Abstract
Children with fetal alcohol spectrum disorders (FASD) may exhibit craniofacial dysmorphology, neurobehavioral deficits, and reduced brain volume. Studies of cortical thickness in FASD have yielded contradictory findings, with 3 reporting thicker cerebral cortex in frontal and temporal brain regions and 2 showing thinner cortex across multiple regions. All 5 studies included subjects spanning a broad age range, and none have examined continuous measures of prenatal alcohol exposure. We investigated the relation of extent of in utero alcohol exposure to cortical thickness in 78 preadolescent children with FASD and controls within a narrow age range. A whole-brain analysis using FreeSurfer revealed no significant clusters where cortical thickness differed by FASD diagnostic group. However, alcohol dose/occasion during pregnancy was inversely related to cortical thickness in 3 regions—right cuneus/pericalcarine/superior parietal lobe, fusiform/lingual gyrus, and supramarginal/postcentral gyrus. The effect of prenatal alcohol exposure on IQ was mediated by cortical thickness in the right occipitotemporal region. It is noteworthy that a continuous measure of maternal alcohol consumption during pregnancy was more sensitive than FASD diagnosis and that the effect on cortical thickness was most evident in relation to a measure of maternal binge drinking.
Keywords: cortical thickness, development, fetal alcohol spectrum disorders, fetal alcohol syndrome, IQ
Introduction
Fetal alcohol spectrum disorders (FASD) encompass a range of physical and neurobehavioral abnormalities. Fetal alcohol syndrome (FAS), the most severe of the FASD, is the most common preventable cause of mental retardation. The highest rates of FAS, ranging from 68 to 89.2 per 1000 live births, have been reported among the Cape Colored (mixed ancestry) community in the Western Cape Province, South Africa (May et al. 2007). Children with FASD may exhibit a characteristic pattern of craniofacial dysmorphology, neurobehavioral deficits, and reduced overall brain volume (Johnson et al. 1996; Swayze et al. 1997; Mattson and Riley 1998; Archibald et al. 2001; Jacobson, Jacobson, et al. 2011; Lebel et al. 2008; Li et al. 2008; Willoughby et al. 2008; Norman et al. 2009). Regional volume reductions are especially pronounced in the frontal, temporal, and parietal lobes and cerebellum (Archibald et al. 2001; Sowell et al. 2002; Astley et al. 2009). In addition to structural brain abnormalities, microcellular and neurochemical alterations of the central nervous system are found (Norman et al. 2009; du Plessis et al. 2014). Neurobehavioral dysfunction, including poorer IQ, hyperactivity, and deficits in attention, verbal learning, arithmetic, and eyeblink conditioning may also occur in individuals with heavy prenatal alcohol exposure who do not have the physical or facial malformations required for a diagnosis of FAS (Streissguth, Sampson et al. 1994; Mattson and Riley 1998; Jacobson et al. 2004; Willford et al. 2004; Jacobson, Stanton, et al. 2008; Suttie et al. 2013).
During typical development from childhood to adolescence, the brain undergoes substantial change in structure and higher cognitive functions. Total brain volume increases with age; white matter volume continues to increase in adulthood, whereas gray matter volume begins to decline during adolescence (Giedd et al. 1999; Thompson et al. 2005). Maturation-related changes in cortical volume and thickness follow a curvilinear trajectory with a period of initial childhood increase and a subsequent period of rapid cortical thinning during adolescence (Giedd 2004; Shaw et al. 2008; Tamnes et al. 2010), although some studies have found a decrease in cortical thickness throughout childhood (Brown et al. 2012; Zhou et al. 2015), with an accelerated decrease in adolescence (Zhou et al. 2015). Most brain regions attain maximum cortical thickness at the age of 8–10 years (Giedd et al. 1999; Sowell et al. 2003; Shaw, Greenstein, et al. 2006), after which thickness begins to decrease, starting in the visual cortex and posterior temporal lobe, followed by the somatosensory cortex (Muftuler et al. 2011). The cortical thickness of motor and sensory areas continues to decrease through the preadolescent period, whereas thinning in the temporal and frontal lobes occurs later in adolescence (Muftuler et al. 2011; van Soelen et al. 2012). This developmental cortical thinning is thought to be a consequence of regionally specific pruning of overabundant synaptic connections (Huttenlocher and Dabholkar 1997), together with trophic glial and vascular changes and possible cell shrinkage (Gogtay et al. 2004), as well as the expansion of white matter volume via proliferation of myelin into the periphery of the cortex (Benes et al. 1994; Sowell et al. 2004). The rate of change in cortical thickness from childhood to adolescence, rather than the thickness itself, has been found to be related to measures of intelligence (Shaw, Greenstein et al. 2006).
Decreased cortical thickness relative to control subjects is a feature of many neurological and psychiatric disorders, including attention deficit hyperactivity disorder (ADHD) (Shaw, Lerch, et al. 2006; Narr et al. 2009), schizophrenia (Kuperberg et al. 2003; Schultz et al. 2010), epilepsy (Widjaja et al. 2011; Overvliet et al. 2013), post-traumatic stress disorder (Bing et al. 2012; Liu et al. 2012), depression (Grieve et al. 2013; van Tol et al. 2013), 22q11.2 deletion syndrome (Bearden et al. 2007), bipolar disorder (Rimol et al. 2010; Elvsåshagen et al. 2013), and mental retardation (Zhang et al. 2011). Differences in cortical thickness between typically developing children and those with neurodevelopmental disorders may be a consequence of abnormal cortical development during embryogenesis, an altered postnatal developmental trajectory of cortical thickening in childhood or thinning in adolescence, or a combination of these factors. Alternatively, differences in cortical thickness at a particular age may manifest due to a delay in cortical maturation, such that peak thickness is reached later than in typically developing children, as has been found in ADHD (Shaw et al. 2007).
Prenatal exposure to teratogens, such as maternal cigarette smoking (Derauf et al. 2012; El Marroun et al. 2013), cocaine (Liu et al. 2013), opiates (Walhovd et al. 2007), and organosphate pesticide (Rauh et al. 2012) has also been associated with thinner cortex. In animal studies, rats prenatally exposed to alcohol have shown thinner cortex relative to controls (Miller and Dow-Edwards 1988; Norton et al. 1988), particularly in layer V (Kotkoskie and Norton 1989), which persists through multiple postnatal time-points (Leigland et al. 2013), particularly in primary sensory areas. Similarly, in adults one of the effects of excessive alcohol consumption is a reduction in cortical thickness compared with healthy controls (Durazzo and Tosun 2011; Fortier and Leritz 2011; Momenan et al. 2012).
Contrary to evidence from other neurodevelopmental disorders and from animal studies and also at odds with the documented reductions in brain volume found in FASD, 3 studies have reported widespread thickening of the cerebral cortex in subjects with FASD relative to controls (Sowell et al. 2008; Fernández-Jaén et al. 2011; Yang et al. 2012). In contrast, 2 other studies on overlapping groups of subjects, have reported thinner cortex across multiple brain regions in subjects with FASD (Zhou et al. 2011; Treit et al. 2014). The samples in all 5 of these initial studies spanned a broad age range (e.g., 9–16, 8–22, 6–30 years). Given the substantial changes in cortical thickness that occur with age during childhood and adolescence, it is important to examine effects on cortical thickness in subjects within a narrow age range. The previous studies all also compared subjects with FASD versus controls; none compared cortical thickness in individuals with different FASD diagnoses. Because prenatal alcohol exposure was determined retrospectively in these studies, effects of extent and pattern of prenatal exposure on cortical thickness have also not previously been considered. Moreover, although at least one study examined the relation of cortical thickness to cognitive outcomes separately in individuals with and without FASD (Sowell et al. 2008), none examined the degree to which differences in thickness may mediate effects of prenatal alcohol exposure on cognitive function.
In this study, we investigated the relation of in utero alcohol exposure to cortical thickness in 3 groups of children—FAS/partial FAS (PFAS), heavily exposed (HE) nonsyndromal, and controls—across a narrow age range in the preadolescent period. Prospective ascertainment of maternal alcohol consumption during pregnancy enabled us to examine effects of average alcohol intake/day as well as dose/occasion. We used multiple regression to examine the degree to which fetal alcohol effects on IQ appear to be mediated by effects on regional cortical thickness.
Material and Methods
Participants
Participants were 78 children from the Cape Colored (mixed ancestry) community in Cape Town, South Africa, who are taking part in the Cape Town Longitudinal FASD Study (Jacobson, Stanton, et al. 2008). The Cape Colored community is composed primarily of descendants of white European settlers, Malaysian slaves, Khoi-San aboriginals, and black African ancestors. The incidence of FASD in this population is exceptionally high due to poor socioeconomic circumstances and historical practices of compensating farm laborers with wine, which have contributed to a tradition of heavy recreational weekend binge drinking (May et al. 2007). Women were recruited during pregnancy at their first visit to an antenatal clinic. Pregnant women who reported consuming at least 14 drinks/week or engaging in binge drinking during pregnancy were invited to participate. Control subjects were recruited from among women from the same community who abstained or drank no more than minimally (<7 drinks/week and no binge drinking) during pregnancy.
