Abstract
Background: Hormone therapy is currently the mainstay in the management of locally advanced and metastatic prostate cancer. We performed a systematic review to compare safety, efficacy and effectiveness of degarelix, a new gonadotropin-releasing hormone (GnRH) antagonist (blocker), versus gonadotropin-releasing hormone (GnRH) agonists.
Methods: MEDLINE, Web of Science and the Cochrane library were searched to identify all of the published Randomized Controlled Trials (RCTs) that used degarelix versus gonadotropin-releasing hormone agonists with or without anti-androgen therapy for the treatment of prostate cancer. We performed meta-analysis of extracted data on safety and efficacy of the target medication.
Results: Six studies were included. They involved a total of 2296 patients which were used in the meta-analysis. Follow-up times after treatment were between 12 weeks and 12 months. Three of six RCTs compared degarelix with goserelin and the others compared it with leuprolide. Meta-analysis on safety outcomes revealed that the only statistically significant difference between the degarelix treated group and GnRH agonists treated group was complication in the injection site which was higher in degarelix-treated group (OR= 46.34, 95% CI: 15.79 to 136, p<0.001). Although general mortality rate was lower in degarelix-treated group (OR= 2.06, 95% CI: 1.08 to 3.93, p=0.03); mortality due to the drug side effects was not different. Meta-analysis of efficacy data also showed that International Prostate Symptom Score (IPSS) reduction at week 12, (MD=-1.85, 95% CI: -2.97 to - 0.72, p=0.001) and Testosterone reduction between day 1-28, (OR=11.58, 95% CI: 5.77 to 23.22, p<0.001) was statistically higher in degarelix-treated group. Testosterone reduction after day 28 and prostate volume reduction did not have significant difference.
Conclusion: Our meta-analysis indicates that, compared with GnRH agonists, degarelix has significantly more effects on lower urinary tract symptoms and also Prostate Specific Antigen (PSA) and testosterone reduction in the first month of the treatment. Except minor complications in the injection site like pain, erythema and swelling, there is no increase in major side effects and mortality due to degarelix. This is while the effect on testosterone and PSA after the first month of treatment is not statistically different between the two groups.
Keywords: Prostate cancer, Degarelix, Leuprolide, Goserelin, Meta-analysis, GnRH Agonists, Safety, Efficacy
Introduction
Prostate cancer is one of the most common malignant cancers around the world. According to the Cancer Statistics2015 in USA (1), prostate, lung and bronchus, and colorectal cancers will account for about one-half of all cancer cases in men, with prostate cancer alone accounting for about one-quarter of new diagnoses. Prostate cancer is also the second cause of cancer death after lung and bronchus cancer in USA (1).
The age-standardized incidence rate of prostate cancer in IRAN is 5.1 per 100,000 person based on the data from population based cancer registries between years 1996-2000, that like other Asian countries is lower than western countries (2). This can partly be due to lack of nationwide screening program, younger age structure and quality of cancer registration system in Iran (2). Prostate cancer is the ninth cause of cancer death in IRAN (3).
After discovering the role of testosterone in prostate cancer in 1940s, suppressing testosterone level has been the main option for management of patients with prostate cancer (4). While orchiectomy is highly effective for suppressing testosterone level, the procedure is unacceptable to many patients and has now largely been replaced by different forms of medical castration, often referred to as androgen-deprivation therapy (ADT). The most common agents used for ADT are Gonadotrophin-releasing hormone (GnRH) receptor agonists that achieve the desired therapeutic goal (serum testosterone≤0.5 ng/ml) in 90–100% of patients, but only after a period of 7–21 days and an initial surge of testosterone level (4,5). This initial surge may stimulate prostate cancer cells in advanced and metastatic cases; therefore, it leads to an exacerbation of clinical symptoms(flare),including spinal cord compression, bone pain and urethral obstruction (6).
GnRH antagonists provide an alternative approach to ADT with a more direct mode of action that causes immediate blockade of GnRH receptors. These drugs produce a more rapid suppression of testosterone (and prostate-specific antigen [PSA]) without a testosterone surge and appear to provide an effective and well tolerated option for ADT of prostate cancer (7).Currently, degarelix, approved in the USA in 2008, is the most extensively studied and widely available agent in this class. Degarelixhas an immediate onset of action, resulting in a rapid reduction in circulating luteinizing hormone and follicle-stimulating hormone with testosterone levels ≤0.5ng/ml achievement within 1 to 3 days after treatment (7,8).
As there are requests for using degarelix instead of GnRH agonists for management of patients with advanced prostate cancer in Iran and the need for scientific evidence to help evidence informed policy making in this field, we performed a systematic review to compare safety and efficacy ofdegarelix, with Gonadotropin-releasing hormone (GnRH) agonists.
Methods
We performed a systematic review of best available evidence using Cochrane Collaboration guidelines for systematic review of interventions (9). Our structured question for this review was as described in Table 1:
Table 1 . Components of structured question .
|
● Population: Patients with advanced prostate cancer;
● Intervention: ADT using degarelix; ● Comparator: ADT usingGnRH agonists (includinggoserelin, leuprolinandtriptorelin) with or without anti-androgen therapy; ● Outcome: reducing prostate volume, health related quality of life, IPSS score, general survival, reducing testosterone level, reducing PSA level, drug induced side effects; ● Type of studies: Randomized Controlled Trials relevant to our PICO; |
Relevant databases including Ovid MEDLINE(R), Scopus (by Elsevier), Web of Science, and Cochrane Central Register of Controlled Trials (CENTRAL) and also Google Scholar and related websites were searched without time limitation up to 2014.Medical subject headings (MeSH) and main key words for search were ‘Prostate cancer’, ‘Androgen Deprivation Therapy’, ‘hormone therapy’, ‘GnRH agonist’, ‘GnRH antagonist’, ‘degarelix’, ‘goserelin’, ‘triptorelin’, and ‘leuprolide’. Variants of the main key words and free text terms were applied. We restricted the language to English. An example search strategy for MEDLINE has been depictedin the appendix.
