Summary
Over the last 5 years, the Chronic Kidney Disease in Children (CKiD) prospective cohort study has enrolled close to 600 children ages 1 to 16 years with mild to moderate chronic kidney disease (CKD). The main purpose of this interim report is to review the initial cross-sectional data and conclusions derived from the clinical studies conducted within CKiD in the context of findings from other pediatric CKD and end-stage renal disease (ESRD) registry and cohort studies. In particular, special emphasis was placed on studying four aspects of chronic kidney disease in children, including the identification of risk factors related to disease progression, the impact of CKD on neurocognition and quality of life (QoL), the cardiovascular morbidity associated with CKD, and identifying the causes and effects of growth failure in the context of mild to moderate kidney failure.
Introduction
Over the last several decades, several studies have reported on the demographic and clinical characteristics of children with CKD. Beginning in the 1990s, two large, prospective registries, The North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) database and the ItalKid Project, provided many important descriptions of the characteristics and comorbidities of children with CKD. These registries provided significant insight into underlying causes of CKD in children and rates of kidney function decline. Registry data are, however, limited by variations in measurement, frequently missing longitudinal data and the absence of direct measures of kidney function. In 2005, in response to a request for applications from the National Institutes of Health (NIH), the Chronic Kidney Disease in Children (CKiD) prospective cohort study was initiated with support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), in collaboration with the National Institute of Neurologic Disorders and Stroke (NINDS), the National Institute of Child Health and Human Development (NICHD) and the National Heart, Lung, and Blood Institute (NHLBI). The CKiD study began by prospectively enrolling children ages 1 to 16 years with chronic kidney disease (CKD) and an estimated GFR (eGFR) by the Schwartz formula (1) of 30 to 90 ml/min per 1.73 m2 from 48 clinical sites in the United States and Canada. The aims of the CKiD initiative were to (1) identify novel and traditional risk factors for the progression of CKD; (2) characterize the impact of a decline in kidney function on neurodevelopment, cognitive abilities, and behavior; (3) identify the prevalence and evolution of cardiovascular disease risk factors in children with CKD; and (4) examine the effects of declining GFR on somatic growth (2). To date, 22 studies have been published from data collected in CKiD, 15 of which address these four clinical domains. The remainder address methodological issues of measurement of kidney function (3–6) or methodologic issues related to the analysis of longitudinal data (7–9). The purpose of this interim review is to summarize these initial reports. Highlights of CKiD findings are presented in Table 1.
Table 1.
CKiD Highlights
| Summary Points |
|---|
|
CKiD, Chronic Kidney Disease in Children; CKD, chronic kidney disease; LVH, left ventricular hypertension.
CKiD offers several advantages over the prior registry reports. These advantages include systematically collected physical examinations, BP measurement and laboratory data, defined follow-up study visits, measured GFRs at study entry, one year later and every other year, systematic assessments of cognitive function and quality of life, ambulatory BP monitoring (ABPM) measurements, echocardiography, and in a subset of children, measures of carotid intimal thickness. The major strength of CKiD is in its systematic measurement and longitudinal follow-up.
