Abstract
Objective
Impairment of the renin-angiotensinogen-aldosterone system (RAAS), one of the characteristics of essential hypertension (EH), imbalances vascular homeostasis. Despite inconsistent reports on individual single nucleotide polymorphisms (SNPs) as a major predictor of EH, interactions among RAAS genetic variants are rarely investigated.
Methods
Using SNP markers, we studied potential interactions between angiotensin 1 converting enzyme (ACE), angiotensinogen (AGT), angiotensin II-type 1 receptor (AGTR1), and α adducin (ADD1) variants and their correlation with clinical endpoints in 545 individuals with hypertension and 400 age- and ethnicity-matched unrelated controls. Generalised multifactor dimensionality reduction (GMDR) analysis identified the models for genotype interaction.
Results
Although the results on single genes were significant, gene-gene interactions were more reliable and promising as markers in predisposing hypertension. The best models to represent association of multi-locus interactions with augmented hypertension susceptibility were: (a) within gene 4-locus model comprised of AGT SNPs −217G/A, −20A/C, −6G/A and 235M/T (p=0.022, OR 6.1); and (b) between genes 5-locus model comprised of AGT −217G/A, −20A/C, −6G/A, 235M/T and ACE I/D (p=0.05, OR 4.6). Stratification of 4- and 5-locus GMDR models on the basis of risk alleles from ≤1 to ≥7 increased the ORs from 2.8 to 36.1 and from 0.9 to 16.1, respectively. Moreover, compared to ≤1 risk alleles the ≥7 interacting risk alleles in both 4- and 5-locus models showed an increment of 14.2% and 11.1% in systolic blood pressure, 7.7% and 1.1% in diastolic blood pressure, and 10.5% and 5.1% in mean arterial pressure, respectively, in patients.
Conclusions
Interactions among the genetic loci of RAAS components may be used as a predictor for susceptibility to hypertension.
Keywords: HYPERTENSION, GENETICS
Introduction
Essential hypertension (EH) represents a progressive global burden due to the amplified incidence of adult morbidity and mortality.1 EH is regarded as a complex disease that results from both genetic and environmental interactions. The genetic components of blood pressure (BP) regulation pathways are implicated in the predisposition to EH, as 40–60% of BP variability is genetically determined.2 3 The last two decades have witnessed remarkable progress in the field of hypertension; however, unifying the global predisposing markers of the disease remains to be accomplished, and the lack of such markers hampers progress in the field of comprehensive personalised medicine. These failures can be attributed to two reasons. First, there is a lack of genetic information from all the ethnicities, because hypertension related traits and genetic mechanisms work differently across races and ethnicities.4 5 Second, there is a lack of reports on interaction studies among multiple loci of EH-implicated pathway(s); single genetic variants have shown modest effects and may not be influential.4 6 7 Of note, non-linear interactions between the different loci likely play a key role in disease susceptibility compared to single variant.8–10
Among the various pathways, the renin-angiotensin-aldosterone system (RAAS) has been well documented in the regulation of BP and vascular homeostasis.11 RAAS components like angiotensinogen, the product of AGT, is the only known precursor protein cleaved by renin to the decapeptide angiotensin (Ang) I, precursor of Ang II. Angiotensin-converting enzyme, encoded by ACE, is a zinc-dependent peptidase responsible for hydrolysing Ang I into the biologically active vasoconstrictor Ang II. The latter, a potent vasopressor hormone, regulates aldosterone secretion and sodium reabsorption, thereby increasing BP through binding to AGTR1 encoded type 1 receptor. In addition, adducin 1 (ADD 1), a family member of heterodimeric cytoskeleton proteins, is known to alter vascular homeostasis by increasing renal sodium reabsorption and thereby may be involved in the pathophysiology of EH.12 Therefore, genetic variations in the RAAS components, angiotensinogen (AGT), angiotensin 1 converting enzyme (ACE), angiotensin II-type 1 receptor (AGTR1), and α adducing (ADD1) are logically oriented toward increasing our understanding BP regulation. We therefore hypothesised that interaction between the loci of RAAS components may be pertinent in understanding the pathophysiological mechanism underlying EH.
To address this issue, 10 genetic variants from AGT, ACE, AGTR1, and ADDI were screened in a well-characterised North Indian cohort with a case–control design. Our emphasis has been to understand the contribution of each allele in the interacting mode. Here we describe the efficiency of the non-linear interaction within and between genes (epistasis), and their influence on clinical endpoints, especially BP, represented by systolic BP (SBP), diastolic BP (DBP), and mean arterial pressure (MAP).
Subjects and methods
Study subjects and recruitment criteria
The study protocol and consent form were approved by the human ethics committees of the Council of Scientific and Industrial Research-Institute of Genomics and Integrative Biology (CSIR-IGIB), Delhi and Govind Ballabh (GB) Pant Hospital, New Delhi; the latter is a hospital specialising in cardiovascular diseases. We recruited 545 patients with EH, and 400 age-, gender- and ethnically-matched unrelated North Indian controls through the hypertension and outpatient clinics of the GB Pant Hospital. A detailed questionnaire about environmental factors, lifestyle, health, clinical history, residential region and haemodynamic parameters was administered by the clinicians. Written informed consent was obtained from each participant.
Recruitment criteria for patients included: age 25–60 years, SBP ≥140 mm Hg and/or DBP ≥90 mm Hg (Joint National Committee VII), and absence of antihypertensive medication. Recruitment criteria for controls included: age 25–60 years, SBP <120 mm Hg and DBP <80 mm Hg, absence of family history of hypertension, and no medication usage. Participants with a history of coronary artery disease, cerebrovascular disease, stroke, diabetes mellitus, renal diseases, and those on antihypertensive medication were excluded from the study. All subjects were asked to remain at rest for 5 min before BP measurement using a calibrated mercury sphygmomanometer with appropriate adult cuff size. Three measurements of BP, with the subject in the supine position, were recorded. Ten millilitres of blood was drawn from each subject after overnight fasting. Isolated DNA and plasma samples from blood were stored at −80°C, if not used immediately.
