Abstract
Tools represent a special class of objects, because they are processed across both the dorsal and ventral visual object processing pathways. Three core regions are known to be involved in tool processing: the left posterior middle temporal gyrus, the medial fusiform gyrus (bilaterally), and the left inferior parietal lobule. A critical and relatively unexplored issue concerns whether, in development, tool preferences emerge at the same time and to a similar degree across all regions of the tool-processing network. To test this issue, we used functional magnetic resonance imaging to measure the neural amplitude, peak location, and the dispersion of tool-related neural responses in the youngest sample of children tested to date in this domain (ages 4–8 years). We show that children recruit overlapping regions of the adult tool-processing network and also exhibit similar patterns of co-activation across the network to adults. The amplitude and co-activation data show that the core components of the tool-processing network are established by age 4. Our findings on the distributions of peak location and dispersion of activation indicate that the tool network undergoes refinement between ages 4 and 8 years.
Keywords: category-specificity, conceptual processing, fMRI, parietal cortex, tools
Introduction
Visual object processing in the brain is divided across multiple parallel processing streams that originate at subcortical and early cortical levels (Ungerleider and Mishkin 1982; Felleman and van Essen 1991; Goodale and Milner 1992; Merigan and Maunsell 1993). The ventral stream, which projects from early visual areas to inferior temporal and lateral occipital areas, processes visual information in the service of categorizing and identifying objects (e.g., shape, texture, and color; Gainotti et al. 1995; Miceli et al. 2001; Grill-Spector et al. 2001; Capitani et al. 2003; Rogers et al. 2005; Cant and Goodale 2007). The dorsal stream, which projects to posterior parietal cortex, processes information relevant for acting on objects (e.g., object orientation and volumetric properties; Perenin and Vighetto 1988; Jeannerod et al. 1994; Pisella et al. 2006; for reviews see Milner and Goodale 1995; Goodale and Humphrey 1998; Kravitz et al. 2011). Although the dorsal stream processes visual information for action, to act upon manipulable objects requires the successful integration of processing represented across the dorsal and ventral visual pathways. As a result, cortical regions within both pathways are involved in processing manipulable objects. These regions are recruited for processing tools even without explicit instructions to act upon or prepare to act upon the objects (Chao and Martin 2000; Fang and He 2005; Mahon et al. 2007; Dekker et al. 2011; for reviews see Lewis 2006; Martin 2007), suggesting that both high-level visual (ventral stream) and action (dorsal stream) knowledge are automatically activated during tool processing. Therefore, studying tools can provide a window into how the brain integrates visual and action knowledge. This is important for understanding the principles that govern communication among distant brain regions generally and provides clues about the constraints that shape object representations in ventral temporal cortex.
Neuroimaging studies have revealed a network for processing tools that includes three principal regions: the inferior parietal lobule, the medial fusiform gyrus (bilaterally), and the posterior middle temporal gyrus (Chao et al. 1999; Chao and Martin 2000; Noppeney et al. 2006; Mahon et al. 2007; for a review see Martin 2007). Each of these 3 regions represents a distinct type of knowledge about tools. For instance, the left posterior middle temporal gyrus has been found to be involved in tool naming, and damage to this structure can result in a loss of lexical-semantic knowledge for tools (Martin et al. 1996; Tranel et al. 2003; Brambati et al. 2006; Campanella et al. 2010). The left inferior parietal lobule is involved in representing high-level action knowledge associated with complex object manipulation (e.g., how to grasp and swing a hammer in order to pound a nail; Binkofski et al. 1999; Rumiati et al. 2004; for a review see Johnson-Frey 2004). By hypothesis, communication among these regions should be instrumental for the successful integration of the different types of information that are necessary to direct the correct actions at the correct objects (for discussion, see Wu 2008). Prior work has shown that the amplitudes of neural responses across participants are correlated between the left fusiform gyrus and the left inferior parietal lobule for tool stimuli, but are not correlated for other stimuli (Mahon et al. 2007). Similar findings have been observed using time-course-based functional connectivity (Noppeney et al. 2006; Mahon et al. 2007, 2013; Almeida et al. 2013; Garcea and Mahon 2014; Stevens et al. 2015 see also Simmons and Martin 2012; Hutchison et al. 2014 for relevant functional connectivity results), suggesting that these regions of parietal and temporal cortex are fundamentally linked. Thus, the tool network includes regions that not only express stimulus preferences for tools but also are functionally coupled with each other (for relevant theoretical discussion, see Martin and Chao 2001; Barsalou et al. 2003; Noppeney et al. 2006).
Functional coupling among brain regions may shape the organization of object representations. According to a “connectivity-constrained account” of the organization of the ventral visual pathway, neural specificity for manipulable objects in the medial fusiform gyrus is caused by that brain region having an innate bias to be connected with action-relevant information in parietal cortex (Mahon et al. 2007, 2009; Mahon and Caramazza 2011; Stevens et al. 2015; for discussion, see Chen and Rogers 2015; Riesenhuber 2007). A similar proposal in the domain of word processing has argued that connectivity with left hemisphere language regions constrains the location of the visual word form area (Dehaene et al. 2005; Martin 2006; Plaut and Behrmann 2011; Bouhali et al. 2014).
Despite the abundance of research on the tool network in adults, there is little evidence from children regarding the development of the tool network. However, knowing the developmental trajectory of tool preferences in the brain could elucidate the organizational constraints of the tool network and the organizational constraints of other networks that exhibit stimulus selectivity. To date, only one previous study examined neural responses to tools in children. Dekker et al. (2011) used functional magnetic resonance imaging (fMRI) to compare the neural responses of 6- to 8-year-old children and adults as they passively viewed intact and scrambled images of tools and animals. They found that, by 6 years of age, children showed a neural preference for tools over animals in regions that were also tool preferring in adults, suggesting that the adult tool network develops tool preferences by at least 6–8 years of age. That study did not test whether there are correlated neural responses across regions of the tool network, nor how stimulus preferences might change (or emerge) over development. Thus, at present we do not know if all of the regions of the adult tool network develop in parallel. In the present study, we study the emergence of tool preferences in younger children (4- to 6-year olds) and test for developmental correlations in neural responses across the tool network.
Materials and Methods
Participants
Twenty-nine typically developing children (4.11–8.77 years, mean age = 6.6 years, 12 females) and 29 adults (18.44–28.09 years, mean age = 22.0 years, 16 females) participated in this study. Adult participants served as a reference group for the developed tool network and thus represented the “end-point” of development. All participants had normal or corrected to normal vision and no history of neurological impairments. All participants or their parents gave informed written consent in accordance with the University of Rochester's Research Subjects Review Board.
fMRI Session
Prior to scanning, children completed a 30-min training session in the mock scanner to familiarize them with the scanner environment and experimental task and to practice staying as still as possible. During scanning, children's heads were stabilized with headphones, foam padding, and medical tape. Adults simply received verbal instructions prior to entering the scanner room and completed a brief practice session during the anatomical scan.