Challenges in CKiD Cohort Study Design
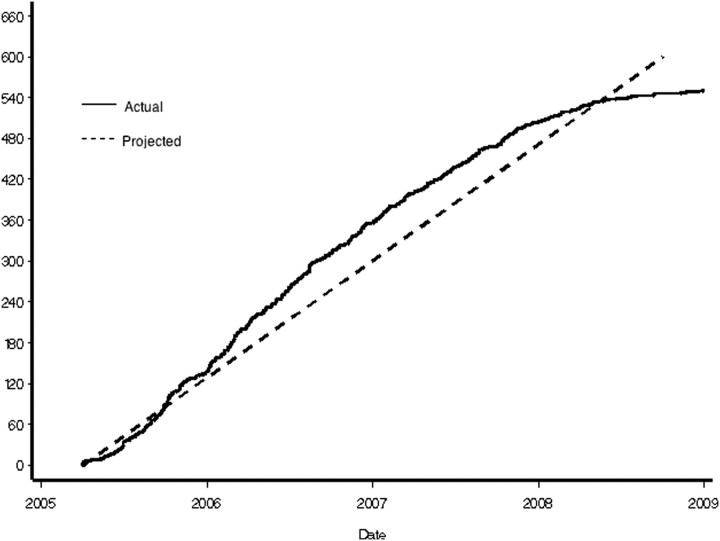
The primary scientific goal of the CKiD study is to determine risk factors for rapid decline of GFR, where one group is considered exposed and the other unexposed to a factor that is putatively associated with faster decline. In studying an uncommon disease, such as kidney disease in children, recruiting and retaining an adequate number of children to assess the association between putative risk factors and GFR decline is a challenge. At the study outset, to assess the power of the study to detect associations between putative risk factors and outcomes with a fixed sample size of 540 children, as outlined in the initial request for application from the NIH, we needed to estimate the average GFR decline and SD, estimates of the within-individual correlation of GFRs 1 year apart, loss to follow-up rates, and the rates of reaching renal replacement therapy in the first few years of the cohort study. We estimated that by the end of the follow-up period, we would have approximately 70% of our initial cohort of active participants with three to eight visits. All power calculations used log-transformed GFR and the observed average GFR decline in previously reported CKD studies, such as the Modification of Diet in Renal Disease and African American Study of Kidney Disease cohorts. Power estimates were based on methods for the calculation of power in longitudinal studies developed and published by the investigators at the data coordinating center (10). The putative exposures of interest in the CKiD cohort have included glomerular diagnosis (estimated at 20% of the cohort), urine protein to creatinine ratio >2 mg/mg (estimated at 15%), and systolic hypertension (20%), for example. In Table 2, we present relative risks of exposed to unexposed to be detected with 80% power at the 5% significance level for exposures with prevalence of 10%, 20%, and 40%, with a sample size of 540 participants. The projected and cumulative enrollment data are summarized in Figure 1.
Table 2.
Time to event analysis: Detectable relative incidence with 80% power
| Exposure Prevalence | Overall Incidence Per 100 Person-Yrs 5% |
|---|---|
| 10% | 2.04 |
| 20% | 1.77 |
| 40% | 1.64 |
Figure 1.
Cumulative enrollment curve.
As chronic kidney disease in children is uncommon, to recruit a large enough sample of children to ascertain the risk of a variety of exposures and accelerated progression, multicenter collaboration was imperative. The organizational structure of the cohort study was designed to facilitate recruitment at a large number of clinical sites across the United States and Canada. Two clinical coordinating centers (CCCs), a central biochemistry laboratory, the data coordinating center, and a representative from the NIDDK Division of Kidney, Urologic and Hematologic diseases led the steering committee. Two CCCs coordinate recruitment and retention at each of the participating clinical sites. The CCCs train data collectors, monitor quality control both centrally and locally, and communicate frequently and directly with recruiting sites to ensure timely follow-up visits. To increase the scientific output of the study, clinical site investigators participate in working groups and are encouraged to lead abstract and manuscript writing groups as well as to propose ancillary studies.
