Abstract
Background:
Reversal learning has proven to be a valuable task in assessing the inhibitory process that is central to executive control. Psycho-stimulants and music are prevalent factors that influence cognition.
Objectives:
The present study aimed at investigating the influences of dexamphetamine and music on inhibitory control.
Materials and Methods:
This experimental study was conducted between May and June 2014 in the laboratory animal center of Shiraz University of Medical Sciences, Shiraz, Iran. Thirty mice were divided to five groups including a control group, a witness group, and three experimental groups. Food availability was restricted in order to maintain the subjects at 85% of their free-feeding body weight for behavioral testing. After discrimination learning, animals received four injections of 2 mg/kg dexamphetamine at two-hour intervals. The music group was exposed to music half an hour before reversal learning.
Results:
According to the results of the repeated measure analysis of variance (ANOVA), music increased errors (mean difference: -2.40, 95% CI: -3.59 to -1.22), yet dexamphetamine had no significant effect on reversal learning. Due to various advantages, we transited to the mixed model that showed increasing (Beta: 2.2 95% CI: 0.26 to 4.13) and borderline (Beta: 1.8 95% CI: -0.13 to 3.73) effects on the number of errors for dexamphetamine and music group, respectively.
Conclusions:
Drug-treated subjects were impaired in their ability to modulate behavior, based upon changing information about stimulus-reward associations, possibly due to the inability to inhibit their response. These effects may have relevance to some mental disorders such as drug-abuse, schizophrenia, and obsessive-compulsive disorder.
Keywords: Dexamphetamine, Inhibitory Control, Mixed Model, Music, Repeated Measure, Reversal Learning
1. Background
Learning is a relatively permanent change in behavior as a result of experience (1). In response to a changing environment, behavior has to be adaptive and flexible and the ability to stop inappropriate response is the key element of executive function that plays an important role in individuals’ adaptation to the changing situational demands (2). Reversal learning, as an experimental paradigm, representing changing environmental conditions (3), is defined as the ability to adapt one’s thinking and behavior in response to a changing environment (4, 5). In reversal learning, the subject has to actively stop ongoing behavior and this ability is an important characteristic of cognitive control-cognitive flexibility (6). Without the ability to inhibit actions, it would be impossible to perform even the simplest of everyday tasks. Failure to adapt to changing environmental demands is observed in various disorders such as schizophrenia, autism, addiction, and obsessive-compulsive disorder (OCD) (7-12) Psycho-stimulants are increasingly used by healthy people as a way of cognitive enhancement (13, 14). At the same time, many studies have revealed their ability to induce cognitive deficits when they are used inappropriately. Users exhibit learning and memory deficits, particularly on tasks requiring response control when situational demands change (15). Besides, there have been numerous claims that music exposure also influences cognitive performance (16) and the potential for music to influence cognitive performance has interested many researchers, therapists and educators. Nevertheless, how does the emotion elicited by auditory stimuli interact with response inhibition has yet to be directly investigated. In addition, in view of the large number of stimulant-users potentially at risk for negative effects and the inconsistency of research findings, further investigation of their routes and dose is warranted, while the effects of psycho-stimulants on conditioned inhibition remain to be fully understood. On the other hand, studies should determine the effect of music and psycho-stimulants, as prevalent factors in our society, on cognitive flexibility.
The repeated measures experiment is a common design for animal science research (17, 18). Two conventional methods are repeated measure analysis of variance (ANOVA) and mixed model. The mixed model has various advantages as a proper method, especially in animal studies compared to traditional repeated measure ANOVA (19). Nevertheless, only a few studies have used the mixed model for analyzing repeated data. Because of many advantages of the mixed model, for our data analysis we transited from ANOVA to the mixed model. The present study aimed to investigate the effect of music, as a basic human function, and dexamphetamine on cognitive flexibility and inhibitory control.
2. Objectives
The present study aimed at investigating the influences of dexamphetamine and music on inhibitory control.
3. Materials and Methods
3.1. Experimental Subjects
This experimental study was conducted between May and June 2014, in the laboratory animal center of Shiraz University of Medical Sciences, Shiraz, Iran. It was conducted in cooperation with the university of Fars science and research and comprised of thirty male mice weighing 25 - 30 g before behavioral testing. The animals were divided into five groups, as follows: G1, control group (no treatment, n = 6); G2, placebo group (received saline, n = 4); G3: treated with dexamphetamine (n = 5) after reaching the criteria of discriminative learning; G4, exposed to music (n = 5) before reversal learning (Beethoven music with frequency of 80); G5, treated with dexamphetamine after reaching the criteria of discriminative learning and exposed to music (n = 4).
