Abstract
Spinocerebellar ataxia 6 (SCA6), an autosomal dominant degenerative disease, is characterized by diplopia, gait ataxia, and incoordination due to severe progressive degeneration of Purkinje cells in the vestibulo- and spinocerebellum. Ocular motor deficits are common, including difficulty fixating on moving objects, nystagmus and disruption of smooth pursuit movements. In presymptomatic SCA6, there are alterations in saccades and smooth-pursuit movements. We sought to assess functional and structural changes in cerebellar connectivity associated with a visual task, hypothesizing that gradual changes would parallel disease progression. We acquired functional magnetic resonance imaging and diffusion tensor imaging data during a passive smooth-pursuit task in 14 SCA6 patients, representing a range of disease duration and severity, and performed a cross-sectional comparison of cerebellar networks compared with healthy controls. We identified a shift in activation from vermis in presymptomatic individuals to lateral cerebellum in moderate-to-severe cases. Concomitantly, effective connectivity between regions of cerebral cortex and cerebellum was at its highest in moderate cases, and disappeared in severe cases. Finally, we noted structural differences in the cerebral and cerebellar peduncles. These unique results, spanning both functional and structural domains, highlight widespread changes in SCA6 and compensatory mechanisms associated with cerebellar physiology that could be utilized in developing new therapies.
Keywords: DTI, eye movements, fMRI, spinocerebellar ataxia, vermis
Introduction
The autosomal dominant spinocerebellar ataxias (SCAs) constitute a genetically heterogeneous group of neurodegenerative disorders characterized by progressive motor incoordination, consisting of either isolated (pure cerebellar) ataxia or ataxia with additional progressive neurological deficits (Maschke et al. 2005). The worldwide prevalence of the SCAs is approximately 10/100 000. SCA, type 6 (SCA6), which is among the most common forms of autosomal dominant SCA represents about 31% of the dominant SCA cases in Japan and 15% of cases in the USA (Geschwind et al. 1997; Leggo et al. 1997; Schols et al. 1998; Matsumura et al. 2003).
SCA6 is caused by a CAG repeat expansion in exon 47 of the CACNA1A gene, encoding the α1A (Cav2.1) subunit, the main pore-forming subunit of the neuronal P/Q-type voltage-gated calcium channel (Zhuchenko et al. (1997) and is one of several clinically distinct, but overlapping dominantly inherited neurological disorders caused by distinct mutations in this gene. Additionally, it is one of 10 neurodegenerative diseases that, like Huntington's disease, are associated with polyglutamine-encoding CAG nucleotide repeat expansions, although the polyQ tract encoded by the CACNA1A gene has a normal range of only 4–18 and in SCA6 is expanded only to 19–33 repeats, considerably smaller than all other polyQ diseases. The age of onset of symptoms correlates inversely with the size of the expansion. SCA6 has been described as the prototype of a pure cerebellar ataxia (Gomez et al. 1997; Yang et al. 2000; Mantuano et al. 2003; Craig et al. 2004), characterized primarily by Purkinje cell degeneration and cerebellar atrophy. In structural magnetic resonance imaging (MRI) of the brain, SCA6 demonstrates cerebellar atrophy affecting mostly the vermis and, to a lesser extent, the cerebellar hemispheres (Murata et al. 1998; Satoh et al. 1998; Butteriss et al. 2005; Lukas et al. 2006). Neuropathological studies show a striking loss of cerebellar Purkinje cells with preservation of other brain regions although more recent reports suggest a more widespread involvement of cell loss (Seidel et al. 2012; Reetz et al. 2013). Cortical or cerebello-olivary atrophy is occasionally noted but attributed to transynaptic degeneration due to loss of Purkinje cells (Koeppen 2005).
SCA6 is characterized by an adult onset, slowly progressive cerebellar ataxia, dysarthria, and nystagmus. Initial symptoms in SCA6 include gait unsteadiness, stumbling and imbalance in about 90% of individuals, with the remainder presenting with diplopia, oscillopsia, dysarthria, or episodic vertigo several years before the gait disturbance (Globas et al. 2008). Symptoms progress slowly, and eventually all affected individuals have gait ataxia, upper limb incoordination, intention tremor, and dysarthria. Dysphagia and choking are common (Gomez et al. 1997).
Multiple ocular motor deficits in SCA6 are also common (Gomez et al. 1997; Buttner et al. 1998; Ackl et al. 2005; Bour et al. 2008; Jacobi et al. 2012), and may precede overt gait and limb ataxia (Oda et al. 2006; Christova et al. 2008). Diplopia occurs in about 50% of individuals. Others experience visual disturbances related to difficulty fixating on moving objects, as well as horizontal gaze-evoked nystagmus (70–100%) and vertical nystagmus (65–83%), compared with fewer than 10% of those with other forms of SCA (Gomez et al. 1997). Other eye movement abnormalities, such as periodic alternating nystagmus and rebound nystagmus, have also been described (Sasaki et al. 2003).
A large-scale study including 107 SCA6 patients (Jacobi et al. 2012) showed that the disruption of smooth-pursuit movements had the highest prevalence (92.5%) of the ocular motor deficits. Additionally, by using sensitive eye movement recording techniques (Christova et al. 2008), alterations in saccadic (velocity and metrics) as well as smooth-pursuit gain of eye movements in presymptomatic SCA6 subjects have been reported. Interestingly, both saccades and smooth-pursuit eye movements are known to place a high computational demand on the cerebellum, particularly the vermis (Konen et al. 2005).
Objective
In this study, we sought to assess functional and structural changes in cerebellar connectivity associated with a visual task. We hypothesized that gradual changes in task-dependent cerebellar network connectivity would parallel progression of disease. To this end, we acquired diffusion tensor imaging (DTI) and functional imaging data during a passive smooth-pursuit task in SCA6 patients and performed a cross-sectional comparison of cerebellar networks from individuals at different stages of disease.
Materials and Methods
Fourteen participants were recruited from The University of Chicago Ataxia Center and 20 age-matched control subjects (9 female/11 male, age range from 30 to 69 years, mean = 49.1 ± 11.91) were recruited from the local community. Individuals with preexisting severe psychiatric or other neurological illnesses were excluded. The diagnosis of SCA6 was established by genetic testing at a commercial laboratory according to standard methods (Athena Diagnostics, Wooster, MA). Clinical disease severity was estimated using a validated ataxia rating scale (SARAII) performed on the same day as the imaging study by a single examiner (C.M.G.) (Schmitz-Hubsch et al. 2006). The age of onset of the 12 affected patients ranged from 44 to 60 years (mean = 50.9 ± 4.7) and disease duration ranged from to 1 to 31 years (mean = 12.3 ± 9.2). The SARAII rating scale scores ranged from 2.0 to 26 (mean = 14.1 ± 7.4). In addition 2 subjects, age 52 and 63, bore a pathological repeat expansion, but were presymptomatic (SARAII = 0). These subjects did not demonstrate balance problems and also exhibited a minimal number of oculomotor problems. The demographics for all SCA6 subjects are listed in Table 1. SCA subjects were classified into 3 groups: “presymptomatic”–“early symptomatic” (SARAII 0–4), “moderate” (9–18), and “severe” (>18.5).
Table 1.
SCA6 patient demographics
| Subject | Age (years) | Sex | SARAII | Repeat expansion | Age at onset (years) | Duration of disease (years) | Oculomotor symptoms (# out of 4) | Oculomotor signs (# out of 4) |
|---|---|---|---|---|---|---|---|---|
| 1 | 55 | F | 10.0 | 22, 13 | 54 | 1 | 3 | 2 |
| 2 | 58 | M | 21.5 | 22, 13 | 48 | 10 | 2 | 3 |
| 3 | 52 | M | 4.0 | 22, 13 | 51 | 1 | 2 | 2 |
| 4 | 61 | F | 17.5 | 22, 14 | 44 | 17 | – | – |
| 5 | 73 | M | 22.0 | Family history | 48 | 24 | 2 | 3 |
| 6 | 63 | F | 18.5 | 22, 14 | 55 | 8 | 4 | 2 |
| 7 | 58 | M | 13.0 | 24, 11 | 48 | 10 | 1 | 3 |
| 8 | 68 | F | 15.0 | 22, 13 | 50 | 18 | 1 | 3 |
| 9 | 81 | F | 26.0 | 22, 16 | 50 | 31 | 3 | 3 |
| 10 | 62 | M | 9.0 | 22, 13 | 60 | 11 | 0 | 2 |
| 11 | 52 | F | 0.0 | 22, 13 | 0 | 0 | 2 | 0 |
| 12 | 59 | F | 2.0 | 22, 13 | 57 | 2 | 4 | 2 |
| 13 | 60 | F | 10.5 | 22, 14 | 46 | 14 | 3 | 2 |
| 14 | 63 | M | 0.0 | 22, 13 | 0 | 0 | 1 | 2 |
This study and all procedures for recruitment and consent were approved by the Institutional Review Board of the Division of Biological Sciences of The University of Chicago.