CKiD Findings to Date
Novel and Traditional Risk Factors for GFR Decline in Childhood CKD
In keeping with the first aim of the study, namely, to identify risk factors for accelerated GFR decline, Schwartz et al. (11) attempted to better classify and follow the progression of children with CKD by developing a new equation for estimating GFR. The original Schwartz formula was devised in the mid-1970s and was designed to estimate GFR in children based on serum creatinine, height, and an empirical constant (1). This formula is currently known to overestimate the true GFR, in part due to a shift in the laboratory creatinine assay from a colorometric reaction with alkaline picrate (Jaffe) to enzymatic methods (12). In an earlier attempt to correct for this discrepancy, Zappitelli et al. (13) derived a local coefficient for the Schwartz formula and dramatically improved the precision, bias, and sensitivity by reducing the constant from 0.55 to 0.47. At enrollment into CKiD, and using a measured GFR derived from the plasma disappearance of iohexol (iGFR) as the gold standard, a method previously reported but refined by the CKiD consortium in a pilot study (12), Schwartz et al. (11) estimated the GFR of 349 CKiD participants. By means of linear regression analyses, the following equation—the so-called CKiD equation—incorporating height (cm), gender, serum creatinine (mg/dl), cystatin C (mg/L), and blood urea nitrogen (mg/dl), was the most precise, the most accurate, and had the best goodness of fit:
eGFR = 39.1[height/Scr]0.516[1.8/cystatin C]0.294×[30/BUN]0.169[1.099]Male[height/1.4]0.188
This new formula yielded 87.7% of eGFR values within 30% of iGFR, and 45.6% within 10%, results similar to the Modification of Diet in Renal Disease (MDRD) equation commonly used in adults. Furthermore, an updated constant of 0.413 was derived as part of a simplified and clinically useful CKiD bedside equation, which yielded 79.4% of eGFR values within 30% of iGFR and 37% within 10%:
eGFR = 0.413[height]/Scr
A total of 168 participants had an iGFR measured 1 year after the baseline visit. The CKiD estimating equation performed well on follow-up with 83% of the eGFR values falling within 30% of iGFR and 41% within 10%. The CKiD bedside equation performed similarly well, with an absolute bias of <2 ml/min per 1.73 m2 and a correlation of 0.84. Three main characteristics of the CKiD cohort preclude prompt generalization of this formula to the general pediatric population. All CKiD patients had moderate CKD, and many had short stature (median height percentile of 22.8%) and evidence of delayed puberty. Recently, Staples et al. (14) attempted to validate the CKiD bedside equation in children with more normal kidney function, all of whom had a GFR measured by iothalamate clearance. The equation performed similarly well; however, the greatest degree of underestimation was in males (−9.2 ml/min per 1.73 m2), in children with a GFR greater than 90 ml/min/1.73m2 (−9.1 ml/min per 1.73 m2), and in children ages 14 to 16 years. These results suggest that the CKiD bedside equation may be most appropriate for children with mild to moderate CKD, and future studies will demonstrate whether or not it may be generalizable to all children.
Proteinuria.
Cross-sectional analyses of baseline data in CKiD have explored a number of known risk factors for GFR decline and have associated these with the level of GFR at study entry. Wong et al. (15) described the baseline distribution of proteinuria in 419 CKiD participants and identified the clinical characteristics associated with varying degrees of proteinuria. In all participants, the mean first morning urine protein:creatinine ratio (Up/c) was 0.53, with an interquartile range of 0.20 to 1.27. Twenty-four percent of the cohort had no proteinuria (Up/c <0.2), 62% had significant proteinuria (Up/c 0.2 to 2), and 14% had nephrotic range proteinuria (Up/c >2). Patients who had a glomerular disorder as the cause of CKD had Up/c levels on average 140% greater than those of nonglomerular patients. Non-Caucasian children had Up/c levels 40% higher than Caucasian children. In both glomerular and nonglomerular cases of CKD, the log-log relationship demonstrated that for every 10% reduction in iGFR, Up/c increased by 14%. In glomerular CKD patients, angiotensin converting enzyme (ACEi)/angiotensin II receptor blocker (ARB) usage was associated with a lower average Up/c levels (54% lower) and a lower prevalence of nephrotic range proteinuria (23% versus 67%) as compared with nonusers. Importantly, these same findings were not observed in patients with nonglomerular CKD. Data from the ItalKid project on children with nonglomerular causes of CKD had previously demonstrated similar findings. Children with Up/c levels <0.9 showed a slower decline of renal function and a higher rate of renal survival than those with baseline Up/c level >0.9 at 5 years (16). Furthermore, ACEi did not significantly delay the progressive decline in renal function in children with lower proteinuria compared with matched controls (17). In the ESCAPE (Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients) trial, ACEi reduced protein excretion by approximately 50% in all forms of nephropathy within the first 6 months in children with CKD (18,19). Longitudinal analyses of the risks of even low-level proteinuria on GFR decline in CKiD are ongoing.
Anemia.
