Abstract
Detailed descriptions of cortical anatomy in youth with Down syndrome (DS), the most common genetic cause of intellectual disability (ID), are scant. Thus, the current study examined deviations in cortical thickness (CT) and surface area (SA), at high spatial resolution, in youth with DS, to identify focal differences relative to typically developing (TD) youth. Participants included 31 youth with DS and 45 age- and sex-matched TD controls (mean age ∼16 years; range = 5–24 years). All participants completed T1-weighted ASSET-calibrated magnetization prepared rapid gradient echo scans on a 3-T magnetic resonance imaging scanner. Replicating prior investigations, cortical volume was reduced in DS compared with controls. However, a novel dissociation for SA and CT was found—namely, SA was reduced (predominantly in frontal and temporal regions) while CT was increased (notably in several regions thought to belong to the default mode network; DMN). These findings suggest that reductions in SA rather than CT are driving the cortical volume reductions reported in prior investigations of DS. Moreover, given the link between DMN functionality and Alzheimer's symptomatology in chromosomally typical populations, future DS studies may benefit from focusing on the cortex in DMN regions, as such investigations may provide clues to the precocious onset of Alzheimer's disease in this at-risk group.
Keywords: Alzheimer's disease, cerebral cortex, intellectual disability, magnetic resonance imaging, Trisomy 21
Introduction
Down syndrome (DS), the most common genetic cause of intellectual disability (ID), occurs in ∼1/700 live births (Parker et al. 2010). It is associated with characteristic physical features, including dysmorphology of the faces, hands, and feet, cardiac and gastrointestinal anomalies, and atypical development of the central nervous system (for a review, see Antonarakis and Epstein 2006). DS is a lifelong neurodevelopmental disorder that is associated with high rates of precocious-onset Alzheimer's disease in adulthood (Zigman and Lott 2007). Thus, characterizing altered brain morphometry in youth with DS is not only relevant to understanding early emerging neurodevelopmental phenotypes, but also provides a foundation for the study of later-emerging degenerative processes.
Despite the fact that DS was first described over 100 years ago (Down 1886) and its genetic origin identified ∼50 years ago (Lejeune et al. 1959), surprisingly little is known about the developing brain in children with DS. This contrasts with advances made in refining the neuropsychological phenotype associated with the disorder in childhood. This substantial body of research has revealed that, in addition to ID (in most but not all cases; for a review, see Pennington et al. 2003), DS is associated with specific language deficits that are in excess of general cognitive delays, including articulation (Dodd 1975) and syntactic weaknesses (Fowler et al. 1994) as well as significant deficits in verbal short-term/working memory (for a review, see Baddeley and Jarrold 2007). Motor weaknesses are also commonly reported (Carr 1970) as are deficits on long-term memory tasks (Pennington et al. 2003). In contrast, some aspects of visual–spatial abilities, particularly visual–spatial short-term memory, and implicit learning are mental age appropriate (Silverstein et al. 1992; Wang and Bellugi 1994; Vicari et al. 2007).
Our understanding of brain morphometry in youth with DS studied in vivo is based upon fewer than 15 original research articles, many of which are characterized by very small sample sizes (<10–15) and descriptions of gross anatomical features of the brain (e.g., total brain volume, lobar volumes) using older magnetic resonance imaging (MRI) technology (e.g., 1.5-Tesla scanners). The primary findings from these studies and those that have included adults with DS (prior to the onset of dementia symptomatology) indicate that the syndrome is associated with reductions in total brain volume (Jernigan and Bellugi 1990; Pearlson et al. 1998; Pinter, Eliez, et al. 2001; Kates et al. 2002; Smigielska-Kuzia et al. 2011) as well as specific reductions in cerebellar (Jernigan and Bellugi 1990; Raz et al. 1995; Pinter, Eliez, et al. 2001; White et al. 2003) and hippocampal volumes (Raz et al. 1995; Aylward et al. 1997; Pearlson et al. 1998; Pinter, Brown, et al. 2001; White et al. 2003; Smigielska-Kuzia et al. 2011). Additionally, reductions in regions of the frontal and temporal lobes have been reported (Raz et al. 1995; Frangou et al. 1997; Kates et al. 2002; White et al. 2003; Smigielska-Kuzia et al. 2011). In contrast, relatively preserved parietal lobar gray matter volumes have been noted (Pinter, Eliez, et al. 2001) along with increases in parahippocampal gyrus volumes (Raz et al. 1995; White et al. 2003). Two recent pediatric neuroimaging studies utilizing voxel-based morphometry (Menghini et al. 2011; Carducci et al. 2013) extend these findings by noting reductions and increases in gray matter density in different regions of the cerebellum, temporal, and frontal lobes. These 2 recent studies support the utility of examining brain morphometry at a much higher level of spatial resolution than has typically been employed in order to make new discoveries about the brain in DS.
Thus, the overarching goal of the current study was to provide detailed descriptions of the neocortex in youth with DS by dissociating the 2 structural determinants of cortical volume—cortical thickness (CT) and cortical surface area (SA)—and measuring these at high spatial resolution (∼41 000 points or vertices across each hemisphere) in order to identify focal differences relative to typically developing (TD) youth. Cortical SA and thickness are thought to have different phylogenetic and ontogenetic origins (Rakic 1995; Panizzon et al. 2009) and developmental trajectories (Raznahan et al. 2011; Wierenga et al. 2014). Distinguishing between CT and SA has also been useful in detecting diagnostic differences among groups (Shaw et al. 2010). Moreover, prior studies have demonstrated that the traditional emphasis on volume as a measure of interest in the analysis of cortical structure can hide opposing alterations of CT and SA in genetically determined disorders of brain development (Raznahan et al. 2010; Meda et al. 2012). Researchers studying heritable psychiatric disorders have come to similar conclusions and have emphasized the importance of examining these 2 neuroanatomic phenotypes separately (Winkler et al. 2010). By dissociating cortical SA and thickness in DS, we aim to contribute to the development of more mechanistic accounts of the possible etiological origins of the DS neuroanatomic phenotype and to identify new aspects of the phenotype which can be manipulated experimentally by researchers studying animal models.
