Abstract
Background: The aim of this study was to directly compare efficacy of atomoxetine and methylphenidate in treatment of children and adolescents 6- 18 years.
Methods: All published, randomized, open label or double blind trials, comparing the efficacy of methylphenidate with atomoxetine in treatment of children diagnosed with ADHD, using DSM-IV criteria were included in this study; ADHD Rating Scale–IV–Parent Version: Investigator Administered and Scored (ADHDRS) scores was used. The standardized mean difference (SMD) was used as a measure of effect size.
Results: Eleven studies were included with a total of 2,772 participants. The meta-analysis did not find a significant difference in the efficacy between methylphenidate and atomoxetine (SMD= 0.09, 95% CI -0.06, 0.25) (Z= 1.18, p= 0.24). Sub group analysis showed a significant standardized mean difference favoring OROS methylphenidate (SMD= 0.31, 95% CI 0.16, 0.47 (Z= 3.91, p< 0.0001); immediate release methylphenidate was not superior to atomoxetine (SMD= -0.05, 95% CI -0.20, 0.10) (Z= 0.68, p= 0.49). Open label trials did not make a difference in the standardized mean difference (SMD= 0.10, 95% CI -0.02, 0.23) (Z= 1.17, p= 0.09). There was significant heterogeneity among the studies (p= 0.003, I2= 63%). Subgroup analysis demonstrated that heterogeneity was because of the open label trials (p= 0.009, I2= 79%).
Conclusion: Atomoxetine and methylphenidate showed comparable efficacy in the treatment of children and adolescents with ADHD. However, Osmotic (Controlled) Release Oral (Delivery) System (OROS) methylphenidate is more effective than atomoxetine in treatment of ADHD in children and adolescents that is suggested as a first-line treatment in ADHD. Moreover, comparing the immediate release (IR) methylphenidate to atomoxetine did not lead to the benefit of IR methylphenidate.
Keywords: Atomoxetine, ADHD, Child, Adolescents, Meta-analysis, Methylphenidate
Introduction
Associated with adverse outcomes, attention deficit hyperactivity disorder (ADHD) is one of the most common psychiatric disorders (1). According to Shelton and Barkley, 30- 40% of the patients seeking treatment at mental health and social service centers are suffering from ADHD (2) The symptoms are included hyperactivity, attention deficit, and impulsivity (3). The specific characteristics of the disease are short attention span, fast distraction, and non-compliance with parents’ requirements. About 75% of ADHD children show symptoms of aggression and disobedience in a relatively constant manner. Educational problems are common in terms of both learning and behavior. It is known that symptoms continue until adolescence or adulthood in approximately 50% of the cases. Most patients somewhat improve in later years, but are still at risk of antisocial behaviors, substance abuse, and mood disorders, with learning difficulties usually continuing throughout their life. A complex interaction between the neuroanatomical and neurochemical systems is involved in the development of ADHD (4). Two sets of diagnostic criteria are available for attention deficit hyperactivity disorder and impulsivity: i.e., the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), and the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). Three subgroups of ADHD patients have been defined in DSM-IV: Hyperactive, inattentive, and combined inattentive-hyperactive (5). ADHD diagnosis is made primarily based on a careful history of the patient and assessment of the current situation and performance level. These criteria stipulate that the symptoms must be present before the age of 7 (6). Estimates of the worldwide prevalence of ADHD among school aged children vary from 2.4-19.8% (7). The prevalence of ADHD in the United States is 2-20% with a boy to girl ratio ranging from 2:1 to as high as 9:1 (4). In a systematic review conducted by Hakim Shushtari et al. in 2010, to determine the prevalence of ADHD and its variants in Iran, Tehran and Sanandaj had the highest and the lowest prevalence of ADHD, as well as the highest (20%) and the lowest (<3%) prevalence of the combined type of the disease, respectively. The overall prevalence of ADHD in Iran has been reported to be about 8-10%, and boys are three times more likely to be diagnosed with ADHD than girls (8). Types of interventions used to treat ADHD might include pharmacological and non-pharmacological interventions such as training parenting behavior as well as psychological, behavioral, and school-based interventions (9). Medication is the first-line treatment for ADHD (4). The goal of any drug therapy is to help children and adolescents with ADHD to have more concentration and to be able to relax (10). Despite concerns about drugs side effects and potential abuse, stimulant medications are widely used (11). Insomnia, tic, irritability, and loss of appetite are some side effects of stimulants, which limit their use. Drug abuse and drug dependence are other serious risks of stimulant consumption. Therefore, substituting stimulants with other drugs and finding more effective medicines for ADHD treatment are necessary (12). Methylphenidate is an FDA-approved stimulant, and atomoxetine is a non-stimulant, both of which were granted permission in the UK in 2004 to be prescribed for children older than 6 years of age (5). Ten to thirty percent of children and adults with ADHD may not respond to stimulants, or may not tolerate their potential side effects such as loss of appetite, sleep disorder, mood change, and exacerbation of abnormal limb movement disorders (tics). Atomoxetine is a good alternative for patients who do not tolerate stimulants, face limitations on their use, or whose family prefers non-stimulant therapy. Atomoxetine is effective in treating ADHD and improves the quality of life (QOL) (13).
