontiers in Ecology and the Environment 7, 409–414. [Google Scholar]
- *Hurteau, M. D. , Stoddard, M. T. & Fulé, P. Z. (2011). The carbon costs of mitigating high‐severity wildfire in southwestern ponderosa pine. Global Change Biology 17, 1516–1521. [Google Scholar]
- Huston, M. A. & Marland, G. (2003). Carbon management and biodiversity. Journal of Environmental Management 67, 77–86. [DOI] [PubMed] [Google Scholar]
- *Hylander, K. & Johnson, S. (2010). In situ survival of forest bryophytes in small‐scale refugia after an intense forest fire. Journal of Vegetation Science 21, 1099–1109. [Google Scholar]
- *Iglay, R. B. , Miller, D. A. , Leopold, B. D. & Wang, G. (2012). Carabid beetle response to prescribed fire and herbicide in intensively managed, mid‐rotation pine stands in Mississippi. Forest Ecology and Management 281, 41–47. [Google Scholar]
- *Imbeau, L. , Savard, J.‐P. L. & Gagnon, R. (1999). Comparing bird assemblages in successional black spruce stands originating from fire and logging. Canadian Journal of Zoology 77, 1850–1860. [Google Scholar]
- Jactel, H. , Nicoll, B. C. , Branco, M. , Gonzalez‐Olabarria, J. R. , Grodzki, W. , Långström, B. , Moreira, F. , Netherer, S. , Orazio, C. , Piou, D. , Santos, H. , Schelhaas, M. J. , Tojic, K. & Vodde, F. (2009). The influences of forest stand management on biotic and abiotic risks of damage. Annals of Forest Science 66, 701. [Google Scholar]
- *James, P. M. A. , Fortin, M.‐J. , Fall, A. , Kneeshaw, D. & Messier, C. (2007). The effects of spatial legacies following shifting management practices and fire on boreal forest age structure. Ecosystems 10, 1261–1277. [Google Scholar]
- Jin, Y. , Randerson, J. T. , Goulden, M. L. & Goetz, S. J. (2012). Post‐fire changes in net shortwave radiation along a latitudinal gradient in boreal North America. Geophysical Research Letters 39, L13403. [Google Scholar]
- *Jones, M. C. , Booth, R. K. , Yu, Z. & Ferry, P. (2013). A 2200–year record of permafrost dynamics and carbon cycling in a collapse‐scar bog, interior Alaska. Ecosystems 16, 1–19. [Google Scholar]
- *Jönsson, M. T. , Fraver, S. & Jonsson, B. G. (2011). Spatio‐temporal variation of coarse woody debris input in woodland key habitats in Central Sweden. Silva Fennica 45, 957–967. [Google Scholar]
- Kajala, L. & Watson, A. (1997). Wilderness‐‐different cultures, different research needs. International Journal of Wilderness 3, 33–36. [Google Scholar]
- *Kang, S. , Kimball, J. S. & Running, S. W. (2006). Simulating effects of fire disturbance and climate change on boreal forest productivity and evapotranspiration. Science of the Total Environment 362, 85–102. [DOI] [PubMed] [Google Scholar]
- *Kashian, D. M. , Romme, W. H. , Tinker, D. B. , Turner, M. G. & Ryan, M. G. (2013). Postfire changes in forest carbon storage over a 300–year chronosequence of Pinus contorta‐dominated forests. Ecological Monographs 83, 49–66. [Google Scholar]
- *Kashian, D. M. , Turner, M. G. & Romme, W. H. (2005). Variability in leaf area and stemwood increment along a 300–year lodgepole pine chronosequence. Ecosystems 8, 48–61. [Google Scholar]
- *Kavgaci, A. , Carni, A. , Basaran, S. , Basaran, M. A. , Košir, P. , Marinšek, A. & Šilc, U. (2010). Long‐term post‐fire succession of Pinus brutia forest in the east Mediterranean. International Journal of Wildland Fire 19, 599–605. [Google Scholar]
- *Kaynas, B. Y. & Gürkan, B. (2005). Changes in Buprestidae (Coleoptera) community with successional age after fire in a Pinus brutia forest. Journal of Pest Science 78, 53–55. [Google Scholar]
- *Keeley, J. E. , Lubin, D. & Fotheringham, C. J. (2003). Fire and grazing impacts on plant diversity and alien plant invasions in the southern Sierra Nevada. Ecological Applications 13, 1355–1374. [Google Scholar]
- *Kennedy, N. & Egger, K. N. (2010). Impact of wildfire intensity and logging on fungal and nitrogen‐cycling bacterial communities in British Columbia forest soils. Forest Ecology and Management 260, 787–794. [Google Scholar]
- *Kennedy, R. S. H. & Wimberly, M. C. (2009). Historical fire and vegetation dynamics in dry forests of the interior Pacific Northwest, USA, and relationships to Northern Spotted Owl (Strix occidentalis caurina) habitat conservation. Forest Ecology and Management 258, 554–566. [Google Scholar]
- Kern, C. C. , Montgomery, R. A. , Reich, P. B. & Strong, T. F. (2014). Harvest‐created canopy gaps increase species and functional trait diversity of the forest ground‐layer community. Forest Science 60, 335–344. [Google Scholar]
- *Kirk, D. A. & Hobson, K. A. (2001). Bird – Habitat relationships in jack pine boreal forests. Forest Ecology and Management 147, 217–243. [Google Scholar]
- *Kirkpatrick, C. & Conway, C. J. (2010). Importance of montane riparian forest and influence of wildfire on nest‐site selection of ground‐nesting birds. Journal of Wildlife Management 74, 729–738. [Google Scholar]
- Klaus, N. A. , Rush, S. A. , Keyes, T. S. , Petrick, J. , Cooper, R. J. , Klaus, N. A. , Rush, S. A. , Keyes, T. I. M. S. , Petrick, J. & Cooper, R. J. (2010). Short‐term effects of fire on breeding birds in southern Appalachian upland forests. The Wilson Journal of Ornithology 122, 518–531. [Google Scholar]
- *Klenner, W. & Arsenault, A. (2009). Ponderosa pine mortality during a severe bark beetle (Coleoptera: Curculionidae, Scolytinae) outbreak in southern British Columbia and implications for wildlife habitat management. Forest Ecology and Management 258, S5–S14. [Google Scholar]
- *Klenner, W. & Walton, R. (2009). Landscape‐level habitat supply modelling to develop and evaluate management practices that maintain diverse forest values in a dry forest ecosystem in southern British Columbia. Forest Ecology and Management 258, S146–S157. [Google Scholar]
- *Knohl, A. , Kolle, O. , Minayeva, T. Y. , Milyukova, I. M. , Vygodskaya, N. N. , Foken, T. & Schulze, E.‐D. (2002). Carbon dioxide exchange of a Russian boreal forest after disturbance by wind throw. Global Change Biology 8, 231–246. [Google Scholar]
- Knox, K. J. E. & Clarke, P. J. (2012). Fire severity, feedback effects and resilience to alternative community states in forest assemblages. Forest Ecology and Management 265, 47–54. [Google Scholar]
- *Koehler, G. M. , Maletzke, B. T. , Von Kienast, J. A. , Aubry, K. B. , Wielgus, R. B. & Naney, R. H. (2008). Habitat fragmentation and the persistence of lynx populations in Washington State. Journal of Wildlife Management 72, 1518–1524. [Google Scholar]
- *Kompa, T. & Schmidt, W. (2005). Plant succession in windthrown beech (Fagus sylvatica) forests on gypsum karst and dolomitic limestone in the Harz Mountain foothills of southern Lower Saxony, Germany [Buchenwald‐Sukzession nach Windwurf auf Zechstein‐Standorten des südwestlichen Harzvorlandes]. Hercynia 38, 233–261. [Google Scholar]
- Kooch, Y. & Hosseini, S. M. (2010). Response of earthworms biomass and diversity to windthrow events and soil properties in Hyrcanian forests of Iran. Folia Oecologica 37, 181–190. [Google Scholar]
- *Kooch, Y. , Hosseini, S. M. & Akbarinia, M. (2008). The ecological effects of pit and mounds created by a windthrow on understory of Hyrcanian forests. Silva Balcanica 9, 13–28. [Google Scholar]
- *Kooch, Y. , Hosseini, S. M. , Mohammadi, J. & Hojjati, S. M. (2012). Effects of uprooting tree on herbaceous species diversity, woody species regeneration status and soil physical characteristics in a temperate mixed forest of Iran. Journal of Forestry Research 23, 81–86. [Google Scholar]
- *Kotliar, N. B. , Kennedy, P. L. & Ferree, K. (2007). Avifaunal responses to fire in southwestern montane forests along a burn severity gradient. Ecological Applications 17, 491–507. [DOI] [PubMed] [Google Scholar]
- *Kucerová, A. , Rektoris, L. , Štechová, T. & Bastl, M. (2008). Disturbances on a wooded raised bog – How windthrow, bark beetle and fire affect vegetation and soil water quality? Folia Geobotanica 43, 49–67. [Google Scholar]
- *Kurz, W. A. & Apps, M. J. (1999). A 70–year retrospective analysis of carbon fluxes in the Canadian Forest Sector. Ecological Applications 9, 526–547. [Google Scholar]
- *Kurz, W. A. , Dymond, C. C. , White, T. M. , Stinson, G. , Shaw, C. H. , Rampley, G. J. , Smyth, C. , Simpson, B. N. , Neilson, E. T. , Trofymow, J. A. , Metsaranta, J. & Apps, M. J. (2009). CBM‐CFS3: a model of carbon‐dynamics in forestry and land‐use change implementing IPCC standards. Ecological Modelling 220, 480–504. [Google Scholar]
- *Kurz, W. A. , Stinson, G. & Rampley, G. (2008). Could increased boreal forest ecosystem productivity offset carbon losses from increased disturbances? Philosophical Transactions of the Royal Society B 363, 2261–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuuluvainen, T. & Aakala, T. (2011). Natural forest dynamics in boreal Fennoscandia: a review and classification. Silva Fennica 45, 823–841. [Google Scholar]
- *Kuuluvainen, T. & Kalmari, R. (2003). Regeneration microsites of Picea abies seedlings in a windthrow area of a boreal old‐growth forest in southern Finland. Annales Botanici Fennici 40, 401–413. [Google Scholar]
- *Lafleur, B. , Parsons, W. F. J. , Bradley, R. L. & Francoeur, A. (2006). Ground‐nesting ant assemblages and their relationships to habitat factors along a chronosequence of postfire‐regenerated lichen‐spruce woodland. Environmental Entomology 35, 1515–1524. [Google Scholar]
- *Lafon, C. W. , Waldron, J. D. , Cairns, D. M. , Tchakerian, M. D. , Coulson, R. N. & Klepzig, K. D. (2007). Modeling the effects of fire on the long‐term dynamics and restoration of Yellow pine and oak forests in the southern Appalachian Mountains. Restoration Ecology 15, 400–411. [Google Scholar]
- *Lain, E. J. , Haney, A. , Burris, J. M. & Burton, J. (2008). Response of vegetation and birds to severe wind disturbance and salvage logging in a southern boreal forest. Forest Ecology and Management 256, 863–871. [Google Scholar]
- *Lane, P. N. J. , Feikema, P. M. , Sherwin, C. B. , Peel, M. C. & Freebairn, A. C. (2010). Modelling the long term water yield impact of wildfire and other forest disturbance in Eucalypt forests. Environmental Modelling and Software 25, 467–478. [Google Scholar]
- *Larrivée, M. , Fahrig, L. & Drapeau, P. (2005). Effects of a recent wildfire and clearcuts on ground‐dwelling boreal forest spider assemblages. Canadian Journal of Forest Research 35, 2575–2588. [Google Scholar]
- *Larsen, K. W. , Adams, I. T. & Haughland, D. L. (2007). Small mammal communities in a pyrogenic habitat mosaic. International Journal of Wildland Fire 16, 728–740. [Google Scholar]
