Abstract
The effects of primary somatosensory cortex (S1) injury on recovery of contralateral upper limb reaching and grasping was studied by comparing the consequences of isolated lesions to the arm/hand region of primary motor cortex (M1) and lateral premotor cortex (LPMC) to lesions of these same areas plus anterior parietal cortex (S1 and rostral area PE). We used multiple linear regression to assess the effects of gray and white matter lesion volumes on deficits in reaching and fine motor performance during the first month after the lesion, and during recovery of function over 3, 6 and 12 months post-injury in 13 monkeys. Subjects with frontoparietal lesions exhibited larger deficits and poorer recovery as predicted, including one subject with extensive peri-Rolandic injury developing learned nonuse after showing signs of recovery. Regression analyses showed that total white matter lesion volume was strongly associated with initial post-lesion deficits in motor performance and with recovery of skill in reaching and manipulation. Multiple regression analyses using percent damage to caudal M1 (M1c), rostral S1 (S1r), LPMC and area PE as predictor variables showed that S1r lesion volumes were closely related to delayed post-lesion recovery of upper limb function, as well as lower skill level of recovery. In contrast, M1c lesion volume was related primarily to initial post-lesion deficits in hand motor performance. Overall, these findings demonstrate that frontoparietal injury impairs hand motor function more so than frontal motor injury alone, and results in slower and poorer recovery than lesions limited to frontal motor cortex.
Keywords: grasp, biomechanics, kinematics, frontal lobe, parietal lobe, brain injury, MCA stroke
Introduction
The contribution of cortical processing of somatosensory information to recovery of dexterous movements after precentral motor cortex injury has rarely been studied and, consequently, is poorly understood. Because the cortical territory served by the middle cerebral artery (MCA) is most commonly affected in stroke, damage frequently occurs to one or both of the major motor and sensory areas, specifically the primary motor cortex (M1) and primary somatosensory cortex (S1). Moreover, other adjacent areas of the frontal and parietal lobes including lateral premotor cortex (LPMC) and posterior parietal cortex (PPC) may also be involved after MCA stroke but the effect of such large lesions on motor recovery has not been a focus of contemporary studies in non-human primates. Indeed, it has been reported that the parietal lobe is the most frequently injured part of the cortical mantle after MCA stroke (Yoo et al., 1998) but injury confined to the precentral motor region continues to be the focus of experimental study(Dancause et al., 2006; Eisner-Janowicz et al., 2008; Moore et al., 2012). Functionally, the anterior and posterior parietal lobe regions are known to play a critical role in processing of somatosensory information for perception (Kaas, 2012) and for control of hand/digit movements for reaching and grasping (Kaas, 1993). Thus, there are ample reasons to study the effects of extensive lesions involving peri-Rolandic sensorimotor areas on recovery of dexterous hand/digit movements, which often recover poorly in stroke patients.
Rarely recognized but classical experimental lesion studies reporting the effects of isolated resection of S1, and larger parietal lobe lesions encompassing both S1 and the PPC in monkeys have shown that such lesions do not cause acute paralysis as is observed after M1 resection, but result in more persistent motor deficits such as hypotonia/weakness, ataxia and reliance on vision when attempting to perform precise upper extremity motor tasks (Kennard and Kessler, 1940; Peele, 1944). Similarly, studies in stroke patients report that functional recovery of hand movements is often poor when localized injury involves the parietal lobe and causes somatosensory dysfunction (Abela et al., 2012; Freund, 2003; Stern et al., 1971; Zeman and Yiannikas, 1989), with recovery associated with changes in gray matter volume of perilesional premotor cortex and subcortical areas including thalamus, caudate nucleus and cerebellum (Abela et al., 2015). In support of these observations is a recent case study of an individual who underwent left S1 resection to manage intractable seizures (Richardson et al., 2016). It was found that this patient had persistent impairments in maintaining right hand power grip force without vision and in initiation of grip forces to visual targets, but feedforward control of grip force during active movement to prevent slip of an object held in precision grip was intact. It seems consistent in the literature that practice of motor tasks and use of vision can ameliorate some of the motor deficits following parietal cortex injury (Carey et al., 2002; Jeannerod et al., 1984; Smania et al., 2003).
We recently reported that frontoparietal lesions in rhesus monkeys caused impaired fine hand/digit grasping and manipulation movements with varying degrees of recovery that are accompanied by striking changes in descending projections from spared medial premotor cortex (Morecraft et al., 2015). Notably, in contrast to our previous observations showing that lesions limited to precentral frontal motor areas results in an enhanced supplementary motor cortex corticospinal projection (CSP) in the form of increased terminal boutons (McNeal et al., 2010), we found that additional lesion of adjacent parietal cortex results in decreased numbers of supplementary motor cortex CSP boutons to neurons controlling hand/digit motion (Morecraft et al., 2015). Thus, parietal cortex may exert trophic influences on frontal lobe motor areas, and contribute to maintaining or enhancing the CSP from those gray matter areas. This may, in part, explain why patients with peri-Rolandic frontoparietal damage show poor recovery of distal upper extremity movements.
In the current report we examined the effects of lesion volume and location within lateral frontal and parietal sensorimotor areas on impairment of fine hand/digit function over the first month post-lesion, and on extended recovery of such function over 3, 6 and 12 months. Previously we demonstrated that initial deficits and recovery of fine hand motor function were strongly correlated with frontal lesion volume involving a wide range of lesions including M1, LPMC, supplementary motor cortex (M2, or the equivalent of MII, SMC, or SMA-proper as used in the literature) (Luppino et al., 1993; Wiesendanger and Wiesendanger, 1984; Woolsey et al., 1951; Woolsey et al., 1952), pre-supplementary motor cortex (pre-SMA), cingulate motor cortex, and medial prefrontal cortex that included both focal and non-focal lesions (Darling et al., 2009). In the present study we assessed hand motor recovery in 7 monkeys with focal lesions limited to M1 and LPMC and in 6 monkeys with combined lesions to M1, LPMC and the anterior parietal lobe (S1, including the rostral-most part of area PE) because this cortical territory is often damaged after MCA stroke in humans (Carrera et al., 2007; Rasmussen et al., 1992; Yoo et al., 1998). Importantly, the medial cortex, including M2, pre-SMA, cingulate motor areas and medial prefrontal region are typically spared following isolated MCA stroke. We hypothesized that lateral peri-Rolandic lesions including the parietal lobe would cause slower and less complete recovery than lateral lesions affecting only frontal lobe motor areas. Moreover, we quantified the percentage of the total volume damage to M1 and S1 controlling arm/hand movements, to test the hypothesis that increasing volume (as a percentage of total volume) of lesions affecting the gray matter surrounding the central sulcus results in greater fine hand motor deficits and slower/less complete recovery. We also tested whether volume of lesions to LPMC and rostral area PE contributed to poorer recovery as these regions are involved in processing of visual and somatosensory information for coordination of reaching and grasping.
Methods
Experimental Animals
Thirteen adult rhesus monkeys (Macaca mulatta) were subjects for these experiments, 7 with lesions limited to the hand/arm area of M1 and LPMC (Category F2 lesion) (Fig. 1) and 6 with lesions of the hand/arm area of M1, LPMC, S1 and rostral part of area PE of the superior parietal lobule (Category F2P2 lesion) (Figs. 2, 3, 4) (Table 1). The animals were housed, cared for, and maintained in a United States Department of Agriculture (USDA) approved and inspected facility. All behavioral and surgical protocols were approved by the University of South Dakota (USD) Institutional Animal Care and Use Committee (IACUC), and conducted in accordance with USDA, National Institutes of Health, and Society for Neuroscience guidelines for the ethical treatment of experimental animals. Prior to beginning the study, each monkey was evaluated by a primate veterinarian and judged to be healthy and free of any neurological deficit. Proximal and distal movements and range of motion at the joints in both upper extremities of all animals were normal with the exception of SDM55. In this case the interphalangeal joints of digit 3 were permanently extended. However, this animal was able to perform precision opposition with digits 1 and 2 to successfully acquire the food rewards in the motor tests.
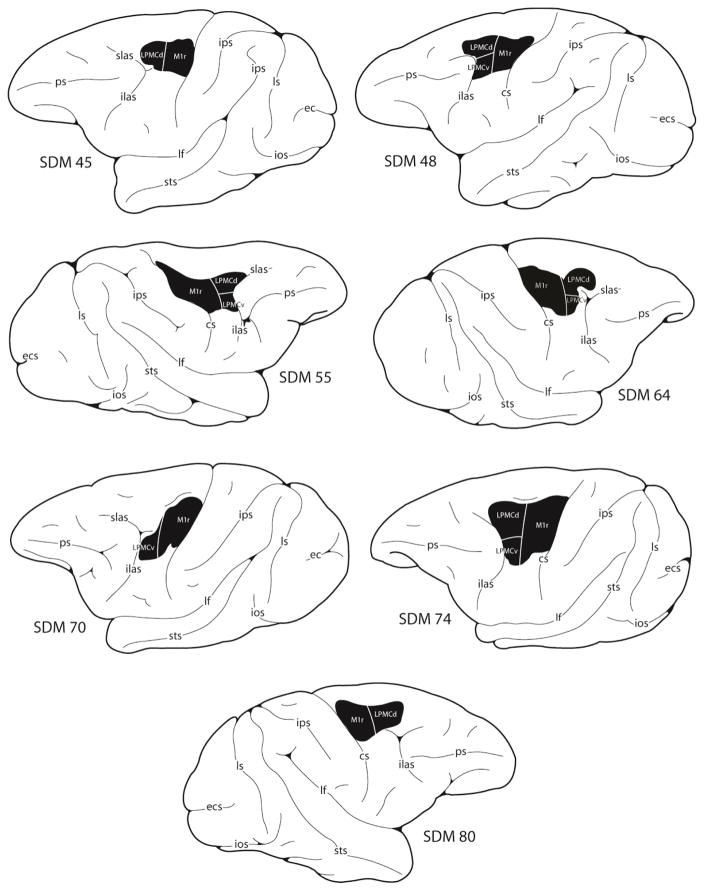
Figure 1.
Line drawings of the lateral surface of the cerebral cortex illustrating the location of the lesion site (blackened area) in the 7 frontal motor lesion cases reported in this study. Detailed descriptions of the histological and cytoarchitectonic characteristics of each experimental lesion site are provided in our previous reports (Darling et al., 2009, 2013; McNeal et al., 2010). Derived microstimulation maps of the cortical surface that were used to guide the placement of each lesion are also provided in these reports. Abbreviations: cs, central sulcus; ecs, ectocalcarine sulcus; ilas, inferior limb of the arcuate sulcus; ios, inferior occipital sulcus; ips, intraparietal sulcus; lf, lateral fissure; ls, lunate sulcus; ots, occipito-temporal sulcus; ps, principal sulcus; superior limb of the arcuate sulcus; sts, superior temporal sulcus.
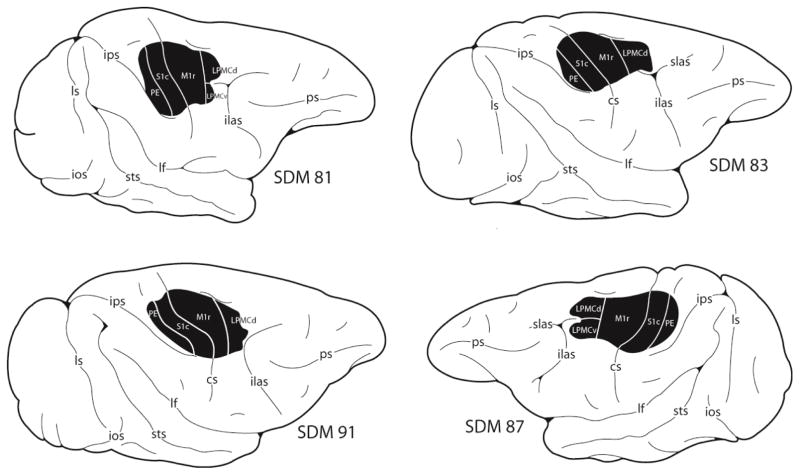
Figure 2.
Line drawings of the lateral surface of the cerebral cortex illustrating the location of the lesion site (blackened area) in 4 of the 6 frontoparietal lesion cases reported in this study. Detailed descriptions of the histological and cytoarchitectonic characteristics of each experimental lesion site, as well as the microstimulation maps that were used to guide lesion site placement are provided in our previous report (Morecraft et al., 2015). The 2 new frontoparietal lesion cases are illustrated in Figures 3 and 4 and detailed histological and cytoarchitectonic descriptions of spared and aspirated cortex affiliated with their lesions are provided in the Results. Abbreviations: see Figure 1.