Thus, the current study sought to answer the following questions:
Do CT reductions, SA reductions, or both morphological features contribute to reductions in cortical gray matter volume in DS?
At the level of the vertex (∼82 000 points across the cerebral cortex), is there regional specificity to differences in CT and SA?
Materials and Methods
All research procedures were completed at the National Institutes of Health Clinical Center in Bethesda, MD. The study was approved by the National Institute of Mental Health (NIMH) Institutional Review Board. Following an explanation of study procedures, TD adult participants provided written consent. Parents of minors and adult participants with DS for whom parents had legal custody (reviewed by NIH legal counsel) provided written consent. In these instances, the participants provided verbal or written assent (depending on the age and cognitive ability level of the participant). For adults with DS whose parents did not have legal custody, study procedures were described to participants and their parents (who accompanied them to the NIH). Then, a quiz was completed to evaluate the participant's understanding of 1) the purpose of the study, 2) the risks and benefits of participation, and 3) the individual's rights as a participant (mostly importantly the right to withdraw from the study at any time). If participants could answer quiz questions competently (n = 2) and were deemed to have capacity to provide consent independently, they provided written consent for the study. If a participant could not competently answer quiz questions, the NIMH Human Subjects Protection Team was consulted. They interviewed the patient and then assisted the patient and parent in completing durable power of attorney (DPA) paperwork. Once DPA was assigned to the participant's parent, then the parent signed the consent form and the adult participant signed the assent form.
Participants
The sample included 31 individuals with DS and 45 TD peers matched on age, gender, and family characteristics (i.e., socioeconomic status). Participants with DS were recruited primarily from the Washington, DC metropolitan area. However, in order to maximize sample size, some participants were included from other regions of the USA. Participants were recruited via advertisements with area and nationwide family organizations focused on providing support and advocacy, including the DS Network of Montgomery County (Maryland), the DS Connection (of Eastern Maryland), the Parents of DS group (of Prince George's County, Maryland), the DS Association of Northern Virginia, the DS Association of Greater Richmond, and both the National DS Society and the National DS Congress. In addition, families were recruited through religious organizations, local Arc chapters, community recreation programs for individuals with disabilities, the National Institutes of Health website, and Twitter.
The 31 participants with DS included in the current study were drawn from a sample of 54 participants who initially enrolled in the study. Of these 54 participants, 6 dropped out prior to completing MRI scans (due to family overcommitment or illness). Of the remaining 48, 3 did not complete scanning due to the inability to complete mock scanning procedures. Forty-five participants completed scanning without sedation. Data for 14 of the 45 participants could not be used due to significant in-scanner motion. Participants with usable scans differed from those without on age (fewer younger participants could complete scans; P < 0.05) and sex (fewer males could complete scans, P < 0.05). There was also a nonsignificant trend toward lower IQ in those who did not provide usable scans (Mean nonverbal IQ of 58 in the group with usable scan data vs. mean nonverbal IQ of 49 in the group without usable scan data. [Note that in the group without usable scan data, IQ data were available for 14 of the 17 participants]).
All participants with DS had chromosomal diagnoses of Trisomy 21 according to parent report. Of these, 22 cases were confirmed via direct testing as a part of this study. None of these participants were found to be mosaic. Of the remaining 9 cases (who elected not to complete genetic testing due to their child's reluctance to give blood), 6 families provided copies of their child's genetic testing results. Three families were not able to locate these results but reported that their child was diagnosed with Trisomy 21 either in utero or shortly after birth. Because we could not confirm that DS was due to Trisomy 21 (as opposed to translocation or mosaicism) in these participants, we ran analyses with and without them and the results were unchanged. Thus, these participants are included in all primary analyses.
In addition to the genetic inclusion criteria, participants were also required be free of any history of acquired head injury or other condition that would cause gross brain abnormalities. We did, however, include one participant with DS who had a well-controlled seizure disorder and who was taking neuroleptic medications. Analyses were run with and without this participant and they remained the same. Thus, this participant's data were included in the final analyses. We did not exclude participants if they were taking psychotropic medications, but it should be noted that 4 participants with DS were on psychotropic medications. All 4 were taking selective serotonin reuptake inhibitors (SSRIs); one participant was taking a stimulant in addition to the SSRI. Analyses were run with and without these participants and results remained largely the same; thus, all participants were included in the sample regardless of whether they were taking psychotropic medication. Participants' parents filled out a questionnaire that probed families about whether the participant had any commonly reported comorbid psychiatric diagnoses, which included autism, attention-deficit/hyperactivity disorder, and obsessive compulsive disorder. Rates according to parent report for these diagnoses were as follows, respectively: ns = 3, 4, 2 (of 30; data missing for one participant). Data were not systematically collected about other psychiatric diagnoses. Lastly, it should be noted that this sample included 2 siblings with DS. Analyses were run with and without one of the siblings, and results were the same. Thus, both siblings are included in the final sample.
Forty-five TD youth served as control participants. These individuals were recruited from the greater Washington, DC, metropolitan area. They included individuals who had previously participated in studies of healthy brain development at the National Institutes of Health as well as participants recruited with the help of the institution's Healthy Volunteer office. These participants were matched to the DS participants group-wise to be similar on age, proportion of males and females, and family characteristics. They were screened by phone prior to study enrollment to exclude those with psychiatric or learning difficulties as well as acquired brain injury (for details, see Lenroot et al. 2007).
Table 1 provides demographic information about the groups. As can be seen, the groups were well matched on age, sex, racial makeup, and socioeconomic status as measured using the Hollingshead 2-factor index (Hollingshead and Redlich 1958). They differed significantly on IQ measures as expected. The DS group also had a smaller proportion of right-handed participants. To address this difference between the groups, analyses were re-run with just right-handed participants from both groups and the results were largely the same. Thus, all participants were included in primary analyses regardless of handedness.