CKiD studies have shown a high prevalence of anemia in moderate CKD, which increased among individuals with lower GFR, despite treatment, and a higher prevalence of anemia among African Americans with CKD. Fadrowski et al. (20) described the relationship between hemoglobin and iGFR in 340 CKiD participants. Above a GFR of 43 ml/min per 1.73 m2, relatively little decline of hemoglobin was seen, with a linear decline in hemoglobin below a threshold iGFR of 43 ml/min per 1.73 m2, independent of age, race, gender, and underlying diagnosis. The hemoglobin declined by 0.3 g/dl for every 5 ml/min per 1.73 m2 decrease in GFR below the 43 ml/min per 1.73 m2 threshold. Atkinson et al. (21) studied 429 CKiD participants to explore the effect of race on hemoglobin levels in children with CKD. Glomerular causes of CKD, lower GFR, lower body mass index, female gender, and prepubertal male gender were all independently associated with lower hemoglobin levels in Caucasian and non-Caucasian subjects. On average, a 20% decrease in GFR was associated with a decrease in hemoglobin level of 0.2 to 0.4 g/dl. A comparison of 338 Caucasian children with 91 African-American children showed that the mean hemoglobin levels tended to be 0.6 mg/dl lower in African-American children with similar anthropometric, socioeconomic, and clinical status characteristics. Erythropoiesis-stimulating agent use and iron supplementation did not differ by race. Interestingly, median hemoglobin levels did not differ between the two groups, suggesting that the lower hemoglobin levels might be explained by greater racial differences at the lower end of hemoglobin level distribution. Generalized gamma modeling confirmed that differences in hemoglobin levels become more pronounced when moving from high to low in the overall hemoglobin distribution level. Noteworthy, however, is the finding that the average racial differences in hemoglobin levels in children with early-stage CKD parallel observed differences in otherwise healthy children (22), whereas the racial disparity widens as the children become more anemic in the context of CKD. Recently, a retrospective review of the NAPRTCS CKD registry identified the prevalence of anemia among children with stage 3 CKD (23). Among 1640 patients, 73% had anemia. Similar to the CKiD report, eGFR and older age were associated with an increased risk for anemia; however, there was no increased risk in African-American or Hispanic children. Additionally, prescription of antihypertensive medications was associated with an increased risk for anemia in longitudinal analysis. This was not studied in the CKiD cohort.
Neurodevelopmental, Cognitive, and Behavioral Aspects of Childhood CKD
Quality of life.
A unique contribution of the CKiD study to the existing literature on childhood CKD is the description of patient- and parent-reported health outcomes. Only a handful of studies have directly assessed the Health Related Quality of Life (HRQoL) in children with CKD (24), and even fewer have studied children before end-stage renal failure (25–27). In keeping with the second aim of the CKiD study, Gerson et al. (28) studied 402 CKiD participants who had an iGFR, a known duration of kidney disease, and a completed Pediatric Inventory of Quality of Life Core Scale (PedsQL) at enrollment. The cross-sectional assessment found a statistically significant difference in the overall HRQoL of CKiD participants, as assessed by both the children and their parents, compared with a published normative sample. The results were consistent across the physical, social, emotional, and school function domains assessed by the PedsQL scale. The most marked differences when comparing CKiD results and normative data were in school functioning. Of interest, there was no significant relationship between the degree of renal dysfunction and the PedsQL scores. Children who had more long-standing CKD were observed by their parents to have better physical and emotional functioning as compared with children who had CKD for a shorter period of time. In addition, patients with CKD for a greater percentage of their lives also reported better physical functioning. Whether improved QoL scores were a reflection of the subset of children who had the mildest disease in this cross-sectional analysis is unclear and will be clarified by longitudinal analysis. Older children self-reported higher physical, emotional, social, and overall QoL compared with their younger peers; however, paradoxically, their parents reported worsening school QoL with age. Finally, short stature was associated with a lower parental perception of physical QoL.
Incontinence.