- *Laughlin, D. C. , Bakker, J. D. , Stoddard, M. T. , Daniels, M. L. , Springer, J. D. , Gildar, C. N. , Green, A. M. & Covington, W. W. (2004). Toward reference conditions: wildfire effects on flora in an old‐growth ponderosa pine forest. Forest Ecology and Management 199, 137–152. [Google Scholar]
- *Laughlin, D. C. & Grace, J. B. (2006). A multivariate model of plant species richness in forested systems: old‐growth montane forests with a long history of fire. Oikos 114, 60–70. [Google Scholar]
- *Law, B. E. , Thornton, P. E. , Irvine, J. , Anthoni, P. M. & Van Tuyl, S. (2001). Carbon storage and fluxes in ponderosa pine forests at different developmental stages. Global Change Biology 7, 755–777. [Google Scholar]
- *Law, B. E. , Turner, D. , Campbell, J. , Sun, O. J. , Van Tuyl, S. , Ritts, W. D. & Cohen, W. B. (2004). Disturbance and climate effects on carbon stocks and fluxes across Western Oregon USA. Global Change Biology 10, 1429–1444. [Google Scholar]
- *Lazaruk, L. W. , Kernaghan, G. , Macdonald, S. E. & Khasa, D. (2005). Effects of partial cutting on the ectomycorrhizae of Picea glauca forests in northwestern Alberta. Canadian Journal of Forest Research 35, 1442–1454. [Google Scholar]
- *Lecomte, N. , Simard, M. & Bergeron, Y. (2006. a). Effects of fire severity and initial tree composition on stand structural development in the coniferous boreal forest of northwestern Québec, Canada. Ecoscience 13, 152–163. [Google Scholar]
- *Lecomte, N. , Simard, M. , Fenton, N. & Bergeron, Y. (2006. b). Fire severity and long‐term ecosystem biomass dynamics in coniferous boreal forests of eastern Canada. Ecosystems 9, 1215–1230. [Google Scholar]
- *Lehmkuhl, J. F. , Kie, J. G. , Bender, L. C. , Servheen, G. & Nyberg, H. (2001). Evaluating the effects of ecosystem management alternatives on elk, mule deer, and white‐tailed deer in the interior Columbia River basin, USA. Forest Ecology and Management 153, 89–104. [Google Scholar]
- *Lehnert, L. W. , Bässler, C. , Brandl, R. , Burton, P. J. & Müller, J. (2013). Conservation value of forests attacked by bark beetles: highest number of indicator species is found in early successional stages. Journal for Nature Conservation 21, 97–104. [Google Scholar]
- *Leitner, L. A. , Dunn, C. P. , Guntenspergen, G. R. , Stearns, F. & Sharpe, D. M. (1991). Effects of site, landscape features, and fire regime on vegetation patterns in presettlement southern Wisconsin. Landscape Ecology 5, 203–217. [Google Scholar]
- *Le Page, Y. , Hurtt, G. , Thomson, A. M. , Bond‐Lamberty, B. , Patel, P. , Wise, M. , Calvin, K. , Kyle, P. , Clarke, L. , Edmonds, J. & Janetos, A. (2013). Sensitivity of climate mitigation strategies to natural disturbances. Environmental Research Letters 8, 015018. [Google Scholar]
- *Leroux, S. J. , Schmiegelow, F. K. A. , Cumming, S. G. , Lessard, R. B. & Nagy, J. (2007). Accounting for system dynamics in reserve design. Ecological Applications 17, 1954–1966. [DOI] [PubMed] [Google Scholar]
- *Lewis, D. (2009). Stand and landscape‐level simulations of mountain pine beetle (Dendroctonus ponderosae) and salvage logging effects on live tree and deadwood habitats in south‐central British Columbia, Canada. Forest Ecology and Management 258, S24–S35. [Google Scholar]
- *Li, C. , Flannigan, M. D. & Corns, I. G. W. (2000). Influence of potential climate change on forest landscape dynamics of west‐central Alberta. Canadian Journal of Forest Research 30, 1905–1912. [Google Scholar]
- Li, X. , He, H. S. , Wu, Z. , Liang, Y. & Schneiderman, J. E. (2013). Comparing effects of climate warming, fire, and timber harvesting on a boreal forest landscape in northeastern China. PLoS One 8, e59747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Liechty, H. O. , Jurgensen, M. F. , Mroz, G. D. & Gale, M. R. (1997). Pit and mound topography and its influence on storage of carbon, nitrogen, and organic matter within an old‐growth forest. Canadian Journal of Forest Research 27, 1992–1997. [Google Scholar]
- *Lilja‐Rothsten, S. , De Chantal, M. , Peterson, C. , Kuuluvainen, T. , Vanha‐Majamaa, I. & Puttonen, P. (2008). Microsites before and after restoration in managed Picea abies stands in southern Finland: effects of fire and partial cutting with dead wood creation. Silva Fennica 42, 165–176. [Google Scholar]
- Lindenmayer, D. B. , Burton, P. J. & Franklin, J. F. (2008. a). Salvage Logging and its Ecological Consequences. Island Press, Washington. [Google Scholar]
- *Lindenmayer, D. B. , Wood, J. T. , Cunningham, R. B. , MacGregor, C. , Crane, M. , Michael, D. , Montague‐Drake, R. , Brown, D. , Muntz, R. & Gill, A. M. (2008. b). Testing hypotheses associated with bird responses to wildfire. Ecological Applications 18, 1967–1983. [DOI] [PubMed] [Google Scholar]
- *Lindenmayer, D. B. , Mackey, B. G. , Mullen, I. C. , McCarthy, M. A. , Gill, A. M. , Cunningham, R. B. & Donnelly, C. F. (1999). Factors affecting stand structure in forests – Are there climatic and topographic determinants? Forest Ecology and Management 123, 55–63. [Google Scholar]
- *Lindenmayer, D. & McCarthy, M. A. (2002). Congruence between natural and human forest disturbance: a case study from Australian montane ash forests. Forest Ecology and Management 155, 319–335. [Google Scholar]
- *Linder, P. , Elfving, B. & Zackrisson, O. (1997). Stand structure and successional trends in virgin boreal forest reserves in Sweden. Forest Ecology and Management 98, 17–33. [Google Scholar]
- Lindner, M. , Maroschek, M. , Netherer, S. , Kremer, A. , Barbati, A. , Garcia‐Gonzalo, J. , Seidl, R. , Delzon, S. , Corona, P. , Kolström, M. , Lexer, M. J. & Marchetti, M. (2010). Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. Forest Ecology and Management 259, 698–709. [Google Scholar]
- *Lindroth, A. , Lagergren, F. , Grelle, A. , Klemedtsson, L. , Langvall, O. , Weslien, P. & Tuulik, J. (2009). Storms can cause Europe‐wide reduction in forest carbon sink. Global Change Biology 15, 346–355. [Google Scholar]
- Littell, J. S. , McKenzie, D. , Peterson, D. L. & Westerling, A. L. (2009). Climate and wildfire area burned in western U.S. ecoprovince, 1916–2003. Ecological Applications 19, 1003–1021. [DOI] [PubMed] [Google Scholar]
- *Littell, J. S. , Oneil, E. E. , McKenzie, D. , Hicke, J. A. , Lutz, J. A. , Norheim, R. A. & Elsner, M. M. (2010). Forest ecosystems, disturbance, and climatic change in Washington State, USA. Climatic Change 102, 129–158. [Google Scholar]
- *Litton, C. M. , Ryan, M. G. & Knight, D. H. (2004). Effects of tree density and stand age on carbon allocation patterns in postfire lodgepole pine. Ecological Applications 14, 460–475. [Google Scholar]
- *Liu, J. , Peng, C. , Apps, M. , Dang, Q. , Banfield, E. & Kurz, W. (2002). Historic carbon budgets of Ontario's forest ecosystems. Forest Ecology and Management 169, 103–114. [Google Scholar]
- *Liu, H. , Randerson, J. T. , Lindfors, J. & Chapin, F. S. III (2005). Changes in the surface energy budget after fire in boreal ecosystems of interior Alaska: an annual perspective. Journal of Geophysical Research 110, D13101. [Google Scholar]
- *Liu, J. , Vogelmann, J. E. , Zhu, Z. , Key, C. H. , Sleeter, B. M. , Price, D. T. , Chen, J. M. , Cochrane, M. A. , Eidenshink, J. C. , Howard, S. M. , Bliss, N. B. & Jiang, H. (2011). Estimating California ecosystem carbon change using process model and land cover disturbance data: 1951–2000. Ecological Modelling 222, 2333–2341. [Google Scholar]
- Loisel, P. (2014). Impact of storm risk on Faustmann rotation. Forest Policy and Economics 38, 191–198. [Google Scholar]
- Lóšková, J. , Ľuptáčik, P. , Miklisová, D. & Kováč, Ľ. (2013). The effect of clear‐cutting and wildfire on soil Oribatida (Acari) in windthrown stands of the High Tatra Mountains (Slovakia). European Journal of Soil Biology 55, 131–138. [Google Scholar]
- *Lygis, V. , Vasiliauskaite, I. , Stenlid, J. & Vasaitis, R. (2010). Impact of forest fire on occurrence of Heterobasidion annosum s.s. root rot and other wood‐inhabiting fungi in roots of Pinus mugo . Forestry 83, 83–92. [Google Scholar]
- *Mabry, C. M. , Brudvig, L. A. & Atwell, R. C. (2010). The confluence of landscape context and site‐level management in determining Midwestern savanna and woodland breeding bird communities. Forest Ecology and Management 260, 42–51. [Google Scholar]
- *Maness, H. , Kushner, P. J. & Fung, I. (2013). Summertime climate response to mountain pine beetle disturbance in British Columbia. Nature Geoscience 6, 65–70. [Google Scholar]
- *Mann, D. , Rupp, T. , Olson, M. & Duffy, P. (2012). Is Alaska's boreal forest now crossing a major ecological threshold? Arctic, Antarctic, and Alpine Research 44, 319–331. [Google Scholar]
- *Marozas, V. , Racinskas, J. & Bartkevicius, E. (2007). Dynamics of ground vegetation after surface fires in hemiboreal Pinus sylvestris forests. Forest Ecology and Management 250, 47–55. [Google Scholar]
- *Martikainen, P. , Kouki, J. & Heikkala, O. (2006). The effects of green tree retention and subsequent prescribed burning on ground beetles (Coleoptera: Carabidae) in boreal pine‐dominated forests. Ecography 29, 659–670. [Google Scholar]
- Martin, P. A. , Newton, A. C. & Bullock, J. M. (2013). Carbon pools recover more quickly than plant biodiversity in tropical secondary forests. Proceedings of the Royal Society B: Biological Sciences 281, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Marzano, R. , Lingua, E. & Garbarino, M. (2012). Post‐fire effects and short‐term regeneration dynamics following highseverity crown fires in a Mediterranean forest. IForest 5, 93–100. [Google Scholar]
- *Mateos, E. , Santos, X. & Pujade‐Villar, J. (2011). Taxonomic and functional responses to fire and post‐fire management of a Mediterranean Hymenoptera community. Environmental Management 48, 1000–1012. [DOI] [PubMed] [Google Scholar]
- *Matsuoka, S. M. & Handel, C. M. (2007). Nesting ecology of boreal forest birds following a massive outbreak of spruce beetles. Journal of Wildlife Management 71, 51–63. [Google Scholar]
- *McCarthy, M. A. & Lindenmayer, D. B. (1998). Multi‐aged mountain ash forest, wildlife conservation and timber harvesting. Forest Ecology and Management 104, 43–56. [Google Scholar]
- *McCarthy, M. A. & Lindenmayer, D. B. (1999). Incorporating metapopulation dynamics of greater gliders into reserve design in disturbed landscapes. Ecology 80, 651–667. [Google Scholar]
- *McFarlane, B. L. & Witson, D. O. T. (2008). Perceptions of ecological risk associated with mountain pine beetle (Dendroctonus ponderosae) infestations in Banff and Kootenay National Parks of Canada. Risk Analysis 28, 203–212. [DOI] [PubMed] [Google Scholar]
