Abstract
Background: Intravenous immunoglobulin (IVIG) is an established treatment of immune mediated demyelinating neuropathy including Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy. Recent trials suggest its efficacy in treating relapsing- remitting multiple sclerosis. IVIG exerts a number of effects, which may be beneficial in treating multiple sclerosis (MS): Reduction of inflammation, inhibition of macrophages, and promotion of remyelination. The aim of this study was to provide an overall assessment of the existing trials of safety and effectiveness of IVIG in relapsing- remitting MS compared to other drugs currently available for the treatment of disease activity in MS.
Methods: A systematic search strategy was applied to MEDLINE (PubMed and Ovid Medline (1990- Nov 2014)), Cochrane Library 2014, and Trip Database 2014, CRD. The reference lists from the identified trials, MS clinical handbooks and guidelines for the use of IVIG were studied. This article was conducted without language restrictions. Randomized controlled trials of IVIG in MS were selected. Sixteen double-blinded trails were randomly selected. Ten trials were excluded and we performed a meta-analysis on the six trials (537 participants) of IVIG in comparison to placebo. The methodological quality of the trials was assessed using Jadad checklist.
Results: The meta-analysis showed a significant beneficial effect on proportion of relapse-free patients (OR: 1.693; 95% CI-1.205-2.380), on the proportion of patients who improved (OR:2.977; 95% CI 1.769-5.010; p=0.0001) and deteriorated (OR:0.522; 95% CI0.330-0.827; p=0.006) between placebo and IVIG-treated patients. In addition, there was a reduction in the annual relapse rate in the IVIG group compared to placebo, which was statistically significant (SMD=-0.218; 95% CI-0.412 to -0.024; p=0.028). The results of the meta-analysis did not show significant differences between Expanded Disability Status Scale (EDSS) changes from baseline (SMD,-0.025; 95% CI,-0.211 to 0.161; p=0.860).
Conclusion: IVIG can be considered as an alternative therapeutic option, second-line therapy or adjuvant therapy, considering its beneficial effects (high tolerance, need to be injected with longer intervals, etc.) for treating relapsing–remitting MS patients.
Keywords: Multiple sclerosis, Relapsing–remitting Multiple Sclerosis, Intravenous Immunoglobulin, Meta-analysis
Introduction
Multiple sclerosis (MS) is a chronic and debilitating, progressive inflammatory and degenerative disease of the central nervous system (CNS) which affects the brain and spinal cord, in which infiltrating lymphocytes, predominantly T-cells and macrophages lead to damage of the myelin sheath (1-5).
The cause and pathogenesis of MS remain to be unknown (6,7), although it is thought to be autoimmune in nature. A variety of genetic, immunological, and environmental factors have been implicated in triggering the onset and progression of the disease, which is characterized by immune- mediated tissue injury directed against CNS myelin antigens(8).
MS is one of the most common contributors to neurological disability among young and middle-aged adults. The peak age of the onset is approximately 30 years, and the disease occurs in twice as many women as men (2,8).
The estimated number of people with MS has increased from 2.1 million in 2008 to 2.3 million in 2013. The MS prevalence rate in Tehran, capital city of Iran, is estimated to be 51.9 per 100,000 population in 2010; and in Isfahan, one of the large cities of Iran, is estimated to be 43.8 per 100,000 population in 2007 (10).
Intravenous immunoglobulin (IVIG) is a blood product, which is prepared from pools of plasma of at least three thousand and up to a hundred thousand healthy blood donors. Initially used at a ‘‘replacement dose’’ for patients who have antibody deficiencies, IVIG is now increasingly being used for the treatment of autoimmune and systemic inflammatory diseases as an immunomodulatory agent (11).
IVIG exerts may have a number of positive effects on multiple sclerosis (MS): Reduction of inflammation, inhibition of macrophages and promotion of remyelination, and increased number of activated pro-inflammatory T-cells (Th1); a reduced number of regulatory T-cells (Th2), and an increased number of B-cells produce IVIG antibodies against myelin proteins (12,13).
A number of trails have indicated effects on MS (Sorensen et al., 1998; Lewanska et al., 2002,….). It is, therefore, important to establish the efficacy of intravenous immunoglobulin treatment to ensure that people with MS are not exposed to a potentially harmful drug without good evidence of efficacy, and that resources are not unnecessarily devoted to an unproven therapy.
Objective
We conducted a meta-analysis of the trials in order to provide an overall evaluation of the safety and effectiveness of IVIG in relapsing–remitting MS compared to other drugs currently available for the treatment of disease activity in MS.
Methods
Criteria for Considering the Studies for Meta-analysis
Types of Studies: We searched randomized controlled trials, which assessed the safety, efficacy and effectiveness of intravenous immunoglobulins for MS.
Types of Participants: Trial participants were diagnosed with definite MS(according to the McDonald diagnostic criteria)(4). Participants may have had relapsing- remitting MS.
Types of Interventions: We limited this meta-analysis to trials of intravenous immunoglobulins for relapsing- remitting MS.
Types of Outcome Measures:
Progression of the disease was evaluated using the Expanded Disability Status Scale (EDSS).
1) The proportion of patients remaining relapse- free at the end of the treatment period
2) Annualized relapse rate, relapse rate, expressed as the number of relapses per annum in each treatment arm
3) The proportion of patients improved in EDSS
4) The proportion of patients deteriorated in EDSS.
