Abstract
Background: Although studies reported diabetes mellitus screening cost effective, the mass screening for type2 diabetes remains controversial. In this study we reviewed the recently evidence about the cost effectiveness of mass screening systematically.
Methods: We reviewed the MEDLINE, Scopus, Web of Science (WOS), and Cochrane library databases by MeSH terms to identify relevant studies from 2000 to 2013. We had 4 inclusion and 6 exclusion criteria and used the Drummond’s checklist for appraising the quality of studies.
Results: The initial search yielded 358 potentially related studies from selected databases. 6 studies met our inclusion and exclusion criteria and included in final review. 3 and 2 of them were conducted in Europe and America and only one of them in Asia. Quality-adjusted life year (QALY) was the main outcome to appraise the effectiveness in the studies. Incremental cost effectiveness ratio (ICER) was computed in range from $516.33 to $126,238 per QALY in the studies.
Conclusion: A review of previous diabetes screening cost effectiveness analysis showed that the studies varied in some aspects but reached similar conclusions. They concluded that the screening may be cost effective, however further studies is required to support the diabetes mass screening.
Keywords: Cost effectiveness, Diabetes, Screening, Economic evaluation
Introduction
Diabetes mellitus is one of the major metabolic and one of the most serious chronic diseases (1–4) with substantial prevalence, incidence and economic burden in the world (3,5). Recent estimates suggest that diabetes mellitus causes 59258034 disability-adjusted life years (DALYs) in 2012 with 89.7% increase in deaths from diabetes since 1990 to 2013 (6). International Diabetes Federation (I DF) estimated about 382 million people (8.3% of adults) have diabetes in 2013 and it rises to 592 million people in 2035. The majority of diseased persons aged between 40 to 59 and this led to higher economic burden of diabetes (7).
Diabetes mellitus is asymptomatic in early stage and can remain undiagnosed for 9 to 12 years (8,9). The asymptomatic and chronic nature of diabetes cause to many individuals have diabetes related complications when in diagnosed (5). Individual with diabetes are at higher risk to have long term dysfunction and failure in heart, kidneys, nerves and damage on eyes and blood vessels (8). Diabetes mellitus cause 5.1 million deaths that half of them occur under age 60 and 11% of total health expenditure (USD 548 billion in health expenditure) (10).
The burden of diabetes can be reduced by many interventions but due to resource limitation they should be prioritized (11). Several studies and organization have recommended diabetes screening to reduce the burden of disease (12,13). Although diabetes meets screening criteria (14), randomized trials have not demonstrated reduction of diabetes’ complications or burden via mass screening (15). Despite the results of the clinical trial, some statistical models-based studies have demonstrated the benefit of mass screening for diabetes (5,15) and others have shown opportunistic screening may be cost effective (13).
Although many studies have shown the diabetes screening is cost effective but their qualities and conclusions are vary (11). Some of them do not support mass screening in all setting and conclude screening for high risk individuals may be worthwhile (16,17). Therefore in this study, we want to appraise individual studies and summarize results using a systematic review, to help policy makers and clinicians in planning and resource allocation, and to finance cost effective interventions for preventing diabetes and its complications.
Methods
The design of the study was approved by the Ethics Committee of Iran University of Medical Sciences.
Search strategy
The review began with systematic search of the main databases. Medical Literature Analysis and Retrieval System Online (MEDLINE), Scopus, Web of Science (WOS), and Cochrane library databases were selected to identify relevant studies from January 2000 to November 2013. We used medical subject headings (MeSH) to identify synonyms of keywords and relevant studies. “diabetes, screening, cost effectiveness and economic evaluation” were the base keywords and these terms combined in different ways based on data bases.
MEDLINE was searched using: MeSH terms of diabetes mellitus AND screening AND “cost effectiveness", diabetes mellitus AND screening AND (key words for costs) AND (keywords for effectiveness), and Diabetes mellitus AND screening AND (“cost-benefit”) OR (“cost-effectiveness”) OR (“cost-utility”) OR (“economic evaluation”) OR (“cost saving”).