Maternal alcohol consumption was assessed using a timeline follow-back approach (Jacobson et al. 2002; Jacobson, Stanton, et al. 2008). Interviews were conducted with each mother at recruitment and mid-pregnancy to determine amount of alcohol consumed around time of conception and during the first and second trimesters of pregnancy, and at 1 month postpartum to determine alcohol consumption during the third trimester. Volume of each type of beverage consumed each day was recorded and converted to oz of absolute alcohol (AA) using multipliers proposed by Bowman et al. (1975) (liquor, 0.4; beer, 0.04; wine, 0.2; wine coolers, 0.05). Each oz of AA is equivalent to about 2 standard drinks. From these data, 3 continuous measures of prenatal alcohol exposure were calculated: average oz AA consumed/day (AA/day), average oz consumed/drinking day (AA/occasion), and frequency of drinking (days/week). AA/occasion is reflects extent of binge drinking; in the present study, most of the mothers of alcohol-exposed children (86%) averaged 4 or more drinks per occasion (binge drinking). Mothers were also asked how many cigarettes they smoked/day and how many days/week they used marijuana or any other illicit drugs, e.g., methaqualone (“mandrax”), during pregnancy.
Each child was examined for growth and FAS dysmorphology by 2 expert dysmorphologists—H. Eugene Hoyme, MD, and Luther Robinson, MD—following the revised Institute of Medicine criteria (Hoyme et al. 2005) during a 6-day clinic held in September 2005 (Jacobson, Stanton, et al. 2008). There was substantial agreement between the 2 examiners in the assessment of all dysmorphic features, including the 3 principal fetal alcohol-related facial characteristics—palpebral fissure length, philtrum, and vermilion—and between them and a Cape Town-based dysmorphologist—Nathaniel Khaole, MD, who examined a small subset (n = 8) who could not attend the clinic. The dysmorphologists (H.E.H. and L.K.R.), S.W.J., J.L.J., and C.D.M. subsequently conducted case conferences to determine which children met criteria for a diagnosis of FAS or partial FAS (PFAS). FAS was diagnosed if the child exhibited at least 2 of the principal facial features, smaller head circumference, and growth retardation (Hoyme et al. 2005); PFAS, if the facial features were present, heavy maternal drinking during pregnancy was confirmed, and the child exhibited either small head circumference or retarded growth.
The mother and child were transported in our research van to our University of Cape Town Child Development Research Laboratory by the research nurse and study driver. A comprehensive neuropsychological assessment was administered individually to each child, including the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler, 2003). The 10-year WISC-IV IQ scores were strongly correlated (r = 0.77, P < 0.001) with IQ scores obtained at 5 years on the Junior South African Individual Scale (Madge et al. 1981), which is normed for South African children (Jacobson, Stanton, et al. 2011). Each child was also assessed for ADHD following research criteria developed in collaboration with 2 experts in ADHD research—Joel Nigg, PhD, and Rafael Klorman, PhD This diagnosis was based on a maternal interview using the Schedule for Affective Disorders and Schizophrenia for School Aged Children (K-SADS) administered by CDM and ratings by the child's classroom teacher on the Disruptive Behavior Disorders Scale (Pelham et al. 1992). An ADHD classification was assigned if (a) at least 6 of the 9 inattention and/or 6 of the 9 hyperactivity/impulsivity symptoms were endorsed (“pretty much” or “very much true”) by one or more informants, and (b) some impairment was reported by 7 years and in 2 or more settings.
All study procedures were performed according to protocols approved by the Wayne State University Institutional Review Board and the University of Cape Town Faculty of Health Sciences Human Research Ethics Committee. Written informed consent was obtained from each mother at recruitment, at the first laboratory visit, and at the 5- and 10-year follow-up visits, and all children provided oral assent.
Imaging
Mothers and children were transported by our research nurse and driver to the Cape Universities Brain Imaging Centre (CUBIC) for neuroimaging. High-resolution motion-corrected multi-echo MPRAGE images (Van der Kouwe et al. 2008; Tisdall et al. 2011) were acquired from 82 children (age range = 9.5–12.0 years). Images were acquired on a 3 T Allegra scanner (Siemens Medical Systems, Erlangen, Germany) at the Cape Universities Brain Imaging Centre with time repetition = 2530 ms, time echo = 1.53, 3.21, 4.89, 6.57 ms, time to inversion = 1100 ms, FA = 7°, 128 sagittal slices of 1.3 mm thickness, field of view = 256 × 256 mm, resolution = 1.3 × 1.0 × 1.3 mm3.
Image Processing and Analysis
Cortical Thickness Computation
Cortical reconstruction and volumetric segmentation were performed with FreeSurfer (version 5.1.0, http://surfer.nmr.mgh.harvard.edu, Last accessed 10 June 2015). This processing included removal of nonbrain tissue using a hybrid watershed/surface deformation procedure (Ségonne et al. 2004), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (Fischl et al. 2002, 2004), intensity normalization (Sled et al. 1998), tessellation of the gray/white matter boundary, automated topology correction (Fischl et al. 2001; Ségonne et al. 2007), and surface deformation (Dale and Sereno 1993; Dale et al. 1999; Fischl and Dale 2000), which allows the creation of surface maps of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface (Fischl and Dale 2000). FreeSurfer morphometric procedures have been demonstrated to show good test–retest reliability across scanner manufacturers and field strengths (Han et al. 2006). Procedures for the measurement of cortical thickness have been validated against histological analysis (Rosas et al. 2002) and manual measurements (Kuperberg et al. 2003; Salat et al. 2004). Outputs from the automated processing stream were visually inspected, and errors were corrected manually prior to the calculation of cortical thickness.
Whole-Brain Statistical Analysis and ROI Creation
Cortical thickness maps for all subjects were smoothed with a 10 mm FWHM kernel, and vertex-by-vertex general linear model analyses across the whole brain were performed with cortical thickness as the dependent variable and prenatal alcohol exposure as the independent variable. The first model was performed in relation to FASD diagnosis; followed by AA/day, AA/occasion, and then drinking days/week. Results were thresholded at P < 0.05, and cluster-size correction for multiple comparisons was performed using Monte Carlo simulation. The cluster-size threshold for a corrected significance level of P < 0.05 was 745 mm2: only clusters larger than this were retained.
In each cluster that survived the significance threshold after correction for multiple comparisons, the mean cortical thickness for each child was calculated using FreeSurfer. The means in these regions of interest (ROIs) were exported to SPSS for further analysis.
Leave-One-Subject-Out (LOSO) Analysis
Post hoc correlation and regression analyses were then run to further examine the relation of prenatal alcohol exposure to cortical thickness in these regions. Because these analyses provide post hoc estimates of effect size in regions that survive multiple comparison correction, they may give inflated estimates of the true effect size. To produce an additional unbiased estimate of the relation between alcohol exposure and cortical thickness, a leave-one-subject-out (LOSO) analysis (Esterman et al. 2010) was performed by conducting 78 separate regressions of whole-brain cortical thickness on alcohol exposure, leaving a different subject out of the analysis each time. Mean thickness was calculated for each subject in the ROI defined in the regression omitting that subject (P < 0.05, cluster-size corrected at P < 0.05).
Statistical Analyses
Statistical analyses were performed using SPSS (version 21). One-way ANOVA was used to compare mean cortical thickness between the FAS/PFAS, HE and control groups in the 3 ROIs defined in the whole-brain analysis. Post hoc tests were performed using Fisher's LSD test.
Seven control variables were considered as potential confounders: child's sex and age at assessment, as well as maternal education, socioeconomic status (Hollingshead 2011), age at delivery, and smoking (cigarettes/day) and marijuana use (days/month) during pregnancy.
The relation of prenatal alcohol exposure to the mean cortical thickness in each ROI identified in the whole-brain cortical thickness analysis was examined in separate hierarchical multiple regression analyses. Any control variable that was related to mean cortical thickness in an ROI at P < 0.20 was considered a potential confounder (Maldonado and Greenland 1993). Selection of confounding variables was performed using the change-in-estimate method, which produces more reliable models than variable selection based on statistical significance (Greenland 1989; Jacobson, Jacobson, et al. 2008). To determine whether the effect of prenatal alcohol on ROI cortical thickness remained significant after statistical adjustment for confounders, prenatal alcohol exposure was entered in the first step of each analysis. Control variables were entered sequentially into the model in order of the strength of their relation to the cortical thickness measure. The control variable was retained only if its entry into the model altered the standardized regression coefficient associated with prenatal alcohol exposure by at least 10%.
Multiple regression was used to investigate whether the relation of prenatal alcohol exposure to child's IQ was mediated by cortical thickness in each of the ROIs identified in the whole-brain analysis. Mediation was inferred if the addition of the cortical thickness measure to the regression of IQ on prenatal alcohol exposure produced a statistically significant reduction in the raw regression coefficient for alcohol exposure, as assessed using the Clogg et al. (1992) test for mediation.