Search Methods to Identify the Studies
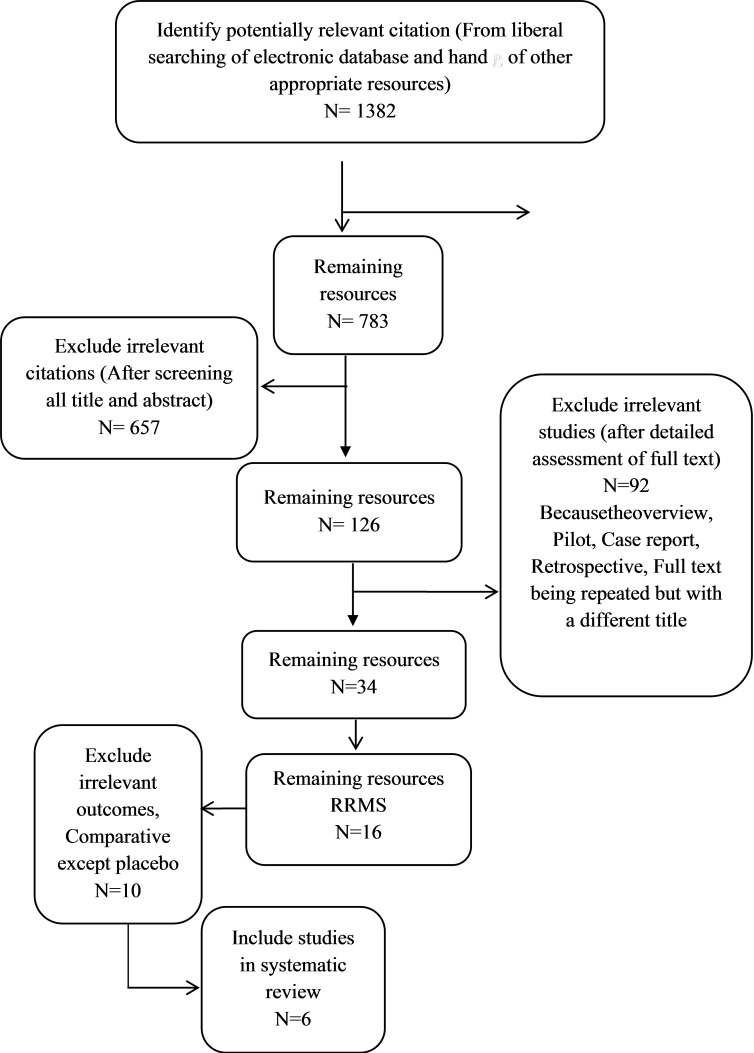
Electronic Searches: A systematic search was conducted (From 1990 to2014, which was updatedin2015/6) to identify all relevant published and unpublished randomized controlled trials (Fig. 5).
Fig. 5 .
The Process of Identifying Related Articles
For this review, the following databases were searched: MEDLINE (PubMed and Ovid Medline), Cochrane library, CRD, Trip Database, FDA, Google scholar, and NAHTA (Table 7).
Table 7 . Ovid MEDLINE(R) 1946 to June Week 2 2014 .
| 39 | 37 or 38 | 18 |
| 38 | 7 and 12 and 32 | 12 |
| 37 | 6 and 11 and 31 | 18 |
| 36 | limit 35 to humans | 106 |
| 35 | 34 or 33 | 108 |
| 34 | 7 and 12 and 20 | 89 |
| 33 | 6 and 11 and 19 | 108 |
| 32 | or/23-30 | 117585 |
| 31 | or/21-29 | 131483 |
| 30 | (Meta Analysis or Systematic Review or Biomedical Technology Assessment or Economic Evaluation).sh. [Embaseterms] | 49981 |
| 29 | (meta regression$ or metaregression$ or mega regression$).ti,ab. | 2431 |
| 28 | (meta analy$ or metaanaly$ or met analy$ or metanaly$ or health technology assessment$ or HTA or HTAs or biomedical technology assessment$ or bio-medical technology assessment$).ti,ab. | 61061 |
| 27 | (handsearch$ or hand search$).ti,ab. | 5121 |
| 26 | (data synthes$ or data extraction$ or data abstraction$).ti,ab. | 12169 |
| 25 | ((integrative adj2 (review$ or overview$)) or (collaborative adj (review$ or overview$)) or pool$ analy$).ti,ab. | 5368 |
| 24 | ((quantitative adj (review$ or overview$ or synthes$)) or (research adj (integration$ or overview$))).ti,ab. | 936 |
| 23 | ((systematic$ adj (literature review$ or review$ or overview$)) or (methodologic$ adj (literature review$ or review$ or overview$))).ti,ab. | 49971 |
| 22 | Meta-Analysis as Topic.sh. or exp Technology Assessment, Biomedical/ [Medlineterms] | 23081 |
| 21 | Meta-Analysis.pt. [Medlineterm] | 49981 |
| 20 | or/15-18 | 950487 |
| 19 | or/13-17 | 1220916 |
| 18 | *Major Clinical Study/ or *Multicenter Study/) or exp Controlled Clinical Trial/ [Embaseterms]) | 88875 |
| 17 | (control$ adj2 (study or studies or trial$)).ti,ab. | 263773 |
| 16 | ((tripl$ adj (blind$ or dumm$ or mask$)) or (trebl$ adj (blind$ or dumm$ or mask$))).ti,ab. | 339 |
| 15 | (random$ or rct$ or sham$ or placebo$ or (singl$ adj (blind$ or dumm$ or mask$)) or (doubl$ adj (blind$ or dumm$ or mask$))).ti,ab. | 786960 |
| 14 | (Multicenter Study or Randomized Controlled Trial or Controlled Clinical Trial).pt. [Medlineterms] | 577370 |
| 13 | exp Controlled Clinical Trials as Topic/ or Double-Blind Method.sh. or Random Allocation.sh. or Single-Blind Method.sh. or Multicenter Studies as Topic.sh. [Medlineterms] | 313388 |
| 12 | or/9-10 | 218492 |
| 11 | or/8-9 | 218492 |
| 10 | multiple sclerosis".sh. [Embaseterm]" | 39830 |
| 9 | (sclerosis Multiple $ or multiple sclerosis$ or MS).ti,ab. | 212879 |
| 8 | multiple sclerosis".sh. [Medlineterm]" | 39830 |
| 7 | or/2-5 | 13515 |
| 6 | or/1-4 | 16941 |
| 5 | Immunoglobulin".sh. [Embaseterm] | 0 |
| 4 | (alphaglobin$ or baygam$ or endobulin$ or gamagard$ or gamimmune$ or gamimune$ or gamunex$ or gammimune$ or gammimmune$ or gam?agard$ or gam?aguard$ or gammaglobulin$ or gammonativ$ or (globulin adj n) or igivnex$ or intraglobin$ or intraglobulin$ or iveegam$ or octagam$ or polygam$ or sandoglobulin$ or venimmune$ or venoglobulin$).ti,ab. | 2936 |
| 3 | (ivig or igiv or igv or ivigg or ivgg).ti,ab. | 5208 |
| 2 | ((intravenous$ adj (antibod$ or gammaglobulin$ or gamma globulin$ or immunoglobulin? or immune globulin?)) or iv immunoglobulin? or intravenous ig or modified immune globulin?).ti,ab. | 9879 |
| 1 | Immunoglobulins Intravenous".sh. [Medlineterm]" | 9826 |