Assessment of biochemical parameters
Plasma ACE activity was measured by a kinetic method.13 Estimations were performed in duplicate on a high-throughput SpectraMax384 Spectrophotometer (Molecular Devices, Sunnyvale, California, USA). Routine biochemical parameters, for example, total cholesterol, triglycerides, glucose, uric acid, and creatinine, were estimated on an Autoanalyzer (Elecsys 2010, Roche, Germany). The intra- and inter-assay coefficients of variation were <5% for all the measurements.
Selection of ACE, AGT, AGTR1, and ADD1 single nucleotide polymorphisms and genotyping
Ten single nucleotide polymorphisms (SNPs) from AGT, ACE, AGTR1, and ADD1 were selected based on their location and clinical and functional relevance. Details of the studied SNPs including the nature of the SNP, its position, base pair change, amino acid change, and importance with respect to the study are portrayed in online supplementary table S1. Briefly, five out of seven AGT SNPs, for example, −532C/T (rs5046), −217G/A (rs5049), −152G/A (rs11568020), −20A/C (rs5050), and −6G/A (rs5051), belong to the promoter region and two non-synonymous SNPs, 174T/M (rs4762) and 235M/T (rs699), belong to exon 2. The selected AGT promoter SNPs are either part of core-promoter element 1 or lie in the upstream of promoter and play a critical role in transcriptional regulation,14 15 whereas amino acid substitution of non-synonymous AGT SNPs increases not only the risk of high BP but also increases the plasma AGT concentration.5 11 The I/D polymorphism (rs4646994) of ACE represents the presence (insertion allele, I) or absence (deletion allele, D) of a 287 bp marker in intron 16. The ACE D allele is associated with elevated ACE concentrations in various cardiovascular diseases and serves as a risk allele for EH.13 16–19 AGTR1 1166A/C (rs5186) polymorphism is located in the 3′ untranslated region. Recently, it has been shown that the C allele of 1166A/C polymorphism interferes with the base-pairing complementariness between AGTR1 mRNA and microRNA-155, and thereby increases AGTR1 protein expression that is associated with increased risk of cardiovascular diseases.20 ADD1 polymorphism 614G/T (rs4961/Gly460Trp) is a non-synonymous polymorphism. It has been shown that this SNP leads to stimulation of sodium–potassium–ATPase activity in renal tubular cells, which increases renal sodium reabsorption and is involved in BP control.21 Genomic DNA was isolated from peripheral blood leucocytes using a modified salting-out protocol.22 Genotyping was performed using PCR-restriction fragment length polymorphism (RFLP) or SNaPshot ddNTP primer extension PCR (Applied Biosystems, Foster City, California, USA). Details of the primers and PCR conditions for the polymorphisms are summarised in online supplementary tables S2 and S3. Two observers independently read and confirmed all the genotypes; discrepancies, if any, were resolved by repeating PCR-RFLP and SNaPshot.
heartasia-2016-010723supp.pdf (187.6KB, pdf)
Gene–gene interactions
Gene–gene interactions among AGT, ACE, AGTR1, and ADD1 were studied using the generalised multifactor dimensionality reduction (GMDR, V.0.9) statistical software package.23 GMDR is a data mining approach for analysing interactions within and between genes. It gives the best genetic models for disease prediction, after adjusting for various confounding factors, for example, age, gender, body mass index (BMI), smoking, alcohol, triglyceride, and cholesterol. It also identifies non-linear interactions among discrete genetic and environmental attributes, adjusting for covariates. The best models obtained by this software were selected on the basis of higher scores of testing accuracy (TA), training balance, and cross validation consistency (CVC). These models were further analysed using multivariate logistic regression analysis.
Correlation analysis
To examine the effect of genetic variants on the clinical endpoints, the correlation of each of the individual and interacting genotypes of AGT, ACE, AGTR1, and ADD1 with clinical parameters—that is, SBP, DBP, and mean arterial pressure—was analysed.
Statistical analysis
The differences in baseline characteristics and demographic features between the two groups were compared by unpaired Student's t tests. A goodness-of-fit test was used for testing the Hardy-Weinberg Equilibrium (HWE). A χ2 test compared the genotype and allele frequencies between the two groups by SPSS v.15 (SPSS Inc, Chicago, Illinois, USA). The risk prediction for EH was estimated by the OR at 95% CI using multivariate logistic regression in SPSS v.15. Gene–gene interactions were looked for using GMDR.23 Further, the genotype–phenotype correlation was analysed with a general linear model (GLM). A value of p<0.05, after adjustment for confounding factors and Bonferroni's correction for multiple testing, was considered statistically significant.
Results
Comparison of demographic and clinical characteristics
The main clinical and demographic characteristics of the two study groups are presented in table 1. Patients had significantly higher BMI (p=0.047), SBP, DBP, and MAP (p<0.0001 each), and higher concentrations of specific and routine biochemical parameters, for example, ACE, cholesterol and triglyceride, when compared with controls (p<0.0001; table 1). Distribution of the clinical and biochemical parameters followed a normal distribution in both controls and patients (p>0.05).
Table 1.
