Abstract
In India, diabetes and cardiovascular disease (CVD) are growing health problems. CVD accounts for much of the increased morbidity and premature mortality associated with type 2 diabetes. Moreover, CVD also occurs 2–3 decades earlier among diabetic subjects and runs a more aggressive course and has a worse prognosis. The pathophysiology of the link between diabetes and CVD is complex and multifactorial and understanding the mechanisms of the disease can help identify and treat CVD in patients with diabetes and vice versa. The current article reviews the common antecedents between type 2 diabetes and CVD including non-modifiable and modifiable risk factors and suggests that future research on diabetes and CVD should focus on searching for risk factors for CVD that may be more specific to diabetes, such as hypoglycaemia or medication related comorbidities. Also, the authors recommend research on common genetic variants which might have stronger effects and hence have a potential role in diabetes and CVD risk prediction. Finally, primary prevention trials trying to prevent both diabetes and CVD are the urgent need of the hour!
Keywords: Endocrinology, coronary artery disease, hypertension, pharmacology, interventional cardiology, valvular disease, genetics, diabetes
According to the recent International Diabetes Federation data, 366 million people now have diabetes globally.1 The recent Indian Council of Medical Research-India Diabetes (ICMR-INDIAB) study estimates that there are currently 62.4 million diabetic individuals in India.2 WHO projects that diabetes related deaths would double between 2005 and 2030. In 2004, an estimated 3.4 million people died from consequences of high blood sugar, of which 50% of deaths were due to cardiovascular complications.3
Diabetes as defined by elevated plasma glucose according to WHO4 is considered as a cardiovascular disease (CVD) equivalent. CVDs, including coronary artery disease (CAD), cerebrovascular and peripheral vascular disease, account for much of the increased morbidity and premature mortality associated with type 2 diabetes.5 CVD is more common in people with diabetes compared with non-diabetic individuals. Moreover, in diabetic individuals CVD runs a more aggressive course and has a worse prognosis.6 Diabetes increases the risk of stroke, myocardial infarction (MI), sudden death and angina pectoris at least twofold compared with those without diabetes.7 Moreover, CVD also occurs 2–3 decades earlier among diabetic subjects.6 Patients with type 2 diabetes without previous MI have as high a risk of MI as non-diabetic patients with previous MI.8 After an acute MI, a considerable number of diabetic subjects die within the first year.9 In the case of subjects with type 1 diabetes, in spite of the fact that the CVD rate is significantly lower compared with the population with type 2 diabetes, their RR for coronary heart mortality is sevenfold higher than in matched counterparts without the disease.10 The aim of the current article is to review the common antecedents between type 2 diabetes and CVD.
Common soil hypothesis
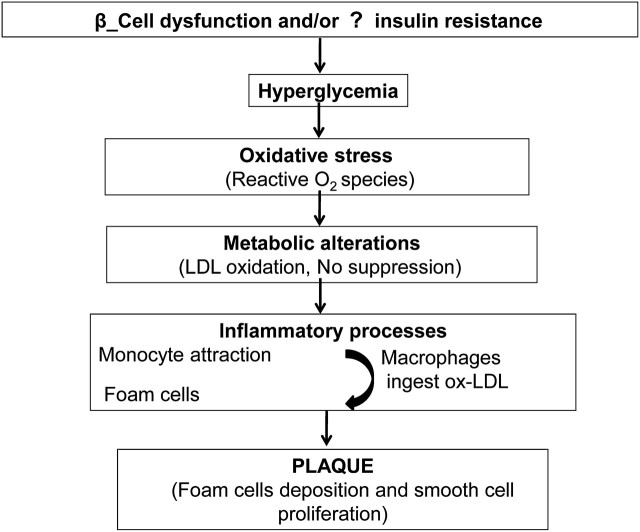
The pathophysiology of the link between diabetes and CVD is complex and multifactorial. Understanding these profound mechanisms of disease can help identify and treat CVD in patients with diabetes and vice versa. Traditionally, impaired insulin action (or ‘insulin resistance’) is considered the initial mechanism driving type 2 diabetic mellitus pathogenesis with subsequent decline in blood glucose regulation and compensatory insulin secretion leading to pancreatic β-cell fatigue and failure, and eventual insulin insufficiency.11 Atherosclerosis is a process by which fibrofatty plaques build up in human arteries. However, recent studies show there is growing evidence that inflammation participates centrally in all stages of this disease, from the initial lesion to the end-stage thrombotic complications.12 It has been observed that hyperglycaemia induces a large number of alterations in vascular tissue that potentially promote accelerated atherosclerosis.13 Figure 1 provides the mechanistic roles of glucose intolerance, inflammation and oxidative stress in the diabetes–CVD link.14 15
Figure 1.
Diabetes and cardiovascular disease link. LDL, low-density lipoprotein; ox-LDL, Oxidized- Low Density Lipoprotein.
Diabetes shares many characteristics and risk factors with CVD,6 and thus the risk for CVD also escalates with the increase in prevalence of diabetes. Type 2 diabetes and coronary heart disease: chicken, egg or neither? This was the question posed by Jarrett in 1984, on the basis of the knowledge available at that time.16 Recognising the commonality between these two diseases, Stern17 in 1995 proposed ‘the common soil hypothesis’ which states that unlike microvascular complications, large-vessel atherosclerosis can precede the development of diabetes, suggesting that rather than atherosclerosis being a complication of diabetes, both conditions may share genetic and environmental antecedents, that is, a ‘common soil’. Following this hypothesis, a plethora of studies emerged in support of the same.18 19 It has also been suggested that the association between type 2 diabetes and atherosclerotic CVD might result from a shared antecedent, which could provide a fundamental link between type 2 diabetes and atherosclerosis via the metabolic (or insulin resistance) syndrome.20 A decrease in the number of functional insulin-producing β-cells contributes to the pathophysiology of type 2 diabetes.
