Abstract
Trisomy 13 (T13) and trisomy 18 (T18) are among the most prevalent autosomal trisomies. Both are associated with a very high risk of mortality. Numerous instances, however, of long-term survival of children with T13 or T18 have prompted some clinicians to pursue aggressive treatment instead of the traditional approach of palliative care. The purpose of this study is to assess current mortality data for these conditions. This multi-state, population-based study examined data obtained from birth defect surveillance programs in nine states on live-born infants delivered during 1999–2007 with T13 or T18. Information on children’s vital status and selected maternal and infant risk factors were obtained using matched birth and death certificates and other data sources. The Kaplan–Meier method and Cox proportional hazards models were used to estimate age-specific survival probabilities and predictors of survival up to age five. There were 693 children with T13 and 1,113 children with T18 identified from the participating states. Among children with T13, 5-year survival was 9.7%; among children with T18, it was 12.3%. For both trisomies, gestational age was the strongest predictor of mortality. Females and children of non-Hispanic black mothers had the lowest mortality. Omphalocele and congenital heart defects were associated with an increased risk of death for children with T18 but not T13. This study found survival among children with T13 and T18 to be somewhat higher than those previously reported in the literature, consistent with recent studies reporting improved survival following more aggressive medical intervention for these children.
Keywords: trisomy 13, trisomy 18, survival, mortality, epidemiology
INTRODUCTION
Next to Down syndrome, trisomy 13 (T13) and trisomy 18 (T18) are the most common autosomal trisomies diagnosed in fetuses and infants. The prevalence of T13 among live born infants in the United States (US) is approximately 0.81 per 10,000, or about 1 in 12,340 live births, and for T18 the birth prevalence is 1.5 per 10,000, or about 1 in 6,670 live births [Parker et al., 2010]. Inclusion of fetal deaths and pregnancy terminations in these numbers increases the estimated prevalence by about 2.5- to fourfold [Crider et al., 2008; Parker et al., 2010]. Among pregnancies that are prenatally diagnosed with T13 only 18.9% have been reported to result in a live birth and, for T18, 13.5% are live-born [Tonks et al., 2013]. The majority of prenatally diagnosed pregnancies are electively terminated [Irving et al., 2011; Tonks et al., 2013]. Affected infants usually have major congenital malformations, including congenital heart defects (CHDs) such as septal defects and aortic and pulmonary valve anomalies, orofacial clefts, omphalocele, renal anomalies, and central nervous system malformations [Jones et al., 2013].
Children born with T13 and T18 have a poor prognosis, with a very high rate of infant mortality [Rasmussen et al., 2003; Vendola et al., 2010; Lakovschek et al., 2011]. Median survival times of 7–10 days for T13 and 10–14.5 days for T18 were reported by Rasmussen et al. [2003] and are consistent with other studies [Lakovschek et al., 2011]. Despite the poor prognosis for these infants, several case reports have been published describing instances of long-term survival of children with T13 and T18, occasionally into the second decade of life [Redheendran et al., 1981; Tunca et al., 2001; Peroos et al., 2012]. In addition, many families of longer term survivors share information on their child’s progress in developmental areas, such as language and communication and motor skills, with researchers and support groups such as the Support Organization for Trisomy 18, 13, and Related Disorders [Baty et al., 1994; Bruns, 2015; SOFT, 2015]. These reports have prompted some clinicians to pursue a more aggressive strategy in managing affected infants, instead of opting for the traditional approach of providing mainly palliative care. A recent analysis of hospital utilization among infants and children with T13 and T18 in the United States seems to support this trend of more aggressive medical management [Nelson et al., 2012]. That study found an increasing number of in-patient hospital stays over time (for T18) and numerous therapeutic procedures being performed on the children, many of whom were over 1 year of age.
In recent years, there has been debate regarding the extent to which technological interventions should be employed in the management of infants with these trisomies [Kaneko et al., 2008; Janvier et al., 2011; Carey, 2012; Merritt et al., 2012]. In order for caregivers and parents to make informed decisions about caring for a child with T13 or T18, accurate and current information regarding infant and childhood survival, including factors influencing survival, is critical. In this study, we estimated survival, and quantified important predictors of survival, using a large, population-based sample of infants born with T13 and T18 from a multi-state collaboration of US birth defect surveillance systems.
MATERIALS AND METHODS
Data Sources
The data for this study were collected as a part of a larger collaborative study of survival among children with selected major birth defects ascertained from 12 state-based birth defect surveillance programs [Wang et al., 2015]. For the present study, we restricted the analysis to nine states that used either active case ascertainment or passive case ascertainment with medical record confirmation of the diagnoses. The participating states in this study, and the inclusive birth cohorts used, are Arizona (1999–2007), Colorado (1999–2006), Georgia (5-county metropolitan Atlanta area) (1999–2007), Illinois (2002–2006), Massachusetts (2000–2007), New Jersey (1999–2005), New York (1999–2007), North Carolina (2003–2007), and Texas (1999–2007). The present study includes all live-born infants diagnosed with T13 or T18, who were residents of one of the participating nine states and were delivered in the years specified above. States using active ascertainment methods to identify cases were Arizona, Georgia, Massachusetts, North Carolina, and Texas; the remaining four states used passive surveillance with follow-up review of the medical record to verify the diagnosis. States were asked to exclude suspected or possible cases of T13 and T18 but, due to local differences in coding and abstraction procedures, we were not able to distinguish between full trisomies, partial or Robertsonian trisomies, and mosaics in most states. Thus, our study sample consisted of all T13 and T18 cytogenetic variants.
Each of the participating states matched their infants born with T13 or T18 to their respective birth certificate records to obtain demographic information and other maternal and infant characteristics that could be associated with survival. Variables included in this analysis were child’s sex (male, female), clinical estimate of gestational age (<32 weeks; 32–36 weeks, ≥37 weeks), plurality (single, multiple), period of birth (1999–2002, 2003–2007), and maternal characteristics, including age (≤19, 20–24, 25–29, 30–34, ≥35 years), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic Asian/Pacific Islander, non-Hispanic other), state of residence, and population density of maternal county of residence at delivery (metropolitan, nonmetropolitan), based on 2003 Rural–Urban Continuum codes [USDA, 2013].
Because infants with T13 and T18 often present with a wide spectrum of other serious birth defects that could alter the course of treatment or survival, we also collected data on selected co-occurring major congenital malformations, which were ascertained by the surveillance program and reported for each infant. We focused on those malformations that are most commonly reported among these infants, and most likely to influence survival. These include omphalocele and selected major CHDs (common truncus, transposition of great arteries, tetralogy of Fallot, atrio-ventricular septal defect, aortic valve stenosis, hypoplastic left heart syndrome, and coarctation of aorta). Although other CHDs, such as patent ductus arteriosus, atrial septal defects, and ventricular septal defects, are quite common in children with T13 and T18, they are not typically associated with an increased risk of mortality; thus, we chose to limit our analysis to the rarer but more severe CHD phenotypes listed above.
To determine the vital status of each child, participating surveillance programs linked their infants born with T13 or T18 to their state death certificate files. To augment the state vital statistics data, Arizona and Texas also used medical records data, and Georgia used National Death Index (NDI) data. For decedent children, participating states provided the month and year of death and age at death (in days). Vital status was ascertained through December 31, 2008; thus the length of the follow-up period varied depending on the birth cohort. If there was no documentation of the child’s death, the child was assumed to be alive for the duration of follow-up, consistent with the approach used by Wang et al. [2015]. One state (Illinois) provided mortality data only up to 1 year of age, and was, therefore, excluded from analyses of survival beyond the first year of life. Each surveillance program removed all personal identifiers from their data and sent a deidentified dataset to the Centers for Disease Control and Prevention (CDC) for consolidation, formatting, and data cleaning prior to analysis.