Assessment of Oculomotor Alterations
To assess severity of oculomotor deficits, the following signs and symptoms were determined during clinical evaluations: Symptoms: vertigo, diplopia, problems focusing, and oscillopsia. Signs: nystagmus, pursuits, saccades, and square wave jerks. For each subject, the number of symptoms and signs present were quantified for a maximum of 4 for each (Table 1). This number was correlated with SARAII score using a Pearson's correlation.
Imaging
Imaging was performed using a 3 T Philips scanner equipped with a SENSE head coil using higher order shims. Subjects were placed in the scanner, with head movement restricted by foam rubber pillows. Electrostatic headphones (Resonance Technologies, Northridge, CA) were placed on the ears and connected to a computer controlled stereo system. The computer was equipped with the PsyScope psychological software system (Cohen et al. 1993), which was used to present the experimental stimuli. Functional scans were acquired while participants passively viewed a 5-min video that alternated between 20 s of rest and 20 s of visual stimuli. The visual stimuli showed different landscapes through the perspective of a walker as seen in the first person. Rest periods were visually neutral showing a static image of a starry sky on a black background. The visual stimuli were presented in videos and did not involve reflexive activity via vestibulo-ocular reflex (VOR) (the head of the participants was immobilized), or optokinetic reflex (i.e., there were no objects moving along the visual field). Hence these global motion stimuli basically involved saccadic and smooth-pursuit movements.
Structural Images
High-resolution T1-weighted 3-dimensional magnetization prepared rapid gradient echo scans were collected: field of view (FOV) = 240 mm2, resolution = 0.75 × 0.75 × 0.75 mm, SENSE reduction factor = 1.5, repetition time (TR)/echo time (TE) = 11/4.5 ms, flip angle = 18°, sagittal orientation, number of slices = 200 covering the whole brain.
fMRI
Blood oxygen level dependent (BOLD) images were collected for the task-dependent functional images using single-shot echo-planar imaging (EPI), with FOV = 250 mm2, TR/TE = 2000/30 ms, flip angle = 70°, in-plane resolution = 2 mm × 2 mm, slice thickness = 5 mm, number of slices = 30 covering the whole brain.
Diffusion Tensor Imaging
DTI images were collected using a single-shot echo-planar spin-echo sequence with the following parameters: total scan time = 8 min 15 s, image volumes acquired using 32 different diffusion sensitizing gradient directions at b = 1000 s/mm2, flip angle = 90°, FOV = 224 mm2, resolution = 0.875 × 0.875 mm2, slice thickness = 2 mm, number of slices = 60, TR/TE = 1031/55 ms, SENSE reduction factor = 2, and EPI factor = 59. Two b = 0 s/mm2 images were acquired. All images were reconstructed to a 256 × 256 matrix.
Image Analysis
fMRI
Following acquisition, fMRI data were reconstructed and preprocessed using AFNI (Cox 1996) as follows: (1) motion correction using a 6-parameter 3D registration of the functional and anatomical data sets (Cox and Jesmanowicz 1999); (2) 3D spatial registration to a reference acquisition from the first fMRI run; (3) registration of functional images to the anatomical volume; (4) De-spiking; (5) mean normalization of the time series; and (6) inspection and censoring of time points occurring during excessive motion (>1 mm) (Johnstone et al. 2006).
Multisubject analysis was performed using an anatomical region-of-interest (ROI) approach that included 8 brain regions selected on the basis of the functional anatomy of the motor system including cerebellum and cerebral cortex. For the cerebellum, the anatomical parcellation was generated based on functional divisions: vermis, paravermal (intermediate, intCRB) region, lateral cerebellum, and flocculus–nodulus, as we have done previously (Solodkin et al. 2011). Cerebral cortex ROIs were generated using the semi-automatic Freesurfer parcellation (Destrieux et al. 2010). Alignment of fMRI to the anatomical images and ROIs was manually confirmed.
Structural Changes
Atrophy of each brain region was calculated based on the T1-weighted structural images normalized by cranial volume as previously reported (de Leon et al. 2001). The linearity of the relationship between the volume of cerebellar regions and disease severity was obtained by Pearson's correlation with probability values corrected for multiple comparisons (Bonferroni) (Armstrong 2014). A 2-tailed Wilcoxon Rank Sum test was used to compare volumes between the control cases and SCA6 cases.
Statistical Maps
Statistical analysis of individual subject data was performed using a multiple linear regression, in which the signal time course for each voxel was compared with a reference waveform. The reference waveform was based on the convolution of a square wave with an empirical model of the hemodynamic response (Cox 1996). Voxels were considered active if the single voxel P-value was <1 × 10−5 and the voxel met a 3-dimensional contiguity requirement, requiring clusters (sets of contiguous voxels) to contain at least 3 voxels (53 mm3). These values were determined following a Monte Carlo simulation that established a corrected whole-brain significance level (α < 0.05) (Forman et al. 1995). The resulting clusters that survived both the initial F-test and the clustering threshold defined the presence of activation in the brain for each subject. Group frequency maps for controls and each stage of SCA6 severity were generated to depict the fraction of subjects per group that had activation in each voxel. Finally, to ensure that atrophy of the cerebellar cortices did not affect the amount of activation seen, we normalized active volumes by the total volume of each of the cerebellar cortices for each individual.
Effective Connectivity: Structural Equation Models
The secondary analysis was the construction of networks for corticocerebellar activity via effective connectivity. We built structural equation models (SEM), covariance structures constrained by known anatomy, using AMOS, as we have done before (Arbuckle 1989, 1992). We measured the fit between predicted and observed covariance matrices using both χ2 and explained variance to generate the SEMs (Solodkin et al. 2004; Walsh et al. 2008). We generated both a group model (e.g., healthy controls) and individual models for each patient. To minimize variance within the group model, we modeled the time series from the peak voxel in each ROI as we have done before (Walsh et al. 2008) (Mashal et al. 2012). To quantify model differences between the group control model and individual SCA6 patients, we used a “stacked model” approach (McIntosh and Gonzalez-Lima 1994) as we have done previously (Solodkin et al. 2004; Walsh et al. 2008). A Bollen–Stine bootstrap analysis was also performed to test the null hypothesis that each model had a good fit with the data. Using this approach, for each model, the data were re-sampled and re-fitted for a total of 2000 iterations. The goodness-of-fit (χ2) obtained from the original model was then compared with those generated from the re-sampled data.
DTI
Preprocessing (FDT diffusion toolbox of FSL; FMRIB Software Library) (Behrens et al. 2003) consisted of (1) correcting for motion and eddy current spatial distortions using an affine transformation with 12 degrees of freedom (eddycorrect function in FSL); (2) improving the tensor estimations by diffusion gradient reorientation (Leemans and Jones 2009); (3) estimating diffusion tensors using a linear least squares approach (dtifit function in FSL) with reconstruction of mean, axial, and radial diffusivities and fractional anisotropy (MD, AD, RD, and FA); and (4) manually segmenting the superior, middle cerebellar peduncles and the cerebral peduncles (ROIs) as we have done before (Walsh et al. 2008). Due to large partial volumes, the inferior peduncle was not included in this analysis. Corrections to the data were performed as follows: SCP, MCP, and CP ROIs were manually drawn carefully to avoid the edges in the T1. We then applied a 12 degrees of freedom transformation to warp the T1 into the b0 of the DTI. All ROIs were then visually inspected to ensure an accurate alignment. In the MCP and CP, because there was minimum distortion, and because we avoided the exterior portions, there were no errors to the alignment of those peduncles. Of the SCP ROI's that were mis-aligned, we then manually shifted those so that they overlapped more with the SCP as viewed in the diffusion images.
All DTI metrics (FA, MD, RD, and AD) were extracted on a voxel-by-voxel basis from each ROI. Mean and median values of the diffusion metrics per group for each ROI were calculated. Voxel-wise frequency distributions for FA and RD in each fiber bundle were also calculated. Differences in distribution between the different SCA6 groups and the controls were performed and corrected for multiple comparisons with the Kolmogorov–Smirnov test.
Deterministic diffusion tensor tractography was performed using a Diffusion Toolkit and Trackvis Software (Wedeen et al. 2008). The fiber assignment by continuous tracking, FACT, algorithm (Mori et al. 1999) was selected to create the tractography with stopping criteria of either a turning angle greater than 60° or an FA value <0.20. From the tractography, the MCP, SCP, and CP were identified automatically and refined manually by removing obvious stray or false-positive tracts from each peduncle. For each peduncle the number of streamlines was calculated. Pathway weights were calculated via 2 methods: by multiplying the number of streamlines by the mean FA and mean inverse RD (1/RD) from all voxels with which each peduncle's streamlines intersected.
Age Correlations
To ensure that age did not affect the results, we performed a Pearson's correlation between age and all measures, including structural volumes, active volumes, and DTI measures, for the healthy control group.
Results
Oculomotor Disorders Along Disease Severity
Severity of SCA6, as assessed by SARAII score, was highly correlated with the number of oculomotor signs present on each patient (r = 0.72, P = 0.0052). However, there was no correlation between number of oculomotor symptoms and SARAII score (r = 0.21, P = 0.4817).