Various genetic and environmental candidates linking CVD and diabetes have been proposed. The genetic variations include PPAR-γ, CD36, tumour necrosis factor α (TNFα), adipocytokines, resistin, leptin, adiponectin; the intrauterine environment encompasses metabolic programming (fetal origins of adult disease hypothesis), the insulin resistance syndrome and chronic inflammation leading to elevated circulating C reactive protein (CRP) levels.21 Adipose tissue is now regarded as a source of pro-inflammatory mediators which may contribute to vascular injury, insulin resistance and atherogenesis. However, some of the adipokines (eg, adiponectin) have a protective role against vascular inflammation and/or insulin resistance.22 Studies in south India have shown that lower adiponectin levels are associated with metabolic syndrome and type 2 diabetes mellitus.23 Adiponectin is believed to play an important role in the development of atherosclerosis;24 adiponectin levels are decreased in adults with advanced stages of atherosclerosis.25 Carotid IMT is associated with lower serum adiponectin levels independent of conventional cardiovascular risk factors in urban South Indians.26 Various biomarkers indicate immune activation in type 2 diabetes and atherosclerosis, and some of these inflammatory markers including CRP have been shown to predict the occurrence of cardiovascular events27 and diabetes.28
Common antecedents of type 2 diabetes and CVD
Type 2 diabetes mellitus and CVD share many common antecedents. Genetic and environmental factors play a major role for both diabetes and CVD. The various risk factors associated with diabetes and CVD are summarised in table 1. The non-modifiable risk factors include age, gender, race and family history and modifiable risk factors include obesity, physical inactivity, unhealthy diet, low birth weight, smoking, dyslipidaemia and psychosocial stress.29 30
Table 1.
Common risk factors of diabetes and CVD
| Risk factors | Diabetes | CVD |
| Age | +++ | +++ |
| Gender (male) | + | ++ |
| South Asians | +++ | +++ |
| Family history | +++ | ++ |
| Physical inactivity | +++ | +++ |
| Unhealthy diet | +++ | +++ |
| Obesity | +++ | +++ |
| Low birth weight | ++ | ++ |
| Inflammation | ++ | ++ |
| Hypertension | + | +++ |
| Dyslipidaemia | + | +++ |
| Smoking | + | +++ |
| Psychosocial stress | ++ | ++ |
CVD, cardiovascular disease.
+ Mild Association; ++ Moderate Association; +++ Strong Association.
Diabetes in South Asians occurs around 10 years earlier than in the Caucasanians.31 Like diabetes, CAD is also more common in South Asians.32 MI occurs at a younger age and is associated with premature CAD mortality in South Asians compared with other populations of the world.33 34 In Asian Indians, half of all heart attacks occur before the age of 50 and a quarter before 40.35 Compared with women, men exhibit higher prevalence of cardiovascular disorders, especially CAD.36 However, the rate of coronary death is twice as high in women as in men after MI and revascularisation procedures.37 Also, men have a higher risk of developing diabetes compared with their female counterparts.2 It is also well recognised that diabetes is common in post-menopausal women and is a predisposing factor for CVD.38 Moreover, Yeung et al 39 suggest that having a family history of CAD confers additional type 2 diabetes risk among those with a parental history of diabetes.
The concept of a syndrome of insulin resistance has featured prominently as a potential link between type 2 diabetes and CVD.40 41 Studies have also elucidated the importance of metabolic syndrome in those with diabetes mellitus. In the NHANES III study, it was observed that when both conditions coexisted, the prevalence of coronary disease rose to 19.2% of the population. However, in the absence of metabolic syndrome, the prevalence of coronary disease in patients with diabetes was low (7.5%), and similar to the population without diabetes (8.7%).42
A study conducted in the urban population of North India reported a low prevalence of multiple cardiovascular risk factors (smoking, hypertension, lipid abnormalities and diabetes) among adolescents and that the prevalence of multiple risk factors increases in the age group 20–29 years with an exponential increase in age group 30–39 years. Increasing risk factors correlated with body mass index (BMI), waist circumference and waist to hip ratio.43 In the four regions studied in India, the take-off point in prevalence of diabetes was at 25–34 years with a decline after 65 years of age and at every age interval, the prevalence of diabetes increased in both urban and rural areas.2 Higher intake of trans fatty acids has been associated with weight gain, increased cardiometabolic risk and insulin resistance.44 Psychosocial stress, depression and shorter sleeping hours have been associated with a higher risk of metabolic syndrome and diabetes in Asian populations.45 It has been shown that acute coronary syndrome and MI in the ICU setting are associated with a very high prevalence of diabetes/glucose intolerance possibly due to stress.46
Hyperglycaemia
The contribution of hyperglycaemia as an independent determinant for developing macrovascular disease in type 2 diabetic patients, CAD in particular, was demonstrated in the United Kingdom Prospective Diabetes Study (UKPDS).47 Brownlee48 reported that hyperglycaemia-induced overproduction of superoxide by the mitochondrial electron transport chain activates four damaging pathways, namely: (1) advanced glycosylation end products formation, (2) polyol pathway (3) hexosamine pathway and (4) protein kinase C pathway. In addition, endothelial dysfunction is caused by superoxide activated pro-inflammatory signal, formed due to insulin resistance. These lead to cardiomyocyte dysfunction and other macrovascular complications.
The three conditions which include insulin resistance, prediabetes (impaired glucose tolerance (IGT) or impaired fasting glucose (IFG)) and overt diabetes appear to be associated, although to a variable degree, with an increased risk of CVD.49 50 The Asia Pacific Cohort Studies Collaboration group has shown that fasting blood glucose is an important determinant of CVD burden, with considerable potential benefit of usual blood glucose lowering down to levels of at least 4.9 mmol/l.51
Studies in non-diabetic Indians have shown a strong association of CVD with increasing plasma glucose levels.52 Prediabetic stages have also been recognised to have pathological consequences leading to macrovascular disease and increased mortality.53 Many prospective studies have shown that IFG is a risk factor for CVD.54 55
Increased plasma glucose concentrations lead to an increase in glycosylation of proteins, particularly lipoproteins, which has been shown to enhance its susceptibility to oxidation, triggering the atherosclerotic processes. The association of hyperglycaemia with CAD is substantiated by the results of the Bedford Study, Whitehall Study and Honolulu Heart Study.56–58 The Bedford study demonstrated a stepwise increase in CAD mortality from normal to diabetes during a 10-year follow-up period.56 The Whitehall study suggested that subjects with glucose intolerance had 1.5- to 2.5-fold higher risk for CAD mortality.57 The Honolulu Study reported that the subjects with baseline glucose values in the fifth quintile had a higher risk for CAD compared with the lowest quintile.58
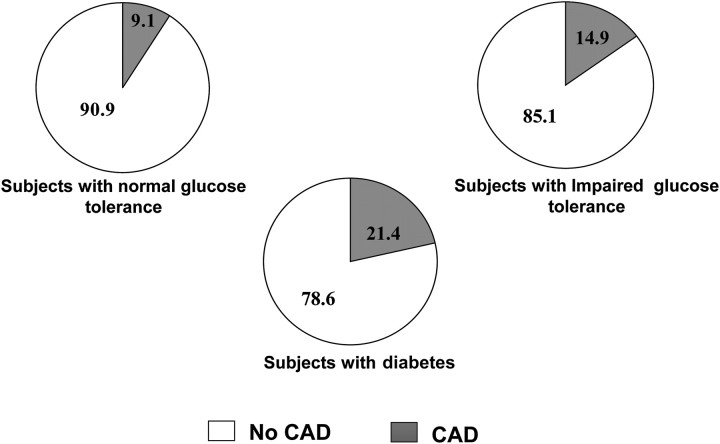
In the Chennai Urban Population Study, it was seen that 9.1% of Asian Indians subjects with normal glucose tolerance, 14.9% of the subjects with IGT and 21.4% of the diabetic subjects had CAD as shown in figure 2.59 In addition, the prevalence of CAD increased with increase in fasting plasma glucose even among non-diabetic subjects. Deepa et al 60 reported that the OR for CAD increased with increase in quartiles of fasting plasma glucose and 2 h postglucose load plasma glucose indicating a strong association of plasma glucose levels with CVD. These studies show that raised plasma glucose in diabetes is in itself a risk factor for CVD.