Dodson et al. (29) studied the specific effects of incontinence on HRQoL in 329 CKiD children using the same PedsQL scale. Using parental responses to questions about toilet training and bedwetting, they categorized children ages 5 to 12 years into three categories: toilet trained and not currently bedwetting (71.4%), previously toilet trained but currently bedwetting (23.1%), and not yet toilet trained (5.5%). Total PedsQL scores, as reported by both the children and their parents, were the lowest in the children who were not yet toilet trained, higher in those who were previously toilet trained but currently bedwetting, and highest in those previously toilet trained and not currently bedwetting. Subscale analysis of the PedsQL scores showed that the greater the degree of incontinence, the lower the physical and school HRQoL by self-report and physical health HRQoL by parental report.
Sleep and fatigue.
Roumelioti et al. (30) studied the prevalence of sleepiness and fatigue and their effects on HRQoL in 301 CKiD participants ages 8 years and older. Sleepiness and fatigue symptoms were measured by surveying individual items pertaining to sleep and fatigue from the PedsQL scale and a CKD-related symptoms list adapted from the Chronic Renal Insufficiency Cohort (CRIC) study. The PedsQL data showed that overall, 29% of CKiD participants reported trouble sleeping or low energy either “often” or “almost always” within 1 month before completing the questionnaire. Parental report of low energy was inversely associated with iGFR. Interestingly, patient's self-reports of low energy and both patient's and parent's reports of trouble sleeping were not significantly associated with iGFR. Similar to the HRQoL data published by Gerson et al. (28), children who had more long-standing CKD had a lower prevalence of low energy compared with those who had CKD <25% of their lifetimes.
According to the CKD-related symptoms list, the prevalence of moderate or severe symptoms of at least one measure of sleep problem or fatigue was 25%. Participants with an iGFR <30 ml/min per 1.73 m2 were almost four times more likely to report severe weakness than those with an iGFR greater or equal to 50 ml/min per 1.73 m2. Patients with an iGFR of 40 to 49 ml/min per 1.73 m2 were three times more likely to report problems of daytime somnolence than those with an iGFR ≥50 ml/min per 1.73 m2. Waking up early and decreased alertness was not significantly associated with iGFR. Notably, reports of low energy (PedsQL) and weakness (CKD-related symptoms list) were independently associated with decreased HRQoL.
Cardiovascular Disease in Childhood CKD
Hypertension.
In line with the third aim of the CKiD study, three reports identified the prevalence of hypertension, left ventricular hypertrophy (LVH), and dyslipidemia in children with CKD. Flynn et al. (31) described the baseline prevalence of hypertension, antihypertensive medication use, and the demographic and clinical characteristics of those children with uncontrolled hypertension in the CKiD cohort. Cross-sectional analysis of 432 children showed that 54% of the children had either systolic or diastolic BP ≥95th percentile or a history of hypertension plus current antihypertensive medication use. Thirty-seven percent of patients had a measured BP greater than or equal to the 90th percentile at enrollment, of whom 39% were not receiving antihypertensive treatment. Sixty-eight percent of patients with elevated systolic BP (>90th percentile) and 53% of patients with elevated diastolic BP (>90th percentile) were taking antihypertensive medications. Of those being treated for hypertension, 48% remained uncontrolled (BP greater than or equal to the 90th percentile). After adjusting for other confounding variables, African-American race, shorter duration of CKD, absence of antihypertensive medication use, and higher serum potassium level were independently associated with elevated BP. Whether individuals with higher BP required more ACEi/ARB usage resulting in higher potassium or those with higher potassium were less likely to receive ACEi/ARB is unclear. Uncontrolled BP in children receiving antihypertensive medications was independently associated with male gender, shorter kidney disease duration, and the absence of ACEi/ARB use. The authors concluded that hypertension in pediatric CKD is frequently undertreated and that ACEi/ARB may be the most effective treatment. These results are similar to reported prevalence data and risk factors for hypertension in the NAPRTCS reports (32). The risk associated with hypertension and benefits of treatment, particularly with ACE inhibitors, have recently been emphasized by the results of the ESCAPE trial. This 5-year follow-up study showed that children with CKD-associated hypertension and receiving treatment with ACEi and intensified BP control (target 24-hour mean ABPM <50th percentile) had delayed progression of renal decline as compared with those in the conventional BP target range (target 24-hour mean ABPM <50th to 90th percentile) regardless of their underlying renal disorder. Preliminary analyses of CKiD data, assessing the association between lower casual BP (<50th percentile for age, gender, and height) and improved renal outcomes have replicated the findings of ESCAPE (33).