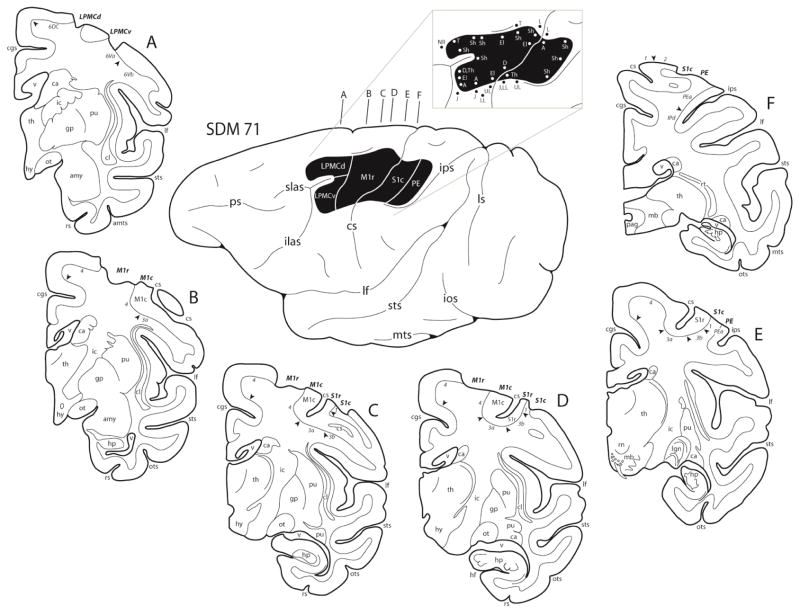
Figure 3.
In the diagram center, a line drawing of the lateral surface of the hemisphere in case SDM71 is illustrated along with the location of the cortical lesion site (blackened area). The affiliated pullout of the lesion site illustrates the microstimulation map used to guide lesion placement. On the map each dot represents a stimulation point with the corresponding movement(s) observed following stimulation. Representative coronal sections through the lesioned hemisphere are also illustrated (A–F). In each coronal section, the regions of lesioned cortex are identified by the bold italicized conventions. Pertinent Brodmann’s areas are indicated on the coronal sections immediately below the cortical gray matter and the respective boundaries are identified by the arrow heads. Abbreviations: A, arm; amts, anterior medial temporal sulcus; amy, amygdala; ca, caudate nucleus; cgs, cingulate sulcus; cl, claustrum; cs, central sulcus; D, digit; ecs, ectocalcarine sulcus; El, elbow; gp, globus pallidus; J, jaw; mb, midbrain; hf, hippocampal fissure; hp, hippocampus; hy, hypothalamus; ic, internal capsule; ilas, inferior limb of the arcuate sulcus; ios, inferior occipital sulcus; ips, intraparietal sulcus; L, leg; lf, lateral fissure; lgn, lateral geniculate nucleus; LL, lower lip; ls, lunate sulcus; NR, no response; ot, optic tract; ots, occipito-temporal sulcus; pag, periaqueductal gray; ps, principal sulcus; pu, putamen; rn, red nucleus; ros, rostral sulcus; rs, rhinal sulcus; rt, reticular thalamic nucleus; Sh, shoulder; slas, superior limb of the arcuate sulcus; sts, superior temporal sulcus; Th, thumb; Tha, thalamus; To, tongue; Tr, trunk; UL, upper lip; v ventricle; Wr, wrist.
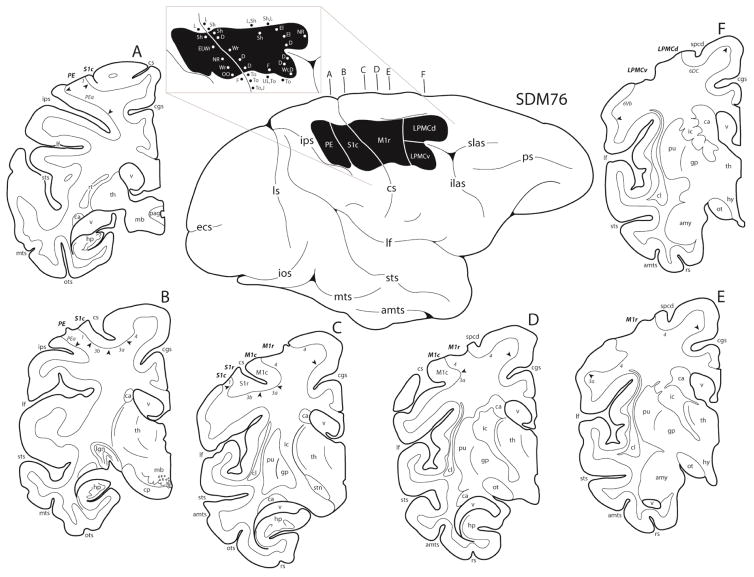
Figure 4.
In the diagram center, a line drawing of the lateral surface of the hemisphere in case SDM76 is illustrated along with the location of the cortical lesion site (blackened area). The affiliated pullout of the lesion site illustrates the microstimulation map. On the map each dot represents a stimulation point with the affiliated movement(s) observed following stimulation. Representative coronal sections through the lesioned hemisphere are also illustrated (A–F). In each coronal section, the regions of lesioned cortex are identified by the bold italicized conventions. Pertinent Brodmann’s areas are indicated on the coronal sections immediately below the cortical gray matter and the respective boundaries are identified by the arrow heads. Abbreviations: cp, cerebral peduncle; F, face; OO, orbicularis oculi; spcd, superior precentral dimple; stn, subthalamic nucleus; To, tongue. For other abbreviations see Figure 3.
Table 1.
Experimental Subjects – Characteristics and Lesion volumes
| Case | Agea | Les. Cat.c | PLDd | GMLVe (mm3) | WMLVf (mm3) | GMLV (%) | GMLV (mm3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDM | (yrs) | Sex | HIb | (mo) | FCg | PCh | FC | PC | M1r | M1c | S1r | S1c | LPMCi | PPCj | |
| 45 | 4.9 | M | 21.3R | F2 | 6 | 212.6 | 0.0 | 23.02 | 0.0 | 100 | 10.7 | 0.0 | 0.0 | 99.6 | 0.0 |
| 48 | 6.8 | F | 6.0R | F2 | 12 | 220.3 | 0.0 | 23.12 | 0.0 | 100 | 7.7 | 0.0 | 0.0 | 110.7 | 0.0 |
| 55 | 11.8 | M | 20.0L | F2 | 12 | 207.7 | 0.0 | 20.51 | 0.0 | 100 | 27.7 | 0.0 | 0.0 | 87.7 | 0.0 |
| 64 | 13.6 | F | 95.3L | F2 | 6 | 217.9 | 0.0 | 43.03 | 0.0 | 96.5 | 8.0 | 0.0 | 0.0 | 111.3 | 0.0 |
| 70 | 7.2 | M | 4.4R | F2 | 6 | 143.2 | 0.0 | 7.76 | 0.0 | 96.5 | 6.2 | 0.0 | 0.0 | 52.3 | 0.0 |
| 74 | 8.5 | M | 93.2R | F2 | 3 | 192.6 | 0.0 | 16.26 | 0.0 | 84.6 | 0.8 | 0.0 | 0.0 | 134.9 | 0.0 |
| 80 | 8.6 | M | 75.7L | F2 | 3 | 150.7 | 0.0 | 10.47 | 0.0 | 78.0 | 0.0 | 0.0 | 0.0 | 79.6 | 0.0 |
| 71 | 8.8 | M | 53.4R | F2P2 | 12 | 191.9 | 105.4 | 22.53 | 8.9 | 100 | 23.0 | 13.0 | 100 | 84.3 | 0.0 |
| 76 | >8.0k | M | 83.2L | F2P2 | 7 | 188.6 | 75.2 | 16.23 | 6.9 | 100 | 6.5 | 7.2 | 100 | 94.3 | 63.7 |
| 81 | 12 | F | 63.6L | F2P2 | 12 | 108.8 | 68.1 | 7.40 | 6.2 | 85.0 | 30.3 | 19.5 | 100 | 34.3 | 14.5 |
| 83 | 3.8 | F | 91.0L | F2P2 | 12 | 181.1 | 76.8 | 8.16 | 8.0 | 100 | 50.2 | 34.4 | 100 | 83.6 | 11.4 |
| 87 | 17 | F | 60.0R | F2P2 | 6 | 224.0 | 102.3 | 44.65 | 11.6 | 100 | 46.1 | 24.4 | 100 | 83.7 | 47.7 |
| 91 | 7.8 | F | 76.0L | F2P2 | 6 | 192.6 | 76.2 | 46.21 | 6.7 | 100 | 100 | 69.9 | 100 | 39.4 | 7.8 |
Abbreviations:
Age at time of lesion;
Handedness Index ((percentage of initial reaches and retrievals with preferred hand – 50)*2), (Nudo et al., 1992); R-right hand preferred, L – left hand preferred) ;
lesion category – F2 (M1+LPMC), F2P2 (M1 + LPMC + S1 + rostral area PE);
PLD – post-lesion duration for recovery;
GMLV – gray matter lesion volume;
WMLV – white matter lesion volume;
FC – frontal cortex;
PC – parietal cortex;
LPMC - lateral premotor cortex;
PPC - posterior parietal cortex (rostral area PE);
estimated age (birthdate unknown, age estimated by an experienced primate veterinarian).
Experimental Apparati
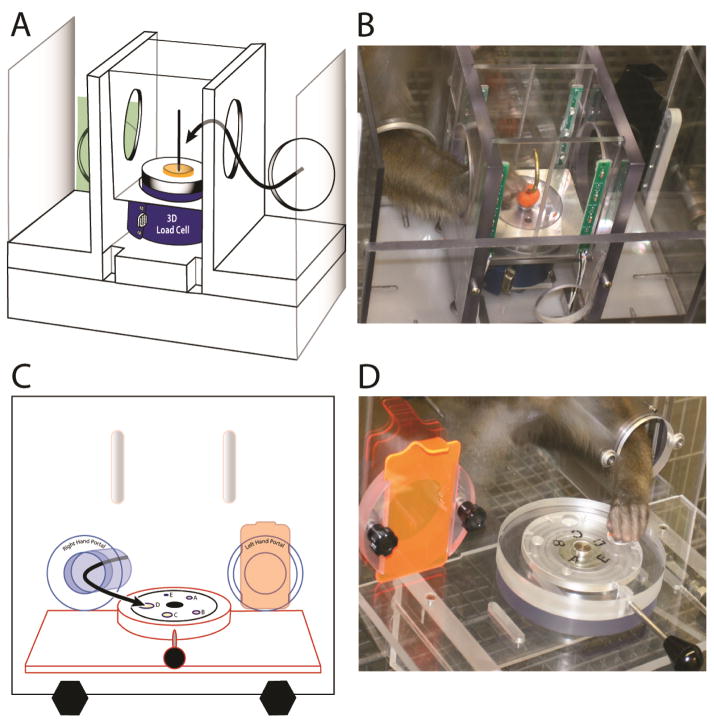
The equipment used to test fine hand motor function has been previously described (modified dexterity board or mDB and modified movement assessment panel or mMAP) (Darling et al., 2006; Darling et al., 2009; Pizzimenti et al., 2007). Both devices were attached to the monkey’s cage and controlled, without restraint, which hand the monkey used to perform the tests (Fig. 5). Each hand was tested both pre- and post-lesion. The monkeys were allowed to move freely about the cage between trials in both tests and palatable food targets were used to minimize training effects. Using the mDB device we assessed duration and accuracy of targeted reaching, manipulation duration as well as the number of times the digit lost contact with the target (a small food pellet inside wells of varying diameter). Using the mMAP device we measured forces applied during grasp and manipulation of a carrot chip (or, in one monkey, a type of cereal due to its preference for the cereal over a carrot chip) from a flat surface or over straight and curved rods.
Figure 5.