Structural Changes in Cerebellum
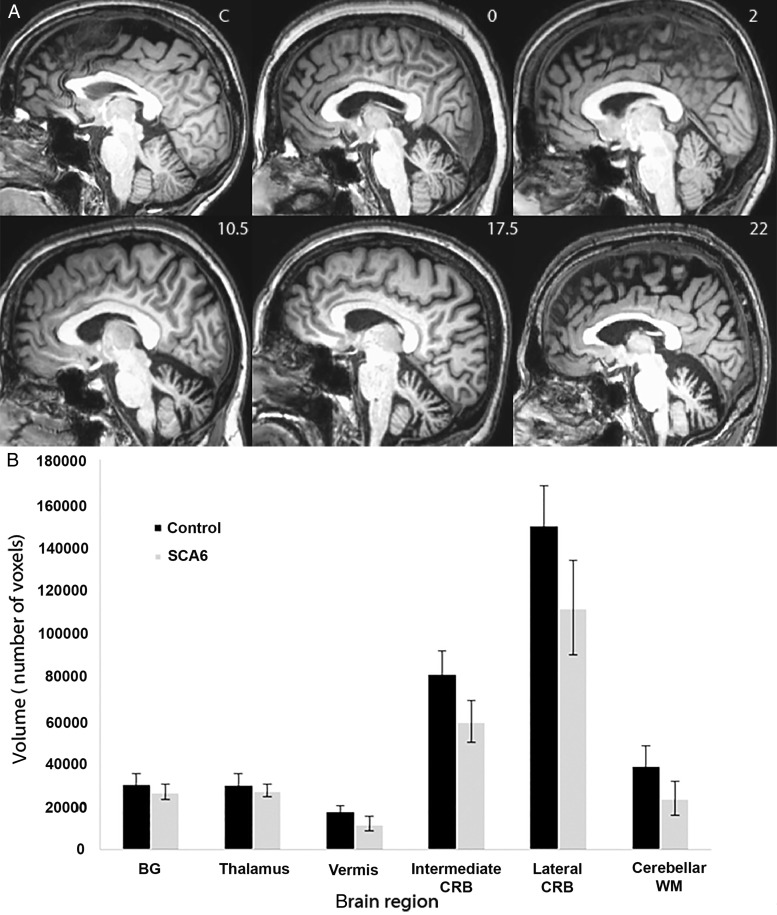
The degree of atrophy based on the normalized T1-weighted ROIs varied among patients (Fig. 1) but on average, all cerebellar regions were atrophic in the SCA6 cases compared with controls (vermis, P = 3.76e−8; intermediate, P = 1.15e−9; lateral, P = 7.66e−9). In addition, Pearson's correlation showed a significant relation (P < 0.02 after Bonferroni correction) between SARAII scores and the volumes of vermis (r = −0.64, P = 0.013) and the lateral cerebellum (r = −0.66; P = 0.011) but not for the intermediate cerebellum (r = −0.58; P = 0.029), as shown previously (Solodkin et al. 2011).
Figure 1.
Volumetric assessment of brain regions in SCA6 and healthy controls. (A) Sagittal view of T1-weighted images in a healthy control (1), a presymptomatic SCA6 case (SARAII = 0, (2)), moderate case (SARAII = 2 (3), SARAII = 10.5 (4), and two severe cases (SARAII = 17.5 (5), and SARAII = 22 (6). Note the increase in cerebellar atrophy along an increase of SARAII scores. (B) Graphical representation of averaged brain region volumes and their standard deviation in healthy controls (black bars) and in SCA6 (gray bars). Abbreviations: BG: basal gangila; CRB: cerebellum; WM: white matter.
Statistical Activation Maps
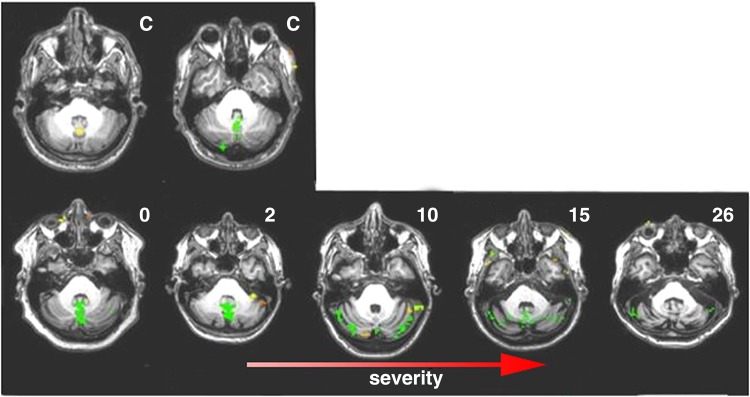
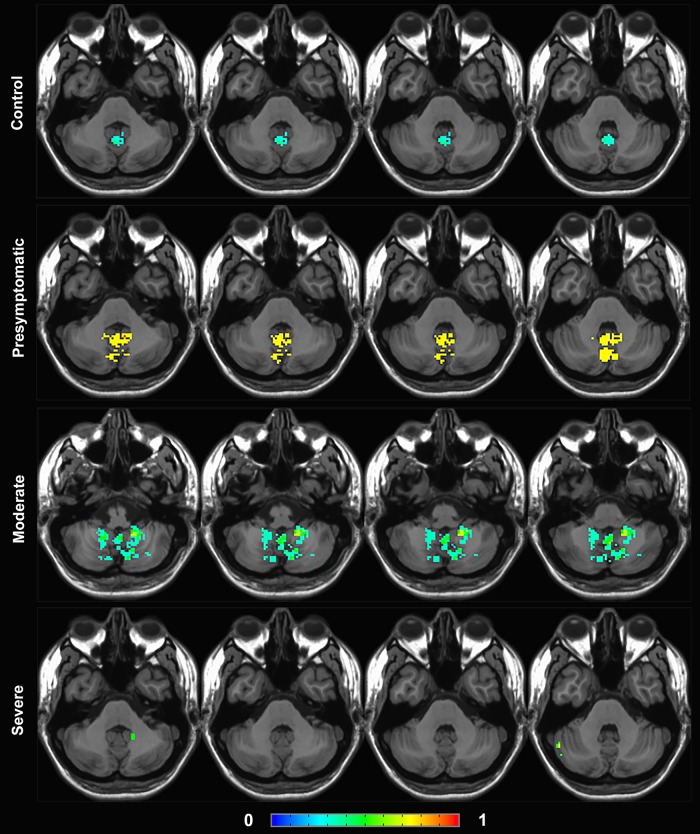
The distribution of brain activation during the visualization of the walking-landscape videos in healthy controls and SCA6 patients showed both similarities and differences (Figs 2 and 3). The stimuli evoked widespread activation in the cerebral cortex in all subjects, including premotor regions, the postcentral sulcus, the medial frontal gyrus (frontal eye fields, FEF), inferior and superior parietal (iPAR, sPAR) lobules (including the intraparietal sulcus) and widespread occipital areas (including MT and MST). In contrast, whereas increased activation in vermis and intermediate cerebellum was seen in healthy controls and presymptomatic cases, individuals with greater disease severity had less activation in these regions, and instead showed increased activity in the lateral cerebellum. Finally, individuals with the highest severity of disease were devoid of this lateral cerebellar activation. Results after volume normalization were consistent with those seen prior to normalization.
Figure 2.
Functional brain activation in cerebellum during an eye movement task. Active volumes (yellow/green) shown in control cases (C) and in increasing severity cases of SCA6. Activation in controls is seen in cerebellar vermis that increases in presympomatic and early cases (SARAII 0–2). Cases with mild severity (SARAII 10–15), see a decreased activation in vermis accompanied by an increase activation in lateral cerebellum which decreases in the more severe cases (SARAII = 26).
Figure 3.
Group frequency maps of functional brain activation in cerebellum. Fraction of subjects with activation in each voxel is shown in control cases and in each stage of SCA6 severity (presymptomatic/early, moderate, and severe), with hotter colors depicting higher fraction of subjects. Note the increase in vermis activation in the presymptomatic/early stage, followed by a spread to lateral cerebellum in the moderate stage, and a decline in activity in the severe stage.
Effective Connectivity: Structural Equation Models
Given that the different functional regions of cerebellum (vermis–intermediate–lateral) are not intrinsically interconnected, the shifting of the activation from spinal to lateral cerebellum should originate extrinsically. Since the main input to lateral cerebellum comes from the cerebral cortex (via both pons and the inferior olives) that was heavily active during the task, we considered the cerebral cortex a suitable candidate mediating this shift. We used SEM analysis to assess effective connectivity between cerebral cortex and cerebellum (Walsh et al. 2008). All SEM models generated had a good fit (χ2 > 0.06) and each node had variance explained between 55 and 94%. Additionally, the Bollen–Stine bootstrap analysis showed that all models had a good fit.