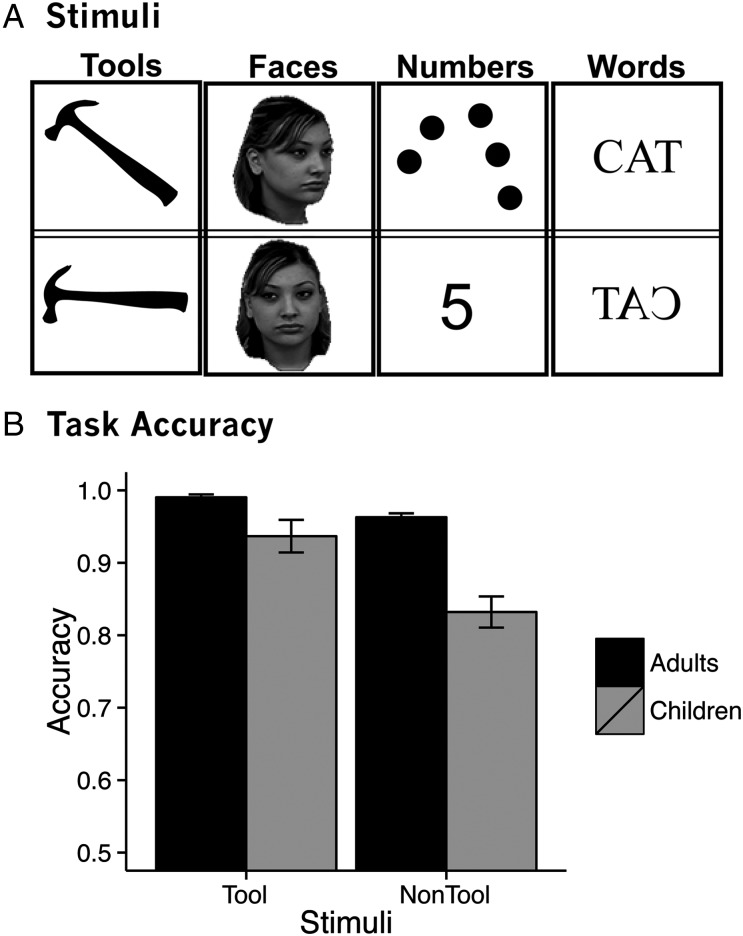
Following an anatomical scan, participants' BOLD contrast in response to pictures of faces, letters, tools, and numbers was measured. Stimuli were presented two at a time, one to the left and one to the right of center as gray-scale (faces) or white (letters, tools, Arabic numerals/dot arrays) images on a green background. Tool stimuli were silhouettes because we wanted children to focus on the global shape properties of the tool stimuli, rather than on internal details. Participants compared pairs of stimuli within-category and pressed a response button when the stimuli matched. Matches were always made across orientation (face, tools) or notation (words, numbers). Faces were presented as a frontal shot paired with an oblique view (45° to the left). Pairs of tool images consisted of one upright image and one image rotated 45 or 90°. Number stimuli (ranging from 1 to 9) were presented as an Arabic numeral paired with a dot array. Pairs of words consisted of one word in capital letters, normally oriented, and one word in capital letters presented as a mirrored image. Example stimuli from each of the 4 categories are shown in Figure 1A.
Figure 1.
(A) Example stimuli and (B) accuracy from fMRI matching task.
Participants completed 2 4.4-min runs of this task. Stimuli were presented in a miniblock design, with each miniblock consisting of three 2-s comparison trials from the same stimulus condition. Between each comparison trial, participants fixated on a central crosshair with a “thumbs-up” image (inter-trial interval duration = 2 s). Eight seconds of fixation occurred between miniblocks.
MR Parameters
Whole-brain BOLD imaging was conducted on a 3-Tesla Siemens MAGNETOM Trio scanner with a 12-channel head coil at the Rochester Center for Brain Imaging. High-resolution structural T1 contrast images were acquired using a magnetization prepared rapid gradient echo pulse sequence at the start of each session [repetition time (TR) = 2530 ms, echo time (TE) = 3.44 ms flip angle = 7°, field of view (FOV) = 256 mm, matrix = 256 × 256, 192 or 176 [depending on head size] 1 × 1 × 1 mm sagittal left-to-right slices].
An echo-planar imaging pulse sequence with online motion correction was used for T2* contrast (TR = 2000ms, TE = 30 ms, flip angle = 90°, FOV = 256 mm, matrix 64 × 64, 30 axial oblique slices, parallel to the AC–PC plane, voxel size = 4 × 4 × 4 mm). There were 2 functional runs of 132 volumes each of the matching task. Total scanning time was approximately 17 min.
Preprocessing
fMRI data were analyzed using BrainVoyager (Goebel et al. 2006) and in-house scripts drawing on the BVQX toolbox (wiki2.brainvoyager.com/BVQXtools). The first 6 volumes of functional data in each run were discarded prior to analysis. Preprocessing consisted of slice scan time correction (cubic spline interpolation), motion correction with respect to the first volume in the first run, and linear trend removal in the temporal domain (cutoff: 2 cycles within the run). Functional data were registered to high-resolution anatomy on a participant-by-participant basis in native space. Echo-planar and anatomical volumes were then transformed into Talairach space (Talairach and Tournoux 1988). Adult and child data were normalized into Talairach space by first aligning images with the stereotactic axes and then transforming them to the Talairach grid using a piecewise affine transformation based on manual identification of anatomical landmarks. Analyses were performed on preprocessed data in Talairach space. A Gaussian spatial filter was applied to each volume of functional data at 1.5 voxels (6 mm) FWHM.
Functional data were analyzed using the general linear model. Experimental events in the matching task were convolved with a standard dual gamma hemodynamic response function. The general linear model included 4 regressors of interest corresponding to the 4 stimulus conditions, 1 regressor of no interest for the button press, and 6 regressors of no interest that corresponded to motion parameters obtained during preprocessing. A random-effects analysis was used to analyze the group data. Adult and child data were modeled separately.
Results
Behavior
Children and adults both performed well above chance on the matching task. To compare performance between groups, we performed a 2 (age group: adults/children) by 2 (stimulus type: tool stimuli/nontool stimuli) ANOVA on task accuracy. This ANOVA revealed a main effect of age group (F1,56 = 20.3, P < 0.01), a main effect of stimulus type (F1,56 = 50.2, P < 0.01), and a significant interaction between age group and stimulus type (F1,56 = 17.2, P < 0.01; Fig. 1B). Post hoc t-tests revealed that adults (mean accuracy = 0.98) were significantly more accurate than children (mean accuracy = 0.88; t(63) = 5.3, P < 0.01) and that accuracy was higher for tool stimuli (mean accuracy = 0.96) compared with nontool stimuli (mean accuracy = 0.90; t(57) = 6.5, P < 0.01). Adults were better at identifying matches for tool stimuli (mean accuracy = 0.99) compared with nontool stimuli (mean accuracy = 0.96; t(28) = 5.6, P < 0.01), and children showed a greater difference in accuracy for tool stimuli (mean accuracy = 0.94) compared with nontool stimuli (mean accuracy = 0.83; t(28) = 5.8, P < 0.01). Outside of the scanner children's accuracy on a tool-naming task, for which the stimulus set largely overlapped with the stimuli from the fMRI task, was on average much lower (mean naming accuracy = 0.70) than their performance during the tool-matching task. This suggests that children were better at matching images of tools than they were at naming similar tool stimuli.