Demographic and clinical phenotypes of the studied participants
| Patients | Controls | ||
|---|---|---|---|
| Parameters | n=545 | n=400 | p Value |
| Gender | |||
| Male | 501 (92%) | 368 (92%) | |
| Female | 44 (08%) | 32 (08%) | |
| Clinical characteristics | |||
| Age, year | 51.6±10.9 | 50.0±9.7 | NS |
| BMI, kg/m2 | 24.3±4.1 | 23.7±4.3 | 0.047 |
| SBP, mm Hg | 160.2±19.3 | 115.9±9.4 | <0.0001 |
| DBP, mm Hg | 97.2±9.6 | 76.4±6.6 | <0.0001 |
| MAP, mm Hg | 117.6±13.7 | 90.5±6.9 | <0.0001 |
| Alcohol consumption | 55 (10%) | 24 (06%) | 0.030 |
| Smoking history | 66 (12%) | 36 (09%) | 0.150 |
| Family history of hypertension | 425 (78%) | (00) 00% | <0.0001 |
| Biochemical parameters | |||
| ACE, U/L | 106.3±32.7 | 86.0±31.2 | <0.0001 |
| Total cholesterol, mmol/L | 3.3±1.2 | 2.6±1.3 | <0.0001 |
| Triglyceride, mmol/L | 1.3±0.8 | 1.0±0.6 | <0.0001 |
| Uric acid, mg/dL | 4.8±1.5 | 4.7±1.5 | NS |
| Glucose, mg/dL | 101.0±23.0 | 99.2±26 | NS |
| Serum creatinine, mg/dL | 1.15±0.45 | 1.14±0.44 | NS |
Data are presented as mean±SD. p Values were calculated using EPIINFO v.6 (Center for Disease Control, Atlanta, Georgia, USA) software. A value of p<0.05 was considered statistically significant.
ACE, angiotensin converting enzyme; BMI, body mass index; DBP, diastolic blood pressure; MAP, mean arterial pressure; n, number of participants; NS, non-significant; SBP, systolic blood pressure.
Single-locus association analyses
The allele and genotype frequencies of the 10 studied polymorphisms were in HWE (p>0.05, see online supplementary table S4) for both the groups. The multiple logistic regression analyses of the single locus are shown in table 2 and online supplementary table S5. As shown in table 2, AGT SNPs −532C/T and −217G/A were associated significantly with EH under the additive model (p=8.4E−04, OR 1.7; p=0.001, OR 1.6, respectively); SNP −20A/C was associated under dominant (p=7.9E−11, OR 3.2), recessive (p=0.001, OR 3.1), and additive (p=1.3E−12, OR 3.2) models; SNP −6G/A was associated under dominant (p=5.8E−06, OR 2.6) and additive (p=2.4E−08, OR 2.8) models, whereas SNP 235M/T was associated under dominant (p=8.6E−04, OR 2.0), recessive (p=2.4E−12, OR 3.9), and additive (p=4.8E−09, OR 3.0) models after adjustment with seven confounding factors and Bonferroni's correction for multiple testing. As a consequence, the alleles −532T, −217A, −20C, −6A, and 235T were significantly prevalent in patients and associated with increased risk of EH (p=0.002, OR 1.5; p=0.004, OR 1.4; p=2.1E−10, OR 2.1; p=1.5E−06, OR 1.7; p=2.6E−14, OR 2.4, respectively, see online supplementary table S5). Further, the ACE I/D polymorphism showed a slight difference under the recessive (p=0.043, OR 1.6) model of inheritance. As a consequence, the D allele was more prevalent in patients, although it did not cross the significance threshold (p=0.123, see online supplementary table S5). The remaining SNPs AGT −152G/A and 174T/M, AGTR1 1166A/C, and ADD1 460G/T did not differ significantly between patients and controls (p>0.05, table 2).
Table 2.
Risk analyses of AGT, ACE, AGTR1, and ADD1 polymorphisms with hypertension under different genetic models
| Genes/SNPs | Models | Comparisons | χ2 | p Value | OR (95% CI) |
|---|---|---|---|---|---|
|
AGT −532C/T (rsID:5046) |
Dominant | CC vs CT+TT | 1.58 | 0.209 | 1.2 (0.9 to 1.8) |
| Recessive | CC+CT vs TT | 0.58 | 0.445 | 1.4 (0.6 to 3.1) | |
| Additive | CC vs CT vs TT | 11.15 | 8.4E−04 | 1.7 (1.2 to 2.3) | |
| −217G/A (rsID:5049) |
Dominant | GG vs GA+AA | 0.99 | 0.319 | 1.2 (0.8 to 1.7) |
| Recessive | GG+GA vs AA | 1.19 | 0.276 | 0.7 (0.4 to 1.3) | |
| Additive | GG vs GA vs AA | 10.46 | 0.001 | 1.6 (1.2 to 2.2) | |
| −152G/A (rsID:11568020) |
Dominant | GG vs GA+AA | 0.01 | 0.925 | 1.0 (0.6 to 1.9) |
| Recessive | GG+GA vs AA | – | – | – | |
| Additive | GG vs GA vs AA | 3.26 | 0.071 | 1.7 (1.0 to 3.0) | |
| −20A/C (rsID:5050) |
Dominant | AA vs AC+CC | 42.27 | 7.9E−11 | 3.2 (2.2 to 4.5) |
| Recessive | AA+AC vs CC | 10.28 | 0.001 | 3.1 (1.6 to 6.3) | |
| Additive | AA vs AC vs CC | 50.27 | 1.3E−12 | 3.2 (2.3 to 4.4) | |
| −6G/A (rsID:5051) |
Dominant | GG vs GA+AA | 20.54 | 5.8E−06 | 2.6 (1.7 to 4.0) |
| Recessive | GG+GA vs AA | 0.23 | 0.631 | 1.1 (0.8 to 1.6) | |