Echocardiography and ambulatory blood pressure monitoring.
Mitsnefes et al. (34) studied 366 CKiD participants to delineate baseline echocardiographic and ABPM data in children with CKD. A confirmed diagnosis of systolic or diastolic hypertension, based on both an elevated casual BP reading and an abnormal ambulatory BP study (load greater than or equal to 25%), was present in 18% of the CKiD population. Notably, 38% of children had masked systolic or diastolic hypertension, based on a normal casual BP and an abnormal ABPM study. Among children with masked hypertension, 29% were not taking antihypertensive medications, compared with only 15% of confirmed hypertensive patients. Importantly, 71% of children with masked hypertension were being treated suboptimally with antihypertensive medications. Seventeen percent of all CKiD participants had LVH and 9% had concentric left ventricular remodeling. Significantly, there was no difference in left ventricular mass index based on iGFR. Multivariate analysis showed that confirmed hypertension, masked hypertension, lower hemoglobin, and female gender were independent predictors of LVH. LVH was more frequent in children with confirmed (34%) and masked (20%) systolic or diastolic hypertension than in children with normal BP (8%). The authors concluded that casual BP measurements alone do not accurately characterize the true prevalence of hypertension in children with CKD. Given masked hypertension's strong association with LVH, the authors recommended early ABPM and echocardiography as part of standard care in children with CKD.
Dyslipidemia.
Before CKiD, data on dyslipidemia in children with CKD had not previously been reported in large cohorts. Saland et al. (35) studied the baseline lipid profile characteristics of 391 CKiD participants. Forty-five percent of the children had at least one measure of dyslipidemia (elevated triglycerides, low HDL cholesterol, elevated non-HDL cholesterol) and 20% had combined dyslipidemia (two or more lipid abnormalities). Thirty-two percent had elevated triglycerides, 21% had low HDL-cholesterol, and 16% had high non-HDL cholesterol. Multivariate analysis showed that lower GFR and obesity were independently associated with elevated triglycerides, low HDL cholesterol, and high non-HDL cholesterol. Mild proteinuria (Up/c 0.2 to 2.0) was independently associated elevated triglycerides and low HDL cholesterol, while nephrotic range proteniuria (Up/c >2.0) was associated with elevated triglycerides and high non-HDL cholesterol. Lower GFR was even more strongly associated with combined dyslipidemia compared with overall dyslipidemia. Children with GFR <30 ml/min per 1.73 m2 were three times more likely to have dyslipidemia and nearly nine times more likely to have combined dyslipidemia than children with GFR >50 ml/min per 1.73 m2.
Growth in Childhood CKD
Birth history.
Consistent with the fourth aim, Greenbaum et al. (36) studied 426 CKiD participants to evaluate whether low birth weight (LBW; <2500 g), prematurity (<36 weeks), small for gestational age (SGA; <10th percentile for gestational age), or intensive care unit (ICU) admission at birth were associated with poor growth outcomes in children with CKD. High prevalences of LBW (17%), SGA (14%), prematurity (12%), and ICU delivery (40%) were observed in the CKiD cohort. Multivariate analysis showed that the current heights and weights were lower in those with a history of LBW or SGA as compared with those whose birth weight was >2500 g or the 10th percentile for their gestational age. Importantly, prematurity and a history of neonatal ICU admission were not significantly associated with a difference in current height or weight. Perhaps surprisingly, subanalysis revealed that the negative effect of SGA on weight was significantly worse in those with a glomerular diagnosis compared with those with nonglomerular causes of CKD. The authors hypothesized that an initial in utero event, which results in SGA status at birth, might also increase the subsequent risk of developing poor weight gain and glomerular diseases such as focal segmental glomerulosclerosis.