Group Model for Healthy Controls
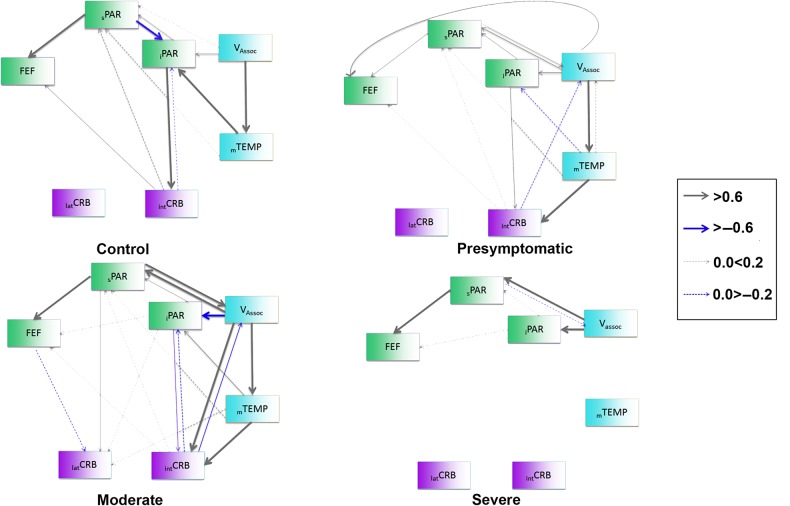
Connectivity among occipital and temporal regions exerted multiple influences over parietal regions that in turn influenced the FEF (Fig. 4). In contrast, connectivity to and from cerebellum was more restricted since the intCRB was the sole locus establishing significant connections with iPAR and the FEF.
Figure 4.
Structural equation model networks depicting the connectivity between cerebral cortex and cerebellum evolve with the development of disease. The nodes (brain regions) have been color coded to denote 3 categories: Turquoise: visual related areas (association visual areas [Vassoc], and visual-temporal MST, MT [mTEMP]); green: sensory-motor areas (superior Parietal [sPAR], inferior Parietal [iPAR] and frontal eye fields [FEF]); magenta: cerebellar regions (intermediate Cerebellum [intCRB] and lateral cerebellum [latCRB]). The thickness of the arrows denotes the weight of connections; gray arrows showing positive correlations and blue arrows negative correlations. The influence of cerebral cortex increases early in the disease, becomes maximal in moderate cases to decrease below control levels in the most severe cases.
Network Changes in SCA6
The networks associated with presymptomatic and early cases (SARAII = 2–4) shared features with the group control model; the connections between occipital and temporal regions and parietal and frontal regions remained constant. However, the strong connectivity between sPAR to iPAR seen in controls was not present, and a new association between the occipital regions and the FEF appeared at this early stage. Additionally, the activation in the intCRB was driven by both the iPAR and the mTEMP (Fig. 4; stacked model, P < 3.6e−12; Bollen–Stine bootstrap, P = 0.219).
In more advanced disease (SARAII 9–18), effective connections associated with the cerebellum showed profound differences compared with the group model (Fig. 4; stacked model, P < 3.2e−35; Bollen–Stine bootstrap, P = 0.142). A new cortical influence on the intCRB included the occipital regions and more importantly, the lateral cerebellum at this point was driven by connectivity from mTEMP, inferior and superior PAR and the FEF. Concomitant with these changes was a disappearance of heavy influence between occipital and the FEF.
In the most advanced cases (SARAII > 18.5), cerebellar networks were less distributed. There was a paucity of connectivity between the cerebellum and the cerebral cortex along some cortico-cortical connections associated with mTEMP (Fig. 4; stacked model, P < 3.9e−26; Bollen–Stine bootstrap, P = 0.401).
White Matter Changes
Peduncle Volumes
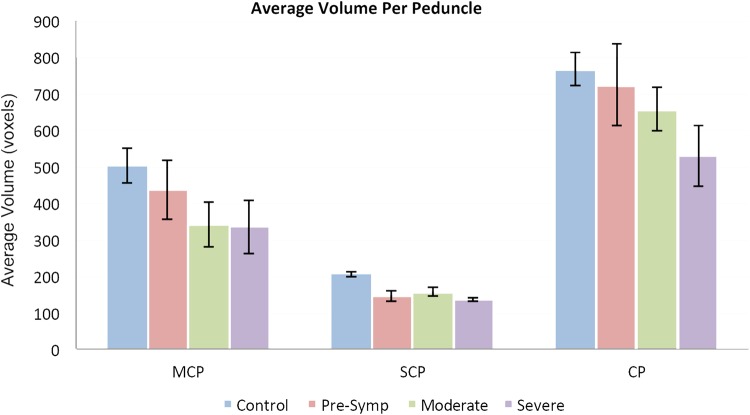
The average volumes of the superior and middle cerebellar peduncles (SCP and MCP) and the cerebral peduncle (CP) were lower in SCA6 cases compared with healthy controls (Wilcoxon Rank Sum Test: P = 0.0, 0.05, 0.05 respectively). In the MCP and CP, a trend toward reduced volume with increased disease severity was observed, beginning with presymptomatic individuals continuing through the more severe cases (SARAII >18.5) (Fig. 5), though these changes were not statistically significant (Spearman's rank correlation).
Figure 5.
Graphical representation of the average size (and SD) of white matter tracts linking (indirectly) the cerebral cortex and the cerebellum. Pathways included are: middle cerebellar peduncle (MCP), superior cerebellar peduncle (SCP) and cerebral peduncle (CP) calculated in controls (blue bars), presymptomatic (pink), moderate (green) and severe SCA6 (purple). Note that all peduncles have progressively reduced volumes in SCA6 compared with controls.
DTI metrics
Superior cerebellar peduncle
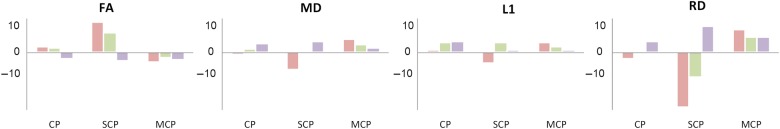
Changes in the SCP were the most salient compared with the other pathways studied (Table 2 and Fig. 6). The main difference when compared with controls was a decrease in RD in presymptomatic cases followed by a progressive increase in moderate and severe cases. This trend was inverted in FA whose values increased in presymptomatic individuals, subsequently decreasing in moderate and severe cases.
Table 2.
Diffusion tensor metrics ± their standard deviation in pathways associated with cerebellum
| FA | MD | L1 | RD | |
|---|---|---|---|---|
| CP | ||||
| Control | 5.74E−01 ± 1.89E−01 | 7.72E−04 ± 2.13E−04 | 1.35E−03 ± 3.20E−04 | 4.87E−04 ± 2.32E−04 |
| Presymptomatic | 5.89E−01 ± 1.93E−01 | 7.67E−04 ± 2.16E−04 | 1.36E−03 ± 3.17E−04 | 4.75E−04 ± 2.40E−04 |
| Moderate | 5.87E−01 ± 1.88E−01 | 7.85E−04 ± 2.82E−04 | 1.40E−03 ± 3.56E−04 | 4.89E−04 ± 3.01E−04 |
| Severe | 5.60E−01 ± 2.10E−01 | 8.03E−04 ± 3.20E−04 | 1.41E−03 ± 3.79E−04 | 5.10E−04 ± 3.42E−04 |
| SCP | ||||
| Control | 6.83E−01 ± 2.21E−01 | 8.59E−04 ± 5.07E−04 | 1.76E−03 ± 5.65E−04 | 4.48E−04 ± 5.32E−04 |
| Presymptomatic | 7.76E−01 ± 1.78E−01 | 7.95E−04 ± 3.27E−04 | 1.68E−03 ± 4.48E−04 | 3.43E−04 ± 3.15E−04 |
| Moderate | 7.44E−01 ± 2.30E−01 | 8.60E−04 ± 5.06E−04 | 1.84E−03 ± 5.34E−04 | 4.01E−04 ± 5.32E−04 |
| Severe | 6.58E−01 ± 2.18E−01 | 9.03E−04 ± 4.19E−04 | 1.78E−03 ± 4.84E−04 | 4.99E−04 ± 4.39E−04 |
| MCP | ||||
| Control | 6.61E−01 ± 1.58E−01 | 7.19E−04 ± 2.68E−04 | 1.37E−03 ± 3.46E−04 | 4.03E−04 ± 2.81E−04 |
| Presymptomatic | 6.34E−01 ± 1.62E−01 | 7.60E−04 ± 3.00E−04 | 1.43E−03 ± 3.66E−04 | 4.44E−04 ± 3.10E−04 |
| Moderate | 6.47E−01 ± 1.72E−01 | 7.46E−04 ± 3.30E−04 | 1.41E−03 ± 3.91E−04 | 4.30E−04 ± 3.47E−04 |
| Severe | 6.40E−01 ± 1.77E−01 | 7.35E−04 ± 2.75E−04 | 1.39E−03 ± 3.43E−04 | 4.30E−04 ± 2.96E−04 |
FA, fractional anisotropy; MD, mean diffusion; L1, lambda 1; RD, radial diffusivity; CP, cerebral peduncle; SCP, superior cerebellar peduncle; MCP, middle cerebellar peduncle.
Figure 6.
Percent change in DTI metrics of pathways of interest in healthy controls and SCA6 cases. FA, fractional anisotropy; MD, mean diffusivity; L1, lambda 1; RD, radial diffusivity; CP, cerebral peduncle, SCP, superior cerebellar peduncle and MCP, middle cerebellar peduncle. Percentage values in presymptomatic cases are depicted in red, moderate cases in green, and severe cases in purple. Note that the larger effects seen in SCP are repeated with less magnitude in the CP.