Statistical Analysis
We used the Kaplan–Meier (K–M) product limit method to estimate unconditional and conditional age-specific survival probabilities (<1 day, <7 days, <28 days, <1 year, <5 years) separately for children with T13 and T18 [Lee, 1980]. Unconditional survival probabilities provided estimates of the likelihood of survival at each age interval measured from the time of birth, whereas conditional survival probabilities estimated the likelihood of survival at a specified age interval given the individual has survived to the preceding interval. We used Greenwood’s method to calculate 95% confidence intervals (CI) for the survival probability estimates [Kalbfleisch and Prentice, 2002]. Using the K–M method and the log-rank test, we performed bivariate analyses and generated survival curves to identify maternal and infant risk factors associated with survival. To identify a set of predictive factors associated with survival, we selected those risk factors that had a P-value of <0.20 in the bivariate analyses and included them in a multivariable analysis using the Cox proportional hazards model [Cox, 1972]. Separate models were run for T13 and T18 to examine survival at ages 1 and 5. Adjusted hazard ratios (aHR) and 95% CIs generated from the Cox models provided risk estimates for each of the explanatory variables while simultaneously controlling for all other variables in the model. We used SAS Version 9.2 to conduct all statistical analyses for this study (SAS Institute, Cary, NC).
RESULTS
There were a total of 693 children with T13 and 1,113 children with T18 identified from the nine states (Table I). Texas contributed the largest percentage of subjects to this study (about 37%). More than one-half of the children were born in the latter time period (2003–2007), which was due to the increased number of states providing data during the latter years. Almost 90% of the children were from metropolitan areas. Children with T13 were evenly split by gender, but a substantially higher proportion of the children with T18 were female (61.2%) compared to male (38.4%). As expected, the maternal age distribution among the infants was skewed toward the older age categories. This was most notable for T18, in which almost 46% of the mothers were 35 years or older. The distribution of maternal race/ethnicity was similar for both phenotypes. About 17% of the children had one or more of the CHDs listed above. Omphalocele was diagnosed in 7.5% of the infants with T13 and 5.2% of the infants with T18.
TABLE I.
Characteristics of Live Born Infants With Trisomy 13 and Trisomy 18 in Nine US States, 1999–2007 Birth Cohort
| Characteristic | Trisomy 13
|
Trisomy 18
|
||
|---|---|---|---|---|
| Number births | Percent | Number births | Percent | |
| Infant sex | ||||
| Male | 347 | 50.1 | 427 | 38.4 |
| Female | 340 | 49.1 | 681 | 61.2 |
| Unknown | 6 | 0.9 | 5 | 0.4 |
| Gestational age | ||||
| <32 weeks | 122 | 17.6 | 206 | 18.5 |
| 32–36 weeks | 223 | 32.2 | 277 | 24.9 |
| ≥37 weeks | 336 | 48.5 | 605 | 54.4 |
| Unknown | 12 | 1.7 | 25 | 2.2 |
| Plurality | ||||
| Single | 675 | 97.4 | 1,065 | 95.7 |
| Multiple | 15 | 2.2 | 41 | 3.7 |
| Unknown | 3 | 0.4 | 7 | 0.6 |
| Maternal race/ethnicity | ||||
| Non-Hispanic White | 327 | 47.2 | 471 | 42.3 |
| Non-Hispanic Black | 110 | 15.9 | 208 | 18.7 |
| Hispanic | 217 | 31.3 | 366 | 32.9 |
| Non-Hispanic Asian/PI | 21 | 3.0 | 38 | 3.4 |
| Other/unknown | 18 | 2.6 | 30 | 2.7 |
| Maternal age | ||||
| <19 years | 53 | 7.6 | 67 | 6.0 |
| 20–24 years | 126 | 18.2 | 156 | 14.0 |
| 25–29 years | 149 | 21.5 | 179 | 16.1 |
| 30–34 years | 155 | 22.4 | 200 | 18.0 |
| ≥35 years | 210 | 30.3 | 511 | 45.9 |
| Geographic area | ||||
| Metropolitan | 618 | 89.2 | 991 | 89.0 |
| Non-metropolitan | 72 | 10.4 | 117 | 10.5 |
| Unknown | 3 | 0.4 | 5 | 0.4 |
| State | ||||
| Arizona | 68 | 9.8 | 118 | 10.6 |
| Colorado | 52 | 7.5 | 59 | 5.3 |
| Georgia | 32 | 4.6 | 61 | 5.5 |
| Illinois | 63 | 9.1 | 127 | 11.4 |
| Massachusetts | 37 | 5.3 | 49 | 4.4 |
| North Carolina | 50 | 7.2 | 92 | 8.3 |
| New Jersey | 50 | 7.2 | 76 | 6.8 |
| New York | 85 | 12.3 | 127 | 11.4 |
| Texas | 256 | 36.9 | 404 | 36.3 |
| Presence of heart defectsa | ||||
| With heart defects | 117 | 16.9 | 193 | 17.3 |
| No heart defects | 576 | 83.1 | 920 | 82.7 |
| Presence of omphalocele | ||||
| With omphalocele | 52 | 7.5 | 58 | 5.2 |
| No omphalocele | 641 | 92.5 | 1,055 | 94.8 |
| Birth period | ||||
| 1999–2002 | 264 | 38.1 | 400 | 35.9 |
| 2003–2007 | 429 | 61.9 | 713 | 64.1 |
| Total | 693 | 100.0 | 1,113 | 100.0 |
Heart defects include common truncus, transposition of great arteries, tetralogy of Fallot, atrioventricular septal defect, aortic valve stenosis, hypoplastic left heart syndrome, and coarctation of aorta.
PI, Pacific Islander.
Median survival for children with T13 and T18 was 5 and 8 days, respectively. Although mortality was quite high for both phenotypes, some differences were evident across the 5-year time span (Table II). Children with T13 had poorer survival than children with T18 at each time point examined, most notably at 7 and 28 days. Survival for infants with T13 fell from 74.6% after the first day of life to 25.5% at 28 days. For infants with T18, survival declined from 78.1% after the first day to 37.2% at 28 days. Among the children with T13, 9.7% survived to age 5, whereas 5-year survival for children with T18 was 12.3%.
TABLE II.
Survival Estimates (Expressed as Percentage) for Children With Trisomy 13 and Trisomy 18 in Nine US States, 1999–2007 Birth Cohort
| Phenotype | Number live births | Number deaths | Survival probability (95%CI)
|
||||
|---|---|---|---|---|---|---|---|
| <1 day | <7 day | <28 day | <1 year | <5 yearsa | |||
| Trisomy 13 | 693 | 625 | 74.6 (71.2, 77.7) | 43.1 (39.4, 46.8) | 25.5 (22.4, 28.8) | 11.5 (9.3, 14.1) | 9.7 (7.2, 12.5) |
| Trisomy 18 | 1,113 | 984 | 78.1 (75.5, 80.4) | 52.5 (49.5, 55.4) | 37.2 (34.4, 40.0) | 13.4 (11.5, 15.5) | 12.3 (10.1, 14.8) |
Includes children born in 1999–2005. Data from IL were excluded because no vital status data beyond 1 year were available.