Only male mice were used in the study, because gender differences can influence cognitive behavior. Mice assigned to the dexamphetamine group received four daily subcutaneous injections of 2 mg/kg at two-hour intervals. The saline control group received the same volume of saline. All experiments were performed during the day from 7 am to 7 pm. All animals were individually housed in temperature-controlled environments under 12: 12 hour light/dark conditions. For discrimination acquisition and reversal learning studies, water was available ad libitum whereas food availability was restricted in order to maintain the animals at 85% of their free-feeding body weight for behavioral testing. Food was given immediately following training, at an amount that maintained body weight at about 85% of the animal’s free feeding weight. Subjects were weighed regularly to make sure they did not lose weight by more than 15%. Animal care and the experiments were conducted in accordance with the guidelines of the national institute of health for the care and use of laboratory animals and the guidelines for proper conduct of animal experiments of the science council of Iran.
3.2. Materials
Reversal learning was studied using a black T-maze. It consisted of a start arm (35 × 10 cm) and two identical goal arms (30 × 10 cm), surrounded by a 15-cm high wall. Goal arms led to goal boxes. The colors of goal arms were changed by inserting 1 mm of thick Plexiglas into the alleys. The position of Plexiglas inserts in the right and left arms changed pseudo-randomly through the 20 daily trials. However, the colors were evenly distributed between the left and right arms through 20 daily trails. Guillotine doors separated boxes from arms. In this experiment, sweetened condensed milk (0.07 mL, measured by a syringe) was used as reinforcement. Reward was put at the end of the targeted goal box.
3.3. Discrimination Learning
First, the habituation phase was performed on two consecutive days. Rewards were available non-contingently in both arms. Each animal was allowed to explore the maze for five minutes. The next day, subjects were trained during 20 daily trial sessions by the experimenter blinded to treatment conditions. Responses (arm choices) and latencies were recorded by the observer. The black arm was the positive reinforced stimuli for half the subjects, whereas the white arm was the reinforced stimuli for the other half. Each animal was placed in the start box and allowed to enter the goal box by opening the Guillotine door. If the subject entered the correct colored arm, it received a reward. After trial completion, the subject was returned to the start box for approximately five seconds, sufficient time to wipe the maze with 70% alcohol, and if necessary change the position of the colored inserts. The criterion of learning secured 80% correct response in 20 trials per session.
3.4. Reversal Learning
After discrimination learning, subjects were given four injections, at two-hour intervals, of dexamphetamine (2 mg free base/kg, SC) or physiological saline solution. The animals were then given five days of rest without behavioral testing followed by a test for retention of the discrimination problem. Mice that did not show the discrimination criteria (80% correct response in 20 trials, during the retention test) were excluded from the study. All the subjects were then tested on a reversal condition. The reinforced contingencies were switched, such that the color previously not associated with reward, was now the reinforced stimulus. Subjects were tested daily (20 trails a day) for eight days. The criterion for reversal learning was 80% correct response in 20 trials during one session.
3.5. Statistical Methods
3.5.1. Repeated Measures Analysis of Variance
For the discrimination acquisition and reversal experiments, repeated measurement analysis of variance was used to determine the effects on different groups (drug and music) across the multiple days of testing (the repeated measure). The dependent variable was the average number of errors per testing day. In this study, we compared differences in reversal learning variability, so called dependent variable, across time (within subject’s factor) by different types of interventions (between subject’s factors). At first, we did our analysis with repeated measure ANOVA and calculated the Mauchly’s test of sphericity results to evaluate whether the sphericity assumption had been violated or not. In order to minimize this problem, Greenhouse and Geisser cited in Abdi (20) suggested the use of an index of deviation to sphericity to correct the number of degrees of freedom of the distribution. In addition, the post hoc least significant difference (LSD) was used for multiple comparisons in different groups. The SPSS software version 19 was used for statistical analysis.