We performed a t-test in each ROI to determine whether cortical thickness differed between subjects with and without a diagnosis of ADHD. To examine whether the observed effects of alcohol exposure on regional cortical thickness might be attributable to ADHD co-morbidity, we reran the regression analyses adding ADHD to the model.
Exclusion Criteria
The data for one control child were discarded because of image artifacts resulting from excessive motion. One mother whose child met all the diagnostic criteria for FAS denied drinking during pregnancy, and one mother who initially denied drinking reported drinking during pregnancy when interviewed at 5 years postpartum. These 2 children were excluded from analyses relating cortical thickness to the continuous measures of prenatal alcohol exposure. In addition, in the FreeSurfer analyses one HE nonsyndromal subject was excluded as an extreme outlier on AA/occasion, leaving a sample of 78 children for analysis.
Results
Sample Characteristics
After applying exclusion criteria, we report data for 78 children (44 male, mean age 10.7 ± 0.6 years): 28 with FAS or PFAS (10 FAS, 18 PFAS), 28 HE nonsyndromal children, and 22 controls.
Table 1 summarizes the demographic information for the children included in the study. As previously reported ( Jacobson, Stanton, et al. 2008), the mothers of the exposed children in this sample did not drink on a daily basis but concentrated their drinking over the weekends, consuming up to 17.9 drinks/occasion (median = 6.5) on 1–2 days/week. The 22 mothers of the control children abstained during pregnancy, except for one who drank 2 drinks on 3 occasions. Mothers of children in the HE and control groups were higher in socioeconomic status, with families of the FAS children averaging in the unskilled labor group compared with HE and controls in the semiskilled group, and were more educated than those in the FAS/ PFAS group. Although there were more smokers among mothers who drank than those who did not, the number of cigarettes and marijuana used did not differ between groups. Only one mother reported using cocaine; none reported using methaqualone (“mandrax”). The low IQ scores among the children in the sample are evidence of the disadvantaged backgrounds and poor education of this population, with the lowest scores seen among the children with FAS and PFAS (Jacobson, Stanton, et al. 2008). Although the intracranial volume for the children with FAS/PFAS was smaller than for the HE and control groups, this difference fell short of conventional levels of significance.
Table 1.
Sample characteristics (N= 78)
| Demographics | FAS/PFAS (N = 28) | Heavily exposed nonsyndromal (N = 28) | Control (N = 22) | F or χ2 |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Education (years)a | 7.1 ± 3.2 | 9.4 ± 2.4 | 10.0 ± 1.5 | 9.18*** |
| Socioeconomic statusa | 15.5 ± 7.8 | 23.4 ± 9.2 | 23.4 ± 7.6 | 7.11** |
| Cigarettes smoked/day during pregnancyb | 9.1 ± 5.1 | 7.7 ± 5.9 | 9.9 ± 12.0 | 0.41 |
| Number (%) smokersc | 25 (89.3) | 22 (78.6) | 11 (50.0) | 10.38** |
| Marijuana use during pregnancy (occasions/month)d | 1.6 ± 1.3 | 0.2 ± 0.2 | 2.7 ± 0 | 2.09 |
| Number (%) marijuana users | 3 (10.7) | 2 (7.1) | 1 (4.5) | 0.68 |
| Alcohol use across pregnancy | ||||
| Absolute alcohol consumed/day (oz)e,f | 1.3 ± 1.4 | 0.5 ± 0.5 | 0.0 ± 0.0 | 13.68*** |
| Absolute alcohol consumed/occasion (oz)g | 4.2 ± 1.8 | 2.9 ± 1.5 | 0.1 ± 0.3 | 54.72*** |
| Frequency (drinking days/week)g | 2.0 ± 1.3 | 1.2 ± 0.9 | 0.0 ± 0.0 | 28.84*** |
| Child characteristics | ||||
| Sex: number (%) males | 16 (57.1) | 17 (60.7) | 11 (50.0) | 0.59 |
| Number (%) left-handers | 3 (10.7) | 0 (0) | 1 (4.5) | 3.33 |
| Child's age at assessmenth | 10.5 ± 0.6 | 11.0 ± 0.6 | 10.6 ± 0.5 | 7.08** |
| WISC-IV IQi,j | 64.3 ± 10.9 | 74.5 ± 11.6 | 72.5 ± 10.8 | 6.55** |
| Intracranial volume (cm3)k | 1289 ± 136 | 1369 ± 141 | 1328 ± 132 | 2.38† |
FAS, fetal alcohol syndrome, PFAS, partial fetal alcohol syndrome.
†P < 0.10, *P < 0.05, **P < 0.01, ***P < 0.001.
aPost hoc Tukey's HSD: FASD < controls (P < 0.01), FASD < HE (P < 0.01).
bMean calculated for smokers only.
cPost hoc Tukey's HSD: FAS/PFAS > controls (P < 0.01), HE > controls (P < 0.05).
dMean for marijuana users only.
e1 oz AA ≈2 standard drinks.
fPost hoc Tukey's HSD: FAS/PFAS > HE (P < 0.01), FAS/PFAS > controls (P < 0.001).
gPost hoc Tukey's HSD: FAS/PFAS > HE (P < 0.01), FAS/PFAS > controls (P < 0.001), HE > controls (P < 0.001).
hPost hoc Tukey's HSD: HE > FAS/PFAS (P < 0.01), HE > controls (P < 0.05).
iWechsler Intelligence Scale for Children—Fourth Edition.
jPost hoc Tukey's HSD: FAS/PFAS < HE (P < 0.01), FAS/PFAS < controls (P < 0.05).
kPost hoc Tukey's HSD: FAS/PFAS < HE (P < 0.10).
Whole-Brain Cortical Thickness Analysis
Relation of Diagnostic Group to Cortical Thickness
A whole-brain vertex-by-vertex analysis using FreeSurfer revealed no significant clusters where cortical thickness differed by FASD diagnostic group.
Relation of Continuous Alcohol Exposure Measures to Cortical Thickness
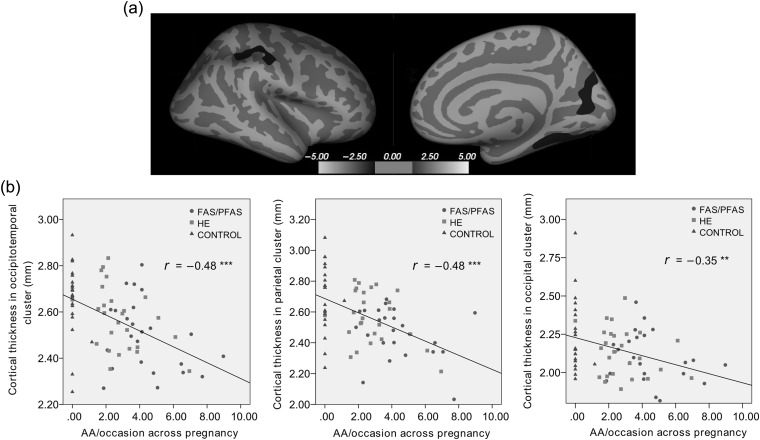
After multiple comparison correction, there were no significant clusters in analyses relating cortical thickness to AA/day or drinking days/week. However, there were 3 regions in the right hemisphere in which AA/drinking occasion was inversely related to cortical thickness. These were an occipital cortex cluster consisting of cuneus and pericalcarine cortex, an occipitotemporal cluster comprising parts of the fusiform and lingual gyri, and a parietal cluster, situated in the supramarginal/postcentral gyrus (Fig. 1a). Similar clusters were seen when brain volume or its cube root were included as regressors in the analysis to control for brain size. Although no regions in the left hemisphere survived multiple comparison correction, AA/drinking occasion was inversely related to cortical thickness at an uncorrected P < 0.05 in similar left hemisphere parietal and occipitotemporal clusters.
Figure 1.
(a) Location of 3 right hemisphere clusters in parietal, occipitotemporal, and occipital regions showing reduced cortical thickness with increased AA/occasion. The statistical map is thresholded at log(p) > 1.3 with cluster-size correction for multiple comparisons applied (P < 0.05). (b) Scatterplots showing the negative relation between AA/occasion and cortical thickness across 78 subjects in occipitotemporal (r = −0.48), parietal (r = −0.48), and occipital (r = −0.35) clusters. The mother of one HE child drank heavily around time of conception (AA/occasion = 2.54, ≈5.8 standard drinks/occasion) but reported drinking no alcohol after the period of conception.
Region of Interest Analysis
Relation of Diagnostic Group to Cortical Thickness in 3 ROIs
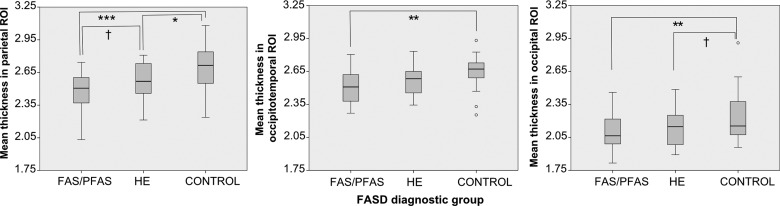
Mean cortical thickness differed significantly by FASD diagnostic group in all 3 of the ROIs identified in the whole-brain analysis— occipitotemporal, F2,75 = 5.31, P < 0.01; parietal, F2,75 = 8.18, P < 0.01; occipital, F2,75 = 3.83, P < 0.05 (Fig. 2). Post hoc comparisons using the Fisher LSD test indicated that mean thickness was significantly greater in the control group than in the FAS/PFAS group in all 3 ROIs (occipitotemporal P = 0.002, occipital P = 0.008, parietal P = 0.0001). In the parietal ROI, mean thickness was significantly smaller in the FAS/PFAS than in the HE group (P = 0.05) and in the HE compared with the controls (P = 0.03); in the occipital cortex, the difference in the HE group compared with controls fell just short of statistical significance (P = 0.06).