Middle cerebellar peduncle
The MCP showed limited changes in DTI metrics along the progression of the disease. The differences were more apparent when comparing patient data with controls (Table 2 and Fig. 6) where we found an increase in RD apparent at all SCA stages.
Cerebral peduncle
The profile of DTI changes in the CP in some ways resembled those in the SCP (Table 2 and Fig. 6), although the magnitude of the changes was smaller.
Statistical distributions
In order to understand the differences seen between SCA6 and controls with more precision, we performed analysis at the single voxel level to determine concrete differences in the statistical distributions as we have done before (Solodkin et al. 2013). This analysis comparing SCA6 to healthy controls with the Kolmogorov–Smirnov test showed significant differences in the distribution of FA and RD. The only comparisons that were not significant included the RD in the CP in the presymptomatic group and FA in the SCP in the severe group (Table 3).
Table 3.
Probability values derived from Kolmogorov–Smirnov comparing SCA-6 groups versus healthy controls
| DTI metrics | Presymptomatic | Moderate | Severe | |
|---|---|---|---|---|
| CP | FA | 4.59e−05 | 2.40e−05 | 2.75e−08 |
| RD | 9.16e−02 | 1.38e−12 | <2.2e−16 | |
| MCP | FA | 4.77e−09 | 1.02e−04 | 4.01e−06 |
| RD | <2.2e−16 | <2.2e−16 | 3.17e−12 | |
| SCP | FA | <2.2e−16 | <2.2e−16 | 7.56e−02 |
| RD | 3.22e−09 | 2.60e−10 | 2.12e−03 |
Correction for multiple comparison level is: 4e−3.
We determined if there was a shift in the distributions of FA and RD between the control and the SCA6 groups. This analysis showed that in the CP, RD distribution compared with controls was shifted to the left in presymptomatic cases (P = 5.79 e−4) and moderate cases (P = 1.01 e−6) and to the right in severe individuals (P = 6.53 e−25). In contrast, in the SCP there was a shift in the distribution of FA to the right in both presymptomatic (P = 5.97 e−20) and moderate individuals (P = 1.0 e−25). In severe cases, the trend inverted but the leftwards shift did not reach significance after correction for multiple comparisons (P = 8.0 e−3).
Peduncle weights
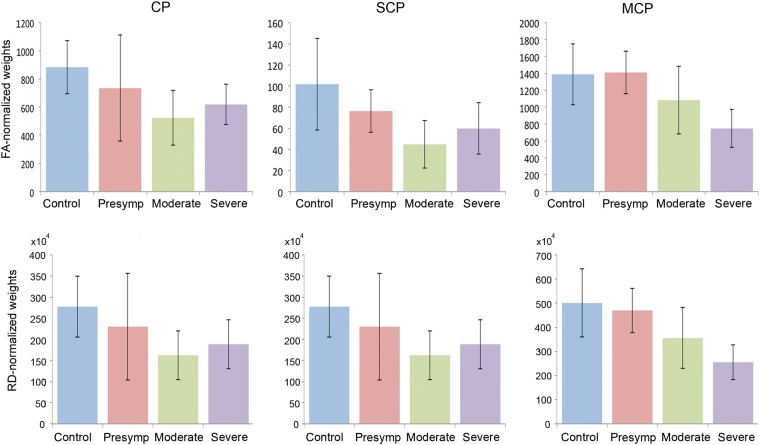
The structural weights of each peduncle normalized by FA (FA × number of streamlines) and by RD (1/RD × number of streamlines) were decreased in SCA6 compared with controls (Fig. 7). In the CP, weights were significantly decreased in SCA6 when compared with controls for both FA-normalized weights (P = 0.0013, t-test) and RD-normalized weights (P = 0.0028, t-test). In the SCP and MCP, the decrease in RD-normalized weights followed a linear trajectory, where the weight correlated with SARAII score significantly for both peduncles (P = 0.0105 and P = 0.0452 respectively, Pearson's correlation). The decrease in FA-normalized weights correlated with SARAII score significantly only in the MCP (P = 0.0073, Pearson's correlation).
Figure 7.
Structural weights of white matter peduncles in healthy controls and SCA6 cases. FA, fractional anisotropy; RD, radial diffusivity; CP, cerebral peduncle, SCP, superior cerebellar peduncle; MCP, middle cerebellar peduncle. FA-normalized weights = FA × number of streamlines. RD-normalized weights = (1/RD) × number of streamlines. Control cases are depicted in blue, presymptomatic cases in red, moderate cases in green, and severe cases in purple. Note that the RD-normalized weights decrease significantly along disease severity for the SCP and MCP whereas FA-normalized weights decrease significantly along disease severity only for the MCP.
Age Assessment
There was no correlation between age and any of the DTI metrics (FA, MD, RD, and L1) for all peduncles in the healthy control group (P > 0.05, Bonferroni correction threshold set at 0.0042 for significance). In addition, there was no relationship between age and total activation in each cerebellar hemisphere (Bonferroni correction, P = 0.0083). Therefore, none of the current results reported were confounded by age effects.
Discussion
Patterns of Cerebellar Activation are Associated with Disease Severity
A salient result of the current study is the increase in BOLD volumes and subsequent shift of activation from vermis (in controls and presymptomatic individuals) to the lateral cerebellum in moderate and severe SCA6 cases during a visual task involving saccades and smooth-pursuit movements. Whereas vermal fMRI activation in controls is not surprising because of its ample role in the regulation of saccades (McDowell et al. 2008; Kheradmand and Zee 2011; Liem et al. 2013), the increase in activation in presymptomatic SCA6 patients could be due to the early alterations in saccadic movements (Christova et al. 2008). In contrast, the shift of activation from spino- to cerebro-cerebellum in moderate and severe SCA6 cases is intriguing since these functional cerebellar regions have marked differences in connectivity: the former with somatosensory nuclei in the spinal cord and medulla and the latter with the cerebral cortex (Stoodley and Schmahmann 2010; Prevosto and Sommer 2013). In order to understand these changes better, we further investigated functional and structural connectivity of cerebellar circuits.
Patterns of Cerebellar Connectivity can be Described in Stages
Effective connectivity models in healthy controls during the visual task show 2 salient links: (1) a feed-forward influence of visual association cortices (occipital and temporal) on parietal regions that in turn influence the FEF and 2) a top-down modulation of the paravermal cerebellar region from the iPAR lobule. This pattern is in agreement with a previously described cortico-cortical network (Lynch and Tian 2006) suggesting that saccadic and smooth-pursuit movements critically depend on 2 hubs, one in the temporal lobule (regions MT, MST) encoding motion in a retinal coordinated system and the FEF representing the motor output (Voogd et al. 2012). Links between and toward these hubs are formed by association regions in the parietal lobule, and by occipital visual regions providing sensory input to the temporal hub.
Early changes in effective connectivity were detected in SCA6 presymptomatic cases and continued throughout disease progression. Based off of these changes, we classified 3 stages of disease severity that also paralleled progressive SARAII scores, average number of oculomotor signs, and patterns of fMRI activation. The progression of connectivity changes linked to each stage is as follows:
Stage 1 (SARAII = 0–4, 1.5 Average Oculomotor Signs)
Contrary to early assumptions regarding the pathology of SCA6 as a uniquely cerebellar ataxia (Zhuchenko et al. 1997), we show that cortico-cortical connectivity is functionally altered early with a decrease in the strength of connections among parietal cortices and increased effective connections between occipital regions and the FEF. This reorganization at the level of cortico-cortical connectivity is accompanied by an increase of novel top-down influences from the temporal regions to the intermediate cerebellum. Because the reorganization of effective connections in presymptomatic and early symptomatic subjects runs parallel to the increase in vermal activation, we explored the possibility of a top-down influence from FEF to vermis using SEM (Voogd et al. 2012). However, we were unable to achieve a good model fit. Thus we hypothesize that an alternative explanation for this increased activation could be the result of a decrease in cerebellar efficacy resulting from the primary damage to Purkinje neurons in vermis that occurs early in the disease (Cricchi et al. 2007). In turn, this additional activation can indirectly generate a systemic modulation of the cerebral cortex via the ascending reticular system (Eccles et al. 1975) producing the cortico-cortical reorganization detected at this stage.