Scopus and Web of Sciences were searched with keywords for (cost) AND (effectiveness) AND (diabetes) AND (screening). In the Cochrane Library search we coded terms and combined them as follows: #1"cost effectiveness, #2"economic evaluation", #3"cost benefit" , #4"cost utility", #5 “cost saving” #6"diabetes mellitus”, #7"diabetes", #8" type 2 diabetes” #9"diabetes mellitus type II” and #10 screening. We combined these cods as follow:#1 and #6and #10, #1 and #7 and#10, #1and #8 and #10, #1and#9 and #10, #2 and #6and #10, #2 and #7 and#10, #2and #8 and #10, #2and#9 and #10, #3 and #6and #10, #3 and #7 and#10, #3and #8 and #10, #3and#9 and #10, #4 and #6and #10, #4 and #7 and#10, #4and #8 and #10, #4and#9 and #10, :#5 and #6and #10, #5 and #7 and#10, #5and #8 and #10, #5and#9 and #10,
For more additional search we used “Google Scholar”, Center for Reviews and Dissemination, CEA Registry and also manually checked reference lists of all publications including original studies and reviews to identify studies not found through systemic searching.
Studies selection
In overall, studies were included in this review if apprise and report both costs and outcomes of screening. We had 4 inclusion and 7 exclusion criteria for selecting of studies and finally only studies which had excellent or good quality rank entered in final analysis.
Inclusion criteria
1) systematic review and original economic evaluation in each three categories of cost benefits or cost effectiveness and cost utility analysis; 2), studies which were apprised type 2 diabetes, 3) outcomes were measured as life years gained (LYGs) or quality-adjusted life years (QALYs) gained and cost saving; and 4) Publication in the English occurred between January 2000 and November 2013.
Excluding criteria
1) Studies which appraise cost or outcome only, 2) studies appraise other type of diabetes than type 2, 3) opportunistic screening economic evaluation, 4) review papers and documents, 5) health economic evaluation studies in children 6) studies appraise only cost per case identification and 7) short term horizon (less than 10 years) to analyzing cost and effectiveness.
Quality assessment
Health economic evaluation studies vary based on their quality of conducting and reporting and there are heterogeneity in their purposes, perspective, conceptual, modeling and measurement issues (18). In order to ensure studies with acceptable quality are included, we used the Drummond’s checklist (19) for appraising the quality of studies. Only those which obtained good and excellent scores according to the Drummond’s checklist were included in the final analysis.
Data extraction
From the selected studies, data were extracted based on the predetermined form. This form contains1) bibliography, including year, country of studies, and authors, 2) study design, including aim and cases of studies, time horizon, interventions and alternatives, costs included in the study, outcome measures for effectiveness, study’s perspective, modeling, discount rate for costs and outcome and sensitivity analysis, and 3) results and conclusion, including incremental cost effectiveness ratio (ICER), cost saving and cost per identification of new diabetic or pre-diabetic.
Analysis process and reporting
The title and abstract of primary results was reviewed by two people to identify repeated and unrelated documents separately. The output of these reviews checked and disagreed cases were identified by referring to the papers’ main text (Kappa was 94%). After reviewing the abstract we reviewed the full text of papers and some of them were excluded based on the inclusion and exclusion criteria. The included papers in the final apprising met the quality assessment criteria.