Figure 2.
Prevalence of coronary artery disease (CAD) in diabetic and non-diabetic subjects in the Chennai Urban Population Study.59
Hypertension
The mechanisms underlying the association between diabetes and hypertension are not yet clearly defined. Savage et al 61 studied IGT/IFG and undiagnosed diabetes among primary care patients with hypertension and patients with ischaemic heart disease and reported that 2% of patients with hypertension had undiagnosed type 2 diabetes and 18.5% had IFG or IGT, and among patients with ischaemic heart disease nearly 2.5% had undiagnosed type 2 diabetes and 12.4% had IFG or IGT. MacMahon et al 62 have demonstrated that the risk for CAD was 3.1 times higher among subjects who were both diabetic and hypertensive.
With regard to diabetic subjects, the UKPDS study47 and the LIFE study63 elegantly demonstrated that blood pressure control decreased the risk of CAD. The ADVANCE trial substantiated that the RR of a major vascular event was decreased by 9% by lowering the blood pressure.64 There was a significant reduction in cardiovascular events in diabetic subjects by optimal control of blood pressure according to the ACCORD blood pressure trial and the Hypertensive Optimal Treatment trial.65 66
Dyslipidaemia
An increased risk of CVD among diabetic patients has been attributed to diabetic dyslipidaemia, particularly increases in small dense low-density lipoprotein (LDL).67 Small dense LDL is considered to be more prone to oxidation and conformational changes, which result in the reduction of LDL clearance by its receptors, triggering immunological changes resulting in atherosclerosis.68 Small dense LDL also showed a positive correlation with fasting plasma glucose, HbA1c, total cholesterol, triglycerides, LDL, total cholesterol to high-density lipoprotein (HDL) ratio and triglycerides to HDL ratio and a negative correlation with HDL cholesterol and insulin sensitivity.
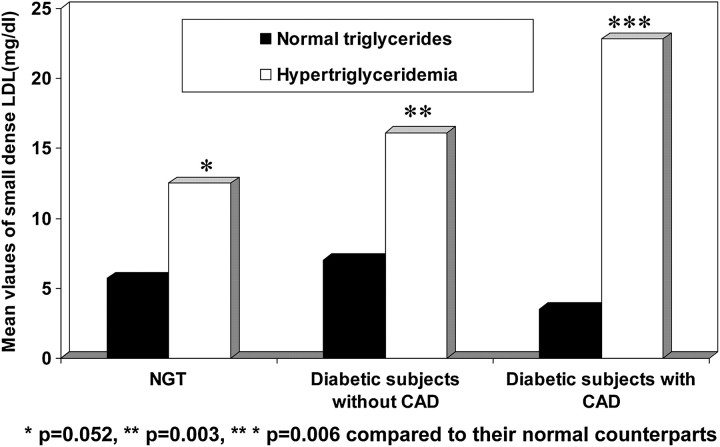
In the Chennai Urban Rural Epidemiology Study, it was noted that subjects with hypertriglyceridaemia had increased levels of small dense LDL compared with those with normal triglycerides and this was more significant in the diabetic groups (NGT: p=0.052, diabetic subjects without CAD: p=0.003, diabetic subjects with CAD: p=0.006; figure 3). In this study, it was also observed that triglycerides to HDL ratio of 3.0 had the optimum sensitivity (80.0%) and specificity (78.0%) for detecting elevated small dense LDL.69
Figure 3.
Mean values of small dense low-density lipoprotein (LDL) in subjects with and without hypertriglyceridaemia in the Chennai Urban Population Study.69 CAD, coronary artery disease; NGT, Normal Glucose Tolerance.
In contrast to LDL cholesterol, HDL cholesterol is a protective lipoprotein with antiatherogenic potential. Low plasma levels of HDLs, which are responsible for the removal of free cholesterol from the blood, are associated with increased cardiovascular and diabetes risk. Low levels of HDL are frequently seen in patients with insulin resistance, from the metabolic syndrome to overt diabetes mellitus.70
A large clinic based study carried out by us on 17 855 type 2 diabetic subjects revealed that the prevalence of CAD was significantly high among patients with isolated hypercholesterolaemia, isolated low LDL and isolated low HDL compared with normal individuals (2.8%), but not in those with isolated hypertriglyceridaemia (3.4%). There was no significant increase in the odd ratios for CAD in relation to quartiles of isolated triglycerides.71 Some studies have indicated that there is an increased risk of CAD in the presence of triglyceride levels of ≥204 mg/dl when the ratio of LDL cholesterol to HDL cholesterol exceeds 5.72 The Fenofibrate Intervention and Event Lowering in Diabetes study showed a decreased incidence of diabetic amputations among those on dyslipidaemia treatment.73
Obesity
It is well known that obesity is one of the major determinants of insulin resistance, diabetes and CAD.74 The role of obesity in the pathogenesis of type 2 diabetes is complex and is confounded by many factors. It has been demonstrated by Chan et al 75 that the RR of type 2 diabetes increases as the BMI increases in the US population. In this study, men with a BMI of ≥35 kg/m2 had a multivariate RR of 42.1 for of type 2 diabetes compared with men with a BMI <23.0 kg/m2. The Chennai Urban Population Study also revealed that in Asian Indians the prevalence of obesity was significantly higher among those with IGT (diabetes+IGT) compared with those with normal glucose tolerance (54.1% vs 23.6%) and a similar trend was observed in the proportion of abdominal obesity (62.2% vs 23.5%).76 Various intervention studies have shown that weight reduction ameliorates insulin resistance77 78 and prevents the development of diabetes.79 80
Both experimental81 and observational studies have suggested that adiposity produces an inflammatory reaction,82 and inflammation could well be the common link between diabetes and CAD.83
Smoking
Smoking has been reported to be one of the strongest risk factors for CAD. Smoking lowers HDL cholesterol, increases fibrinogen and results in aggregation of platelets.84 Smokers have two to four times higher risk for developing CAD. Sudden heart attacks are more common among smokers than non-smokers.