Stage 2 (SARAII = 9–18, 2.4 Average Oculomotor Signs)
Characterized by the appearance of new functional descending control from several cortical regions (FEF, mTEMP, iPAR, sPAR) onto the lateral cerebellum, this is the critical point at which the activity originally located in the vermis during the visual task is now decreased and instead, lateral cerebellum is now active. Previous fMRI studies have shown that saccadic movements produce consistent activation of the vermis and the paravermal regions (for review, see Voogd et al. 2012) and the lateral hemispheres only if the visual task implies a cognitive load such as attention or memory demands (Stephan et al. 2002; Nitschke et al. 2004). This perspective fits with the traditional view that the modulation of eye movements by the cerebellum is associated with the vestibulocerebellum for reflexive modulation (e.g. VOR), the control of dynamics of saccades and smooth pursuit by the spinocerebellum (McDowell et al. 2008; Kheradmand and Zee 2011), and modulation of complex eye movements involving cognitive demands by the cerebro-cerebellum (Schmahmann and Caplan 2006; Strick et al. 2009; Prevosto and Sommer 2013; Koziol et al. 2014). Thus the mechanism for the shift in activation from spino- to cortico-cerebellum is intriguing since anatomically there is no intrinsic connectivity linking them and behaviorally there is a clear difference in the roles associated with each cerebellar domain. In Stage 2, although volumes of activation in the cerebral cortex do not change, cortico-cortical effective connections do change, increasing in number and strength between many regions and thus generating a different neural context (Walsh et al. 2008). This stronger cortico-cortical network can in turn produce a concomitant increase in top-down regulation in widespread regions in cerebellum. That is, as the weight of the cortico-cortical connections increases, these cortical regions expand their influence on to the lateral cerebellum. In turn, this up-regulation of the activity in lateral cerebellum can produce positive feedback back to cerebral cortex via the SCP.
Stage 3 (SARAII >18, 2.75 Average Oculomotor Signs)
This stage is characterized by a paucity of any significant influence from the cerebral cortex to the cerebellum despite the persistence of some relatively stable cortico-cortical connections. At this stage, cerebellar degeneration becomes generalized and in consequence, it is possible that transneuronal degeneration affects additional brain regions including primary motor cortex, brainstem nuclei, thalamus, and basal ganglia (Gierga et al. 2009; Wang et al. 2010). As a consequence of this widespread cerebellar degeneration, its influence over cortical upper motor neurons can decrease and hence along the descending influences. This decrease in effective connectivity has also been related to atrophy associated with other neurodegenerative disorders (Dennis and Thompson 2014; Thomas et al. 2014).
The Activation Shift may be a Compensatory Mechanism Against Neurodegeneration
It is interesting to speculate whether the activation shift has an adaptive, functional role reminiscent to what occurs after motor stroke where brain regions are recruited to control upper extremity movements (Ward and Cohen 2004; Grefkes et al. 2008) or to increase activity in degenerative disorders that modulate the progression of symptoms (Kloppel et al. 2009; Savioz et al. 2009; Scheller et al. 2013). The involvement of the lateral cerebellum in SCA6 may be interpreted as a compensatory response to either maintain functionally sound eye motor control in the presence of vermal degeneration (Kloppel et al. 2009) or by prolonging the anatomical integrity of regions not yet in overt degeneration.
Because vermal degeneration appears early in the disease (Cricchi et al. 2007) followed by other cerebellar regions (Geschwind and Galaburda 1987; Sasaki et al. 1998), we suggest a compensatory mechanism associated with the survival of cerebellar regions not yet in degeneration (also known as lateral cerebellum). Several mechanisms are thought to drive compensatory activity, including synaptic scaling, increased size of surviving synapses, neuritic outgrowth, and firing rate regulation (Savioz et al. 2009; Abuhassan et al. 2014). Another alternative is the recruitment of silent synapses, which refer to connections without physiological effect when the presynaptic neuron is activated, that become active under certain pathological conditions (Jahromi and Atwood 1974) (Atwood and Wojtowicz 1999). One of the proposed mechanisms by which silent synapses become active is based on homeostatic plasticity (Wojtowicz et al. 1991; Kerchner and Nicoll 2008; Cabezas and Buno 2011; Funahashi et al. 2013), where global activity modulates the synaptic strength (Nakayama et al. 2005; Savioz et al. 2009).
In the cerebellum, silent synapses have been suggested to account for 80 to 98% of synapses between parallel fibers and Purkinje cells (Losi et al. 2002; Ito 2006; Dean et al. 2010). These become functional with repeated stimulation (Ito 2006; Porrill and Dean 2008) elicited by either LTP or LTD (Hess et al. 1996; Hoffland et al. 2012). Hence, the novel top-down activation from cerebral cortex to the lateral cerebellum could in fact activate these silent synapses and thus delay the ongoing disease-related damage.
Functional Network Changes are Accompanied by Structural Pathway Changes
While pathway degeneration associated with overt neuronal death is detectable, methods to assess structural changes in pathways associated with functional regulation were not obvious until recently (Hagmann et al. 2008). One can expect that overt degeneration of Purkinje cells in moderate and severe SCA6 cases produces increased RD, decreased FA and decreased number of streamlines in cerebellar peduncles with the consequent decrease in the weight of these pathways. Smaller degenerating pathways in turn can result in lower levels of cerebral cortex activation.
In contrast, expected changes in presymptomatic cases or in the cerebral peduncles (far removed from Purkinje degeneration) are harder to imagine. Yet the SCP, the main output from cerebellum to the cerebral cortex via thalamus, shows not only the largest changes in presymptomatic and early symptomatic SCA6 patients, but also these results are counterintuitive. Opposite to the effects of degeneration, in the SCP, RD decreases more than 20% and FA increases >10%. Concomitant to this, there is a decrease in peduncle volume, number of streamlines and pathway weight that could be explained by the presence of thinner axons compared with those in healthy controls. Thinner axons would act as barriers to water flow in the radial direction both intra and extra-cellularly, causing the decreased RD and increased FA seen in early cases (Beaulieu 2002; Song et al. 2002; Nilsson et al. 2013) and potentially producing an increase in variance of activation in cerebellar outputs (Swindale 2003; Seidl 2013). Afterward, the typical increase in RD and decrease in FA is detected. This result is not surprising in the most severe cases since a recent report showed degeneration of the dentate nucleus in SCA6 where the pathway originates (Stefanescu et al. 2015). The pathway size and number of streamlines does not decrease parallel to this, perhaps due to partial volume effects in this small pathway.
Surprisingly, the CP shows similar trajectories to those seen in the SCP, although on a smaller scale probably due to the direct contact of SCP with the primarily degenerating Purkinje neurons on the one hand, and the heterogeneous anatomical composition of the CP on the other (Wakana et al. 2004; Ramnani 2006; Ramnani et al. 2006; Ramnani 2012). Of the many pathways comprising the CP, it may be expected that the cortico-pontine fibers would be the most affected. As the illness progresses, the remaining pathways within the CP may be affected (Smith 1975; Uchino et al. 2006) as degeneration in SCA6 spreads including layer V neurons in M1 (Gierga et al. 2009). At this point, the MCP, which forms the input from pons to cerebellum, is also affected perhaps in the anterograde and retrograde directions as a consequence of cerebellar degeneration (Sasaki et al. 1998; Ishikawa et al. 1999).
When detecting decreased FA and increased RD, it is worth addressing a limitation of the diffusion tensor model, the effect of partial volume voxels containing tissue and cerebrospinal fluid (CSF), which would exaggerate RD values and reduce FA (Vos et al. 2011). Under these conditions, the number of streamlines can be underestimated by the premature termination of the tracking algorithm. However, 2 key points suggest this was not the case in our calculations. First, although the peduncle most likely affected by CSF is the SCP due to its location and small size, early cases display an initial decrease in RD followed by a decrease with disease progression. Second, this trend is also seen in the CP, which because of location and size should not have this potential artifact.
Summary
Key findings in this study reveal parallel structural and functional changes in different stages of SCA6. Importantly, although SCA6 is commonly referred to as a pure cerebellar ataxia, our observations demonstrate widespread functional changes involving the cerebral cortex even before the first clinical symptoms have manifested. Finally, we propose that those changes have a compensatory nature, allowing the cerebellum to counteract or delay the effects of the neurodegeneration, making them essential in understanding the nature of SCA6 pathology.
Funding
This work was supported by the Center for Integrative Neuroscience and Neurogineering Research (CINNR), the National Institutes of Health (NIH RO1-NS-54942), and the James McDonnell Foundation (NRG Group).
Notes
The authors thank Victoria M. Staszak, RN, MBA Ataxia Center Coordinator for her work to make the transition between clinic and MRI easy for the patients. We also thank Mr R. Lyons for technical help in performing the scan sessions. Conflict of Interest: None declared.