Research Question
We sought to compare the efficacy and safety of atomoxetine with those of methylphenidate in children and adolescent 6 to 18 years of age in treatment of ADHD.
Study Objective
The original objective of the present study was to conduct a systematic review to compare efficacy and safety of atomoxetine to methylphenidate however because the trials were not individually powerful enough to detect differences in the therapeutic effects of the drugs, a meta-analysis was conducted by extracting and analyzing data from all available clinical trials. This study provides evidence to help physicians selecting suitable drug for the treatment of patients with ADHD.
Methods
The meta-analysis was based on all published randomized blinded and non-blinded studies in any language, in which a comparison had been performed between atomoxetine and methylphenidate in the treatment of 6-18 year old ADHD diagnosed children and adolescents according to DSM-IV criteria.
Search Strategy
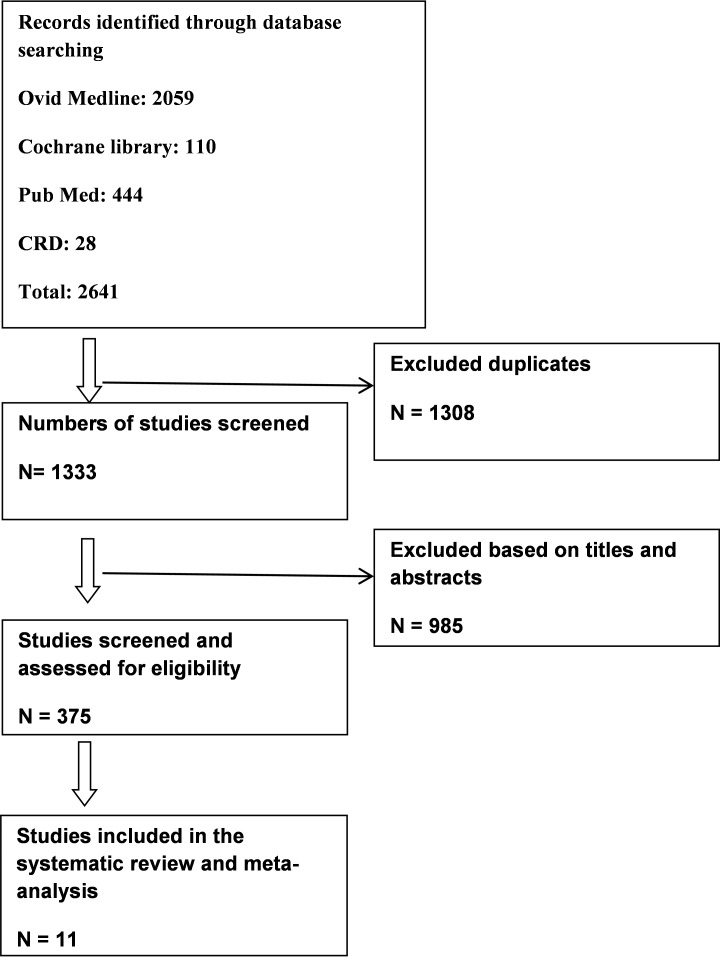
First, a pilot search was performed in PubMed to find the keywords and the synonyms for each keyword in order to use them in the final search strategy. A search strategy was designed for each citation database. Search was performed broadly to avoid publication bias, and multiple databases were manually searched upon completion of the search. Initially, no language limitation was imposed on the search. However, upon checking the relevance of the non-English articles through reading their English abstracts, these articles were ultimately excluded from the study. The searched articles had been published from January 1999 to March 2015. Citation databases of Cochrane Library, PubMed, CRD, and Ovid Medline were searched on September 2014 and updated on 4/9/2015. Search strategies were developed using the keywords “Atomoxetine” and “Methylphenidate” or the synonyms extracted for these keywords from PubMed. In order to expand the scope of the search and to keep papers, only the “Atomoxetine” and “Methylphenidate” keywords were used and the other PICO components such as population and outcomes were considered in the screening of the titles and abstracts. The full electronic search strategy for PubMed is shown in the appendix (Table 1). A manual search was also carried out in Google Scholar. A trial flow summary is given in Figure 1. The titles and abstracts were reviewed independently by two colleagues, and any disagreements were resolved through dialogue. Subsequently, the original articles were included. In cases where the original articles were not available, they were obtained either by emailing the author or by ordering and paying for the article. The quality of the original articles was assessed after being included in the study based on the Cochrane index scoring scale. Any difference in the evaluation between the colleagues was resolved through dialogue to reach final agreement.
Table 1 . ID Search for PubMed Database .
|
ID Search Search Query #1 Atomoxetine #2 Methylphenidate #3 Metadate #4 Equasym #5 Methylin #6 Concerta #7 Phenidylate #8 Ritalin #9 Ritaline #10 Ritalin-SR #11 Ritalin SR #12 Tsentedrin #13 Centedrin #14 Daytrana #15 Methylphenidate Hydrochloride #16: # 2 or #3 or#4 or...#15 #17: #1 AND#16 |
Fig. 1 .