Motor testing Apparati: drawing of the modified movement assessment panel (mMAP) from an angled frontal/side view (A); picture of the mMAP showing the monkey reaching for the carrot chip on the curved rod (B); drawing of the modified dexterity board (mDB) from a front view (C); and picture of the mDB showing a monkey reaching to grasp the food pellet. The curved lines in A, C show the path of the hand to the target. Note that the devices allow independent testing of each hand by blocking exit from the cage to the testing apparatus from one side. With the mMAP device we measure applied 3D forces and duration of manipulation and use these to compute a performance score (higher performance scores for trials with lower applied forces and shorter duration). With the mDB device we measure duration and accuracy of reaching (distance of index tip to target) as well as duration of manipulation of the food pellet target
Video and Force Data Acquisition
mMAP Task
Forces applied during manipulation of the carrot chip in the mMAP task (Fig 5A, B) were recorded at 200 samples/s using Datapac 2k2 (Run Technologies). Movements of the hand during the mMAP task were recorded using a single digital video camera (Sony, model DCR-DVD301) placed directly in front of the cage. These recordings were used for qualitative ratings of the movement strategy and to assess success/failure of the animal on each trial.
mDB Task
Quantitative video recordings of hand movements during the mDB task (Fig. 5C, D) were used to assess spatial and temporal variables (e.g., accuracy and duration of the initial reach, grip aperture at touchdown of the hand, etc.) as described previously (Darling et al., 2009). These recordings used four digital video cameras interfaced with the SIMI Motion data acquisition package (SIMI Reality Motion Systems, Unterschleissheim, Germany). Video data collection began when the portal door was opened to allow the monkey to reach toward the food pellet and continued until the pellet was retrieved into the cage, the pellet was knocked off of the platform, or a 60 s time limit had expired. Video collection was manually triggered and single trial video clips of each trial were manually created and verified. Details of the video collection protocol, data acquisition, and data analysis of the mDB task are provided in our previous work (Pizzimenti, et. al, 2007).
Behavioral Procedures
Details of the behavioral protocols used in the current investigation have been described previously. Before the motor testing sessions, monkeys were food restricted for 18–24 hours (Darling et al., 2009). To determine which hemisphere would receive the lesion, hand preference was assessed. This was accomplished using a standard dexterity board (sDB) device that allowed the monkey to choose which hand to use on 150 trials over 3 days. The number of initial reaches and subsequent reaches with each hand on each trial was assessed from video recordings of the two sessions and used to compute an index of hand preference as described previously (Nudo et al., 1992) (Table 1). Training on the mMAP and mDB devices began after hand preference was determined. This was followed by a series of pre-lesion full testing sessions until stable performance was achieved for the mDB (5 trials on each of 5 wells, totaling 25 trials with each hand) and for the mMAP (5 trials for each test level (flat surface, straight rod, curved rod), totaling 15 trials with each hand). Post-lesion testing was performed weekly for 8 weeks, then every other week for 3–12 months (Table 1). During the first few post-lesion tests, the more impaired hand was tested first on the flat surface task (easiest task) to ensure high motivation. Once the monkey began successfully retrieving the carrot chips, the more impaired hand was tested first on the curved rod task to ensure high motivation on this difficult task.
Surgical Procedure
The frontal surgical lesions included the arm area of primary motor cortex (M1) and the adjacent arm area of the lateral premotor cortex (LPMC) (F2 lesion – 7 cases). The frontoparietal surgical lesions included the same parts of M1 and LMPC plus the arm area of primary somatosensory motor cortex (S1) and adjacent part of area PE (F2P2 lesion – 6 cases). All lesions were placed in the hemisphere contralateral to the preferred limb using surgical procedures approved by the University of South Dakota (USD) Institutional Animal Care and Use Committee (IACUC). Preoperatively, each monkey was injected with either glycopyrrolate (0.2 mg/kg IM) or atropine sulfate (0.05mg/kg), immobilized with ketamine hydrochloride (10mg/kg), intubated then placed on a mechanical respirator where it was deeply anesthetized with isoflurane inhalation (1.2–1.5%) and a surgical grade air/oxygen mixture. The monkey was placed into a head holding device and administered mannitol (1.5–2g/kg) intravenously to reduce brain volume and tissue accessibility during surgery and reduce risk of brain swelling and cortical injury during craniotomy. Using aseptic sterile surgical technique, a skin incision was made over the lateral frontoparietal region and an elliptical section of bone was removed using sharped-tip bone cutters and periosteal elevators then placed in chilled sterile physiological saline solution for the duration of the surgery. A –U-shaped incision was made in the dura over the lateral convexity of the cerebral hemisphere and reflected, thus exposing the cortical surface. The animal was then transferred to intravenous ketamine anesthetic sedation for electrophysiological mapping of M1 and LPMC or of M1, LPMC and S1. Intracortical microstimulation (ICMS) was used to localize the arm areas of M1 and LPMC, as previously described (Morecraft et al., 2015, 2016; Morecraft et al., 2013), and S1, which can also elicit upper limb motion in primates with surface stimulation (Woolsey et al, 1958) and ICMS (Burnish et al., 2008, Widener and Cheney, 1997). To accomplish this, a tungsten electrode (impedance 0.5–1.5 MΩ) was inserted 200 μm below the pial surface then advanced at 500 μm intervals. Movements were evoked using a train duration of 50 ms and pulse duration of 0.2 ms delivered at 330 Hz. Current intensity ranged between 0.1 and 90 μA. Threshold currents were determined and the evoked movements were recorded when agreed upon by two observers. After defining the location and borders of the forelimb representations and non-excitable cortex (i.e., the rostral part of frontal area 6DR and parietal area PE), the animal was returned to isoflurane anesthesia. Surface vessels supplying the targeted lesion areas were ligated using electrocauterization, which was followed approximately 5–10 minutes later by subpial aspiration to extract the affected gray matter and assure a thorough lesion (McNeal et al., 2010; Morecraft et al., 2015). The aspirated area was covered for approximately 15–20 minutes with saline soaked cottonoid padding to control bleeding and promote injury homeostasis. The cottonoid padding was carefully removed and the dura was sutured closed. The retracted bone was then replaced and anchored with 3-0 suture passed through opposing burr holes made in the bone graft and intact cranium. Finally, the epicranial aponeurosis and temporalis muscle were reattached and the skin closed using standard neurosurgical techniques. Each animal was monitored post-surgically throughout the survival period. Buprenorphine (0.01–0.5mg/kg) was administered immediately after surgery for 48–72 hours. Twenty four hours prior to the surgery, and for at least 9 days post-surgery, each animal was administered an antibiotic (bicillin –LA, 60–100 units/kg every 3 days; or amoxicillin, 11mg/kg daily; or baytril, 5mg/kg daily which was discontinued due to interference with dextran tract tracer transport in 4 animal experiments). In all cases, a second surgery (also approved by the USD IACUC) was performed 33–34 days prior to sacrifice when neural tract tracers were injected into cortical areas of interest for post-mortem study of the reorganized neural connections that occur following brain injury.
Histological Procedures
Following the predetermined survival period, each monkey was deeply anesthetized with an overdose of pentobarbital (50 mg/kg or more) and perfused transcardially with 0.9% saline. This was immediately followed by 2 liters of 4% paraformaldehyde in 0.1M phosphate buffer (PB) at pH 7.4, then one liter each of 10% and 30% sucrose in 0.1M PB at pH 7.4 for cryoprotection. The brain was removed, blocked into cortical, brainstem and spinal cord regions, placed in 30% sucrose in 0.1M PB, and refrigerated for 2 to 5 days at 4° C. Post-mortem digital electronic images of the central nervous system were taken for data reconstruction. The cortical tissue was frozen sectioned in the coronal plane on a sliding microtome (American Optical 860, Buffalo, NY, USA) at a thickness of 50 μm in cycles of 10, forming 10 complete series of evenly spaced tissue sections respectively. SDM56 was cut at a thickness of 60 μm in cycles of 10. One series of tissue sections was processed for Nissl substance using our previously described histochemical methods (Morecraft et al., 2004; Morecraft et al., 1992; Morecraft et al., 2012) and used for the lesion volume analysis in the present report. Additional series of tissue sections were used for immunohistochemical visualization of neural tract tracers for neuroanatomical tract tracing studies (McNeal et al., 2010; Morecraft et al., 2015, 2016).
Estimation of Lesion Volume
The methods for estimating gray and white matter lesion volume were described previously (Darling et al., 2009; McNeal et al., 2010). Briefly, Nissl stained tissue sections at 500 (or 600 in case SDM56) μm intervals through the lesion site were examined for tissue damage. Effects of atrophic distortions on lesion volume (Denny-Brown et al., 1975; Marshall et al., 2000) were minimized by superimposing an outline of the lesion site onto the undamaged hemisphere to calculate the total gray matter and total white lesion volume as described previously (Darling et al., 2009). We also estimated percentage of the upper limb areas of M1 rostral (M1r), M1 caudal (M1c), S1 rostral (S1r) and S1 caudal (S1c) damaged by the lesion for three reasons: (1) to normalize lesion volume to upper limb map volume in each animal; (2) corticospinal projection neurons in M1c (which line the anterior bank of the central sulcus) may be particularly important in control of hand movements due to presence of monosynaptic connections with lamina IX motoneurons (Lemon, 2008; Lemon and Griffiths, 2005; Rathelot and Strick, 2006; 2009) and; (3) the importance of short somatosensory affiliated projections from S1r neurons to M1c neurons for fine motor control (Kaneko et al., 1994a, b). Thus, larger percentage lesion volumes of M1c and S1r may be particularly important in creating long-term deficits in hand motor function. Percentage of total gray matter of these areas that was damaged was computed from gray matter lesion volume within the defined area and total volume of the area defined using ICMS (Table 1). In most cases, near complete removal of the hand/arm region of M1r and S1c on the lateral aspect of the cortex was accomplished, but variable amounts of M1c and S1r deep within the central sulcus were removed (Table 1).
Data Analysis
We quantified pre- and post-lesion performance of the contralesional hand on the mDB and mMAP tasks using performance scores (see Supplementary Methods) derived from measures such as reach duration and accuracy (reach performance – mDB), manipulation duration and number of grasp attempts (manipulation performance – mDB) and manipulation duration and total absolute impulse during manipulation (manipulation performance – mMAP) as described previously (Darling et al., 2006; Darling et al., 2009; Pizzimenti et al., 2007). We also recorded the first attempt, first success and consistent success (all 5 trials at a single level of difficulty) on the mDB (any well, best well, 2nd well) and mMAP (flat surface, straight rod, curved rod) to assess rate of recovery (Table 2). Skill was quantified as the mean performance score divided by standard deviation of performance score on the best well and a 2nd well of the mDB (with pre-lesion performance scores about 50% of those on the best well) and on the curved rod task of the mMAP over the last 5 complete testing sessions before the lesion (pre-lesion skill) and the best 5 consecutive testing sessions after the lesion (post-lesion skill). Recovery of skill was measured as the ratio of the post-lesion to pre-lesion skill. We also quantified post-lesion decrement in performance as the ratio of the mean performance score over the first 4 post-lesion tests (over 4 weeks) to the last 5 pre-lesion tests.
Table 2.
Post-lesion duration of impairment in the modified dexterity board and modified movement assessment panel tasks
| Post-lesion week of: | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| Case | 1st attempt | 1st success | consistent successa | |||||||||||||||
| mDB | mMAP | mDB | mMAP | mDB | mMAP | |||||||||||||
| AWb | BWc | W2d | flat | stre | crvd | AW | BW | W2 | flat | str | crvd | AW | BW | W2 | flat | str | crvd | |
| SDM45 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
|
| ||||||||||||||||||
| SDM48 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 3 | 2 | 3 | 5 | 6 | 2 | 3 | 4 |
|
| ||||||||||||||||||
| SDM55 | 1 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 4 | 4 | 3 | 4 | 4 | 1 | 4 | 5 |
|
| ||||||||||||||||||
| SDM64 | 4 | 4 | 4 | 2 | 3 | 4 | 4 | 4 | 4 | 2 | 5 | 7 | 5 | 6 | 5 | 3 | 7 | 8 |
|
| ||||||||||||||||||
| SDM70 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 4 | 1 | 3 | 2 |
|
| ||||||||||||||||||
| SDM74 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
|
| ||||||||||||||||||
| SDM80 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
|
| ||||||||||||||||||
| SDM71 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 3 | 3 | 3 | 5 | 2 | 3 | 4 |
|
| ||||||||||||||||||
| SDM76 | 1 | 2 | 2 | 4 | 4 | 3 | 2 | 2 | 2 | 4 | 4 | 4 | 2 | 2 | 5 | NAg | NA | 4 |
|
| ||||||||||||||||||
| SDM81 | 1 | 1 | 2 | 3 | 1 | 1 | 2 | 2 | 4 | 3 | 3 | 3 | 6 | 28* | 6 | 3 | 3 | 3 |
|
| ||||||||||||||||||
| SDM83 | 1 | 3 | 3 | 2 | 1 | 2 | 3 | 3 | 7 | 2 | 2 | 2 | 3 | 3 | NA | 2 | 2 | 2 |
|
| ||||||||||||||||||
| SDM87 | 3 | 3 | 4 | 1 | 2 | 5 | 3 | 3 | 4 | 2 | 5 | 6 | 4 | 4 | 6 | 5 | 7 | 6 |
|
| ||||||||||||||||||
| SDM91 | 4 | 6 | NA | 5 | 1 | 4 | 5 | 6 | NA | 5 | NA | NA | 7 | NA | NA | NA | NA | NA |
Abbreviations:
Consistent Success - successful acquisition on all 5 trials for a mDB well or mMAP task; bAW – any well of mDB;
BW – mDB best well (with highest pre-lesion skill);
W2 – smaller well of mDB with lower pre-lesion skill than best well;
str – straight rod task of mMAP;
crvd – curved rod task of mMAP;
NA – not applicable (i.e., no post-lesion attempts or successful acquisitions or consistent successful acquisitions).