The extracted articles were organized in Endnote software. After deleting the duplicate articles, two reviewers independently assessed the titles and abstracts of search results and selected potentially relevant studies according to our main question (Table 1).
The studies were excluded if they either were not in English or the study designs were not Randomized Controlled trial. Disagreements were settled by examination of the full paper.
The Cochrane Collaboration’s tool for assessing risk of bias was applied to assess methodological quality and detect potential sources of bias. The scale of Jadad (10)was used to quantify the quality of included studies; this scale assesses randomization, blinding and description of withdrawals. Relevant data on study design, including patients, interventions and reported outcome measurements were recorded on an extraction form designed for this review by two reviewers. Disagreements were settled by consensus. Following agreement, the data were entered into Review Manager Software.
We performed meta-analysis using Review Manager Software (version 5) for outcome measures when the heterogeneity of data was not significant. Heterogeneity was assessed using the I2 statistic, which describes the variability in effect estimate across studies that is due to differences between studies rather than random sampling error. Heterogeneity was deemed substantial if the I2 analysis suggested more than 50 percent of the variability in an analysis was due to differences between trials. In such outcome measures we did qualitative analysis(0).We used random effect model for meta-analysis. The Mantel-Haenszel method was used for dichotomous outcomes and the Inverse Variance method for continuous outcomes.
Results
The initial search yielded 581 articles, of which 96 duplicated ones were deleted. From the 485 remaining papers, 421were excluded by reviewing their titles and abstracts. Studying the full report of the 64 remaining papers led to the inclusion of 6 papers. (Fig. 1).Of course two papers (11,12) were additional analysis on data from the leuprolide-controlled pivotal Phase 3 trial of degarelix, including 610 patients treated with these agents for 12 months (13).The total number of patients was 1080. The age of patients ranged from 68 to 72 years and follow up time after treatment varied between 3 months to 14 months. Characteristics of included papers demonstrated in Tables 2 and 3.
Fig. 1 .
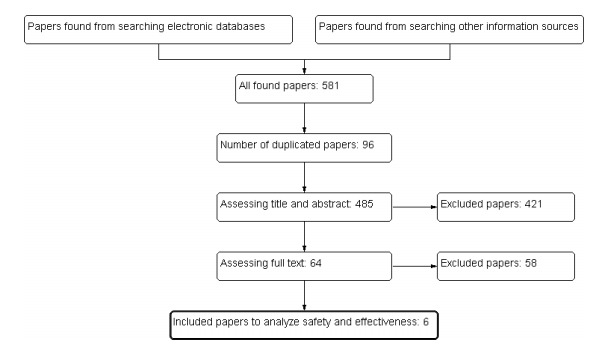
Process of search and selection of studies
Table 2 . Intervention and control groups in 6 included papers .
| Reference | Intervention treatment | No. of patients randomized | Comparison treatment | No. of patients randomized | Followup time |
| Klotz (13) (CS21,global) | Degarelix: 240mg initial dose 80mg Monthly or Degarelix: 240mgintial dose 16mg monthly | Number: 210 Number: 206 | Leuprorelin: 7.5mg Monthly with or without bicalutamide | Number: 204 (11 % received flare protection) | 12 months |
| Anderson (14) (CS28,United Kingdom) | Degarelix: 240mg initial dose 80mg Monthly | Number: 29 | Goserelin 3.6mg on days 3, 31, and 59 and bicalutamide on days 0-17 | Number: 13 (all cases received flare protection ) | 3 months |
| Mason (15) (CS30,USA&W estern Europe) | Degarelix: 240mg initial dose 80mg Monthly | Number: 181 | Goserelin 3.6mg on days 3, 31, and 59 andbicalutamide 50mg daily on days 0-16 | Number: 65 (all cases received flare protection) | 3 months |
| Axcrona (16) (CS31,Scandina via) | Degarelix: 240mg initial dose 80mg Monthly | Number: 84 | Goserelin 3.6mg on day 0, 28, and 56 + bicalutamide 50mg daily on days 0-28 | Number: 98 (all cases received flare protection) | 3 months |
| Tombal (11) (global) | Degarelix: 240mg initial dose 80mg Monthly or Degarelix: 240mg intial dose 16mg monthly | Number: 210 Number: 206 | Leuprorelin: 7.5mg Monthly with or without bicalutamide | Number: 204 (11 % received flare protection) | 12 months |
| Iversen (12) (global) | Degarelix: 240mg initial dose 80mg Monthly or Degarelix: 240mg intial dose 16mg monthly | Number: 210 Number: 206 | Leuprorelin: 7.5mg Monthly with or without bicalutamide Number:204 (11 % received flare protection) | 12 months |
Table 3 . Patient characteristics .