Study Flow Summary
Data Extraction
A form was designed for data extraction using the available literature. This form was in several parts. The first part included the main information of the articles such as title, code, name of the first author, year of study, year of publication, DOI number, location (country), ethics committee approval, patients’ consent, and declaration of conflict of interest. Other parts included items on the studied population, intervention group, control group, and outcomes. For data extraction, studies included in the meta-analysis were divided between two colleagues who independently extracted data from the studies. After completing the data extraction forms, the colleagues examined each other’s completed forms. Ultimately, common outcomes of the articles were entered into Rev Man 5.0. The outcomes of this practice were entered into the data analysis software in a quantitative, continuous manner.
Statistical Analysis
The data of relevant studies were entered under the produced outcomes, and the mean difference was calculated based on the random effects model at the confidence interval of 95%. Heterogeneity was assessed with I2 test in accordance with the p-value. Outcomes of the studies were collected using the ADHD Rating Scale-IV: Parent Version, Turgay DSM-IV which screens behavioral disorders in children and adolescents, and T-DSM-IV-S scoring scale (14,15). The standardized mean difference (SMD) was used to measure the effect size. The ADHD Rating Scale-IV: Parent Version, which was filled by an interviewer through questioning the parents of ADHD children, composed of 18 items. These items included main symptoms of the disease designed as questions, and each item was rated from 0= never/rarely to 3= often/very. The total score ranged from zero to 54 (14). Data were analyzed using the Rev Man 5.0 for Windows (16). The meta-analysis was performed using the random effects model of Der Simonian and Laird (17). Given the different methodology used in the blinded and non-blinded studies, a sensitivity analysis was conducted. To this end, the meta-analysis was performed twice: Once after excluding the non-blinded clinical trials and once through excluding the blinded clinical trials.
Results
Upon the elimination of the duplicate articles, of the 2,641 articles originally searched, 1333 articles remained. The titles and abstracts of these articles were reviewed. Abstracts were reviewed only when the articles were found relevant to the meta-analysis. As, in certain cases, the names of the desired medicines did not appear in titles, abstracts were reviewed. The assessments were conducted independently to prevent bias. Of the 1,333 studies found, only 16 articles were randomized clinical trials which have been fully examined. Finally, 11 randomized trials were identified that compared atomoxetine and methylphenidate in 6-18 year old children and adolescents with ADHD. The total number of people enrolled in the analysis and received either atomoxetine or methylphenidate was 2,772, of which 1,397 and 1,525 patients received atomoxetine and methylphenidate, respectively. The articles included in the study had an acceptable quality in terms of Cochrane criteria and Jadad scoring scale. Table 2 summarizes the characteristics of patients. The study by Spencer et al. (18) in fact included two trials; and hence, it was considered two studies. Six trials compared immediate-release methylphenidate (IR-MPH) with atomoxetine (18-22), four trials compared extended-release methylphenidate (OROS-MPH) with atomoxetine (23-25), and one trial evaluated both forms of methylphenidate (IR-MPH and OROs-MPH) (27). Nine and two trials had parallel group (18-22,23-25,27) and cross-over (20,26) design, respectively. In a study which compared atomoxetine with standard therapy, only the data of patients who received methylphenidate at the beginning of the treatment were included in the analysis. In five studies, atomoxetine had been administered twice-daily (18-20,24), in four studies once a day (21-23,25), and in one study, the dosage was not stated (26). Methylphenidate was administrated twice a day in three studies (18,21), once a day in three studies (23-25), and three times a day in two studies (19,20). In one study, methylphenidate was consumed once or twice a day (22). Table 3 summarizes the study characteristics. Duration of the trials ranged from 3 weeks to 12 weeks. The severity of the disease was assessed at baseline using ADHD-RS instrument which was 38.6-45.5 for atomoxetine and 37.4-40.0 for methylphenidate. The outcomes were measured in one trial using PT-DSM-IV; the severity of the symptoms at baseline was 44.2 and 47.3 for atomoxetine and methylphenidate, respectively (25). In another trial which used VADPRS, the outcome at baseline was 55.03 (14.44) for atomoxetine and 51.18 for methylphenidate (22). In all studies, patients with a history of bipolar and psychological disorders, anxiety, convulsion, and Tourette syndrome were excluded.
Table 2 . Summary of Patients’ Characteristics .
| Characteristic | Atomoxetine | Methylphenidate | Total |
| Gender n (%) | |||
| Male | 1059 (79.8) | 1000 (75.6) | 2059 (77.7) |
| Female | 267 (20.2) | 323 (25.4) | 590 (22.3) |
| Ethnic origin | |||
| Caucasian | 618 (66.8) | 673 (63.5) | 1291 (65.1) |
| Others | 306 (33.1) | 386 (36.4) | 692 (34.8) |
| ADHD subtype | |||
| Hyperactive/impulsive | 30 (40.2) | 14 (24.6) | 44 (11.5) |
| Inattentive | 185 (24.7) | 158 (27.8) | 343 (28.2) |
| Combined | 531 (71.1) | 396 (69.7) | 927 (76.3) |
| Prior stimulant use- yes | 489 (36.8) | 606 (45.8) | 1059 (39.9) |
| Comorbid with oppositional defiant disorder | 317 (23.9) | 120 (9.07) | 437 (16.4) |
Table 3 . Study Characteristics .