Table 1.
Demographic information about Down syndrome and typically developing control groups
| Down syndrome (N = 31) |
Control (N = 45) |
||||||
|---|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | Stat. significance | |
| Age | 15.18 | 5.64 | 5–24 | 15.72 | 5.87 | 5–24 | n.s. |
| IQ | 54.26 | 14.32 | 24–92 | 116.47 | 15.33 | 85–151 | t(74) = −17.9, P < 0.001 |
| Verbal IQ | 54.07 | 16.37 | 29–93 | 116.14 | 15.21 | 82–156 | t(73) = −16.9, P < 0.001 |
| Nonverbal IQ | 57.81 | 16.74 | 24–95 | 112.7 | 15.8 | 81–151 | t(73) = −14.52, P < 0.001a |
| Hollingshead SES | 42.23 | 14.17 | 20–63 | 37.91 | 22.95 | 20–134 | n.s. |
| n | % | n | % | Stat. significance | |||
| Male, n (%) | 14 | 45 | 22 | 49 | n.s. | ||
| Right hand, n (%) | 15 | 48 | 32 | 73 | χ2 (1) = 4.6, P < 0.05 | ||
| Caucasian, n (%) | 23 | 74 | 27 | 60 | n.s.b | ||
aDown syndrome, n = 31; Control, n = 44.
bDown syndrome, n = 30; Control, n = 38.
Procedures
Intelligence Testing
Participants completed developmentally appropriate testing to estimate overall intellectual abilities. For participants under the age of 18, the Differential Ability Scales, Second Edition (Elliott 2007) was administered. For participants 18 and older, the Kaufman Brief Intelligence Test, Second Edition (Kaufman and Kaufman 2004) was administered. Both tests have good to excellent test–retest reliability. In addition, criterion validity was established for the 2 instruments by the publishers by comparing scores of TD participants with those found for groups with prior diagnoses of intellectual or other learning disabilities. Results of these evaluations suggested that both tests were sensitive to detecting cognitive differences in these groups (as reflected in lower scores by the intellectual and learning disabilities groups). Moreover, both tests have been found to have moderate to high external validity (as evaluated relative to the Wechsler scales, for example).
MRI Scan Acquisition and Data Processing
Imaging was completed without sedation on the same 3-Tesla General Electric Scanner using an 8-channel head coil. High-resolution (0.94 × 0.94 × 1.2 mm) T1-weighted images were acquired utilizing an ASSET-calibrated magnetization prepared rapid gradient echo sequence (128 slices; 224 × 224 acquisition matrix; flip angle = 12°; field of view [FOV] = 240 mm). Tissue classification and CT/SA measurements were completed using Montreal Neurological Institute's automated Civet pipeline. This pipeline first registers the MRI scans into standardized stereotaxic space and corrects for nonuniformity artifacts (Sled et al. 1998) using a linear transformation (Collins et al. 1994). Then tissue is classified into gray or white matter, cerebrospinal fluid, or background with a neural net classifier (Zijdenbos et al. 2002). Subsequently, the inner (i.e., white matter) and outer (i.e., pial) cortical surfaces are extracted using deformable surface-mesh models (MacDonald et al. 2000; Kim et al. 2005). These are then aligned nonlinearly toward a standard template surface (Robbins et al 2004).
CT was calculated by measuring the linked distance between the white and pial surfaces (t-link metric) in native space (MacDonald et al. 2000; Lerch and Evans 2005) at 40 962 vertices in each hemisphere. A 20-mm surface-based diffusion-smoothing kernel (Chung et al. 2003) was utilized. Cortical SA was measured at 40 962 vertices along the middle cortical surface, which is the geometric center between the pial and white matter surfaces. The middle cortical surface offers some advantages over measuring either the pial or white matter surface, as it is thought to provide “…a relatively unbiased representation of sulcal versus gyral regions” (Im et al. 2008, p. 2183). SA was measured at each vertex by averaging the SA of 6 triangles surrounding a particular vertex (Jubault et al. 2011). A 40-mm smoothing kernel was utilized for vertex-level SA measurements.
Statistical Analysis
Prior to conducting primary analyses focused on group differences in CT and SA, 3 steps were taken. First, the effects of age were evaluated in order to identify how best to model age in the current analyses. Second, the effects of sex and age on volume, SA, and CT measurements were evaluated in order to determine if there were any statistically significant group differences in these factors (i.e., sex × group or age × group interactions). Third, whole-brain, cortical, and lobar-level volumetric analyses were completed in order to permit comparison of the current study's results to prior pediatric investigations. Finally, analyses to answer primary study questions were completed.
The effects of age on brain morphometry were evaluated separately in each group in order to determine if a linear or quadratic age function best fit volumetric, SA, and CT data. In general, linear age effects appeared to capture the data best. This was not surprising, given the small sample size in the current investigation and the fact that studies that have tended to identify quadratic (or cubic) age effects on different morphometric characteristics of the developing brain have tended to use longitudinal study designs with many more participants (e.g., Shaw et al. 2008; Raznahan et al. 2011). Thus, for all analyses, linear age (centered) was the only age term included.
Following this step, group differences in age and sex effects on brain morphometry were evaluated for whole-brain and lobar-level volumetric measurements, total SA, mean CT, and for vertex-level measurements of cortical SA and thickness. These effects were evaluated with linear regression utilizing the following equations: Sex: Morphometric measurement ∼ intercept + β1 (sex) + β2 (age centered) + β3 (group) + β4 (sex × group). Age: Morphometric measurement ∼ intercept + β1 (sex) + β2 (age centered) + β3 (group) + β4 (age centered × group).