Tool Preferences
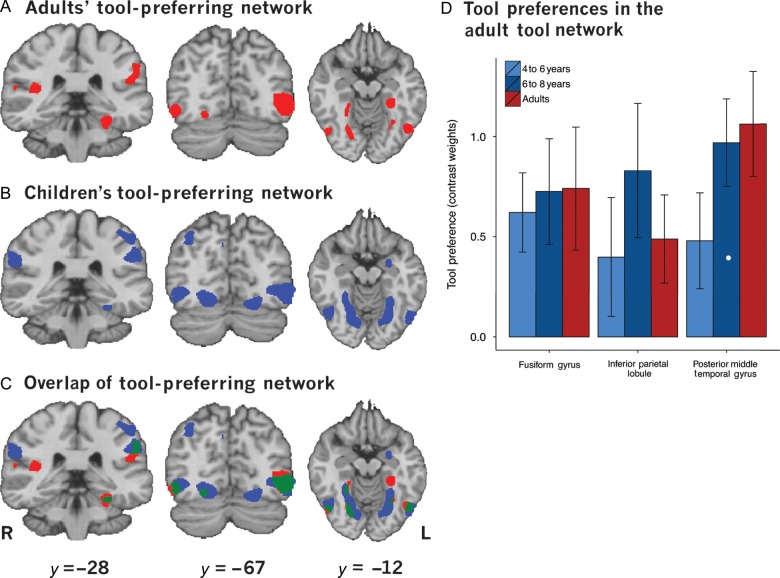
The adult tool-preferring network was identified with a whole-brain statistical contrast of tool stimuli versus nontool stimuli (faces, numbers, and words weighted equally against tool stimuli). This revealed tool-preferring regions in the bilateral medial fusiform gyrus, bilateral posterior middle temporal gyrus, and left inferior parietal lobule (P < 0.005; Fig. 2A), replicating previous work (e.g., Chao et al. 1999; Chao and Martin 2000; Noppeney et al. 2006; Mahon et al. 2007). The same contrast performed on data from children revealed that children recruited a network of regions that overlapped with the adult network. Specifically, like adults, children recruited regions of the inferior parietal lobule, posterior middle temporal gyrus, and medial fusiform gyrus (Fig. 2B). Thus, adults and children recruited a common tool-processing network (Fig. 2C). In addition, children demonstrated tool preferences in larger regions around the fusiform gyrus as well as more superior regions of the parietal lobule. Table 1 presents a full list of tool-preferring regions from adults and children. Although whole-brain analyses revealed bilateral activation of the fusiform gyrus and posterior middle temporal gyrus, the remaining analyses will focus on left hemisphere regions because the left hemisphere is known to be critical for mature, adult tool processing (for reviews see Johnson-Frey 2004; Lewis 2006; Martin 2007). We suggest that the bilateral recruitment seen in the main contrast of tool stimuli over nontool stimuli is driven by the bilateral presentation of stimuli during the fMRI sessions, and thus the development of tool processing should be assessed by the maturity of the left hemisphere tool representations.
Figure 2.
Tool-preferring regions identified by a contrast of tools > faces, numbers, and words at P < 0.005. (A) The tool-preferring network in adults. (B) The tool-preferring network in children. (C) Overlap of the adult and child tool-preferring networks. (D) Contrast weights in the adult tool network.
Table 1.
Regions recruited during Tools > Else at P < 0.005 in adults and children
| Region | Hemisphere | Number of voxels | TAL coordinates |
||
|---|---|---|---|---|---|
| Peak X | Peak Y | Peak Z | |||
| Adults | |||||
| Frontal lobe | Left | 12 | −19 | −14 | 47 |
| Inferior temporal cortex | Left | 303 | −26 | −54 | −15 |
| Right | 1601 | 23 | −47 | −19 | |
| Right | 433 | 21 | −66 | −13 | |
| Parahippocampal gyrus | Left | 2141 | −27 | −24 | −19 |
| Right | 602 | 28 | −16 | −26 | |
| Cingulate gyrus | Right | 386 | 13 | −24 | 44 |
| Right | 2 | 17 | −17 | 35 | |
| Occipital lobe | Right | 754 | 44 | −66 | −9 |
| Right | 33 | 44 | −77 | −5 | |
| Left | 4474 | −46 | −61 | −6 | |
| Parietal lobe | Left | 762 | −54 | −29 | 33 |
| Left | 11 | −29 | −35 | 39 | |
| Claustrum | Left | 1127 | −35 | −15 | 0 |
| Left | 6 | −31 | −21 | 9 | |
| Temporal lobe | Left | 38 | −38 | −9 | −14 |
| Right | 2877 | 38 | −22 | 5 | |
| Right | 140 | 42 | −8 | −12 | |
| Right | 8 | 62 | 8 | −3 | |
| Children | |||||
| Cerebellum | Right | 24 | 20 | −70 | −33 |
| Frontal lobe | Left | 849 | −20 | 49 | 26 |
| Left | 642 | −6 | 60 | 17 | |
| Left | 196 | −21 | −21 | 58 | |
| Right | 213 | 17 | 55 | 30 | |
| Right | 84 | 6 | 61 | 16 | |
| Right | 67 | 60 | 2 | 7 | |
| Right | 9 | 53 | 31 | 5 | |
| Right | 6 | 2 | 58 | 33 | |
| Inferior temporal cortex | Left | 155 | −46 | −8 | −22 |
| Left | 3 | −52 | −11 | −27 | |
| Parahippocampal gyrus | Left | 304 | −28 | −28 | −18 |
| Left | 219 | −31 | −15 | −19 | |
| Uncus | Right | 1239 | 31 | −14 | −25 |
| Occipital lobe | Left | 2124 | −20 | −88 | 23 |
| Right | 1455 | 16 | −85 | 13 | |
| Occipital lobe and inferior | Left | 10 814 | −47 | −69 | −4 |
| Temporal cortex | Right | 10 154 | 32 | −49 | −6 |
| Parietal lobe | Left | 36 | −43 | −41 | 60 |
| Right | 178 | 23 | −48 | 55 | |
| Parietal lobe and claustrum | Left | 14 459 | −55 | −23 | 28 |
| Claustrum | Right | 2412 | 34 | −4 | 1 |
| Insula | Left | 7 | −43 | −20 | 12 |
| Temporal lobe | Left | 26 | −39 | 6 | −27 |
| Left | 8 | −40 | −22 | 9 | |
| Left | 4 | −46 | −2 | −13 | |
| Left | 3 | −46 | 10 | −24 | |
| Right | 6023 | 61 | −11 | 12 | |
| Right | 296 | 59 | 11 | −4 | |
| Right | 22 | 65 | −14 | −3 | |
To test for developmental changes in the strength of tool-preferring activation, contrast-weighted t-values for the contrast of tool stimuli over nontool stimuli were extracted from adult-defined regions of interest (ROIs) (left fusiform gyrus, left posterior middle temporal gyrus, and left inferior parietal lobule). A leave-one-out strategy was adopted to extract contrast-weighted t-values for adults without introducing bias between voxel selection and the extracted contrast-weighted t-values (for discussion, see Kriegeskorte et al. 2009). Specifically, n − 1 (i.e., 28) adults were used to define at the group level (random effects) all regions of the tool network, and then the contrast-weighted t-values for those regions were extracted for the adult who had been left out of the group analysis. This procedure was iterated 29 times, each time leaving a different adult participant out of the analysis. This ensures that all contrast-weighted t-values were extracted from regions defined without bias. Children were split into 2 age groups: one consisting of younger children (4–6 years) and one consisting of older children (6–8 years). We performed one-way ANOVAs across age (4–6 years, 6–8 years, adults) within each ROI to determine if there were any differences in the strength of tool preferences (contrast-weighted t-values) across age groups (Fig. 2D). No effect of age was found in any ROI (fusiform gyrus: F2,55 < 1; posterior middle temporal gyrus F2,55 = 1.2, P > 0.1; inferior parietal lobule: F2,55 < 1), suggesting that the strength of tool preferences in adult regions of the tool network are relatively adult-like by 4 or 5 years. Because no age-related differences were found, the remaining analyses collapse the data across all children.