| Study | Number of Participants | Blinding | Design | Follow up | Baseline Severity ADHD-RS Total Score | Mean Daily Dose of Amoxetine and Frequency | Mean Daily Dose Methylphenidate and Frequency |
| Spencer et al. 2002 | 163 | Double Blind | Parallel group | 9 weeks | Atomoxetine= 39.5 | 1.56mg/kg Twice a day | IR MPH= 1.12mg/kg Twice a day |
| Kratochvil et al. 2002 | 228 | Open Label | Parallel group | 10 weeks | Atomoxetine= 39.4 MPH= 37.6 | 0.48mg/kg or 1.4mg/kg/kg Twice daily | Final mean dose 0.85mg/kg Three times a day |
| Sangal et al. 2005 | 85 | Double Blind | Cross over | 7 weeks | Atomoxetine= 39.6 MPH not stated | 1.56mg/kg/day Twice a day IR MPH= | 1.12mg/kg Three times a day |
| Kemner et al. 2005 | 1323 | Open Label | Parallel group | 3 weeks | Atomoxetine= 38.6 (SD 8.1) MPH= 39 | 1.08mg/kg/Once a day | OROS-MPH 1.01mg/kg/day (IR equivalent 0.841mg/kg) Once a day |
| Wang et al. 2007 | 330 | Double Blind | Parallel group | 8 weeks | Atomoxetine= 38.6 (SD 7.6) MPH= 37.4 (SD 7.6 | Final range 0.8mg/kg-1.8 mg/kg Once a day | IR MPH= 17.8/mg/day Twice a day |
| Prasad et al. 2007 | 180 | Open Label | Parallel group | 10 weeks | Atomoxetine= 45.5 (SD 8.7) MPH not stated | 1.5mg/kg Once a daily 8pts got twice daily | IR MPH= 0.8mg/kg OROS-MPH= 1.03mg/kg (IR equivalent= 0.858mg/kg) |
| Newcorn et al. 2008 | 442 | Double Blind | Parallel group | 6 weeks | Atomoxetine= 40.9 (SD 8.8) MPH= 40 (SD 8.8) | 1.45mg/kg Twice a day | OROS-MPH= 1.16mg/kg (IR equivalent= 0.966mg/kg Once a day |
| Yildiz et al. 2010 | 25 | Open Label | Parallel group | 12 weeks | Parents TDSM- IV inattention scores atomoxetine= 16.72 MPH= 17.72 | 1.28mg/kg/day Once a day | OROS-MPH= 1.07mg/kg (IR= equivalent 0.89mg/kg) Once a day |
| Bedard et al. 2014 | 102 | Double Blind | Cross over | 4-6 weeks | Atx= 36.5±11.09 MPH= 37.9±10.96 | 1.4±0.5 mg/kg/day | OROS-MPH= 52.4±16.6mg |
| Garg et al. 2014 | 69 | Open label | Parallel group | 8 weeks | Atx= 55.03 (14.44) MPH= 51.18 (0.86) | 17.46 (7.22) mg/day (or 0.7mg/kg/day) Once a day | IR MPH=17.35 (7.52) mg/ day (or 0.62mg/kg/day) Once or Twice a day |
Studies Homogeneity
Studies homogeneity showed that the difference between the results of studies was due to random error rather than difference in their conditions. As a homogeneity index, I2 showed how much of the total variance aroused from the variance between the studies. This index was equal to 63%, representing that of the total difference between the studies, 63% of distribution and the difference was attributed to the differences between the studies, suggesting that the different heterogeneous results were probably due to the difference in their procedures and conditions. The obtained p-value also proved heterogeneity. This heterogeneity justified performance of the meta-analysis as random-effects (I2= 63%, p= 0.003). Analysis of subgroups revealed that the heterogeneity was due to non-blinded trials with I2= 79%; p= 0.009. In addition, no statistically significant heterogeneity existed among the blinded trials with I2= 0%; p= 0.45.
Meta-analysis
The results of the primary outcome are summarized in Figure 2. Given the heterogeneity of the studies, the random-effects model was applied in this meta-analysis, in which no statistical significance was observed between the two drugs. SMD was used to measure the effect size, and there was no statistically significant difference between methylphenidate and atomoxetine in terms of the effectiveness when SMD was used to express the effect size i.e. 9% at 95% CI (-6%, 25%) and (Z= 1.18, p= 4).
Fig. 2 .
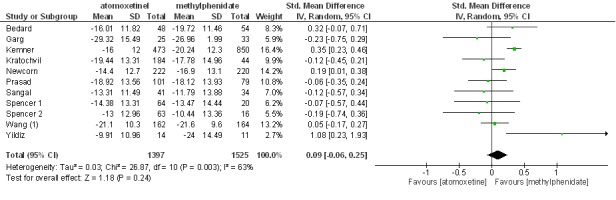
Standardized Mean Difference in ADHDRS-IV Scores for Methylphenidate and Atomoxetine
Analysis of the Subgroups
Subgroup analysis was performed for various forms of the drugs; six studies used IR-MPH (18-22). By entering these studies into the analysis and comparing with atomoxetine, the effect size was -5% at 95%CI (-0.20, 0.10) and (Z= 68%, p= 0.49) which meant that IR-MPH had no preference to atomoxetine. Five studies compared OROS-MPH with atomoxetine (23-26). By entering these studies and comparing with atomoxetine, a significant superiority of OROS-MPH to atomoxetine was found. The effect size of the analysis was 0.031 at 95%CI (0.16, 0.47) and (Z= 3.91, p< 0.0001). The following Figures represent the analysis of the two forms of methylphenidate.