| Paper | patients | ||
| Klotz |
Degarelix 240/160mg (n=202) |
Degarelix 240/80mg (n=207) |
Leuprorelin 7.5mg (n=201) |
| Mean age (range), year | 72 (50-88) | 72 (51-89) | 74 (52-98) |
| Median (range) testosterone level(IQR), ng/ml | 3.78 (2.86, 5.05) | 4.11(3.05,5.32) | 3.84(2.91,5.01) |
| Median (range) PSA level (IQR), ng/ml | 19.9 (8.2, 68) | 19.8 (9.4, 46) | 17.4 (8.4, 56) |
| Stage of prostate cancer: number( % ) | |||
| Localized | 59 (29) | 69 (33) | 63 (31) |
| Locally advanced | 62 (31) | 64 (31) | 52 (26) |
| Metastatic | 41 (20) | 37 (18) | 47 (23) |
| Not classifiable | 40 (20) | 37 (18) | 39 (19) |
| Gleason score: number( % ) | |||
| 2-4 | 21 (11) | 20 (10) | 24 (12) |
| 5-6 | 67 (34) | 68 (33) | 63 (32) |
| 7 | 56 (28) | 63 (30) | 62 (31) |
| 8-10 | 56 (28) | 56 (27) | 51 (26) |
| Anderson | Degarelix (n=27) | Goserelin (n=13) | |
| Mean age (range), year | 68 (53, 87) | 72 (57, 85) | |
| Median (range) testosterone level(IQR), ng/ml | 4.2 (1.1, 6.7) | 3.9 (2.7, 7.4) | |
| Median (range) PSA level (IQR), ng/ml | 54.8 (8, 1914) | 41.1 (14.6, 348) | |
| Stage of prostate cancer: number( % ) | |||
| Localized | 4 (15) | 0 (0) | |
| Locally advanced | 4 (15) | 1 (8) | |
| Metastatic | 10 (37) | 4 (31) | |
| Not classifiable | 9 (33) | 8 (62) | |
| Gleason score: number( % ) | |||
| 5-6 | 2 (7) | 0 (0) | |
| 7-10 | 25 (93) | 13 (100) | |
| Mean (standard error) of IPSS general score | 20.1 (1.1) | 21.1 (1.6) | |
| Mean (standard error) of IPSS QoL score | 3.6 (0.3) | 3.2 (0.5) | |
| Mean (standard error) of prostate volume, ml | 53.5 )5.5( | 50.3 (4.5) | |
| Mason | Degarelix (n=180) | Goserelin (n=64) | |
| Mean (standard deviation)age, year | 70.6 (6.37) | 70.8 (5.96) | |
| Mean (standard deviation) testosterone level, ng/ml | 4.18 (1.72) | 4.45 (1.49) | |
| Median (range) testosterone, ng/ml | 3.92 (0.58, 11.2) | 4.42 (0.19, 8.16) | |
| Mean (standard deviation) PSA, ng/ml | 17.4 (30.1) | 13.4 (12.9) | |
| Median (range) PSA, ng/ml | 10.0 (2.5, 339) | 9.75 (2.9, 80) | |
| Stage of prostate cancer: number( % ) | |||
| Localized | 111 (62) | 41 (64) | |
| Locally advanced | 63 (35) | 20 (31) | |
| Not classifiable | 6 (3) | 3 (5) | |
| Gleason score: number( % ) | |||
| 2-6 | 41 (23) | 12 (19) | |
| 7 | 97 (54) | 42 (66) | |
| 8-10 | 42 (23) | 10 (16) | |
| Mean (standard error) of IPSS general score | 9.5 (6.71) | 8.5 (6.3) | |
| Mean (standard error) of IPSS QoL score | 2.27 (1.63) | 1.94 (1.56) | |
| Mean (standard error) of prostate volume, ml | 50.9 (20.3) | 52.5 (18.8) | |
| Axcrona | Degarelix (n=82) | Goserelin (n=97) | |
| Mean (standard deviation) age, year | 71.9 (7.71) | 73 (7.1) | |
| Mean (standard deviation) testosterone level, ng/ml | 4.25 (1.88) | 4.43 (1.64) | |
| Median (range) testosterone, ng/ml | 4.08 (0.32, 10.8) | 4.33 (0.13, 9.61) | |
| Mean (standard deviation) PSA, ng/ml | 277 (937) | 148 (438) | |
| Median (range) PSA, ng/ml | 27.8 (1.9, 6206) | 15.6 (3, 2829) | |
All included studies obtained acceptable scores on Jadad scale. One paper scored 5, four papers scored 4 and the other one scored 3. The risk of bias according to the Cochrane Collaboration’s tool has been showed in Fig. 2.
Fig. 2 .
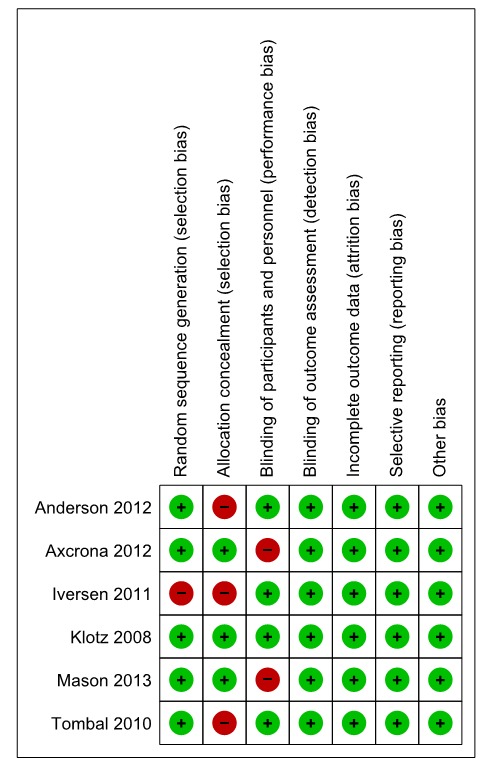
Included studies risk of bias based on results of critical appraisal
Efficacy outcomes
Prostate volume reduction
Three papers (14-16) reported the prostate volume after 12 weeks. These three studies involved 435 patients, 273 received degarelix and 162received GnRH agonists.Pooled data from these studies suggested that there was no significant difference in the prostate volume reduction among the groups (MD=-0.97, 95% CI: -5.32 to 3.39, p= 0.66) within 12 weeks of ADT (Fig. 3). Heterogeneity assessment did not show significant heterogeneity (I2=38%, p=0.2).
Fig. 3 .