| Additive | GG vs GA vs AA | 31.16 | 2.4E−08 | 2.8 (2.0 to 4.1) | |
| 174T/M (rsID:4762) |
Dominant | TT vs TM+MM | 3.48 | 0.062 | 0.6 (0.4 to 1.0) |
| Recessive | TT+TM vs MM | 1.89 | 0.169 | 0.3 (0.1 to 1.6) | |
| Additive | TT vs TM vs MM | 0.69 | 0.406 | 0.8 (0.6 to 1.3) | |
| 235M/T (rsID:699) |
Dominant | MM vs MT+TT | 11.12 | 8.6E−04 | 2.0 (1.3 to 3.0) |
| Recessive | MM+MT vs TT | 49.16 | 2.4E−12 | 3.9 (2.7 to 5.8) | |
| Additive | MM vs MT vs TT | 34.26 | 4.8E−09 | 3.0 (2.1 to 4.4) | |
|
ACE I/D (rsID:4646944) |
Dominant | II vs ID+DD | 0.00 | 0.983 | 1.0 (0.7 to 1.4) |
| Recessive | II+ID vs DD | 4.10 | 0.043 | 1.6 (1.0 to 2.4) | |
| Additive | II vs ID vs DD | 1.01 | 0.315 | 1.2 (0.9 to 1.6) | |
|
AGTR1 1166A/C (rsID:5186) |
Dominant | AA vs AC+CC | 0.60 | 0.439 | 1.2 (0.8 to 1.8) |
| Recessive | AA+AC vs CC | 1.31 | 0.253 | 0.5 (0.1 to 1.7) | |
| Additive | AA vs AC vs CC | 0.11 | 0.740 | 1.1 (0.7 to 1.6) | |
|
ADD1 614G/T (rsID:4961) |
Dominant | GG vs GT+TT | 2.38 | 0.123 | 1.3 (0.9 to 1.8) |
| Recessive | GG+GT vs TT | 0.14 | 0.710 | 0.9 (0.4 to 1.9) | |
| Additive | GG vs GT vs TT | 3.30 | 0.069 | 1.3 (1.0 to 1.8) |
p Value, χ2 and OR were calculated using multivariate logistic regression analysis after adjustment for age, gender, BMI, smoking, alcohol, triglyceride and cholesterol. Dominant model: A single copy of mutant allele is enough to modify the risk; hence, heterozygous and homozygous (mutant) genotypes have the same risk. Recessive model: Two copies of mutant allele are necessary to change the risk. Hence, heterozygous and homozygous (wild-type) genotypes have the same effect. Additive model: Each copy of mutant allele modifies the risk in an additive form. Hence, a combination of the two genotypes with weights 2 and 1, respectively, homozygous (mutant) and heterozygous to homozygous (wild-type), is compared. A value of p<0.05 was considered statistically significant.
BMI, body mass index; SNPs, single nucleotide polymorphisms.
Multi-locus interaction analyses
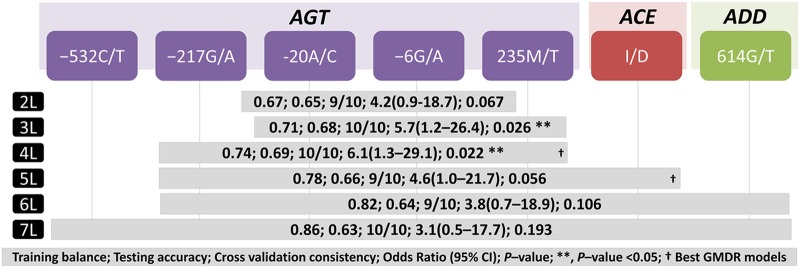
GMDR analysis was used to evaluate the impact of interactions among the genotypes of the 10 SNPs of AGT, ACE, AGTR1, and ADD1 in EH. GMDR analysis after adjustment with seven confounding factors revealed a 4-locus model comprised of AGT SNPs, for example, −217G/A, −20A/C, −6G/A, and 235M/T, as the best within-gene disease predicting model with a prediction error of 0.26 (TA=0.69, CVC=10/10, OR 6.1, 95% CI 1.3 to 29.1, p=0.022; figure 1). Similarly, GMDR analysis after adjustment with seven confounding factors revealed a 5-locus model comprised of AGT −217G/A, −20A/C, −6G/A, 235M/T, and ACE I/D as the best between-genes disease predicting model with a prediction error of 0.22 (TA=0.66, CVC=9/10, OR 4.6, 95% CI 1.0 to 21.7, p=0.05; figure 1).
Figure 1.
Identification of the best interacting genetic models of within- and between-genes, AGT, ACE, AGTR1, and ADD1 using generalised multifactor dimensionality reduction (GMDR). † Best GMDR models for each analysis, on the basis of higher testing accuracy and cross validation consistency. AGT, angiotensinogen gene; ACE, angiotensin 1 converting enzyme gene; ADD1, α adducin gene; 2L to 7L, 2-locus to 7-locus GMDR models carrying best interacting genotypes after adjustment for age, gender, body mass index, smoking, alcohol, triglyceride and cholesterol. p Values and OR at 95% CI were calculated by permuting the patients and controls 1000 times.
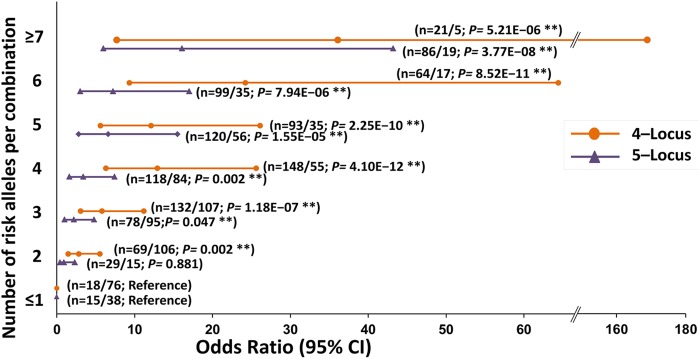
Furthermore, as shown in figure 2, the 4- and 5-locus GMDR models having 8 and 10 alleles, respectively, were stratified on the basis of risk alleles in increasing number (n ≤1 to ≥7). The multivariate logistic regression analysis after adjustment with seven confounding factors and Bonferroni's correction test for multiple testing revealed a significant and continuous increase in the OR from 2.8 to 36.1 as we moved from n=2 to ≥7 risk alleles, respectively, in the 4-locus model (p=0.002–5.21E−06) compared to OR for n ≤1 risk allele. Similarly, a continuous increase in the OR from 0.9 to 16.1 was observed as we moved from n=2 to ≥7 risk alleles, respectively, in the 5-locus model (p=0.881–3.77E−08) compared to OR for n ≤1 risk allele.