Results
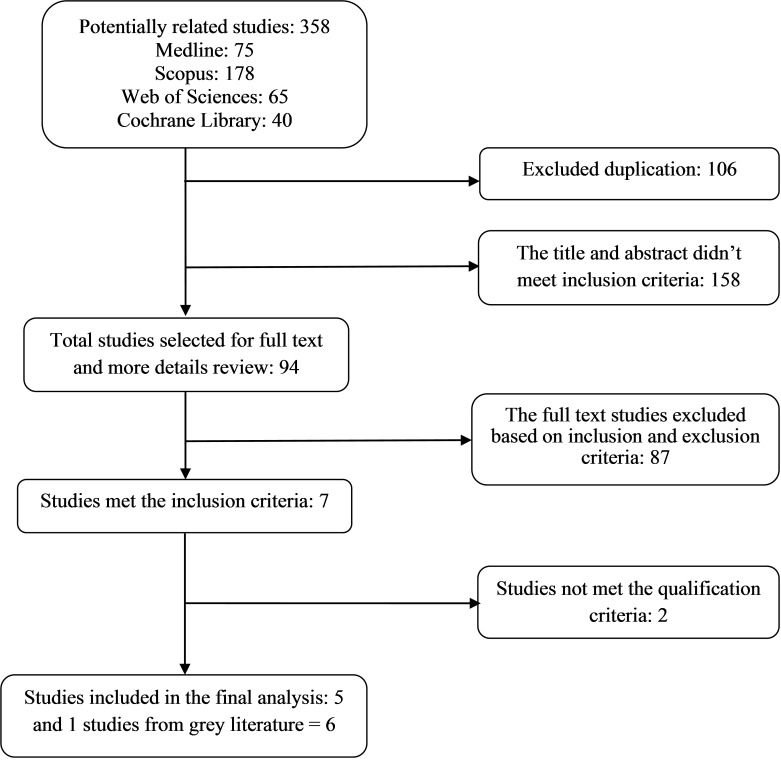
The initial search from selected databases yielded 358 potentially related studies. 106 papers were duplicated and excluded from the analysis. 158 papers did not meet inclusion criteria based on title and abstract review and 94 possible original papers remained for full screen. Further review of the full text resulted in 7 CE studies that met our inclusion criteria. One studies met the inclusion criteria from the grey literature. Two studies recognized as poor quality papers and excluded from the final analysis. Error! Reference source not found. shows the detailed description of studies selection process and data abstraction which we included in the study.
In this review, studies were described based on their country setting and year of the study, study population, intervention and alternative options for comparison, the outcome (effectiveness) that study focuses on, costs included in the analysis, analytical time horizon, perspective, discount rate for outcome and costs, cost effectiveness ratio and modeling of study that used for long term outcome and cost estimation in the long term studies.
Review yielded six studies with our inclusion and exclusion criteria. The studies took the long term time horizon and assessed the long-term costs and consequences of screening (5,14,15,20–22). The characteristics of these are summarized in Error! Reference source not found. Four of 6 studies took the life time (5,15,20,21) and one of them took 14 years (14) and other one took 50 years analytical horizon (22). The studies were conducted in the United States, United Kingdom, Germany, and Thailand. In this review we did not find any study for recent years and all included studies were conducted before 2011. All studies assessed the cost effectiveness of screening in the populations aged over 30 years (two studies over 30; two others over 35; one 45, and another one over 65 years).
All studies assessed the cost effectiveness of mass screening by different interventions for screening and diagnostic tests in comparison with no screening (Error! Reference source not found). Five of 6 studies used Fasting Plasma Glucose (FPG) as screening or diagnostic test. Moreover some studies have applied additional tests such as hemoglobin test (15); Oral Glucose Tolerance Test (OGTT) (15,22), and Capillary Blood Glucose (CBG) (21) as screening or diagnostic tests. In one study (20) only OGTT was used for screening and diagnostic of diabetic individuals.
Different screening intervals are the prevalent strategy which was used as an alternative option for comparison. Chen, et al (15) conducted 2 and 5 year intervals and Kahn et al (5) developed 8 strategies based on screening interval and age at initiation for comparison. Except one (21) which compared universal screening with targeted screening in people with hypertension, all the rest compared the interventions and strategies with no screening.
QALY is the most prevalent outcome used for measuring the effectiveness of interventions. Five of 6 studies used QALY and one the cost saving for measuring the effectiveness. Chen et al applied LYGs in addition to QALY.
Fig. 1 .