Sensitivity Analysis
Excluding non-blinded studies from the analysis had no impact on standardized mean differences. The effect size in blinded trials was 0.10 at 95%CI (-0.02, 0.23) and (Z= 1.70, p= 0.09). This analysis is displayed in Figure 3. Besides, excluding blinded studies from the analysis made no difference. In this analysis, the effect size was 0.12 at 95%CI (-0.20, 0.44) and (Z= 0.75, 0.45). This analysis is displayed in Figure 4.
Fig. 3 .
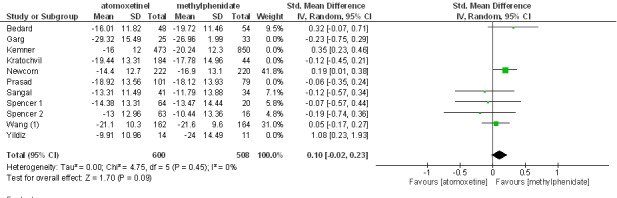
Excluding non-blinded studies from the analysis had no impact on standardized mean differences.
Fig. 4 .
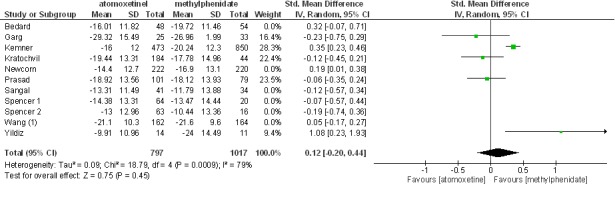
Excluding with-blinded studies from the analysis had no impact on standardized mean differences.
Publication Bias
Publication bias occurs when the effect size is small or when those with no differences between the two drugs are not published. In the following funnel plot resulted from the studies, no asymmetry was found. Funnel plot into the meta-analysis studies is presented in Figure 5.
Fig. 5 .
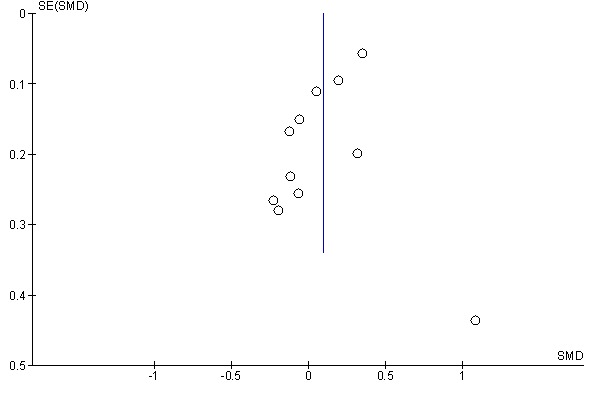
Funnel Plot into the Meta-analysis Studies
Discussion
The present meta-analysis was carried out through synthesis of data from all available randomized clinical trials which compared methylphenidate and atomoxetine for the treatment of ADHD in children and adolescents. The effectiveness of treatment with these drugs in children and adolescents was approved during the controlled clinical trials with both medications (28-30). Although the effect size in children and adolescents was in favor of methylphenidate, no statistically significant difference was observed between the two drugs in the analysis. Analysis of subgroups pointed to the greater significance of standardized mean difference (SMD) in methylphenidate group; however, IR-MPH showed no preference for atomoxetine, and the analysis was in favor of atomoxetine.
Studies by Gibson et al. (31) with five articles, Hazell et al. (32) with seven articles, with inclusion criteria similar to our study, and Hanwella et al. with nine articles concluded that atomoxetine was not better than methylphenidate and their analyses were in favor of methylphenidate (33). Hazell et al. excluded the large clinical trial of Kemner et al., which had lasted three weeks, from their meta-analysis. The present study is consistent with the findings of Gibson et al. and Hanwella et al. who stated that OROS-MPH had a relative superiority to atomoxetine, that the comparison of the two drugs in the meta-analysis was in favor of methylphenidate, and that no statistical significance existed between the two drugs. The comparison of IR-MPH and atomoxetine was in favor of atomoxetine, although there was no statistically significant difference between the two drugs studied (31-33).
Some evidence from blinded clinical trials comparing atomoxetine and methylphenidate show the preference of OROS-MPH compared to IR-MPH (34,35). However, other studies do not confirm the significant difference in effectiveness between the two forms (36-38).
Several methodological factors may have affected the results of each trial. Lower effectiveness of IR-MPH may be related to the planning of drug dosage; IR-MPH was prescribed in evening doses only in two studies (39,40). The symptoms severity was assessed through ADHD Rating Scale-IV: Parent Version; and parents may have similar assessment with respect to the behaviors occurred at out-school hours, while symptoms should be evaluated during activities in school. Because the effect of IR-MPH may decrease later in the day and this may be attributed to the lower effectiveness of this form of the drug, although IR-MPH was prescribed only once or twice a day.