Figure 2.
Risk analyses of best generalised multifactor dimensionality reduction (GMDR) models with essential hypertension corresponding to the increasing number of risk alleles. 4-locus and 5-locus represent within angiotensinogen (AGT) and between AGT and angiotensin 1 converting enzyme (ACE) best models, respectively. n, number of the classified risk alleles in the respective models. N, number of cases and controls in each classified risk alleles. p Value and OR at 95% CI were calculated after adjustment for age, gender, body mass index, alcohol, smoking, triglyceride and cholesterol using multivariate logistic regression analysis and Bonferroni's correction for multiple testing.
Correlation analyses
Single-locus versus clinical characteristics
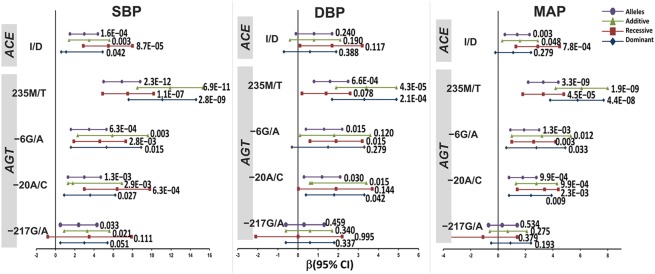
As shown in figure 3, GLM after adjustment for seven confounding factors and Bonferroni's correction for multiple testing revealed a significant positive correlation for AGT SNPs −217G/A, −20A/C, and −6G/A, and ACE I/D polymorphism with BP phenotypes at dominant, recessive, and additive models. As a consequence, the risk allele −217A correlated with 2.4 mm Hg higher SBP (p=0.033), whereas risk alleles −20C, −6A, and 235T correlated with 3.0, 3.5, and 6.9 mm Hg higher SBP (p=1.3E−03; p=6.3E−04, p=2.3E−12, respectively); 1.2, 1.3, and 1.6 mm Hg higher DBP (p=0.03; p=0.015; p=6.6E−04, respectively); and 1.8, 1.9, and 3.3 mm Hg higher MAP (p=9.9E−04; p=1.3E−03; p=3.3E−09, respectively). The ACE risk allele D correlated with 3.0 mm Hg higher SBP (p=1.6E−04) and 1.4 mm Hg higher MAP (p=0.003).
Figure 3.
Correlation of clinical parameters with AGT, ACE, AGTR1, and ADD1 polymorphisms under different genetic models. p Values and correlation coefficient (β) at 95% CI were calculated after adjustment for age, gender, smoking, alcohol, triglyceride and cholesterol, and Bonferroni's correction for multiple testing using SPSS v.15. DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure.
Multi-locus interactions versus clinical characteristics
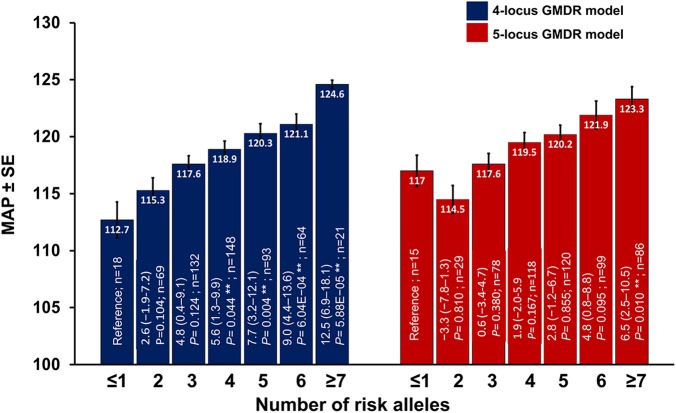
The analysis of covariance after adjustment with seven confounding factors revealed significant differences between the SBP, DBP, and MAP when analysed against 4- and 5-locus models with both having risk alleles n ≤1 to ≥7 (pANCOVA<0.0001, each). As shown in figure 4, stratification of the interacting 4-locus model with risk alleles in increasing number (n=2 to ≥7) revealed elevation of 2.6–12.5 mm Hg MAP (p=0.104–5.88E−05) relative to the values of respective parameters in the model with ≤1 risk allele. Similarly, stratification of the 5-locus model with risk alleles in increasing number (n=2 to ≥7) revealed an increase of −3.3 to 6.5 mm Hg in MAP (p=0.810–0.010) relative to the model with ≤1 risk allele.
Figure 4.
Influence of the increasing number of risk alleles in the 4-locus and 5-locus best generalised multifactor dimensionality reduction (GMDR) model on clinical parameters. n, number of cases in each classified risk alleles. Inset in bars represents correlation coefficient (β) at 95% CI after adjustment for age, gender, smoking, alcohol, triglyceride and cholesterol, and Bonferroni's correction for multiple testing and p value. The general linear model was used to calculate β and p values. MAP, mean arterial pressure.