CI, confidence interval.
Although the cumulative probability of survival for both T13 and T18 decreased precipitously throughout infancy, conditional survival probabilities showed a less marked decline after the first day of life (Table III). Among infants with T13, those who survived the first day of life had approximately a 58% chance of surviving to seven days, and those who lived to seven days had about the same chance of surviving to 28 days. Infants with T18 who survived the first week had about a 71% chance of surviving through the neonatal period. Children with either condition who survived the first year of life had better than an 80% chance of surviving to age 5.
TABLE III.
Conditional * Survival Estimates (Expressed as Percentage) for Children With Trisomy 13 and Trisomy 18 in Nine US States, 1999–2007 Birth Cohort
| Phenotype | Number live births | Number deaths | Survival probability (95%CI)
|
||||
|---|---|---|---|---|---|---|---|
| <1 day | <7 day | <28 day | <1 year | <5 yearsa | |||
| Trisomy 13 | 693 | 625 | 74.6 (71.2, 77.7) | 57.8 (53.5, 62.0) | 59.2 (53.4, 64.5) | 45.2 (37.8, 52.3) | 82.5 (69.8, 90.2) |
| Trisomy 18 | 1.113 | 984 | 78.1 (75.5, 80.4) | 67.2 (64.0, 70.2) | 70.9 (67.0, 74.4) | 36.0 (31.4, 40.6) | 86.8 (78.7, 92.0) |
Likelihood of survival to time (t) given child survived to time (t-1).
Includes children born in 1999–2005. Data from IL were excluded because no vital status data beyond 1 year were available.
CI, confidence interval.
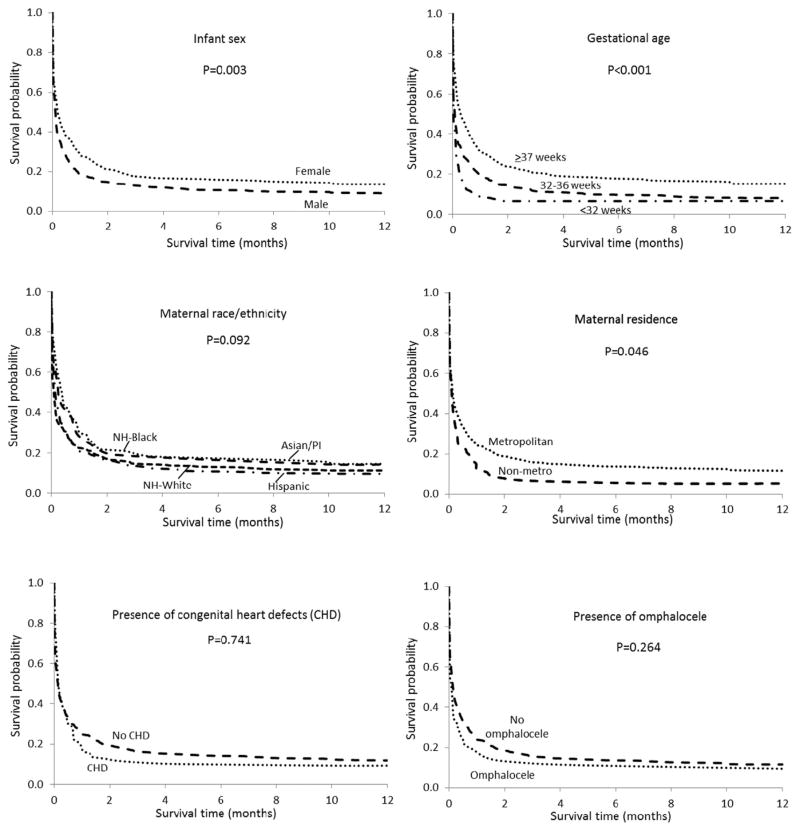
Table IV shows 1-month and 1-year survival probabilities for infants with T13 by selected maternal and infant risk factors. For most factors examined there were no statistically significant differences in survival, including maternal age, presence of selected major CHDs or omphalocele, and birth period. Female infants had significantly higher survival probabilities than males at both 1 month and 1 year of age. Infants born full term (≥37 weeks gestation) had substantially better survival compared to their counterparts born either moderately preterm (32–36 weeks) or very preterm (<32 weeks). Infants of non-Hispanic black mothers had the highest survival probability at 1 month, whereas Hispanic infants had the lowest survival at both 1 month and 1 year of age. Infants whose mothers resided in a metropolitan area at the time of delivery had a higher probability of survival than those from non-metropolitan areas, particularly at 1 year. Although the differences were not statistically significant, we also found differences in survival by state of residence. The lowest 1-month survival probabilities were among infants from Arizona and Illinois. One-year survival was lowest for Arizona and North Carolina. Infants from Georgia and New Jersey had the highest survival at both 1 month and 1 year.
TABLE IV.
One-Month and 1-Year Survival Probabilities (Expressed as Percentage) for Trisomy 13 in Nine US States, 1999–2007 Birth Cohort
| Characteristica | 1-month survival probability (95%CI) | P-valueb for difference in 1-month survival | 1-year survival probability (95%CI) | P-valueb for difference in 1-year survival |
|---|---|---|---|---|
| Infant sex | ||||
| Male | 18.7 (14.8, 23.0) | 0.001 | 8.9 (6.2, 12.2) | 0.003 |
| Female | 28.8 (24.1, 33.7) | 13.5 (10.1, 17.4) | ||
| Gestational age | ||||
| <32 weeks | 9.0 (4.8, 14.9) | <0.001 | 6.6 (3.1, 11.9) | <0.001 |
| 32–36 weeks | 19.7 (14.8, 25.2) | 8.1 (5.0, 12.1) | ||
| ≥37 weeks | 31.2 (26.4, 36.2) | 15.2 (11.6, 19.2) | ||
| Plurality | ||||
| Single | 23.7 (20.6, 27.0) | 0.702 | 11.0 (8.7, 13.5) | 0.751 |
| Multiple | 20.0 (4.9, 42.4) | 20.0 (4.9, 42.4) | ||
| Maternal race/ethnicity | ||||
| Non-Hispanic White | 22.6 (18.3, 27.3) | 0.030 | 11.3 (8.2, 15.0) | 0.092 |
| Non-Hispanic Black | 31.8 (23.4, 40.6) | 14.5 (8.7, 21.8) | ||
| Hispanic | 21.2 (16.0, 26.9) | 9.7 (6.2, 14.1) | ||
| Non-Hispanic Asian/PI | 28.6 (11.7, 48.2) | 14.3 (3.6, 32.1) | ||
| Other/unknown | 27.8 (10.1, 48.9) | 16.7 (4.1, 36.5) | ||
| Maternal age | ||||
| <19 years | 28.3 (17.0, 40.7) | 0.450 | 11.3 (4.6, 21.4) | 0.439 |
| 20–24 years | 19.0 (12.7, 26.3) | 9.5 (5.2, 15.4) | ||
| 25–29 years | 20.8 (14.7, 27.6) | 6.0 (3.0, 10.6) | ||
| 30–34 years | 29.7 (22.7, 37.0) | 14.8 (9.8, 20.9) | ||
| ≥35 years | 23.8 (18.3, 29.7) | 14.3 (10.0, 19.4) | ||
| Geographic area | ||||
| Metropolitan | 24.6 (21.3, 28.1) | 0.088 | 11.8 (9.4, 14.5) | 0.046 |
| Non-Metropolitan | 15.3 (8.1, 24.5) | 5.6 (1.8, 12.5) | ||
| State | ||||
| Arizona | 14.7 (7.5, 24.1) | 0.132 | 4.4 (1.2, 11.2) | 0.076 |
| Colorado | 26.9 (15.8, 39.3) | 13.5 (5.9, 24.1) | ||
| Georgia | 37.5 (21.3, 53.7) | 21.9 (9.6, 37.2) | ||
| Illinois | 19.0 (10.5, 29.5) | 9.5 (3.9, 18.2) | ||
| Massachusetts | 24.3 (12.1, 38.8) | 10.8 (3.4, 23.0) | ||
| North Carolina | 26.0 (14.9, 38.6) | 8.0 (2.6, 17.5) | ||
| New Jersey | 32.0 (19.7, 45.0) | 22.0 (11.8, 34.2) | ||
| New York | 22.4 (14.2, 31.7) | 15.3 (8.6, 23.7) | ||
| Texas | 23.8 (18.8, 29.2) | 9.8 (6.5, 13.8) | ||
| Presence of heart defectsc | ||||
| With heart defects | 18.8 (12.3, 26.3) | 0.773 | 9.4 (5.0, 15.5) | 0.741 |
| No heart defects | 25.0 (21.5, 28.6) | 12.0 (9.5, 14.8) | ||
| Presence of omphalocele | ||||
| With omphalocele | 19.2 (9.9, 30.9) | 0.198 | 9.6 (3.5, 19.4) | 0.246 |
| No omphalocele | 24.3 (21.1, 27.7) | 11.7 (9.4, 14.3) | ||
| Birth period | ||||
| 1999–2002 | 25.8 (20.6, 31.1) | 0.292 | 11.4 (7.9, 15.5) | 0.477 |
| 2003–2007 | 22.8 (19.0, 26.9) | 11.7 (8.8, 14.9) | ||
| Total | 24.0 (20.8, 27.2) | 11.5 (9.3, 14.1) | ||
Unknown was excluded except as indicated.