Figure 2.
Boxplots of mean cortical thickness in parietal, occipitotemporal and occipital ROIs for each diagnostic group. In all 3 clusters the mean thickness was significantly greater in the control group than in the FAS/PFAS group. In the parietal ROI the mean thickness was significantly smaller in the HE group than in the controls. Significant differences between groups using the Fisher LSD test are marked with †P < 0.10, *P < 0.05, **P < 0.01, ***P < 0.001.
Relation of Continuous Alcohol Exposure to Cortical Thickness in 3 ROIs
Correlations between AA/drinking occasion and cortical thickness were r = −0.35, P = 0.002 in the occipital ROI, r = −0.48, P = 0.00001 in the occipitotemporal ROI and r = −0.48, P = 0.000009 in the parietal ROI. It should be noted that these correlations (shown in Fig. 1b) are post hoc estimates of effect size in regions that survive multiple comparison correction. The parallel LOSO analysis allows assessment of the degree to which these correlations are biased estimates of the true effect size.
Each subject for whom no anatomically appropriate cluster was produced in the regression using all other subjects was omitted from the calculation of the correlation coefficient for the LOSO analysis, resulting in different Ns for each region. From the LOSO analysis, the unbiased correlations between AA/drinking occasion and cortical thickness were r = 0.06, P = 0.6, N = 70 in the occipital region, r = −0.34, P = 0.003, N = 78 in the occipitotemporal region and r = −0.22, P = 0.06, N = 73 in the parietal region.
All 3 measures of extent of prenatal alcohol exposure were negatively correlated with mean cortical thickness in all 3 ROIs (Table 2), and all the correlations remained significant even after one child with FAS whose AA/day value was an extreme outlier was removed from the analysis.
Table 2.
Relation of prenatal alcohol exposure to cortical thickness in 3 regions of interest, controlling for potential confounding variables
| ROI | Occipitotemporal N = 78 (LOSO N = 78) |
Parietal N = 78 (LOSO N = 73) |
Occipital N = 78 (LOSO N = 70) |
|||
|---|---|---|---|---|---|---|
| r | β | r | β | r | β | |
| AA/day | −0.35** (−0.30**) | −0.25*,a (−0.22†,a) | −0.44*** (−0.24**) | −0.44*** (−0.22†,b) | −0.27* (0.02) | −0.22†,c (0.02) |
| AA/occasion | −0.48*** (−0.34**) | −0.41***,a (−0.27*,a) | −0.48*** (−0.22†) | −0.48*** (−0.19b) | −0.35** (0.06) | −0.32**,c (0.06) |
| Drinking Days/week | −0.29** (−0.24*) | −0.14a,b (−0.13a) | −0.36** (−0.16) | −0.35**,a,b (−0.12b) | −0.24* (0.09) | −0.20†,c (0.09) |
r represents the simple correlation between alcohol exposure and cortical thickness in the ROI.
β is the standardized regression coefficient, after adjustment for the potential confounding variables listed below. Control variables indicated with superscripts were retained in the regression only if their entry in the model altered the standardized regression coefficient associated with prenatal alcohol exposure by at least 10%.
Regression coefficients in parentheses are those obtained from the LOSO analysis before and after controlling for confounding variables. Ns differ where no cluster survived threshold in the appropriate anatomical region for one of the LOSO analyses and thickness for the left-out subject could not be obtained.
aMaternal education.
bSocioeconomic status.
cCigarettes/day.
†P < 0.10, *P < 0.05, **P < 0.01, ***P < 0.001.
Similarly, in the LOSO analysis, all 3 measures of extent of prenatal alcohol exposure were negatively correlated with mean cortical thickness in the occipitotemporal region, however, only AA/day remained significantly negatively correlated with mean cortical thickness in the parietal region and there was no relation between prenatal alcohol exposure and mean cortical thickness in the occipital region.
Adjustment for Confounding Variables
Table 3 shows the Pearson correlations for mean cortical thickness in each ROI with each of the control variables, and Table 2 shows the results of the multiple regression analyses before and after controlling for potential confounders. Most associations of alcohol exposure with cortical thickness remained significant after adjustment for confounders. When the analyses were repeated excluding the one cocaine-exposed and 6 marijuana-exposed children, the results were essentially unchanged except that, after exclusion of the marijuana-exposed children, the regression coefficient of occipital thickness on drinking frequency dropped to β = −0.19, P = 0.10. Because ADHD is frequently seen in children with FASD, we also examined whether the observed effects on regional cortical thickness might be attributable to ADHD comorbidity. The alcohol-exposed children with and without ADHD did not differ in cortical thickness in the occipitotemporal (t = 0.31, P = 0.76, df = 76) or parietal (t = 0.33, P = 0.75, df = 76) regions; in the occipital region, the group difference fell just short of conventional levels of statistical significance (t = 1.91, P = 0.06, df = 76). When ADHD was added to the regressions in Table 2, the coefficients for the alcohol measures were essentially unchanged, indicating that ADHD status did not account for the relation of prenatal alcohol to thickness in these regions.
Table 3.
Relation of control variables to mean cortical thickness in 3 regions of interest (N= 78)
| ROI | Occipitotemporal | Parietal | Occipital |
|---|---|---|---|
| Age of child | −0.03 | 0.02 | −0.04 |
| Sex of child | 0.16†† | −0.13 | 0.17†† |
| Mother's age at delivery | −0.12 | −0.06 | −0.12 |
| Maternal education | 0.34** | 0.15†† | 0.14 |
| Socioeconomic statusa | 0.30** | 0.27* | 0.16†† |
| Cigarettes/day during pregnancy | −0.11 | −0.15†† | −0.21† |
aBased on Hollingshead Scale (2011).
††P < 0.20; †P < 0.10; *P < 0.05, **P < 0.01.
Mediation of Alcohol Exposure to IQ by Cortical Thickness
AA/day and drinking days/week were associated with lower IQ scores (both rs = −0.32, P < 0.01), and the relation of AA/occasion to IQ fell just short of statistical significance (r = −0.21, P = 0.06). Decreased cortical thickness in the occipitotemporal and parietal ROIs was significantly associated with lower IQ (r = 0.34, P = 0.002, and r = 0.24, P = 0.036, respectively). In multiple regression analyses to test for mediation, the regression coefficients for AA/day and drinking days/week were significantly reduced by the addition of cortical thickness in the occipitotemporal ROI to the model (Table 4), suggesting that the relation of prenatal alcohol to IQ is mediated, in part, by the effects of the alcohol exposure on cortical thickness in the fusiform area. Mediation for AA/day remained significant (t = 1.73, P = 0.04) when confounders were included in the analysis.
Table 4.
Mediation of the effect of prenatal alcohol exposure on WISC-IV IQa by regional cortical thickness (N = 78)
| Mediator | β1 | β2 | tb |
|---|---|---|---|
| Occipitotemporal | |||
| AA/day | −0.32** | −0.22† | −2.35** |
| AA/occasion | −0.21† | −0.07 | −4.75*** |
| Drinking days/week | −0.32** | −0.24* | −2.48** |
| Parietal | |||
| AA/day | −0.32** | −0.27* | −1.00 |
| AA/occasion | −0.21† | −0.13 | −1.38† |
| Drinking days/week | −0.32** | −0.26* | −1.20 |
β1, standardized regression coefficient for alcohol in the regression of IQ on alcohol during pregnancy before controlling for mediator.
β2, standardized coefficient for alcohol in the regression of IQ on alcohol during pregnancy after controlling for mediator.
aWechsler Intelligence Scale for Children—Fourth Edition.
bTests the significance of the change in the magnitude of the effect of the alcohol measure on IQ after the mediator has been entered into the model, using the difference in coefficients method (Clogg et al. 1992).
†P < 0.10, *P < 0.05, **P < 0.01.
Discussion
Thinner Cortex in FASD
This is the first study to examine the effect of degree of prenatal alcohol exposure on cerebral cortical thickness in a homogeneous prepubertal cohort. We observed regional cortical thinning, rather than thickening, with increased prenatal alcohol exposure. Although inconsistent with the findings of 3 previous studies on cortical thickness in FASD (Sowell et al. 2008; Fernández-Jaén et al. 2011; Yang et al. 2012), our findings are consistent with Zhou et al. (2011), who found thinner cortex in areas of the bilateral middle frontal lobe, pre- and postcentral areas, lateral and inferior temporal and occipital lobes in FASD subjects compared with controls, and with the Treit et al. (2014) longitudinal study, which found lower total mean cortical thickness in FASD compared with controls at their initial scan. Like Zhou et al. (2011) we found thinner cortex in right superior parietal and middle temporal fusiform, calcarine, and lingual gyri. These findings are also consistent with the animal studies showing thinner cortex in alcohol-exposed animals relative to controls (Miller and Dow-Edwards 1988; Norton et al. 1988; Kotkoskie and Norton 1989; Leigland et al. 2013).