Searching other Resources: To find more resources and studies that may have not been found in the electronic search, we searched the reference lists of the identified trials. Moreover, the clinical handbooks of Multiple sclerosis and guidelines for the use of intravenous immunoglobulin were searched.
Data Collection and Analysis
From 748 sources of the initial search, we identified 16 trials. To be included in the review, a trial had to meet the following criteria: (1) Allocation concealment had to be adequate (Unclear allocation concealment was accepted if every other methodological factor was of the best possible standard); (2) Treated and non-treated groups had adequate baseline comparability; (3) Patients and the treating physicians were blind to the treatment allocation; (4) All analyses had to be intended- to- treat analyses.
We excluded 10 trials: Five trials differed in the type of drug used in the control group, and three trials did not measure the desired outcomes, one trial was conducted only on pregnant women, and one trial did not clearly specify the target group. We performed a quality evaluation of evidence for trails in this review by Cochrane Reviewer’s Handbook, which discusses the methodological quality under the following headings: (1) Selection bias; (2) Performance bias; (3) Attrition bias; and (4) Detection bias. In addition, we performed a quality evaluation of evidence for trials included in this review by Jadad checklist.
For the quantitative outcomes of EDSS score and annual relapse rate, the combination of results was based on the use of the effect size (SMD), Δ/σ, where Δ is the difference in the mean results for IVIG and placebo and σ is the common (population) standard deviation (SD). These quantities are estimated for each study by the difference in the mean scores and the pooled estimated SD, σ. Note that the ratio of these estimates is biased and a bias adjustment as described by Begg's test was considered in the analysis. These results were pooled together using weights based on the sample size for each trial and study preparation, using basic techniques described by Begg's test. However, before pooling all of the results, a chi-square test for homogeneity was conducted. If this test was significant, an attempt was made to eliminate the result(s) that may have caused the heterogeneity, and the remaining findings were then combined.
For the qualitative outcomes of relapse-free, improved and deterioration rates, effect size is simply the difference in the proportions between the active and placebo groups. These values are weighted based on the difference in proportions. A test of homogeneity of results was also used following a simple modification of the chi-square procedure mentioned above. In addition, odds ratios were computed for each study and overall, 95% confidence interval was assumed. These calculations were implemented using Stata / SE 11.
Results
Patients and Study Design
We identified six published studies, which met our inclusion criteria (Fazekas et al., 1997; Achiron et al., 1998; Strasser et al., 2000; Lewanska et al., 2002; Kocer et al., 2004; Fazekas et al., 2008). Also, we considered a meta-analysis by Sorensen et al., (2002) which included four trials, and a meta-analysis by Gray et al., (2009) which included 10 randomized trials of intravenous immunoglobulins for the treatment of MS (4,14). Thus, we performed a more comprehensive meta-analysis.
In the six included trials, 537 patients were enrolled. Patient characteristics, IVIG dosage, and trial duration, etc. are presented in Table 1. The dosage of IVIG varied considerably from 0.15 to 0.2g/kg bodyweight monthly in the study by Fazekas et al. (1997) to 0.4 g/kg bodyweight monthly in the study by Fazekas et al. (2008) and Lewanska et al. (2002). All the six trials had used a parallel-group design.