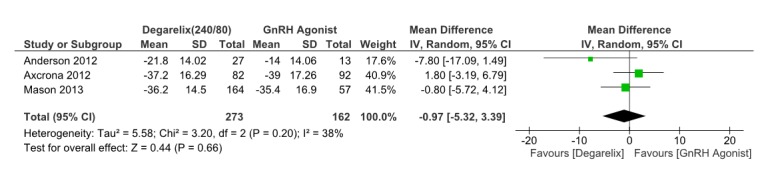
Comparison of Prostate volume reduction between degarelix and GnRH agonist treated group
IPSS score
The severity of and changes in Lower Urinary Tract Symptoms (LUTS) due to prostate cancer is assessed by the International Prostate Symptom Score (IPSS) questionnaire (17).
Three papers (14-16) reported the IPSS score after 12weeks.These three studies involved 435 patients, 273 receiving degarelix and 162 controls. Pooled data from these studies suggested that IPSS score in degarelix treated groups is significantly lower than control groups (MD=-1.85, 95% CI: -2.97 to -0.72, p=0.001) within 12 weeks of ADT(Fig. 4). So improving in LUTS in degarelix treated patients is obvious. Heterogeneity did not account for a significant proportion of the variability between studies (I2 = 0 percent).
Fig. 4 .
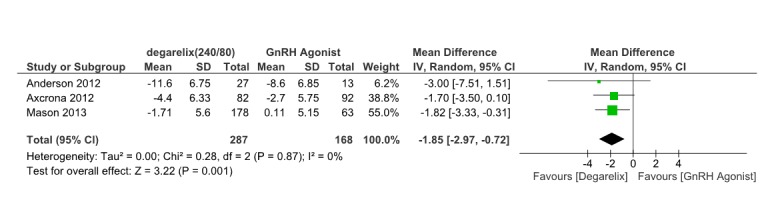
Comparison of IPSS score reduction between degarelix and GnRH agonist treated group
Quality of life
Quality of life related to urinary symptoms was assessed by the separate eighth IPSS question in three papers (14-16) after 12 weeks. As the heterogeneity was significant (I2=78%, p= 0.01), we did not pooled data. In two studies (15,16), more patients in degarelix treated groups reported improvement in QOL score but this difference was not statistically significant. Anderson study (18)showed that Significantly more degarelix patients had improved quality of life at week 12 (85 vs. 46%; p = 0.01).
Testosterone level reduction
Two studies (13,18)reported the serum testosterone level ≤0.5ng/ml between 1-28 days of treatment. These involved 364 patients, 224 receiving degarelix and 140 received GnRH agonists. Pooled data from these studies suggested that testosterone reduction≤0.5ng/ml is significantly more in degarelix treated group compared to control group. (OR= 11.58, 95% CI: 5.77 to 23.22, p< 0.001) in first 28 days of ADT (Fig. 5). Heterogeneity did not account for a significant proportion of the variability between studies (I2 = 0 percent).
Fig. 5 .
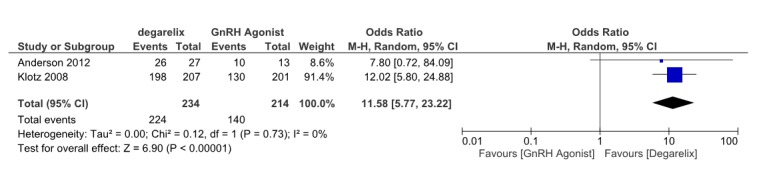
Comparison of testosterone level reduction between degarelix and GnRH agonist treated group between 1-28 days
Three papers (13,15,18)reported the serum testosterone level ≤0.5ng/ml after 28 days. These studies involved 663 patients, 400 receiving degarelix and 263 received GnRH agonists. Pooled data from these studies suggested that although testosterone reduction≤0.5ng/ml is more in degarelix treated group compared to control group (OR= 1.87, 95% CI: 0.89 to 3.96, p=0.1) after 28 days of ADT, but this difference is not statistically significant (Fig. 6). Heterogeneity did not account for a significant proportion of the variability between studies (I2 = 0 percent).
Fig. 6 .
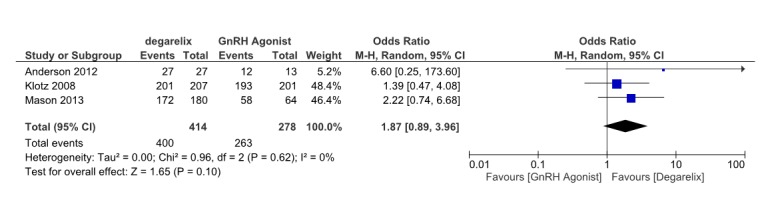
Comparison of testosterone level reduction between degarelix and GnRH agonist treated group after28 days
PSA level reduction
Due to different measures for reporting PSA level reduction in different studies and high heterogeneity, there was no possibility to pool data and performmeta-analysis. In Axcrona’s (16)RCT,82 patients received degarelix and 93 patients received goserelin (GnRH agonist) plus bicalutamide(anti androgen). The median percentage changes in PSA level were similar; for degarelix the decrease from baselineat weeks 4, 8 and 12 were− 80.6%, − 89.7% and − 92.0%, respectively whilst for goserelin they were − 85.2%,− 96.6% and − 97.3%.
In Anderson’s (18)experience, PSA levels were reduced by 90% at week 12 in both the degarelix(27 patients) and goserelin plus bicalutamide (13 patients) groups (mean reduction 92% versus 97%, respectively). Median PSA levels at this time point were less than 2ng/ml in both groups. This result indicates that there is no significant difference between intervention and control groups.
In Mason’s (15) experience, like previously mentioned studies, there was no significant difference among degarelix treated (180 patients) and goserelin plus bicalutamide treated (64 patients) groups. The median percentage changes in PSA were also comparable; for degarelix the decrease from baseline at weeks 4, 8and 12 were −71.6, −84.8 and −89.2%, respectively, whereas for goserelin they were −72.2, −93.1 and −93.0%.