3.5.2. Repeated Measure Mixed Model
The general linear mixed model or group means are considered as fixed effects while simultaneous modeling for individual subject variables are regarded as random effects. Regarding the analysis described here, the mixed model also allowed us to model for higher order, nonlinear changes in the dependent measure (reversal learning) across time. The mixed model, with its broad possibilities for modeling longitudinal data, is becoming immensely popular as a framework for the analysis of bio-behavioral data. These include multiple procedures that handle mixed modeling in STATA, which generally begin with the XT command, and follow the procedures for the ordinary version of the statistical model, using the STATA software version 13.
3.6. Ethics
Animal care and the experiments were conducted in accordance with the guidelines of the national institute of health for the care and use of laboratory animals and the guidelines for proper conduct of animal experiments of the science council of Iran.
4. Results
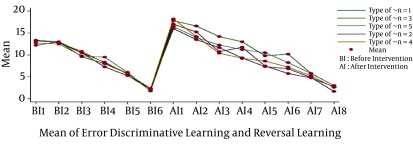
The descriptive statistics of error in learning are depicted in Figure 1, where the mean of error for direct, and reversal learning showed decreasing trend by passage of time (Table 1). The maximum and minimum error belonged to the first and last days of follow up. In the first step, we check for sphericity assumption by Machualy’s test. As P = 0, the null hypothesis was not rejected, and therefore the results were consistent with the Greenhouse and Geisser repeated measure.
Figure 1. The Descriptive Statistics of Error in Learning.
Table 1. Descriptive Statistics of Error in Discriminative Learning and Reversal Learning.
| Time | Mean ± SD | Range |
|---|---|---|
| Before the Intervention (Error) | ||
| Day 1 | 12.95 ± 0.90 | 11 - 15 |
| Day 2 | 12.83 ± 1.12 | 11 - 15 |
| Day 3 | 10.25 ± 1.03 | 9 - 13 |
| Day 4 | 8.25 ± 1.15 | 7 - 11 |
| Day 5 | 5.70 ± 0.75 | 5 - 7 |
| Day 6 | 2.08 ± 0.65 | 1 - 3 |
| After the Intervention (Error) | ||
| Day 1 | 17.08 ± 1.44 | 13 - 19 |
| Day 2 | 14.66 ± 1.68 | 11 - 18 |
| Day 3 | 11.87 ± 1.94 | 8 - 17 |
| Day 4 | 10.79 ± 2.08 | 6 - 14 |
| Day 5 | 8.66 ± 2.42 | 5 - 13 |
| Day 6 | 7.66 ± 2.59 | 4 - 14 |
| Day 7 | 5.25 ± 1.29 | 3 - 8 |
| Day 8 | 2.54 ± 0.97 | 1 - 4 |
In the direct learning ascribed to before the intervention, there was no significant effect on the treatment group (F = 1.38, P = 0.27), nor was there any statistically significant difference between the interaction of time and treatments (F = 1.06, P = 0.40). However, as we expected, direct and reversal learning changed over time. On the other hand, in the reversal learning, there was a significant difference between interaction of treatments and time with repeated measures ANOVA (Table 2).
Table 2. Repeated Measure Analysis of Variance: Within and Between Subjects For Discriminative Learning (Before the Intervention) and Reversal Learning (After the Intervention).
| Type of responses | df | MS | F | P |
|---|---|---|---|---|
| Before the Intervention | ||||
| Between subjects | ||||
| Treatment | 4 | 1.17 | 1.38 | 0.27 |
| Error | 19 | 0.85 | ||
| Within subjects | ||||
| Time | 5 | 423.93 | 468.42 | 0.0001 |
| Time treatment | 20 | 0.95 | 1.06 | 0.40 |
| Error | 95 | 0.90 | ||
| After the Intervention | ||||
| Between subjects | ||||
| Treatment | 4 | 35.77 | 5.10 | 0.0001 |
| Error | 19 | 7.00 | ||
| Within subjects | ||||
| Time | 7 | 538.74 | 264.75 | 0.0001 |
| Time treatment | 28 | 3.71 | 1.82 | 0.012 |
| Error | 133 | 2.03 | ||
Post hoc LSD test was done for multiple comparisons of repeated measure of ANOVA with each of the interventional groups and control group. As shown in Table 3, increasing error in reversal learning was related to music (mean difference -2.40, 95% CI -3.59 to -1.22, P = 0.0001), and music plus dexamphetamine (mean difference 1.29, 95% CI -2.56 to -0.03, P = 0.046).