References
- Abuhassan K, Coyle D, Belatreche A, Maguire L. 2014. Compensating for synaptic loss in Alzheimer's disease. J Comput Neurosci. 36:19–37. [DOI] [PubMed] [Google Scholar]
- Ackl N, Ising M, Schreiber YA, Atiya M, Sonntag A, Auer DP. 2005. Hippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 384:23–28. [DOI] [PubMed] [Google Scholar]
- Arbuckle JL. 1992. AMOS 3.1 Documentation Package. Philadelphia: Temple University. [Google Scholar]
- Arbuckle JL. 1989. Analysis of Moment Structures. Am Stat. 43:66–67. [Google Scholar]
- Armstrong RA. 2014. When to use the Bonferroni correction. Ophthalmic Physiol Opt. 34:502–508. [DOI] [PubMed] [Google Scholar]
- Atwood HL, Wojtowicz JM. 1999. Silent synapses in neural plasticity: current evidence. Learn Mem. 6:542–571. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. 2002. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 15:435–455. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. 2003. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 50:1077–1088. [DOI] [PubMed] [Google Scholar]
- Bour LJ, van Rootselaar AF, Koelman JH, Tijssen MA. 2008. Oculomotor abnormalities in myoclonic tremor: a comparison with spinocerebellar ataxia type 6. Brain. 131:2295–2303. [DOI] [PubMed] [Google Scholar]
- Butteriss D, Chinnery P, Birchall D. 2005. Radiological characterization of spinocerebellar ataxia type 6. Br J Radiol. 78:694–696. [DOI] [PubMed] [Google Scholar]
- Buttner N, Geschwind D, Jen JC, Perlman S, Pulst SM, Baloh RW. 1998. Oculomotor phenotypes in autosomal dominant ataxias. Arch Neurol. 55:1353–1357. [DOI] [PubMed] [Google Scholar]
- Cabezas C, Buno W. 2011. BDNF is required for the induction of a presynaptic component of the functional conversion of silent synapses. Hippocampus. 21:374–385. [DOI] [PubMed] [Google Scholar]
- Christova P, Anderson JH, Gomez CM. 2008. Impaired eye movements in presymptomatic spinocerebellar ataxia type 6. Arch Neurol. 65:530–536. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. 1993. PsyScope: a New graphic interactive environment for Designing Psychology Experiments. Behav Res Methods Instrum Comput. 25:257–271. [Google Scholar]
- Cox RW. 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29:162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. 1999. Real-time 3D image registration for functional MRI. Magn Reson Med. 42:1014–1018. [DOI] [PubMed] [Google Scholar]
- Craig K, Keers SM, Archibald K, Curtis A, Chinnery PF. 2004. Molecular epidemiology of spinocerebellar ataxia type 6. Ann Neurol. 55:752–755. [DOI] [PubMed] [Google Scholar]
- Cricchi F, Di Lorenzo C, Grieco GS, Rengo C, Cardinale A, Racaniello M, Santorelli FM, Nappi G, Pierelli F, Casali C. 2007. Early-onset progressive ataxia associated with the first CACNA1A mutation identified within the I-II loop. J Neurol Sci. 254:69–71. [DOI] [PubMed] [Google Scholar]
- de Leon M, Bobinski M, Convit A, Wolf O, Insausti R. 2001. Usefulness of MRI measures of entorhinal cortex versus hippocampus in AD. Neurology. 56:820–821. [DOI] [PubMed] [Google Scholar]
- Dean P, Porrill J, Ekerot CF, Jorntell H. 2010. The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat Rev Neurosci. 11:30–43. [DOI] [PubMed] [Google Scholar]
- Dennis EL, Thompson PM. 2014. Functional brain connectivity using fMRI in aging and Alzheimer's disease. Neuropsychol Rev. 24:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. 2010. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Nicoll RA, Taborikova H, Willey TJ. 1975. Medial recticular neurons projecting Rostrally. J Neurophysiol. 38:531–538. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. 1995. Improved Assessment of significant change in functional magnetic resonance imaging (fMRI): use of a cluster size threshold. Magn Reson Med. 33:636–647. [DOI] [PubMed] [Google Scholar]
- Funahashi R, Maruyama T, Yoshimura Y, Komatsu Y. 2013. Silent synapses persist into adulthood in layer 2/3 pyramidal neurons of visual cortex in dark-reared mice. J Neurophysiol. 109:2064–2076. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Perlman S, Figueroa KP, Karrim J, Baloh RW, Pulst SM. 1997. Spinocerebellar ataxia type 6. Frequency of the mutation and genotype–phenotype correlations. Neurology. 49:1247–1251. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. 1987. Cerebral lateralization: biological mechanisms, associations, and pathology. Cambridge: (MA: ): MIT Press. [DOI] [PubMed] [Google Scholar]
- Gierga K, Schelhaas HJ, Brunt ER, Seidel K, Scherzed W, Egensperger R, de Vos RA, den Dunnen W, Ippel PF, Petrasch-Parwez E et al. 2009. Spinocerebellar ataxia type 6 (SCA6): neurodegeneration goes beyond the known brain predilection sites. Neuropathol Appl Neurobiol. 35:515–527. [DOI] [PubMed] [Google Scholar]
- Globas C, du Montcel ST, Baliko L, Boesch S, Depondt C, DiDonato S, Durr A, Filla A, Klockgether T, Mariotti C et al. 2008. Early symptoms in spinocerebellar ataxia type 1, 2, 3, and 6. Mov Disord. 23:2232–2238. [DOI] [PubMed] [Google Scholar]
- Gomez CM, Thompson RM, Gammack JT, Perlman SL, Dobyns WB, Truwit CL, Zee DS, Clark HB, Anderson JH. 1997. Spinocerebellar ataxia type 6: gaze-evoked and vertical nystagmus, Purkinje cell degeneration, and variable age of onset. Ann Neurol. 42:933–950. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Kust J, Karbe H, Fink GR. 2008. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 63:236–246. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. 2008. Mapping the structural core of human cerebral cortex. PLoS Biol. 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G, Aizenman CD, Donoghue JP. 1996. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 75:1765–1778. [DOI] [PubMed] [Google Scholar]
- Hoffland BS, Bologna M, Kassavetis P, Teo JT, Rothwell JC, Yeo CH, van de Warrenburg BP, Edwards MJ. 2012. Cerebellar theta burst stimulation impairs eyeblink classical conditioning. J Physiol. 590:887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Watanabe M, Yoshizawa K, Fujita T, Iwamoto H, Yoshizawa T, Harada K, Nakamagoe K, Komatsuzaki Y, Satoh A et al. 1999. Clinical, neuropathological, and molecular study in two families with spinocerebellar ataxia type 6 (SCA6). J Neurol Neurosurg Psychiatry. 67:86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. 2006. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 78:272–303. [DOI] [PubMed] [Google Scholar]
- Jacobi H, Hauser TK, Giunti P, Globas C, Bauer P, Schmitz-Hubsch T, Baliko L, Filla A, Mariotti C, Rakowicz M et al. 2012. Spinocerebellar ataxia types 1, 2, 3 and 6: the clinical spectrum of ataxia and morphometric brainstem and cerebellar findings. Cerebellum. 11:155–166. [DOI] [PubMed] [Google Scholar]
- Jahromi SS, Atwood HL. 1974. Three-dimensional ultrastructure of the crayfish neuromuscular apparatus. J Cell Biol. 63:599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, Oakes TR. 2006. Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Hum Brain Mapp. 27:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. 2008. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 9:813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradmand A, Zee DS. 2011. Cerebellum and ocular motor control. Front Neurol. 2:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppel S, Chu C, Tan GC, Draganski B, Johnson H, Paulsen JS, Kienzle W, Tabrizi SJ, Ashburner J, Frackowiak RS et al. 2009. Automatic detection of preclinical neurodegeneration: presymptomatic Huntington disease. Neurology. 72:426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen AH. 2005. The pathogenesis of spinocerebellar ataxia. Cerebellum. 4:62–73. [DOI] [PubMed] [Google Scholar]
- Konen CS, Kleiser R, Seitz RJ, Bremmer F. 2005. An fMRI study of optokinetic nystagmus and smooth-pursuit eye movements in humans. Exp Brain Res. 165:203–216. [DOI] [PubMed] [Google Scholar]
- Koziol LF, Budding D, Andreasen N, D'Arrigo S, Bulgheroni S, Imamizu H, Ito M, Manto M, Marvel C, Parker K et al. 2014. Consensus paper: the cerebellum's role in movement and cognition. Cerebellum. 13:151–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A, Jones DK. 2009. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med. 61:1336–1349. [DOI] [PubMed] [Google Scholar]
- Leggo J, Dalton A, Morrison PJ, Dodge A, Connarty M, Kotze MJ, Rubinsztein DC. 1997. Analysis of spinocerebellar ataxia types 1, 2, 3, and 6, dentatorubral-pallidoluysian atrophy, and Friedreich's ataxia genes in spinocerebellar ataxia patients in the UK. J Med Genet. 34:982–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem EI, Frens MA, Smits M, van der Geest JN. 2013. Cerebellar activation related to saccadic inaccuracies. Cerebellum. 12:224–235. [DOI] [PubMed] [Google Scholar]
- Losi G, Prybylowski K, Fu Z, Luo JH, Vicini S. 2002. Silent synapses in developing cerebellar granule neurons. J Neurophysiol. 87:1263–1270. [DOI] [PubMed] [Google Scholar]
- Lukas C, Schols L, Bellenberg B, Rub U, Przuntek H, Schmid G, Koster O, Suchan B. 2006. Dissociation of grey and white matter reduction in spinocerebellar ataxia type 3 and 6: a voxel-based morphometry study. Neurosci Lett. 408:230–235. [DOI] [PubMed] [Google Scholar]
- Lynch JC, Tian JR. 2006. Cortico-cortical networks and cortico-subcortical loops for the higher control of eye movements. Prog Brain Res. 151:461–501. [DOI] [PubMed] [Google Scholar]
- Mantuano E, Veneziano L, Jodice C, Frontali M. 2003. Spinocerebellar ataxia type 6 and episodic ataxia type 2: differences and similarities between two allelic disorders. Cytogenet Genome Res. 100:147–153. [DOI] [PubMed] [Google Scholar]