P-values based on log-rank test.
Heart defects include common truncus, transposition of great arteries, tetralogy of Fallot, atrioventricular septal defect, aortic valve stenosis, hypoplastic left heart syndrome, and coarctation of aorta.
CI, confidence interval; PI, Pacific Islander.
Kaplan–Meier survival curves displaying differences in 1-year survival by sex, gestational age, maternal race/ethnicity, maternal residence (metropolitan/non-metropolitan), presence of CHDs, and presence of omphalocele for infants with T13 are presented in Figure 1. Survival was consistently better for females, full term infants and for infants from metropolitan areas at each time point throughout the first year of life. The trend in survival patterns by race/ethnicity during the first year was not as clear, and the differences were not statistically significant. Children with CHDs or omphalocele had slightly lower survival compared to those without these anomalies, but the differences were not statistically significant.
FIG. 1.
Kaplan–Meier 1-year survival curves for infants with trisomy 13 by selected risk factors in nine US states, 1999–2007 birth cohort.
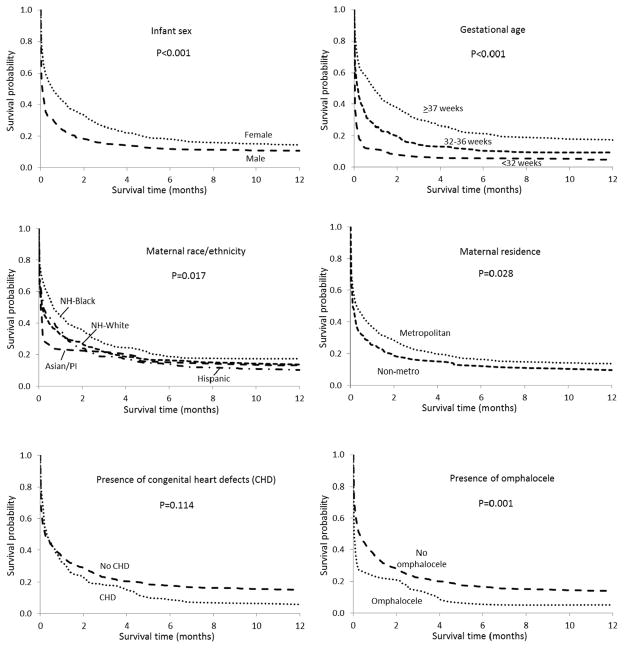
The 1-month and 1-year survival probabilities for infants with T18 by selected maternal and infant risk factors are shown in Table V. Similar to the pattern seen for T13, among infants with T18, survival was highest for females, full term infants and for infants whose mothers resided in a metropolitan area. Survival was also higher for singleton infants compared to multiples. Infants born to non-Hispanic black mothers, and infants of other/ unknown race, had the highest survival probabilities at both 1 month and 1 year. Differences in survival by state were also apparent, with Arizona and Illinois infants having the lowest 1-month survival, and Arizona and North Carolina having the lowest survival at age 1. Georgia and Massachusetts had the highest survival probabilities at both 1 month and 1 year. Infants with trisomy 18 and omphalocele had lower survival than their counterparts without omphalocele. Infants with major CHDs had a lower probability of survival at one year of age compared to those infants without, although the difference was not statistically significant. Similar to T13, for children with T18 there were no differences in survival by birth period or maternal age.
TABLE V.