- *McGlinn, D. J. , Churchwell, R. & Palmer, M. W. (2010). Effects of a tornado on birds in a cross timbers community. Southwestern Naturalist 55, 460–466. [Google Scholar]
- *McLeod, R. F. & Gates, J. E. (1998). Response of herpetofaunal communities to forest cutting and burning at Chesapeake Farms, Maryland. American Midland Naturalist 139, 164–177. [Google Scholar]
- *McWethy, D. B. , Whitlock, C. , Wilmshurst, J. M. , McGlone, M. S. , Fromont, M. , Li, X. , Dieffenbacher‐Krall, A. , Hobbs, W. O. , Fritz, S. C. & Cook, E. R. (2010). Rapid landscape transformation in South Island, New Zealand, following initial Polynesian settlement. Proceedings of the National Academy of Sciences of the United States of America 107, 21343–21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *McWethy, D. B. , Whitlock, C. , Wilmshurst, J. M. , McGlone, M. S. & Li, X. (2009). Rapid deforestation of South Island, New Zealand, by early Polynesian fires. Holocene 19, 883–897. [Google Scholar]
- MEA (2005). Ecosystems and human well‐being: current state and trends In Millennium Ecosystem Assessment (Volume 1, eds Hassan R., Scholes R. and Ash N.), p. 917 Island Press, Washington. [Google Scholar]
- Meddens, A. J. H. , Hicke, J. A. & Ferguson, C. A. (2012). Spatiotemporal patterns of observed bark beetle‐caused tree mortality in British Columbia and the western United States. Ecological Applications 22, 1876–1891. [DOI] [PubMed] [Google Scholar]
- *Mehr, M. , Brandl, R. , Kneib, T. & Müller, J. (2012). The effect of bark beetle infestation and salvage logging on bat activity in a national park. Biodiversity and Conservation 21, 2775–2786. [Google Scholar]
- Meigs, G. W. , Donato, D. C. , Campbell, J. L. , Martin, J. G. & Law, B. E. (2009). Forest fire impacts on carbon uptake, storage, and emission: the role of burn severity in the eastern Cascades, Oregon. Ecosystems 12, 1246–1267. [Google Scholar]
- *Messier, M. S. , Shatford, J. P. A. & Hibbs, D. E. (2012). Fire exclusion effects on riparian forest dynamics in southwestern Oregon. Forest Ecology and Management 264, 60–71. [Google Scholar]
- *Miller, M. T. & Douglas, G. W. (1999). Status of Lyall's Mariposa Lily, Calochortus lyallii (Liliaceae), in Canada. Canadian Field‐Naturalist 113, 652–658. [Google Scholar]
- Miller, A. D. , Roxburgh, S. H. & Shea, K. (2011). How frequency and intensity shape diversity‐disturbance relationships. Proceedings of the National Academy of Sciences of the United States of America 108, 5643–5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Minchinton, T. E. (2001). Canopy and substratum heterogeneity influence recruitment of the mangrove Avicennia marina . Journal of Ecology 89, 888–902. [Google Scholar]
- *Minshall, G. W. , Robinson, C. T. , Lawrence, D. E. , Andrews, D. A. & Brock, J. T. (2001). Benthic macroinvertebrate assemblages in five central Idaho (USA) streams over a 10–year period following disturbance by wildfire. International Journal of Wildland Fire 10, 201–213. [Google Scholar]
- Møller, A. P. & Jennions, M. D. (2001). Testing and adjusting for publication bias. Trends in Ecology & Evolution 16, 580–586. [Google Scholar]
- *Monserud, R. A. , Lowell, E. C. , Becker, D. R. , Hummel, S. S. , Donoghue, E. M. , Barbour, R. J. , Kilborn, K. A. , Nicholls, D. L. , Roos, J. & Cantrell, R. A. (2004). Contemporary wood utilization research needs in the Western United States. USDA Forest Service ‐ General Technical Report PNW 616, 1–49. [Google Scholar]
- *Moore, D. J. P. , Trahan, N. A. , Wilkes, P. , Quaife, T. , Stephens, B. B. , Elder, K. , Desai, A. R. , Negron, J. & Monson, R. K. (2013). Persistent reduced ecosystem respiration after insect disturbance in high elevation forests. Ecology Letters 16, 731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Moretti, M. , Conedera, M. , Duelli, P. & Edwards, P. J. (2002). The effects of wildfire on ground‐active spiders in deciduous forests on the Swiss southern slope of the Alps. Journal of Applied Ecology 39, 321–336. [Google Scholar]
- *Moretti, M. , De Bello, F. , Roberts, S. P. M. & Potts, S. G. (2009). Taxonomical vs. functional responses of bee communities to fire in two contrasting climatic regions. Journal of Animal Ecology 78, 98–108. [DOI] [PubMed] [Google Scholar]
- *Moretti, M. , De Cáceres, M. , Pradella, C. , Obrist, M. K. , Wermelinger, B. , Legendre, P. & Duelli, P. (2010). Fire‐induced taxonomic and functional changes in saproxylic beetle communities in fire sensitive regions. Ecography 33, 760–771. [Google Scholar]
- *Moretti, M. , Duelli, P. & Obrist, M. K. (2006). Biodiversity and resilience of arthropod communities after fire disturbance in temperate forests. Oecologia 149, 312–327. [DOI] [PubMed] [Google Scholar]
- *Moretti, M. & Legg, C. (2009). Combining plant and animal traits to assess community functional responses to disturbance. Ecography 32, 299–309. [Google Scholar]
- *Moretti, M. , Obrist, M. K. & Duelli, P. (2004). Arthropod biodiversity after forest fires: winners and losers in the winter fire regime of the southern Alps. Ecography 27, 173–186. [Google Scholar]
- *Mori, A. S. & Lertzman, K. P. (2011). Historic variability in fire‐generated landscape heterogeneity of subalpine forests in the Canadian Rockies. Journal of Vegetation Science 22, 45–58. [Google Scholar]
- *Moroni, M. T. , Shaw, C. H. & Otahal, P. (2010). Forest carbon stocks in Newfoundland boreal forests of harvest and natural disturbance origin I: field study. Canadian Journal of Forest Research 40, 2135–2145. [Google Scholar]
- *Moss, M. & Hermanutz, L. (2010). Monitoring the small and slimy – Protected areas should be monitoring native and non‐native slugs (Mollusca: Gastropoda). Natural Areas Journal 30, 322–327. [Google Scholar]
- *Müller, J. , Bußler, H. , Goßner, M. , Rettelbach, T. & Duelli, P. (2008). The European spruce bark beetle Ips typographus in a national park: from pest to keystone species. Biodiversity and Conservation 17, 2979–3001. [Google Scholar]
- *Müller, M. & Job, H. (2009). Managing natural disturbance in protected areas: tourists' attitude towards the bark beetle in a German national park. Biological Conservation 142, 375–383. [Google Scholar]
- *Myers‐Smith, I. H. , Harden, J. W. , Wilmking, M. , Fuller, C. C. , McGuire, A. D. & Chapin, F. S. III (2008). Wetland succession in a permafrost collapse: interactions between fire and thermokarst. Biogeosciences 5, 1273–1286. [Google Scholar]
- *Myronidis, D. I. , Emmanouloudis, D. A. , Mitsopoulos, I. A. & Riggos, E. E. (2010). Soil erosion potential after fire and rehabilitation treatments in Greece. Environmental Modeling and Assessment 15, 239–250. [Google Scholar]
- Nachtergale, L. , Ghekiere, K. , De Schrijver, A. , Muys, B. , Luyssaert, S. & Lust, N. (2002). Earthworm biomass and species diversity in windthrow sites of a temperate lowland forest. Pedobiologia 46, 440–451. [Google Scholar]
- *Nalder, I. A. & Wein, R. W. (1999). Long‐term forest floor carbon dynamics after fire in upland boreal forests of Western Canada. Global Biogeochemical Cycles 13, 951–968. [Google Scholar]
- *Nealis, V. G. , Noseworthy, M. K. , Turnquist, R. & Waring, V. R. (2009). Balancing risks of disturbance from mountain pine beetle and western spruce budworm. Canadian Journal of Forest Research 39, 839–848. [Google Scholar]
- *Neill, C. , Patterson, W. A. III & Crary, D. W. Jr. (2007). Responses of soil carbon, nitrogen and cations to the frequency and seasonality of prescribed burning in a Cape Cod oak‐pine forest. Forest Ecology and Management 250, 234–243. [Google Scholar]
- *Nelson, J. L. , Groninger, J. W. , Battaglia, L. L. & Ruffner, C. M. (2008. b). Bottomland hardwood forest recovery following tornado disturbance and salvage logging. Forest Ecology and Management 256, 388–395. [Google Scholar]
- *Nelson, C. R. , Halpern, C. B. & Agee, J. K. (2008. a). Thinning and burning result in low‐level invasion by nonnative plants but neutral effects on natives. Ecological Applications 18, 762–770. [DOI] [PubMed] [Google Scholar]
- *Newmaster, S. G. , Belland, R. J. , Arsenault, A. , Vitt, D. H. & Stephens, T. R. (2005). The ones we left behind: comparing plot sampling and floristic habitat sampling for estimating bryophyte diversity. Diversity and Distributions 11, 57–72. [Google Scholar]
- Newton, A. C. , Echeverría, C. , Cantarello, E. & Bolados, G. (2011). Projecting impacts of human disturbances to inform conservation planning and management in a dryland forest landscape. Biological Conservation 144, 1949–1960. [Google Scholar]
- *Niemuth, N. D. & Boyce, M. S. (2004). Influence of landscape composition on sharp‐tailed grouse lek location and attendance in Wisconsin pine barrens. Ecoscience 11, 209–217. [Google Scholar]
- *Niklasson, M. & Drakenberg, B. (2001). A 600–year tree‐ring fire history from Norra Kvills National Park, southern Sweden: implications for conservation strategies in the hemiboreal zone. Biological Conservation 101, 63–71. [Google Scholar]
- *Noormets, A. , Chen, J. & Crow, T. R. (2007). Age‐dependent changes in ecosystem carbon fluxes in managed forests in northern Wisconsin, USA. Ecosystems 10, 187–203. [Google Scholar]
- *North, M. P. & Hurteau, M. D. (2011). High‐severity wildfire effects on carbon stocks and emissions in fuels treated and untreated forest. Forest Ecology and Management 261, 1115–1120. [Google Scholar]
- *Nowak, S. , Kershaw, G. P. & Kershaw, L. J. (2002). Plant diversity and cover after wildfire on anthropogenically disturbed and undisturbed sites in Subarctic upland Picea mariana forest. Arctic 55, 269–280. [Google Scholar]
- *Nuttle, T. , Royo, A. A. , Adams, M. B. & Carson, W. P. (2013). Historic disturbance regimes promote tree diversity only under low browsing regimes in eastern deciduous forest. Ecological Monographs 83, 3–17. [Google Scholar]
- *Odion, D. C. & Hanson, C. T. (2006). Fire severity in conifer forests of the Sierra Nevada, California. Ecosystems 9, 1177–1189. [Google Scholar]
- *O'Donnell, J. A. , Harden, J. W. , McGuire, A. D. & Romanovsky, V. E. (2011). Exploring the sensitivity of soil carbon dynamics to climate change, fire disturbance and permafrost thaw in a black spruce ecosystem. Biogeosciences 8, 1367–1382. [Google Scholar]
- Ogris, N. , Hauptman, T. & Jurc, D. (2009). Chalara fraxinea causing common ash dieback newly reported in Slovenia. Plant Pathology 58, 1173. [Google Scholar]