Our meta-analysis was performed with a relatively small SMD in ADHD scores between atomoxetine and methylphenidate using parents scoring, but if the scoring of teachers was used at school hours, SMD might have been larger. However, only two studies have evaluated children’s behavior at school (22,25).
In a meta-analysis by Cheng et al., it was shown that the effect size of atomoxetine using parents scoring was 0.34 which was half of that of teachers scoring. However, in our meta-analysis, it was much smaller for atomoxetine compared to methylphenidate (29). Therefore, it seems that the advantage of methylphenidate to atomoxetine was higher in school evaluations than house evaluations, therefore suggesting that school evaluations should not be ignored.
In some cases, the special design of the studies might have resulted in favor of or against a particular treatment. For example, in the large trial of Kemner et al., a great number of subjects participated in a short period of treatment, but it usually requires four to six weeks for the optimal efficacy of atomoxetine to be evident in evaluations. (41). Two trials have excluded participants who had a history of poor response to methylphenidate, and this design can be in favor of methylphenidate (19,24). In one study, patients with previous treatment with methylphenidate who have not responded to treatment with stimulants were excluded; this type of design can be also in favor of methylphenidate (18). Although ADHD has a high rate of comorbidity, people with a history of tics and a family history of Tourette syndrome and anxiety were excluded because methylphenidate is contraindicated in these cases. Therefore, this design can be in favor of methylphenidate, because methylphenidate cannot be used in such cases, and exclusion of these people can actually be in favor of methylphenidate. Atomoxetine can be administrated to people in whom methylphenidate is contraindicated; therefore, methylphenidate should not be used in people with abnormal heart condition, arrhythmia, psychosis, and those who have suicidal thoughts. However, there are reports about suicidal thoughts and hepatic disorders in patients treated with atomoxetine which is considered as warnings (11).
Limitations
Considering the limitations of this study, the results should be interpreted cautiously, because the interpretation of the findings is difficult due to observed heterogeneity. Sensitivity analysis showed that non-blinded studies are the source of the heterogeneity. Non-blinding in these studies will result in patient and investigator bias and will affect patients’ and researchers’ expectation and eventually the results. Thus, due to the small number of studies in each subgroup, there will not be enough power to detect the difference between the two treatments. There is also a publication bias due to not including non-published data such as conference abstracts, dissertations, and those of unpublished studies by pharmaceutical companies. Since some trials had excluded population subgroups with associated conditions, the results of this meta-analysis could not be generalized for these subgroups. In addition, features of different study designs affect the outcome of trials, such as not including teachers scoring that can be against methylphenidate. In general, findings of this meta-analysis revealed that atomoxetine and methylphenidate have comparable efficiencies. These results also suggest that the OROS-MPH is more effective and can be used as the first-line treatment for children and adolescents. Atomoxetine is used in patients who are poor responders to methylphenidate or populations who are stimulants abusers. The use of higher doses of atomoxetine (twice a day) may have better results in terms of outcomes. Of the 11 articles, two studies have not declared the conflict of interest, one study was sponsored by McNeil Consumer, and eight studies were conducted by Eli Lilly and Company. Each trial had a different protocol and was conducted independently. From trials included in the meta-analysis, those of Kratochvil et al., Sangal et al., and Wang et al. stated that the effectiveness of methylphenidate did not differ with that of atomoxetine. Prasad et al. stated in his study that atomoxetine was superior to stimulant drugs; and according to Newcorn et al. study, the effectiveness of atomoxetine was not lower than methylphenidate. In a study by Spencer et al. although methylphenidate was the conducted treatment arm of trial, comparison of the effectiveness of methylphenidate and atomoxetine was not directly reported in the analyses. According to a study by Garg et al., the effectiveness of methylphenidate and atomoxetine was comparable, and methylphenidate was recommended as the first-line treatment and atomoxetine as the second-line. In the large trial of Kemner et al., further improvement of the disease was observed in the group receiving OROS-MPH. It should also be noted that in the previous meta-analysis such as the study of Hazell et al., the authors were employees, consultants, and shareholders of Eli Lilly and Company. As stated in the meta-analysis of Hanwella et al., there was no dependence and conflict of interest in relation to a particular company or individual.
Conclusion
In general, atomoxetine and methylphenidate have a comparable efficacy in the treatment of children and adolescents with ADHD. However, the sustained-release methylphenidate is more effective than atomoxetine and can be the first-line treatment for ADHD in children and adolescents with ADHD. The immediate-release form of methylphenidate has no preference to atomoxetine, and the meta-analysis is in favor of atomoxetine. The results should be interpreted cautiously due to the heterogeneity.
Funding/ Support
This study is a part of a thesis in a course in Master of Science.
Conflict of interests
All authors report having no potential conflicts of interest.
Cite this article as: Rezaei G, Hosseini SA, Akbari Sari A, Olyaeemanesh A, Lotfi MH, Yassini M, Bidaki R, Nouri B. Comparative efficacy of methylphenidate and atomoxetine in the treatment of attention deficit hyperactivity disorder in chil-dren and adolescents: A systematic review and meta-analysis. Med J Islam Repub Iran 2016 (10 February). Vol. 30:325.