Co-activation of the Tool Network
To test whether adults exhibit co-activation in tool-preferring regions, we extracted β values from our 3 ROIs: the left inferior parietal lobule, the left fusiform gyrus, and the left posterior middle temporal gyrus. We then tested for inter-region correlations in β values across subjects for tool as well as for nontool stimuli. Inter-region correlations were found for tool stimuli between the left inferior parietal lobule and the left medial fusiform gyrus (r = 0.43, P < 0.02), between the left inferior parietal lobule and the left posterior middle temporal gyrus (r = 0.56, P < 0.01), and between the left medial fusiform gyrus and the left posterior middle temporal gyrus (r = 0.49, P < 0.01). No significant correlations were found for nontool stimuli between the inferior parietal lobule and the fusiform gyrus or between the fusiform gyrus and the posterior middle temporal gyrus (Ps > 0.1). There was a nonsignificant trend toward significant co-activation for nontool stimuli between the left parietal lobule and the left posterior middle temporal gyrus (r = 0.33, P = 0.08) (Fig. 3, top row of panels A, B, C).
Figure 3.
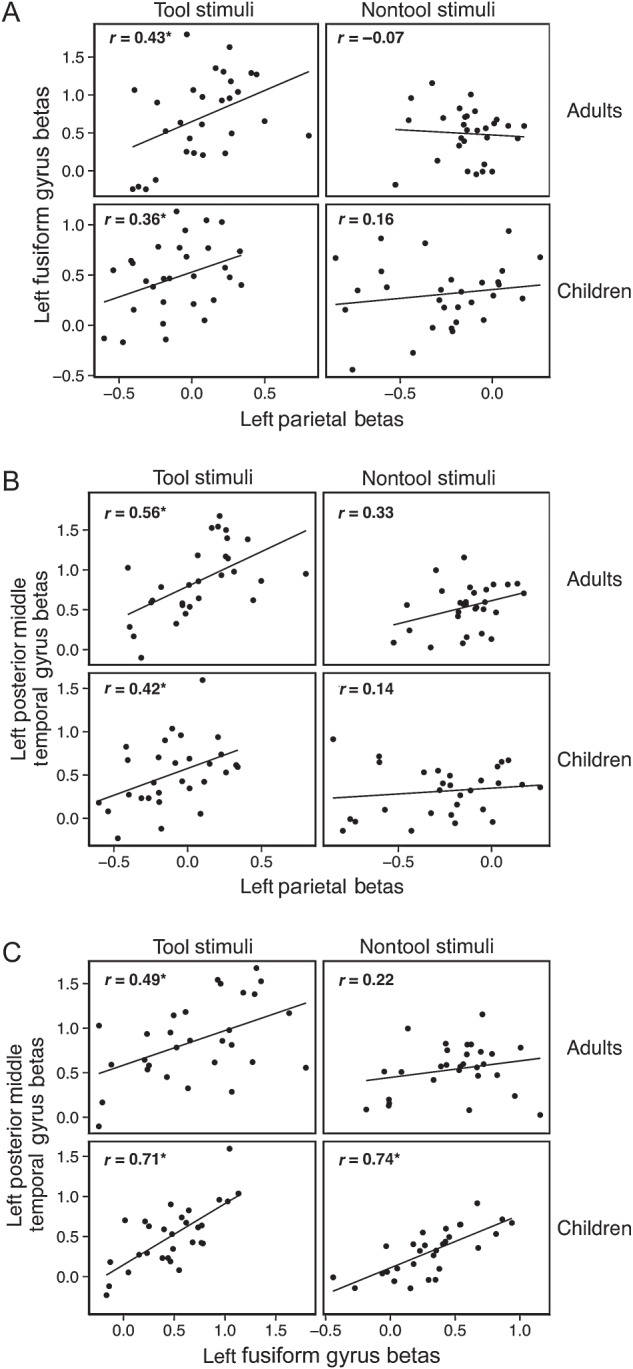
Inter-region correlations for adult data between the left inferior parietal lobule and the left fusiform gyrus (A), the left posterior middle temporal gyrus (B), and between the left fusiform gyrus and the left posterior middle temporal gyrus (C) for tool stimuli (column 1) and nontool stimuli (column 2). Correlation values (r) are indicated on each scatterplot. Significant correlations are indicated by asterisks following the R-values. Adult data are depicted in the top row of each panel, and the data from children are in the bottom row of each panel. Within the adult tool network, significant correlations were observed for tool-related stimuli in children and adults. Nontool stimuli were only correlated for children between the left posterior middle temporal gyrus and the left fusiform gyrus.
We repeated this analysis in children by extracting children's β weights from the same 3 regions of the adult tool-processing network. Inter-region correlations revealed that children show similar patterns of co-activation as adults. Children's neural responses to tools in the left inferior parietal lobule were significantly correlated with their neural responses to tools in the left medial fusiform gyrus (r = 0.36, P = 0.05) and the left posterior middle temporal gyrus (r = 0.42, P < 0.03). In contrast, children's neural responses to nontool stimuli in the left inferior parietal lobule were not correlated with their nontool neural responses in the left medial fusiform gyrus (P > 0.1) or left posterior middle temporal gyrus (P > 0.1). This replicates the patterns of co-activation seen in adults. Interestingly, children's neural responses in the left medial fusiform gyrus were significantly correlated with their responses in the left posterior middle temporal gyrus for tool stimuli (r = 0.71, P < 0.01) and nontool stimuli (r = 0.74, P < 0.01) (Fig. 3, bottom row of panels A,B,C), indicating that co-activation of these regions is not restricted to tool stimuli. This suggests that the pattern of co-activation seen for these regions in adults (i.e., co-activation of the left medial fusiform gyrus and the left middle temporal gyrus for tool stimuli, but not for nontool stimuli) develops later than the tool-preferring co-activation of other regions of the tool-processing network.
A regression analysis was conducted to determine whether the correlation of neural responses between the medial fusiform gyrus and the inferior parietal lobule was present only for tool stimuli. This analysis was conducted over the subject-level β estimates for tool and nontool stimuli. Neural responses in the left medial fusiform gyrus were the dependent variable, and neural responses in the left inferior parietal lobule, age group (child, adult), and stimulus type (tool stimuli, nontool stimuli) were predictors. Also included as predictors in the model were the interaction between stimulus type and parietal activation, and the three-way interaction of age group × parietal activation × stimulus type. The model was significant overall (R2 = 0.22, F6, 109 = 5.06, P < 0.01) with a significant main effect of stimulus type (β = 0.18, P < 0.05) and a significant interaction between stimulus type and activation in the left parietal lobule (β = 0.96, P = 0.056). Follow-up tests revealed that activation in the left parietal lobule significantly predicted activation in the left fusiform gyrus for tool stimuli (R2 = 0.20, F1, 56 = 13.70, P < 0.01), but not for nontool stimuli (R2 = 0.02, F1, 56 = 1.15, P > 0.1). There was no main effect of age group or an interaction between age group, stimulus type, and parietal activation (all Ps > 0.16). This replicates the pattern of results we observed from the correlation analyses and indicates that the left inferior parietal lobule and the left medial fusiform gyrus exhibit co-activation in their neural responses only for tool stimuli, and beginning, at least, at 4–5 years of age.