SDM81 often dropped the food pellet in the best well (E) task during the post-lesion phase, presumably due to lack of an adequate grasp. Note that well E is only a small dimple to hold the food pellet, thus the sides of the well cannot be used to assist with grasping the food pellet.
Regression Analyses
The overall goal of these analyses was to determine whether lesions to specific brain sensorimotor areas predicted lesion effects on initial performance deficits in the 1st post-lesion month and on long-term recovery of skill better than a simple model of total gray and white matter lesion volumes. Thus, we used forward stepwise multiple linear regression with dependent variables relevant to deficits and recovery of fine motor function with the following sets of independent (predictor) variables: (1) total gray and total white matter lesion volumes (TGMLV, TWMLV); (2) frontal gray white matter lesion volumes (FGMLV, FWMLV) and parietal gray and white matter lesion volumes (PGMLV, PWMLV); and (3) percent of M1c hand/arm area lesion volume (M1cLV%), LPMC lesion volume (LPMCLV), percent of S1r lesion volume (S1rLV%) and area PE lesion volume (PELV). Since collinearity of predictor variables may influence regression coefficients for these variables we computed the variance inflation factors (VIFs) and tolerances (Ts) for the predictor variables for each regression analysis and report these in Tables 4, 5 and 6. The purpose of the latter analysis was to examine whether lesion induced initial deficits and recovery could be primarily attributed to damage to the peri-Rolandic areas M1c and S1r and the higher order premotor area (LPMC) and sensory association area (PE) or more generally to total gray matter lesion volume or to frontal and parietal gray matter lesion volume. Variables were added to the multiple regression equation only if they increased the coefficient of determination by at least 0.05 (i.e., percentage of explained variance increased by 5%). We compared coefficients of determination to identify appropriate models for prediction of post-lesion deficits in motor performance and recovery of fine motor skill.
We did not include additional predictor variables of percentage lesion volumes of M1r and S1c despite their potential importance in recovery of hand function because almost 100% of M1r was damaged/removed in all monkeys and 100% of S1c was removed in monkeys with frontoparietal lesions (Table 1). We also did not include damage to LMPC in these regression analyses because we estimated that nearly 100% of the arm area of LPMC, as determined by intracortical microstimulation, was resected in all cases. We completed a large number of regression analyses performed on possibly correlated variables (e.g., r = 0.76, p = 0.003 for correlation of total white and gray matter lesion volumes). However, because this is an exploratory study in a relatively small number of subjects to assess how lesion volumes affect initial deficits after the lesion and recovery of hand motor performance, we report uncorrected p-values and variables that contributed significantly to prediction of dependent variables.
Results
Histological Lesion Site Analysis
We have previously provided detailed histological/cytoarchitectonic descriptions of the lesions of all F2 lesion monkeys (SDM45, SDM48, SDM55, SDM64, SDM70, SDM74, SDM80 – see (Darling et al., 2013; Darling et al., 2009; McNeal et al., 2010) and 4 of the 6 F2P2 lesion monkeys (Morecraft et al., 2015). Anatomical and cytoarchitectonic descriptions of the lesions of the other two F2P2 lesioned monkeys included in this study are provided below.
SDM71 (F2P2 Lesion)
Overall, the lesion site in case SDM71 involved the arm portion of the frontal motor cortex and parietal somatosensory cortex on the lateral surface of the hemisphere (Fig. 3). Specifically, the lesion at the rostral end of the excised frontal cortex involved the caudal and inferior part of the dorsal lateral premotor cortex (LPMCd) located above the superior limb and spur on the arcuate sulcus (corresponding to cytoarchitectonic area 6DC). Ventrally, the lesion involved dorsal part of the ventral lateral premotor cortex (LPMCv) located immediately below the arcuate spur (corresponding to cytoarchitectonic area 6Va). In both premotor regions, layers I–III were removed in the rostral-most part of the lesion site (i.e., rostral 1–3 mm of the lesion site), but through the mid- to caudal part of the premotor injury, layers I–VI were completely excised. Cortex in close proximity to the spur of the arcuate sulcus, including the depths of the arcuate spur, was spared. The frontal lobe lesion extended caudally from LPMC to involve all gray matter layers (i.e., I–VI) that corresponded to the arm part of M1r on the gyral surface. In contrast, most of the cortex forming M1c, which lines the anterior bank of the central sulcus, was spared with the exception of the upper 1–2 mm that was removed. The M1 leg and face regions were both found to be fully intact. In the parietal lobe region, most of the cortex forming rostral S1 (S1r), which lines the posterior bank of the central sulcus (cytoarchitectonic areas 3a, 3b and 1), was spared. However, all gray matter (i.e., layers I–VI) forming the targeted part of S1c (cytoarchitectonic areas 1 and 2) on the gyral surface was removed, including the adjacent cortex forming the rostral part of cytoarchitectonic area PE of the superior parietal lobule. The parietal lesion extended posteriorly to the intraparietal sulcus, involving only the upper 1 mm of the cortex forming the dorsal bank of the sulcus thus sparing area PEa. Damage to the white matter located below the aspirated gray matter was minimal, as it was confined to a small portion of white matter located immediately below the resected gray matter field. Thus, all major white matter pathways were spared including the superior longitudinal fasciculus and frontal occipital fasciculus.
SDM76 (F2P2 Lesion)
The lesion site in case SDM76 was similar to case SDM71 as it involved the arm region the frontal motor cortex and parietal somatosensory cortex (Fig. 4). The major difference between these experimental cases was that nearly all of the cortex forming M1c (lining the anterior bank of the central sulcus) and S1r (lining the posterior bank of the central sulcus) was spared. Damage to the white matter underlying the aspiration lesion was also confined to a small portion of white matter located immediately below the resected gray matter field, thus sparing all major subcortical white matter pathways.
Behavioral Analyses
As described previously, observations of cage behavior in the days immediately following the F2 lesions indicated that the contralesional hand was not used for postural support or other motor tasks. The F2P2 lesioned monkeys did not use the contralesional hand immediately following the lesion and, in all cases, when moving about the testing cage dragged the affected hand with their finger-tips in contact with the cage floor, demonstrating lack of awareness of tactile inputs from the hand. This behavior was not observed in the F2 lesion animals. This lack of tactile awareness when moving about the cage persisted throughout the entire post-lesion survival/testing period.
The initial upper extremity paresis of F2P2 lesioned monkeys was followed by some spontaneous use of the contralesional hand in the 2nd post-lesion week after the first testing session in which they experienced “forced use” of the contralesional hand if they were motivated to attempt to acquire the food targets in the mDB and mMAP tasks. Notably, 5 of the 7 F2 and 4 of the 6 F2P2 lesioned monkeys attempted to acquire the food target in the mDB (any well) and mMAP (flat surface) tasks in the 1st post-lesion test one week after the lesion (Table 2). At least one food target in the 1st post-lesion test was successfully acquired by 3 F2 and 1 F2P2 lesioned monkeys in the 1st post-lesion test. Interestingly, as described previously (Morecraft et al., 2015), the F2P2 lesioned monkeys exhibited additional evidence of a somatosensory deficit in that they would attentively look at the hand, presumably to confirm that they actually had the food in possession, before taking it to the mouth. During the 2nd post-lesion week gradual increase in spontaneous use of the contralesional hand occurred in all monkeys and 6 of 7 F2 lesioned monkeys successfully acquired at least one food target at the 2nd post-lesion test two weeks after the lesion (Table 2). In contrast, only 3 of 6 F2P2 lesioned monkeys successfully acquired at least one food target at the 2nd post-lesion test (Table 2). Consistent success (all 5 trials on at least one level of difficulty) in the mDB or mMAP tasks occurred by the 3rd post-lesion week in 6 of 7 (and by the 5th post-lesion week in all 7) F2 lesioned monkeys and by the 7th post-lesion week in all F2P2 lesioned monkeys. However, one F2P2 lesioned monkey (SDM91) developed learned nonuse (LNU) after consistent success on well E in the mDB task in the 7th post-lesion test and never used the contralesional hand on either the mDB or mMAP after the test on post-lesion week 8 (Fig. 6F). Video recordings of post-lesion performances of the mDB task showed that this monkey was able to acquire small food targets using precision grip from wells B and C in the 6th and 7th post-lesion week motor tests but often lost the food pellet due to bumping of the hand on the side of the cage portal, which probably occurred due to poor awareness of hand location during the movement back into the cage (see supplemental videos). Subsequently, this monkey would attempt to use only the ipsilesional hand on both the mDB and mMAP tasks during subsequent post-lesion tests, often with success in the mMAP task (which would probably reinforce learned nonuse) but was never successful on the mDB task (note that performance scores were assigned as zero when attempts were made with only the ipsilesional hand when the device was set for testing the contralesional hand).
Figure 6.
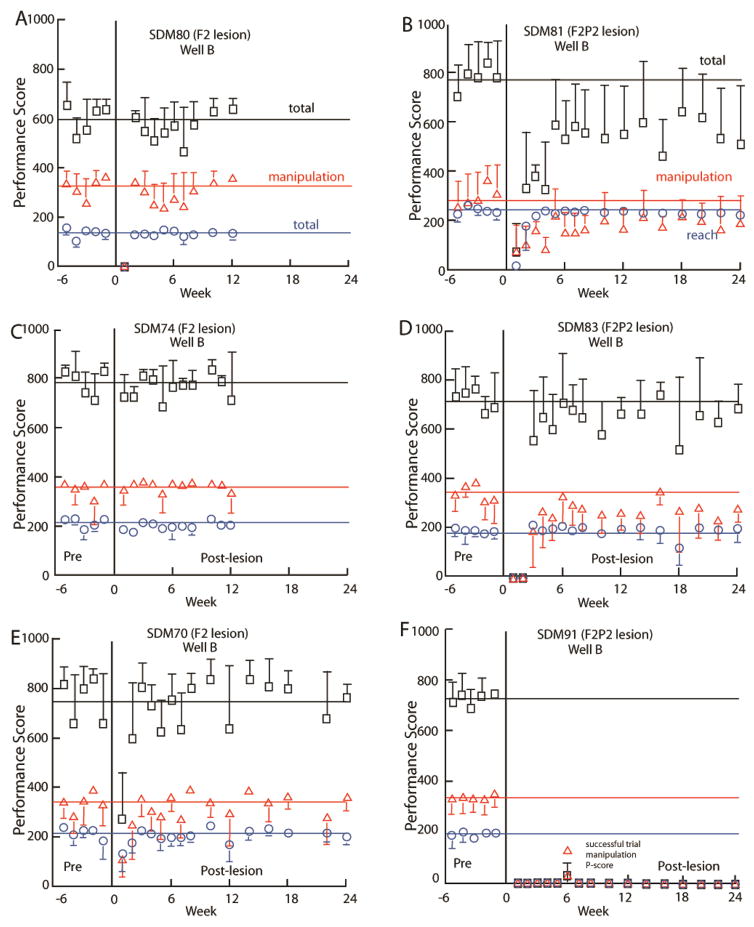
Pre- and post-lesion performance scores in the mDB well B task by 6 monkeys – 3 with F2 lesions (A, C, E) and 3 with F2P2 lesions (B, D, F). Total, reach and manipulation performance scores are shown. The horizontal lines represent average scores from the last 5 pre-lesion tests. Each plotted point is the average performance score for 5 trials to well B. Error bars are 1 S.D. Note that F includes the score for a successful trial by SDM91 on well B at post-lesion week 6.