Smoking has been linked with insulin resistance in clinical studies and with markers of insulin resistance including central obesity and dyslipidaemia.85 There is emerging evidence that cigarette smoking could also predispose type 2 diabetes.85 – 87 Findings from the US Cancer Prevention Study involving over 275 000 men and 434 000 women aged ≥30 years demonstrated that there is a positive association between the number of cigarettes smoked per day and the incidence of diabetes mellitus in both men and women.86 It has been reported in the UKPDS study that the risk of stroke increases by 1.55 (95% CI 1.08 to 2.01) if a person is smoking at the time of the diagnosis of diabetes.87 It has been reported that the absolute risk of smoking is greater in diabetic, compared with non-diabetic subjects.88
Low birth weight
Birth weight has been found to be inversely associated with CVD89 as well as considered a risk factor for type 2 diabetes mellitus.90 The Barker hypothesis suggests that diabetes and CVD could be related to low birth weight.91 According to this hypothesis, low birth weight followed by obesity in adolescence or early adulthood could lead to insulin resistance, diabetes and CVD. A strong association for low birth weight with insulin resistance has also been shown in Indian children.92 Bhargava et al 93 reported that there was an association between thinness in infancy and progression to higher categories of BMI after age of 2, and with IGT or diabetes in young adulthood.
Coagulation factors
Diabetes is associated with various abnormalities of the haemostatic and fibrinolytic system. High levels of fibrinogen and Plasminogen Activator Inhibitor-1 (PAI-1) have been indicated by both clinical and epidemiological studies among diabetic subjects.94 Fibrinogen has been shown to be associated with enhanced platelet aggregation and smooth muscle cell proliferation. Furthermore, there is a strong association of fibrinogen with blood viscosity and thrombus formation and circulating levels of fibrinogen have been known to have a strong and consistent relationship with CAD.95 We reported that in subjects with type 2 diabetes that PAI-1 antigen and tPA antigen levels were significantly elevated among those with CAD compared with those without.96
Inflammatory markers
Evidence suggests that inflammatory markers are high among subjects with diabetes and CAD.97 Inflammation is considered to be a part of the insulin resistance syndrome98 and this, to some extent, explains the high risk for CAD among diabetic subjects. Studies on pro-inflammatory makers have revealed that cytokines like TNFα, CRP and interleukin-6 are strongly associated with CAD. It has been demonstrated that TNFα plays a key role in mediating insulin resistance as a result of obesity.99
The inflammatory marker, high sensitivity CRP (hs-CRP), has received a lot of attention recently in the field of cardiology. Though the exact pathological sequel is not clearly understood, several studies have shown an association between hs-CRP and the various components of insulin resistance syndrome, especially diabetes, obesity and CAD.98 99
In a study done by us we found that hs-CRP levels were higher among diabetic subjects with CAD compared with non-diabetic subjects without CAD.100 The study also showed that hs-CRP values increased with increase in tertiles of body fat and HbA1c. Regression analysis revealed hs-CRP to be strongly associated with CAD and diabetes even after adjusting for age and gender. This study suggested that association of body fat with diabetes seems to be mediated through hs-CRP.
Management
Management of type 2 diabetes and CVD includes non-pharmacological and pharmacological therapies. For both CVD and type 2 diabetes, diet and exercise play a major role in management. Unhealthy dietary practices, sedentary lifestyle and obesity, all of which are major risk factors for both CVD and diabetes are all lifestyle related. A survey done in urban, urban slum and rural population of Haryana, India, reported that a very high proportion of all the three populations reported inadequate intake of fruits and vegetables, rural men reported five times the amount of physical activity as compared with urban and urban slum men and rural women reported seven times physical activity as compared with women in the other two settings. Prevalence of obesity was the highest for urban population followed by urban slum and rural populations.101
Medications, that is, oral hypoglycaemic agents or insulin injections are used to reduce the blood sugar levels in type 2 diabetic subjects if diet and exercise fail to achieve adequate control. Over the past few years, several newer oral agents have become available for the treatment of type 2 diabetes. Oral hypoglycaemic agents are of three groups; the first group includes sulphonylureas (eg, glibenclamide, glipizide or gliclazide) and newer sulphonylureas like glimiperide and metiglinides (eg, repaglinide or nateglinide), which stimulate the pancreatic β-cells to secrete or release insulin. The second group is ‘insulin sensitisers’, which increase the uptake of glucose by the peripheral tissues, thus enhancing the action of insulin in the body. These include drugs like metformin and glitazones (eg, rosiglitazne and pioglitazone). The third type of drug is called alphaglucosidase inhibitors (eg, acarbose), which acts by reducing the glucose absorption from the gut after meals. For type 2 diabetes patients, oral monotherapy may be initially effective for controlling blood glucose, but it is associated with a high secondary failure rate. A wide range of combinations (eg, sulfonylurea plus metformin, a thiazolidinedione or acarbose; metformin plus a thiazolidinedione or acarbose) has been used effectively to achieve glycaemic control in patients in whom oral monotherapy has failed.102 A wide range of insulin preparations and therapeutic strategies have also been developed in attempts to mimic natural insulin profiles.