- *von Oheimb, G. , Friedel, A. , Bertsch, A. & Härdtle, W. (2007). The effects of windthrow on plant species richness in a Central European beech forest. Plant Ecology 191, 47–65. [Google Scholar]
- *Ohlson, D. W. & Serveiss, V. B. (2007). The integration of ecological risk assessment and structured decision making into watershed management. Integrated Environmental Assessment and Management 3, 118–128. [PubMed] [Google Scholar]
- *Ohlson, M. , Söderström, L. , Hörnberg, G. , Zackrisson, O. & Hermansson, J. (1997). Habitat qualities versus long‐term continuity as determinants of biodiversity in boreal old‐growth swamp forests. Biological Conservation 81, 221–231. [Google Scholar]
- Olsson, J. & Jonsson, B. G. (2010). Restoration fire and wood‐inhabiting fungi in a Swedish Pinus sylvestris forest. Forest Ecology and Management 259, 1971–1980. [Google Scholar]
- Overbeck, M. & Schmidt, M. (2012). Modelling infestation risk of Norway spruce by Ips typographus (L.) in the Lower Saxon Harz Mountains (Germany). Forest Ecology and Management 266, 115–125. [Google Scholar]
- *Palik, B. J. , Mitchell, R. J. & Hiers, J. K. (2002). Modeling silviculture after natural disturbance to sustain biodiversity in the longleaf pine (Pinus palustris) ecosystem: balancing complexity and implementation. Forest Ecology and Management 155, 347–356. [Google Scholar]
- *Palmer, M. W. , McAlister, S. D. , Arévalo, J. R. & DeCoster, J. K. (2000). Changes in the understory during 14 years following catastrophic windthrow in two Minnesota forests. Journal of Vegetation Science 11, 841–854. [Google Scholar]
- *Parro, K. , Köster, K. , Jogiste, K. & Vodde, F. (2009). Vegetation dynamics in a fire damaged forest area: the response of major ground vegetation species. Baltic Forestry 15, 206–215. [Google Scholar]
- *Pasch, B. & Koprowski, J. L. (2011). Impacts of fire suppression on space use by Mexican fox squirrels. Journal of Mammalogy 92, 227–234. [Google Scholar]
- *Paterson, A. M. , Gumming, B. F. , Smol, J. P. , Blais, J. M. & France, R. L. (1998). Assessment of the effects of logging, forest fires and drought on lakes in northwestern Ontario: a 30–year paleolimnological perspective. Canadian Journal of Forest Research 28, 1546–1556. [Google Scholar]
- *Patoine, A. , Pinel‐Alloul, B. & Prepas, E. E. (2002). Effects of catchment perturbations by logging and wildfires on zooplankton species richness and composition in Boreal Shield lakes. Freshwater Biology 47, 1996–2014. [Google Scholar]
- *Patriquin, M. N. , Lantz, V. A. , Stedman, R. C. & White, W. A. (2008). Working together: a reciprocal wood flow arrangement to mitigate the economic impacts of natural disturbance. Forestry 81, 227–242. [Google Scholar]
- *Patriquin, M. N. , Wellstead, A. M. & White, W. A. (2007). Beetles, trees, and people: regional economic impact sensitivity and policy considerations related to the mountain pine beetle infestation in British Columbia, Canada. Forest Policy and Economics 9, 938–946. [Google Scholar]
- *Payette, S. & Delwaide, A. (2004). Dynamics of subarctic wetland forests over the past 1500 years. Ecological Monographs 74, 373–391. [Google Scholar]
- *Pearson, D. , Shine, R. & Williams, A. (2005). Spatial ecology of a threatened python (Morelia spilota imbricata) and the effects of anthropogenic habitat change. Austral Ecology 30, 261–274. [Google Scholar]
- *Peay, K. G. , Garbelotto, M. & Bruns, T. D. (2009). Spore heat resistance plays an important role in disturbance‐mediated assemblage shift of ectomycorrhizal fungi colonizing Pinus muricata seedlings. Journal of Ecology 97, 537–547. [Google Scholar]
- *Pekin, B. K. , Boer, M. M. , Macfarlane, C. & Grierson, P. F. (2009). Impacts of increased fire frequency and aridity on eucalypt forest structure, biomass and composition in southwest Australia. Forest Ecology and Management 258, 2136–2142. [Google Scholar]
- *Pekin, B. K. , Wittkuhn, R. S. , Boer, M. M. , Macfarlane, C. & Grierson, P. F. (2012). Response of plant species and life form diversity to variable fire histories and biomass in the jarrah forest of south‐west Australia. Austral Ecology 37, 330–338. [Google Scholar]
- *Pellerin, S. & Lavoie, C. (2003). Reconstructing the recent dynamics of mires using a multitechnique approach. Journal of Ecology 91, 1008–1021. [Google Scholar]
- *Peltzer, D. A. , Bast, M. L. , Wilson, S. D. & Gerry, A. K. (2000). Plant diversity and tree responses following contrasting disturbances in boreal forest. Forest Ecology and Management 127, 191–203. [Google Scholar]
- *Peng, C. , Liu, J. , Dang, Q. , Zhou, X. & Apps, M. (2001). Developing carbon‐based ecological indicators to monitor sustainability of Ontario's forests. Ecological Indicators 1, 235–246. [Google Scholar]
- *Penman, T. D. , Binns, D. L. , Shiels, R. J. , Allen, R. M. & Kavanagh, R. P. (2008). Changes in understorey plant species richness following logging and prescribed burning in shrubby dry sclerophyll forests of south‐eastern Australia. Austral Ecology 33, 197–210. [Google Scholar]
- Perry, D. A. , Hessburg, P. F. , Skinner, C. N. , Spies, T. A. , Stephens, S. L. , Taylor, A. H. , Franklin, J. F. , McComb, B. & Riegel, G. (2011). The ecology of mixed severity fire regimes in Washington, Oregon, and Northern California. Forest Ecology and Management 262, 703–717. [Google Scholar]
- *Peter, B. & Nelson, J. (2005). Estimating harvest schedules and profitability under the risk of fire disturbance. Canadian Journal of Forest Research 35, 1378–1388. [Google Scholar]
- *Peters, E. B. , Wythers, K. R. , Bradford, J. B. & Reich, P. B. (2013). Influence of disturbance on temperate forest productivity. Ecosystems 16, 95–110. [Google Scholar]
- *Peterson, D. W. & Reich, P. B. (2008). Fire frequency and tree canopy structure influence plant species diversity in a forest‐grassland ecotone. Plant Ecology 194, 5–16. [Google Scholar]
- *Pfeifer, E. M. , Hicke, J. A. & Meddens, A. J. H. (2011). Observations and modeling of aboveground tree carbon stocks and fluxes following a bark beetle outbreak in the western United States. Global Change Biology 17, 339–350. [Google Scholar]
- *Pharo, E. J. & Beattie, A. J. (2002). The association between substrate variability and bryophyte and lichen diversity in eastern Australian forests. Bryologist 105, 11–26. [Google Scholar]
- *Pilz, D. , Weber, N. S. , Carol Carter, M. , Parks, C. G. & Molina, R. (2004). Productivity and diversity of morel mushrooms in healthy, burned, and insect‐damaged forests of northeastern Oregon. Forest Ecology and Management 198, 367–386. [Google Scholar]
- *Pinzon, J. , Spence, J. R. & Langor, D. W. (2013). Effects of prescribed burning and harvesting on ground‐dwelling spiders in the Canadian boreal mixedwood forest. Biodiversity and Conservation 22, 1513–1536. [Google Scholar]
- *Pollock, S. L. & Payette, S. (2010). Stability in the patterns of long‐term development and growth of the Canadian spruce‐moss forest. Journal of Biogeography 37, 1684–1697. [Google Scholar]
- *Porto, M. , Correia, O. & Beja, P. (2011). Long‐term consequences of mechanical fuel management for the conservation of Mediterranean forest herb communities. Biodiversity and Conservation 20, 2669–2691. [Google Scholar]
- *Potter, C. , Gross, P. , Klooster, S. , Fladeland, M. & Genovese, V. (2008). Storage of carbon in U.S. forests predicted from satellite data, ecosystem modeling, and inventory summaries. Climatic Change 90, 269–282. [Google Scholar]
- *Potter, C. , Klooster, S. , Crabtree, R. , Huang, S. , Gross, P. & Genovese, V. (2011). Carbon fluxes in ecosystems of Yellowstone National Park predicted from remote sensing data and simulation modeling. Carbon Balance and Management 6, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Potts, S. G. , Petanidou, T. , Roberts, S. , O'Toole, C. , Hulbert, A. & Willmer, P. (2006). Plant‐pollinator biodiversity and pollination services in a complex Mediterranean landscape. Biological Conservation 129, 519–529. [Google Scholar]
- Powell, K. (2012). Publishing: foreign tongues. Nature 487, 129–131. [DOI] [PubMed] [Google Scholar]
- *Premoli, A. C. & Kitzberger, T. (2005). Regeneration mode affects spatial genetic structure of Nothofagus dombeyi forests. Molecular Ecology 14, 2319–2329. [DOI] [PubMed] [Google Scholar]
- *Premoli, A. C. & Steinke, L. (2008). Genetics of sprouting: effects of long‐term persistence in fire‐prone ecosystems. Molecular Ecology 17, 3827–3835. [DOI] [PubMed] [Google Scholar]
- *Priewasser, K. , Brang, P. , Bachofen, H. , Bugmann, H. & Wohlgemuth, T. (2013). Impacts of salvage‐logging on the status of deadwood after windthrow in Swiss forests. European Journal of Forest Research 132, 231–240. [Google Scholar]
- *Proença, V. , Pereira, H. M. & Vicente, L. (2010). Resistance to wildfire and early regeneration in natural broadleaved forest and pine plantation. Acta Oecologica 36, 626–633. [Google Scholar]
- *Radeloff, V. C. , Mladenoff, D. J. & Boyce, M. S. (2000). Effects of interacting disturbances on landscape patterns: budworm defoliation and salvage logging. Ecological Applications 10, 233–247. [Google Scholar]
- *Radeloff, V. C. , Mladenoff, D. J. , He, H. S. & Boyce, M. S. (1999). Forest landscape change in the northwestern Wisconsin Pine Barrens from pre‐European settlement to the present. Canadian Journal of Forest Research 29, 1649–1659. [Google Scholar]
- *Rajora, O. P. , Mann, I. K. & Shi, Y.‐Z. (2005). Genetic diversity and population structure of boreal white spruce (Picea glauca) in pristine conifer‐dominated and mixedwood forest stands. Canadian Journal of Botany 83, 1096–1105. [Google Scholar]
- *Rammig, A. , Fahse, L. , Bebi, P. & Bugmann, H. (2007). Wind disturbance in mountain forests: simulating the impact of management strategies, seed supply, and ungulate browsing on forest succession. Forest Ecology and Management 242, 142–154. [Google Scholar]
- *Rammig, A. , Fahse, L. , Bugmann, H. & Bebi, P. (2006). Forest regeneration after disturbance: a modelling study for the Swiss Alps. Forest Ecology and Management 222, 123–136. [Google Scholar]
- *Randall, L. A. , Barclay, R. M. R. , Reid, M. L. & Jung, T. S. (2011). Recent infestation of forest stands by spruce beetles does not predict habitat use by little brown bats (Myotis lucifugus) in southwestern Yukon, Canada. Forest Ecology and Management 261, 1950–1956. [Google Scholar]
- *Ratchford, J. S. , Wittman, S. E. , Jules, E. S. , Ellison, A. M. , Gotelli, N. J. & Sanders, N. J. (2005). The effects of fire, local environment and time on ant assemblages in fens and forests. Diversity and Distributions 11, 487–497. [Google Scholar]