There were no sex × group or age × group interactions that survived false discovery rate (FDR; Benjamini and Hochberg 1995) correction for multiple comparisons (q < 0.05). Use of a relaxed statistical threshold without correction for multiple comparisons (nominal P < 0.05) identified 3 foci of difference in age-related anatomical variation between the DS and control groups (see Supplementary Fig. 1). As a result of these preliminary analyses, additive models for group differences were utilized for all subsequent analyses with the following regression equation: Morphometric measurement ∼ intercept + β1 (sex) + β2 (age centered) + β3 (group).
Lastly, because of differences in total brain volume and physical height between the groups (and the fact that recent research suggests a correlation between height and both brain volumes and intelligence; Taki et al. 2012), supplementary analyses were completed that added total brain volume and height as covariates.
Results
Volumetric Differences in Brain Morphometry
Consistent with prior investigations, the results of linear regression analyses revealed a significant effect of group on total brain volume, gray and white matter volumes, and cortical gray matter volume (see Table 2). Specifically, cortical gray matter volume was reduced significantly (7% reduction). Moreover, lobar-level volumetric analyses revealed reduced frontal, temporal, and occipital gray matter volumes, but similar parietal gray matter volume relative to the TD group (replicating prior investigations).
Table 2.
Total and regional morphometric measurements for Down syndrome and typically developing controls
| Down syndrome (n = 31) |
Typical dev. control (n = 45) |
||||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | % Diff. | D | Fa | |
| Volume measures (cm3) | |||||||
| ICV (GM + WM + CSF) | 1232.39 | (121.68) | 1394.65 | (113.7) | −11.63 | −1.43 | F1,72 = 52.63, P < 0.001b |
| TBV (GM + WM) | 1125.45 | (111.96) | 1276.39 | (108.33) | −11.83 | −1.39 | F1,72 = 49.04, P < 0.001b |
| GM | 725.03 | (63.41) | 797.19 | (77.29) | −9.05 | −0.93 | F1,72 = 30.06, P < 0.001b |
| Cortical GM | 544.34 | (52.82) | 586.27 | (60.23) | −7.15 | −0.70 | F1,72 = 16.45, P < 0.001b,c,d,* |
| Frontal GM | 193.38 | (19.58) | 217.71 | (23.33) | −11.18 | −1.04 | F1,72 = 36.82, P < 0.001b |
| Parietal GM | 105.82 | (12.79) | 108.55 | (14.55) | −2.51 | −0.19 | F1,72 = 1.26, P > 0.26c,d,* |
| Temporal GM | 179.88 | (16.88) | 191.68 | (16.96) | −6.16 | −0.70 | F1,72 = 11.92, P < 0.001b,c,d,* |
| Occipital GM | 65.26 | (7.88) | 68.34 | (10.29) | −4.51 | −0.30 | F1,72 = 3.82, P < 0.05c,d,* |
| WM | 400.42 | (68.33) | 479.2 | (61.36) | −16.44 | −1.28 | F1,72 = 48.35, P < 0.001b |
| Cortical WM | 333.53 | (55.01) | 387.22 | (48.41) | −13.87 | −1.11 | F1,72 = 33.14, P < 0.001b |
| Frontal WM | 133.06 | (21.95) | 165.81 | (20.15) | −19.75 | −1.63 | F1,72 = 71.46, P < 0.001b,c,d |
| Parietal WM | 78.06 | (13.27) | 86.46 | (12.03) | −9.72 | −0.70 | F1,72 = 12.04, P < 0.001b,c,d,* |
| Temporal WM | 86.61 | (15.47) | 94.13 | (12.32) | −7.99 | −0.61 | F1,72 = 7.55, P < 0.01b,c,d,* |
| Occipital WM | 35.79 | (6.45) | 40.82 | (6.47) | −12.32 | −0.78 | F1,72 = 14.68, P < 0.001b |
| Cerebrospinal fluid | 106.93 | (23.71) | 118.26 | (24.98) | −9.58 | −0.45 | F1,72 = 4.40, P < 0.05b |
| Total surface area (cm2) | 1607.65 | (145.67) | 1832.96 | (134.88) | −12.29 | −1.67 | F1,72 = 61.67, P < 0.001b,c |
| Mean cortical thickness (mm) | 3.59 | (0.19) | 3.45 | (0.17) | 4.06 | 0.82 | F1,72 = 15.06, P < 0.001b,c,d |
ICV, intracranial volume; TBV, total brain volume; GM, gray matter; WM, white matter; D, Cohen's d.
aF-value (and corresponding unadjusted P-value) is for model that includes sex and centered age as covariates; note, however, that the groups means and SDs in the table are unadjusted.
bSurvives FDR correction, q < 0.05 (with sex and centered age in the model).
cSurvives FDR correction, q < 0.05 (with sex, centered age, and total GM + WM in the model).
dSurvives FDR correction, q < 0.05 (with sex, centered age, total GM + WM and height in the model; note missing data on 7 participants).
*For these findings, controlling for total GM + WM or total GM + WM and height, the estimated marginal means are actually larger in the DS than control groups, unlike the adjusted values.
To compare the magnitude of the lobar-level gray matter reductions in the DS group, raw lobar volumes were converted to z-scores (using the control group mean and standard deviation [SD] as a reference) and a within-subject analysis of variance (ANOVA) was run for the DS group only. A significant effect of lobe was found (F3,90 = 29.0, P < 0.001). Pairwise comparisons revealed that the frontal lobe was the most reduced (Ps < 0.01), followed by the temporal lobe (which was more reduced than the parietal and occipital lobes; Ps < 0.01). The parietal and occipital lobe z-scores did not differ significantly from one another (P = 0.24).
Lastly, given the reduction in total brain volume in DS as well as physical height differences between the groups (in inches, DS M = 55; SD = 6; Control M = 62; SD = 9), results of raw lobar comparisons were re-run with both total brain volume (gray and white matter total) and height included as additional covariates. See Table 2.
Dissociations in Cortical Thickness and Surface Area in DS
QUESTION 1. Do CT reductions, SA reductions, or both morphological features contribute to the reduction in cortical gray matter volume in DS?