Previous studies of cortical thickness in FASD have assessed samples that included both preadolescent and adolescent subjects. In the studies reporting increased thickness in FASD (Sowell et al. 2008; Fernández-Jaén et al. 2011; Yang et al. 2012), this increase was attributed to a delay or absence of the age-related cortical thinning that occurs in normally developing children. This developmental cortical thinning is thought to be the result of both synaptic pruning and increased myelination accompanying cognitive maturation (Sowell et al. 2004; Shaw, Greenstein, et al. 2006). We did not observe increased cortical thinning with age within the narrow preadolescent age range of our study cohort (9–12 years of age). Although this is considered by some to be the age range during which peak cortical thickness is attained, it is important to note that there is substantial regional variation in the age at which gray matter volume peaks (Muftuler et al. 2011; van Soelen et al. 2012) and some studies suggest that cortical thickness may peak much earlier in life (Sowell et al. 2004; Brown et al. 2012; Zhou et al. 2015). Nevertheless, our sample may have included some participants in the early stages of adolescent thinning, as well as those in the late stages of preadolescent cortical thickening.
The Treit et al. (2014) study cited above was the first to examine cortical thickness longitudinally in FASD. As noted, the authors found thinner cortex in the FASD group at the initial scan (mean age = 8 years). They also found reduced cortical thinning compared with controls between this scan and a second scan 2–4 years later. The finding of a reduced rate of maturation-related cortical thinning may explain the findings of greater cortical thickness in individuals with FASD in some of the studies that combined preadolescent and adolescent subjects within the same sample. Other factors that might contribute to the discrepant findings among these studies include sample differences in the proportion of subjects with FASD who met diagnostic criteria for FAS and in the incidence of ADHD comorbidity, timing and amount of prenatal alcohol exposure, and unmeasured potential confounding variables.
Effects of FASD Diagnosis and Continuous Measures of Alcohol Exposure
Mapping relations at the vertex level across the whole brain requires a large number of subjects to provide sufficient statistical power to yield significant results corrected for multiple comparisons. Comparing mean thickness within the clusters generated from the whole-brain analysis is potentially more powerful and can improve the signal-to-noise ratio compared with vertex-by-vertex analysis. Although in the whole-brain analysis the relation of prenatal alcohol exposure with cortical thinning was found only for the measure of average alcohol/occasion, analyses of mean thickness within the ROIs that showed thinner cortex in relation to dose/occasion revealed differences in thickness between diagnostic groups (Fig. 2) and associations with the measures of average alcohol/day and frequency of drinking (Table 2).
The finding that the association between increased prenatal alcohol exposure and thinner cortex was most evident for alcohol dose/occasion is consistent with evidence from animal studies that have shown that ingestion of a concentrated dose of alcohol over a short period of time causes greater neuronal (Bonthius and West 1990) and behavioral impairment (Goodlett et al. 1987) than a larger dose ingested over several days. Similarly, human studies have frequently found that a higher dose/occasion, the key feature of binge drinking, leads to more severe adverse effects (Streissguth, Barr, et al. 1994). In our Detroit Longitudinal Cohort study, we have found that pregnant women typically do not drink daily but concentrate their drinking over the weekends, often resulting in a binge drinking pattern (Jacobson et al. 2013). In this Cape Town cohort, we also found that all the women in the FAS/PFAS and HE groups concentrated their drinking on 1–2 days/week, thus engaging in a binge drinking pattern that places the fetus at greater risk.
Although the previous studies did not examine the degree to which cortical thickness may vary among FASD diagnostic groups, it is noteworthy that the proportion of participants with FAS or PFAS differed among studies. The studies showing cortical thickening consisted exclusively (Fernández-Jaén et al. 2011) or mostly of FAS/PFAS cases (67% (Sowell et al. 2008); 60% (Yang et al. 2012)); whereas the FASD group in the study indicating cortical thinning (Zhou et al. 2011) consisted of only 15% FAS/PFAS cases. In contrast, Rajaprakash et al. (2014) found no differences in cortical thickness between controls and non-FAS subjects with alcohol-related neurodevelopmental disorder (ARND). Although it has been suggested that more severely affected individuals (FAS/PFAS) may show thickening and others may show thinning, when the dysmorphic and nondysmorphic groups were compared directly, (Yang et al. 2012) found no difference in cortical thickness between their FAS and nondysmorphic FASD groups. Moreover, our data indicate a dose-dependent reduction in regional cortical thickness, with thickness values for the HE nonsyndromal group that were intermediate between those of the FAS/PFAS and control groups.
Functional Significance of Regional Cortical Thinning
Our findings of reduced cortical thickness with increased prenatal alcohol exposure in right superior parietal and middle temporal fusiform, calcarine, and lingual gyri may partially account for functional deficits found in FASD, which include attention (Coles et al. 2002; Burden, Jacobson, Sokol, et al. 2005; Mattson et al. 2006), verbal learning and memory (e.g., Mattson and Roebuck 2002; Willford et al. 2004; Lewis et al. 2015) and visuospatial attention and memory (Streissguth, Sampson, et al. 1994; Willoughby et al. 2008; Green et al. 2009). The parietal lobe has previously been shown to be relatively more affected by prenatal alcohol exposure than other cortical regions (Archibald et al. 2001; Spadoni et al. 2007), and morphological alterations in this region may be associated with the difficulties in number processing frequently seen in FASD (Kopera-Frye et al. 1996; Burden, Jacobson, Jacobson, 2005; Meintjes et al. 2010; Jacobson et al. 2011). The parietal and fusiform regions that we found to be thinner in children with alcohol exposure are heteromodal association areas that integrate information from surrounding somatosensory, auditory, and visual cortices. These areas are important for higher level information processing and are components of both the dorsal and ventral visual processing pathways.
The temporal fusiform gyrus (BA 37) is involved in processing of sensory information and is relevant for recognition of face, body, numbers, letters, and category information. The fusiform and lingual gyrus are also involved in spatial information processing (Maguire et al. 1998; Bohbot et al. 2000; Menon et al. 2000), memory (Druzgal and D'Esposito 2001; Linden et al. 2011), and encoding (Kraft et al. 2012), especially of visual memories (Machielsen et al. 2000; Leshikar et al. 2012). A morphometric study has shown that structural features of the fusiform gyrus are associated with both spatial intelligence and attention (Colom et al. 2013). Zhou et al. (2011) found increased bilateral thinning of the fusiform with age in FASD, which did not occur in control subjects. Our findings of reduced cortical thickness in this region are also consistent with reports of reductions in gray matter volume (Sowell et al. 2002; Li et al. 2008) and surface area (Rajaprakash et al. 2014) in the occipitotemporal area in subjects prenatally exposed to alcohol. Similarly, fetal alcohol-related functional abnormalities in this area have been observed during shape recognition tasks involving sustained visual attention (Li et al. 2008).
Decreased cortical thickness in the fusiform, lingual, and supramarginal gyri has also been associated with inattention and impulsivity in institutionalized children who are at increased risk of developing ADHD (McLaughlin et al. 2013). Given that some features of ADHD may be attributable to prenatal alcohol exposure (Mick et al. 2002; Jacobson, Jacobson, et al. 2011), it is of interest that decreased cortical thickness in these regions in our Cape Town cohort were related to prenatal alcohol exposure and not to ADHD.
Rajaprakash et al. (2014) found decreased surface area, but not cortical thickness, in subjects with ARND in a right hemisphere occipitotemporal region similar to that identified in the current study. Surface area is thought to be established early in embryogenesis when progenitor cells divide symmetrically at the ventricular zone (Chenn and Walsh 2002), whereas cortical thickness is believed to develop later via asymmetric division (Rakic 1995). The timing of alcohol exposure in gestation may, therefore, influence whether cortical surface area or cortical thickness is more greatly affected (Rajaprakash et al. 2014), which may account for the differences between Rajaprakash et al. (2014) and the current study.
Mediation of Alcohol to IQ by Regional Cortical Thickness
Cortical thickness in the occipitotemporal cluster (comprising fusiform and lingual gyrus) and parietal cluster (comprising supramarginal and postcentral gyrus) was related to WISC-IV IQ. These temporal and parietal regions are components of the parieto-frontal integration theory (PFIT) model of intelligence (Jung and Haier 2007), according to which the structure and function of a distributed network of multimodal association areas are associated with general intellectual abilities. In the PFIT model, sensory information is first processed by temporal and occipital areas for subsequent integration and abstraction in parietal areas. Problem evaluation and response selection are then mediated by the prefrontal cortex and anterior cingulate, respectively.