Aspirin, β-blockers, ACE inhibitors and statins have been used in the management of CVD.103–106 Wald and Law107–109 extended this hypothesis in several ways by combining agents and estimated that such a polypill would reduce CVD by more than 80%. The effect and tolerability of the polypill on blood pressure, lipids, heart rate and urinary thromboxane was assessed in the Indian Polycap Study, which concluded that polypill formulation could be conveniently used to reduce multiple risk factors and cardiovascular risk.110
Prevention strategies
Primary prevention of diabetes and CVD refers to the prevention before the disorder/disease develops and should begin in childhood or early adulthood and risk reduction education should be directed at the entire family. One should regularly screen for risk factors and encourage a healthy lifestyle, including a healthy diet, adequate exercise and weight control. Secondary prevention refers to therapy to prevent the progression of disease to stages of complications while tertiary prevention is aimed at limiting physical disability and rehabilitation measures in those who have already developed complications. CVD and diabetes prevention are a continuum that encompasses the life-course of the individual.
Evidence regarding the health benefits of traditional diet and physical activity is overwhelming and they play a critical role in both the primary and secondary prevention of CVD and diabetes. Reddy and Katan111 reported that there is adequate evidence available, from studies conducted within and across Indian population, to link several nutrients, minerals, food groups and dietary patterns with an increased or decreased risk of CVD. Dietary fats associated with an increased risk of CVD and diabetes include trans-fats and saturated fats, while polyunsaturated fats are known to be protective. Dietary sodium is associated with elevation of blood pressure, while dietary potassium lowers the risk of hypertension and stroke. Regular intake of fruits and vegetables is protective against CVD and diabetes. Diets such as DASH diets, Mediterranean diet and ‘prudent’ diet have been demonstrated to reduce the risk of CVD. A study conducted among Indians in New Delhi (in northern India) and Bangalore (in southern India) has reported that diets rich in vegetables and use of mustard oil could lower the risk of CAD among Indians.112
The Chennai Urban Rural Epidemiology Study has reported on the deleterious effect of refined grains (predominantly white rice) among the Chennai population and showed a strong association with type 2 diabetes and also with metabolic syndrome.113 The same study demonstrated that the diet of the urban South Indian population consisted mainly of refined cereals with low intake of fish, fruit and vegetables, and all of these could possibly contribute to the increased risk of non-communicable diseases.114 In a study undertaken to explore the association of dietary patterns with cardiovascular risk factors in an urban population of women in West Bengal, India, it was reported that the vegetable, fruits and pulses pattern was inversely associated with serum total cholesterol, LDL cholesterol and non-HDL cholesterol concentrations, and hydrogenated and saturated fat and vegetable oil pattern was positively associated with BMI, waist circumference and HDL cholesterol concentration.115
The INTERHEART study, an international case-control study carried out in 52 countries involving 15 152 cases of incident acute MI and 14 820 controls, reported that leisure-time physical activity and mild-to-moderate occupational physical activity, but not heavy physical labour, were associated with a reduced risk, while ownership of a car and TV was associated with an increased risk of MI across all economic regions.116 In a study conducted at New Delhi and Bangalore, leisure-time exercise, including as much as 35–40 min per day of brisk walking, was shown to be protective for CHD risk and sedentary lifestyles were positively associated with a risk of CAD.117 Thus, healthier dietary choices, along with increased physical activity, play a vital role in reducing the burden of diabetes/CVD epidemic in India.118
Suggestions on future research
In India, diabetes and CVD are growing health problems, and therefore the prevention and treatment of these diseases remain challenges for the future. Future research on diabetes and CVD should focus on searching for risk factors for CVD that may be more specific to diabetes, such as hypoglycaemia or medication related comorbidities. In addition, research should also focus on the common genetic variants, which might have stronger effects and hence have a potential role in diabetes and CVD risk prediction. Finally, primary prevention trials trying to prevent both diabetes and CVD are the urgent need of the hour!
Footnotes
Contributors: RP conceived and drafted the article, AN helped in literature search and drafting the manuscript and VM critically revised the manuscript.
Competing interest: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.International Diabetes Federation. Diabetes Atlas. 5th edn Brussels, Belgium: International diabetes Federation, 2011. http://www.idf.org/new-idf-data-reveals-diabetes-epidemic-continues-escalate (accessed 11 Oct 2011). [Google Scholar]
- 2.Anjana RM, Pradeepa R, Deepa M, et al. ; ICMR–INDIAB Collaborative Study Group. Prevalence of diabetes and prediabetes (impaired fasting glucose or/and impaired glucose tolerance) in rural and urban India: phase 1 results of the Indian Council of Medical Research-INdiaDIABetes (INDIAB) study. Diabetologia 2011;54:3022–7. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Factsheet. http://www.who.int/mediacentre/factsheets/fs312/en/ (accessed 12 Aug 2011).