- *Raybuck, A. L. , Moorman, C. E. , Greenberg, C. H. , DePerno, C. S. , Gross, K. , Simon, D. M. & Warburton, G. S. (2012). Short‐term response of small mammals following oak regeneration silviculture treatments. Forest Ecology and Management 274, 10–16. [Google Scholar]
- R Development Core Team (2014). A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna: Electronic file available at http://www.r‐project.org/ Accessed 2.4.2014. [Google Scholar]
- *Rees, D. C. & Juday, G. P. (2002). Plant species diversity on logged versus burned sites in central Alaska. Forest Ecology and Management 155, 291–302. [Google Scholar]
- Reichstein, M. , Bahn, M. , Ciais, P. , Frank, D. , Mahecha, M. D. , Seneviratne, S. I. , Zscheischler, J. , Beer, C. , Buchmann, N. , Frank, D. C. , Papale, D. , Rammig, A. , Smith, P. , Thonicke, K. , van der Velde, M. , Vicca, S. , Walz, A. & Wattenbach, M. (2013). Climate extremes and the carbon cycle. Nature 500, 287–295. [DOI] [PubMed] [Google Scholar]
- *Reilly, M. J. , Wimberly, M. C. & Newell, C. L. (2006. a). Wildfire effects on ß‐diversity and species turnover in a forested landscape. Journal of Vegetation Science 17, 447–454. [Google Scholar]
- *Reilly, M. J. , Wimberly, M. C. & Newell, C. L. (2006. b). Wildfire effects on plant species richness at multiple spatial scales in forest communities of the southern Appalachians. Journal of Ecology 94, 118–130. [Google Scholar]
- *Reinhardt, E. & Holsinger, L. (2010). Effects of fuel treatments on carbon‐disturbance relationships in forests of the northern Rocky Mountains. Forest Ecology and Management 259, 1427–1435. [Google Scholar]
- *Reyes, G. P. & Kneeshaw, D. (2008). Moderate‐severity disturbance dynamics in Abies balsamea‐Betula spp. forests: the relative importance of disturbance type and local stand and site characteristics on woody vegetation response. Ecoscience 15, 241–249. [Google Scholar]
- *Richardson, E. , Stirling, I. & Kochtubajda, B. (2007). The effects of forest fires on polar bear maternity denning habitat in western Hudson Bay. Polar Biology 30, 369–378. [Google Scholar]
- *Robichaud, P. R. , Wagenbrenner, J. W. & Brown, R. E. (2010). Rill erosion in natural and disturbed forests: 1. Measurements. Water Resources Research 46, W10506. [Google Scholar]
- *Robinson, R. M. , Mellican, A. E. & Smith, R. H. (2008). Epigeous macrofungal succession in the first five years following a wildfire in karri (Eucalyptus diversicolor) regrowth forest in Western Australia. Austral Ecology 33, 807–820. [Google Scholar]
- *Ross, K. A. , Fox, B. J. & Fox, M. D. (2002). Changes to plant species richness in forest fragments: fragment age, disturbance and fire history may be as important as area. Journal of Biogeography 29, 749–765. [Google Scholar]
- *Ross, C. S. , Kaye, J. P. , Kaye, M. W. , Kurth, V. J. , Brimmer, R. , Hart, S. C. & Fulé, P. Z. (2012). Ecosystem carbon remains low for three decades following fire and constrains soil CO 2 responses to precipitation in southwestern ponderosa pine forests. Ecosystems 15, 725–740. [Google Scholar]
- *Ross, K. A. , Taylor, J. E. , Fox, M. D. & Fox, B. J. (2004). Interaction of multiple disturbances: importance of disturbance interval in the effects of fire on rehabilitating mined areas. Austral Ecology 29, 508–529. [Google Scholar]
- *Royo, A. A. , Collins, R. , Adams, M. B. , Kirschbaum, C. & Carson, W. P. (2010). Pervasive interactions between ungulate browsers and disturbance regimes promote temperate forest herbaceous diversity. Ecology 91, 93–105. [DOI] [PubMed] [Google Scholar]
- *Ruiz‐Benito, P. , Gómez‐Aparicio, L. & Zavala, M. A. (2012). Large‐scale assessment of regeneration and diversity in Mediterranean planted pine forests along ecological gradients. Diversity and Distributions 18, 1092–1106. [Google Scholar]
- *Rumbaitis Del Rio, C. M. (2006). Changes in understory composition following catastrophic windthrow and salvage logging in a subalpine forest ecosystem. Canadian Journal of Forest Research 36, 2943–2954. [Google Scholar]
- Ruokolainen, L. & Salo, K. (2009). The effect of fire intensity on vegetation succession on a sub‐xeric heath during ten years after wildfire. Annales Botanici Fennici 46, 30–42. [Google Scholar]
- *Russell, R. E. , Royle, J. A. , Saab, V. A. , Lehmkuhl, J. F. , Block, W. M. & Sauer, J. R. (2009). Modeling the effects of environmental disturbance on wildlife communities: avian responses to prescribed fire. Ecological Applications 19, 1253–1263. [DOI] [PubMed] [Google Scholar]
- *Ryu, S.‐R. , Chen, J. , Zheng, D. , Bresee, M. K. & Crow, T. R. (2006). Simulating the effects of prescribed burning on fuel loading and timber production (EcoFL) in managed northern Wisconsin forests. Ecological Modelling 196, 395–406. [Google Scholar]
- *Sackmann, P. & Farji‐Brener, A. (2006). Effect of fire on ground beetles and ant assemblages along an environmental gradient in NW Patagonia: does habitat type matter? Ecoscience 13, 360–371. [Google Scholar]
- *Saint‐Germain, M. , Drapeau, P. & Hébert, C. (2004). Xylophagous insect species composition and patterns of substratum use on fire‐killed black spruce in central Quebec. Canadian Journal of Forest Research 34, 677–685. [Google Scholar]
- *Saint‐Germain, M. , Drapeau, P. & Hibbert, A. (2013). Saproxylic beetle tolerance to habitat fragmentation induced by salvage logging in a boreal mixed‐cover burn. Insect Conservation and Diversity 6, 381–392. [Google Scholar]
- *Saint‐Germain, M. , Larrivée, M. , Drapeau, P. , Fahrig, L. & Buddle, C. M. (2005). Short‐term response of ground beetles (Coleoptera: Carabidae) to fire and logging in a spruce‐dominated boreal landscape. Forest Ecology and Management 212, 118–126. [Google Scholar]
- *Sano, T. , Hirano, T. , Liang, N. , Hirata, R. & Fujinuma, Y. (2010). Carbon dioxide exchange of a larch forest after a typhoon disturbance. Forest Ecology and Management 260, 2214–2223. [Google Scholar]
- *Santana, J. , Porto, M. , Reino, L. & Beja, P. (2011). Long‐term understory recovery after mechanical fuel reduction in Mediterranean cork oak forests. Forest Ecology and Management 261, 447–459. [Google Scholar]
- *Santos, X. , Bros, V. & Miño, À. (2009). Recolonization of a burned Mediterranean area by terrestrial gastropods. Biodiversity and Conservation 18, 3153–3165. [Google Scholar]
- *Santos, X. , Bros, V. & Ros, E. (2012). Contrasting responses of two xerophilous land snails to fire and natural reforestation. Contributions to Zoology 81, 167–180. [Google Scholar]
- *Sarà, M. , Bellia, E. & Milazzo, A. (2006). Fire disturbance disrupts co‐occurrence patterns of terrestrial vertebrates in Mediterranean woodlands. Journal of Biogeography 33, 843–852. [Google Scholar]
- *Sarriquet, P. E. , Delettre, Y. R. & Marmonier, P. (2006). Effects of catchment disturbance on stream invertebrates: comparison of different habitats (vegetation, benthic and interstitial) using bio‐ecological groups. Annales de Limnologie 42, 205–219. [Google Scholar]
- *Savage, M. , Sawhill, B. & Askenazi, M. (2000). Community dynamics: what happens when we rerun the tape? Journal of Theoretical Biology 205, 515–526. [DOI] [PubMed] [Google Scholar]
- *Schäfer, K. V. R. , Clark, K. L. , Skowronski, N. & Hamerlynck, E. P. (2010). Impact of insect defoliation on forest carbon balance as assessed with a canopy assimilation model. Global Change Biology 16, 546–561. [Google Scholar]
- *Schaffhauser, A. , Curt, T. , Véla, E. & Tatoni, T. (2012). Fire recurrence effects on the abundance of plants grouped by traits in Quercus suber L. woodlands and maquis. Forest Ecology and Management 282, 157–166. [Google Scholar]
- *Scharenbroch, B. C. & Bockheim, J. G. (2008). The effects of gap disturbance on nitrogen cycling and retention in late‐successional northern hardwood‐hemlock forests. Biogeochemistry 87, 231–245. [Google Scholar]
- Schelhaas, M. J. (2008). The wind stability of different silvicultural systems for Douglas‐fir in the Netherlands: a model‐based approach. Forestry 81, 399–414. [Google Scholar]
- *Schelhaas, M. J. , Hengeveld, G. , Moriondo, M. , Reinds, G. J. , Kundzewicz, Z. W. , ter Maat, H. & Bindi, M. (2010). Assessing risk and adaptation options to fires and windstorms in European forestry. Mitigation and Adaptation Strategies for Global Change 15, 681–701. [Google Scholar]
- Schelhaas, M. J. , Nabuurs, G.‐J. & Schuck, A. (2003). Natural disturbances in the European forests in the 19th and 20th centuries. Global Change Biology 9, 1620–1633. [Google Scholar]
- *Scheller, R. M. , Hua, D. , Bolstad, P. V. , Birdsey, R. A. & Mladenoff, D. J. (2011. a). The effects of forest harvest intensity in combination with wind disturbance on carbon dynamics in Lake States Mesic Forests. Ecological Modelling 222, 144–153. [Google Scholar]
- *Scheller, R. M. , Spencer, W. D. , Rustigian‐Romsos, H. , Syphard, A. D. , Ward, B. C. & Strittholt, J. R. (2011. b). Using stochastic simulation to evaluate competing risks of wildfires and fuels management on an isolated forest carnivore. Landscape Ecology 26, 1491–1504. [Google Scholar]
- *Scheller, R. M. , van Tuyl, S. , Clark, K. L. , Hom, J. & La Puma, I. (2011c). Carbon sequestration in the New Jersey Pine Barrens under different scenarios of fire management. Ecosystems 14, 987–1004. [Google Scholar]
- *Schmalholz, M. , Hylander, K. & Frego, K. (2011). Bryophyte species richness and composition in young forests regenerated after clear‐cut logging versus after wildfire and spruce budworm outbreak. Biodiversity and Conservation 20, 2575–2596. [Google Scholar]
- *Schmidt, W. & Heinrichs, S. (2012). 13 years after windthrow – Vegetation dynamics of the strict beech forest reserve “Königsbuche” (southwestern Harz Mountain foothills, Lower Saxony) [13 Jahre nach dem Sturm – Vegetationsentwicklung im Buchen‐Naturwald “Königsbuche” (südwestliches Harzvorland, Niedersachsen)]. Hercynia 45, 81–110. [Google Scholar]
- *Schmidt, K. M. , Roering, J. J. , Stock, J. D. , Dietrich, W. E. , Montgomery, D. R. & Schaub, T. (2001). The variability of root cohesion as an influence on shallow landslide susceptibility in the Oregon Coast Range. Canadian Geotechnical Journal 38, 995–1024. [Google Scholar]
- *Schönenberger, W. , Noack, A. & Thee, P. (2005). Effect of timber removal from windthrow slopes on the risk of snow avalanches and rockfall. Forest Ecology and Management 213, 197–208. [Google Scholar]
- SciVerse Scopus (2013). Scopus – Document search. Elsevier. Electronic file available at http://www.scopus.com Accessed 6.6.2013.