References
- 1.Fabiano GA, Chacko A, Pelham WE, Robb J, Walker KS, Wymbs F. et al. A comparison ofbehavioral parent training programs for fathers of children with attention-deficit/hyperactivity disorder. Behav Ther. 2009;40(2):190–204. doi: 10.1016/j.beth.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Shelton TL, Barkley RA. Critical Issues in the Assessment of Attention Deficit Disorders in Children. Topics in Language Disorders. 1994;14(4):26–41. [Google Scholar]
- 3. NICE. Attention Deficit Hyperactivity Disorder. The NICE Guideline on Diagnosis and management of ADHD in children, young people and adults. Section 7.2.14 from evidence to recommendations: psychological interventions for children and young people with ADHD. In: NICE Technology Appraisal 72. London: National Institute for Health and Clinical Excellence. 2009.
- 4. Sadock BJ, Sadock VA, Kaplan HI. Kaplan and Sadock's concise textbook of child and adolescent psychiatry: Lippincott Williams & Wilkins; 2009.
- 5.King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G. et al. A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents. Health Technology Assessment. 2006;10:23. doi: 10.3310/hta10230. [DOI] [PubMed] [Google Scholar]
- 6.Post RE, Kurlansik SL. Diagnosis and management of attention- deficit/ hyperactivity disorder in adults. American Family Physician 2012 May. 1;85:890–6. [PubMed] [Google Scholar]
- 7. Association AP. DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders, 4th edn, text revision. Washington, DC: American Psychiatric Association 2000;739-99.
- 8.Hakim Shooshtary M, Chimeh N, Najafi M, Mohamadi MR, Yousefi-Nouraie R, Rahimi-Mvaghar A. The prevalence of Attention Deficit Hyperactivity Disorder in Iran: A systematic review. Iran J Psychiatry. 2010;5:88–92. [PMC free article] [PubMed] [Google Scholar]
- 9. Charach A, Carson P, Fox S, Ali MU, Beckett J, Lim CG. Interventions for preschool children at high risk for ADHD: a comparative effectiveness review. Pediatrics 2013: Peds. 2012-0974. [DOI] [PubMed]
- 10.Taylor E, Döpfner M, Sergeant J, Asherson P, Banaschewski T, Buitelaar J. et al. European clinical guidelines for hyperkinetic disorder–first upgrade. European child & adolescent psychiatry. 2004;13(1):i7–i30. doi: 10.1007/s00787-004-1002-x. [DOI] [PubMed] [Google Scholar]
- 11. Pringsheim T, Steeves T. Pharmacological treatment for Attention Deficit Hyperactivity Disorder (ADHD) in children with comorbid tic disorders. The Cochrane Library. 2011. [DOI] [PubMed]
- 12.Pierce K. Treatment of attention-deficit/ hyperactivity disorder. Pediatr Ann. 2011;40(11):556–62. doi: 10.3928/00904481-20111007-06. [DOI] [PubMed] [Google Scholar]
- 13. Barkley RA. Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment. New York: Guildford Press; 1998.
- 14. DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD rating scale-IVchecklists, norms, and clinical interpretation New York: The Guilford Press; 1998.
- 15. Turgay A. Disruptive Behavior Disorders Child and Adolescent Screening and Rating Scales for Children, Adolescents, Parents, and Teachers West Blomfield: Integrative Therapy Institute Publication; 1994.
- 16. The Nordic Cochrane Centre: Review Manager (RevMan) [Computer Program] Version 5.0. Copenhagen: The Cochrane Collaboration; Version 5.0 2008.
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Spencer T, Heiligenstein JH, Biederman J, Faries DE, Kratochvil CJ, Conners CK. et al. Results from 2 proof-of-concept, placebocontrolledstudies of atomoxetine in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2002;63(12):1140–1147. doi: 10.4088/jcp.v63n1209. [DOI] [PubMed] [Google Scholar]
- 19.Kratochvil CJ, Heiligenstein JH, Dittmann R, Spencer TJ, Biederman J, Wernicke J. et al. Atomoxetine and methylphenidate treatment in children with ADHD: a prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry. 2002;41(7):776–784. doi: 10.1097/00004583-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Sangal RB, Owens J, Allen AJ, Sutton V, Schuh K, Kelsey D. Effects of atomoxetine and methylphenidate on sleep in children with ADHD. Sleep. 2006;29(12):1573–1585. doi: 10.1093/sleep/29.12.1573. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Zheng Y, Du Y, Song DH, Shin YJ, Cho SC. et al. Atomoxetine versus methylphenidate in paediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Aust N Z J Psychiatry. 2007;41(3):222–230. doi: 10.1080/00048670601057767. [DOI] [PubMed] [Google Scholar]
- 22.Garg J, Arun P, Chavan BS. Comparative short term efficacy and tolerability of methylphenidate and atomoxetine in attention deficit hyperactivity disorder. Indian pediatrics. 2014;51(7):550–4. doi: 10.1007/s13312-014-0445-5. Epub 2014/07/18. [DOI] [PubMed] [Google Scholar]