Table 3. Post Hoc Least Significant Difference Test for Multiple Comparisons with Repeated Measure of Analysis of Variance in Different Groups.
| Treatment | Mean ± SE | P Value | 95% CI |
|---|---|---|---|
| Saline | -0.54 ± 0.604 | 0.381 | -1.81 - 0.72 |
| Music | -2.40 ± 0.567 | 0.000 | -3.59 - -1.22 |
| Dexamphetamine | -0.55 ± 0.567 | 0.340 | -1.74 - 0.63 |
| Music and dexamphetamine | -1.29 ± 0.604 | 0.046 | -2.56 - -0.03 |
On the other hand, multiple comparisons of repeated measure with mixed model showed that only dexamphetamine had a significant effect on increasing error in reversal learning compared with the control group (Beta: 2.2 95% CI: 0.26 to 4.13, and P = 0.02). Also, music showed borderline effect with Beta 1.8, 95% CI -0.13 to 3.73, and P = 0.06 (Table 4).
Table 4. Multiple Comparisons of Repeated Measure Mixed Model in Different Groups.
| Treatment | Coefficient | Standard Error | z | P1 [z] | 95% CI |
|---|---|---|---|---|---|
| Saline | 1 | 1.05 | 0.95 | 0.34 | -1.06 - 3.06 |
| Music | 1.8 | 0.98 | 1.82 | 0.06 | -0.13 - 3.73 |
| Dexamphetamine | 2.2 | 0.98 | 2.23 | 0.02 | 0.26 - 4.13 |
| Music and dexamphetamine | 0.5 | 1.05 | 0.48 | 0.63 | -1.56 - 2.56 |
| Time | |||||
| 2 | -2.5 | 0.82 | -3.04 | 0.002 | -4.11 - -0.88 |
| 3 | -4.33 | 0.82 | -5.26 | 0.0001 | -5.94 - -2.71 |
| 4 | -6.66 | 0.82 | -8.09 | 0.0001 | -8.28 - -5.05 |
| 5 | -8.66 | 0.82 | -10.52 | 0.0001 | -10.28 - -7.05 |
| 6 | -9.16 | 0.82 | -11.13 | 0.0001 | -10.78 - -7.55 |
| 7 | -11.16 | 0.82 | -13.56 | 0.0001 | -12.78 - -9.55 |
| 8 | -14.33 | 0.82 | -17.40 | 0.0001 | -15.94 - -12.71 |
5. Discussion
According to the results of repeated measure ANOVA, subjects exposed to music, committed more errors during reversal learning, which was not significantly affected by dexamphetamine. While the results of the mixed model analysis showed binge dose-induced deficits in conditioned reversal learning. On the other hand, music had a borderline effect on reversal learning. In addition, no significant difference was found between drug-treated subjects exposed to music and the control group.
The use of mixed models represents a substantial difference from the traditional analysis of variance, but the results were comparable regarding balanced designs including equal sample sizes in different groups, indicative of the appropriateness of statistical analysis. However, the actual statistical approach is quite different and ANOVA and mixed models will lead to different results if the data are not balanced (21) or we try to use different, and often more logical, covariance structures. One of the reasons for obtaining different results in this study was unbalanced data represented by different number of subjects in different groups.
The principal virtue of the ANOVA approach to longitudinal data analysis is its technical simplicity, which outweighs its inherent limitations. For example, statistical assumptions related to a complete dataset, randomization, and a common set of time periods cannot be frequently met in bio behavioral research. The mixed model has several unique abilities such as automatically computing correct standard errors for each effect, allowing unbalance or missing observations within-subject and incorporating additional covariates (22). Although the repeated measures ANOVA requires a fixed time schedule among all individual units, the mixed model can accommodate flexible time schedules. This adaptation of continuous treatment time allows for varied entry of participants into the study, which also allows for several, generally nonequivalent possibilities for modeling behavior.
In this study there were compelling reasons for transitioning from ANOVA to the mixed model, these include, unbalanced and missing data, randomized block design, the overtime change of learning as a bio-behavioral variable and probability of carryover effect. Therefore, for data analysis we had to focus on the results obtained from the mixed model.
The mixed model is an efficient method to cope with data missing at random (23). Therefore, in our study because of missing data, it was preferred to use the mixed model. Designed experiments usually involve blocking as well as several nested or crossed levels of randomization, giving rise to multiple block and error effects. It is not obvious how such effects should be treated in repeated measures settings. Only a few publications explicitly address this problem in the context of randomized block experiments in agriculture and biology (24, 25). Despite the advantages of the mixed model approach in randomized experiments with blocking design, this model is not in frequent use. Therefore, another compelling reason for using the mixed model was the randomized block design of the experimental subject.