- *Scrimgeour, G. J. , Tonn, W. M. , Paszkowski, C. A. & Aku, P. M. K. (2000). Evaluating the effects of forest harvesting on littoral benthic communities within a natural disturbance‐based management model. Forest Ecology and Management 126, 77–86. [Google Scholar]
- Seedre, M. & Chen, H. Y. H. (2010). Carbon dynamics of aboveground live vegetation of boreal mixedwoods after wildfire and clear‐cutting. Canadian Journal of Forest Research 40, 1862–1869. [Google Scholar]
- *Seely, B. , Welham, C. & Kimmins, H. (2002). Carbon sequestration in a boreal forest ecosystem: results from the ecosystem simulation model, FORECAST. Forest Ecology and Management 169, 123–135. [Google Scholar]
- *Segerström, U. (1997). Long‐term dynamics of vegetation and disturbance of a southern boreal spruce swamp forest. Journal of Vegetation Science 8, 295–306. [Google Scholar]
- Seidl, R. (2014). The shape of ecosystem management to come: anticipating risks and fostering resilience. BioScience 64, 1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Seidl, R. & Blennow, K. (2012). Pervasive growth reduction in Norway spruce forests following wind disturbance. PLoS One 7, E33301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl, R. , Fernandes, P. M. , Fonseca, T. F. , Gillet, F. , Jönsson, A. M. , Merganičová, K. , Netherer, S. , Arpaci, A. , Bontemps, J.‐D. , Bugmann, H. , González‐Olabarria, J. R. , Lasch, P. , Meredieu, C. , Moreira, F. , Schelhaas, M.‐J. & Mohren, F. (2011a). Modelling natural disturbances in forest ecosystems: a review. Ecological Modelling 222, 903–924. [Google Scholar]
- Seidl, R. , Schelhaas, M.‐J. & Lexer, M. J. (2011b). Unraveling the drivers of intensifying forest disturbance regimes in Europe. Global Change Biology 17, 2842–2852. [Google Scholar]
- *Seidl, R. , Rammer, W. , Jäger, D. & Lexer, M. J. (2008). Impact of bark beetle (Ips typographus L.) disturbance on timber production and carbon sequestration in different management strategies under climate change. Forest Ecology and Management 256, 209–220. [Google Scholar]
- Seidl, R. , Schelhaas, M.‐J. , Rammer, W. & Verkerk, P. J. (2014). Increasing forest disturbances in Europe and their impact on carbon storage. Nature Climate Change 4, 806–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Seitz, D. (2010). Influence of different structures of windthrows on bird habitats – investigations in the National Park 'Kellerwald‐Edersee' [Nutzung von Windwurfflächen durch Vögel ‐ Strukturelle Untersuchungen im Nationalpark Kellerwald‐Edersee]. Naturschutz und Landschaftsplanung 42, 267–274. [Google Scholar]
- Seymour, R. S. , White, A. S. & DeMaynadier, P. G. (2002). Natural disturbance regimes in northeastern North America—evaluating silvicultural systems using natural scales and frequencies. Forest Ecology and Management 155, 357–367. [Google Scholar]
- *Shrestha, B. M. & Chen, H. Y. H. (2010). Effects of stand age, wildfire and clearcut harvesting on forest floor in boreal mixedwood forests. Plant and Soil 336, 267–277. [Google Scholar]
- *Signell, S. A. & Abrams, M. D. (2006). Influence of rocky landscape features and fire regime on vegetation dynamics in Appalachian Quercus forests. Journal of Vegetation Science 17, 675–684. [Google Scholar]
- *Simard, D. G. , Fyles, J. W. , Paré, D. & Nguyen, T. (2001). Impacts of clearcut harvesting and wildfire on soil nutrient status in the Quebec boreal forest. Canadian Journal of Soil Science 81, 229–237. [Google Scholar]
- *Simon, A. , Gratzer, G. & Sieghardt, M. (2011). The influence of windthrow microsites on tree regeneration and establishment in an old growth mountain forest. Forest Ecology and Management 262, 1289–1297. [Google Scholar]
- *Simon, N. P. P. , Schwab, F. E. & Otto, R. D. (2002). Songbird abundance in clear‐cut and burned stands: a comparison of natural disturbance and forest management. Canadian Journal of Forest Research 32, 1343–1350. [Google Scholar]
- Siry, J. P. , Cubbage, F. W. & Ahmed, M. R. (2005). Sustainable forest management: global trends and opportunities. Forest Policy and Economics 7, 551–561. [Google Scholar]
- *Sklodowski, J. & Garbalinska, P. (2007). Ground beetle assemblages (Coleoptera, Carabidae) in the third year of regeneration after a hurricane in the Puszcza Piska pine forests. Baltic Journal of Coleopterology 7, 17–36. [Google Scholar]
- *Sklodowski, J. & Garbalinska, P. (2011). Ground beetle (Coleoptera, Carabidae) assemblages inhabiting Scots pine stands of Puszcza Piska Forest: six‐year responses to a tornado impact. ZooKeys 100, 371–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Smirnova, E. , Bergeron, Y. & Brais, S. (2008). Influence of fire intensity on structure and composition of jack pine stands in the boreal forest of Quebec: live trees, understory vegetation and dead wood dynamics. Forest Ecology and Management 255, 2916–2927. [Google Scholar]
- *Smith, D. M. , Kelly, J. F. & Finch, D. M. (2007). Avian nest box selection and nest success in burned and unburned southwestern riparian forest. Journal of Wildlife Management 71, 411–421. [Google Scholar]
- *Smith, J. E. , McKay, D. , Brenner, G. , McIver, J. & Spatafora, J. W. (2005). Early impacts of forest restoration treatments on the ectomycorrhizal fungal community and fine root biomass in a mixed conifer forest. Journal of Applied Ecology 42, 526–535. [Google Scholar]
- *Smith, M. A. , Turner, M. G. & Rusch, D. H. (2002). The effect of military training activity on eastern lupine and the Karner blue butterfly at Fort McCoy, Wisconsin, USA. Environmental Management 29, 102–115. [DOI] [PubMed] [Google Scholar]
- *Smithwick, E. A. H. , Harmon, M. E. & Domingo, J. B. (2007). Changing temporal patterns of forest carbon stores and net ecosystem carbon balance: the stand to landscape transformation. Landscape Ecology 22, 77–94. [Google Scholar]
- *Smithwick, E. A. H. , Ryan, M. G. , Kashian, D. M. , Romme, W. H. , Tinker, D. B. & Turner, M. G. (2009). Modeling the effects of fire and climate change on carbon and nitrogen storage in lodgepole pine (Pinus contorta) stands. Global Change Biology 15, 535–548. [Google Scholar]
- *Sorensen, T. , Mcloughlin, P. D. , Hervieux, D. , Dzus, E. , Nolan, J. , Wynes, B. & Boutin, S. (2008). Determining sustainable levels of cumulative effects for boreal caribou. Journal of Wildlife Management 72, 900–905. [Google Scholar]
- *Spaulding, S. E. & Rothstein, D. E. (2009). How well does Kirtland's warbler management emulate the effects of natural disturbance on stand structure in Michigan jack pine forests? Forest Ecology and Management 258, 2609–2618. [Google Scholar]
- *Spring, D. A. , Kennedy, J. , Lindenmayer, D. B. , McCarthy, M. A. & Mac Nally, R. (2008). Optimal management of a flammable multi‐stand forest for timber production and maintenance of nesting sites for wildlife. Forest Ecology and Management 255, 3857–3865. [Google Scholar]
- *Sprugel, D. G. & Bormann, F. H. (1981). Natural disturbance and the steady state in high‐altitude balsam fir forests. Science 211, 390–393. [DOI] [PubMed] [Google Scholar]
- *Stark, K. E. , Arsenault, A. & Bradfield, G. E. (2006). Soil seed banks and plant community assembly following disturbance by fire and logging in interior Douglas‐fir forests of south‐central British Columbia. Canadian Journal of Botany 84, 1548–1560. [Google Scholar]
- *Stark, K. E. , Arsenault, A. & Bradfield, G. E. (2008). Variation in soil seed bank species composition of a dry coniferous forest: spatial scale and sampling considerations. Plant Ecology 197, 173–181. [Google Scholar]
- *Stephens, S. S. & Wagner, M. R. (2006). Using ground foraging ant (Hymenoptera: Formicidae) functional groups as bioindicators of forest health in northern Arizona ponderosa pine forests. Environmental Entomology 35, 937–949. [Google Scholar]
- *Steventon, J. D. & Daust, D. K. (2009). Management strategies for a large‐scale mountain pine beetle outbreak: modelling impacts on American martens. Forest Ecology and Management 257, 1976–1985. [Google Scholar]
- Stewart‐Oaten, A. , Murdoch, W. W. & Parker, K. R. (1986). Environmental impact assessment: “pseudoreplication” in time? Ecology 67, 929–940. [Google Scholar]
- *Stinson, G. , Kurz, W. A. , Smyth, C. E. , Neilson, E. T. , Dymond, C. C. , Metsaranta, J. M. , Boisvenue, C. , Rampley, G. J. , Li, Q. , White, T. M. & Blain, D. (2011). An inventory‐based analysis of Canada's managed forest carbon dynamics, 1990 to 2008. Global Change Biology 17, 2227–2244. [Google Scholar]
- *Storms, D. , Said, S. , Fritz, H. , Hamann, J.‐L. , Saint‐Andrieux, C. & Klein, F. (2006). Influence of hurricane Lothar on red and roe deer winter diets in the Northern Vosges, France. Forest Ecology and Management 237, 164–169. [Google Scholar]
- *Strand, E. K. , Bunting, S. C. & Vierling, L. A. (2012). Landscape composition in aspen woodlands under various modeled fire regimes. USDA Forest Service ‐ General Technical Report PNW‐GTR 869, 197–214. [Google Scholar]
- *Streby, H. M. & Miles, D. B. (2010). Assessing ecosystem restoration alternatives in eastern deciduous hardwood forests using avian nest survival. Open Environmental Sciences 4, 31–40. [Google Scholar]