Table 1 . Design, Patient Characteristics, IVIG Doses and Trial Duration .
| RCT Study | Design | N |
MS duration (years) |
Mean age (years) |
EDSS |
Trial duration |
Dose (months) |
|
Fazekas et al. (1997) |
PG | IVIG:75 Placebo:73 |
IVIG:6.8 Placebo:7.3 |
IVIG:36.7±2.4 Placebo:37.2±2.3 |
IVIG:3.3 Placebo:3.3 |
24 | 0.15–0.2g/kg |
|
Achiron et al. (1998) |
PG | IVIG:20 Placebo:20 |
IVIG:4.1 Placebo:3.95 |
IVIG:35.4±2.1 Placebo:33.8±2.4 | IVIG:2.9 Placebo:2.82 | 24 | 0.4g/kg |
|
Strasser et al. (2000) |
PG | IVIG:75 Placebo:75 | IVIG:6.8±4.6 Placebo:7.3±5.7 | IVIG:36.7±10.4 Placebo:37.3±9.8 | IVIG:3.33±1.39 Placebo:3.37±1.67 | 24 | 0.2g/kg |
|
Lewanska et al. (2002) |
PG | IVIG:17 IVIG:15 Placebo:17 | IVIG:10.7±7.57 IVIG:7.2±5.48 Placebo:7.5±4.7 | IVIG:38±6.96 IVIG:31.1±6.08 Placebo:41.8±6.98 | IVIG:3.0±1.35 IVIG:3.0±2.08 Placebo:2.97±1.58 | 12 |
0.2g/kg 0.4g/kg |
|
Kocer et al. (2004) |
PG | IVIG:12 Placebo:12 | IVIG:2-14 Placebo:2-14 | IVIG:22-56 Placebo:22-39 | IVIG:2.46±1.82 Placebo:1.96±1.60 | 9 | 0.4g/kg |
|
Fazekas et al. (2008) |
PG | IVIG:44 IVIG:42 Placebo:41 | IVIG:2.8±1.9 IVIG:2.7±2.1 Placebo:2.3±1.5 | IVIG:31.9±7.5 IVIG:34.4±7.9 Placebo:33.0±8.7 | IVIG:1.8±0.9 IVIG:2.1±1.1 Placebo:2.1±1.2 | 12 |
0.2g/kg 0.4g/kg |
PG= parallel groups; IVIG= intravenous immunoglobulin; EDSS= Expanded Disability Status Scale; MS= multiple sclerosis
Description of Studies
In the study by Fazekas et al. (1997), the primary outcome measures were the change in EDSS and the proportion of patients who improved, remained stable or worsened in disability, defined as an increase or decrease of at least 1.0 point in the EDSS score by the end of the study. Secondary outcome measures included the annual relapse rate and the proportion of relapse-free patients (15).
In the Achiron et al. (1998) study, they used the annual relapse rate, proportion of relapse-free patients and median time to first relapse as the primary study endpoints. The changes in neurological disability measured on the EDSS scale, mean annual severity of exacerbations and mean MRI lesion score were used as the secondary outcome endpoint. Alloutcomes were measured at baseline and after 1 and 2 years (16).
In the study of Strasser et al. (2000), the change in EDSS and the proportion of patients who improved, remained stable or worsened in disability were the primary outcomes measured. The secondary endpoints were the number of confirmed relapses, the annual relapse rate, time to first relapse during the study period and proportion of relapse-free patients (17).
In the study of Lewanska et al. (2002), two groups were treated with two doses of IVIG 0.4 g/kg and 0.2 g/kg, and one group with placebo. The annual relapse rate and a comparison between pre-study relapse rate and relapse rate during the study period were considered as primary outcomes. The secondary clinical outcomes included the proportion of relapse-free patients, mean changes in the EDSS, and the proportion of patients with worsening, mean changes in the NRSS (Neurological Rating Scale Score), change of mean lesion volume on T2-weighted, the mean number of new lesions on T2-weighted and the mean number of gadolinium-enhancing lesions on T1–weighted scans every 3 months (18).
In the study of Kocer et al. (2004), endpoints measured included the proportion of patients who improved, remained stable or deteriorated of clinical disability, changes in the EDSS, the number and volume of lesions on MRI in all sites (19).
In the Fazekaset al. (2008) study, they used the proportion of relapse-free patients and annualized relapse rate as the primary efficacy endpoint. Secondary outcome measures included change in burden of disease volume, change in brain parenchymal fraction, ratio and cumulative number of unique newly active lesions (20).
Progression of Disease (Disability)
The five trials enrolling patients had not showed significant differences between EDSS from baseline (SMD,-0.025; 95% CI, -0.211 to 0.161; p= 0.860; Table 2, Fig. 1), Thus, the trials were comparable in this respect. There were no evidence of heterogeneity among the studies (p= 0.860, χ2= 2.57, I2=0.0%).