Multiple regression analysis showed that the association of prenatal alcohol exposure with lower IQ scores appears to be mediated, in part, by cortical thinning in the occipitotemporal region. This finding is consistent with studies linking greater intellectual ability in adults to greater cortical thickness in the prefrontal (Narr et al. 2007; Hartberg et al. 2010) and lingual (Narr et al. 2007) gyri and with the finding that cortical thickness is decreased in mental retardation (Zhang et al. 2011). In children and adolescents, IQ scores have also been found to be positively correlated with cortical thickness, particularly in the frontal lobes, as well as in parietal, temporal, and occipital association areas (Shaw, Greenstein et al. 2006a; Karama et al. 2009; Misaki et al. 2012; Menary et al. 2013). Thinner cortex is also associated with decreased cognitive performance in aging (Dickerson et al. 2008; Burzynska et al. 2012).
These data suggest that AA/occasion, which is a measure of the degree to which the mother concentrates her alcohol consumption, is the best predictor of brain damage in these occipitotemporal and parietal regions, even though it is not the strongest predictor of IQ, which is presumably affected by impairment in multiple brain regions. AA/day is the most consistent predictor of deficits across a broad range of neurobehavioral domains, possibly because it provides the best overall summary of total alcohol exposure across pregnancy. These data show that alcohol-related damage in the occipitotemporal region, which was highly sensitive to dose/occasion, partially mediates the alcohol effect on IQ, which was most strongly related to total alcohol ingested during the course of the pregnancy.
Influence of Confounding Variables and Comorbidities
Although children with FASD have frequently also been exposed prenatally to cigarette smoking and other drugs, previous studies have not considered potential confounding factors beyond age, sex, and child's IQ in the investigation of cortical thickness in FASD. Exposure to maternal cigarette smoking in utero has been associated with thinner cortex in the frontal (Toro et al. 2008; Lotfipour et al. 2009; El Marroun et al. 2013), superior parietal, precentral (El Marroun et al. 2013), and lateral occipital cortices (Derauf et al. 2012). In this study, when prenatal exposure to cigarette smoking was controlled statistically in the analysis, the relation between alcohol exposure and cortical thickness remained significant. Cortical thickness in children is also related to parental and social factors, including parental education (Lawson et al. 2013) and maternal age (Shaw et al. 2012). In our study, the relation between prenatal alcohol exposure and cortical thickness also persisted when socioeconomic status and maternal education were adjusted statistically. Given the high rate of comorbidity of FASD with ADHD (Mick et al. 2002; Jacobson, Dodge, et al. 2011) and that global and regional cortical thinning has also been observed in participants with ADHD (Shaw, Lerch, et al. 2006; Narr et al. 2009; Almeida Montes et al. 2012), it was of particular interest that multiple regression analyses showed that the regional cortical thinning observed in this study was not attributable to ADHD.
Previous studies (Zhou et al. 2011; Yang et al. 2012) have corrected cortical thickness for brain volume. Because cortical thickness does not scale with brain volume (Barnes et al. 2010), we did not include brain volume as a covariate in the statistical analysis. However, even when brain volume or its cube root was included to control for brain size, we observed thinner cortex with increasing alcohol exposure in the same 3 regions.
Conclusions
The previous studies of cortical thickness in FASD, which yielded contradictory findings, spanned a broad age range including periods during which cortical thickening and thinning are both normative. The data in this first study to address FASD-related variations in cortical thickness in a homogeneous, prepubertal, prospectively recruited sample support a fetal alcohol-related reduction in regional cortical thickness in middle childhood. The children with greater prenatal alcohol exposure had thinner cortex at an age when cortical thickness is expected to be at its maximum. Based on data obtained during pregnancy, average maternal alcohol dose/occasion emerged as the measure of exposure most predictive of cortical thinning in parietal and temporo-occipital hetero-modal association regions, suggesting that maternal binge drinking is particularly detrimental to cortical development. These results are consistent with one prior study (Zhou et al. 2011) showing FASD-related reductions in cortical thickness in similar parietal, temporal, and occipital association areas, as well as a recent report of thinner total mean cortex in children with FASD at an initial scan in preadolescence (Treit et al. 2014). The observation of reduced cortical thickness is consistent with studies in animals prenatally exposed to alcohol, as well as with findings of reduced cortical thickness in other neurodevelopmental disorders. Notably, the effect of prenatal alcohol exposure on IQ appeared to be mediated, in part, by reduced cortical thickness in the right occipitotemporal region.
Funding
This work was supported by National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism (grant numbers R01-AA016781, R21-AA017410, U01-AA014790); South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa; Medical Research Council of South Africa; Fulbright South Africa Research/Scholar Award; Joseph Young, Sr., Fund, State of Michigan; and Ellison Medical Foundation.
Notes
We thank Bruce Spottiswoode, PhD, the CUBIC radiographers Nailah Maroof and Marie-Louise de Villiers, and our UCT and WSU research staff Nicolette Hamman, Mariska Pienaar, Maggie September, Emma Makin, Renee Sun, and Neil Dodge. We also thank H. Eugene Hoyme, Luther K. Robinson, and Nathaniel C.O. Khaole, who conducted the Cape Town dysmorphology examinations in conjunction with the NIAAA Collaborative Initiative on Fetal Alcohol Spectrum Disorders. We appreciate the input from Joel Nigg, PhD, and Rafael Klorman, PhD, who consulted on the ADHD diagnoses. We wish to express our appreciation to the mothers and children who participated in our Cape Town longitudinal research. Conflict of Interest: None declared.
References
- Almeida Montes LG, Prado Alcantara H, Martinez Garcia RB, De La Torre LB, Avila Acosta D, Duarte MG. 2012. Brain cortical thickness in ADHD: age, sex, and clinical correlations. J Atten Disord. 17:641–654. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. 2001. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 43:148–154. [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T et al. 2009. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 33:1671–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Ridgway GR, Bartlett J, Henley SMD, Lehmann M, Hobbs N, Clarkson MJ, MacManus DG, Ourselin S, Fox NC. 2010. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 53:1244–1255. [DOI] [PubMed] [Google Scholar]
- Bearden CE, van Erp TGM, Dutton RA, Tran H, Zimmermann L, Sun D, Geaga JA, Simon TJ, Glahn DC, Cannon TD et al. 2007. Mapping cortical thickness in children with 22q11.2 deletions. Cereb Cortex. 17:1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. 1994. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 51:477–484. [DOI] [PubMed] [Google Scholar]
- Bing X, Ming-guo Q, Ye Z, Jing-na Z, Min L, Han C, Yu Z, Jia-jia Z, Jian W, Wei C et al. 2012. Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with post-traumatic stress disorder. Brain Res. 1490:225–232. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Allen JJ, Nadel L. 2000. Memory deficits characterized by patterns of lesions to the hippocampus and parahippocampal cortex. Ann N Y Acad Sci. 911:355–368. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. 1990. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 14:107–118. [DOI] [PubMed] [Google Scholar]
- Bowman RS, Stein LI, Newton JR. 1975. Measurement and interpretation of drinking behavior. I. On measuring patterns of alcohol consumption. II. Relationships between drinking behavior and social adjustment in a sample of problem drinkers. J Stud Alcohol. 36:1154–1172. [DOI] [PubMed] [Google Scholar]
- Brown TT, Kuperman JM, Chung Y, Erhart M, McCabe C, Hagler DJ, Venkatraman VK, Akshoomoff N, Amaral DG, Bloss CS et al. 2012. Neuroanatomical assessment of biological maturity. Curr Biol. 22:1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Jacobson JL. 2005. Relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcohol Clin Exp Res. 29:1473–1483. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL. 2005. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res. 29:443–452. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Nagel IE, Preuschhof C, Gluth S, Bäckman L, Li S-C, Lindenberger U, Heekeren HR. 2012. Cortical thickness is linked to executive functioning in adulthood and aging. Hum Brain Mapp. 33:1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. 2002. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 297:365–369. [DOI] [PubMed] [Google Scholar]
- Clogg CC, Petkova E, Shihadeh ES. 1992. Statistical methods for analyzing collapsibility in regression models. J Educ Behav Stat. 17:51–74. [Google Scholar]
- Coles CD, Platzman KA, Lynch ME, Freides D. 2002. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcohol Clin Exp Res. 26:263–271. [PubMed] [Google Scholar]
- Colom R, Burgaleta M, Román FJ, Karama S, Alvarez-Linera J, Abad FJ, Martínez K, Quiroga MÁ, Haier RJ. 2013. Neuroanatomic overlap between intelligence and cognitive factors: morphometry methods provide support for the key role of the frontal lobes. Neuroimage. 72:143–152. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 9:179–194. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. 1993. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction. J Cogn Neurosci. 5:162–176. [DOI] [PubMed] [Google Scholar]