Performance scores on the mDB and mMAP tasks decreased at the 1st post-lesion test (1 week post-lesion) and then recovered relatively quickly in monkeys with F2 lesions (e.g., Fig. 6A, C, E) but more slowly in monkeys with F2P2 lesions (Fig. 6B, D, F). For example, SDM80 and SDM70 (F2 lesion) made no attempts in the first post-lesion testing session on well B of the mDB task but then performed with reach and manipulation scores similar to pre-lesion scores in the 2nd post-lesion week and generally maintained that level of performance thereafter (Fig. 6A) whereas SDM74 performed similarly to pre-lesion at the first post-lesion test and maintained that performance throughout the 3 month post-lesion period (Fig. 6C). In contrast, the F2P2 lesion monkeys showed much poorer recovery. For example, SDM81 made some feeble attempts in the first post-lesion testing session on some wells and then showed some improvement over the 1st post-lesion month but exhibited lower than pre-lesion and highly variable performance scores throughout the recovery period (Fig. 6B). SDM83 made attempts only on larger wells in the first two weeks post-lesion and began to perform fairly well on smaller wells at post-lesion week 3, but with clearly lower and more variable performance scores than in pre-lesion testing and with little improvement thereafter (Fig. 6D). SDM91 made no attempts during testing on post-lesion weeks 1 through 5 on the largest well (D), then showed some ability to successfully grasp the pellet over post-lesion weeks 6 and 7 (see supplemental videos) on wells B, C, D, E, after which learned nonuse set in and no more attempts were made on well D or other wells (e.g., Fig. 6F).
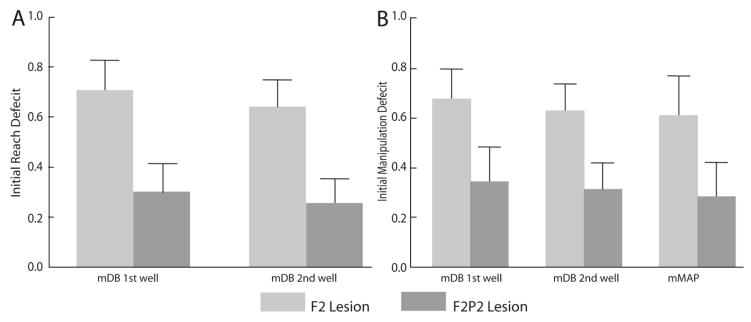
Initial deficits in both reaching and manipulation performance scores over the 1st post-lesion month were far greater in F2P2 lesioned monkeys than in F2 lesioned monkeys (Table 3, Fig. 7). During the first month after the lesion, all 6 F2P2 lesioned cases averaged less than 70% of manipulation performance scores on the last 5 pre-lesion tests in the mDB best well and 2nd well tasks (e.g., Fig. 6D, F), whereas only one F2 lesioned monkey (SDM64, which compared to the other F2 cases had the extensive subcortical white matter damage) had similarly poor performance on the mDB tasks in the first post-lesion month. Similarly, on the mMAP curved rod task, two of the seven F2 lesion cases and five of six F2P2 lesion cases averaged less than 70% of manipulation performance scores on the last 5 pre-lesion tests during the 1st 4 post-lesion tests.
Table 3.
Initial deficits in performance scores (ratio of performance scores during the 1st post-lesion month to last 5 pre-lesion trials) and Recovery of Reach and Manipulation Skill (ratio of highest skill over 5 consecutive post-lesion tests to skill over last 5 pre-lesion tests)
| Best Wella | 2nd Wellb | mMAP Curved Rod | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case SDM | Rch Idefc | Man Idefd | Rch Skl Recove | Man Skl Recovf | Rch Idef | Man Idef | Rch Skl Recov | Man Skl Recov | Idef | Skl Recov |
| 45 | 0.98 | 0.92 | 0.98 | 1.18 | 0.84 | 0.68 | 0.39 | 1.30 | 0.806 | 1.663 |
| 48 | 0.65 | 0.46 | 1.51 | 0.48 | 0.44 | 0.38 | 1.30 | 1.09 | 0.834 | 1.163 |
| 55 | 0.51 | 0.60 | 1.05 | 1.09 | 0.55 | 0.62 | 1.13 | 1.31 | 0.114 | 0.911 |
| 64 | 0.17 | 0.18 | 0.41 | 0.88 | 0.17 | 0.23 | 0.52 | 0.81 | 0.005 | 0.346 |
| 70 | 0.93 | 0.78 | 1.57 | 1.09 | 0.82 | 0.86 | 0.96 | 1.28 | 0.775 | 0.947 |
| 74 | 0.93 | 1.06 | 1.46 | 7.37* | 0.94 | 0.97 | 1.26 | 0.70 | 0.970 | 1.115 |
| 80 | 0.80 | 0.76 | 1.16 | 1.06 | 0.72 | 0.70 | 2.25 | 0.72 | 0.791 | 2.337 |
| M F2g | 0.708 | 0.679 | 1.163 | 1.879 | 0.640 | 0.635 | 1.169 | 1.032 | 0.613 | 1.212 |
| 71 | 0.53 | 0.79 | 1.34 | 1.42 | 0.49 | 0.53 | 0.97 | 1.75 | 0.311 | 0.519 |
| 76 | 0.27 | 0.65 | 0.58 | 0.57 | 0.30 | 0.45 | 0.39 | 0.41 | 0.214 | 0.506 |
| 81 | 0.68 | 0.37 | 2.73 | 0.97 | 0.53 | 0.58 | 2.45 | 1.33 | 0.365 | 0.498 |
| 83 | 0.17 | 0.15 | 2.31 | 0.72 | 0.07 | 0.22 | 0.1 | 0.31 | 0.817 | 1.227 |
| 87 | 0.15 | 0.11 | 1.21 | 0.90 | 0.14 | 0.11 | 0.14 | 0.11 | 0.010 | 0.658 |
| 91 | 0 | 0 | 0.06 | 0.05 | 0 | 0 | 0 | 0 | 0.009 | 0.064 |
| M F2P2h | 0.300 | 0.344 | 1.372 | 0.771 | 0.253 | 0.313 | 0.763 | 0.698 | 0.288 | 0.579 |
Abbreviations:
well with highest overall pre-lesion skill;
smaller well with about 50% lower skill level;
initial deficit in reach performance scores;
initial deficit in manipulation performance scores;
recovery of reach skill;
recovery of manipulation skill;
Mean of F2 lesion cases;
Mean of F2P2 lesion cases
This case developed a highly stereotyped method of grasping the pellet from the best well (B) that resulted in very low variability of performance scores in the post-lesion phase and resulted in a very high post-lesion skill, but mean performance scores averaged only 7.5% higher during the post-lesion period of highest skill than during the last 5 pre-lesion tests.
Figure 7.
Initial deficits in reach (A) and manipulation (B) performance scores in the mDB best and 2nd well tasks and mMAP curved rod task. Each bar is the average of ratios of average post-lesion performance scores over the 1st post-lesion month to the average scores of the last 5 pre-lesion tests for 7 F2 lesioned monkeys and 6 F2P2 lesioned monkeys (higher ratios indicate a lower deficit). Error bar are 1 S.E
All F2 lesioned monkeys except SDM64 showed good to excellent recovery on the mDB and/or mMAP tasks. That is, they recovered to within at least 80% of pre-lesion manipulation skill in at least one of the mDB best well or 2nd well tasks and/or mMAP curved rod task (Table 3). In contrast, among the 6 F2P2 lesioned monkeys, only 3 recovered to at least 80% of pre-lesion manipulation skill in the mDB best well or 2nd well tasks (Table 3). Notably, only one of the F2P2 lesioned monkeys recovered to within 80% of pre-lesion skill in the difficult mMAP curved rod task, which usually involve manipulation of hand and carrot chip orientation while attempting to apply appropriate forces to maintain the carrot chip within grasp. However, it should be noted that some of the F2 and F2P2 lesioned monkeys used quite different strategies in this task such as lifting the carrot chip partially up the rod and then placing two digits under the carrot chip and flinging the carrot upward, around the curve and off the rod. This technique, and others, permitted successful acquisition but with relatively high applied forces and poorer performance scores because the carrot chip was not maintained within grasp. Regardless of technique used in the mMAP task, frontoparietal injury clearly created larger and more lasting deficits in fine motor performance than those occurring after isolated frontal motor area lesions.
Regression Analyses – Total Gray and White Matter Lesions
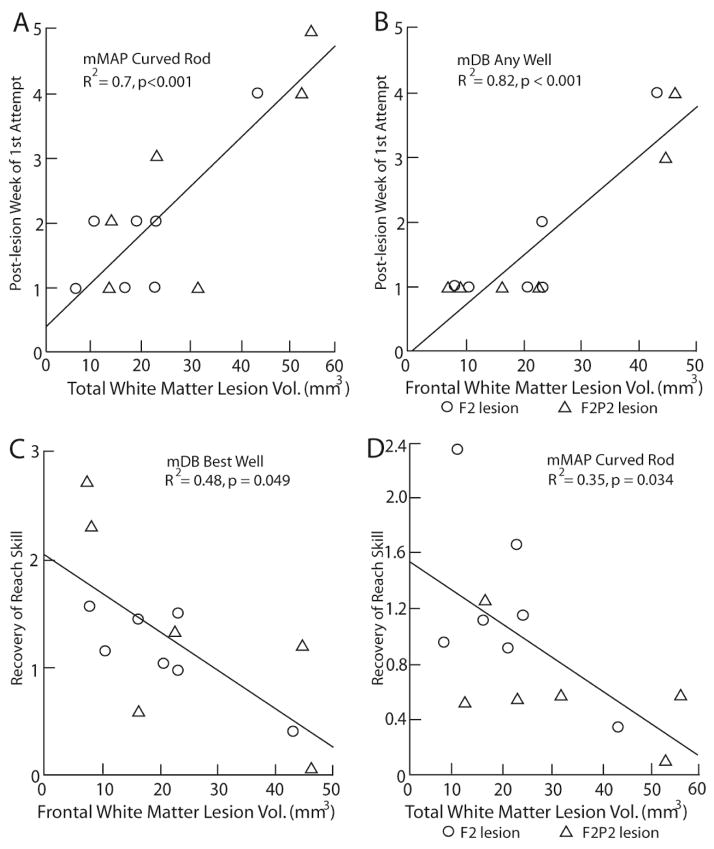
Post-lesion durations (i.e., post-injury time interval) until the 1st attempt, 1st successful acquisitions and consistent acquisition of the food targets in the mDB and mMAP tasks increased with increases in total white matter lesion volume (TWMLV) (Fig. 8A, Table 4). Total gray matter lesion volume (TGMLV) contributed partially to the prediction of duration until 1st attempt in any well of the mDB task and to duration until first and consistent successful acquisitions of food targets in some tasks (Table 4). Curiously, the coefficients for TGMLV were negative in all these cases, indicating that larger gray matter lesion volumes were associated with shorter post-lesion durations until the 1st attempt, success and consistent success in these fine motor tasks. Deficits in reaching and manipulation performance scores over the 1st post-lesion month also increased with increasing TWMLV (Table 4). TGMLV also contributed somewhat to prediction of reaching, but not manipulation, deficits. In this case, larger TGMLVs were associated with greater deficits in performance of reaching (Table 4). Recovery of reaching skill in the mDB 2nd well task decreased with increases in TGMLV whereas recovery of manipulation skill in the mMAP curved rod task decreased with increases in TWMLV (Table 4).
Figure 8.
Scatterplots of: post-lesion week of first attempt on the mMAP curved rod task versus total white matter lesion volume (A), post-lesion week of first attempt on the mDB (any well) task versus frontal white matter lesion volume (B), recovery of reach skill in the mDB (best well) task vs. frontal white matter lesion volume (C) and recovery of manipulation skill in the mMAP curved rod task versus total white lesion volume (D). Each plotted point is data from a single monkey with a F2 (circle) or F2P2 (triangle) lesion
Table 4.