In Klotz’s (13) study, 610 patients were randomized to 210 patients received degarelix240/80 mg, 206 patients received degarelix 240/160 mg and 204 patients received leuprolide (22 of them received concomitantanti-androgen). After 14 days, PSA levels had declined by 64%,65% and 18% from baseline in the degarelix240/80 mg, degarelix 240/160 mg and leuprolide groups, respectively; after 28 days, the PSA declines were 85%, 83% and 68%,respectively. The differences in the reduction in PSA from baseline betweendegarelix and leuprolide patients at days 14and 28 were statistically significant (p<0.001).In the subgroup of patients receiving leuprolide and concomitant bicalutamide, thePSA reduction was more rapid than in those who only received leuprolide, and similar to that of those on degarelix.
Safety outcomes
Drug induced mortality
Four studies (13,15,16,18) reported drug induced deaths. Pooling the drug induced mortality data was done on these four studies which involved 875 patients, 479 receiving degarelix and 396 received GnRH agonists. There was not significant difference among groups (OR=0.76, 95% CI: 0.2 to 2.96, p= 0.69) (Fig. 7) and There was some heterogeneity that was not significant (I2=42%, p= 0.18).
Fig. 7 .
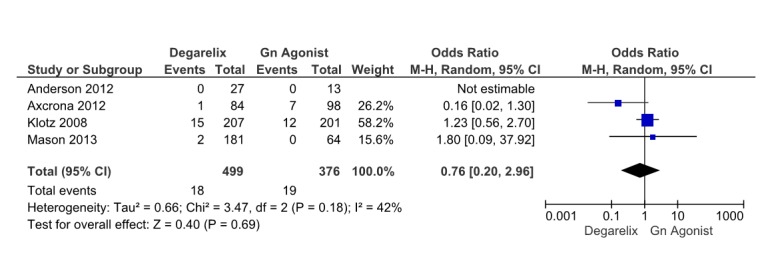
Comparison of drug induced mortality between degarelix and GnRH agonist treated group
Treatment-emergent adverse events (AEs)
Four studies (13,15,16,18) reported treatment-emergent adverse events. These four studies involved 875 patients, 479 receiving degarelix and 396 received GnRH agonists. Pooled data from these studies revealed that generally drug and treatment induced adverse events are similar among groups (OR=0.97, 95% CI: 0.7 to 1.33, p= 0.84) (Fig. 8). Heterogeneity did not account for a significant proportion of the variability between studies (I2= 0 percent).
Fig. 8 .
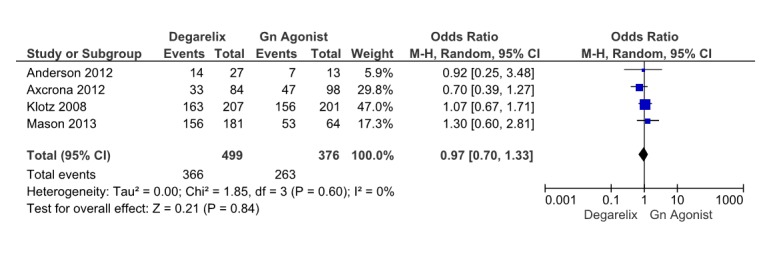
Comparison of treatment emergent adverse events between degarelix and GnRH agonist treated group
Hot flashes
We pooled data from four studies (13,15,16,18), (875 patients, 479 receiving degarelix and 396 received GnRH agonists) which reported hot flashes as an important treatment induced adverse event. Meta-analysis results revealed that deagrelix and GnRHagonists are similar in this outcome (OR=1.04, 95% CI: 0.75 to 1.44, p= 0.83) (Fig. 9). Theirheterogeneity was not important. (I2=13%).
Fig. 9 .
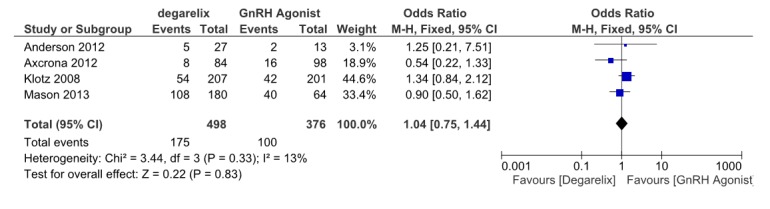
Comparison of hot flashes between degarelix and GnRH agonist treated group
Injection site reaction
Injection site reactions including pain, erythema, pruritus, swelling and induration; are the most prevalent and important adverse event. Four studies (13,15,16,18) reported injection site reactions. These four studies involved 875 patients, 479 receiving degarelix and 396 received GnRH agonists. Pooled data from these studies revealed that the degarelix S.C. injection was associated with a higher rate of injectionsitereactions than with the GnRH agonist injection (OR=46.34, 95% CI: 15.79 to 136, p≤0.001) (Fig. 10).Heterogeneity did not account for a significant proportion of the variability between studies (I2=0 percent).
Fig. 10 .
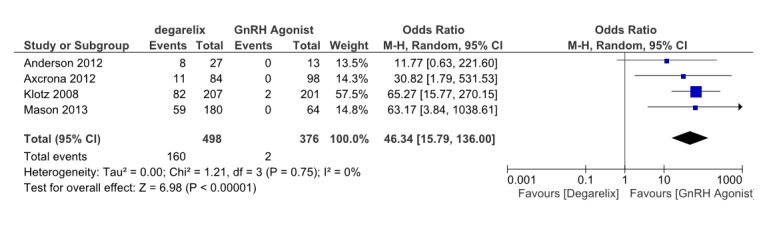
Comparison of injection site reactions between degarelix and GnRH agonist treated group
Other adverse events
Besides above mentioned important treatment induced adverse events, there are some rare adverse events which reported in different studies. According to Klotz study (13), urinary tract infection (9% versus 3%, respectively; p<0.01) and musculoskeletal and connective tissue AEs (26% versus 17%; p< 0.05) were more common with leuprolide. Other complications like cardiovascular and cerebrovascular accidents, weight gain and arthralgia were the same among different groups. In Axcrona study (16), erectile dysfunction (degarelix, 5%; goserelin, 4%) and hyperhidrosis (degarelix, 4%; goserelin, 5%) were reported besides other prevalent AEs, which were similar among groups. The Anderson study (18), also showed lower incidence of urinary tract infection in degarelix treated group.