- Derauf C, Lester BM, Neyzi N, Kekatpure M, Gracia L, Davis J, Kallianpur K, Efird JT, Kosofsky B. 2012. Subcortical and cortical structural central nervous system changes and attention processing deficits in preschool-aged children with prenatal methamphetamine and tobacco exposure. Dev Neurosci. 34:327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, Pacheco J, Quinn BT, Van der Kouwe A, Greve DN et al. 2008. Detection of cortical thickness correlates of cognitive performance: reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 39:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzgal TJ, D'Esposito M. 2001. Activity in fusiform face area modulated as a function of working memory load. Cogn Brain Res. 10:355–364. [DOI] [PubMed] [Google Scholar]
- Du Plessis L, Jacobson JL, Jacobson SW, Hess AT, van der Kouwe A, Avison MJ, Molteno CD, Stanton ME, Stanley JA, Peterson BS et al. 2014. An in vivo (1)H magnetic resonance spectroscopy study of the deep cerebellar nuclei in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 38:1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo T, Tosun D. 2011. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res. 1:1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H, Schmidt MN, Franken IH, Jaddoe VW, Hofman A, van der Lugt A, Verhulst FC, Tiemeier H, White T. 2013. Prenatal tobacco exposure and brain morphology: a prospective study in young children. Neuropsychopharmacology. 39:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvsåshagen T, Westlye LT, Bøen E, Hol PK, Andreassen OA, Boye B, Malt UF. 2013. Bipolar II disorder is associated with thinning of prefrontal and temporal cortices involved in affect regulation. Bipolar Disord. 15:855–864. [DOI] [PubMed] [Google Scholar]
- Esterman M, Tamber-Rosenau BJ, Chiu YC, Yantis S. 2010. Avoiding non-independence in fMRI data analysis: Leave one subject out. Neuroimage. 50:572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Jaén A, Fernández-Mayoralas DM, Quiñones Tapia D, Calleja-Pérez B, García-Segura JM, Arribas SL, Muñoz Jareño N. 2011. Cortical thickness in fetal alcohol syndrome and attention deficit disorder. Pediatr Neurol. 45:387–391. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. 2001. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 20:70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S et al. 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Van Der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM. 2004. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 23(Suppl 1):S69–S84. [DOI] [PubMed] [Google Scholar]
- Fortier C, Leritz E. 2011. Reduced cortical thickness in abstinent alcoholics and association with alcoholic behavior. Alcohol Clin Exp Res. 35:2193–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. 2004. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 1021:77–85. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 2:861–863. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW et al. 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett C, Kelly S, West J. 1987. Early postnatal alcohol exposure that produces high blood alcohol levels impairs development of spatial navigation learning. Psychobiology. 15:64–74. [Google Scholar]
- Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP, Reynolds JN. 2009. Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB). J Child Psychol Psychiatry. 50:688–697. [DOI] [PubMed] [Google Scholar]
- Greenland S. 1989. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 79:340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. 2013. Widespread reductions in gray matter volume in depression. NeuroImage Clin. 3:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, Van Der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R et al. 2006. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 32:180–194. [DOI] [PubMed] [Google Scholar]
- Hartberg CB, Lawyer G, Nyman H, Jönsson EG, Haukvik UK, Saetre P, Bjerkan PS, Andreassen OA, Hall H, Agartz I. 2010. Investigating relationships between cortical thickness and cognitive performance in patients with schizophrenia and healthy adults. Psychiatry Res. 182:123–133. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. 2011. Four factor index of social status. Yale J Sociol. 8:21–52. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N et al. 2005. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 115:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. 1997. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 387:167–178. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Dodge NC, Burden MJ, Klorman R, Jacobson SW. 2011. Number processing in adolescents with prenatal alcohol exposure and ADHD: differences in the neurobehavioral phenotype. Alcohol Clin Exp Res. 35:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, Dewailly E. 2008. Beneficial effects of a polyunsaturated fatty acid on infant development: evidence from the Inuit of Arctic Quebec. J Pediatr. 152:356–364. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Carter RC, Jacobson JL. 2013. Commentary on Day and colleagues : the association between prenatal alcohol exposure and behavior at 22 years of age—adverse effects of risky patterns of drinking among low to moderate alcohol-using pregnant women. Alcohol Clin Exp Res. 37:1069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. 2002. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 109:815–825. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R. 2004. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcohol Clin Exp Res. 28:1732–1745. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Stanton ME, Meintjes EM, Molteno CD. 2011. Biobehavioral markers of adverse effect in fetal alcohol spectrum disorders. Neuropsychol Rev. 21:148–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N et al. 2011. Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res. 35:250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. 2008. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 32:365–372. [DOI] [PubMed] [Google Scholar]
- Johnson VP, Swayze VW II, Sato Y, Andreasen NC. 1996. Fetal alcohol syndrome: craniofacial and central nervous system manifestations. Am J Med Genet. 61:329–339. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. 2007. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 30:135–154. discussion 154–187. [DOI] [PubMed] [Google Scholar]
- Karama S, Ad-Dab'bagh Y, Haier R, Deary I, Lyttelton O, Lepage C, Evans A. 2009. Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 37:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopera-Frye K, Dehaene S, Streissguth AP. 1996. Impairments of number processing induced by prenatal alcohol exposure. Neuropsychologia. 34:1187–1196. [DOI] [PubMed] [Google Scholar]
- Kotkoskie LA, Norton S. 1989. Cerebral cortical morphology and behavior in rats following acute prenatal ethanol exposure. Alcohol Clin Exp Res. 13:776–781. [DOI] [PubMed] [Google Scholar]
- Kraft A, Grimsen C, Kehrer S, Bahnemann M, Spang K, Prass M, Irlbacher K, Köhnlein M, Lipfert A, Brunner F et al. 2012. Neurological and neuropsychological characteristics of occipital, occipito-temporal and occipito-parietal infarction. Cortex. 56:38–50. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SCR, van der Kouwe AJW et al. 2003. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 60:878–888. [DOI] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, Farah MJ. 2013. Associations between children's socioeconomic status and prefrontal cortical thickness. Dev Sci. 16:641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J, Beaulieu C. 2008. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 32:1732–1740. [DOI] [PubMed] [Google Scholar]
- Leigland La, Ford MM, Lerch JP, Kroenke CD. 2013. The influence of fetal ethanol exposure on subsequent development of the cerebral cortex as revealed by magnetic resonance imaging. Alcohol Clin Exp Res. 37:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshikar ED, Duarte A, Hertzog C. 2012. Task-selective memory effects for successfully implemented encoding strategies. PLoS One. 7:e38160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CE, Thomas KGF, Dodge NC, Molteno CD, Meintjes EM, Jacobson JL, Jacobson SW. 2015. Verbal learning and memory impairment in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 39:724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ma X, Peltier S, Hu X, Coles CD, Lynch ME. 2008. Occipital-temporal reduction and sustained visual attention deficit in prenatal alcohol exposed adults. Brain Imaging Behav. 2:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DEJ, Oosterhof NN, Klein C, Downing PE. 2011. Mapping brain activation and information during category-specific visual working memory. J Neurophysiol. 107:628–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lester BM, Neyzi N, Sheinkopf SJ, Gracia L, Kekatpure M, Kosofsky BE. 2013. Regional brain morphometry and impulsivity in adolescents following prenatal exposure to cocaine and tobacco. JAMA Pediatr. 167:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li Y-J, Luo E-P, Lu H-B, Yin H. 2012. Cortical thinning in patients with recent onset post-traumatic stress disorder after a single prolonged trauma exposure. PLoS One. 7:e39025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfipour S, Ferguson E, Leonard G, Perron M, Pike B, Richer L, Séguin JR, Toro R, Veillette S, Pausova Z et al. 2009. Orbitofrontal cortex and drug use during adolescence: role of prenatal exposure to maternal smoking and BDNF genotype. Arch Gen Psychiatry. 66:1244–1252. [DOI] [PubMed] [Google Scholar]
- Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Witter MP. 2000. FMRI of visual encoding: reproducibility of activation. Hum Brain Mapp. 9:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madge EM, Van Den Berg AR, Robinson M, Landman J. 1981. Junior South African individual scales. Pretoria, South Africa: Human Sciences Research Council.