One-Month and 1-Year Survival Probabilities (Expressed as Percentage) for Trisomy 18 in Nine US states, 1999–2007 Birth Cohort
| Characteristica | 1-month survival probability (95%CI) | P-valueb for difference in 1-month survival | 1-year survival probability (95%CI) | P-valueb for difference in 1-year survival |
|---|---|---|---|---|
| Infant sex | ||||
| Male | 24.6 (20.6, 28.8) | <0.001 | 10.8 (8.1, 13.9) | <0.001 |
| Female | 42.9 (39.1, 46.6) | 14.4 (11.9, 17.1) | ||
| Gestational age | ||||
| <32 weeks | 11.7 (7.7, 16.5) | <0.001 | 4.9 (2.5, 8.4) | <0.001 |
| 32–36 weeks | 26.4 (21.3, 31.6) | 9.4 (6.3, 13.2) | ||
| ≥37 weeks | 48.8 (44.7, 52.7) | 17.2 (14.3, 20.3) | ||
| Plurality | ||||
| Single | 36.5 (33.6, 39.4) | 0.036 | 13.2 (11.3, 15.4) | 0.077 |
| Multiple | 19.5 (9.2, 32.7) | 7.3 (1.9, 17.8) | ||
| Maternal race/ethnicity | ||||
| Non-Hispanic White | 33.1 (28.9, 37.4) | 0.002 | 13.6 (10.7, 16.9) | 0.017 |
| Non-Hispanic Black | 44.7 (37.9, 51.3) | 17.3 (12.5, 22.7) | ||
| Hispanic | 35.8 (30.9, 40.7) | 10.1 (7.3, 13.5) | ||
| Non-Hispanic Asian/PI | 23.7 (11.8, 37.9) | 13.2 (4.8, 25.8) | ||
| Other/unknown | 43.3 (25.6, 59.9) | 23.3 (10.3, 39.4) | ||
| Maternal age | ||||
| <19 years | 46.3 (34.1, 57.6) | 0.419 | 10.4 (4.6, 19.1) | 0.924 |
| 20–24 years | 31.4 (24.3, 38.8) | 14.7 (9.7, 20.8) | ||
| 25–29 years | 34.1 (27.2, 41.0) | 12.3 (8.0, 17.6) | ||
| 30–34 years | 34.5 (28.0, 41.1) | 12.0 (8.0, 16.9) | ||
| ≥35 years | 37.6 (33.4, 41.8) | 14.3 (11.4, 17.5) | ||
| Geographic area | ||||
| Metropolitan | 37.0 (34.0, 40.0) | 0.008 | 13.4 (11.4, 15.6) | 0.028 |
| Non-metropolitan | 25.6 (18.1, 33.8) | 9.4 (5.0, 15.5) | ||
| State | ||||
| Arizona | 22.0 (15.1, 29.9) | <0.001 | 9.3 (4.9, 15.4) | 0.002 |
| Colorado | 30.5 (19.4, 42.4) | 20.3 (11.2, 31.4) | ||
| Georgia | 49.2 (36.2, 60.9) | 24.6 (14.7, 35.9) | ||
| Illinois | 26.8 (19.4, 34.7) | 10.2 (5.7, 16.2) | ||
| Massachusetts | 55.1 (40.2, 67.7) | 22.4 (12.0, 34.8) | ||
| North Carolina | 42.4 (32.2, 52.2) | 9.8 (4.8, 16.9) | ||
| New Jersey | 42.1 (30.9, 52.8) | 14.5 (7.7, 23.3) | ||
| New York | 43.3 (34.6, 51.7) | 18.9 (12.6, 26.1) | ||
| Texas | 34.9 (30.3, 39.6) | 10.6 (7.9, 13.9) | ||
| Presence of heart defectsc | ||||
| With heart defects | 33.2 (26.6, 39.8) | 0.866 | 5.7 (3.0, 9.6) | 0.114 |
| No heart defects | 36.7 (33.6, 39.9) | 15.0 (12.8, 17.4) | ||
| Presence of omphalocele | ||||
| With omphalocele | 24.1 (14.1, 35.7) | 0.002 | 5.2 (1.4, 13.0) | 0.001 |
| No omphalocele | 36.8 (33.9, 39.7) | 13.8 (11.8, 16.0) | ||
| Birth period | ||||
| 1999–2002 | 37.8 (33.0, 42.5) | 0.318 | 14.0 (10.8, 17.6) | 0.397 |
| 2003–2007 | 35.2 (31.7, 38.7) | 13.0 (10.7, 15.6) | ||
| Total | 36.1 (33.3, 38.9) | 13.4 (11.5, 15.5) | ||
Unknown was excluded except as indicated.
P-values based on log-rank test.
Heart defects include common truncus, transposition of great arteries, tetralogy of Fallot, atrioventricular septal defect, aortic valve stenosis, hypoplastic left heart syndrome, and coarctation of aorta.
CI, confidence interval; PI, Pacific Islander.
Figure 2 presents 1-year survival curves for infants with T18 by selected risk factors. Significantly higher survival throughout the first year of life is evident for females, full term infants, and infants from metropolitan areas. Likewise, higher survival for infants of non-Hispanic black mothers is apparent during the first year. Children with T18 and who had major CHDs or omphalocele had worse survival compared to their counterparts without these malformations. This difference was most apparent for omphalocele.
FIG. 2.
Kaplan–Meier 1 year survival curves for infants with trisomy 18 by selected risk factors in nine US states, 1999–2007 birth cohort.
Table VI presents adjusted hazard ratios for mortality at 1 and 5 years of age for selected risk factors for children with T13 and T18. Controlling for all other risk factors in the model, being born preterm, particularly <32 weeks, was associated with the highest hazard ratios at both 1 and 5 years. This was true for both T13 and T18, although the association was stronger among the latter. For children with T18, being diagnosed with omphalocele and, to a lesser extent, CHDs, was associated with an increased mortality risk at age 1 and 5. For both T13 and T18, hazard ratios were less than unity for females, with a slightly stronger association for T18. Children of non-Hispanic black mothers had a lower mortality risk compared to non-Hispanic whites for both phenotypes. After controlling for other risk factors, there were also some survival differences by state. Compared to the referent state (Texas), residents of Arizona and Massachusetts had elevated aHRs for T13 at age 5, whereas for T18, New York residents had a lower hazard ratio at both ages. Georgia residents had a lower hazard ratio for both T13 and T18 at age 1, although these differences were not statistically significant.
TABLE VI.
Adjusted Hazard Ratios (aHR) and 95% Confidence Intervals (CI) for 1-Year and 5-Year Survival for Children With Trisomy 13 and Trisomy 18 From Nine US States, 1999–2007 Birth Cohort
| Characteristic | Trisomy 13
|
Trisomy 18
|
||
|---|---|---|---|---|
| 1-year survival aHR (95%CI) | 5-year survivala aHR (95%CI) | 1-year survival aHR (95%CI) | 5-year survivala aHR (95%CI) | |
| Infant sex | ||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 0.8 (0.7, 1.0) | 0.8 (0.7, 1.0) | 0.7 (0.6, 0.8) | 0.7 (0.6, 0.9) |
| Gestational age | ||||
| <32 weeks | 1.9 (1.6, 2.4) | 1.9 (1.5, 2.5) | 2.6 (2.2, 3.1) | 2.7 (2.2, 3.4) |
| 32–36 weeks | 1.4 (1.2, 1.7) | 1.3 (1.0, 1.6) | 1.5 (1.3, 1.8) | 1.5 (1.2, 1.8) |
| ≥37 weeks | 1.0 | 1.0 | 1.0 | 1.0 |
| Plurality | ||||
| Single | Excluded from model | 1.1 (0.8, 1.6) | 0.9 (0.6, 1.4) | |
| Multiple | 1.0 | 1.0 | ||
| Maternal race/ethnicity | ||||
| Non-Hispanic White | 1.0 | 1.0 | 1.0 | 1.0 |
| Non-Hispanic Black | 0.8 (0.6, 1.0) | 0.7 (0.5, 1.0) | 0.8 (0.7, 1.0) | 0.7 (0.6, 0.9) |
| Hispanic | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.2) | 1.0 (0.9, 1.2) | 0.9 (0.8, 1.1) |
| Non-Hispanic Asian/PI | 0.7 (0.4, 1.2) | 0.8 (0.5, 1.5) | 1.0 (0.7, 1.4) | 0.8 (0.5, 1.2) |
| Other/unknown | 1.4 (0.8, 2.5) | 1.4 (0.7, 2.7) | 1.1 (0.7, 1.7) | 1.0 (0.6, 1.7) |
| Geographic area | ||||
| Metropolitan | 1.0 | 1.0 | 1.0 | 1.0 |
| Non-Metropolitan | 1.2 (0.9, 1.5) | 1.3 (0.9, 1.8) | 1.1 (0.9, 1.4) | 1.1 (0.8, 1.4) |
| State | ||||
| Arizona | 1.3 (1.0, 1.8) | 1.4 (1.0, 1.9) | 1.2 (0.9, 1.5) | 1.0 (0.8, 1.3) |
| Colorado | 1.0 (0.7, 1.4) | 1.1 (0.7, 1.6) | 1.0 (0.8, 1.4) | 1.1 (0.8, 1.6) |
| Georgia | 0.7 (0.5, 1.1) | 0.8 (0.5, 1.3) | 0.7 (0.5, 1.0) | 0.9 (0.6, 1.3) |
| Illinoisb | 1.1 (0.8, 1.5) | – | 1.2 (1.0, 1.5) | – |
| Massachusetts | 1.2 (0.8, 1.7) | 1.7 (1.1, 2.6) | 0.8 (0.6, 1.1) | 0.9 (0.6, 1.3) |
| North Carolina | 1.0 (0.7, 1.3) | 1.0 (0.6, 1.5) | 1.0 (0.8, 1.3) | 0.9 (0.6, 1.2) |
| New Jersey | 0.9 (0.6, 1.2) | 1.0 (0.7, 1.4) | 0.9 (0.7, 1.2) | 1.0 (0.7, 1.3) |
| New York | 1.0 (0.7, 1.3) | 1.2 (0.9, 1.6) | 0.8 (0.6, 1.0) | 0.7 (0.6, 1.0) |
| Texas | 1.0 | 1.0 | 1.0 | 1.0 |
| Heart defectsc | ||||
| With heart defects | Excluded from model | 1.2 (1.0, 1.4) | 1.3 (1.1, 1.6) | |
| No heart defects | 1.0 | 1.0 | ||
| Omphalocele | ||||
| With omphalocele | Excluded from model | 1.5 (1.1, 1.9) | 1.6 (1.1, 2.3) | |
| No omphalocele | 1.0 | 1.0 | ||
Based on children born 1999–2005; excludes Illinois, which provided mortality data only up to 1 year of age.