Studies review process for systematic review of cost effectiveness of diabetes screening
Table 1 . Description of long-term cost effectiveness studies characteristics .
|
Sources/ Authors |
Country and year of study | Study population | Intervention |
Alternative options for comparison |
Outcome measure | Study time horizon |
| Chen and e.tal | Taiwan /2001 | Aged over 30 years | mass screening in 2 and 5 year interval | Over 30 years not screening | life year gained and QALY | Life time |
| Lee, D. S. et al |
USA/ 2000 |
Wisconsin Medicare population (65 and Older) |
Mass screening |
No screening |
cost saving per diabetic Detected | 14 years |
| Kahn R. and et al |
USA/ 2010 |
Aged 30 years | 9 different screening strategies |
No screening |
QALY | Life time |
| Schaufler, T. M. and et al | Germany/2010 | Aged 35–75 | Screening with OGTT | Current status quo (No Screen) | QALY | Life time |
| Hoerger, T. J. et al |
UK/ 2004 |
Over 35 aged and people with hypertension |
Universal and targeted screening | With together and no screening |
QALY |
Life time |
| Gillies CL. et al |
UK/ 2008 |
Age 45 at screen time | screening to early detection and treatment for type 2 diabetes, (b) screening for type 2 diabetes and IGT, intervening with lifestyle (c) as for (b) but with pharmacological interventions, | No screening | QALY | 50 Years |
Table 1 . Cntd .
| Sources/ Authors | Included Cost | Study perspective | Cost Effective Ratio | Discount Rate for cost and outcome | Study Model |
| Chen and et.al | Direct medical cost | N/A | Biennial: $26 750 (34903a) per life-year gained, and $17 833(23268a) per QALY. fiveyearly screening: $10 531(13741a) per lifeyear gained and $17 113(22329a) per QALY | 3% | Markov Monte Carlo Simulation Model |
| Lee, D. S. et al | Direct medical cost | Health system perspective | The cost of community screening are greater than the cost of diabetes without screening ICER(-) | 3% | Monte Carlo simulation Mode |
| Kahn R. and et al | Direct medical Cost | Health service or delivery system | Five screening strategies had costs per QALY of about US$10 500. 45 years and every year $15 509, at 60 years and every 3 years $25 738, at 30 years and repeated every 6 months; $40 778 3% | Archimedes model | |
| Schaufler, T. M. and et al | Direct medical cost | German system of health | insurance ICER: $892.5 per QALY for lifestyle intervention, $316.33 per QALY for prevention with metformin | Cost 5% / Outcome (0 | Markov Monte Carlo Simulation Mode |
| Hoerger, T. J. et al | Direct medical cost | Health care system | ICER for universal screening $126238 (150735a), $121965 (145633a), $62934 (75146a), $59183(70668a) and $48146 (57489a) and targeting screening $87,096(103997a), $46,881 (55978a), $34,375(41046a), $31,228 (37288a) and $32,106 (38336a) for age at 35, 45, 55, 65 and 75 years respectively | 3% | Markov model |
| Gillies CL. et al | Direct Medical Cost | UK health Care System | ICER: £14 150 (€17 560; $27 860 (29557a)) for screening DM, £6242 ($12290(13038a)) for screening for DM and IGT followed by lifestyle interventions, and £7023 ($13828 (14670a)) for screening for DM and IGT followed by pharmacological intervention | 3.5% | hybrid decision tree/Markov model was developed to simulate the long term effects |
Markov simulation models were conducted to assess the long-term outcomes and costs of screening for type 2 diabetes mellitus in 4 studies. Two studies have developed the Archimedes model (5) and hybrid decision tree/Markov model (21) to simulate the long-term costs and effects.
All studies included the direct medical costs in their models. They did not included direct non-medical costs and used the health system perspective to identify the costs. Four studies used 3% rate to discount the future costs and effectiveness of interventions (5,14,15,21). One (20) used the 5% and zero to discount costs and outcome, respectively, and the other (22) discounted the costs and outcome by 3.5%.