- Maguire EA, Frith CD, Burgess N, Donnett JG, O'Keefe J. 1998. Knowing where things are parahippocampal involvement in encoding object locations in virtual large-scale space. J Cogn Neurosci. 10:61–76. [DOI] [PubMed] [Google Scholar]
- Maldonado G, Greenland S. 1993. Simulation study of confounder-selection strategies. Am J Epidemiol. 138:923–936. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Calarco KE, Lang AR. 2006. Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology. 20:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. 1998. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 22:279–294. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM. 2002. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 26:875–882. [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais A-S, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NCO, Snell C, Kalberg WO et al. 2007. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 88:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, Nelson CA. 2013. Widespread reductions in cortical thickness following severe early-life deprivation: a neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biol Psychiatry. 76:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes EM, Jacobson JL, Molteno CD, Gatenby JC, Warton C, Cannistraci CJ, Hoyme HE, Robinson LK, Khaole N, Gore JC et al. 2010. An FMRI study of number processing in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 34:1450–1464. [DOI] [PubMed] [Google Scholar]
- Menary K, Collins PF, Porter JN, Muetzel R, Olson EA, Kumar V, Steinbach M, Lim KO, Luciana M. 2013. Associations between cortical thickness and general intelligence in children, adolescents and young adults. Intelligence. 41:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, White CD, Eliez S, Glover GH, Reiss AL. 2000. Analysis of a distributed neural system involved in spatial information, novelty, and memory processing. Hum Brain Mapp. 11:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. 2002. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolesc Psychiatry. 41:378–385. [DOI] [PubMed] [Google Scholar]
- Miller MW, Dow-Edwards DL. 1988. Structural and metabolic alterations in rat cerebral cortex induced by prenatal exposure to ethanol. Brain Res. 474:316–326. [DOI] [PubMed] [Google Scholar]
- Misaki M, Wallace G, Dankner N. 2012. Characteristic cortical thickness patterns in adolescents with autism spectrum disorders: interactions with age and intellectual ability revealed by canonical correlation. Neuroimage. 60:1890–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momenan R, Steckler LE, Saad ZS, Van Rafelghem S, Kerich MJ, Hommer DW. 2012. Effects of alcohol dependence on cortical thickness as determined by magnetic resonance imaging. Psychiatry Res. 204:101–111. [DOI] [PubMed] [Google Scholar]
- Muftuler LT, Davis EPE, Buss C, Head K. 2011. Cortical and subcortical changes in typically developing preadolescent children. Brain Res. 1399:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Lin J, Kim J, Phillips OR, Del'Homme M, Caplan R, Toga AW, McCracken JT, Levitt JG. 2009. Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 48:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM. 2007. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 17:2163–2171. [DOI] [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP. 2009. Neuroimaging and fetal alcohol spectrum disorders. Dev Disabil Res Rev. 15:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton S, Terranova P, Na JY, Sancho-Tello M. 1988. Early motor development and cerebral cortical morphology in rats exposed perinatally to alcohol. Alcohol Clin Exp Res. 12:130–136. [DOI] [PubMed] [Google Scholar]
- Overvliet GM, Besseling RMH, Jansen JF, van der Kruijs SJM, Vles JSH, Hofman PA, Ebus SCM, de Louw A, Aldenkamp AP, Backes WH. 2013. Early onset of cortical thinning in children with rolandic epilepsy. NeuroImage Clin. 2:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Evans SW, Gnagy EM, Greenslade KE. 1992. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders—prevalence, factor-analyses, and conditional probabilities in a special-education sample. School Psych Rev. 21:285–299. [Google Scholar]
- Rajaprakash M, Chakravarty MM, Lerch JP, Rovet J. 2014. Cortical morphology in children with alcohol-related neurodevelopmental disorder. Brain Behav. 4:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. 1995. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 18:383–388. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, Liu J, Barr DB, Slotkin TA, Peterson BS. 2012. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc Natl Acad Sci USA. 109:7871–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvåg R, Fennema-Notestine C, Hagler DJ, Pung CJ, Jennings RG, Haukvik UK, Lange E, Nakstad PH et al. 2010. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 68:41–50. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, Van Der Kouwe A, Jenkins BG, Dale AM, Fischl B. 2002. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 58:695–701. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B. 2004. Thinning of the cerebral cortex in aging. Cereb Cortex. 14:721–730. [DOI] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C, Nenadic I, Reichenbach JR, Sauer H, Schlösser RGM. 2010. Reduced cortical thickness in first episode schizophrenia. Schizophr Res. 116:204–209. [DOI] [PubMed] [Google Scholar]
- Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. 2004. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 22:1060–1075. [DOI] [PubMed] [Google Scholar]
- Ségonne F, Pacheco J, Fischl B. 2007. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 26:518–529. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. 2007. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 104:19649–19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Gilliam M, Malek M, Rodriguez N, Greenstein D, Clasen L, Evans A, Rapoport J, Giedd J. 2012. Parental age effects on cortical morphology in offspring. Cereb Cortex. 22:1256–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. 2006. Intellectual ability and cortical development in children and adolescents. Nature. 440:676–679. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL et al. 2008. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci Off J Soc Neurosci. 28:3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J. 2006. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 63:540–549. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. 1998. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 17:87–97. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. 2008. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 18:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. 2003. Mapping cortical change across the human life span. Nat Neurosci. 6:309–315. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. 2004. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 24:8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Peterson BS, Mattson SN, Welcome SE, Henkenius AL, Riley EP, Jernigan TL, Toga AW. 2002. Mapping cortical gray matter asymmetry patterns in adolescents with heavy prenatal alcohol exposure. Neuroimage. 17:1807–1819. [DOI] [PubMed] [Google Scholar]
- Spadoni A, McGee C, Fryer S, Riley E. 2007. Neuroimaging and fetal alcohol spectrum disorders. Neurosci Biobehav Rev. 15:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Olson HC, Sampson PD, Bookstein FL, Burgess DM. 1994. Drinking during pregnancy decreases word attack and arithmetic scores on standardized tests: adolescent data from a population-based prospective study. Alcohol Clin Exp Res. 18:248–254. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Sampson PD, Olson HC, Bookstein FL, Barr HM, Scott M, Feldman J, Mirsky AF. 1994. Maternal drinking during pregnancy: attention and short-term memory in 14-year-old offspring—a longitudinal prospective study. Alcohol Clin Exp Res. 18:202–218. [DOI] [PubMed] [Google Scholar]
- Suttie M, Foroud T, Wetherill L, Jacobson JL, Molteno CD, Meintjes EM, Hoyme HE, Khaole N, Robinson LK, Riley EP et al. 2013. Facial dysmorphism across the fetal alcohol spectrum. Pediatrics. 131:e779–e788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayze VW, Johnson VP, Hanson JW, Piven J, Sato Y, Giedd JN, Mosnik D, Andreasen NC. 1997. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 99:232–240. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. 2010. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 20:534–548. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Sowell ER, Gogtay N, Giedd JN, Vidal CN, Hayashi KM, Leow A, Nicolson R, Rapoport JL, Toga AW. 2005. Structural MRI and brain development. Int Rev Neurobiol. 67:285–323. [DOI] [PubMed] [Google Scholar]
- Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, Van Der Kouwe AJW. 2011. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med. 68:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Leonard G, Lerner JV, Lerner RM, Perron M, Pike GB, Richer L, Veillette S, Pausova Z, Paus T. 2008. Prenatal exposure to maternal cigarette smoking and the adolescent cerebral cortex. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 33:1019–1027. [DOI] [PubMed] [Google Scholar]
- Treit S, Zhou D, Lebel C, Rasmussen C, Andrew G, Beaulieu C. 2014. Longitudinal MRI reveals impaired cortical thinning in children and adolescents prenatally exposed to alcohol. Hum Brain Mapp. 35:4892–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kouwe AJW, Benner T, Salat DH, Fischl B. 2008. Brain morphometry with multiecho MPRAGE. Neuroimage. 40:559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Soelen ILC, Brouwer RM, van Baal GCM, Schnack HG, Peper JS, Collins DL, Evans AC, Kahn RS, Boomsma DI, Hulshoff Pol HE. 2012. Genetic influences on thinning of the cerebral cortex during development. Neuroimage. 59:3871–3880. [DOI] [PubMed] [Google Scholar]
- Van Tol M-J, Li M, Metzger CD, Hailla N, Horn DI, Li W, Heinze HJ, Bogerts B, Steiner J, He H et al. 2013. Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psychol Med. 1:1–13. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Moe V, Slinning K, Due-Tønnessen P, Bjørnerud A, Dale AM, van der Kouwe A, Quinn BT, Kosofsky B, Greve D et al. 2007. Volumetric cerebral characteristics of children exposed to opiates and other substances in utero. Neuroimage. 36:1331–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 2003. WISC-IV Administration Manual., The Wechsler intelligence scale for children—fourth edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Widjaja E, Mahmoodabadi SZ, Snead OC, Almehdar A, Smith ML. 2011. Widespread cortical thinning in children with frontal lobe epilepsy. Epilepsia. 52:1685–1691. [DOI] [PubMed] [Google Scholar]
- Willford JA, Richardson GA, Leech SL, Day NL. 2004. Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Alcohol Clin Exp Res. 28:497–507. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J. 2008. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc. 14:1022–1033. [DOI] [PubMed] [Google Scholar]
- Yang Y, Roussotte F, Kan E, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O'Connor MJ et al. 2012. Abnormal cortical thickness alterations in fetal alcohol spectrum disorders and their relationships with facial dysmorphology. Cereb Cortex. 22:1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wu Y, Zhu M, Wang C, Wang J, Zhang Y, Yu C, Jiang T. 2011. Reduced cortical thickness in mental retardation. PLoS One. 6:e29673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Lebel C, Lepage C, Rasmussen C, Evans A, Wyper K, Pei J, Andrew G, Massey A, Massey D et al. 2011. Developmental cortical thinning in fetal alcohol spectrum disorders. Neuroimage. 58:16–25. [DOI] [PubMed] [Google Scholar]
- Zhou D, Lebel C, Treit S, Evans A, Beaulieu C. 2015. Accelerated longitudinal cortical thinning in adolescence. Neuroimage. 104:138–145. [DOI] [PubMed] [Google Scholar]