Quantitative Analysis of Locations of Tool Preferring Peaks
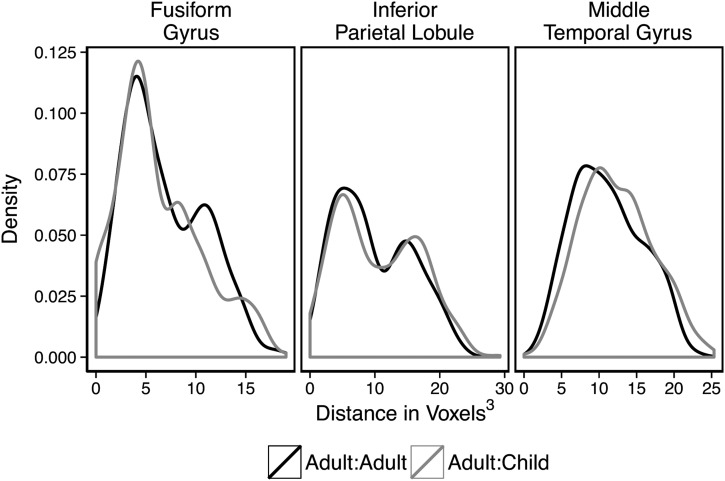
To quantify the degree of overlap between children and adults, we calculated the distances among each individual's peak voxel and every other individual's peak voxel in each left hemisphere ROI. If adults and children recruit similar regions, then the distances among peak voxels when comparing an adult's peak to a child's peak should be no different than when comparing within adults. In contrast, if adults and children consistently recruit somewhat different regions, then the distances among peak voxels when comparing adults' peaks to children's peaks should be significantly greater than when comparing within adults.
As in the extraction of contrast weights above, to maintain independence, peaks were extracted from ROIs defined by an n − 1 subset of adult data that excluded the adult whose peaks we were currently extracting. Children's peaks were extracted from each of the ROIs, for each iteration of the n − 1 strategy in adults, and averaged across the 29 data folds, for each child. Distances were calculated from each adult's peak to every other adult's peak and from each adult's peak to every child's peak. Figure 4 plots the distributions of distances from each adult's peak to every other adult's peak and from each adult's peak to every child's peak by region of interest. These density plots suggest that the distances between one individual's peak to every other individual's peak were fairly consistent across calculation type (i.e., adult-to-adult and adult-to-child distance calculations) (It should be noted that, particularly in the left inferior parietal lobule, the distribution seems to be bimodal. This suggests that there may be subgroups of participants that systematically differ in the location of their peak tool-preferring voxels within the tool-preferring ROIs. For present purposes, what is relevant is that the same pattern is observed in adults and children. For a discussion of different approaches for parcellating tool-responsive areas of parietal cortex, and an empirical demonstration of why there may be subclusters within the large parietal tool-preferring ROI, see Garcea and Mahon (2014)). This suggests that the distances among locations of peak activations for each adult to every other adult are similar to the distances when comparing the locations of peak activity in each adult to those of individual children.
Figure 4.
Distributions of distances between peak voxels as calculated from one adult's peak to another's peak and from one adult's peak to a child's peak. Adult-to-child peak distance distributions were similar to adult-to-adult peak distance distributions in the inferior parietal lobule. In the medial fusiform gyrus, average adult-to-child distance was lower than the average for adult-to-adult. However, in the left posterior middle temporal gyrus, the average of adult-to-child was larger than the average for adult-to-adult.
To explore this similarity, we conducted one-way ANOVAs on data from each of the three ROIs. The ANOVAs tested for variation in peak locations for each calculation type (adult–adult, adult–child, and for completeness we also included child–child calculations of distance between each child's peak to every other child's peak). These ANOVAs revealed a main effect of calculation type in the left fusiform gyrus (F2,1650 = 11.7, P < 0.01), such that adult-to-adult distance calculations were significantly greater than both the adult-to-child distance calculations (t(856) = 2.2, P = 0.03) and the child-to-child distance calculations (t(639) = 5.7, P < 0.01), while the adult-to-child distance calculations were significantly greater than the child-to-child distance calculations (t(1237) = 4.0, P < 0.01). This indicates that the peak voxels within the fusiform gyrus were significantly more variable when comparing within the group of adults than when comparisons were made between adults and children or between individual children. Because the distances calculated from adult to child were smaller than those calculated from adult to adult, this indicates overlap among adult and child peaks in the fusiform gyrus. A significant effect of calculation type was also found in the left middle temporal gyrus (F2,1650 = 12.4, P < 0.01), but here the adult-to-adult variability was significantly less than both the adult-to-child calculations(t(833) = −4.2, P = 0.03) and the child-to-child calculations (t(783) = −4.2, P < 0.01). There was no difference between the child-to-child distance calculations and the adult-to-child distance calculations (t(984) = −0.9, P > 0.1). This indicates that although the locations of peak voxels in the left posterior middle temporal gyrus are closer together in adults than in children, the variability in distances between adults and children and within children is similar. The lack of a significant difference in the distance calculated from adults to children compared with the distances calculated form child to child, suggests a high degree of similarity in the peak responses for adults and children. There was no significant effect of distance calculation type in the left inferior parietal lobule (F2,1650 = 2.0, P > 0.10), suggesting that the locations of peak voxels have similar variability across children and adults.
Quantitative Analysis of the Extent of Tool-Preferring Activation
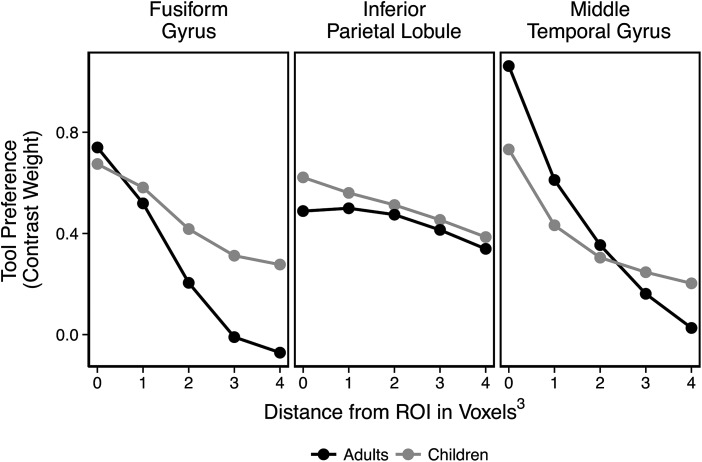
Visual inspection of group contrast maps (see Fig. 2) suggests that tool preferences are more diffuse (i.e., spread out) in children compared with adults. To test this in individual participants, we used the same contrast-weighted t-values from the analysis of tool preferences, and then extracted additional contrast-weighted t-values from voxels adjacent to, but outside of, the original adult ROIs. These 1-voxel thick “shells” were defined by taking the edge of the ROI, and expanding it by one functional voxel (i.e., 3 mm3), removing the center (i.e., everything but the shell), and then extracting t-values from that shell. Contrast weights were extracted from 4 such shells: one immediately surrounding the ROI, a shell that was 2 voxels from edge of the original ROI, a shell 3 voxels from the original ROI, and a shell 4 voxels away from the original ROI. If children's tool-preferring activation is in fact more extensive than adults' tool-preferring activation, then children should maintain higher levels of tool-preferring activation across the cortex surrounding the adult ROIs. To test this prediction, we compared the average t-value for the adults and children in each of the original ROIs and the 4 shells surrounding the original ROIs. This analysis was modeled after a similar analysis comparing face selectivity in adults and children (Golarai et al. 2007).