Linear regression analyses revealed significant effects of group for both total SA (F1,72 = 61.67, P < 0.001) and mean CT (F1,72 = 15.06, P < 0.001). However, there was a dissociation in the direction of the effects—namely, cortical SA was reduced by 12% in DS while CT was increased by 4%. Both of these effects were “large” in magnitude using Cohen's d (Cohen 1988); see Table 2) and suggest that cortical SA (and not thickness) reduction is driving the cortical volume reduction found here and in other investigations of DS.
QUESTION 2: At the level of the vertex, is there regional specificity to differences in SA and CT?
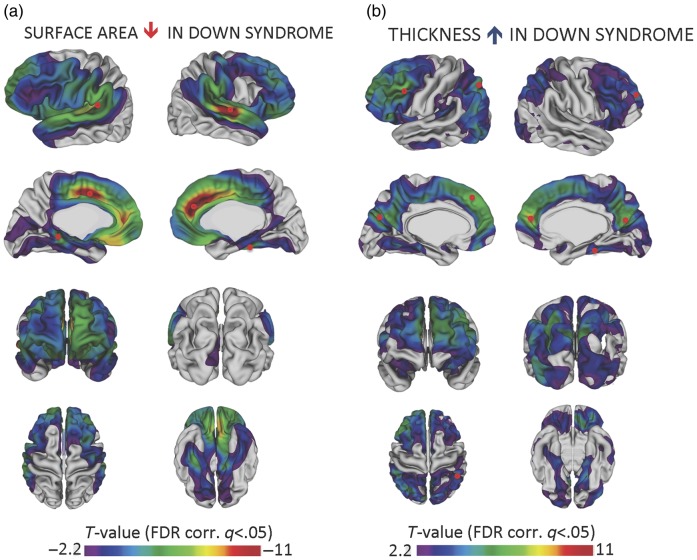
To provide a more refined spatial description of SA and CT differences, a series of regression analyses were completed at 40 962 vertices in each hemisphere (using the regression equation described in the Statistical Analysis section above) and an FDR adjustment was applied (q < 0.05) to control for multiple comparisons. Results are summarized in Figure 1. (Effect size estimates at the vertex level can be viewed in Supplementary Fig. 2.) Significant reductions in cortical SA were identified primarily in the frontal and temporal lobes, consistent with volumetric findings in which these were the 2 regions of greatest reduction. With regard to CT, increases were noted in the frontal, parietal, and occipital lobes. In contrast, largely similar thickness values were found on the lateral surface of the temporal lobes (and there were also 2 small regions in which the DS group had thinner cortex relative to controls on the medial surface of the temporal lobes).
Figure 1.
Reductions in cortical surface area and increases in cortical thickness in DS relative to typical controls. A series of regression analyses were completed at 40 962 vertices in each hemisphere using the following regression equation: Cortical thickness or surface area (vertexj) ∼ intercept + β1(Sex) + β2 (age centered) + β3 (group). T-statistics associated with the group term that exceeded the FDR-corrected threshold of ±2.2 were projected onto the cortex. Panel (a) displays surface area findings; panel (b) displays cortical thickness findings. Note that the range of T-values for surface area is negative 2.2 to negative 11 while the range for thickness is positive 2.2 to positive 11. This is because DS is associated with largely decreased surface area relative to controls but largely increased cortical thickness. To identify regions in which cortical surface area and thickness were most deviant from controls, the t-scores associated with the group term in the regression equation were ranked and those vertices above the 90th percentile for thickness and below the 10th percentile for surface area were identified. The approximate locations of the peak vertices within these regions are marked with small red circles in panels (a) and (b).
To identify regions in which SA and CT were most deviant from controls, the t-scores associated with the group term in the regression equation were ranked and those vertices above the 90th percentile for thickness and below the 10th percentile for SA were identified. The peak vertices within these regions are noted with small red circles in Figure 1. Vertices in the dorsal medial frontal lobe and cingulate were above the 90th percentile for thickness and below the 10th percentile for SA. For SA, the other region of great reduction was the superior temporal gyrus. For thickness, vertices in the parietal and lateral frontal lobes were also ranked above the 90th percentile for greater thickness relative to controls.
As a follow-up to primary analyses, vertex-level analyses were then re-run with total brain volume (total gray matter + white matter) and physical height covaried (see Supplementary Fig. 3). With these additional covariates, the SA reductions were largely reduced to the superior temporal gyrus, medial frontal lobe, and cingulate (regions which were the most reduced as described above). Moreover, a portion of the superior parietal lobe and inferior temporal gyrus were increased relative to controls. In contrast, the CT findings remained largely unchanged (if not slightly strengthened and more extensive) with these additional covariates. This pattern of findings—that is, SA being significantly changed with these covariates and thickness being relatively preserved—is not surprising, given the much stronger relationship between total brain volume and total SA (in DS and control groups, Pearson's rs = 0.92) than between total brain volume and mean thickness (in DS group, Pearson's r = 0.14; in the control group, Pearson's r = 0.36).
Discussion
The current study sought to delineate further the nature of previously reported cortical volume reductions in DS by examining the 2 ontogenetically and phylogenetically distinct structural determinants of the cerebral cortex—SA and CT. In the first report of its kind with the largest pediatric sample studied to date, we describe dissociations in the thickness and SA of the cortex in youth with DS. Specifically, significant SA reductions were observed alongside increases in CT at the global and vertex level relative to sex- and age-matched TD peers. Statistically significant SA reductions in DS were localized to the frontal and temporal lobes, with the most prominent reductions in the medial frontal and cingulate cortex as well as the superior temporal gyrus. With regard to CT increases, the frontal lobes were again implicated. However, unlike SA findings, thickness increases were also noted in large swaths of the parietal and occipital lobes. In contrast, the majority of the vertices measured on the lateral surface of the temporal lobe did not differ with respect to CT from controls. When the increase in the thickness of regions of the parietal lobes is evaluated in relation to the SA findings (i.e., no statistically significant reductions), it is not surprising that prior investigations have reported “sparing” of the parietal lobes in DS. However, by dissociating these 2 aspects of cortical morphology, we detected deviations in CT that may relate to aspects of the DS cognitive phenotype that have been ignored previously.