- Maschke M, Oehlert G, Xie TD, Perlman S, Subramony SH, Kumar N, Ptacek LJ, Gomez CM. 2005. Clinical feature profile of spinocerebellar ataxia type 1–8 predicts genetically defined subtypes. Mov Disord. 20:1405–1412. [DOI] [PubMed] [Google Scholar]
- Mashal N, Solodkin A, Dick AS, Chen EE, Small SL. 2012. A network model of observation and imitation of speech. Front Psychol. 3:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura R, Futamura N, Ando N, Ueno S. 2003. Frequency of spinocerebellar ataxia mutations in the Kinki district of Japan. Acta Neurol Scand. 107:38–41. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Dyckman KA, Austin BP, Clementz BA. 2008. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn. 68:255–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. 1994. Structural equation modelling and its application to network analysis in functional brain imaging. Hum Brain Mapp. 2:2–22. [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. 1999. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 45:265–269. [DOI] [PubMed] [Google Scholar]
- Murata Y, Kawakami H, Yamaguchi S, Nishimura M, Kohriyama T, Ishizaki F, Matsuyama Z, Mimori Y, Nakamura S. 1998. Characteristic magnetic resonance imaging findings in spinocerebellar ataxia 6. Arch Neurol. 55:1348–1352. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Kiyosue K, Taguchi T. 2005. Diminished neuronal activity increases neuron-neuron connectivity underlying silent synapse formation and the rapid conversion of silent to functional synapses. J Neurosci. 25:4040–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, van Westen D, Stahlberg F, Sundgren PC, Latt J. 2013. The role of tissue microstructure and water exchange in biophysical modelling of diffusion in white matter. MAGMA. 26:345–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke MF, Binkofski F, Buccino G, Posse S, Erdmann C, Kompf D, Seitz RJ, Heide W. 2004. Activation of cerebellar hemispheres in spatial memorization of saccadic eye movements: an fMRI study. Hum Brain Mapp. 22:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda R, Takemoto T, Kawai M, Yamashita H. 2006. [Study of oculomotor disorders in spinocerebellar ataxia genotype]. Nihon Jibiinkoka Gakkai Kaiho. 109:30–35. [DOI] [PubMed] [Google Scholar]
- Porrill J, Dean P. 2008. Silent synapses, LTP, and the indirect parallel-fibre pathway: computational consequences of optimal cerebellar noise-processing. PLoS Comput Biol. 4:e1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevosto V, Sommer MA. 2013. Cognitive control of movement via the cerebellar-recipient thalamus. Front Syst Neurosci. 7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N. 2012. Frontal lobe and posterior parietal contributions to the cortico-cerebellar system. Cerebellum. 11:366–383. [DOI] [PubMed] [Google Scholar]
- Ramnani N. 2006. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 7:511–522. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Behrens TE, Johansen-Berg H, Richter MC, Pinsk MA, Andersson JL, Rudebeck P, Ciccarelli O, Richter W, Thompson AJ et al. 2006. The evolution of prefrontal inputs to the cortico-pontine system: diffusion imaging evidence from Macaque monkeys and humans. Cereb Cortex. 16:811–818. [DOI] [PubMed] [Google Scholar]
- Reetz K, Costa AS, Mirzazade S, Lehmann A, Juzek A, Rakowicz M, Boguslawska R, Schols L, Linnemann C, Mariotti C et al. 2013. Genotype-specific patterns of atrophy progression are more sensitive than clinical decline in SCA1, SCA3 and SCA6. Brain. 136:905–917. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Kojima H, Yabe I, Tashiro K, Hamada T, Sawa H, Hiraga H, Nagashima K. 1998. Neuropathological and molecular studies of spinocerebellar ataxia type 6 (SCA6). Acta Neuropathol (Berl). 95:199–204. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Yabe I, Tashiro K. 2003. The hereditary spinocerebellar ataxias in Japan. Cytogenet Genome Res. 100:198–205. [DOI] [PubMed] [Google Scholar]
- Satoh JI, Tokumoto H, Yukitake M, Matsui M, Matsuyama Z, Kawakami H, Nakamura S, Kuroda Y. 1998. Spinocerebellar ataxia type 6: MRI of three Japanese patients. Neuroradiology. 40:222–227. [DOI] [PubMed] [Google Scholar]
- Savioz A, Leuba G, Vallet PG, Walzer C. 2009. Contribution of neural networks to Alzheimer disease's progression. Brain Res Bull. 80:309–314. [DOI] [PubMed] [Google Scholar]
- Scheller E, Abdulkadir A, Peter J, Tabrizi SJ, Frackowiak RS, Kloppel S. 2013. Interregional compensatory mechanisms of motor functioning in progressing preclinical neurodegeneration. Neuroimage. 75:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Caplan D. 2006. Cognition, emotion and the cerebellum. Brain. 129:290–292. [DOI] [PubMed] [Google Scholar]
- Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS et al. 2006. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 66:1717–1720. [DOI] [PubMed] [Google Scholar]
- Schols L, Kruger R, Amoiridis G, Przuntek H, Epplen JT, Riess O. 1998. Spinocerebellar ataxia type 6: genotype and phenotype in German kindreds. J Neurol Neurosurg Psychiatry. 64:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rub U. 2012. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 124:1–21. [DOI] [PubMed] [Google Scholar]
- Seidl AH. 2013. Regulation of conduction time along axons. Neuroscience. 276:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MC. 1975. Histological findings after hemicerebellectomy in man: anterograde, retrograde and transneuronal degeneration. Brain Res. 95:423–442. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Chen EE, Van Hoesen GW, Heimer L, Shereen A, Kruggel F, Mastrianni J. 2013. In vivo parahippocampal white matter pathology as a biomarker of disease progression to Alzheimer's disease. J Comp Neurol. 521:4300–4317. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Chen EE, Small SL. 2004. Fine modulation in network activation during motor execution and motor imagery. Cereb Cortex. 14:1246–1255. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Peri E, Chen EE, Ben-Jacob E, Gomez CM. 2011. Loss of intrinsic organization of cerebellar networks in spinocerebellar ataxia type 1: correlates with disease severity and duration. Cerebellum. 10:218–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. 2002. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 17:1429–1436. [DOI] [PubMed] [Google Scholar]
- Stefanescu MR, Dohnalek M, Maderwald S, Thurling M, Minnerop M, Beck A, Schlamann M, Diedrichsen J, Ladd ME, Timmann D. 2015. Structural and functional MRI abnormalities of cerebellar cortex and nuclei in SCA3, SCA6 and Friedreich's ataxia. Brain. 138:1182–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan T, Mascolo A, Yousry TA, Bense S, Brandt T, Dieterich M. 2002. Changes in cerebellar activation pattern during two successive sequences of saccades. Hum Brain Mapp. 16:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. 2010. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 46:831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. 2009. Cerebellum and nonmotor function. Annu Rev Neurosci. 32:413–434. [DOI] [PubMed] [Google Scholar]
- Swindale NV. 2003. Neural synchrony, axonal path lengths, and general anesthesia: a hypothesis. Neuroscientist. 9:440–445. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Bateman RJ, Snyder AZ, Benzinger TL, Xiong C, Raichle M, Holtzman DM, Sperling RA, Mayeux R et al. 2014. Functional connectivity in autosomal dominant and late-onset Alzheimer disease. JAMA Neurol. 71:1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino A, Takase Y, Nomiyama K, Egashira R, Kudo S. 2006. Brainstem and cerebellar changes after cerebrovascular accidents: magnetic resonance imaging. Eur Radiol. 16:592–597. [DOI] [PubMed] [Google Scholar]
- Voogd J, Schraa-Tam CK, van der Geest JN, De Zeeuw CI. 2012. Visuomotor cerebellum in human and nonhuman primates. Cerebellum. 11:392–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos SB, Jones DK, Viergever MA, Leemans A. 2011. Partial volume effect as a hidden covariate in DTI analyses. Neuroimage. 55:1566–1576. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. 2004. Fiber tract-based atlas of human white matter anatomy. Radiology. 230:77–87. [DOI] [PubMed] [Google Scholar]
- Walsh RR, Small SL, Chen EE, Solodkin A. 2008. Network activation during bimanual movements in humans. Neuroimage. 43:540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Yang X, Xia K, Hu ZM, Weng L, Jin X, Jiang H, Zhang P, Shen L, Guo JF et al. 2010. TGM6 identified as a novel causative gene of spinocerebellar ataxias using exome sequencing. Brain. 133:3510–3518. [DOI] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. 2004. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 61:1844–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D'Arceuil H, de Crespigny AJ. 2008. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 41:1267–1277. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Smith BR, Atwood HL. 1991. Activity-dependent recruitment of silent synapses. Ann N Y Acad Sci. 627:169–179. [DOI] [PubMed] [Google Scholar]
- Yang Q, Hashizume Y, Yoshida M, Wang Y, Goto Y, Mitsuma N, Ishikawa K, Mizusawa H. 2000. Morphological Purkinje cell changes in spinocerebellar ataxia type 6. Acta Neuropathol (Berl). 100:371–376. [DOI] [PubMed] [Google Scholar]
- Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Dobyns WB, Subramony SH, Zoghbi HY, Lee CC. 1997. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 15:62–69. [DOI] [PubMed] [Google Scholar]