Conclusion
As the CKiD study continues to accumulate longitudinal data, CKiD investigators will focus on the determination and quantification of traditional and novel risk factors for CKD progression identified during the key period spanning the early decline in renal function (i.e., GFR 30 to 90 ml/min/1.73m2) to the development of ESRD. By concurrently collecting data on growth, neurocognitive deficits, and cardiovascular risk factors using standardized criteria, the study will elucidate the sequence of associations between CKD progression and the development of growth abnormalities and neurologic and cardiovascular comorbidities. Understanding the epidemiology and evolution of kidney disease and its sequelae in childhood will provide insight for targeting intervention strategies designed to prevent or ameliorate the frequently observed adverse outcomes.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 2. Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) Prospective Cohort Study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwartz GJ, Furth SL: Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol 22: 1839–1848, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Abraham AG, Schwartz GJ, Furth SL, Warady BA, Muñoz A: Longitudinal formulas to estimate glomerular filtration rate in children with CKD. Clin J Am Soc Nephrol 4: 1724–1730, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schwartz GJ, Abraham AG, Furth SL, Warady BA, Muñoz A: Optimizing iohexol plasma disappearance curves to measure the glomerular filtration rate in children with chronic kidney disease. Kidney Int 77: 65–71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Checkley W, Brower RG, Muñoz A: Inference for mutually exclusive competing events through a mixture of generalized gamma distributions. Epidemiology 21: 557–565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ng DK, Schwartz GJ, Jacobson LP, Palella FJ, Margolick JB, Warady BA, Furth SL, Muñoz A: GFR Estimation for diverse populations based on only two time points of plasma disappearance curves. Kidney Int. 2011. June 8 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pierce CB, Cox C, Saland JM, Furth SL, Muñoz A: Methods for characterizing differences in longitudinal GFR changes between children with glomerular and non-glomerular chronic kidney disease. Am J Epidemiol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abraham AG, Muñoz A, Furth SL, Warady BA, Schwartz GJ: Extracellular volume and disease progression in children with chronic kidney disease. Clin J Am Soc Nephrol 6: 741–747, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirby AJ, Galai N, Muñoz A: Sample size estimation using repeated measurements on biomarkers as outcomes. Control Clin Trials 15: 165–172, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Schwartz GJ, Muñoz A, Schneiderb MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwartz GJ, Furth S, Cole SR, Warady B, Mñnoz A: Glomerular filtrate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Zappitelli M, Parvex P, Joseph L, Paradis G, Grey V, Lau S, Bell M: Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kid Dis 48: 221–230, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Staples A, Leblond R, Watkins S, Wong C, Brandt J: Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol 25: 2321–2326, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Wong CS, Pierce CB, Cole SR, Warady BA, Mak RH, Benador NM, Kaskel F, Furth SL, Schwartz GJ: Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in chronic kidney disease in children study. Clin J Am Soc Nephrol 4: 812–819, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ardissino G, Testa S, Dacco, et al. : Proteinuria as a predictor of disease progression in children with hypoplastic nephropathy. Pediatr Nephrol 19: 172–177, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Ardissino G, Viganò S, Testa S, Daccò V, Paglialonga F, Leoni A, Belingheri M, Avolio L, Ciofani A, Claris-Appiani A, Cusi D, Edefonti A, Ammenti A, Cecconi M, Fede C, Ghio L, La Manna A, Maringhini S, Papalia T, Pela I, Pisanello L, Ratsch IM; ItalKid Project: No clear evidence of ACEi efficacy on the progression of chronic kidney disease in children with hypodysplastic nephropathy–report from the ItalKid Project database. Nephrol Dial Transplant 22: 2525–2530, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Wuhl E, Mehls O, Schaefer F, and the ESCAPE Trial Group: Antihypertensive and antiproteinuric efficacy of ramipril in children with chronic renal failure. Kidney International 66: 768–776, 2004 [DOI] [PubMed] [Google Scholar]