- 4.World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. Geneva: World Health Org., 2006. [Google Scholar]
- 5.Day C. Metabolic syndrome or what you will: definitions and epidemiology. Diab Vasc Dis Res 2007;4:32–8. [DOI] [PubMed] [Google Scholar]
- 6.Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–34. [DOI] [PubMed] [Google Scholar]
- 7.Laakso M, Lehto S. Epidemiology of macro vascular disease in diabetes. Diabetes Rev 1997;5:294–315. [Google Scholar]
- 8.Schramm TK, Gislason GH, Køber L, et al. Diabetes patients requiring glucose-lowering therapy and non-diabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation 2008;117:1945–54. [DOI] [PubMed] [Google Scholar]
- 9.Miettinen H, Lehto S, Salomaa V, et al. Impact of diabetes on mortality after the first myocardial infarction: the FINMONICA Myocardial Infarction Register Study Group. Diabetes Care 1998;21:69–75. [DOI] [PubMed] [Google Scholar]
- 10.Laing SP, Swerdlow AJ, Slater SD, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003;46:760–5. [DOI] [PubMed] [Google Scholar]
- 11.Kasuga M. Insulin resistance and pancreatic beta cell failure. J Clin Invest 2006;116:1756–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 2006;83:456S–60S. [DOI] [PubMed] [Google Scholar]
- 13.Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Adv Cardiol 2008;45:1–16. [DOI] [PubMed] [Google Scholar]
- 14.Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag 2007;3:853–76. [PMC free article] [PubMed] [Google Scholar]
- 15.Andor M. The role of inflammation in endothelial dysfunction and progression of atherosclerosis in metabolic syndrome. TMJ 2005;55:30–4. [Google Scholar]
- 16.Jarrett RJ. Type 2 (non-insulin-dependent) diabetes mellitus and coronary heart disease-chicken, egg or neither? Diabetologia 1984;26:99–102. [DOI] [PubMed] [Google Scholar]
- 17.Stern MP. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes 1995;44:369–74. [DOI] [PubMed] [Google Scholar]
- 18.Stolar MW, Chilton RJ. Type 2 diabetes, cardiovascular risk, and the link to insulin resistance. Clin Ther 2003;25(Suppl B):B4–31. [DOI] [PubMed] [Google Scholar]
- 19.Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J 2002;23:831–4. [DOI] [PubMed] [Google Scholar]
- 20.Krentz AJ. Type 2 diabetes and atherosclerotic cardiovascular disease: do they share common antecedents? Br J Diabetes Vasc Dis 2002;2:37. [Google Scholar]
- 21.Calle MC, Fernandez ML. Inflammation and type 2 diabetes. Diabetes Metab. 2012 Jan 15. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.El-Mesallamy HO, Hamdy NM, Salman TM, et al. Adiponectin and E-selectin concentrations in relation to inflammation in obese type 2 diabetic patients with coronary heart disease(s). Minerva Endocrinol 2011;36:163–70. [PubMed] [Google Scholar]
- 23.Mohan V, Deepa R, Pradeepa R, et al. Association of low adiponectin levels with the metabolic syndrome—the Chennai Urban Rural Epidemiology Study (CURES-4). Metabolism 2005;54:476–81. [DOI] [PubMed] [Google Scholar]
- 24.Shimada K, Miyazaki T, Daida H. Adiponectin and atherosclerotic disease. Clin Chim Acta 2004;344:1–12. [DOI] [PubMed] [Google Scholar]
- 25.Kumada M, Kihara S, Sumitsuji S, et al. ; Osaka CAD Study Group: Coronary artery disease. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 2003;23:85–9. [DOI] [PubMed] [Google Scholar]
- 26.Gokulakrishnan K, Indulekha K, Ganesan S, et al. Adiponectin and carotid intimal medial thickness in subjects with and without glucose intolerance (CURES-82). Diabetes Technol Ther 2010;12:109–15. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003;107:363–9. [DOI] [PubMed] [Google Scholar]
- 28.Festa A, D'Agostino Jr R, Tracy RP, et al. ; Insulin Resistance Atherosclerosis Study. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: The Insulin Resistance Atherosclerosis Study. Diabetes 2002;51:1131–7. [DOI] [PubMed] [Google Scholar]
- 29.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782–7. [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S, Hawken S, Ounpuu S, et al. ; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- 31.Mukhopadhyay B, Forouhi NG, Fisher BM, et al. A comparison of glycaemic and metabolic control over time among South Asian and European patients with Type 2 diabetes: results from follow-up in a routine diabetes clinic. Diabet Med 2006;23:94–8. [DOI] [PubMed] [Google Scholar]
- 32.Mukhopadhyay B, Sattar N, Fisher BM. Diabetes and cardiac disease in South Asians. Br J Diabetes Vasc Dis 2005;5:253–9. [Google Scholar]
- 33.Joshi P, Islam S, Pais P, et al. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA 2007;297:286–94. [DOI] [PubMed] [Google Scholar]
- 34.Harding S, Rosato M, Teyhan A. Trends for coronary heart disease and stroke mortality among migrants in England and Wales, 1979–2003: slow declines notable for some groups. Heart 2008;94:463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enas EA, Senthilkumar A. Coronary artery disease in Asian Indians: an update and review [online]. Internet J Cardiol. 2001:1 (accessed 11 Oct 2011). http://www.ispub.com/ostia/index.php?xmlFilePath=journals/ijc/vol1n2/cadi.xml [Google Scholar]
- 36.McCarthy JJ. Gene by sex interaction in the etiology of coronary heart disease and the preceding metabolic syndrome. Nutr Metab Cardiovasc Dis 2007;17:153–61. [DOI] [PubMed] [Google Scholar]
- 37.Solimene MC. Coronary heart disease in women: a challenge for the 21st century. Clinics (Sao Paulo) 2010;65:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wedisinghe L, Perera M. Diabetes and the menopause. Maturitas 2009;63:200–3. [DOI] [PubMed] [Google Scholar]
- 39.Yeung EH, Pankow JS, Astor BC, et al. Increased risk of type 2 diabetes from a family history of coronary heart disease and type 2 diabetes. Diabetes Care 2007;30:154–6. [DOI] [PubMed] [Google Scholar]
- 40.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest 2000;106:453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rett K. The relation between insulin resistance and cardiovascular complications of the insulin resistance syndrome. Diabetes Obes Metab 1999;1(Suppl 1):S8–16. [DOI] [PubMed] [Google Scholar]
- 42.Alexander CM, Landsman PB, Teutsch SM, et al. ; National Cholesterol Education Program (NCEP). NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 2003;52:1210–14. [DOI] [PubMed] [Google Scholar]
- 43.Gupta R, Misra A, Vikram NK, et al. Younger age of escalation of cardiovascular risk factors in Asian Indian subjects. BMC Cardiovasc Disord 2009;9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haag M, Dippenaar NG. Dietary fats, fatty acids and insulin resistance: short review of a multifaceted connection. Med Sci Monit.2005;11:RA359–67. [PubMed] [Google Scholar]