Discussion
The present study investigated genetic variants of AGT, ACE, AGTR1, and ADD1, and their interactions and influence on clinical traits related to EH. Individual loci of the AGT genes showed association with EH in dominant, recessive, and additive models. As a consequence, a higher OR was observed for alleles −532T, −217A, −20C, −6A, and 235T and these are considered as risk conferring alleles. Furthermore, using GMDR analysis, augmented risk for EH was observed for the within-gene interacting 4-locus model of AGT and the between-genes interacting 5-locus model comprising AGT and ACE. The interactions among the loci were more apparent when patients were classified on the basis of increasing numbers of risk alleles. The increment in the number of risk alleles in patients correlated with increased OR for the disease (EH). Furthermore, the correlation with consistent increases in clinical phenotypes like SBP, DBP, and MAP strengthened the outcome of this study.
Our findings on individual components of RAAS were significant as they revealed higher OR for EH in dominant, recessive, and additive modes of inheritance, specifically for AGT polymorphisms after adjustment with potential seven confounding factors. ACE I/D polymorphism also differed significantly under the recessive model. Accumulating literature reveals the AGT and ACE genetic variants as an important area of research in hypertension;24–28 and a good amount of work has been done in the Indian population, especially in those in South Indian.29–32 Importantly, the current observations are in accordance with our previous reports on cohorts of the same ethnicity.25 26 The literature on RAAS components is consistently in agreement with our findings, but with certain conflicts.15 16 24 33–35 However, these conflicts could be attributed to the ethnic variations and/or the lack of non-linear interactions among the loci of gene(s).5 36
Although the single locus results on the RAAS components were encouraging (but for the fact that complex diseases like EH are multigenic and multifactorial), we looked for gene–gene interactions using the GMDR approach. Of note, the attributed inconsistencies and the magnitude of low power to detect an association with individual SNPs can be improved using gene–gene interaction analysis.36 37 The epistatic evaluation in multifactorial disease like EH further increases the predictive accuracy of genotype–phenotype correlations.7 In our analyses, the best within-gene disease-conferring model was the 4-locus model of AGT comprising the SNPs −217G/A, −20A/C, −6G/A, and 235M/T. Compared to the individual risk alleles, the interaction among risk alleles further increased the OR for EH. Next, the best disease-conferring model between genes was the 5-locus model comprising the variants AGT −217G/A, −20A/C, −6G/A, 235M/T, and ACE I/D. Similar to the 4-locus model, the stratification of the 5-locus model on the basis of increased numbers of risk alleles revealed a higher OR for EH compared to individual risk alleles; it thus supported the role of RAAS epistasis in the regulation of BP.8 33 34 Of relevance, the appearance of AGT −217G/A, −20A/C, −6G/A, 235M/T, and ACE ID variants in the best models can be attributed to the notion of loci-specific interactions.8 Our results suggested that interactions between the risk variants of the AGT gene might be enough to impair vascular homeostasis.
The significant association of multi-locus interactions and the increase in the risk for EH at the genetic and clinical level support the notion that gene–gene and gene–environment interactions may play fundamental roles in the development of EH and other complex diseases.6 8–10 26 36 38 This study exposed the interaction between loci of AGT and ACE as a potential disease modifier to influence the biological and biochemical pathways underlying the disease pathophysiology compared to individual loci of respective genes. With regard to correlation analysis, our findings signified a major contribution of epistasis towards compromised BP phenotypes. The key clinical endpoint that determines hypertension phenotypes is SBP ≥140 mm Hg and/or DBP ≥90 mm Hg, therefore their correlation with genetic variants seems pertinent.39 Although the single locus correlation with BP phenotypes was apparent, the GLM revealed a significant linear correlation of multi-locus interactions with clinical endpoints, for example, SBP, DBP, and MAP. Compared to ≤1 risk allele the ≥7 interacting risk alleles in both 4- and 5-locus models showed increments in SBP (14.2% and 11.1%), DBP (7.7% and 1.1%), and MAP (10.5% and 5.1%), suggesting that the interactions of genetic loci played an important role in determining the observed phenotypes.6 40 The elevated RAAS-related biochemical parameters ACE, aldosterone and Ang II and their correlation with increased BP phenotypes are implicated in disease pathophysiology.11 Overall, our findings not only support previous reports but also provide an insight into the significant interaction of risk variants of the AGT gene and show the possible role of gene-to-gene interaction with the ACE gene in the susceptibility for EH.
Inconsistencies in genetic association studies are attributed mainly to population stratification and limited sample size. To disparage population stratification, we recruited patients and controls from the same region. The multi-locus analyses also highlighted the need to have a much higher sample size to strengthen the findings, so as to define the underlying mechanisms in the pathophysiology of hypertension. Although the plasma concentrations of ACE were measured, measurement of other RAAS related biochemical parameters and their correlation with genetic and clinical parameters would have further substantiated the study's findings.
In conclusion, the interactions among genetic loci of RAAS have a notable interactive effect and are associated with altered clinical phenotypes and consequently EH. This study advocates further investigation of the potential interactions among genetic loci in order to contribute robust information toward deciphering the pathophysiological mechanisms underlying complex diseases.
Key messages.
What is already known about this subject?
The biological regulation of blood pressure comes, to a greater extent, from the complex interactions between environmental and genetic factors. Large numbers of studies have reported the association of various genetic variants of the renin-angiotensin-aldosterone system (RAAS) with essential hypertension (EH). However, the attempt towards unifying global predisposing markers for EH has failed due to the lack of genetic information on interactions among multiple loci of EH-implicated pathway(s); a single genetic variant due to its modest effect may not be influential.
What does this study add?
The interactions among risk alleles of RAAS genetic loci, in particular AGT, has a notable interactive effect and is associated with increased blood pressure phenotypes, for example, systolic blood pressure, diastolic blood pressure, and mean arterial pressure. Conversely, the interaction among protective alleles showed a profound reduction in blood pressure phenotypes. We also showed that potential interaction among genetic loci, for example, AGT and ACE genes, holds robust information in deciphering pathophysiological mechanisms underlying complex diseases.
How might this impact on clinical practice?