Interpreting Findings Within the Context of Typical Brain Development
How do the current findings for DS fit within the context of typical developmental trajectories? Unfortunately, our cross-sectional study design and sample size preclude a direct test of this question. However, it is interesting to note that, in our DS group with a mean age of 16 years, we found thicker cortex in regions that are characterized by pronounced thinning during adolescence for typical youth (Shaw et al. 2008; Raznahan et al. 2011; Wierenga et al. 2014; Vandekar et al. 2015). In contrast, the single large swath of cortex in which the DS group did not have significantly thicker cortex than controls, the lateral surface of the temporal lobes, is the only brain region characterized by cortical thickening in early adolescence in typical youth (Vandekar et al. 2015).
These findings could suggest a delay in cortical thinning in DS. However, the data are also congruent with a “static” difference between the DS and TD groups, such that a largely thicker cortex is present in childhood and persists into adolescence in individuals with DS. To compare these models would require longitudinal data, with a greater sampling of younger ages in particular. Lastly, while we cannot speak to the developmental origins of the increased CT found here for DS, we note that the thinning of the cortex observed in typical youth is thought to contribute to the maturation of cognitive abilities from late childhood and into young adulthood (e.g., Squeglia et al. 2013). From this perspective, it may be the case that having a thicker cortex is disadvantageous, as the development of higher level cognitive abilities may be dependent on a thinner cortex that maintains only the most essential connections necessary for efficient neural communication (i.e., one characterized by effective synaptic pruning among other factors; Petanjek et al. 2011).
Interpreting Findings Within the Context of Other Intellectual Disability Syndromes
Turning to the existing literature on the developing cortex in youth with other ID syndromes, we find mixed results for both CT and SA. For example, in work done examining CT in idiopathic ID, one paper reported reductions, particularly in inferior and medial regions of the temporal lobe (Zhang et al. 2011). This contrasts with findings for Fragile X syndrome, in which cortical volume and thickness increases have been observed alongside similar SA measurements (Meguid et al. 2012). The general pattern of findings observed here for DS (i.e., regions of SA reduction and increased CT) is most consistent with findings reported for Williams syndrome (Meda et al. 2012) in which there is evidence for regions of reduced cortical volume and SA in the face increases in CT.
Thus, across 4 groups of youth with ID, different patterns for CT and SA deviations emerge, underscoring the fact that the path from brain morphology to complex behavioral phenotypes, such as ID, is not a direct one. Furthermore, given the differences in the neuropsychological profiles of these disorders, it is expected that the path from brain morphology to cognition would be somewhat different. However, we hope that this points to the need for future research to compare directly the neuroanatomic characteristics of youth with different ID syndromes in order to identify phenotypically similar and divergent morphometric features that may be contributing to the differences and similarities in the cognitive profiles associated with these disorders. Such research may identify new targets of study for animal models of ID syndromes, permitting a controlled examination of the path from genetic abnormalities to brain abnormalities. This may, in turn, lead to novel biomedical treatment approaches to correct these abnormalities based on the genetic etiology of ID. In addition, by identifying shared morphometric features across different genetic forms of ID, scientists may be able to identify shared molecular abnormalities that could be the target of treatment for a number of different forms of ID.
Interpreting Findings with Reference to the DS Cognitive Phenotype
The foci of maximal SA alteration in DS, regions of the superior temporal gyrus and the dorsal medial prefrontal cortex/dorsal anterior cingulate, have been found in studies of TD individuals to be associated with phonological processing/speech perception abilities (Binder et al. 2000; Mesgarani et al. 2014) and effortful control/inhibition (Bush et al. 2000), respectively. These cognitive domains are noteworthy given neuropsychological evidence of significant weaknesses in phonological processing (Jarrold et al. 1999; Lee et al. 2010) and cognitive inhibition (Borella et al. 2013) in DS.
A similar convergence is noted between the literature on brain–behavior relations in chromosomally typical populations and the pattern of CT deviations found here for DS. Specifically, several of the most structurally deviant regions of CT in DS overlap with nodes in the default mode network (DMN; Raichle and Snyder 2007). This convergence is particularly noteworthy for DS, a disorder associated with the precocious onset of Alzheimer's disease (Zigman and Lott 2007), as research links dysfunction in the DMN to Alzheimer's symptomatology in chromosomally typical older adults. Specifically, studies have shown that reductions in functional connectivity in the DMN may help distinguish healthy aging from that found in Alzheimer's disease (Greicius et al. 2004). Moreover, research has linked amyloid deposition to impaired DMN activity in dementia (Sperling et al. 2009). Given the rates of elevated amyloid in adults with DS (Head and Lott 2004), it is interesting to note correlations between DMN activity and amyloid in chromosomally typical adults with dementia. Thus, the deviations in CT in these regions in DS may be particularly relevant to our understanding of the precocious onset of Alzheimer's disease in this at-risk population.
An important topic for future research will be to examine the extent to which anatomical atypicality within these regions correlates with the severity of the neuropsychological phenotype in DS. While crucial for our understanding of brain–behavior relations in DS, this research is not without its challenges, which include the need to maximize sample size while minimizing age-related anatomical variation. Thus, future research may benefit from oversampling participants from a particular age range in order to begin to test directly relations between atypical brain anatomy and neuropsychological functioning in DS.
Limitations
While the current study reports on the largest pediatric sample with DS examined to date using structural MRI, the sample size and large age range precluded a rigorous investigation of brain–behavior correlations, an important topic for future research. Moreover, given the sample size, we were not powered to detect group differences that were smaller in magnitude. Thus, we draw the reader's attention to Supplementary Figure 2 to examine effect size maps for CT and SA, as these display the range of group differences including small to medium effects.