The results of this analysis, shown in Figure 5, suggest developmental differences in the extent of tool preferences in the medial fusiform gyrus and posterior middle temporal gyrus, but not in the inferior parietal lobule. A 2 (age group: children/adults) × 5 (distance from original ROI in voxels, with zero being the original ROI) ANOVA was performed in each of the 3 ROIs. Main effects of the distance from the original ROI were found in all 3 regions (left fusiform gyrus: F1,56 = 18.7, P < 0.01; left posterior middle temporal gyrus: F1,56 = 54.5, P < 0.01; left inferior parietal lobule: F1,56 = 4.12, P = 0.047). The main effects of distance show that, for both children and adults, the magnitude of tool preferences decreases as measurements move farther from the core ROI. The left posterior middle temporal gyrus exhibited an interaction between age group and distance (F1,56 = 6.26, P = 0.015), which indicated that adults exhibited a larger decrease in activation across shells than did children (adult mean decrease = 1.04, child mean decrease = 0.53, t(39) = 2.44, P < 0.02). This indicates that children maintained stronger tool preferences over more cortex surrounding the posterior middle temporal gyrus than did adults (but not within the core ROI). Although Figure 5 suggests a similar trend in the fusiform gyrus, the interaction between age group and distance from the original ROI did not reach significance (P = 0.15).
Figure 5.
Contrast-weighted t-values in ROIs and surrounding concentric rings of 1–4 voxels from the original ROI. Children activated more diffuse regions than adults in the middle temporal gyrus, but not in the inferior parietal lobule or fusiform gyrus. There was a notable, but nonsignificant, trend in the fusiform gyrus for more diffuse tool preferences in children compared with adults.
One concern that may be raised is that the extent of tool-preferring activation in children could be due to greater movement during the scan or differences in the signal-to-noise ratio between children and adults. While this is unlikely to explain the data, as the phenomenon was category-specific (i.e., tools versus nontools) and region-specific (i.e., in the left posterior middle temporal gyrus), we sought to empirically address those possible concerns. First, we tested for effects of motion by calculating average frame-wise displacement (FD; Grill-Spector et al. 2008; Power et al. 2012) across the whole brain for each participant. This allowed us to test whether the increased extent of tool-preferring activation around the posterior middle temporal gyrus could be due to differences in motion between adults and children. To that end, we tested for correlations between FD and the number of voxels with t-values that survived each of 5 thresholds (corresponding to P values <0.1, 0.05, 0.01, 0.005, and 0.001 on a single-subject whole-brain map). No significant correlations were found (all Ps > 0.1). We then conducted a similar analysis, in which we calculated the average temporal signal-to-noise ratio (tSNR, whole brain) for each participant (as calculated by Simmons et al. 2010). We first compared the average tSNR across the whole brain to the number of voxels with t-values that survived the same 5 thresholds. Again, no significant correlations were found (all Ps, > 0.1). Finally, to look more closely at tSNR in our ROIs and the 1-voxel shells that we defined to examine the extent of tool-preferring activation, we extracted the mean tSNR from those ROIs and shells. Plotting the mean tSNR values across participants for these regions (Supplementary Fig. 1) revealed a very different pattern than the pattern observed when evaluating the extent of tool-preferring activation (Fig. 5). This indicates that the pattern of results, and specifically the differences seen between adults and children in the extent of tool preferences, cannot be explained by differences in tSNR. These control analyses indicate that the greater extent of activation around the posterior middle temporal gyrus in children can be attributed to development of the tool network rather than to head motion during scanning or differences in signal to noise.
Discussion
Adults and children were presented with tool and nontool stimuli during fMRI. Our results indicate that adults and children recruit a common tool network consisting of the left inferior parietal lobule, the left medial fusiform gyrus, and the left posterior middle temporal gyrus during visual processing of tool images. Reliable tool preferences were evident in these regions in children and adults. Some evidence of developmental changes in tool preferences was observed, particularly, in the left posterior middle temporal gyrus, which exhibited more diffuse activation in children than in adults. Regions of the adult tool network exhibited correlated neural responses to tool but not nontool stimuli in both adults and children. That is, participants (children and adults) with high tool activation in the inferior parietal lobule also had relatively high tool-preferring activity in the medial fusiform gyrus and the posterior middle temporal gyrus (see Mahon et al. 2007 for a similar observation in adults). This demonstrates that not only are these regions preferentially engaged during tool processing, but also the patterns of neural responses to tools within these regions are correlated across participants, even in young children. Interestingly, a different pattern emerged in relation to co-activation of the posterior middle temporal gyrus and the medial fusiform gyrus. We found that children who had a high response to stimuli in the posterior middle temporal gyrus also had a high response to stimuli in the medial fusiform gyrus, regardless of the type of stimulus. Children's activation pattern in the middle temporal gyrus also differed from adult activation in that it was more diffuse across the region. These findings suggest that at least one component of the adult tool-processing network undergoes significant developmental change during early childhood. Taken together, our findings show that tool preferences emerge early in development, and there is a common trajectory of development among regions of the tool network.
Our findings are in line with previous work in adults (e.g., Martin et al. 1996; Binkofski et al. 1999; Chao et al. 1999; Chao and Martin 2000; Johnson-Frey 2004; Noppeney et al. 2006; Mahon et al. 2007, 2010, 2013; Almeida et al. 2013; Garcea and Mahon 2014) that has identified a tool-processing network consisting of the left inferior parietal lobule, the left posterior middle temporal gyrus, and the left medial fusiform gyrus. Our findings also accord with the previous findings of Dekker et al. (2011), who found that school-age children exhibit adult-like tool preferences. However, our data show that tool preferences are present in neural regions that form the adult tool network by at least 4 or 5 years of age and that children not only recruit similar regions as adults for processing tools, but they also exhibit correlated neural responses for tools but not for nontool stimuli between the inferior parietal lobule and the other regions of the tool-processing network.
The observation that both the fusiform gyrus and the inferior parietal lobule demonstrated adult-like levels of tool preferences in early childhood bears on accounts of the causes of neural specificity for manipulable objects in the medial fusiform gyrus. According to connectivity-constrained accounts (Mahon et al. 2007; Riesenhuber 2007; Stevens et al. 2015), neural specificity in the ventral stream for manipulable objects arises because of innate connectivity between the medial fusiform gyrus and regions of parietal cortex that process action properties of objects. That proposal is consistent with the known white matter connectivity between the inferior parietal lobule and the medial temporal lobe (e.g., Kravitz et al. 2011; for review and discussion, see Garcea and Mahon 2014). An alternative account of the causes of neural specificity for manipulable objects in the ventral stream appeals to domain-general properties of the visual system interacting with statistical regularities in the visual input (Haxby et al. 2001; Levy et al. 2001). There is no expectation for there to be privileged connectivity between the ventral stream and the parietal lobule under that domain-general account. Thus, a domain-general account would not predict that children as young as 4 years of age co-activate the medial fusiform gyrus and the inferior parietal lobule while viewing tools. The observation of very early specialization in the ventral stream for tools, together with yoked patterns of stimulus-evoked activity across ventral temporal and parietal cortex, is strongly suggestive of a connectivity-constrained account (for discussion, see Mahon and Caramazza 2011). Similar arguments have been articulated for connectivity constraints shaping neural specificity for faces and words in the ventral temporal cortex (Plaut and Behrmann 2011; Behrmann and Plaut 2013). A recent computational model (Chen and Rogers, 2015) has shown that connectivity-based constraints can yield neural specificity in the ventral stream. Finally, it has recently been shown that there is privileged functional connectivity between category-preferring regions of the ventral stream and regions outside of the ventral stream with congruent category preferences (Hutchison et al. 2014; Stevens et al. 2015).