- *Stromberg, J. C. , Rychener, T. J. & Dixon, M. D. (2009). Return of fire to a free‐flowing desert river: effects on vegetation. Restoration Ecology 17, 327–338. [Google Scholar]
- Stuart, S. N. , Chanson, J. S. , Cox, N. A. , Young, B. E. , Rodrigues, A. S. L. , Fischman, D. L. & Waller, R. W. (2004). Status and trends of amphibian declines and extinctions worldwide. Science (New York, N.Y.) 306, 1783–1786. [DOI] [PubMed] [Google Scholar]
- *Suárez‐Seoane, S. & García‐Rovés, P. (2004). Do disturbances in surrounding areas affect a core population of Cantabrian capercaillie Tetrao urogallus cantabricus? The case of the The Natural Reserve of Muniellos (Asturias, NW Spain). Ardeola 51, 395–409. [Google Scholar]
- *Sullivan, T. P. , Lautenschlager, R. A. & Wagner, R. G. (1999). Clearcutting and burning of northern spruce‐fir forests: implications for small mammal communities. Journal of Applied Ecology 36, 327–344. [Google Scholar]
- *Surrette, S. B. & Brewer, J. S. (2008). Inferring relationships between native plant diversity and Lonicera japonica in upland forests in north Mississippi, USA. Applied Vegetation Science 11, 205–214. [Google Scholar]
- *Svoboda, M. , Fraver, S. , Janda, P. , Bace, R. & Zenáhlíková, J. (2010). Natural development and regeneration of a Central European montane spruce forest. Forest Ecology and Management 260, 707–714. [Google Scholar]
- *Takakai, F. , Desyatkin, A. R. , Lopez, C. M. L. , Fedorov, A. N. , Desyatkin, R. V. & Hatano, R. (2008). Influence of forest disturbance on CO2, CH4 and N2O fluxes from larch forest soil in the permafrost taiga region of eastern Siberia. Soil Science and Plant Nutrition 54, 938–949. [Google Scholar]
- Temperli, C. , Bugmann, H. & Elkin, C. (2013). Cross‐scale interactions among bark beetles, climate change and wind disturbances: a landscape modeling approach. Ecological Monographs 83, 383–402. [Google Scholar]
- Thom, D. , Seidl, R. , Steyrer, G. , Krehan, H. & Formayer, H. (2013). Slow and fast drivers of the natural disturbance regime in Central European forest ecosystems. Forest Ecology and Management 307, 293–302. [Google Scholar]
- Thomas, C. D. , Cameron, A. , Green, R. E. , Bakkenes, M. , Beaumont, L. J. , Collingham, Y. C. , Erasmus, B. F. N. , Ferreira de Siqueira, M. , Grainger, A. , Hannah, L. , Hughes, L. , Huntley, B. , van Jaarsveld, A. S. , Midgley, G. F. , Miles, L. , Ortega‐Huerta, M. A. , Peterson, A. T. , Phillips, O. L. & Williams, S. E. (2004). Extinction risk from climate change. Nature 427, 145–148. [DOI] [PubMed] [Google Scholar]
- Thomas, S. C. & MacLellan, J. (2002). Boreal and temperate forests In Encyclopedia of Life Support Systems, 11pp. EOLSS Publishers Co., UNESCO, Paris. [Google Scholar]
- *Thompson, C. M. , Zielinski, W. J. & Purcell, K. L. (2011). Evaluating management risks using landscape trajectory analysis: a case study of California fisher. Journal of Wildlife Management 75, 1164–1176. [Google Scholar]
- Thorn, S. , Bässler, C. , Gottschalk, T. , Hothorn, T. , Bussler, H. , Raffa, K. & Müller, J. (2014). New insights into the consequences of post‐windthrow salvage logging revealed by functional structure of saproxylic beetles assemblages. PLoS One 9, e101757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Thornton, P. E. , Law, B. E. , Gholz, H. L. , Clark, K. L. , Falge, E. , Ellsworth, D. S. , Goldstein, A. H. , Monson, R. K. , Hollinger, D. , Falk, M. , Chen, J. & Sparks, J. P. (2002). Modeling and measuring the effects of disturbance history and climate on carbon and water budgets in evergreen needleleaf forests. Agricultural and Forest Meteorology 113, 185–222. [Google Scholar]
- *Thürig, E. , Palosuo, T. , Bucher, J. & Kaufmann, E. (2005). The impact of windthrow on carbon sequestration in Switzerland: a model‐based assessment. Forest Ecology and Management 210, 337–350. [Google Scholar]
- Toivanen, T. & Kotiaho, J. S. (2007). Mimicking natural disturbances of boreal forests: the effects of controlled burning and creating dead wood on beetle diversity. Biodiversity and Conservation 16, 3193–3211. [Google Scholar]
- *Toivanen, T. & Kotiaho, J. S. (2010). The preferences of saproxylic beetle species for different dead wood types created in forest restoration treatments. Canadian Journal of Forest Research 40, 445–464. [Google Scholar]
- *Tonn, W. M. , Paszkowski, C. A. , Scrimgeour, G. J. , Aku, P. K. M. , Lange, M. , Prepas, E. E. & Westcott, K. (2003). Effects of forest harvesting and fire on fish assemblages in Boreal Plains lakes: a reference condition approach. Transactions of the American Fisheries Society 132, 514–523. [Google Scholar]
- *Trofymow, J. A. , Stinson, G. & Kurz, W. A. (2008). Derivation of a spatially explicit 86–year retrospective carbon budget for a landscape undergoing conversion from old‐growth to managed forests on Vancouver Island, BC. Forest Ecology and Management 256, 1677–1691. [Google Scholar]
- Turner, M. G. (2010). Disturbance and landscape dynamics in a changing world. Ecology 91, 2833–2849. [DOI] [PubMed] [Google Scholar]
- *Turner, P. A. M. , Balmer, J. & Kirkpatrick, J. B. (2009). Stand‐replacing wildfires? The incidence of multi‐cohort and single‐cohort Eucalyptus regnans and E. obliqua forests in southern Tasmania. Forest Ecology and Management 258, 366–375. [Google Scholar]
- *Turner, D. P. , Ritts, W. D. , Law, B. E. , Cohen, W. B. , Yang, Z. , Hudiburg, T. , Campbell, J. L. & Duane, M. (2007). Scaling net ecosystem production and net biome production over a heterogeneous region in the western United States. Biogeosciences 4, 597–612. [Google Scholar]
- Turner, M. G. , Romme, W. H. , Gardnerl, R. H. , Neill, R. V. O. & Kratz, T. K. (1993). A revised concept of landscape equilibrium: disturbance and stability on scaled landscapes. Landscape Ecology 8, 213–227. [Google Scholar]
- *Twieg, B. D. , Durall, D. M. & Simard, S. W. (2007). Ectomycorrhizal fungal succession in mixed temperate forests. New Phytologist 176, 437–447. [DOI] [PubMed] [Google Scholar]
- *Uchiyama, K. , Goto, S. , Tsuda, Y. , Takahashi, Y. & Ide, Y. (2006). Genetic diversity and genetic structure of adult and buried seed populations of Betula maximowicziana in mixed and post‐fire stands. Forest Ecology and Management 237, 119–126. [Google Scholar]
- *Uriarte, M. & Papaik, M. (2007). Hurricane impacts on dynamics, structure and carbon sequestration potential of forest ecosystems in Southern New England, USA. Tellus 59A, 519–528. [Google Scholar]
- *Uys, C. , Hamer, M. & Slotow, R. (2009). Turnover in flightless invertebrate species composition over different spatial scales in Afrotemperate forest in the Drakensberg, South Africa. African Journal of Ecology 47, 341–351. [Google Scholar]
- *Vallecillo, S. , Brotons, L. & Herrando, S. (2008). Assessing the response of open‐habitat bird species to landscape changes in Mediterranean mosaics. Biodiversity and Conservation 17, 103–119. [Google Scholar]
- *Vandermast, D. B. & Van Lear, D. H. (2002). Riparian vegetation in the southern Appalachian mountains (USA) following chestnut blight. Forest Ecology and Management 155, 97–106. [Google Scholar]
- *Vayreda, J. , Gracia, M. , Canadell, J. G. & Retana, J. (2012). Spatial patterns and predictors of forest carbon stocks in Western Mediterranean. Ecosystems 15, 1258–1270. [Google Scholar]
- *Vedrova, E. F. , Shugalei, L. S. & Stakanov, V. D. (2002). The carbon balance in natural and disturbed forests of the southern taiga in central Siberia. Journal of Vegetation Science 13, 341–350. [Google Scholar]
- *Vidal, O. J. & Reif, A. (2011). Effect of a tourist‐ignited wildfire on Nothofagus pumilio forests at Torres del Paine biosphere reserve, Chile (Southern Patagonia). Bosque 32, 64–76. [Google Scholar]
- *Viedma, O. , Torres, I. , Pérez, B. & Moreno, J. M. (2012). Modeling plant species richness using reflectance and texture data derived from QuickBird in a recently burned area of Central Spain. Remote Sensing of Environment 119, 208–221. [Google Scholar]
- *Villa‐Castillo, J. & Wagner, M. R. (2002). Ground beetle (Coleoptera: Carabidae) species assemblage as an indicator of forest condition in northern Arizona ponderosa pine forests. Environmental Entomology 31, 242–252. [Google Scholar]
- *Wales, B. C. , Suring, L. H. & Hemstrom, M. A. (2007). Modeling potential outcomes of fire and fuel management scenarios on the structure of forested habitats in northeast Oregon, USA. Landscape and Urban Planning 80, 223–236. [Google Scholar]
- *Wang, C. , Gower, S. T. , Wang, Y. , Zhao, H. , Yan, P. & Bond‐Lamberty, B. P. (2001). The influence of fire on carbon distribution and net primary production of boreal Larix gmelinii forests in north‐eastern China. Global Change Biology 7, 719–730. [Google Scholar]
- *Ward, B. , Robinson, R. M. , Cranfield, R. J. & Williams, M. R. (2011). Forestcheck: the response of vascular flora to silviculture in jarrah (Eucalyptus marginata) forest. Australian Forestry 74, 276–287. [Google Scholar]