Results of forward stepwise multiple linear regression (FSMR) analyses to predict initial deficits and recovery of function with independent variables: total gray matter lesion volume (TGMLV) and total white matter lesion volume (TWMLV). The positive or negative superscript by the variable indicates whether the relationship is as expected (i.e., +larger lesion volume associated with larger initial deficits and poorer recovery) or opposite to expected (i.e., −larger lesion volume associated with smaller initial deficits and better recovery). The analyses were included in this table only if the analysis for the primary independent variable or the final regression model was statistically significant (p < 0.05). Collinearity analyses of the 2 predictor variables indicated a VIF of 2.37 and tolerance of 0.43
| Dependent Variable | Primary Independent Variablea (R2, p) | Additional Independent Variableb (R2, R2adj, p) |
|---|---|---|
| mDB 1st attc AWd | TWMLV+ (0.73,<0.001) | TGMLV− (0.89, 0.87, <0.001) |
| mMAP 1st att CRe | TWMLV+ (0.70,<0.001) | |
| mDB 1st succf BWg | TWMLV+ (0.55,0.003) | |
| mMAP 1st succ CR | TWMLV+ (0.64,0.002) | |
| mMAP 1st succ SRh | TWMLV+ (0.5,0.010) | |
| mDB Csucci AW | TWMLV+ (0.36,0.030) | |
| mMAP Csucc CR | TWMLV+ (0.58,0.004) | TGMLV− (0.63, 0.55, 0.012) |
| mMAP Csucc SR | TWMLV+ (0.64, <0.001) | TGMLV− (0.74,0.68,0.005) |
| mMAP Csucc FSj | TWMLV+ (0.56,0.008) | |
| mDB Rchj Idef l BW | TWMLV+ (0.53,0.005) | TGMLV− (0.59, 0.51, 0.012) |
| mDB Manm Idef W2n | TWMLV+ (0.62,0.001) | |
| mMAP Man Idef CR | TWMLV+ (0.59,0.035) | |
| mDB Rch Skilln BW | TWMLV+ (0.48,0.008) | |
| mDB Rch Skill W2 | TGMLV+ (0.36,0.031) | |
| mMAP Man Skill CR | TWMLV+ (0.55,0.003) |
Abbreviations:
First variable added to FSMR,
additional independent variables included in final FSMR model,
attempt,
any well of mDB task,
curved rod mMAP task,
successful acquisition of food target,
best well of mDB task,
straight rod mMAP task,
successful acquisition of target on all 5 trials of a single well of mDB or level of mMAP in the testing session,
flat surface mMAP task,
Reach,
Idef - ratio of performance scores in the 1st post-lesion month to those of last 5 pre-lesion tests,
Man - manipulation,
2nd (smaller) well of mDB task,
ratio of highest skill during post-lesion period to skill during last 5 pre-lesion tests.
Regression Analyses – Frontal and parietal lobe lesion volumes
These analyses were conducted to assess whether strength of associations to lesion effects on motor performance and recovery of skill were greater than for total gray and white matter lesion volumes. Post-lesion durations (i.e., post-injury time interval) until the 1st attempt, 1st success and consistent success in the mDB task and the curved rod mMAP task increased with frontal lobe WMLV (Fig. 8B, C, Table 5). Frontal lobe WMLV was a stronger predictor of post-lesion duration until the 1st attempt on any well of the mDB task (Fig. 8B, R2 = 0.82) and until consistent success on any well in the mDB task (R2 = 0.5) than TWMLV (R2 = 0.73, 0.36 respectively) (Tables 4, 5). However, the full regression models using frontal and parietal lesion volumes did not predict post-lesion duration until 1st and consistent success better than the full regression models using total lesion volumes. Moreover, most regression models for prediction of initial deficits and skill recovery in reaching and manipulation from frontal and parietal lesion volumes did not exhibit higher coefficients of determination than models using total gray and white matter lesion volumes (Tables 4, 5). The only exceptions were that recovery of reach skill in the mDB tasks were better predicted from frontal and parietal white matter lesion volumes than from total gray or total white matter lesion volume (compare Tables 4, 5). Overall, these regressions indicated that frontal and parietal white matter volumes were closely associated with initial motor deficits and recovery whereas gray matter lesion volumes made relatively small contributions to the regression models. However, in general the initial deficits and recovery in the mDB and mMAP curved rod task were most closely associated with total white matter lesion volume and, as observed for total gray matter lesion volume, coefficients for gray matter lesion volumes were sometimes opposite to the expected effects of greater lesion volumes being associated with larger initial post-lesion deficits and poorer recovery of skill (Table 5).
Table 5.
Results of forward stepwise multiple regression (FSMR) with independent variables: frontal lobe gray matter (FGMLV) and white matter (FWMLV) lesion volumes, parietal lobe gray matter (PGMLV) and white matter (PWMLV) lesion volumes. The positive or negative superscript by the variable indicates whether the relationship is as expected (i.e., +larger lesion volume associated with larger initial deficits and poorer recovery) or opposite to expected (i.e., −larger lesion volume associated with smaller initial deficits and better recovery). The analyses were included in this table only if either the analysis for the primary independent variable or the final regression model was statistically significant (p < 0.05). Collinearity analyses of predictor variables indicated VIFs < 2.0 and Ts > 0.4 except for PGMLV and PWMLV (VIF=41.4, Ts < 0.03)
| Dependent Variable | Primary Independent Variable (R2, p) | FSMR Additional Independent Variable(s) (R2, R2adj, p) |
|---|---|---|
| mDB 1st att, AW | FWMLV+ (0.82, 0.001) | FGMLV−, PGMLV− (0.89, 0.85, <0.001) |
| mMAP 1st att, CR | FWMLV+ (0.65,0.001) | PWMLV+, PGMLV− (0.79, 0.72, 0.002) |
| mDB 1st succ, AW | FWMLV+ (0.46,0.004) | FGMLV− (0.56, 0.47, 0.006) |
| mMAP 1st succ, CR | FWMLV+ (0.59,0.001) | FGMLV− (0.69, 0.62, 0.005) |
| mMAP 1st succ SR | FWMLV+ (0.45,0.017) | |
| mDB Csucc AW | FWMLV+ (0.50,0.014) | FGMLV− (0.64, 0.56, 0.006) |
| mDB Csucc BW | FGMLV− (0.35,0.043) | FWMLV+ (0.51,0.40,0.040) |
| mMAP Csucc CR | FWMLV+ (0.60,0.001) | FGMLV(0.65, 0.57, 0.009) |
| mMAP Csucc SR | FWMLV+ (0.62,0.001) | FGMLV− (0.77,0.72,0.003) |
| mMAP Csucc FS | PWMLV+ (0.46, 0.051) | FWMLV+, PGMLV− (0.84, 0.78,0.003) |
| mDB Rch Idef BW | PWMLV+ (0.40,0.088) | FWMLV(0.63, 0.55, 0.007) |
| mDB Rch Idef W2 | PWMLV+ (0.26,0.075) | FWMLV+ (0.59, 0.51,0.011) |
| mDB Man Idef BW | FWMLV+(0.30,0.051) | PWMLV+(0.45, 0.34, 0.059) |
| mDB Man Idef W2 | PWMLV+ (0.36,0.113) | FWMLV+(0.59, 0.51, 0.011) |
| mMAP Man Idef CR | FWMLV+ (0.46,0.012) | PGMLV+, FGMLV− (0.62, 0.50, 0.027) |
| mDB Rch Skill BW | FWMLV+ (0.48,0.049) | PWMLV− (0.58, 0.49, 0.013) |
| mDB Rch Skill W2 | FWMLV+ (0.32,0.044) | PWMLV+ (0.46,0.36,0.045) |
Abbreviations: See Table 4.
Regression Analyses – Percent of Caudal M1 and Rostral S1 Lesion Volume, LPMC and area PE Lesion Volumes
These multiple regression analyses showed that percent damage to S1r hand/arm area was closely associated with post-lesion duration until 1st attempt and 1st success in the mDB and mMAP tasks while percent damage to M1c hand/arm area was closely associated with post-lesion duration until consistent success in the mDB task and initial deficits in reaching and manipulation in the mDB task. Interestingly, recovery of manipulation skill in the mDB best well and difficult mDB 2nd well tasks were primarily associated with percentage damage to S1r with some contribution of volume of damage to LPMC, M1c and area PE. Notably, the coefficients of determination were generally lower than for regression models using white matter lesion volumes (compare R2 values in Tables 6 to Tables 4, 5), suggesting again that white matter lesion volume is a major contributor to post-lesion deficits and recovery of hand motor function. However, it is clear that gray matter lesion volume in specific functional areas are strongly associated with effects of the lesion as coefficients of determination for these models often exceed 0.5 (Table 6). It is also evident that M1c and S1r are the primary gray matter areas associated with lesion effects with some contribution from volume of damage to LPMC and area PE. Moreover, in some cases volume of gray matter damage to specific areas were more strongly correlated with lesion effects than volume of white matter damage. For example, post-lesion duration until consistent success on any well of the mDB task was more strongly associated with percentage of M1c damage (R2 = 0.52) than with total white matter lesion volume (R2 = 0.36) and with similar association to frontal white matter damage (R2 = 0.5). However, as described above, there were coefficients for gray matter lesion volumes that were sometimes opposite to the expected effects of greater lesion volumes being associated with slower and poorer recovery (Table 6).
Table 6.
Results of forward stepwise multiple regression (FSMR) with independent variables: Percentage of M1c arm/hand area damaged by the lesion (M1cLV%), percentage of S1r arm/hand area damaged by the lesion (S1rLV%), LPMC lesion volume (LPMCLV) and area PE lesion volume (PELV). These analyses were included in this table only if the analysis for the primary independent variable or final regression model was statistically significant (p < 0.05). Collinearity analyses of predictor variables indicated VIFs < 2.0 and Ts > 0.6 except for M1CLV% and S1RLV% (VIF=13.7, Ts < 0.08)
| Dependent Variable | Primary Independent Variable (R2, p) | FSMR Additional Independent Variable(s) (R2, R2adj, p) |
|---|---|---|
| mDB 1st att BW | S1rLV%+ (0.58,0.002) | LPMCLV(0.67, 0.60, 0.004) |
| mMAP 1st att FS | S1rLV%+ (0.42,0.008) | M1cLV%(0.55, 0.45, 0.019) |
| mDB 1st succ AW | M1cLV%+ (0.52,0.006) | |
| mDB 1st succ BW | S1rLV%+ (0.64,0.001) | |
| mDB 1st succ W2 | S1rLV%+ (0.64,0.001) | PELV(0.77, 0.72, 0.001) |
| mMAP 1st succ FS | S1rLV%+ (0.49,0.008) | M1cLV%(0.59, 0.5, 0.012) |
| mDB Csucc AW | M1cLV%+ (0.52,0.006) | |
| mDB Csucc BW | LPMCLV− (0.37,0.035) | |
| mDB Rch Idef BW | M1cLV%+ (0.46,0.011) | LPMCLV, PELV(0.58, 0.44, 0.042) |
| mDB Rch Idef W2 | M1cLV%+ (0.48,0.009) | |
| mDB Man Idef BW | M1cLV%+ (0.53,0.005) | |
| mDB Man Idef W2 | M1cLV%+ (0.52,0.005) | LPMCLV(0.57, 0.48, 0.015) |
| mDB Man Skill BW* | S1rLV%+ (0.41, 0.025) | PELV−, LPMCLV+, M1CLV%−(0.68,0.50,0.060) |
| mDB Man Skill W2 | S1rLV%+ (0.36,0.03) | LPMCLV+, M1cLV%−, PELV− (0.63,0.45,0.066) |
Discussion
Combined lateral frontoparietal injury clearly increases initial hand movement control deficits and results in slower and poorer recovery compared with what occurs following isolated lateral frontal motor lesions. However, it is not clear whether these findings can be attributed primarily to the additional parietal gray and white matter damage because total white matter lesion volume was the best overall predictor of lesion effects on hand motor function and some monkeys with F2P2 lesions (SDM81, SDM83) had lower total white matter lesion volume than many monkeys with F2 lesions (Table 1). Similarly, in regression analyses with parietal and frontal gray and white matter lesion volumes as predictors it was apparent that frontal and parietal lobe white matter lesion volumes had stronger associations than gray matter lesion volumes with post-lesion hand motor function. These findings are consistent with the idea that damage to white matter located just below sensorimotor cortex has important effects of increased initial deficits and poorer recovery of hand motor function. This region contains corticocortical, commissural, corticofugal and corticopetal projection fibers that provide communication among a vast number of gray matter areas/structures in the injured and contralesional CNS, of which many may contribute to contralateral hand motor function. However, the importance of functional gray matter area lesions was evident in regression models using only the gray matter lesion volumes of M1c, S1r, LPMC and area PE as predictors. Specifically, percent damage to M1c and S1r arm/hand areas were important primary predictors of lesion volume effects on initial post-lesion deficits and recovery of hand motor function, with volume of damage to LPMC and area PE making relatively minor contributions (Table 6). Notably, recovery of manipulation skill in the difficult mDB smaller well (W2) task was most closely associated with percentage of damage to S1r arm/hand area with LPMC, M1c and area PE damage also contributing to this association (Table 6). This finding suggests that the cerebral injury of these areas, along with white matter damage, strongly affect recovery of dexterous hand/digit motor function.