In this study, there were two phases of learning, which probably interfered with each other, probably due to carryover effect. Care must be taken to account for carryover effects, either by allowing sufficient time between treatments or by using a special design, so called cross-over design (26, 27). We consider a five-day interval between the two phases to eliminate the effect of the first phase. On the other hand, in this study we entered the first day of the intervention as a covariance in the model to investigate the carry over effect in the second phase (reversal learning), which was not statistically significant.
Results showed that drug-treated subjects confronted with changes in contingencies, made more mistakes in favor of the previously rewarded learning eventualities. Binge regimen of dexamphetamine (4 × 2 mg/kg) impaired reversal learning as assessed in the T-maze task. The drug impaired the ability of subjects to shift responding away from a previously rewarded, yet currently unrewarded stimulus. Here, we found that animals that received dexamphetamine treatment subsequently displayed impulsivity when tested on tasks that required inhibiting a conditioned response, when the stimulus-response contingencies changed. Many psychiatric diseases, most obviously drug abuse, schizophrenia, and obsessive-compulsive disorders are characterized by increased impulsivity. Each of these pathological states is associated with the inability to inhibit inappropriate behaviors (12). This behavioral deficit is indicative of deficit in inhibitory control. Our findings were consistent with earlier reports of reversal impairment after sensitizing regimens of either amphetamine or cocaine (28, 29) This finding is in contrast to the findings of Schoenbaum et al. (29). , who reported no impairment in subjects treated with a neurotoxic dose of methamphetamine (30). They reported that large dopaminergic depletion (55%) in striatum of subjects treated with methamphetamine did not impair reversal learning. Some methodological differences between these two studies can be accounted for the different results. These include differences in doses of drug administrations, routes of drug administrations, treatment times, and strains and kinds of animals used. Studies of the effect of amphetamine and its analogues on reversal learning in rodent models have generated variable results including improvement (31), impairment (32) and no effect (33). The differences between these findings can be explained by variability in different doses of drugs, routes of drug administration, and different types of tasks. The effect of dopaminergic drugs often seems paradoxical, as both improvement and impairments are observed. These paradoxical effects are observed across different individuals, who performed the same task, or within the same individual across different tasks (34). Some studies have reported decreases in impulsive behavior following chronic use of dexamphetamine (35) and others have shown increases in impulsive behavior (36). Dexamphetamine produces a dose-related change in dopamine accumulation in the striatum. In this study, mice were tested on their retention of a pretreatment conditioned problem and they were found to be unimpaired. This suggests that the resulting impairment in inhibitory control might have more to do with disturbance in behavior flexibility. A variety of psychological impairments can be argued to be responsible for inflexible behavior exhibited by drug-treated subjects. Here, in this study we hypothesized that behavioral inflexibility may be characteristic of these abnormalities. One possible explanation for our finding is based on the effect of interventions on brain circuitry especially dopamine pathways. The impairment of other psychological constructs such as motor process, required for accurate or optimum performance in this kind of task, seems unlikely, because subjects showed no abnormal response during the paradigm. It is also important to consider the possible role of working memory in the observed behavior impairment (37). This is based on the hypothesis that impulsivity, perseveration and pre-potent responding can result from the failure of working memory. In the absence of regulation of responding by working memory, the default is due to exhibiting conditioned and over-learned behavior. This hypothesis posits that inhibitory control is not an active process of prefrontal cortex, but rather, a result of deficit in working memory. Alternatively, prefrontal lobe lesion and exposure to some psycho-stimulants can also impair reversal learning and produce exaggerated control of behavior by conditioned reinforcement (38). According to the results of the mixed model, music had a borderline effect on reversal learning, possibly caused by the small sample size. There was no significant difference between drug-treated subjects exposed to music half an hour before reversal learning and the control group. Listening to music strongly affects activity in a network