Table 2 . EDSS in Baseline (Mean ± SD) .
| Study | SMD | [95% Conf. Interval] | % Weight | |
| Achiron (1998) | 0.199 | -0.422 | 0.821 | 8.95 |
| Strasser (2000) | -0.026 | -0.348 | 0.296 | 33.30 |
| Lewanska (2002) 0.4g/kg | 0.016 | -0.678 | 0.711 | 7.17 |
| Lewanska (2002) 0.2g/kg | 0.041 | -0.632 | 0.713 | 7.65 |
| Koçer (2004) | 0.292 | -0.513 | 1.097 | 5.34 |
| Fazekas (2008) 0.4g/kg | 0.000 | -0.430 | 0.430 | 18.68 |
| Fazekas (2008) 0.2g/kg | -0.284 | -0.712 | 0.143 | 18.91 |
| I-V pooled SMD | -0.025 | -0.211 | 0.161 | p=0.860 |
SMD= Standardized mean difference; IVIG=Intravenous immunoglobulin; EDSS= Expanded Disability Status Scale
Fig .1 .
EDSS in Baseline (Mean ± SD)
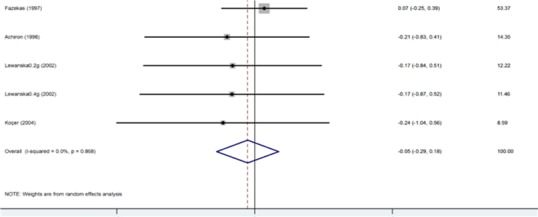
Four trials showed a trend towards a reduction in EDSS score during the IVIG treatment when compared to the baseline. The decrease in EDSS score was defined as an effect size (SMD) -0.052 with a 95% confidence interval from -0.288 to 0.183 (p= 0.663), and the difference was not statistically significant (Table 3, Fig. 2). There was evidence of heterogeneity among the studies although it was not statistically significant.
Table 3 . Change in EDSS (Mean ± SD) .
| Study | SMD | [95% Conf. Interval] | % Weight | |
| Fazekas (1997) | 0.073 | -0.250 | 0.395 | 53.37 |
| Achiron (1998) | -0.209 | -0.831 | 0.412 | 14.35 |
| Lewanska (2002) 0.4g/kg | -0.168 | -0.842 | 0.505 | 12.22 |
| Lewanska (2002) 0.2g/kg | -0.172 | -0.868 | 0.523 | 11.46 |
| Koçer (2004) | -0.240 | -1.044 | 0.563 | 8.59 |
| I-V pooled SMD | -0.052 | -0.288 | 0.183 | P=0.633 |
SMD= Standardized mean difference; IVIG=Intravenous immunoglobulin; EDSS= Expanded Disability Status Scale
Fig. 2 .
Change in EDSS (Mean ± SD)
Four studies reported the proportion of patients who improved, and five studies provided an analysis of the proportion of patients who deteriorated. Fazekas et al. (1997)and Achiron et al. (1998) defined improvement and deterioration by a change of one point or more in the EDSS at the end of the study; Strasser et al. (2000) changed at least 1.0 EDSS grade during the trail and Kocer et al. (2004) did not determine the definition improvement. The meta-analysis revealed a significant difference in the proportion of patients who improved on IVIG compared to placebo treatment. A test for heterogeneity across the studies was significant for this variable (p= 0.0001). Also, the meta-analysis revealed a strong trend in the proportion that deteriorated between placebo and IVIG-treated patients (Table 4). However, there was a large degree of heterogeneity in these results (p=0.009).
Table 4 . Proportion of Patients with Improvement or Deterioration in EDSS .
| Study | IVIG | Placebo | OR (95%CI) | % Weight | |||
| Improved | |||||||
| Fazekas (1997) | 0.31 | 0.14 | 2.787 (1.217-6.379) | 42.20 | |||
| Achiron (1998) | 0.24 | 0.11 | 3.000 (0.507-17.740) | 9.43 | |||
| Strasser (2000) | 0.34 | 0.14 | 3.343 (1.473-5.585) | 46.65 | |||
|
Koçer (2004) M-H pooled OR |
0.25 | 0.083 | 3.667 (0.323-41.590) | 4.72 | |||
| Deteriorated | 3.080 (1.769-5.282) | ||||||
| Fazekas et al. (1997) | 0.16 | 0.23 | 0.627 (0.276-1.427) | 30.31 | |||
| Achiron et al. (1998) | 0.14 | 0.17 | 0.706 (0.136-3.658) | 7.12 | |||
| Strasser et al.(2000) | 0.18 | 0.23 | 0.756 (0.333-0.857) | 29.35 | |||
| Lewanska et al. (2002) 0.2g/kg | 0.24 | 0.47 | 0.346 (0.080-1.507) | 12.81 | |||
| Lewanska et al. (2002) 0.4g/kg | 0.07 | 0.47 | 0.080 (0.009-0.756) | 14.66 | |||
| Kocer (2004) | 0.08 | 0.25 | 0.273 (0.024-3.093) | 5.76 | |||
| M-H pooledOR | 0.534 (0.333-0.857) | ||||||
IVIG= Intravenous immunoglobulin; EDSS=Expanded Disability Status Scale; OR= odds ratio
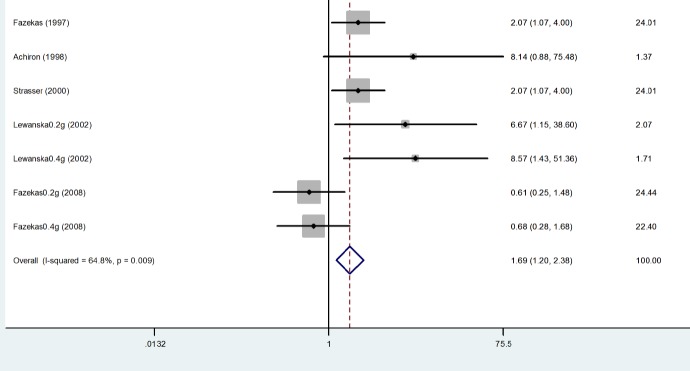
Proportion of Relapse-free Patients: Four trails reported the proportion of relapse-free patients (Fazekas et al. 1997; Achiron et al. 1998; Lewanska et al. 2000; Fazekas et al. 2008). This proportion was statistically higher for those treated with IVIg with a pooled odds Ratio (OR) of 1.693 with a 95% confidence interval of 1.205- 2.380. The difference was statistically significant with a p-value of 0.002. In addition, no evidence of heterogeneity was found among the studies (p= 0.007), indicating that the results were consistent over the trials. The odds-ratios for being relapse-free with 95% confidence intervals for each study and the overview results are given in Table 5, Fig. 3.