Studies yielded very different range of ICER. In order to make them comparable we inflate to 2010 with 3% yearly. Chen et al.(15) had estimated the ICER for biennial and 5-yearly screening $26750 and $10531 per LYGs and also $17833 and $17113 per QALY gained, respectively. Lee et al used the CDC diabetes cost effectiveness study group(23) results and assumptions for their estimation. They estimated the cost of screening $100 per diagnosed diabetes. They presented three scenarios to estimate the lifetime costs and assumed screening could reduce cardiovascular risk zero and 30%. In this way excess lifetime costs associated with screening would be $4471, and $3246 greater than no screening per identification, respectively. In addition to cardiovascular risk reduction, if screening lead to 30% reduction in routine medical care costs, it can save an average of $619 per detected diabetes. Kahn et al appraised 8 strategies to identify the cost effectiveness of screening.
Five screening strategies had about $10500 costs per QALY, screening at age 45 years and repeat every year had $15509, age at 60 years and repeated every 3 years $25738, and age at 30 years and repeated every 6 months had $40,778 cost per QALY compared with no screening. Schaufler et al estimated the ICER for screening and lifestyle and metformin intervention €562.54 ($892.50) and €325.44 ($516.33) per QALY compared with no screening. Hoerger et al estimated the ICER for universal and targeted screening with intensive glycemic control and intensive hypertension control at 5 age initiation screening. They calculated ICER for universal screening $126238, $121965, $62934, $59183 and $48146 and targeting screening $87,096, $46,881, $34,375, $31,228 and $32,106 for age at 35, 45, 55, 65 and 75 years, respectively. Gillies et al computed ICER for diabetes and IGT screening by two preventive interventions. They estimated ICER $27,860 for screening type2 diabetes, $12,290 for diabetes and IGT screening by lifestyle interventions, and $13828 for diabetes and IGT screening followed by pharmacological intervention.
Conclusion
We found that all studies conclude the screening for diabetes is cost effective. However country setting can lead to different ICER for same interventions. For example screening for diabetes and glycemic control in new diabetic detected individuals is low cost effective in the US in comparison with other developed countries (7). On the other hand, the health outcome measures that have been used to estimate QALYs may overestimate or underestimate health utilities. Our study showed that cost effectiveness ratio has very wide range in the different countries. It varies from $516.33 (€325.44) in Germany (20) to $126,238 in UK per QALY(21). But it varies by some factors such as initiation age for screening, cutoff point for diagnosis, screening interval and prevention strategies from diabetes complications. Therefore it is difficult to aggregate data or to compare the ICER from different studies.
However the cost-effectiveness of diabetes screening and modification intervention varies by age at the time of screening. Current studies are uncertain on how the cost effectiveness of diabetes screening would change with the age at initiation. Some studies concluded the incremental costs per QALY increased with initial screening age (5,15), whereas others found that screening for diabetes may be more cost effective if it initiated at high ages like as 55, 65 years than 35(21).
All studies except one used the FPG as screening test (20). However some of them only used this test for screening and diagnosis without repetition (5,14), others used the extra tests as diagnosis test and this could rise the screening costs (15,21,22). Schaufler et al used the OGTT as screening test but they estimated lowest ICER in the studies. It seems diversity in screening and diagnosis tests are not very strong determinant in cost effectiveness of diabetes screening.
Mass screening, opportunistic and targeted screening is discussed in the studies. Chen et al concluded that incremental cost of opportunistic screening is higher than mass screening in both per LYGs and QALY gained. Their results showed mass screening with 5-year interval is more cost-effective than opportunistic screening when diabetes prevalence is between 6–12%. On the other hand Kahn found that opportunistic screening strategies at the time of visits had the lowest costs per QALY.
Chen and colleagues and Hoerger and colleagues assumed treatment and preventive intervention can control glycemic level and accordingly reduce micro and macro-vascular complications. They did not have alternative treatment options for comparison and include costs and effectiveness of preventive and treatment intervention in model based on previous studies. Lee and colleagues had three scenarios for preventive and treatment intervention effectiveness. They assume zero and 30% reduction in cardiovascular risk and 30% reduction in cardiovascular risk and 3% reduction in cost of routine care with preventive and treatment intervention. They concluded in third scenario we have cost saving. Kahn and colleagues included the lifestyle and metformin modification as preventive intervention in their model. They also had some other interventions in the next stage of diabetes development. But they did not report the separated ICER for every of them. Schuafler and colleagues compared the cost effectiveness ratio in metformin and lifestyle as diabetes preventive interventions. They estimated prevention with metformin is more cost effective than lifestyle. In contrast other studies found the diet and lifestyle interventions are more cost effective than pharmacological interventions (22,24). However, it is likely the models not taking full account of the benefits of the diet and lifestyle changes on cardiovascular risk factors and had underestimated benefit of lifestyle due to focus mainly on prevention of diabetes.