This study unravels the interaction among the RAAS genetic variants, and their influence on clinical traits related to EH. The outcome may pave the way in unifying ethnicity-based global predisposing markers for EH that might assist towards development of comprehensive personalised medicines.
Acknowledgments
We greatly appreciate the support and constant encouragement of the Director, CSIR-Institute of Genomics and Integrative Biology, Dr Daniel Hernandez-Saavedra, Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado, Denver for editing and valuable suggestions, and the staff at the Department of Cardiology, GB Pant Hospital, New Delhi.
Footnotes
Contributors: Conception and design: SK, RK, MAQP. Analysis and interpretation: SK, RK, MG, ST, MAQP. Drafting the manuscript for important intellectual content: SK, RK, MAQP. All the authors reviewed and approved the manuscript.
Funding: The Council of Scientific and Industrial Research, India under the Taskforce BSC0122 supported this work.
Competing interests: None declared.
Ethics approval: The Human Ethics Committees of Council of Scientific and Industrial Research-Institute of Genomics and Integrative Biology (CSIR-IGIB), Delhi and Govind Ballabh (GB) Pant Hospital, New Delhi.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kearney PM, Whelton M, Reynolds K et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217–23. doi:10.1016/S0140-6736(05)17741-1 [DOI] [PubMed] [Google Scholar]
- 2.Hong Y, de Faire U, Heller DA et al. Genetic and environmental influences on blood pressure in elderly twins. Hypertension 1994;24:663–70. doi:10.1161/01.HYP.24.6.663 [DOI] [PubMed] [Google Scholar]
- 3.Harrap SB, Stebbing M, Hopper JL et al. Familial patterns of covariation for cardiovascular risk factors in adults: The Victorian Family Heart Study. Am J Epidemiol 2000;152:704–15. doi:10.1093/aje/152.8.704 [DOI] [PubMed] [Google Scholar]
- 4.Kato N. Ethnic differences in genetic predisposition to hypertension. Hypertens Res 2012;35:574–81. doi:10.1038/hr.2012.44 [DOI] [PubMed] [Google Scholar]
- 5.Campbell CY, Fang BF, Guo X et al. Associations between genetic variants in the ACE, AGT, AGTR1 and AGTR2 genes and renal function in the Multi-ethnic Study of Atherosclerosis. Am J Nephrol 2010;32:156–62. doi:10.1159/000315866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore JH. The ubiquitous nature of epistasis in determining susceptibility to common human diseases. Hum Hered 2003;56:73–82. doi:73735 [DOI] [PubMed] [Google Scholar]
- 7.Moore JH, Williams SM. New strategies for identifying gene-gene interactions in hypertension. Ann Med 2002;34:88–95. doi:10.1080/07853890252953473 [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Nejatizadeh A, Gupta M et al. The epistasis between vascular homeostasis genes is apparent in essential hypertension. Atherosclerosis 2012;220:418–24. doi:10.1016/j.atherosclerosis.2011.10.036 [DOI] [PubMed] [Google Scholar]
- 9.Kumar R, Kohli S, Alam P et al. Interactions between the FTO and GNB3 genes contribute to varied clinical phenotypes in hypertension. PLoS One 2013;8:e63934 doi:10.1371/journal.pone.0063934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar R, Kohli S, Mishra A et al. Interactions between the genes of vasodilatation pathways influence blood pressure and nitric oxide level in hypertension. Am J Hypertens 2015;28:239–47. doi:10.1093/ajh/hpu130 [DOI] [PubMed] [Google Scholar]
- 11.Jeunemaitre X, Soubrier F, Kotelevtsev YV et al. Molecular basis of human hypertension: role of angiotensinogen. Cell 1992;71:169–80. doi:10.1016/0092-8674(92)90275-H [DOI] [PubMed] [Google Scholar]
- 12.Hughes CA, Bennett V. Adducin: a physical model with implications for function in assembly of spectrin-actin complexes. J Biol Chem 1995;270:18990–6. doi:10.1074/jbc.270.32.18990 [DOI] [PubMed] [Google Scholar]
- 13.Rigat B, Hubert C, Alhenc-Gelas F et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 1990;86:1343–6. doi:10.1172/JCI114844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanai K, Saito T, Hirota K et al. Molecular variation of the human angiotensinogen core promoter element located between the TATA box and transcription initiation site affects its transcriptional activity. J Biol Chem 1997;272:30558–62. doi:10.1074/jbc.272.48.30558 [DOI] [PubMed] [Google Scholar]
- 15.Wu SJ, Chiang FT, Jiang JR et al. The G-217A variant of the angiotensinogen gene affects basal transcription and is associated with hypertension in a Taiwanese population. J Hypertens 2003;21:2061–7. doi:10.1097/01.hjh.0000098122.00558.8e [DOI] [PubMed] [Google Scholar]
- 16.Danser AH, Schalekamp MA, Bax WA et al. Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation 1995;92:1387–8. [DOI] [PubMed] [Google Scholar]
- 17.Mohana VU, Swapna N, Reddy SS et al. Risk of angiotensin converting enzyme (ACE) gene I/D and G2350A polymorphism in causing susceptibility to essential hypertension. Asian Biomedicine J 2011;6:255–64. [Google Scholar]
- 18.Bhavani BA, Padma T, Shastry BK et al. The insertion I/deletion D polymorphism of angiotensin converting enzyme (ACE) gene increases the susceptibility to hypertension and /or diabetes. Int J Hum Genet 2005;5:247–52. [Google Scholar]