Illinois provided mortality data up to age 1 only.
Heart defects include common truncus, transposition of great arteries, tetralogy of Fallot, atrioventricular septal defect, aortic valve stenosis, hypoplastic left heart syndrome, and coarctation of aorta.
PI, Pacific Islander.
DISCUSSION
This is the largest population-based study of survival among children with T13 or T18 published to date, and the first study with sufficient statistical power to characterize an array of maternal and infant factors potentially related to survival. After adjusting for other explanatory factors, we found that children with T13 and T18 who were born very preterm (<32 weeks gestation) had at least a two fold increased risk of mortality compared to their counterparts born at term. Gestational age was the strongest independent determinant of survival in our study, which is noteworthy because about one-half of the children with T13 and T18 were born preterm. Children with either condition who were born very preterm had the poorest survival, but those born between 32 and 36 weeks also had increased mortality compared to term infants. At least two other studies found a survival differential among children with T18 by gestational age [Niedrist et al., 2006; Wu et al., 2013]. Both of these studies found that infants born ≥37 weeks gestation had better survival compared to infants born at earlier gestational ages. To our knowledge, the current study is the first with sufficient statistical power to demonstrate that gestational age is an important predictor of survival in both T13 and T18, independent of other factors such as gender, race/ethnicity and state of residence. In a recent multicenter study comparing outcomes among a cohort of very low birth weight (VLBW) infants with T13, T18, Down syndrome, and infants without major birth defects, Boghossian et al. [2014] found that mortality was substantially higher among infants with T13 and T18 compared to the other two groups. However, that study was limited to infants born VLBW who were treated at neonatal intensive care units, and did not include a referent group of normal weight infants for comparison. It is possible that the high mortality among infants with T13 or T18 and who are VLBW may be due, in part, to a tendency to withhold aggressive neonatal care which would typically be provided to other very preterm infants [Boghossian et al., 2014].
Although the median survival of children with T13 (5 days) and T18 (8 days) in our study was comparable to previously reported findings, survival rates at 1 year of age were higher than those reported in some other recent studies, which ranged from about 3% to 8% [Rasmussen et al., 2003; Vendola et al., 2010; Irving et al., 2011]. There are several possible explanations for this. First, our study population included infants with various expressions of T13 and T18, including cytogenetic mosaics and Robertsonian translocations. Greatly improved long-term survival has been observed for children with partial or mosaic trisomies compared to children with full trisomy. For example, Wu et al. [2013] reported 1-year survival rates of 29% and 70% for children with partial T13 and partial T18, respectively. Although the proportion of such cytogenetic variations relative to full trisomy 13 or 18 is relatively small [Parker et al., 2003; Wu et al., 2013], it is likely that inclusion of these variants in our study population had at least some impact on the observed survival rates. Another possible explanation for the relatively higher survival in our study compared to earlier reports could be increased prenatal detection and termination of trisomy fetuses with major malformations or pregnancy complications such as holoprosencephaly, complex congenital heart defects, oligohydramnios or severe fetal growth restriction. Selective termination of fetuses with the poorest prognosis could result in a higher proportion of less severely affected fetuses with T13 and T18 being born alive, thus increasing median and overall survival rates. Our study found a slightly lower prevalence of major CHDs and omphalocele compared to some other studies, which is consistent with such an explanation.
Similar to previously published reports, our study found very high mortality within the first week of life. For infants who survived the first week, however, the survival outlook improved. Infants with T13 who survived the first week had nearly a 60% chance of neonatal survival, and children with trisomy 18 who survived the first week had better than a 70% chance. Children with either phenotype who survived the first year had a very good chance of living to age 5. These findings, although seemingly encouraging and potentially useful for patient counseling, should be interpreted with caution because overall prolonged survival in T13 and T18 remains rare, and is likely to be largely dependent on genotypic variation and the child’s associated medical complications. For example, in an analysis of conditional survival of infants with full T13 or T18, Brewer et al. [2002] found improving chances of survival only up to 1 month of age. For infants who survived one month, survival to 1 year was only about 10% in both groups.
Our study found that infants with T18 and omphalocele had a 50% increased risk of mortality at 1 and 5 years compared to those without an omphalocele, after adjusting for other risk factors. Omphalocele was not associated with survival among children with T13. About 5% of the children with T18 in our study had an omphalocele, compared to 14.3% from a Swiss population [Niedrist et al., 2006], 7–9% as found by Baty et al. [1994] from a sample of families participating in a national support group, 5% from a hospital-based sample in Taipei [Lin et al., 2006] and <1% as reported in analysis of the national Healthcare Cost and Utilization project (HCUP) [Pont et al., 2006].
Among children with T13, presence of selected major CHDs showed no substantial effect on either 1-month or 1-year survival. For children with T18, the presence of CHD was associated with an increased risk of death at both 1 and 5 years of age. These findings contrast with those of some previous studies that found no survival differences between affected children with and without CHDs, although those studies included CHD subtypes that were not captured in our study, and presented with a wider range of severity, such as ventricular septal defects [Rasmussen et al., 2003; Niedrist et al., 2006; Wu et al., 2013].
As reported in several previous studies [Weber, 1967; Baty et al., 1994; Rasmussen et al., 2003; Lin et al., 2006; Niedrist et al., 2006], we found a survival advantage for female children relative to males for both T13 and T18. After controlling for potential confounders, this association held for T18 but was not as strong for children with T13. The reasons for the higher survival among females are unclear, but this finding is generally consistent across the majority of previous studies. Additionally, as our study shows, the gender difference persists even after controlling for factors such as gestational age and occurrence of co-morbidities such as omphalocele and CHDs.