Given that the current study included TD individuals as a comparison group, we were unable to test the syndromic specificity of these findings (i.e., we could not evaluate if the deviations in cortical anatomy observed here are specific to DS or if they reflect the nonspecific effects of ID). Future investigations may benefit from comparing individuals with DS with those with other forms of ID in order to test the specificity of these findings. As stated earlier, this research may provide important targets of study for animal models of DS that have greater experimental control in their investigations of the pathway from genotype to phenotype. With these advances, it is hoped that the field will get closer to identifying more effective biomedical interventions to lessen the impact of disability on individuals with DS and their families.
Conclusions
In closing, the current investigation provides further evidence for atypical cortical development in youth with DS. To the best of our knowledge, this is the first study to report dissociations in SA and CT in this group. The current findings underscore the need to examine these 2 structural determinants of cortical anatomy separately in DS, as these deviations may have been obscured in other studies that have focused on cortical volume in this group. Moreover, by describing SA and CT in this young sample, we hope to lay a foundation for future studies seeking to understand which aspects of the phenotype may be most vulnerable in individuals who go on to develop Alzheimer's disease later in life.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Mental Health (1 ZIA MH002794-13; Protocol ID 89-M-0006).
Supplementary Material
Notes
We thank the families who made this research possible. Conflict of Interest: None declared.
References
- Antonarakis SE, Epstein CJ. 2006. The challenge of Down syndrome. Trends Mol Med. 12:473–479. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Habbak R, Warren AC, Pulsifer MB, Barta PE, Jerram M, Pearlson GD. 1997. Cerebellar volume in adults with Down syndrome. Arch Neurol. 54:209–212. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Jarrold C. 2007. Working memory and Down syndrome. J Intellect Disabil Res. 51:925–931. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B (Methodological). 57:289–300. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, Possing ET. 2000. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 10:512–528. [DOI] [PubMed] [Google Scholar]
- Borella E, Carretti B, Lanfranchi S. 2013. Inhibitory mechanisms in Down syndrome: is there a specific or general deficit? Res Dev Disabil. 34:65–71. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 4:215–222. [DOI] [PubMed] [Google Scholar]
- Carducci F, Onorati P, Condoluci C, Di Gennaro G, Quarato PP, Pierallini A, Sara M, Miano S, Cornia R, Albertini G. 2013. Whole-brain voxel-based morphometry study of children and adolescents with Down syndrome. Funct Neurol. 28:19–28. [PMC free article] [PubMed] [Google Scholar]
- Carr J. 1970. Mental and motor development in young mongol children. J Ment Defic Res. 14:205–220. [DOI] [PubMed] [Google Scholar]
- Chung MK, Worsley KJ, Robbins S, Paus T, Taylor J, Giedd JN, Rapoport JL, Evans AC. 2003. Deformation-based surface morphometry applied to gray matter deformation. Neuroimage. 18:198–213. [DOI] [PubMed] [Google Scholar]
- Cohen J. 1988. Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale: (NJ: ): Erlbaum. [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. 1994. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 18:192–205. [PubMed] [Google Scholar]
- Dodd B. 1975. Recognition and reproduction of words by Down's syndrome and non-Down's syndrome retarded children. Am J of Ment Defic. 80:306–311. [PubMed] [Google Scholar]
- Elliott CD. 2007. The Differential Ability Scales, 2nd ed. San Antonio: (TX: ): The Psychological Corporation. [Google Scholar]
- Fowler AE, Gelman R, Gleitman LA. 1994. The course of language learning in children with Down syndrome: longitudinal and language level comparisons with young normally developing children. In: Tager-Flusberg H, editor. Constraints on language acquisition. Hillsdale: (NJ: ): Erlbaum; pp. 91–140. [Google Scholar]
- Frangou S, Aylward E, Warren A, Sharma T, Barta P, Pearlson G. 1997. Small planum temporale volume in Down's syndrome: a volumetric MRI study. Am J Psychiatry. 154:1424–1429. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. 2004. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E, Lott IT. 2004. Down syndrome and beta-amyloid deposition. Curr Opin Neurol. 17:95–100. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. 1958. Social class and mental illness. New York: (NY: ): Wiley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI. 2008. Brain size and cortical structure in the adult human brain. Cereb Cortex. 18:2181–2191. [DOI] [PubMed] [Google Scholar]
- Jarrold C, Baddeley AD, Phillips C. 1999. Down syndrome and the phonological loop: the evidence for, and importance of, a specific verbal short-term memory deficit. Downs Syndr Res Pract. 6:61–75. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Bellugi U. 1990. Anomalous brain morphology on magnetic resonance images in Williams syndrome and Down syndrome. Arch Neurol. 47:529–533. [DOI] [PubMed] [Google Scholar]
- Jubault T, Gagnon JF, Karama S, Ptito A, Lafontaine AL, Evans AC, Monchi O. 2011. Patterns of cortical thickness and surface area in early Parkinson's disease. Neuroimage. 55:462–467. [DOI] [PubMed] [Google Scholar]
- Kates WR, Folley BS, Lanham DC, Capone GT, Kaufmann WE. 2002. Cerebral growth in Fragile X syndrome: review and comparison with Down syndrome. Microsc Res Tech. 57:159–167. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. 2004. Kaufman Brief Intelligence Test, 2nd ed San Antonio, TX: Pearson. [Google Scholar]
- Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab'bagh Y, MacDonald D, Lee JM, Kim SI, Evans AC. 2005. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 27:210–221. [DOI] [PubMed] [Google Scholar]
- Lee NR, Pennington BF, Keenan JM. 2010. Verbal short-term memory deficits in Down syndrome: phonological, semantic, or both? J Neurodev Disord. 2:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune J, Gauthier M, Turpin R. 1959. Human chromosomes in tissue cultures. C R Hebd Seances Acad Sci. 248:602–603. [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, et al. 2007. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 36:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Evans AC. 2005. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 24:163–173. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Kabani N, Avis D, Evans AC. 2000. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 12:340–356. [DOI] [PubMed] [Google Scholar]