An adult-like network for tool processing in early childhood seems surprising given strong behavioral evidence that object representations continue to develop throughout childhood. For instance, behavioral evidence suggests that young children are more likely to categorize objects based on physical features of the objects (e.g., material of the object) rather than features related to function (Smith et al. 1996; Landau et al. 1998). Furthermore, Mounoud et al. (2007) found that 9-year-old children were more strongly affected by tool-related action primes than 10-year-old children during a tool-picture naming task. Mounoud et al. (2007) argue that action primes were more facilitative to the 9-year olds because their representations of tools and their respective actions were less integrated than the 10-year olds, and thus, the 9-year olds had to rely more on visually presented tool-related action information than their 10-year-old counterparts. The ability to recognize tools from unusual viewpoints also improves between the ages of 6 and 11 (Bova et al. 2007; Dekker et al. 2011). Because object-directed actions are represented in the inferior parietal lobule, some researchers have proposed that tool preferences might first develop in the dorsal stream (Dekker et al. 2011). However, it is likely the case that high-level praxis knowledge in the inferior parietal lobule is not given bottom up by the visual input but must be accessed contingent on identification of the object (for data and discussion, see Almeida et al. 2013; Mahon et al. 2013). Mutual interactions between action-related neural representations in parietal cortex and visual object representations in the ventral stream likely both contribute to the development of the tool network. Our observation of co-activation of regions of the tool network in young children suggests that the left inferior parietal lobule and the left medial fusiform gyrus are in fact functionally interdependent during tool processing from a very young age. This raises the question of whether the types of information that are communicated between the inferior parietal lobule and the fusiform gyrus might change across development as children become adept at manipulating objects according to their function, as well as recognizing and naming those objects.
Although our study revealed relatively mature patterns of tool preferences in children, it is important to note that the degree to which neural regions are “tool selective” likely depends on the baseline against which tool stimuli are contrasted. Our study employed a baseline of faces, numbers, and words. It remains an open question whether children's tool preferences would show the same degree of maturity with a different baseline condition, such as nonmanipulable objects or novel manipulable objects. Future studies that conduct such contrasts will be critical for a full description of the development of the tool network.
We found that adults and children recruit overlapping regions during tool processing and that even in very young children neural responses to tools in all regions of the adult tool network are correlated. However, patterns of tool preferences were more diffuse in children than adults. At the group level, regions of the ventral stream that preferentially responded to tools in children were much larger than those recruited by the adults, and tool-preferring regions of the inferior parietal lobule recruited in the children extended more superiorly than adult activation. Thus, as has been shown for other stimulus categories in the ventral visual pathway, it could be that as tool concepts develop, tool-preferring neural activation in children is pruned or refined into the more focal adult tool network (e.g., Durston et al. 2006; Cantlon et al. 2011). This developmental refinement of cortical regions based on experience is known as interactive specialization (Johnson 2001, 2011), and for manipulable objects may depend on interactions between parietal-based action representations and ventral stream visual representations. This hypothesis can be directly and definitively tested with a longitudinal design that assesses tool knowledge behaviorally in the same cohort of young children that are studied with fMRI.
Supplementary material
Supplementary Material can be found at http://www.cercor.oxfordjournals.org/online.
Funding
This work was supported by the National Institutes of Health (R21 NS076176 and R01 NS089609 to B.Z.M. and R01 HD064636 to J.F.C.), the Alfred P. Sloan Foundation Fellowship (#220020300 to J.F.C.), the James S. McDonnell Foundation, and the National Sciences Foundation (DRL1459625 to J.F.C. and Graduate Research Fellowship DGE 1419118 to A.J.K.).
Supplementary Material
Notes
The authors thank Frank Garcea for his help with analyses. Conflict of Interest: None declared.
References
- Almeida J, Fintzi AR, Mahon BZ. 2013. Tool manipulation knowledge is retrieved by way of the ventral visual object processing pathway. Cortex. 49:2334–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou LW, Kyle Simmons W, Barbey AK, Wilson CD. 2003. Grounding conceptual knowledge in modality-specific systems. Trends Cogn Sci. 7:84–91. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Plaut DC. 2013. Distributed circuits, not circumscribed centers, mediate visual recognition. Trends Cogn Sci. 17:210–219. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H. 1999. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI study. Eur J Neurosci. 11:3276–3286. [DOI] [PubMed] [Google Scholar]
- Bouhali F, Thiebaut de Schotten M, Pinel P, Poupon C, Mangin J-F, Dehaene S, Cohen L. 2014. Anatomical connections of the visual word form area. J Neurosci. 34:15402–15414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bova SM, Fazzi E, Giovenzana A, Montomoli C, Signorini SG, Zoppello M, Lanzi G. 2007. The development of visual object recognition in school-age children. Dev Neuropsychol. 31:79–102. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Myers D, Wilson A, Rankin KP, Allison SC, Rosen HJ, Miller BL, Gorno-Tempini ML. 2006. The anatomy of category-specific object naming in neurodegenerative diseases. J Cogn Neurosci. 18:1644–1653. [DOI] [PubMed] [Google Scholar]
- Campanella F, D'Agostini S, Skrap M, Shallice T. 2010. Naming manipulable objects: anatomy of a category specific effect in left temporal tumours. Neuropsychologia. 48:1583–1597. [DOI] [PubMed] [Google Scholar]
- Cant JS, Goodale MA. 2007. Attention to form or surface properties modulates different regions of human occipitotemporal cortex. Cereb Cortex. 17:713–731. [DOI] [PubMed] [Google Scholar]
- Cantlon JF, Pinel P, Dehaene S, Pelphrey KA. 2011. Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cereb Cortex. 21:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitani E, Laiacona M, Mahon B, Caramazza A. 2003. What are the facts of semantic category-specific deficits? A critical review of the clinical evidence. Cogn Neuropsychol. 20:213–261. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. 1999. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 2:913–919. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. 2000. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 12:478–484. [DOI] [PubMed] [Google Scholar]
- Chen L, Rogers T. Forthcoming 2015. A model of emergent category-specific activation in the posterior fusiforn gyrus of sighted and congenitally blind populations. J Cogn Neurosci. doi:10.1162/jocn_a_00834 [DOI] [PubMed]
- Dehaene S, Cohen L, Sigman M, Vinckier F. 2005. The neural code for written words: a proposal. Trends Cogn Sci. 9:335–341. [DOI] [PubMed] [Google Scholar]
- Dekker T, Mareschal D, Sereno MI, Johnson MH. 2011. Dorsal and ventral stream activation and object recognition performance in school-age children. Neuroimage. 57:659–670. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. 2006. A shift from diffuse to focal cortical activity with development. Dev Sci. 9:1–8. [DOI] [PubMed] [Google Scholar]
- Fang F, He S. 2005. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nat Neurosci. 8:1380–1385. [DOI] [PubMed] [Google Scholar]