- 45.Takeuchi T, Nakao M, Nomura K, et al. Association of metabolic syndrome with depression and anxiety in Japanese men. Diabetes Metab 2009;35:32–6. [DOI] [PubMed] [Google Scholar]
- 46.Umpierez GE. How to manage type 2 diabetes in medical and surgical patients in the hospital. Cleve Clin J Med 2011;78:379–84. [DOI] [PubMed] [Google Scholar]
- 47.Anonymous. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38 UK Prospective Diabetes Study Group. BMJ 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 48.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–25. [DOI] [PubMed] [Google Scholar]
- 49.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002;288:2709–16. [DOI] [PubMed] [Google Scholar]
- 50.Balkau B, Bertrais S, Ducimetiere P, et al. Is there a glycaemic threshold for mortality risk? Diabetes Care 1999;22:696–9. [DOI] [PubMed] [Google Scholar]
- 51.Lawes CM, Parag V, Bennett DA, et al. Asia Pacific Cohort Studies Collaboration. Blood glucose and risk of cardiovascular disease in the Asia Pacific Region. Diabetes Care 2004;27:2836–42. [DOI] [PubMed] [Google Scholar]
- 52.Pais P, Pogue J, Gerstein H, et al. Risk factors for acute myocardial infarction in Indians: a case-control study. Lancet 1996;348:358–63. [DOI] [PubMed] [Google Scholar]
- 53.Jesudason DR, Dunstan K, Leong D, et al. Macrovascular risk and diagnostic criteria for type 2 diabetes: implications for the use of FPG and HbA1c for cost-effective screening. Diabetes Care 2003;26:485–90. [DOI] [PubMed] [Google Scholar]
- 54.Haffner SM. Impaired glucose tolerance, insulin resistance, and cardiovascular disease. Diabet Med 1997;14(Suppl 3):S12–18. [DOI] [PubMed] [Google Scholar]
- 55.Laakso M, Lehto S. Epidemiology of risk factors for cardiovascular disease in diabetes and impaired glucose tolerance. Atherosclerosis 1998;137:S65–73. [DOI] [PubMed] [Google Scholar]
- 56.Jarrett RJ, McCartney P, Keen H. The Bedford Survey: ten year mortality rates in newly diagnosed diabetics, borderline diabetics, and normoglycaemic controls and risk indices for coronary heart disease in borderline diabetics. Diabetologia 1982;22:79–84. [DOI] [PubMed] [Google Scholar]
- 57.Fuller JH, Shipley MJ, Rose G, et al. Mortality from coronary heart disease and stroke in relation to degree of glycaemia: the Whitehall study. Br Med J (Clin Res Ed) 1983;287:867–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donahue RP, Abbott RD, Reed DM, et al. Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry. Honolulu Heart Program. Diabetes 1987;36:689–92. [DOI] [PubMed] [Google Scholar]
- 59.Mohan V, Deepa R, Shanthirani S, et al. Prevalence of coronary artery disease and its relationship to lipids in a selected population in South India: The Chennai Urban population Study (CUPS No. 5). J Am Coll Cardiol 2001;38:682–7. [DOI] [PubMed] [Google Scholar]
- 60.Deepa R, Arvind K, Mohan V. Diabetes and risk factors for coronary artery disease. Curr Sci 2002;83:1497–505. [Google Scholar]
- 61.Savage G, Ewing P, Kirkwood H, et al. Are undiagnosed IGT/IFG and type 2 diabetes common in heart disease and hypertension? Br J Diabetes Vasc Dis 2003;6:414–16. [Google Scholar]
- 62.MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990;335:765–74. [DOI] [PubMed] [Google Scholar]
- 63.Lindholm LH, Ibsen H, Dahlof B, et al. LIFE Study Group. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:1004–10. [DOI] [PubMed] [Google Scholar]
- 64.Patel A, MacMahon S, Chalmers J. ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascula outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomized controlled trial. Lancet 2007;370:829–40. [DOI] [PubMed] [Google Scholar]
- 65.Cushman WC, Evans GW, Byington RP; The ACCORD Study group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zanchetti A, Hansson L, Clement D, et al. Benefits and risks of more intensive blood pressure lowering in hypertensive patients of the HOT study with different risk profiles: does a J-shaped curve exist in smokers? J Hypertens 2003;21:797–804. [DOI] [PubMed] [Google Scholar]
- 67.Syvanne M, Taskinen MR. Lipids and lipoproteins as coronary risk factors in non-insulin-dependent diabetes mellitus. Lancet 1997;350(Suppl 1):SI20–3. [DOI] [PubMed] [Google Scholar]
- 68.Tan KC, Ai VH, Chow WS, et al. Influence of low-density lipoprotein (LDL) subfraction profile and LDL oxidation on endothelium-dependent and independent vasodilation in patients with type 2 diabetes. J Clin Endocrinol Metab 1999;84:3212–16. [DOI] [PubMed] [Google Scholar]
- 69.Mohan V, Deepa R, Velmurugan K, et al. Association of small dense LDL with coronary artery disease and diabetes in urban Asian Indians- The Chennai Rural Epidemiology Study (CURES 8). J Assoc Physicians India 2005;53:95–100. [PubMed] [Google Scholar]
- 70.Moreno PR, Fuster V. New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol 2004;44:2293–300. [DOI] [PubMed] [Google Scholar]
- 71.Rajmohan L, Deepa R, Mohan A, et al. Association between Isolated hypercholesterolemia, isolated hypertriglycerideia and coronary artery disease in south Indian type 2 diabetic patients. Indian Heart J 2000;52:400–6. [PubMed] [Google Scholar]
- 72.Manninen V, Tenkanen L, Koskinen P, et al. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study: implications for treatment. Circulation 1992;85:37–45. [DOI] [PubMed] [Google Scholar]
- 73.Rajamani K, Colman PG, Li LP, et al. ; FIELD study investigators. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a pre specified analysis of a randomized controlled trial. Lancet 2009;373:1780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Forouhi NG, Sattar N, McKeigue PM. Relation of C-reactive protein to body fat distribution and features of the metabolic syndrome in Europeans and South Asians. Int J Obes Relat Metab Disord 2001;25:1327–31. [DOI] [PubMed] [Google Scholar]
- 75.Chan JM, Rimm EB, Colditz GA, et al. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994;17:961–9. [DOI] [PubMed] [Google Scholar]
- 76.Mohan V, Shanthirani CS, Deepa R. Glucose intolerance (diabetes and IGT) in a selected south Indian population with special reference to family history, obesity and life style factors – The Chennai Urban Population Study (CUPS 14). J Assoc of Physicians India 2003;51:771–7. [PubMed] [Google Scholar]
- 77.Monzillo LU, Hamdy O, Horton ES, et al. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res 2003;11:1048–54. [DOI] [PubMed] [Google Scholar]
- 78.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 2003;289:1799–804. [DOI] [PubMed] [Google Scholar]