Table 5 . Proportion of Relapse-free Patients .
| Study | IVIG | Placebo | OR (95%CI) | % Weight | |||
| Events | NonEvents | Events | NonEvents | ||||
| Fazekas (1997) | 40 | 35 | 26 | 47 | 2.066 (1.068-3.996) | 24.01 | |
| Achiron (1998) | 6 | 14 | 1 | 19 | 8.143 (0.878-75.479) | 1.37 | |
| Strasser (2000) | 40 | 35 | 26 | 49 | 2.066 (1.068-3.996) | 24.01 | |
| Lewanska (2002) 0.2g/kg | 8 | 9 | 2 | 15 | 6.667 (1.151-38.598) | 2.07 | |
| Lewanska (2002) 0.4g/kg | 8 | 7 | 2 | 15 | 8.571 (1.430-51.362) | 1.71 | |
| Fazekas (2008) 0.2g/kg | 25 | 19 | 28 | 31 | 0.611 (0.251-1.485) | 24.44 | |
| Fazekas (2008) 0.4g/kg | 25 | 25 | 28 | 31 | 0.611 (0.251-1.485) | 22.40 | |
| D+L pooled OR | 152 | 136 | 113 | 207 | 1.690 (1.202-2.375) | ||
Fig. 3 .
Proportion of Relapse-Free Patients
Relapse Rate: Four studies reported the annual relapse rate in patients at baseline, which the meta-analysis did not show any significant difference between IVIG and placebo groups (SMD= 0.136; 95%CI, -0.090 to 0.361; p= 0.238) (Fig. 4).
Fig .4 .
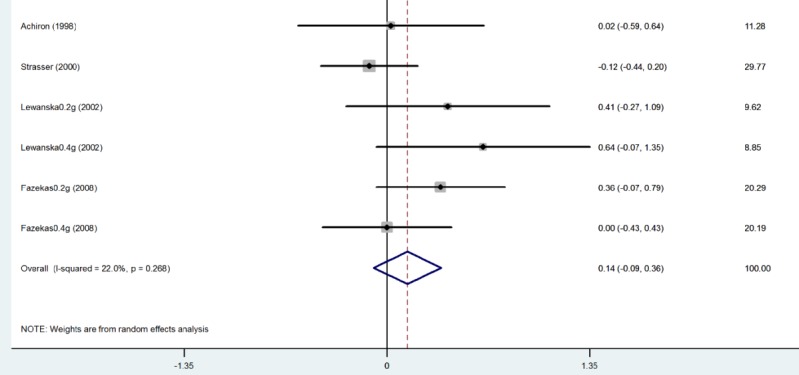
Annual Relapse Rate at Baseline
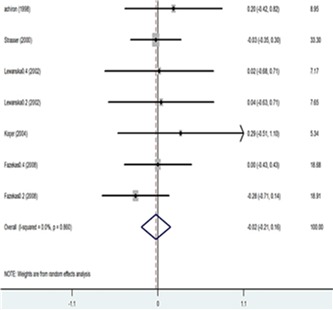
Table 6 demonstrates the changes in the annual relapse rate in four trails (Fazekas et al. 1997; Achiron et al. 1998; Lewanska et al. 2000; Fazekas et al. 2008) after treatment with IVIG, or placebo. The pooled results revealed significant differences in the number of relapses experienced on an annual basis in favor of IVIG (SMD= -0.218; 95% CI -0.412 to -0.024; p=0.028) (Fig. 5).
Table 6 . Annual Relapse Rate .
| Study | SMD | [95% Conf. Interval] | % Weight |
| Fazekas (1997) | -0.482 | -0.809 -0.155 | 35.23 |
| Achiron (1998) | -1.215 | -1.892 -0.538 | 8.21 |
| Lewanska (2002) 0.2g/kg | -0.347 | -1.025 0.330 | 8.20 |
| Lewanska (2002) 0.4g | -0.425 | -1.128 0.277 | 7.63 |
| Fazekas (2008) 0.2g | 0.315 | -0.113 0.744 | 20.54 |
| Fazekas (2008) 0.4g | 0.235 | -0.197 0.667 | 20.19 |
| I-V pooled SMD | -0.218 | -0.412 -0.024 | p=0.001 |
SMD=Standardized mean difference; IVIG=Intravenous immunoglobulin
Discussion
The present research confirms that intravenous immunoglobulin has a beneficial effect on the annual relapse rate, proportion of relapse-free patients, on the proportion of patients deteriorated and improved, and on clinical disability in patients with relapsing–remitting MS. Despite the difference between the included trials (design, duration, dose and endpoint), the results seemed remarkably consistent in the meta-analysis.