of mesolimbic structures including nucleus accumbens (39). Nucleus accumbens and medial prefrontal cortex are important parts of the circuit that regulates the control of adaptive behavior (40). The nucleus accumbens and its dopaminergic innervation are known to be involved in reward processes and conditioned reinforcement (41). Multiple studies have also demonstrated that working memory can be modulated by mood and music, as an inducer of mood, influences working memory through mood modulation (42). Music impacts listeners’ emotional states (43) and the induced emotional state affects the performance of subjects in cognitive tasks. According to arousal and mood hypothesis (44), listening to music affects listener’s cognition through changing their arousal or mood, which can both positively and negatively affect their cognition (45). From a neuroscience perspective, the influence of music has been detected in many parts of the cortex. However, the frontal lobe of the brain is known to be the center for controlling mood and emotion. Ashby et al.(1999) confirmed the influence of music on mental flexibility and suggested a mechanism by which music-induced mood can influence executive function (46). It is possible that mice were impaired at learning a new discrimination (in this study it was not assessed), or that impairment in reversal learning might have manifested with higher treatment doses of dexamphetamine. Future studies should investigate the effect of different doses and routes of dexamphetamine treatment on conditioned reversal learning. Moreover, further studies examining the effect of pharmacological treatments on inhibitory control problems are required to see if these treatments could be beneficial. Further studies are also recommended to investigate stress indices such as corticosterone, as stress can be a possible contributor to reversal learning impairment (47). Moreover, one factor that may influence the effect of music on performance is stress (48, 49). Some studies have confirmed the effect of music on psychological stress response. These findings can help with better understanding of the beneficial effect of music on inhibitory control or behavioral flexibility as a component of executive function. Whether this functional dysfunction would be deteriorated by multiple binge doses exposure has yet to be determined. The small sample size of the group exposed to music is one of the methodological shortcomings of this study. Further studies should follow-up on this explanation and dissect the effect of music on reversal learning as a measure of behavior flexibility. Neurobiological studies have assumed that impulsivity, as a trait might be associated with vulnerability of people for the onset of drug abuse, whereas exposure to drugs may induce permanent deficits in memory, attention and different executive functions (11). Therefore, understanding changes in cognition and behavior, which occur as a result of listening to music or using psycho stimulants also has major implications for public health. Clinicians need to be aware of cognitive dysfunctions of patients with substance-related disorders. They need to do thorough neuropsychological assessment, and choose the most appropriate rehabilitation therapies.
Using the mixed model can be regarded as the strength of this study, which measures, for the first time, the effect of dexamphetamine and music on conditioned reversal learning in a T-maze task. It also provides the first experimental assessment of the sustained effect of binge dose of dexamphetamine.
This study had some methodological limitations including different number of subjects in various groups that may constrain generalization of the results. Cognitive testing with touch-screen operant box is becoming popular. Using a popular device in studies aids in interpreting and reconciling pharmacological effects across studies, but because of the lack of required expertise to make this device, we used the T-maze task in our study.
5.1. Conclusion
The current data indicates that binge dose of dexamphetamine increases errors committed by healthy subjects during reversal learning. Music also influences conditioned inhibitory control of experimental subjects. This has important implications for music therapists choosing music in clinical settings. As it was mentioned, further research is needed to determine the relative importance of the type of music. The mixed model has begun to play an important role in statistical analysis and has many advantages over traditional analyses.
Footnotes
Authors’ Contribution:Naghshvarian and Yazdani conceived and designed the evaluation; Yazdani, collected the experimental data; Salehi and Marzban interpreted the data and performed the statistical analysis; Marzban, Nahshvarian, Saheki and Yazdani drafted the manuscript and revised it critically for important intellectual content, all authors read and approve the final manuscript.
Funding/Support:There was no funding for this study.
Declaration of Interest:None.