Discussion
Hormone therapy using GnRH receptor agonists and recently antagonists is the main treatment for locally advanced and metastatic prostate cancer. There are many studies assessing and comparing ADT desired and adverse effects on patients (9,19-24).We performed this study to compare safety and efficacy of degarelix, with Gonadotropin-releasing hormone (GnRH) agonists using best available evidence to provide further evidence for informed policy making about using degarelix instead of GnRH agonists.
This systematic review provides some evidence that, for patients with locally advanced and metastatic prostate cancer, the only statistically significant treatment effect in degarelix treated groups (compared with GnRH agonists group), which lasts beyond first month of treatment is improvement in LUTS. This is measured by International Prostate Symptom Score (IPSS) at week 12, (MD=-1.85, 95% CI: -2.97 to -0.72, p=0.001). However, the QOL of patients, which is measured as a single question in the IPSS questionnaire, was not different among groups.
In first 28 days of treatment the testosterone reduction≤0.5 ng/ml is significantly more in degarelix treated groups compared to control groups. (OR=11.58, 95%CI: 5.77 to 23.22, p<0.001) but after first month, this difference is not statistically significant anymore (OR= 1.87, 95% CI: 0.89 to 3.96, p=0.1). The PSA reduction like testosterone reduction is more in degarelix treated groups at days 14 and 28 of treatment. These important outcomes were the same in both GnRH agonists and antagonists after first month of treatment and also in the subgroup of GnRH agonists treated patients receiving concomitant anti androgen from the first days of treatment.
Most reported AEs were of mild to moderate intensity. Generally, the incidence of drug and treatment induced adverse events and the number of discontinuations due to AEs were similar among groups (OR=0.97, 95% CI: 0.7 to 1.33, p= 0.84). The most frequently reported AEs in studies were flushing and injection-site reactions. Meta-analysis results revealed that deagrelix and GnRH agonists are similar in hot flashes occurrence (OR=1.04, 95% CI: 0.75 to 1.44, p= 0.83).Additional analysis also showed that except for a more rapid onset with the GnRH antagonist, there were no major differences in the overall pattern of hot flushes between treatment options (12). Degarelix S.C. injection was associated with a higher rate of injection site reactions than with GnRH agonist injections (OR=46.34, 95% CI: 15.79 to 136, p≤0.001).
Degarelix is generally well tolerated, without systemic allergic reactions. With the exception of injection site reactions, most AEs reflect androgen suppression or the underlying condition and are similar between degarelix and GnRH agonist groups (25). A pooled analysis of data from 1704 men in nine clinical trials showed that the rate of CVD events was similar before and after degarelix treatment in the overall patient population (26). Multivariate analysis showed that traditional CV risk factors of age, obesity and baseline CVD were associated with a higher CVD risk (p< 0.05). Degarelix dose and treatment duration were not independently associated with CVD events (26).
Limitations
Several problems exist in this review which needs to be addressed. Only 4 RCTs and 6 papers were suitable for inclusion in this study (11-13,15,16,18). While quality of studies were acceptable, but one RCT had been stopped recruiting patients early and so the sample size of that study was small (18). Considering the different mechanism of action between GnRH agonists and antagonists, measuring the outcomes in first days of treatment is important and some studies did not assess them. Also measurement of some outcomes like PSA level reduction was not the same in different studies and this prevented pooling data and performing Meta-analysis. Another issue was about receiving concomitant anti-androgen in GnRH agonist treated groups. Except for the Klotz study, the control group in other studies received anti-androgen. In Klotz study however just a small subgroup of the control group (22 from 202) received concomitant anti-androgen, it was not clearly mentioned that what has been the rational for selecting these patients. It is also to be mentioned that clinical trials included in this review were sponsored by Ferring Pharmaceuticals.
Conclusion
The different pharmacological profile of degarelix causes rapid testosterone and PSA suppression without the initial testosterone surge or microsurges associated with GnRH agonists. Degarelix efficacy in reducing testosterone and PSA level are not less than those of GnRh agonists (27-29). The point is that when we compare degarelix with GnRH plus anti-androgen, this rapid onset is not significant and the only difference is about more pronounced effects on lower urinary tract symptoms by degarelix (30). This difference despite similar effects on PSA and testosterone suppression, may suggest the mechanism on LUTS is independent of these markers (29-32).
When one compares different aspects of these drugs, the cost of treatment accounts as a key factor to make a cost effective decision for using degarelix instead of GnRh agonists in patients with metastatic and locally advanced prostate cancer. Performing an economic evaluation of degarelix will also provide further evidence for better decision making in our setting.
The authors state that there is no conflict of interest to declare.
Acknowledgment
This research has been supported by National Institute of Health Research of Iran grant number 91321/م/241.
Appendix 1
MEDLINE search strategy
1. (degarelix OR firmagon OR abarelix OR plenaxis).tw.
2. exp Gonadotropin-Releasing Hormone/
3. exp Hormone Antagonists/
4. 2 AND 3
5. ((luteinising OR luteinizing OR LHRH OR gonadotrop$ OR GNRH) AND (agonist$ OR antagonist$ OR blocker$)).tw.
6. (androgen deprivation OR ADT OR androgen suppression).tw.