- 79.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance.The DaQing IGT and Diabetes Study. Diabetes Care 1997;20:537–44. [DOI] [PubMed] [Google Scholar]
- 80.The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bullo M, Garcia-Lorda P, Megias I, et al. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res 2003;11:525–31. [DOI] [PubMed] [Google Scholar]
- 83.Festa A, Haffner SM. Inflammation and cardiovascular disease in patients with diabetes: lessons from the Diabetes Control and Complications Trial. Circulation 2005;111:2414–15. [DOI] [PubMed] [Google Scholar]
- 84.Lakier JB. Smoking and cardiovascular disease. Am J Med 1992;93:8S–12S. [DOI] [PubMed] [Google Scholar]
- 85.Wili C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007;298:2654–64. [DOI] [PubMed] [Google Scholar]
- 86.Sargeant LA, Khaw KT, Bingham S, et al. Cigarette smoking and glycaemia: the EPIC-Norkfolk Study. European Prospective Investigation into Cancer. Int J Epidemiol 2001;30:547–54. [DOI] [PubMed] [Google Scholar]
- 87.Stevens RJ, Kothari V, Adler AI, et al. ; United Kingdom Prospective Diabetes Study (UKPDS) Group. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin Sci (Lond) 2001;101:671–9. [PubMed] [Google Scholar]
- 88.Fagard RH. Smoking amplifies cardiovascular risk n patients with hypertension and diabetes. Diabetes Care 2009;32(Suppl 2):S429–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Risnes KR, Vatten LJ, Baker JL, et al. Birth weight and mortality in adulthood: a systematic review and meta-analysis. Int J Epidemiol 2011;40:647–61. [DOI] [PubMed] [Google Scholar]
- 90.Whincup PH, Kaye SJ, Owen CG, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA 2008;300:2886–97. [DOI] [PubMed] [Google Scholar]
- 91.Hales CN, Barker DJ. Type-II (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992;35:595–601. [DOI] [PubMed] [Google Scholar]
- 92.Yajnik CS. The insulin resistance epidemic in India: fetal origins, later lifestyle, or both? Nutr Rev 2001;59:1–9. [DOI] [PubMed] [Google Scholar]
- 93.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body mass index to impaired glucose tolerance in young adulthood. N Engl J Med 2004;350:865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schneider DJ, Nordt TK, Sobel BE. Attenuated fibrinolysis and accelerated atherosclerosis in type II diabetic patients. Diabetes 1993;42:1–7. [DOI] [PubMed] [Google Scholar]
- 95.Thompson SG, Kienast J, Pyke SD, et al. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. New Eng J Med 1995;332:635–41. [DOI] [PubMed] [Google Scholar]
- 96.Deepa R, Velmurugan K, Saravanan G, et al. Relationship of tissue plasminogen activator, plasminogen activator inhibitor-1 and fibrinogen with coronary artery disease in South Indian male subjects. J Assoc Physicians India 2002;50:901–6. [PubMed] [Google Scholar]
- 97.Jialal I, Devaraj S. Inflammation and atherosclerosis: the value of the high-sensitivity C-reactive protein assay as a risk marker. Am J Clin Pathol 2001;116:S108–15. [DOI] [PubMed] [Google Scholar]
- 98.Festa A, D'Agostino R, Jr, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000;102:42–7. [DOI] [PubMed] [Google Scholar]
- 99.Nicaud V, Raoux S, Poirier O, et al. The TNF alpha/G-308A polymorphism influences insulin sensitivity in offspring of patients with coronary heart disease: the European Atherosclerosis Research Study II. Atherosclerosis 2002;161:317–25. [DOI] [PubMed] [Google Scholar]
- 100.Mohan V, Deepa R, Velmurugan K, et al. Association of C-reactive protein with body fat, diabetes and coronary artery disease in Asian Indians the Chennai Urban Rural Epidemiology Study (CURES-6), Diabet Med 2005;22:863–70. [DOI] [PubMed] [Google Scholar]
- 101.Yadav K, Krishnan A. Changing patterns of diet, physical activity and obesity among urban, rural and slum populations in north India. Obes Rev 2008;9:400–8. [DOI] [PubMed] [Google Scholar]
- 102.Sheehan MT. Current therapeutic options in type 2 diabetes mellitus: a practical approach. Clin Med Res 2003;1:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yusuf S, Peto R, Lewis J, et al. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985;27:335–71. [DOI] [PubMed] [Google Scholar]
- 105.Yusuf S, Sleight P, Pogue J, et al. Eff ects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000;342:145–53. [DOI] [PubMed] [Google Scholar]
- 106.Baigent C, Keech A, Kearney PM; Cholesterol Treatment Trialists' (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomized trials of statins. Lancet 2005;366:1267–78. [DOI] [PubMed] [Google Scholar]
- 107.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ 2003;326:1419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326:1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Law MR, Wald NJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ 2003;326:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yusuf S, Pais P, Afzal R, et al. ; Indian Polycap Study (TIPS). Effects of a polypill (Polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a phase II, double-blind, randomised trial. Lancet 2009;373:1341–51. [DOI] [PubMed] [Google Scholar]
- 111.Reddy KS, Katan MB. Diet, nutrition and the prevention of hypertension and cardiovascular diseases. Public Health Nutr 2004;7:167–86. [DOI] [PubMed] [Google Scholar]
- 112.Rastogi T, Reddy KS, Vaz M, et al. Diet and risk of ischemic heart disease in India. Am J Clin Nutr 2004;79:582–92. [DOI] [PubMed] [Google Scholar]
- 113.Radhika G, Van Dam RM, Sudha V, et al. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai urban rural epidemiology study 57). Metabolism 2009;58:675–81. [DOI] [PubMed] [Google Scholar]
- 114.Radhika G, Sathya RM, Ganesan A, et al. Dietary profile of urban adult population in South India in the context of chronic disease epidemiology (CURES-68). Public Health Nutr 2011;14:591–8. [DOI] [PubMed] [Google Scholar]
- 115.Ganguli D, Das N, Saha I, et al. Major dietary patterns and their associations with cardiovascular risk factors among women in West Bengal, India. Br J Nutr 2011;105:1520–9. [DOI] [PubMed] [Google Scholar]
- 116.Held C, Iqbal R, Lear SA, et al. Physical activity levels, ownership of goods promoting sedentary behaviour and risk of myocardial infarction: results of the INTERHEART study. Eur Heart J 2012;33:452–66. [DOI] [PubMed] [Google Scholar]
- 117.Rastogi T, Vaz M, Spiegelman D, et al. Physical activity and risk of coronary heart disease in India. Int J Epidemiol 2004;33:759–67. [DOI] [PubMed] [Google Scholar]
- 118.Mohan V, Radhika G, Vijayalakshmi P, et al. Can the diabetes/cardiovascular disease epidemic in India be explained, at least in part, by excess refined grain (rice) intake? Indian J Med Res 2010;131:369–72. [PubMed] [Google Scholar]