References
- 1.Robbins SP, Judge TA. Organization behavior. Farhangi research unit; 2001. [Google Scholar]
- 2.Yuan J, He Y, Qinglin Z, Chen A, Li H. Gender differences in behavioral inhibitory control: ERP evidence from a two-choice oddball task. Psychophysiology. 2008;45(6):986–93. doi: 10.1111/j.1469-8986.2008.00693.x. [DOI] [PubMed] [Google Scholar]
- 3.Jocham G, Klein TA, Neumann J, von Cramon DY, Reuter M, Ullsperger M. Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. J Neurosci. 2009;29(12):3695–704. doi: 10.1523/JNEUROSCI.5195-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cools R, Robbins TW. Chemistry of the adaptive mind. Philos Trans A Math Phys Eng Sci. 2004;362(1825):2871–88. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- 5.Stemme A, Deco G, Busch A, Schneider WX. Neurons and the synaptic basis of the fMRI signal associated with cognitive flexibility. Neuroimage. 2005;26(2):454–70. doi: 10.1016/j.neuroimage.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 6.Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl). 2012;219(2):607–20. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bissonette GB, Powell EM. Reversal learning and attentional set-shifting in mice. Neuropharmacology. 2012;62(3):1168–74. doi: 10.1016/j.neuropharm.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceaser AE, Goldberg TE, Egan MF, McMahon RP, Weinberger DR, Gold JM. Set-shifting ability and schizophrenia: a marker of clinical illness or an intermediate phenotype? Biol Psychiatry. 2008;64(9):782–8. doi: 10.1016/j.biopsych.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ. Motor Inhibition and Cognitive Flexibility in Obsessive-Compulsive Disorder and Trichotillomania. Am J Psych. 2006;163(7):1282–4. doi: 10.1176/ajp.2006.163.7.1282. [DOI] [PubMed] [Google Scholar]
- 10.Klanker M, Feenstra M, Denys D. Dopaminergic control of cognitive flexibility in humans and animals. Frontiers in Neuroscience. 2013;7. doi: 10.3389/fnins.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garci M. Executive dysfunction in substance dependent individuals during drug use and abstinence: An examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsycho Soci. 2006;12(03) doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- 12.Yerys BE, Wallace GL, Harrison B, Celano MJ, Giedd JN, Kenworthy L. Set-shifting in children with autism spectrum disorders: Reversal shifting deficits on the Intradimensional/Extradimensional Shift Test correlate with repetitive behaviors. Autism. 2009. doi: 10.1177/1362361309335716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Schaaf ME, Fallon SJ, ter Huurne N, Buitelaar J, Cools R. Working memory capacity predicts effects of methylphenidate on reversal learning. Neuropsychopharmacology. 2013;38(10):2011–8. doi: 10.1038/npp.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith ME, Farah MJ. Are prescription stimulants "smart pills"? The epidemiology and cognitive neuroscience of prescription stimulant use by normal healthy individuals. Psychol Bull. 2011;137(5):717–41. doi: 10.1037/a0023825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood S, Sage JR, Shuman T, Anagnostaras SG. Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol Rev. 2014;66(1):193–221. doi: 10.1124/pr.112.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schellenberg EG. Music and Cognitive Abilities. Curr Direc Psycho Sci. 2005;14(6):317–20. doi: 10.1111/j.0963-7214.2005.00389.x. [DOI] [Google Scholar]
- 17.Melinda S, Lawrence R, Jeanne S. Antidepressants: What you need to know about depression medication. Retrieved from. 2011.
- 18.Sodocm BJ, Sodoc V, Pedro R. Comprehensive text book of psychiatry. 9th ed. USA: Lw. Com; 2009. [Google Scholar]
- 19.Abedini S, Davachi A, Sahbaei F, Mahmoudi M, Safa O. Depression in medical and nursing students, Bandar Abbas. Med J Hormozgan Univ. 2007;2(11):6. [Google Scholar]
- 20.Abdi H. The greenhouse-geisser correction. Encyclopedia of Research Design: Sage Publications; 2010. pp. 544–8. [Google Scholar]
- 21.Jennrich RI, Schluchter MD. Unbalanced repeated-measures models with structured covariance matrices. Biometrics. 1986;42(4):805–20. [PubMed] [Google Scholar]
- 22.Krueger C, Tian L. A comparison of the general linear mixed model and repeated measures ANOVA using a dataset with multiple missing data points. Biol Res Nurs. 2004;6(2):151–7. doi: 10.1177/1099800404267682. [DOI] [PubMed] [Google Scholar]
- 23.Follmann D, Wu M. An approximate generalized linear model with random effects for informative missing data. Biometrics. 1995;51(1):151–68. [PubMed] [Google Scholar]
- 24.Gotlib IH, Hammen CL. Handbook of depression. Guilford Press; 2008. [Google Scholar]