7. Goserelin/
8. Leuprolide/
9. TriptorelinPamoate/
10. Buserelin/
11. (goserelin OR zoladex OR leuprORelin OR leuprolide OR triptorelin OR trelstar).tw.
12. (bicalutamide OR casodex OR bicalox).tw.
13. exp Androgen Antagonists/
14. 1 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13
15. 1 OR 5 OR 6 OR 11 OR 12
16. exp Prostatic Neoplasms/
17. ((prostate OR prostatic) AND (cancer OR carcinoma OR adenocarcinoma OR tumour OR tumor OR neoplasm$)).tw.
18. 16 OR 17
19. 14 AND 18
20. 15 AND 19
21. 19 not 20
MEDLINE (adverse events search strategy)
1. (degarelix OR firmagon).tw.
2. exp Gonadotropin-Releasing Hormone/
3. exp Hormone Antagonists/
4. 2 AND 3
5. ((luteinising OR luteinizing OR LHRH OR gonadotrop$ OR GnRH) AND (agonist OR
antagonist OR blocker$)).tw.
6. (androgen deprivation OR ADT OR androgen suppression).tw.
7. Goserelin/
8. Leuprolide/
9. Triptorelin /
10. Buserelin/
11. (goserelin OR zoladex OR leuprorelin OR leuprolide OR triptorelin OR buserelin).tw.
12. (bicalutamide).tw.
13. exp Androgen Antagonists/
14. 1 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13
15. exp Prostatic Neoplasms/
16. ((prostate or prostatic) AND (cancer or carcinoma or adenocarcinoma or tumour or tumor or neoplasm$)).tw.
17. 15 or 16
18. 14 AND 17
19. (safe or safety or side-effect or tolerability or toxicity).ti,ab.
20. (adverse adj2 (effect or reaction$ or event or outcome)).tw.
21. 19 or 20
22. 14 AND 17 AND 21
23. 18 or 22
Cite this article as: Hosseini SA, Rajabi F, Akbari Sari A, Ayati M, Heidari S, Ghamary F. Degarelix for the treatment of advanced prostate cancer com-pared with GnRh-Agonists: a systematic review and meta-analysis. Med J Islam Repub Iran 2016 (9 January). Vol. 30:317.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: A Cancer Journal for Clinicians. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Sadjadi A, Nooraie M, Ghorbani A, Alimohammadian M, Zahedi MJ, Darvish-Moghadam S. et al. The incidence of prostate cancer in Iran: results of a population-based cancer registry. Arch Iran Med. 2007;10(4):481–5. [PubMed] [Google Scholar]
- 3.Kolahdoozan S, Sadjadi A, Radmard A R, Kahademi H. Five common cancers in Iran. Arch Iran Med. 2010;13(2):143–6. [PubMed] [Google Scholar]
- 4.Conn PM, Crowley WF Jr. Gonadotropin-releasing hormone and its analogues. N Engl J Med. 1991;324(2):93–103. doi: 10.1056/NEJM199101103240205. [DOI] [PubMed] [Google Scholar]
- 5.Lepor H. Comparison of single-agent androgen suppression for advanced prostate cancer. Rev Urol. 2005;7(5):S3–S12. [PMC free article] [PubMed] [Google Scholar]
- 6.Waxman J, Man A, Hendry WF, Whitfield HN, Besser GM, Tiptaft RC. et al. Importance of early tumour exacerbation in patients treated with long acting analogues of gonadotrophin releasing hormone for advanced prostatic cancer. Br Med J. 1985;291(6506):1387–8. doi: 10.1136/bmj.291.6506.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boccon-Gibod L, Iversen P, Persson B-E. Degarelix 240/80 mg: a new treatment option for patients with advanced prostate cancer. Expert Review of Anticancer Therapy. 2009;9(12):1737–43. doi: 10.1586/era.09.150. [DOI] [PubMed] [Google Scholar]
- 8.Boccon-Gibod L, van der Meulen E, Persson B-E. An update on the use of gonadotropin-releasing hormone antagonists in prostate cancer. Therapeutic Advances in Urology. 2011;3(3):127–40. doi: 10.1177/1756287211414457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cochrane Handbook for Systematic Reviews of Interventions. Higgins JPT, Green S, editors: John Wiley & Sons, Ltd; 2008.