- 19.Bhavani BA, Padma T, Sastry BKS et al. Gender specific association of insertional/deletion polymorphisms of the human angiotensin converting enzyme gene with essential hypertension. Int J Hum Genet 2004;4:207–13. [Google Scholar]
- 20.Jin Y, Kuznetsova T, Thijs L et al. Association of left ventricular mass with the AGTR1 A1166C polymorphism. Am J Hypertens 2012;25:472–8. doi:10.1038/ajh.2011.244 [DOI] [PubMed] [Google Scholar]
- 21.Staessen JA, Bianchi G. Adducin and hypertension. Pharmacogenomics 2005;6:665–9. doi:10.2217/14622416.6.7.665 [DOI] [PubMed] [Google Scholar]
- 22.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988;16:1215 doi:10.1093/nar/16.3.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lou XY, Chen GB, Yan L et al. A generalized combinatorial approach for detecting gene-by-gene and gene-by-environment interactions with application to nicotine dependence. Am J Hum Genet 2007;80:1125–37. doi:10.1086/518312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Zagato L, Kuznetsova T et al. Angiotensin-converting enzyme I/D and alpha-adducin Gly460Trp polymorphisms: from angiotensin-converting enzyme activity to cardiovascular outcome. Hypertension 2007;49:1291–7. doi:10.1161/HYPERTENSIONAHA.106.085498 [DOI] [PubMed] [Google Scholar]
- 25.Nejatizadeh A, Kumar R, Stobdan T et al. Significance of angiotensinogen gene haplotypes and genotypes combinations in hypertension. J Hypertens 2008;26:1094–101. doi:10.1097/HJH.0b013e3282fad951 [DOI] [PubMed] [Google Scholar]
- 26.Kumar R, Nejatizadeh A, Arif E et al. Multi-locus interactions of vascular homeostasis genes in essential hypertension: a gender-based study. Clin Chim Acta 2009;405:87–93. doi:10.1016/j.cca.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 27.Mohana VU, Swapna N, Surender RS et al. Gender-related association of AGT gene variants (M235T and T174M) with essential hypertension—a case-control study. Clin Exp Hypertens 2012;34:38–44. doi:10.3109/10641963.2011.618207 [DOI] [PubMed] [Google Scholar]
- 28.Padma G, Bhupatiraju C, Srinivas B et al. High risk for essential hypertension in males conferred by g.15241A>G polymorphism in intron 3 of AGT gene. Clin Exp Hypertens 2013;35:108–11. doi:10.3109/10641963.2012.702828 [DOI] [PubMed] [Google Scholar]
- 29.Mohana Vamsi U, Swapna N, Usha G et al. Contribution of REN gene MBbo I polymorphism in conferring risk for essential hypertension: a case control study from South India. J Renin Angiotensin Aldosterone Syst 2013;14:242–7. doi:10.1177/1470320312459981 [DOI] [PubMed] [Google Scholar]
- 30.Padma G, Swapna N, Mamata M et al. Risk conferred by tagged SNPs of AGT gene in causing susceptibility to essential hypertension. Clin Exp Hypertens 2014;36:579–85. doi:10.3109/10641963.2014.881845 [DOI] [PubMed] [Google Scholar]
- 31.Padma G, Charita B, Swapna N et al. Novel variants detected in AGT gene among patients with essential hypertension. J Renin Angiotensin Aldosterone Syst 2015;16:642–6. doi:10.1177/1470320313513483 [DOI] [PubMed] [Google Scholar]
- 32.Bhupatiraju C, Patkar S, Pandharpurkar D et al. Association and interaction of −58C>T and ±9 bp polymorphisms of BDKRB2 gene causing susceptibility to essential hypertension. Clin Exp Hypertens 2012;34:230–5. doi:10.3109/10641963.2011.631653 [DOI] [PubMed] [Google Scholar]
- 33.Tsai CT, Fallin D, Chiang FT et al. Angiotensinogen gene haplotype and hypertension: interaction with ACE gene I allele. Hypertension 2003;41:9–15. doi:10.1161/01.HYP.0000045080.28739.12 [DOI] [PubMed] [Google Scholar]
- 34.Fatini C, Sticchi E, Sofi F et al. Multilocus analysis in candidate genes ACE, AGT, and AGTR1 and predisposition to peripheral arterial disease: role of ACE D/-240T haplotype. J Vasc Surg 2009;50:1399–404. doi:10.1016/j.jvs.2009.07.075 [DOI] [PubMed] [Google Scholar]
- 35.Niu W, Qi Y, Cen W et al. Genetic polymorphisms of angiotensinogen and essential hypertension in a Tibetan population. Hypertens Res 2007;30:1129–37. doi:10.1291/hypres.30.1129 [DOI] [PubMed] [Google Scholar]
- 36.Cordell HJ. Detecting gene-gene interactions that underlie human diseases. Nat Rev Genet 2009;10:392–404. doi:10.1038/nrg2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charita B, Padma G, Sushma P et al. Estimation of risk and interaction of single nucleotide polymorphisms at angiotensinogen locus causing susceptibility to essential hypertension: a case control study. J Renin Angiotensin Aldosterone Syst 2012;13:461–71. doi:10.1177/1470320312444650 [DOI] [PubMed] [Google Scholar]
- 38.Zhao Q, Wang L, Yang W et al. Interactions among genetic variants from contractile pathway of vascular smooth muscle cell in essential hypertension susceptibility of Chinese Han population. Pharmacogenet Genomics 2008;18:459–66. doi:10.1097/FPC.0b013e3282f97fb2 [DOI] [PubMed] [Google Scholar]
- 39.Fox ER, Young JH, Li Y et al. Association of genetic variation with systolic and diastolic blood pressure among African Americans: the Candidate Gene Association Resource study. Hum Mol Genet 2011;20:2273–84. doi:10.1093/hmg/ddr092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma L, Clark AG, Keinan A. Gene-based testing of interactions in association studies of quantitative traits. PLoS Genet 2013;9:e1003321 doi:10.1371/journal.pgen.1003321 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartasia-2016-010723supp.pdf (187.6KB, pdf)