- 23.Kemner JE, Starr HL, Ciccone PE, Hooper-Wood CG, Crockett RS. Outcomes of OROS methylphenidate compared with atomoxetine in children with ADHD: a multicenter, randomized prospective study. AdvTher. 2005;22(5):498–512. doi: 10.1007/BF02849870. [DOI] [PubMed] [Google Scholar]
- 24.Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ. et al. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry. 2008;165(6):721–730. doi: 10.1176/appi.ajp.2007.05091676. [DOI] [PubMed] [Google Scholar]
- 25.Yildiz O, Sismanlar SG, Memik NC, Karakaya I, Agaoglu B. Atomoxetine and Methylphenidate Treatment in Children with ADHD: The Efficacy, Tolerability and Effects on Executive Functions. Child Psychiatry Hum Dev. 2010 doi: 10.1007/s10578-010-0212-3. [DOI] [PubMed] [Google Scholar]
- 26.Bedard AC, Stein MA, Halperin JM, Krone B, Rajwan E, Newcorn JH. Differential impact of methylphenidate and atomoxetine on sustained attention in youth with attention-deficit/hyperactivity disorder. Journal of child psychology and psychiatry, and allied disciplines. 2014 doi: 10.1111/jcpp.12272. Epub 2014/06/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasad S, Harpin V, Poole L, Zeitlin H, Jamdar S, Puvanendran K. A multicentre, randomised, open-label study of atomoxetine compared with standard current therapy in UK children and adolescents with attentiondeficit/ hyperactivity disorder (ADHD) Curr Med Res Opin. 2007;23(2):379–394. doi: 10.1185/030079906X167309. [DOI] [PubMed] [Google Scholar]
- 28.Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention- deficit disorder in children and adolescents? A meta-analysis. CMAJ. 2001;165(11):1475–1488. [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng JY, Chen RY, Ko JS, Ng EM. Efficacy and safety of atomoxetine for attention-deficit/hyperactivity disorder in children and adolescentsmeta- analysis and meta-regression analysis. Psychopharmacology (Berl) 2007;194(2):197–209. doi: 10.1007/s00213-007-0840-x. [DOI] [PubMed] [Google Scholar]
- 30.Kratochvil CJ, Wilens TE, Greenhill LL, Gao H, Baker KD, Feldman PD. et al. Effects of long-term atomoxetine treatment for young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(8):919–927. doi: 10.1097/01.chi.0000222788.34229.68. [DOI] [PubMed] [Google Scholar]
- 31.Gibson AP, Bettinger TL, Patel NC, Crismon ML. Atomoxetine versus stimulants for treatment of attention deficit/hyperactivity disorder. Ann Pharmacother. 2006;40(6):1134–1142. doi: 10.1345/aph.1G582. [DOI] [PubMed] [Google Scholar]
- 32.Hazell PL, Kohn MR, Dickson R, Walton RJ, Granger RE, van Wyk GW. Core ADHD Symptom Improvement With Atomoxetine Versus Methylphenidate: A Direct Comparison Meta-Analysis. J Atten Disord. 2010 doi: 10.1177/1087054710379737. [DOI] [PubMed] [Google Scholar]
- 33.Hanwella R, Senanayake M, de Silva V. Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis. BMC psychiatry. 2011;11:176. doi: 10.1186/1471-244X-11-176. Epub 2011/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steele M, Weiss M, Swanson J, Wang J, Prinzo RS, Binder CE. A randomized, controlled effectiveness trial of OROS-methylphenidate compared to usual care with immediate-release methylphenidate in attention deficithyperactivity disorder. Can J ClinPharmacol. 2006;13(1):e50–62. [PubMed] [Google Scholar]
- 35.Remschmidt H, Hoare P, Ettrich C, Rothenberger A, Santosh P, Schmidt M. et al. Symptom control in children and adolescents with attention-deficit/hyperactivity disorder on switching from immediate-release MPH to OROS MPH Results of a 3- week open-label study. Eur Child Adolesc Psychiatry. 2005;14(6):297–304. doi: 10.1007/s00787-005-0467-6. [DOI] [PubMed] [Google Scholar]
- 36.Pelham WE, Gnagy EM, Burrows-Maclean L, Williams A, Fabiano GA, Morrisey SM. et al. Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics. 2001;107(6):E105. doi: 10.1542/peds.107.6.e105. [DOI] [PubMed] [Google Scholar]
- 37.Wolraich ML, Greenhill LL, Pelham W, Swanson J, Wilens T, Palumbo D. et al. Randomized, controlled trial of oros methylphenidate once a day in children with attention-deficit/ hyperactivity disorder. Pediatrics. 2001;108(4):883–892. doi: 10.1542/peds.108.4.883. [DOI] [PubMed] [Google Scholar]
- 38.Favreau A, Deseille-Turlotte G, Brault F, Giraudeau B, Krier C, Barthez MA. et al. [Benefit of the extended-release methylphenidate formulations: a comparative study in childhood] Arch Pediatr. 2006;13(5):442–448. doi: 10.1016/j.arcped.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 39. American Psychiatric Association: Diagnostic and statistical manual of mental disorders. 4 edition. AmericanPsychiatric Association, Washington, DC; 2000.
- 40.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 41.Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C. et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry. 2002;159(11):1896–1901. doi: 10.1176/appi.ajp.159.11.1896. [DOI] [PubMed] [Google Scholar]