- 25.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-5. ManMag; 2003. [Google Scholar]
- 26.Auerbach RP, Abela JRZ, Zhu X, Yao S. Understanding the role of coping in the development of depressive symptoms: Symptom specificity, gender differences, and cross-cultural applicability. Clinc Psycho. 2010;49(4):547–61. doi: 10.1348/014466509x479681. [DOI] [PubMed] [Google Scholar]
- 27.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff C. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher PJ, Tenn CC, Rizos Z, Lovic V, Kapur S. Sensitization to amphetamine, but not PCP, impairs attentional set shifting: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Psychopharmacology. 2005;183(2):190–200. doi: 10.1007/s00213-005-0157-6. [DOI] [PubMed] [Google Scholar]
- 29.Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19(7):1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- 30.Daberkow DP, Riedy MD, Kesner RP, Keefe KA. Effect of methamphetamine neurotoxicity on learning-induced Arc mRNA expression in identified striatal efferent neurons. Neurotox Res. 2008;14(4):307–15. doi: 10.1007/BF03033855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiner I, Feldon J. Reversal and nonreversal shifts under amphetamine. Psychopharmacology. 1986;89(3):355–9. doi: 10.1007/BF00174374. [DOI] [PubMed] [Google Scholar]
- 32.Idris NF, Repeto P, Neill JC, Large CH. Investigation of the effects of lamotrigine and clozapine in improving reversal-learning impairments induced by acute phencyclidine and D-amphetamine in the rat. Psychopharmacology (Berl). 2005;179(2):336–48. doi: 10.1007/s00213-004-2058-5. [DOI] [PubMed] [Google Scholar]
- 33.Wilpizeski C, Hamilton C. Drug-induced decrement in spatial reversal learning in the white rat. Psychopharmacologia. 1964;6(6):475–9. doi: 10.1007/bf00429574. [DOI] [PubMed] [Google Scholar]
- 34.Mehta MA, Calloway P, Sahakian BJ. Amelioration of specific working memory deficits by methylphenidate in a case of adult attention deficit/hyperactivity disorder. Journal of Psychopharmacology. 2000;14(3):299–302. doi: 10.1177/026988110001400314. [DOI] [PubMed] [Google Scholar]
- 35.de Wit H. Acute Administration of d-Amphetamine Decreases Impulsivity in Healthy Volunteers. Neuropsychopharmacology. 2002;27(5):813–25. doi: 10.1016/s0893-133x(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 36.Hurst PM, Weidner MF, Radlow R. The effects of amphetamines upon judgments and decisions. Psychopharmacologia. 1967;11(5):397–404. doi: 10.1007/bf00405023. [DOI] [PubMed] [Google Scholar]
- 37.Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1445–53. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- 38.Weissenborn R, Robbins TW, Everitt BJ. Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacology. 1997;134(3):242–57. doi: 10.1007/s002130050447. [DOI] [PubMed] [Google Scholar]
- 39.Albano JD, Ward E, Jemal A, Anderson R, Cokkinides VE, Murray T, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99(18):1384–94. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- 40.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuro Bio Rev. 2002;26(3):321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 41.Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology. 1984;84(3):405–12. doi: 10.1007/bf00555222. [DOI] [PubMed] [Google Scholar]
- 42.Lima CF, Castro SL. Emotion recognition in music changes across the adult life span. Cogn Emot. 2011;25(4):585–98. doi: 10.1080/02699931.2010.502449. [DOI] [PubMed] [Google Scholar]
- 43.Thayer JF, Levenson RW. Effects of music on psychophysiological responses to a stressful film. Psychomusicology. 1983;3(1):44–52. doi: 10.1037/h0094256. [DOI] [Google Scholar]
- 44.Husain G, Thompson WF, Schellenberg EG. Effects of Musical Tempo and Mode on Arousal, Mood, and Spatial Abilities. Music Perception. 2002;20(2):151–71. doi: 10.1525/mp.2002.20.2.151. [DOI] [Google Scholar]
- 45.Sofia CYT. Should you listen to music while you study? 2012. [Google Scholar]
- 46.Ashby FG, Isen AM, Turken AU. A neuropsychological theory of positive affect and its influence on cognition. Psycho Rev. 1999;106(3):529–50. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- 47.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33(6):773–83. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradt J, Dileo C. Music for stress and anxiety reduction in coronary heart disease patients. The Cochrane Library. 2009. doi: 10.1002/14651858.CD006577.pub2. [DOI] [PubMed] [Google Scholar]
- 49.Rupp CI, Kemmler G, Kurz M, Hinterhuber H, Wolfgang FW. Cognitive remediation therapy during treatment for alcohol dependence. J Stud Alcohol Drugs. 2012;73(4):625–34. doi: 10.15288/jsad.2012.73.625. [DOI] [PubMed] [Google Scholar]