- 10.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 11.Tombal B, Miller K, Boccon-Gibod L, Schroder F, Jensen JK, Olesen TK. et al. Degarelix vs leuprolide treatment in patients with advanced prostate cancer: PSA failures during a randomised, phase 3 trial (CS21) European Urology Supplements. 2009;8(4):130. [Google Scholar]
- 12.Iversen P- Karup C, van der Meulen E, Tanko LB, Huhtaniemi I. Hot flushes in prostatic cancer patients during androgen-deprivation therapy with monthly dose of degarelix or leuprolide. Prostate Cancer and Prostatic Diseases. 2011;14(2):184–90. doi: 10.1038/pcan.2011.11. [DOI] [PubMed] [Google Scholar]
- 13.Klotz L, Boccon Gibod L, Shore ND, Andreou C, Persson B-E, Cantor P. et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU International. 2008;102(11):1531–8. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- 14.Anderson J, Al-Ali G, Wirth M, Gual JB, Gomez Veiga F, Colli E. et al. Degarelix versus goserelin (+ anti-androgen flare protection) in the relief of lower urinary tract symptoms secondary to prostate cancer: results from a phase IIIb study ( NCT00831233) Urol Int. 2013;90(3):321–8. doi: 10.1159/000345423. [DOI] [PubMed] [Google Scholar]
- 15.Mason M, Maldonado Pijoan X, Steidle C, Guerif S, Wiegel T, van der Meulen E. et al. Neoadjuvant Androgen Deprivation Therapy for Prostate Volume Reduction, Lower Urinary Tract Symptom Relief and Quality of Life Improvement in Men with Intermediate- to High-risk Prostate Cancer: A Randomised Non-inferiority Trial of Degarelix versus Goserelin plus Bicalutamide. Clinical Oncology. 2013;25(3):190–6. doi: 10.1016/j.clon.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 16. Axcrona K , Aaltomaa S , da Silva CM, Ozen H, Damber J-E, Tanko LB, Colli E. et al. Androgen deprivation therapy for volume reduction, lower urinary tract symptom relief and quality of life improvement in patients with prostate cancer: degarelix vs goserelin plus bicalutamide. BJU International. 2012;110(11):1721–8. doi: 10.1111/j.1464-410X.2012.11107.x. [DOI] [PubMed] [Google Scholar]
- 17.Madersbacher S, Alivizatos G, Nordling J, Sanz CR, Emberton M, de la Rosette JJ. EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines) Eur Urol. 2004;46(5):547–54. doi: 10.1016/j.eururo.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 18. Anderson John , Al-Ali G , Wirth M, Gual JB, Gomez Veiga F, Colli E, van der Meulen E. et al. Degarelix versus Goserelin (+ Antiandrogen Flare Protection) in the relief of lower urinary tract symptoms secondary to prostate cancer: results from a phase IIIb study ( NCT00831233) Urologia Internationalis. 2013;90(3):321–8. doi: 10.1159/000345423. [DOI] [PubMed] [Google Scholar]
- 19.Kunath F, Grobe HR, Rucker G, Motschall E, Antes G, Dahm P. et al. Non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer. Cochrane Database Syst Rev. 2014;2014(30) doi: 10.1002/14651858.CD009266.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirby RS, Fitzpatrick JM, Clarke N. Abarelix and other gonadotrophin-releasing hormone antagonists in prostate cancer. BJU International. 2009;104(11):1580–4. doi: 10.1111/j.1464-410X.2009.08924.x. [DOI] [PubMed] [Google Scholar]
- 21.Isbarn H, Boccon-Gibod L, Carroll PR, Montorsi F, Schulman C, Smith MR. et al. Androgen Deprivation Therapy for the Treatment of Prostate Cancer: Consider Both Benefits and Risks. European Urology. 2009;55(1):62–75. doi: 10.1016/j.eururo.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson J, Abrahamsson P-A, Crawford D, Miller K, Tombal B. Management of advanced prostate cancer: can we improve on androgen deprivation therapy? BJU International. 2008;101(12):1497–501. doi: 10.1111/j.1464-410X.2008.07590.x. [DOI] [PubMed] [Google Scholar]
- 23.Crawford ED, Tombal B, Miller K, Boccon-Gibod L, Schröder F, Shore N. et al. A Phase III Extension Trial with a 1-Arm Crossover From Leuprolide to Degarelix: Comparison of Gonadotropin-Releasing Hormone Agonist and Antagonist Effect on Prostate Cancer. The Journal of Urology. 2011;186(3):889–97. doi: 10.1016/j.juro.2011.04.083. [DOI] [PubMed] [Google Scholar]
- 24.Heidenreich A. Management of Advanced Prostate Cancer: Gonadotropin-Releasing Hormone Blockers Might Improve Prognosis. European Urology. 2008;54(4):726–7. doi: 10.1016/j.eururo.2008.04.101. [DOI] [PubMed] [Google Scholar]
- 25.Smith MR, Klotz L, Persson BE, Olesen TK, Wilde AA. Cardiovascular safety of degarelix: results from a 12-month, comparative, randomized, open label, parallel group phase III trial in patients with prostate cancer. J Urol. 2010;184(6):2313–9. doi: 10.1016/j.juro.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith MR, Klotz L, van der Meulen E, Colli E, Tankó LB. Gonadotropin-Releasing Hormone Blockers and Cardiovascular Disease Risk: Analysis of Prospective Clinical Trials of Degarelix. The Journal of Urology. 2011;186(5):1835–42. doi: 10.1016/j.juro.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doehn C - Sommerauer M, Jocham D. Degarelix and its therapeutic potential in the treatment of prostate cancer. Clinical Interventions in Aging 2009; 4(1178-1998 (Electronic)):215-523. [DOI] [PMC free article] [PubMed]
- 28.Shore ND. Experience with degarelix in the treatment of prostate cancer. Therapeutic Advances in Urology. 2013;5(1):11–24. doi: 10.1177/1756287212461048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gittelman M, Pommerville PJ, Persson B-E, Jensen J-K, Olesen TK. A 1-Year, Open Label, Randomized Phase II Dose Finding Study of Degarelix for the Treatment of Prostate Cancer in North America. The Journal of Urology. 2008;180(5):1986–92. doi: 10.1016/j.juro.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 30.Cui Y, Zong H, Yan H, Li N, Zhang Y. Degarelix versus goserelin plus bicalutamide therapy for lower urinary tract symptom relief, prostate volume reduction and quality of life improvement in men with prostate cancer: a systematic review and meta-analysis. Urol Int. 2014;93(2):152–9. doi: 10.1159/000356272. [DOI] [PubMed] [Google Scholar]
- 31.Gil T, Aoun F, Cabri P, Maisonobe P, van Velthoven R. A prospective, observational grouped analysis to evaluate the effect of triptorelin on lower urinary tract symptoms in patients with advanced prostate cancer. Ther Adv Urol. 2015;7(3):116–24. doi: 10.1177/1756287215574480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peltier A, Aoun F, De Ruyter V, Cabri P, Van Velthoven R. Triptorelin in the Relief of Lower Urinary Tract Symptoms in Advanced Prostate Cancer Patients: The RESULT Study. Prostate Cancer. 2015;978194:28–36. doi: 10.1155/2015/978194. [DOI] [PMC free article] [PubMed] [Google Scholar]