The results of the present overview revealed that IVIg does not have an effect on the EDSS scores compared to placebo; and although it was decreased, it was not statistically significant. The difference in the annual relapse rate between IVIG and placebo-treated patients was a result of a strong reduction in the annual relapse rate on therapy compared to baseline in the IVIG group. The proportion of relapse-free patients increased in the IVIg compared to placebo treatment. The increase in the proportion of the patients improved clinically indicated a positive association with receiving IVIg treatment compared to placebo in patients.
The results of similar studies that have been done in this field in other countries (Sorensen 2002 (14), Fergusson 2004 (21) and Gary 2009 (4)) are largely similar to the results obtained in this study.
The studies that were entered into the meta- analysis used different doses of IVIG and only two trails compared two different doses of IVIG with placebo (Lewanska et al. 2000; Fazekas et al. 2008), and these two trails can help select the optimal dose of IVIG. However, the decisions made considering the use of each of the doses are associated with the frequent and severe side effects. Unfortunately, the two mentioned studies did not show any difference between the two doses.
Several studies have shown that immunoglobulin was well tolerated, and did not report any severe related side effects; the most common mild side effects included headache, nausea, fever, dizziness, chills, rash and fatigue.
Most of the studies compared the effectiveness of this drug with placebo. However, to determine the effectiveness of this drug, it needs to be compared with other MS drugs. However, unfortunately, few studies (3 trials and one intervention study) have done this comparison. The study conducted by Kalanie et al. 2004 (22) compared the efficacy of this drug with interferon beta-1a (Avonex) in RRMS patients. Thisstudy showed a reduction of relapse rates by using IVIG compared to interferon beta-1a better safety profile for IVIG in the studiedIranian population. In the two other studies (Visser et al. 2004 and Sorensen et al. 2004), IVIG was used as add-on to methylprednisolone and was compared to IV methylprednisolone only in the treatment of relapses in MS, and in both studies there was no significant difference between the groups in terms of relapse and measured outcomes (23,24).
Although studies have shown beneficial effects of immunoglobulin in measuring variable diseases activities, the evidence, with regards to the short duration and the number of participants in the trials, was not sufficient, and more accurate assessment of the patients is needed. Thus, based on the results of this meta-analysis, immunoglobulin can be considered as an alternative option, second-line therapy, or adjuvant therapy considering its beneficial effect (high tolerance, need to be injected with longer intervals, etc.) for treating relapsing–remitting MS patients.
Acknowledgments
We thank the National Institute of Health Research and Yazd University of Medical Sciences for providing facilities for this review. It should also be noted that this article has been adapted from a Master's thesis.
Cite this article as: Olyaeemanesh A, Rahmani M, Goudarzi R, Rahimdel A. Safety and effectiveness assessment of intravenous immunoglobulin in the treatment of relapsing-remitting multiple sclerosis: A meta-analysis. Med J Islam Repub Iran 2016 (23 February). Vol. 30:336.
References
- 1.Achiron A, Kishner I, Sarova-Pinhas I, Raz H, Faibel M, Stern Y. et al. Intravenous immunoglobulin treatment following the first demyelinating event suggestive of multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Archives of Neurology. 2004;61(10):1515–20. doi: 10.1001/archneur.61.10.1515. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto D, Campbell JD. Cost-effectiveness of multiple sclerosis disease-modifying therapies: a systematic review of the literature. Autoimmune diseases. 2012:2012. doi: 10.1155/2012/784364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsang BK, Macdonell R. Multiple sclerosis- diagnosis, management and prognosis. Aust Fam Physician . 2011 Dec;40(12):948–55. [PubMed] [Google Scholar]
- 4. Gray O MG, Forbes RB. Intravenous immunoglobulins for multiple sclerosis. Cochrane Database of Systematic Reviews 2010. [DOI] [PMC free article] [PubMed]
- 5.Sorensen PS. The role of intravenous immunoglobulin in the treatment of multiple sclerosis. J Neurol Sci. 2003 Feb 15;206(2):123–30. doi: 10.1016/s0022-510x(02)00343-x. [DOI] [PubMed] [Google Scholar]
- 6.Lewanska M, Selmaj K. [Immunotherapy of intravenous immunoglobulin preparations in neurologic diseases] Postepy Hig Med Dosw. 2002;56 Suppl:69–83. [PubMed] [Google Scholar]
- 7.Clegg A, Bryant J, Milne R. Disease-modifying drugs for multiple sclerosis: a rapid and systematic review. Health Technology Assessment (Winchester, England) 2000;4(9):i–iv, 1-101. [PubMed] [Google Scholar]
- 8.Achiron A, Miron S. Intravenous immunoglobulin and multiple sclerosis. Clin Rev Allergy Immunol. 2005 Dec;29(3):247–54. doi: 10.1385/CRIAI:29:3:247. [DOI] [PubMed] [Google Scholar]
- 9. Organization WH. Multiple scherosis resources in the world 2013. 2013.