There are very few studies on cost effectiveness analysis of diabetes screening in developing countries, while more than 80% of people with diabetes live in low- and middle-income countries (25). In our review we found only one study for the Asian countries.
In this study we tried to review the cost effectiveness studies about the mass screening of type 2 diabetes. In conclusion, studies did not demonstrate that the mass screening for type2 diabetes is not cost effective. However some of them concluded that the ratio of cost effectiveness may be altered by some considerations such as age and screening interval.
There were some limitations in our study. Firstly, despite of abundant studies on cost effectiveness of diabetes prevention strategies, there were only a small number of papers on cost effectiveness of screening in type 2 diabetes. Secondly, the efficacy of global diabetes screening depend on many parameters including the screening and diagnosing tests or tools, the natural history of disease, and the follow up protocol of disease (15). On the other hand the expenditure of health care, devices and opportunity cost of human resource are varied by country. Thus, these results could not be generalized to the other settings. Thirdly, we include only English publication in our study so it means some publication in other languages may be missed. And finally there were several factors led to heterogeneity among the studies. Firstly, the studies did not describe the treatment process very well, but it seemed the process of treatment was not same in the studies. Secondly, some studies include the preventive interventions in their model while other neglected them. Thirdly, there is very diversity in screening and diagnostic process among the studies. Therefore, it is difficult to integrate the results and interpretation of should be made by caution.
Acknowledgment
This study was part of PhD. dissertation supported by Iran University of Medical Sciences (Grant NO: IUMS/SHMIS-778-2012).
Conflict of Interests
All authors declare that they have no significant competing interests.
Cite this article as: Najafi B, Farzadfar F, Ghaderi H, Hadian M. Cost effectiveness of type 2 diabetes screening: A systematic review. Med J Islam Repub Iran 2015 (13 February). Vol. 29:326.
References
- 1. Durand CP. A systematic review and meta-analysis of diabetes disease management programs [MSc Thesis] [Internet]. ProQuest; 2008. Available from: Texas Medical Center Dissertations (via ProQuest). /http://digitalcommons.library.tmc.edu/dissertations/AAI1454501.
- 2.Roglic G, Unwin N. Mortality attributable to diabetes: estimates for the year 2010Diabetes Res. Clin Pract Elsevier. 2010;87(1):15–19. doi: 10.1016/j.diabres.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Kiadaliri A, Najafi B. Obesity in type 2 diabetes mellitus: a review of health economics’ evidences. Int J Heal Insur Equity. 2013;1(1):2–13. [Google Scholar]
- 4.Lawrence J, Robinson A. Screening for diabetes in general practice. Prev Cardiol. 2003;6(2):78–84. doi: 10.1111/j.1520-037x.2003.01662.x. [DOI] [PubMed] [Google Scholar]
- 5. Kahn R, Alperin P, Eddy D, Borch-Johnsen K, Buse J, Feigelman J, et al. Age at initiation and frequency of screening to detect type 2 diabetes: a cost-effectiveness analysis. Lancet. 2010/04/02 ed. 2010;375(9723):1365–74. [DOI] [PubMed]
- 6.Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M. et al. Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M, et alGlobal, regional, and national age–sex specifi c all-cause and cause-specifi c mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013Lancet. Elsevier. 2015;385(9963):117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Internatinal Diabetes Federation. IDF Diabetes Atlas, sixth edition. Hallado en http//www.idf.org/diabetesatlas/6e. International Diabetes Federation; 2013;.