- 19. The ESCAPE Trial Group: Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Fadrowski JJ, Pierce CB, Cole SR, Moxey-Mims M, Warady BA, Furth SL: Hemoglobin decline in children with chronic kidney disease: Baseline results from the chronic kidney disease in children prospective cohort study. Clin J Am Soc Nephrol 3: 457–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Atkinson MA, Pierce CB, Zack RM, Barletta GM, Yadin O, Mentser M, Warady BA, Furth SL: Hemoglobin differences by race in children with CKD. Am J Kid Dis 55: 1009–1017, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robins EB, Blum S: Hematologic reference values for African American children and adolescents. Am J Hematol 82: 611–614, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Atkinson MA, Martz K, Warady BA, Neu AM: Risk for anemia in pediatric chronic kidney disease patients: a report of NAPRTCS. Pediatr Nephrol 25: 1699–1706, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Gerson AC, Butler R, Moxey-Mims M, Wentz A, Shinnar S, Lande MB, Mendley SR, Warady BA, Furth SL, Hooper SR: Neurocognitive outcomes in children with chronic kidney disease: Current findings and contemporary endeavors. MRDD Research Reviews 12: 208–215, 2006 [DOI] [PubMed] [Google Scholar]
- 25. McKenna AM, Keating LE, Vigneux A, Stevens S, Williams A, Geary DF: Quality of life in children with chronic kidney disease: Patient and caregiver assessments. Nephrol Dial Transplant 21: 1899–1905, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Fadrowski J, Cole SR, Hwang W, Fiorenza J, Weiss RA, Gerson A, Furth SL: Changes in physical and psychosocial functioning among adolescents with chronic kidney disease. Pediatr Nephrol 21: 394–399, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Gerson AC, Riley A, Fivush BA, Pham N, Fiorenza J, Robertson J, Chandra M, Trachtman H, Weiss R, Furth SL; Council on Pediatric Nephrology and Urology of New York/New Jersey; Kidney and Urology Foundation of America: Assessing health status and health care utilization in adolescents with chronic kidney disease. J Am Soc Nephrol 16: 1427–1432, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Gerson AA, Wentz MA, Abraham AG, Mendley SR, Hooper SR, Butler RW, Gipson DS, Lande MB, Shinnar S, Moxey-Mims M, Warady BA, Furth SL: Health-related quality of life of children with mild to moderate chronic kidney disease. Pediatrics 125: e349–e357, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dodson JL, Cohn SE, Cox C, Hmiel PS, Wood E, Mattoo TK, Warady BA, Furth SL: Urinary incontinence in the CKiD cohort and health related quality of life. J Urology 182: 2007–2014, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roumelioti ME, Wentz A, Schneider MF, Gerson AC, Hooper S, Benfield M, Warady BA, Furth SL, Unruh ML: Sleep and fatigue symptoms in children and adolescents with CKD: A cross-sectional analysis from the Chronic Kidney Disease in Children (CKiD). Am J Kidney Dis 55: 269–280, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh SR, Furth SL, Warady BA: Blood pressure in children with chronic kidney disease. A report from the Chronic Kidney Disease in Children Study. Hypertension 52: 631–637, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mitsnefes M, Ho PL, McEnrey PT: Hypertension and progression of chronic renal insufficiency in children: A report of the North American Renal Transplant Cooperative Study (NAPRTCS). J Am Soc Nephrol 14: 2618–2622, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Furth SL, Flynn JT, Pierce CB, Mitsnefes M, Wong CS, Saland JM, Moxey-Mims MM, Abraham AG, Warady BA: Lower systolic BP associated with slower CKD progression in the CKiD study. [Poster]. ASN Renal Week 2010, November 19, 2010, Denver, Colorado [Google Scholar]
- 34. Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansem T, Saland J, Kimball T, Furth SL, Warady BA: Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol 21: 137–144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saland JM, Pierce CB, Mintsnefes MM, Flynn JT, Goebel J, Kupferman JC, Warady BA, Furth SL: Dyslipidemia in children with chronic kidney disease. Kidney Int 78: 1154–1163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greenbaum LA, Muñoz A, Schneider MF, Kaskel FJ, Ashkenazi DJ, Jenkins R, Hitchkiss H, Moxey-Mims M, Furth SL, Warady BA: The association between abnormal birth history and growth in children with CKD. Clin J Am Soc Nephrol 6: 14–21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]