We found survival to be slightly higher among children of non-Hispanic black mothers compared to non-Hispanic whites after adjusting for other variables, but no other racial/ethnic differences of note were evident. This finding is consistent with that of Rasmussen et al. [2003], which used data from the metropolitan Atlanta area. On the other hand, Vendola et al. [2010] found no association between race/ethnicity and survival in a cohort from Texas. The findings of higher survival among females and among children of non-Hispanic black mothers warrant further investigation.
After controlling for other risk factors, our study also found some survival differences among the nine states. It is possible that local differences in the aggressiveness of medical management of infants with T13 or T18 may contribute to the variation in survival among states. Some studies suggest that cardiac surgery and more intensive neonatal treatment may prolong survival of some infants with T13 and T18 [Kosho, 2006; Kaneko et al., 2008, 2009; Maeda et al., 2011; Tsukada et al., 2012]. There continues to be much discussion and varied opinion within the medical community about when to use more aggressive treatment options, and how much care is “the right amount” given the poor prognosis of children with these disorders [Carey, 2012]. Treatment practices for these patients do vary among hospitals and physicians, but whether differences in medical care contributed to the observed differences in survival between the states in this study is unclear. Furthermore, as noted by Hsu and Hou [2007], other studies have found that many children with T13 have experienced prolonged survival without receiving more aggressive care, and suggest that variability in the clinical spectrum of T13 is an important determinant in long-term survival.
It is also possible that the observed state-specific differences in survival may be due, at least in part, to differences in completeness of ascertaining vital status. The specific data linkage methods used to match case information with state death certificates varied by state, and this might have led to differences in completeness of ascertainment. Additionally, some of the states used other data sources to supplement state mortality files for ascertaining vital status, including hospital records and NDI searches; however, there did not appear to be a clear pattern associated with the search methods used and survival among the states. Finally, as discussed above, it is also possible that differences in prenatal detection and termination rates exist among the nine states, which could result in state differences in childhood survival.
This study had several strengths. The large sample size enabled us to investigate several maternal and infant risk factors potentially related to survival that have not been previously studied, such as maternal age, state of residence and gestational age (identified as a new predictor of mortality among infants with T13). The study population also comprises a national sample of a geographically and racial/ethnically diverse population that was obtained from well-established state-based birth defect surveillance systems throughout the United States. This allowed us to examine regional and urban/rural differences in survival, which previous studies have not been able to address. Also, in our study, diagnoses were verified by medical record review in all of the participating states, which included karyotype confirmation or other cytogenetic findings when available in the record, in addition to clinical exams and other medical documentation supporting the diagnosis. According to data available from five of the states, approximately 80% of the children’s medical charts contained a karyotype report. We found no significant difference in survival among those children whose record did or did not include karyotype confirmation (data not shown).
This study was subject to some limitations. The lack of specificity in the birth defect coding systems used and differences in local case abstraction procedures precluded us from distinguishing between full trisomy and partial or mosaic variants. As noted above, although the proportion of children with partial or mosaic trisomies is expected to be small, our study population was clinically heterogeneous compared with study populations with detailed cytogenetic results on all participants. Although this may have contributed to somewhat higher long-term survival estimates in our study, it does not appear to have caused substantial bias with regard to identification of risk factors associated with survival, as our findings with respect to risk factors such as gender and gestational age are largely consistent with earlier reports. Also, as previously noted, there was potential for underascertainment of deaths in this study, due to children moving out of state during the follow up period, or missed matches between the child’s birth and death records. This may have resulted in our study overstating the true survival rates, particularly after infancy. However, the one site in our study that used NDI to identify deaths (Georgia) had the same or higher survival rates as those states that employed state death certificates alone. Another limitation of our study is the lack of information on the clinical care provided to the children, preventing us from examining the extent to which factors such as cardiac surgery or other therapies may have affected survival. This is a critical gap in our current knowledge that could be addressed in several ways—with administrative cohorts developed through longitudinal linkages of large population-based data sources, or with multicenter hospital-based cohort studies that focus on health services utilization and long-term survival.
This multi-state population-based study provides new and updated information on the 5-year survival of children born with T13 or T18, including factors that influence survival. Our findings support the emerging view that, despite their severity and high mortality, these conditions should no longer be routinely regarded as uniformly lethal malformations. This information can be helpful to clinicians, parents and other caregivers involved in the treatment and decision-making around the care and well-being of children born with T13 or T18.
Acknowledgments
The authors thank the following birth defects surveillance programs for contributing data to this study: Arizona Birth Defects Monitoring Program, Colorado Responds to Children with Special Needs, Metropolitan Atlanta Congenital Defects Program, Illinois Adverse Pregnancy Outcomes Reporting System, Massachusetts Birth Defects Monitoring Program, New Jersey Special Health Services Registry, New York State Congenital Malformations Registry, North Carolina Birth Defects Monitoring Program, Texas Birth Defects Epidemiology, and Surveillance Branch. We also thank the National Birth Defects Prevention Network Data Committee, particularly Dr. Russell Kirby and Mr. Russel Rickard, for facilitating the data collection process. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflicts of interest: None to report.
Financial disclosure: None to report.
References
- Baty BJ, Blackburn BL, Carey JC. Natural history of trisomy 18 and trisomy 13: 1. Growth, physical assessment, medical histories, survival, and recurrence risk. Am J Med Genet. 1994;49:175–188. doi: 10.1002/ajmg.1320490204. [DOI] [PubMed] [Google Scholar]