- Felleman D, van Essen D. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1:1–47. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Silveri MC, Daniele A, Giustolisi L. 1995. Neuroanatomical correlates of category-specific semantic disorders: a critical survey. Memory. 3:247–264. [DOI] [PubMed] [Google Scholar]
- Garcea FE, Mahon BZ. 2014. Parcellation of left parietal tool representations by functional connectivity. Neuropsychologia. 60:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. 2006. Analysis of functional image analysis contest (FIAC) data with BrainVoyagerQX: from single-subject to cortically aligned group GLM analysis and self-organizing group independent component analysis. Hum Brain Mapp. 27:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Ghahremani DG, Whitfield-Gabrieli S, Reiss A, Eberhardt JL, Gabrieli JDE, Grill-Spector K. 2007. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat Neurosci. 10:512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, Humphrey GK. 1998. The objects of action and perception. Cognition. 67:181–207. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. 1992. Separate visual pathways for perception and action. Trends Neurosci. 15:20–25. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Golarai G, Gabrieli J. 2008. Developmental neuroimaging of the human ventral visual cortex. Trends Cogn Sci. 12:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. 2001. The lateral occipital complex and its role in object recognition. Vision Res. 41:1409–1422. [DOI] [PubMed] [Google Scholar]
- Haxby J, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. 2001. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 293:2425–2430. [DOI] [PubMed] [Google Scholar]
- Hutchison RM, Culham JC, Everling S, Flanagan JR, Gallivan JP. 2014. Distinct and distributed functional connectivity patterns across cortex reflect the domain-specific constraints of object, face, scene, body, and tool category-selective modules in the ventral visual pathway. Neuroimage. 96:216–236. [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Decety J, Michel F. 1994. Impairment of grasping movements following a bilateral posterior parietal lesion. Neuropsychologia. 32:369–380. [DOI] [PubMed] [Google Scholar]
- Johnson MH. 2001. Functional brain development in humans. Nat Rev Neurosci. 2:475–483. [DOI] [PubMed] [Google Scholar]
- Johnson MH. 2011. Interactive specialization: a domain-general framework for human functional brain development? Dev Cogn Neurosci. 1:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Frey SH. 2004. The neural bases of complex tool use in humans. Trends Cogn Sci. 8:71–78. [DOI] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Mishkin M. 2011. A new neural framework for visuospatial processing. Nat Rev Neurosci. 12:217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. 2009. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 12:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau B, Smith L, Jones S. 1998. Object shape, object function, and object name. J Mem Lang. 38:1–27. [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. 2001. Center-periphery organization of human object areas. Nat Neurosci. 4:533–539. [DOI] [PubMed] [Google Scholar]
- Lewis JW. 2006. Cortical networks related to human use of tools. Neurosci. 12:211–231. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Anzellotti S, Schwarzbach J, Zampini M, Caramazza A. 2009. Category-specific organization in the human brain does not require visual experience. Neuron. 63:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. 2011. What drives the organization of object knowledge in the brain? Trends Cogn Sci. 15:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Kumar N, Almeida J. 2013. Spatial frequency tuning reveals interactions between the dorsal and ventral visual systems. J Cogn Neurosci. 25:862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Milleville SC, Negri GAL, Rumiati RI, Caramazza A, Martin A. 2007. Action-related properties shape object representations in the ventral stream. Neuron. 55:507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Schwarzbach J, Caramazza A. 2010. The representation of tools in left parietal cortex is independent of visual experience. Psychol Sci. 21:764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. 2007. The representation of object concepts in the brain. Annu Rev Psychol. 58:25–45. [DOI] [PubMed] [Google Scholar]
- Martin A. 2006. Shades of Déjerine—forging a causal link between the visual word form area and reading. Neuron. 50:173–175. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. 2001. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 11:194–201. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. 1996. Neural correlates of category-specific knowledge. Nature. 379:649–652. [DOI] [PubMed] [Google Scholar]
- Merigan W, Maunsell J. 1993. How parallel are the primate visual pathways? Annu Rev Neurosci. 16:369–402. [DOI] [PubMed] [Google Scholar]
- Miceli G, Fouch E, Capasso R, Shelton JR, Tomaiuolo F, Caramazza A. 2001. The dissociation of color from form and function knowledge. Nat Neurosci. 4:662–667. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. 1995. The visual brain in action. New York: (NY: ): Oxford University Press. [Google Scholar]
- Mounoud P, Duscherer K, Moy G, Perraudin S. 2007. The influence of action perception on object recognition: a developmental study. Dev Sci. 10:836–852. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ, Penny WD, Friston KJ. 2006. Two distinct neural mechanisms for category-selective responses. Cereb Cortex. 16:437–445. [DOI] [PubMed] [Google Scholar]
- Perenin MT, Vighetto A. 1988. Optic ataxia: a specific disruption in visuomotor mechanisms. I Different aspects of the deficit in reaching for objects Brain. 111:643–674. [DOI] [PubMed] [Google Scholar]
- Pisella L, Binkofski F, Lasek K, Toni I, Rossetti Y. 2006. No double-dissociation between optic ataxia and visual agnosia: multiple sub-streams for multiple visuo-manual integrations. Neuropsychologia. 44:2734–2748. [DOI] [PubMed] [Google Scholar]
- Plaut DC, Behrmann M. 2011. Complementary neural representations for faces and words: a computational exploration. Cogn Neuropsychol. 28:251–275. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenhuber M. 2007. Appearance isn't everything: news on object representation in cortex. Neuron. 55:341–344. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Hocking J, Mechelli A, Patterson K, Price C. 2005. Fusiform activation to animals is driven by the process, not the stimulus. J Cogn Neurosci. 17:434–445. [DOI] [PubMed] [Google Scholar]
- Rumiati RI, Weiss PH, Shallice T, Ottoboni G, Noth J, Zilles K, Fink GR. 2004. Neural basis of pantomiming the use of visually presented objects. Neuroimage. 21:1224–1231. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Martin A. 2012. Spontaneous resting-state BOLD fluctuations reveal persistent domain-specific neural networks. Soc Cogn Affect Neurosci. 7:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Reddish M, Bellgowan PSF, Martin A. 2010. The selectivity and functional connectivity of the anterior temporal lobes. Cereb Cortex. 20:813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LB, Jones SS, Landau B. 1996. Naming in young children: a dumb attentional mechanism? Cognition. 60:143–171. [DOI] [PubMed] [Google Scholar]
- Stevens WD, Tessler MH, Peng CS, Martin A. 2015. Functional connectivity constrains the category-related organization of human ventral occipitotemporal cortex. Hum Brain Mapp. 36:2187–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 1988. Co-planar stereotaxic atlas of the human brain. New York: (NY: ): Thieme. [Google Scholar]
- Tranel D, Kemmerer D, Adolphs R, Damasio H, Damasio AR. 2003. Neural correlates of conceptual knowledge for actions. Cogn Neuropsychol. 20:409–432. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. 1982. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. Cambridge: (MA: ): MIT Press; p. 549–586. [Google Scholar]
- Wu W. 2008. Visual attention, conceptual content, and doing it right. Mind. 117:1003–1029. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.