- *Wardell‐Johnson, G. & Williams, M. (2000). Edges and gaps in mature karri forest, south‐western Australia: logging effects on bird species abundance and diversity. Forest Ecology and Management 131, 1–21. [Google Scholar]
- *Wardell‐Johnson, G. W. , Williams, M. R. , Mellican, A. E. & Annells, A. (2004). Floristic patterns and disturbance history in karri forest, south‐western Australia: 1. Environment and species richness. Forest Ecology and Management 199, 449–460. [Google Scholar]
- *Wardell‐Johnson, G. W. , Williams, M. R. , Mellican, A. E. & Annells, A. (2007). Floristic patterns and disturbance history in karri (Eucalyptus diversicolor: Myrtaceae) forest, south‐western Australia: 2. Origin, growth form and fire response. Acta Oecologica 31, 137–150. [Google Scholar]
- *Wardle, D. A. , Jonsson, M. , Bansal, S. , Bardgett, R. D. , Gundale, M. J. & Metcalfe, D. B. (2012). Linking vegetation change, carbon sequestration and biodiversity: insights from island ecosystems in a long‐term natural experiment. Journal of Ecology 100, 16–30. [Google Scholar]
- *Wayman, R. B. & North, M. (2007). Initial response of a mixed‐conifer understory plant community to burning and thinning restoration treatments. Forest Ecology and Management 239, 32–44. [Google Scholar]
- *Wayne, A. F. , Cowling, A. , Lindenmayer, D. B. , Ward, C. G. , Vellios, C. V. , Donnelly, C. F. & Calver, M. C. (2006). The abundance of a threatened arboreal marsupial in relation to anthropogenic disturbances at local and landscape scales in Mediterranean‐type forests in south‐western Australia. Biological Conservation 127, 463–476. [Google Scholar]
- *Webb, S. L. & Scanga, S. E. (2001). Windstorm disturbance without patch dynamics: twelve years of change in a Minnesota forest. Ecology 82, 893–897. [Google Scholar]
- Weber, M. G. & Flannigan, M. D. (1997). Canadian boreal forest ecosystem structure and function in a changing climate: impact on fire regimes. Environmental Reviews 5, 145–166. [Google Scholar]
- *Wei, X. , Kimmins, J. P. & Zhou, G. (2003). Disturbances and the sustainability of long‐term site productivity in lodgepole pine forests in the central interior of British Columbia–an ecosystem modeling approach. Ecological Modelling 164, 239–256. [Google Scholar]
- *Welch, N. T. , Waldrop, T. A. & Buckner, E. R. (2000). Response of southern Appalachian table mountain pine (Pinus pungens) and pitch pine (P. rigida) stands to prescribed burning. Forest Ecology and Management 136, 185–197. [Google Scholar]
- *Wermelinger, B. , Duelli, P. & Obrist, M. K. (2002). Dynamics of saproxylic beetles (Coleoptera) in windthrow areas in alpine spruce forests. Forest Snow and Landscape Research 77, 133–148. [Google Scholar]
- *Werner, R. A. (2002). Effect of Ecosystem Disturbance on Diversity of Bark and Wood‐Boring Beetles (Coleoptera: Scolytidae, Buprestidae, Cerambycidae) in White Spruce (Picea glauca (Moench) Voss) Ecosystems of Alaska. Research Papers RMRS 546. USDA Forest Service, Portland. [Google Scholar]
- Westerling, A. L. , Hidalgo, H. G. , Cayan, D. R. & Swetnam, T. W. (2006). Warming and earlier spring increase western U.S. forest wildfire activity. Science 313, 940–943. [DOI] [PubMed] [Google Scholar]
- *Whicker, J. J. , Pinder, J. E. III & Breshears, D. D. (2008). Thinning semiarid forests amplifies wind erosion comparably to wildfire: implications for restoration and soil stability. Journal of Arid Environments 72, 494–508. [Google Scholar]
- *Whicker, J. J. , Pinder, J. E. III , Breshears, D. D. & Eberhart, C. F. (2006). From dust to dose: effects of forest disturbance on increased inhalation exposure. Science of the Total Environment 368, 519–530. [DOI] [PubMed] [Google Scholar]
- White, P. S. & Jentsch, A. (2001). The search for generality in studies of disturbance and ecosystem dynamics. Progress in Botany 62, 399–449. [Google Scholar]
- Whittaker, R. J. (2010). Meta‐analyses and mega‐mistakes: calling time on meta‐analysis of the species richness‐productivity relationship. Ecology 91, 2522–2533. [DOI] [PubMed] [Google Scholar]
- *Wienk, C. L. , Sieg, C. H. & McPherson, G. R. (2004). Evaluating the role of cutting treatments, fire and soil seed banks in an experimental framework in ponderosa pine forests of the Black Hills, South Dakota. Forest Ecology and Management 192, 375–393. [Google Scholar]
- *Wilmshurst, J. M. , McGlone, M. S. & Partridge, T. R. (1997). A late holocene history of natural disturbance in lowland podocarp/hardwood forest, Hawke's Bay, New Zealand. New Zealand Journal of Botany 35, 79–96. [Google Scholar]
- *Wilson, C. J. (1999). Effects of logging and fire on runoff and erosion on highly erodible granitic soils in Tasmania. Water Resources Research 35, 3531–3546. [Google Scholar]
- *Wimberly, M. C. & Reilly, M. J. (2007). Assessment of fire severity and species diversity in the southern Appalachians using Landsat TM and ETM+ imagery. Remote Sensing of Environment 108, 189–197. [Google Scholar]
- *Winford, E. M. & Gaither, J. C. (2012). Carbon outcomes from fuels treatment and bioenergy production in a Sierra Nevada forest. Forest Ecology and Management 282, 1–9. [Google Scholar]
- *Wittkuhn, R. S. , McCaw, L. , Wills, A. J. , Robinson, R. , Andersen, A. N. , Van Heurck, P. , Farr, J. , Liddelow, G. & Cranfield, R. (2011). Variation in fire interval sequences has minimal effects on species richness and composition in fire‐prone landscapes of south‐west Western Australia. Forest Ecology and Management 261, 965–978. [Google Scholar]
- *Wolff, J. M. , Battaglia, L. , Carter, T. C. , Rodman, L. B. , Britzke, E. R. & Feldhamer, G. A. (2009). Effects of tornado disturbance on bat communities in Southern Illinois. Northeastern Naturalist 16, 553–562. [Google Scholar]
- *Xiao, J. , Zhuang, Q. , Law, B. E. , Baldocchi, D. D. , Chen, J. , Richardson, A. D. , Melillo, J. M. , Davis, K. J. , Hollinger, D. Y. , Wharton, S. , Oren, R. , Noormets, A. , Fischer, M. L. , Verma, S. B. , Cook, D. R. , Sun, G. , McNulty, S. , Wofsy, S. C. , Bolstad, P. V. , Burns, S. P. , Curtis, P. S. , Drake, B. G. , Falk, M. , Foster, D. R. , Gu, L. , Hadley, J. L. , Katul, G. G. , Litvak, M. , Ma, S. , Martin, T. A. , Matamala, R. , Meyers, T. P. , Monson, R. K. , Munger, J. W. , Oechel, W. C. , Paw, U. K. T. , Schmid, H. P. , Scott, R. L. , Starr, G. , Suyker, A. E. & Torn, M. S. (2011). Assessing net ecosystem carbon exchange of U.S. terrestrial ecosystems by integrating eddy covariance flux measurements and satellite observations. Agricultural and Forest Meteorology 151, 60–69. [Google Scholar]
- *Youngman, J. A. & Gayk, Z. G. (2011). High density nesting of black‐backed woodpeckers (Picoides arcticus) in a post‐fire great lakes jack pine forest. Wilson Journal of Ornithology 123, 381–386. [Google Scholar]
- *Yu, Z. , Apps, M. J. & Bhatti, J. S. (2002). Implications of floristic and environmental variation for carbon cycle dynamics in boreal forest ecosystems of central Canada. Journal of Vegetation Science 13, 327–340. [Google Scholar]
- *Zamora, R. , Molina‐Martínez, J. R. , Herrera, M. A. & Rodríguez y Silva, F. (2010). A model for wildfire prevention planning in game resources. Ecological Modelling 221, 19–26. [Google Scholar]
- *Zhao, D. , Allen, B. & Sharitz, R. R. (2006). Twelve year response of old‐growth southeastern bottomland hardwood forests to disturbance from Hurricane Hugo. Canadian Journal of Forest Research 36, 3136–3147. [Google Scholar]
- *Zhou, L. , Dai, L. , Wang, S. , Huang, X. , Wang, X. , Qi, L. , Wang, Q. , Li, G. , Wei, Y. & Shao, G. (2011). Changes in carbon density for three old‐growth forests on Changbai Mountain, Northeast China: 1981–2010. Annals of Forest Science 68, 953–958. [Google Scholar]
- *Zinck, R. D. , Johst, K. & Grimm, V. (2010). Wildfire, landscape diversity and the Drossel‐Schwabl model. Ecological Modelling 221, 98–105. [Google Scholar]
- *Zmihorski, M. (2010). The effect of windthrow and its management on breeding bird communities in a managed forest. Biodiversity and Conservation 19, 1871–1882. [Google Scholar]
- *Zmihorski, M. (2012). The effects of anthropogenic and natural disturbances on breeding birds of managed Scots pine forests in northern Poland. Ornis Fennica 89, 63–73. [Google Scholar]
- *Zozaya, E. L. , Brotons, L. & Vallecillo, S. (2011). Bird community responses to vegetation heterogeneity following non‐direct regeneration of Mediterranean forests after fire. Ardea 99, 73–84. [Google Scholar]
- *Zwolak, R. & Foresman, K. R. (2007). Effects of a stand‐replacing fire on small‐mammal communities in montane forest. Canadian Journal of Zoology 85, 815–822. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Reported disturbance effects on biodiversity and ecosystem service categories (following the definition of the Millenium Ecosystem Assessment, 2005): (A) biodiversity, (B) supporting services, (C) provisioning services, (D) regulation services and (E) cultural services. N indicates the number of observations.
Appendix S1. Indicators of biodiversity and ecosystem services and their respective synonyms used in the literature search.
Appendix S2. Database of disturbance impacts on ecosystem services and biodiversity derived from the literature.