- 8. American Dibetes Association. Screening for type 2 diabetes. Diabetes Care 2003;26(SUPPL. 1): S21–S24. [DOI] [PubMed]
- 9.Brown J, Nicolas G, Glauber H, Bakst A. incremental medical care costs during the first 8 years after diagnosis. Diabetes Care. 1999;22:1116–24. doi: 10.2337/diacare.22.7.1116. [DOI] [PubMed] [Google Scholar]
- 10. International Diabetes Federation. Diabetes Atlas, fifth edition. Hallado en http//www.idf.org/diabetesatlas/5e/es/prologo.2013.
- 11.Li R, Zhang P, Barker LE, Chowdhury FM, Zhang X. Cost-effectiveness of interventions to prevent and control diabetes mellitus: A systematic review. Diabetes Care. 2010;33(8):1872–94. doi: 10.2337/dc10-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Diabetes Association. screening for type 2 diabetes. Diabetes Care 2004;27 (suppl (SUPPL. 1):s11–14. [DOI] [PubMed]
- 13.Calonge N, Petitti DB, DeWitt TG, Dietrich AJ, Gordis L, Gregory KD. et al. Screening for type 2 diabetes mellitus in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(11):846–54. doi: 10.7326/0003-4819-148-11-200806030-00007. [DOI] [PubMed] [Google Scholar]
- 14.Lee DS, Remington P, Madagame J, Blustein J. A cost analysis of community screening for diabetes in the central Wisconsin Medicare population (results from the MetaStar pilot project in Wausau) Wmj. 2000;99(3):39–43. [PubMed] [Google Scholar]
- 15. Chen THH, Yen MF, Tung TH. A computer simulation model for cost-effectiveness analysis of mass screening for Type 2 diabetes mellitus. Diabetes Res Clin Pract 2001;54(SUPPL. 1):S37–S42. [DOI] [PubMed]
- 16.O’Connor PJ, Rush WA, Cherney LM, Pronk NP. Screening for diabetes mellitus in high-risk patients: cost, yield, and acceptability. Eff Clin Pract. 2001;4(6):271–7. [PubMed] [Google Scholar]
- 17. canadian diabetes association. Canadian Diabetes Association 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada 2008;32(September):1–215.
- 18. Langer A. A framework for assessing Health Economic Evaluation ( HEE ) quality appraisal instruments. BMC Health Serv. Res. [Internet] 2012;12(253). Available from: http://www.biomedcentral.com/1472-6963/12/253. [DOI] [PMC free article] [PubMed]
- 19.Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJThe BMJ Economic Evaluation Working Party. BMJ Br. 1996;313(7052):275–83. doi: 10.1136/bmj.313.7052.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaufler TM, Wolff M. Cost effectiveness of preventive screening programmes for type 2 diabetes mellitus in Germany. Appl Heal Econ Heal Policy. 2010;8(3):191–202. doi: 10.2165/11532880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost-effectiveness analysis. Ann Intern Med. 2004;140(9):689–99. doi: 10.7326/0003-4819-140-9-200405040-00008. [DOI] [PubMed] [Google Scholar]
- 22.Gillies CL, Lambert PC, Abrams KR, Sutton AJ, Cooper NJ, Hsu RT. et al. Different strategies for screening and prevention of type 2 diabetes in adults: Cost effectiveness analysis. BMJ. 2008;336(7654):1180–4. doi: 10.1136/bmj.39545.585289.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC Diabetes. the cost effectiveness of screening for type 2 diabetes. JAMA. 1998;280:1757–63. [PubMed] [Google Scholar]
- 24.Vijgen SMC, Hoogendoorn M, Baan CA, De Wit GA, Limburg W, Feenstra TL. Cost effectiveness of preventive interventions in type 2 diabetes mellitus: A systematic literature review. Pharmacoeconomics. 2006;24(5):425–41. doi: 10.2165/00019053-200624050-00002. [DOI] [PubMed] [Google Scholar]
- 25. IDF. IDF Annual Report 2013. Annu. Rep. 2013: p. 14.