- 10.Ebrahimi HA, Sedighi B. Prevalence of multiple sclerosis and environmental factors in Kerman province, Iran. Neurology Asia. 2013;18(4) [Google Scholar]
- 11.Sibéril S, Elluru S, Negi V-S, Ephrem A, Misra N, Delignat S. et al. Intravenous immunoglobulin in autoimmune and inflammatory diseases: more than mere transfer of antibodies. Transfusion and Apheresis Science. 2007;37(1):103–7. doi: 10.1016/j.transci.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Sorensen PS. Treatment of multiple sclerosis with intravenous immunoglobulin: review of clinical trials. Neurol Sci. 2003 Oct;24 Suppl 4:S227–30. doi: 10.1007/s10072-003-0083-5. [DOI] [PubMed] [Google Scholar]
- 13. Fernández Liguori N, Rojas JI, Klajn DS, Ciapponi A. Intravenous immunoglobulin to prevent relapses during pregnancy and postpartum in multiple sclerosis. The Cochrane Library. 2013.
- 14.Sorensen PS, Fazekas F, Lee M. Intravenous immunoglobulin G for the treatment of relapsing-remitting multiple sclerosis: a meta-analysis. Eur J Neurol. 2002 Nov;9(6):557–63. doi: 10.1046/j.1468-1331.2002.00501.x. [DOI] [PubMed] [Google Scholar]
- 15.Fazekas F, Deisenhammer F, Strasser-Fuchs S, Nahler G, Mamoli B. Randomised placebo-controlled trial of monthly intravenous immunoglobulin therapy in relapsing-remitting multiple sclerosis Austrian Immunoglobulin in Multiple Sclerosis Study Group. Lancet. 1997 Mar 1;349(9052):589–93. doi: 10.1016/s0140-6736(96)09377-4. [DOI] [PubMed] [Google Scholar]
- 16.Achiron A, Gabbay U, Gilad R, Hassin-Baer S, Barak Y, Gornish M. et al. Intravenous immunoglobulin treatment in multiple sclerosis Effect on relapses. Neurology. 1998;50(2):398–402. doi: 10.1212/wnl.50.2.398. [DOI] [PubMed] [Google Scholar]
- 17.Strasser-Fuchs S, Fazekas F, Deisenhammer F, Nahler G, Mamoli B. The Austrian Immunoglobulin in MS (AIMS) study: final analysis. Mult Scler. 2000 Oct;6 Suppl 2:S9–13. [PubMed] [Google Scholar]
- 18.Lewańska M, SigerZajdel M, Selmaj K. No difference in efficacy of two different doses of intravenous immunoglobulins in MS: clinical and MRI assessment. European Journal of Neurology. 2002;9(6):565–72. doi: 10.1046/j.1468-1331.2002.00500.x. [DOI] [PubMed] [Google Scholar]
- 19.Kocer B, Yildirim-Gurel S, Tali ET, Irkec C, Isik S. The role of qualitative and quantitative MRI assessment of multiple sclerosis lesions according to their in evaluating the efficacy of intravenous immunoglobulin G. Neuroradiology. 2004 Apr;46(4):287–90. doi: 10.1007/s00234-003-1088-8. [DOI] [PubMed] [Google Scholar]
- 20.Fazekas F, Lublin FD, Li D, Freedman MS, Hartung HP, Rieckmann P. et al. Intravenous immunoglobulin in relapsing-remitting multiple sclerosis: a dose-finding trial. Neurology 2008 Jul. 22;71(4):265–71. doi: 10.1212/01.wnl.0000318281.98220.6f. [DOI] [PubMed] [Google Scholar]
- 21.Fergusson D, Hutton B, Sharma M, Tinmouth A, Wilson K, Cameron DW. et al. Use of intravenous immunoglobulin for treatment of neurologic conditions: a systematic review. Transfusion. 2005 Oct;45(10):1640–57. doi: 10.1111/j.1537-2995.2005.00581.x. [DOI] [PubMed] [Google Scholar]
- 22.Kalanie H, Gharagozli K, Hemmatie A, Ghorbanie M, Kalanie AR. Interferon Beta-1a and intravenous immunoglobulin treatment for multiple sclerosis in Iran. Eur Neurol. 2004;52(4):202–6. doi: 10.1159/000082036. [DOI] [PubMed] [Google Scholar]
- 23.Visser LH, Beekman R, Tijssen CC, Uitdehaag BM, Lee ML, Movig KL. et al. A randomized, double-blind, placebo-controlled pilot study of iv immune globulins in combination with iv methylprednisolone in the treatment of relapses in patients with MS. Mult Scler. 2004 Feb;10(1):89–91. doi: 10.1191/1352458504ms978sr. [DOI] [PubMed] [Google Scholar]
- 24.Sorensen PS, Haas J, Sellebjerg F, Olsson T, Ravnborg M. IV immunoglobulins as add-on treatment to methylprednisolone for acute relapses in MS. Neurology. 2004;63(11):2028–33. doi: 10.1212/01.wnl.0000145798.61383.39. [DOI] [PubMed] [Google Scholar]