- Boghossian NS, Hansen NI, Bell EF, Stoll BJ, Murray JC, Carey JC, Adams-Chapman I, Shankaran S, Walsh MC, Laptook AR, Faix RG, Newman NS, Hale EC, Das A, Wilson LD, Hensman AM, Grisby C, Collins MV, Vasil DM, Finkle J, Maffett D, Ball MB, Lacy CB, Bara R, Higgins RD. Mortality and morbidity of VLBW infants with trisomy 13 or trisomy 18. Pediatrics. 2014;133:226–235. doi: 10.1542/peds.2013-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer CM, Holloway SH, Stone DH, Carothers AD, FitzPatrick DR. Survival in trisomy 13 and trisomy 18 cases ascertained from population based registers. J Med Genet. 2002;39:e54. doi: 10.1136/jmg.39.9.e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns DA. Developmental status of 22 children with trisomy 18 and eight children with trisomy 13: Implications and recommendations. Am J Med Genet Part A. 2015;167A:1807–1815. doi: 10.1002/ajmg.a.37102. [DOI] [PubMed] [Google Scholar]
- Carey JC. Perspectives on the care and management of infants with trisomy 18 and trisomy 13: Striving for balance. Curr Opin Pediatr. 2012;24:672–678. doi: 10.1097/MOP.0b013e3283595031. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables. J Royal Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- Crider KS, Olney RS, Cragan JD. Trisomies 13 and 18: Population prevalences, characteristics, and prenatal diagnosis, Metropolitan Atlanta, 1994–2003. Am J Med Genet Part A. 2008;146A:820–826. doi: 10.1002/ajmg.a.32200. [DOI] [PubMed] [Google Scholar]
- Hsu H-F, Hou J-W. Variable expressivity in Patau syndrome is not all related to trisomy 13 mosaicism. Am J Med Genet Part A. 2007;143A:1739–1748. doi: 10.1002/ajmg.a.31835. [DOI] [PubMed] [Google Scholar]
- Irving C, Richmond S, Wren C, Longster C, Embleton ND. Changes in fetal prevalence and outcomes for trisomies 13 and 18: A population-based study over 23 years. J Matern Fetal Neonat Med. 2011;24:137–141. doi: 10.3109/14767051003758879. [DOI] [PubMed] [Google Scholar]
- Janvier A, Okah F, Farlow B, Lantos JD. Ethics rounds: An infant with trisomy 18 and a ventricular septal defect. Pediatrics. 2011;127:754–758. doi: 10.1542/peds.2010-1971. [DOI] [PubMed] [Google Scholar]
- Jones KL, Jones MC, del Campo M. Smith’s recognizable patterns of human malformation, 7th edition. Philadelphia: Elsevier/Saunders; 2013. p. 976. [Google Scholar]
- Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2. New York: John Wiley and Sons; 2002. p. 462. [Google Scholar]
- Kaneko Y, Kobayashi J, Yamamoto Y, Yoda H, Kanetaka Y, Nakajima Y, Endo D, Tsuchiya K, Sato H, Kawakami T. Intensive cardiac management in patients with trisomy 13 or trisomy 18. Am J Med Genet Part A. 2008;146A:1372–1380. doi: 10.1002/ajmg.a.32311. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Kobayashi J, Achiwa I, Yoda H, Tsuchiya K, Nakajima Y, Endo D, Sato H, Kawakami T. Cardiac surgery in patients with trisomy 18. Pediatr Cardiol. 2009;30:729–734. doi: 10.1007/s00246-009-9427-0. [DOI] [PubMed] [Google Scholar]
- Kosho T, Nakamura T, Kawame H, Baba A, Tamura M, Fukushima Y. Neonatal management of trisomy 18: Clinical details of 24 patients receiving intensive treatment. Am J Med Genet Part A. 2006;140A:937–944. doi: 10.1002/ajmg.a.31175. [DOI] [PubMed] [Google Scholar]
- Lakovschek IC, Streubel B, Ulm B. Natural outcome of trisomy 13, trisomy 18, and triploidy after prenatal diagnosis. Am J Med Genet Part A. 2011;155A:2626–2633. doi: 10.1002/ajmg.a.34284. [DOI] [PubMed] [Google Scholar]
- Lee ET. Statistical methods for survival data analysis. Belmont, CA: Lifetime Learning Publications; 1980. p. 557. [Google Scholar]
- Lin H-Y, Lin S-P, Chen Y-J, Hung H-Y, Kao H-A, Hsu C-H, Chen M-R, Chang J-H, Ho C-S, Huang F-Y, Shyur S-D, Lin D-S, Lee H-C. Clinical characteristics and survival of trisomy 18 in a medical center in Taipei, 1988–2004. Am J Med Genet Part A. 2006;140A:945–951. doi: 10.1002/ajmg.a.31173. [DOI] [PubMed] [Google Scholar]
- Maeda J, Yamagishi H, Furutani Y, Kamisago M, Waragai T, Oana S, Kajino H, Matsuura H, Mori K, Matsuoka R, Nakanishi T. The impact of cardiac surgery in patients with trisomy 18 and trisomy 13 in Japan. Am J Med Genet Part A. 2011;155:2641–2646. doi: 10.1002/ajmg.a.34285. [DOI] [PubMed] [Google Scholar]
- Merritt DA, Catlin A, Wool C, Paverini R, Goldstein M, Oshiro B. Trisomy 18 and trisomy 13: Treatment and management decisions. NeoReviews. 2012;3:540–548. [Google Scholar]
- Nelson KE, Hexem KR, Feudtner C. Inpatient hospital care of children with trisomy 13 and trisomy 18 in the United States. Pediatrics. 2012;129:868–876. doi: 10.1542/peds.2011-2139. [DOI] [PubMed] [Google Scholar]
- Niedrist D, Riegel M, Achermann J, Schinzel A. Survival with trisomy 18—Data from Switzerland. Am J Med Genet Part A. 2006;140A:952–959. doi: 10.1002/ajmg.a.31172. [DOI] [PubMed] [Google Scholar]
- Parker MJ, Budd JLS, Draper ES, Young ID. Trisomy 13 and trisomy 18 in a defined populations: Epidemiological, genetic and prenatal observations. Prenatal Diagn. 2003;23:856–860. doi: 10.1002/pd.707. [DOI] [PubMed] [Google Scholar]
- Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, Correa A. Updated national prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res Part A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- Peroos S, Forsythe E, Pugh JH, Arthur-Farraj P, Hodes D. Longevity and Patau syndrome: What determines survival? BMJ Case Rep. 2012 doi: 10.1136/bcr-06-2011-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pont SJ, Robbins JM, Bird TM, Gibson JB, Cleves MA, Tilford JM, Aitken ME. Congenital malformations among liveborn infants with trisomies 18 and 13. Am J Med Genet Part A. 2006;140A:1749–1756. doi: 10.1002/ajmg.a.31382. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Wong LC, Yang Q, May KM, Friedman JM. Population-based analysis of mortality in trisomy 13 and trisomy 18. Pediatrics. 2003;111:777–784. doi: 10.1542/peds.111.4.777. [DOI] [PubMed] [Google Scholar]
- Redheendran R, Neu RL, Bannerman RM. Long survival in trisomy 13 syndrome: 21 cases including prolonged survival in two patients 11 and 19 years old. Am J Med Genet. 1981;8:167–172. doi: 10.1002/ajmg.1320080207. [DOI] [PubMed] [Google Scholar]
- SOFT. [Accessed July 20, 2015];2015 http://trisomy.org/
- Tonks AM, Gornall AS, Larkins SA, Gardosi JO. Trisomies 18 and 13: Trends in prevalence and prenatal diagnosis—Population based study. Prenat Diagn. 2013;33:742–750. doi: 10.1002/pd.4117. [DOI] [PubMed] [Google Scholar]
- Tsukada K, Imataka G, Suzumura H, Arisaka O. Better prognosis in newborns with trisomy 13 who received intensive treatments: A retrospective study of 16 patients. Cell Biochem Biophys. 2012;63:191–198. doi: 10.1007/s12013-012-9355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunca Y, Kadandale JS, Pivnick EK. Long-term survival in Patau syndrome. Clin Dysmorphol. 2001;10:149–150. doi: 10.1097/00019605-200104000-00014. [DOI] [PubMed] [Google Scholar]
- USDA. [Accessed May 22, 2015];2013 http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx.
- Vendola C, Canfield M, Daiger SP, Gambello M, Hashimi SS, King T, Noblin SJ, Waller DK, Hecht JT. Survival of Texas infants born with trisomies 21, 18, and 13. Am J Med Genet Part A. 2010;152A:360–366. doi: 10.1002/ajmg.a.33156. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu G, Canfield MA, Mai CT, Gilboa SM, Meyer RE, Anderka M, Copeland GE, Kucik JE, Nembhard WN, Kirby RS. Racial/ethnic differences in survival of United States children with birth defects: A population-based study. J Pediatr. 2015;166:819–826. doi: 10.1016/j.jpeds.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber WW. Survival and sex ratio in trisomy 17–18. Am J Hum Genet. 1967;19:369–377. [PMC free article] [PubMed] [Google Scholar]
- Wu J, Springett A, Morris JK. Survival of trisomy 18 (Edwards syndrome) and trisomy 13 (Patau syndrome) in England and Wales, 2004–2011. Am J Med Genet Part A. 2013;161A:2512–2518. doi: 10.1002/ajmg.a.36127. [DOI] [PubMed] [Google Scholar]