We expected that increased damage to the peri-Rolandic regions M1c and S1r would be strongly correlated with increased initial motor deficits and poorer recovery because this region contains corticospinal neurons with monosynaptic connections onto motoneurons (M1c) (Rathelot and Strick, 2009) (Lemon et al., 1998) and somatosensory processing areas thought to be crucial for control of digit motions for tactile exploration and acquiring small objects (Freund, 2003; Weiss et al., 2001). Indeed, our experimental case with the greatest damage to the peri-Rolandic region, estimated as 100% of the M1c and 69.9% of the S1r arm/hand area, had the poorest recovery and developed learned nonuse (LNU) after the motor testing in post-lesion week 8. However, in post-lesion weeks 6 and 7, SDM91 did show evidence of ability to perform precision grasping of small food objects and remove such objects from small diameter wells (B – 13 mm dia., C – 19 mm dia. – see supplementary videos) before learned nonuse set in. The limited ability to perform such dexterous movements in the absence of the M1c monosynaptic projections onto motor neurons suggests that other spared motor areas that give rise to such connections, such as M2 (Boudrias et al., 2006; Boudrias et al., 2010; McNeal et al., 2010), may have supported such precision grasping before learned non-use set in. The delayed onset of learned nonuse in SDM91 may also be a consequence of degraded corticospinal innervation of motoneurons and interneurons from undamaged M2 that has recently been found to accompany lateral frontoparietal injury (Morecraft et al., 2015). This delayed onset may also be influenced by degradation of descending projections from other cortical and subcortical sources. Disrupted cortical processing of tactile and proprioceptive information from the contralateral upper limb may have also contributed to the manifestation of LNU, since it was first demonstrated that lack of sensation without damage to cortical motor or sensory areas is sufficient to cause LNU (Knapp et al., 1963; Taub et al., 1966). Also of note is that SDM91 had the smallest lesion to area PE of all F2P2 lesioned monkeys (Table 1). Other F2P2 lesioned cases with 1.5X to 8X larger lesions to area PE but smaller lesions to S1r (Table 1) did not exhibit LNU and clearly used precision grasping after the lesion. Thus, area PE injury may have little effect on development of LNU and also may not be a major contributor to precision grasping of small objects if most of S1r arm/hand area is spared (all F2P2 lesion cases except SDM91 had over 50% of this area spared).
There was additional evidence that greater damage to sensorimotor gray matter areas is associated with poorer recovery of hand function than to total gray and white matter damage. Specifically, recovery of manipulation skill in the mDB best and difficult mDB 2nd well task was closely associated with the extent of injury to S1r, with some relation to volume of damage to LPMC, M1c and area PE (Table 6, Fig. 8) whereas total and frontal/parietal gray and white matter lesion volumes were not significantly associated with recovery of manipulation skill (Tables 4, 5). These effects of parietal cortex lesions are also consistent with classical work indicating powerful negative effects of isolated postcentral gyrus lesions on recovery of digit fine motor functions that were more severe and persistent than the motor deficits following localized M1 lesions (Kennard and Kessler, 1940) and with work in human subjects showing substantial impairment of digit motions for tactile exploration of objects (Binkofski et al., 2001; Weiss et al., 2001). Loss of cortical processing of tactile and proprioceptive inputs as a result of postcentral injury apparently greatly impairs recovery of hand/digit motions, even in the absence of precentral motor cortex lesions (Kennard and Kessler, 1940). Kennard and Kessler also reported that enhanced visual attention to the motor act was clearly insufficient to compensate for loss of parietal cortex somatosensory processing in the case of fine digit movements. Similarly, it has been reported that in humans, stroke lesions extending into parietal lobe that damage area 2 and the intraparietal sulcus cortex, result in slower recovery and chronic impairment of fine hand motor function (Abela et al., 2012). In contrast, lesions limited to frontal motor areas that spare area 2 and the intraparietal sulcus are associated with good recovery of hand/digit movements (Abela et al., 2012). Notably, severe tactile deficits resulting in poor abilities in tactile object recognition were associated with very poor recovery of motor function. However, it should also be noted that recovery of skill in the mMAP curved rod task, which is also a difficult task but involves control of pronation/supination muscles rather than the control of digit muscles required in the mDB task, was associated only with total white matter lesion volume (Tables 4) and not with lesion of gray matter areas (Tables 5, 6 – note lack of significant multiple regression models involving recovery of mMAP curved rod manipulation skill).
The present work corroborates some of our previous conclusions that increasing volume of damage to frontal lobe white matter subadjacent to motor cortex areas increases the duration of severe impairment of hand fine motor function (i.e., until first attempts and successful acquisitions in the mDB and mMAP tasks) (Darling et al., 2009). In our previous work, most lesions were limited to the lateral frontal lobe, but also included monkeys with damage on the medial surface that involved M2, the pre-SMA and cingulate motor cortex. Furthermore, several of these cases were associated with very large volumes of white matter damage located below the frontal gray matter injury. The current finding that frontal lobe WMLV was a stronger predictor than total WMLV of post-lesion duration until the 1st attempt, 1st success and consistent success on any well of the mDB task (compare Tables 4, 5) is consistent with the previous findings. Moreover, larger volumes of frontal and parietal gray matter damage and parietal white matter damage had relatively little contribution to prediction of duration of impairment (Table 5). However, percentage damage to S1r and M1c were also closely associated with longer duration of impairment (Table 6) which is consistent with the idea that both peri-Rolandic frontal and parietal cortical areas contribute to fine motor coordination.
There are some limitations that should be considered in the findings and interpretations of the current study. First, an ideal study design to address differential effects of lesion location would have included additional subjects with lesions limited to M1 alone, S1 alone, and S1 plus area PE. Related to this issue, the multiple regression approach to assess contributions of gray matter lesion location/volume and gray vs. white matter lesions effects on initial deficits and recovery is complex and can reveal unexpected relationships with gray matter lesion volume. Specifically, multiple regression assumes independence of the predictor variables but there were significant correlations of total gray and white matter lesion volumes and among lesion volumes in some functional areas (e.g., M1c and S1r lesion volumes, parietal gray and white matter lesion volumes). This is known as multicollinearity, which can influence the regression coefficients associated with the collinear predictors, but does not reduce reliability or predictive power of the regression model (O’Brien, 2007). Thus, the regression coefficients of collinear predictors must be interpreted with caution. For example, as we observed, white matter lesion volume is strongly associated with lesion effects on motor function, gray matter contributions to lesion effects are minimized and can appear opposite to their true effects in that larger gray matter lesions seem to contribute to smaller initial post-lesion deficits and better recovery of motor function in the multiple regression model (see Tables 4, 5 and compare to Table 6 which considers only gray matter lesions). Similar problems may occur when assessing effects of gray matter damage if lesion volumes in the different functional areas are correlated and one functional area is primarily associated with lesion effects (see Table 6). However, regression analyses with a single dependent variable always revealed the expected larger deficits and poorer recovery with larger gray matter lesion volumes. Overall, a separate group of monkeys with each type of lesion, as indicated above, would provide a better test of gray matter lesion effects. However, given present realities in primate research, completion of such an ideal experiment is uncertain. The present approach provides for useful conclusions concerning effects of white matter lesion volume, and the role of select areas of gray matter because the associations with lesion effects in both cases are generally quite strong (Tables 4, 5, 6). Also related to lesion location/volume measures is that these were assessed 12 to 48 weeks post-injury and were based on comparison to the intact hemisphere due to structural changes occurring in the lesioned hemisphere such as tissue cavitation. However, the relatively localized damage to the superficial cortical mantle using the aspiration method minimized such structural changes in the F2 and F2P2 experimental cases. Furthermore, the relatively simple sulcal organization that is often very similar in pattern on both hemispheres of a given Macaca mulatta cortex (particularly with respect to the primary sulci of which the central, arcuate and intaparietal sulci were key landmarks for orientation in the present study) allows for relatively accurate contralesional transfer of the lesion site estimate. Moreover, the microscopic capability to precisely identify the linear interface between the bottom of gray matter layer VI and adjacent subcortical white matter zone all help to minimize inaccurate lesion volume estimates when using the intact (contralesional) hemisphere for this purpose. It is also clear that a longitudinal assessment of lesion location/volume using MRI methods shortly after injury and at various time points throughout the recovery process would help verify this estimate, and may contribute additional information relevant to gradual changes in lesion site shape and volume, initial post-lesion deficits, and temporal aspects of changes of hand motor function recovery. Use of both approaches would be optimal.
A second limitation is that we did not verify a somatosensory deficit with objective testing of tactile and proprioceptive function of the contralesional upper limb. Thus, the extent of any sensorimotor deficit is not clear in the F2P2 lesion experiments. However, there was evidence that subjects consistently used vision to verify acquisition of the food targets in these cases, which was also observed in previous studies with somatosensory processing deficits due to spinal dorsal column transection (Qi et al., 2014) or parietal cortex ablation (Kennard and Kessler, 1940). Importantly, the more affected hand of all 6 F2P2 lesion cases was often dragged, with the finger-tips in constant contact with the metal cage floor when moving about the testing cage. This behavior was not observed in any of the F2 lesion cases. We also did not record evoked potentials in S1 prior to the lesion to verify that the regions removed received sensory inputs from the upper limb. Instead we used ICMS to identify areas in anterior parietal cortex that evoked upper limb motion and surgically removed those areas. However, this approach is consistent with previous work showing that stimulation of postcentral gyrus elicits movements of the same body parts that are activated during sensory stimulation in macaques (Woolsey, 1958).
In conclusion, we have observed that lesion of anterior parietal cortex and adjacent frontal motor areas delay recovery onset and result in greater and more persistent impairments in hand motor function (reaching and grasping) than damage limited to lateral frontal lobe motor areas. Thus, subtotal injury to cortex known to be involved in neural processing of somatosensory inputs from the upper limb appears to contribute to poorer recovery of reaching and grasping following frontoparietal injury than injury limited to the frontal motor cortex. Surprisingly, recovery of frontoparietal cortex lesioned monkeys is also generally poorer than large frontal cortex lesions involving the arm/hand areas of M1, LPMC and M2 as reported previously (Darling et al., 2009). This suggests that when lateral frontal motor areas are damaged in the monkey model, additional injury to cortical areas processing somatosensory information (i.e., S1, rostral area PE), which commonly occurs in MCA stroke, may impair motor function more so than additional damage to medial motor areas (i.e., M2). From a neurological perspective, there is an emerging picture of more serious, and widespread motor recovery complications that appears to develop after frontoparietal injury when compared to isolated frontal motor injury. The possible loss of parietal lobe trophic effects on corticospinal projections from spared M2 in the ipsilesional hemisphere (Morecraft et al., 2015) and spared M1 of the contralesional hemisphere (Morecraft et al., 2016), in combination with the probable impairment of cortical somatosensory processing after anterior parietal lesions, may partially explain the poor recovery of hand motor function observed here and in survivors of MCA stroke with injury to the lateral frontal and parietal cortex.
Supplementary Material
Figure 9.
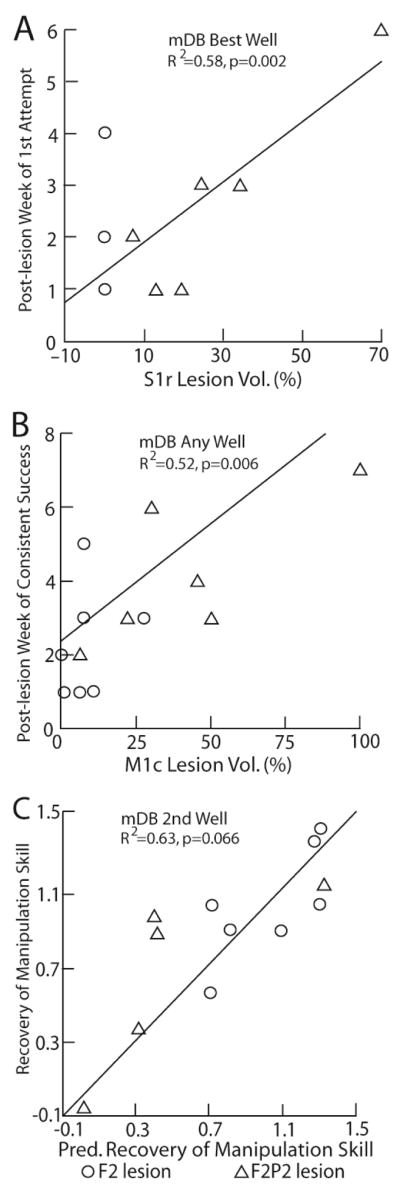
Scatterplots of post-lesion week of first attempt versus percentage of S1r arm/hand area lesion volume in the mDB best well task (A), of post-lesion week of consistent success (on all trials in any well) versus percentage of M1c arm/hand area lesion volume (B) and recovery of manipulation skill on the 2nd well versus vs predicted recovery of manipulation (C). Each plotted point is data from a single monkey with a F2 (circle) or F2P2 (triangle) lesion. Predicted recovery of manipulation skill in C is from the best fit forward stepwise multiple regression model (equation: = 0.045 x S1rLV% - 0.0085xLPMCLV + 0.017xM1cLV% + 0.006*PELV + 1.682).