- Meda SA, Pryweller JR, Thornton-Wells TA. 2012. Regional brain differences in cortical thickness, surface area and subcortical volume in individuals with Williams syndrome. PLoS One. 7:e31913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguid NA, Fahim C, Sami R, Nashaat NH, Yoon U, Anwar M, El-Dessouky HM, Shahine EA, Ibrahim AS, Mancini-Marie A, et al. 2012. Cognition and lobar morphology in full mutation boys with fragile X syndrome. Brain Cogn. 78:74–84. [DOI] [PubMed] [Google Scholar]
- Menghini D, Costanzo F, Vicari S. 2011. Relationship between brain and cognitive processes in Down syndrome. Behav Genet. 41:381–393. [DOI] [PubMed] [Google Scholar]
- Mesgarani N, Cheung C, Johnson K, Chang EF. 2014. Phonetic feature encoding in human superior temporal gyrus. Science. 343:1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, et al. 2009. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 19:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, et al. 2010. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 88:1008–1016. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Breiter SN, Aylward EH, Warren AC, Grygorcewicz M, Frangou S, Barta PE, Pulsifer MB. 1998. MRI brain changes in subjects with Down syndrome with and without dementia. Dev Med Child Neurol. 40:326–334. [PubMed] [Google Scholar]
- Pennington BF, Moon J, Edgin J, Stedron J, Nadel L. 2003. The neuropsychology of Down syndrome: evidence for hippocampal dysfunction. Child Dev. 74:75–93. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Šimic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. 2011. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 108:13281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter JD, Brown WE, Eliez S, Schmitt JE, Capone GT, Reiss AL. 2001. Amygdala and hippocampal volumes in children with Down syndrome: a high-resolution MRI study. Neurology. 56:972–974. [DOI] [PubMed] [Google Scholar]
- Pinter JD, Eliez S, Schmitt JE, Capone GT, Reiss AL. 2001. Neuroanatomy of Down's syndrome: a high-resolution MRI study. Am J Psychiatry. 158:1659–1665. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. 2007. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 37:1083–1090. [DOI] [PubMed] [Google Scholar]
- Rakic P. 1995. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 18:383–388. [DOI] [PubMed] [Google Scholar]
- Raz N, Torres IJ, Briggs SD, Spencer WD, Thornton AE, Loken WJ, Gunning FM, McQuain JD, Driesen NR, Acker JD. 1995. Selective neuroanatomical abnormalities in Downs-syndrome and their cognitive correlates—evidence from MRI morphometry. Neurology. 45:356–366. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Cutter W, Lalonde F, Robertson D, Daly E, Conway GS, Skuse DH, Ross J, Lerch JP, Giedd JN, et al. 2010. Cortical anatomy in human X monosomy. Neuroimage. 49:2915–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN. 2011. How does your cortex grow? J Neurosci. 31:7174–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins S, Evans AC, Collins DL, Whitesides S. 2004. Tuning and comparing spatial normalization methods syndrome. Med Image Anal. 8:311–323. [DOI] [PubMed] [Google Scholar]
- Shaw P, Gogtay N, Rapoport J. 2010. Childhood psychiatric disorders as anomalies in neurodevelopmental trajectories. Hum Brain Mapp. 31:917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, et al. 2008. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 28:3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein AB, Legutski G, Friedman SL, Tayakama DL. 1992. Performance of Down syndrome individuals on the Stanford-Binet intelligence scale. Am J Ment Defic. 86:548–551. [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. 1998. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 17:87–97. [DOI] [PubMed] [Google Scholar]
- Smigielska-Kuzia J, Bockowski L, Sobaniec W, Sendrowski K, Olchowik B, Cholewa M, Lukasiewicz A, Lebkowska U. 2011. A volumetric magnetic resonance imaging study of brain structures in children with Down syndrome. Neurol Neurochir Pol. 45:363–369. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, et al. 2009. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 63:178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Sorg SF, Jernigan TL, Tapert SF. 2013. Early adolescent cortical thinning is related to better neuropsychological performance. J Int Neuropsychol Soc. 19:962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y, Hashizume H, Sassa Y, Takeuchi H, Asano M, Asano K, Kotozaki Y, Nouchi R, Wu K, Fukuda H, et al. 2012. Correlation among body height, intelligence, and brain gray matter volume in healthy children. Neuroimage. 59:1023–1027. [DOI] [PubMed] [Google Scholar]
- Vandekar SN, Shinohara RT, Raznahan A, Roalf DR, Ross M, DeLeo N, Ruparel K, Verma R, Wolf DH, Gur RC, et al. 2015. Topologically dissociable patterns of development of the human cerebral cortex. J Neurosci. 35:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S, Verucci L, Carlesimo GA. 2007. Implicit memory is independent from IQ and age but not from etiology: evidence from Down and Williams syndromes. J Intellect Disabil Res. 51:932–941. [DOI] [PubMed] [Google Scholar]
- Wang PP, Bellugi U. 1994. Evidence from two genetic syndromes for a dissociation between verbal and visual-spatial short-term memory. J Clin Exp Neuropsychol. 16:317–322. [DOI] [PubMed] [Google Scholar]
- White NS, Alkire MT, Haier RJ. 2003. A voxel-based morphometric study of nondemented adults with Down syndrome. Neuroimage. 20:393–403. [DOI] [PubMed] [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S. 2014. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 87:120–126. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. 2010. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 53:1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wu Y, Zhu M, Wang C, Wang J, Zhang Y, Yu C, Jiang T. 2011. Reduced cortical thickness in mental retardation. PLoS One. 6:e29673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman WB, Lott IT. 2007. Alzheimer's disease in Down syndrome: neurobiology and risk. Ment Retard Dev Disabil Res Rev. 13:237–246. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. 2002. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 21:1280–1291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.