Research Highlights.
Frontoparietal lesions impair hand function more than frontal lobe lesions.
Caudal M1 injury affects initial post-lesion deficits.
Rostral S1 injury contributes to delayed recovery and poorer motor skill recovery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abela E, Missimer J, Wiest R, Federspiel A, Hess C, Sturzenegger M, Weder B. Lesions to primary sensory and posterior parietal cortices impair recovery from hand paresis after stroke. PLoS One. 2012;7:e31275. doi: 10.1371/journal.pone.0031275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abela E, Seiler A, Missimer JH, Federspiel A, Hess CW, Sturzenegger M, Weder BJ, Wiest R. Grey matter volumetric changes related to recovery from hand paresis after cortical sensorimotor stroke. Brain Struct Func. 2015;220:2533–2550. doi: 10.1007/s00429-014-0804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Kunesch E, Classen J, Seitz RJ, Freund HJ. Tactile apraxia: unimodal apractic disorder of tactile object exploration associated with parietal lobe lesions. Brain. 2001;124:132–144. doi: 10.1093/brain/124.1.132. [DOI] [PubMed] [Google Scholar]
- Boudrias MH, Belhaj-Saif A, Park MC, Cheney PD. Contrasting properties of motor output from the supplementary motor area and primary motor cortex in rhesus macaques. Cereb Cortex. 2006;16:632–638. doi: 10.1093/cercor/bhj009. [DOI] [PubMed] [Google Scholar]
- Boudrias MH, Lee SP, Svojanovsky S, Cheney PD. Forelimb muscle representations and output properties of motor areas in the mesial wall of rhesus macaques. Cereb Cortex. 2010;20:704–719. doi: 10.1093/cercor/bhp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, Ugurbil K. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- Carrera E, Maeder-Ingvar M, Rossetti AO, Devuyst G, Bogousslavsky J, Lausanne Stroke R. Trends in risk factors, patterns and causes in hospitalized strokes over 25 years: The Lausanne Stroke Registry. Cerebrovasc Dis. 2007;24:97–103. doi: 10.1159/000103123. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Zoubina EV, Plautz EJ, Mahnken JD, Nudo RJ. Effects of Small Ischemic Lesions in the Primary Motor Cortex on Neurophysiological Organization in Ventral Premotor Cortex. J Neurophysiol. 2006;96:3506–3511. doi: 10.1152/jn.00792.2006. [DOI] [PubMed] [Google Scholar]
- Darling WG, Helle N, Pizzimenti MA, Rotella DL, Hynes SM, Ge J, Stilwell-Morecraft KS, Morecraft RJ. Laterality affects spontaneous recovery of contralateral hand motor function following motor cortex injury in rhesus monkeys. Exp Brain Res. 2013;228:9–24. doi: 10.1007/s00221-013-3533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling WG, Peterson CR, Herrick JL, McNeal DW, Stilwell-Morecraft KS, Morecraft RJ. Measurement of coordination of object manipulation in non-human primates. J Neurosci Methods. 2006;154:38–44. doi: 10.1016/j.jneumeth.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Darling WG, Pizzimenti MA, Rotella DL, Peterson CR, Hynes SM, Ge J, Solon K, McNeal DW, Stilwell-Morecraft KS, Morecraft RJ. Volumetric effects of motor cortex injury on recovery of dexterous movements. Exp Neurol. 2009;220:90–108. doi: 10.1016/j.expneurol.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny-Brown D, Yanagisawa N, Kirk E. The localization of hemispheric mechanisms of visually directed reaching and grasping. In: Zulch KJ, Kruetzfeldt O, Galbraith GC, editors. Cerebral localization. Springer; Berlin: 1975. pp. 62–65. [Google Scholar]
- Eisner-Janowicz I, Barbay S, Hoover E, Stowe AM, Frost SB, Plautz EJ, Nudo RJ. Early and late changes in the distal forelimb representation of the supplementary motor area after injury to frontal motor areas in the squirrel monkey. J Neurophysiol. 2008;100:1498–1512. doi: 10.1152/jn.90447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund HJ. Somatosensory and motor disturbances in patients with parietal lobe lesions. Adv Neurol. 2003:179–193. [PubMed] [Google Scholar]
- Jeannerod M, Michel F, Prablanc C. The control of hand movements in a case of hemianaesthesia following a parietal lesion. Brain. 1984;107(Pt 3):899–920. doi: 10.1093/brain/107.3.899. [DOI] [PubMed] [Google Scholar]
- Kaas JH. The functional organization of somatosensory cortex in primates. Ann Anat. 1993;175:509–518. doi: 10.1016/s0940-9602(11)80212-8. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Somatosensory System. In: Mai JK, Paxinos G, editors. The Human Nervous System. Elsevier Academic Press; London: 2012. pp. 1064–1099. [Google Scholar]
- Kaneko T, Caria MA, Asanuma H. Information processing within the motor cortex. I Responses of morphologically identified motor cortical cells to stimulation of the somatosensory cortex. J Comp Neurol. 1994a;345:161–171. doi: 10.1002/cne.903450202. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Caria MA, Asanuma H. Information processing within the motor cortex. II Intracortical connections between neurons receiving somatosensory cortical input and motor output neurons of the cortex. J Comp Neurol. 1994b;345:172–184. doi: 10.1002/cne.903450203. [DOI] [PubMed] [Google Scholar]
- Kennard M, Kessler M. Studies of motor performance after parietal ablations in monkeys. J Neurophysiol. 1940;3:248–257. [Google Scholar]
- Knapp HD, Taub E, Berman AJ. Movements in monkeys with deafferented forelimbs. Exp Neurol. 1963;7:305–315. doi: 10.1016/0014-4886(63)90077-3. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Baker SN, Davis JA, Kirkwood PA, Maier MA, Yang HS. The importance of the cortico-motoneuronal system for control of grasp. Novartis Found Symp. 1998;218:202–215. doi: 10.1002/9780470515563.ch11. discussion 215-208. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–661. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- McNeal DW, Darling WG, Ge J, Stilwell-Morecraft KS, Solon KM, Hynes SM, Pizzimenti MA, Rotella DL, Vanadurongvan T, Morecraft RJ. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J Comp Neurol. 2010;518:586–621. doi: 10.1002/cne.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Pessina MA, Moss MB, Finklestein SP, Rosene DL. Recovery from ischemia in the middle-aged brain: a nonhuman primate model. Neurobiol Aging. 2012;33:619.e619–619.e624. doi: 10.1016/j.neurobiolaging.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Cipolloni PB, Stilwell-Morecraft KS, Gedney MT, Pandya DN. Cytoarchitecture and cortical connections of the posterior cingulate and adjacent somatosensory fields in the rhesus monkey. J Comp Neurol. 2004;469:37–69. doi: 10.1002/cne.10980. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Hynes SM, Pizzimenti MA, Rotella DL, Darling WG. Vulnerability of the medial frontal corticospinal projection accompanies combined lateral frontal and parietal cortex injury in rhesus monkey. J Comp Neurol. 2015;523:669–697. doi: 10.1002/cne.23703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Hynes SM, Pizzimenti MA, Rotella DL, Darling WG. Frontal and frontoparietal injury differentially affect the ipsilateral corticospinal projection from the nonlesioned hemisphere in monkey (Macaca mulatta) J Comp Neurol. 2016;524:380–407. doi: 10.1002/cne.23861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Pizzimenti MA, Darling WG. Terminal distribution of the corticospinal projection from the hand/arm region of the primary motor cortex to the cervical enlargement in rhesus monkey. J Comp Neurol. 2013;521:4205–4235. doi: 10.1002/cne.23410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Cipolloni PB, Ge J, McNeal DW, Pandya DN. Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Res Bull. 2012;87:457–497. doi: 10.1016/j.brainresbull.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992;12:2918–2947. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RM. A caution regarding rules of thum for variance inflation factors. Quantity & Quantity. 2007;41:673–690. [Google Scholar]
- Peele TL. Acute and chronic parietal lobe ablations in monkeys. J Neurophysiol. 1944;7:269–286. [Google Scholar]
- Pizzimenti MA, Darling WG, Rotella DL, McNeal DW, Herrick JL, Ge J, Stilwell-Morecraft KS, Morecraft RJ. Measurement of reaching kinematics and prehensile dexterity in nonhuman primates. J Neurophysiol. 2007;98:1015–1029. doi: 10.1152/jn.00354.2007. [DOI] [PubMed] [Google Scholar]
- Qi HX, Kaas JH, Reed JL. The reactivation of somatosensory cortex and behavioral recovery after sensory loss in mature primates. Front Syst Neurosci. 2014;8:84. doi: 10.3389/fnsys.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen D, Kohler O, Worm-Petersen S, Blegvad N, Jacobsen HL, Bergmann I, Egeblad M, Friis M, Nielsen NT. Computed tomography in prognostic stroke evaluation. Stroke. 1992;23:506–510. doi: 10.1161/01.str.23.4.506. [DOI] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci U S A. 2006;103:8257–8262. doi: 10.1073/pnas.0602933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci U S A. 2009;106:918–923. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AG, Attiah MA, Berman JI, Chen HI, Liu X, Zhang M, Van der Spiegel J, Lucas TH. The effects of acute cortical somatosensory deafferentation on grip force control. Cortex. 2016;74:1–8. doi: 10.1016/j.cortex.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smania N, Montagnana B, Faccioli S, Fiaschi A, Aglioti SM. Rehabilitation of somatic sensation and related deficit of motor control in patients with pure sensory stroke. Arch Phys Med Rehabil. 2003;84:1692–1702. doi: 10.1053/s0003-9993(03)00277-6. [DOI] [PubMed] [Google Scholar]
- Stern PH, McDowell F, Miller JM, Robinson M. Factors influencing stroke rehabilitation. Stroke. 1971;2:213–218. doi: 10.1161/01.str.2.3.213. [DOI] [PubMed] [Google Scholar]
- Taub E, Ellman SJ, Berman AJ. Deafferentation in monkeys: effect on conditioned grasp response. Science. 1966;151:593–594. doi: 10.1126/science.151.3710.593. [DOI] [PubMed] [Google Scholar]
- Weiss PH, Dohle C, Binkofski F, Schnitzler A, Freund HJ, Hefter H. Motor impairment in patients with parietal lesions: disturbances of meaningless arm movement sequences. Neuropsychologia. 2001;39:397–405. doi: 10.1016/s0028-3932(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M, Wiesendanger R. The supplementary motor area in the light of recent investigations. Exp Brain Res Suppl. 1984;9:382–392. [Google Scholar]
- Woolsey CN. Organization of somatic sensory and motor areas of the cerebral cortex. In: Harlow HF, Woolsey CN, editors. Biological and Biochemical Bases of Behavior. University of Wisconsin Press; Madison, WI, U.S.A: 1958. pp. 63–82. [Google Scholar]
- Woolsey CN, Settlage PH, Meyer DR, Sencer W, Hamuy TP, Travis AM. Patterns of localization in precentral and \supplementary” motor areas and their relation to the concept of a premotor area. Res Publ Assoc Res Nerv Ment Dis. 1951;30:238–264. [PubMed] [Google Scholar]
- Woolsey CN, Settlage PH, Meyer DR, Sencer W, Pinto Hamuy T, Travis AM. Patterns of localization in precentral and “supplementary” motor areas and their relation to the concept of a premotor area. Res Publ Assoc Res Nerv Ment Dis. 1952;30:238–264. [PubMed] [Google Scholar]
- Yoo KM, Shin HK, Chang HM, Caplan LR. Middle cerebral artery occlusive disease: the New England Medical Center Stroke Registry. J Stroke Cerebrovasc Dis. 1998;7:344–351. doi: 10.1016/s1052-3057(98)80053-0. [DOI] [PubMed] [Google Scholar]
- Zeman BD, Yiannikas C. Functional prognosis in stroke: use of somatosensory evoked potentials. J Neurol Neurosurg Psychiatry. 1989;52:242–247. doi: 10.1136/jnnp.52.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.