Abstract
Energy balance, the relationship between energy intake and expenditure, is regulated by a complex interplay of hormones, brain circuits and peripheral tissues. Leptin is an adipocyte-derived cytokine that suppresses appetite and increases energy expenditure. Ironically, obese individuals have high levels of plasma leptin and are resistant to leptin treatment. Neurotrophic factors, particularly ciliary neurotrophic factor (CNTF) and brain-derived neurotrophic factor (BDNF), are also important for the control of body weight. CNTF can overcome leptin resistance to reduce body weight, although CNTF and leptin activate similar signalling cascades. Mutations in the gene for BDNF lead to insatiable appetite and severe obesity.
If energy intake exceeds energy expenditure for a long period, obesity can result. A key factor in the regulation of energy intake is the sensation of satiety, and energy expenditure mainly occurs through basal metabolism, physical activity, thermoregulation, and digestion and processing of ingested foods1,2. A highly complex system has been evolved to control the balance between these two processes. The system is composed of several organs, including the liver, the pancreas, adipose tissues, the gastrointestinal tract, and the brain. The peripheral tissues produce hormones and other signals in response to changes in feeding state and the size of the energy store of the body (adipose tissue). Examples of this are leptin produced in white adipocytes3, insulin produced in the pancreas4, ghrelin produced in the stomach5–8, PYY3-36 produced in the intestine9–11, and certain nutrients obtained from ingested food (e.g. glucose, fatty acids, and amino acids)12–15. These signals are integrated by a variety of neurons in the hypothalamus and brainstem, which then act to control food intake and energy expenditure16–18. Among these neurons, those carrying out the anorexigenic effect of the following three ligand-receptor pairs are particularly important: leptin and its receptor, alpha-melanocyte-stimulating hormone (αMSH) and one of its receptors the melanocortin-4 receptor (MC4R), and brain-derived neurotrophic factor (BDNF) and the tropomyosin receptor kinase B (TrkB).
Neurotrophic factors are well known for their activity of promoting neuronal differentiation and survival, axonal and dendritic growth, and synaptic formation and plasticity19,20. In addition to BDNF, studies have implicated another neurotrophic factor, ciliary neurotrophic factor (CNTF), in the control of human body weight. Mutations in the genes for BDNF and its receptor TrkB (encoded by the NTRK2 gene; NTRK2 stands for neurotrophic tyrosine kinase, receptor, type 2) lead to severe obesity in humans (Box 1)21–24. Subcutaneous administration of recombinant CNTF has been found to reduce food intake and cause weight loss in obese humans25. In this review, we focus on the evidence for BDNF and CNTF as regulators of satiety and body weight, discuss the recent progress on our understanding of the neural basis of the anorexigenic effect of these two factors, and present our opinions on the future directions of this research. We start with a brief overview of the central mechanism of energy balance regulation, focusing on the two well-studied signalling pathways, leptin and melanocortin, which are likely to interact with BDNF and CNTF to regulate body weight.
Box 1. Obesity in humans with BDNF or NTRK2 mutations.
A de novo Y722C missense mutation in TrkB was found in a boy with severe obesity and impairment in learning, memory and nociception21. Y722 is a key tyrosine residue in the activation loop of the TrkB kinase domain80, and the mutation markedly impairs activation of the TrkB receptor21. Furthermore, a de novo mutation in a girl with hyperphagia and severe obesity was found to cause BDNF haploinsufficiency22. Interestingly, one study shows that BDNF haploinsufficiency is the cause for hyperphagia and obesity associated with some patients with the Wilms’ tumor, aniridia, genitourinary anomalies, and mental retardation (WAGR) syndrome24. The WAGR syndrome is a rare disorder and results from heterozygous, variably sized, contiguous deletions on chromosome 11, which extend into the BDNF gene in some cases146. Careful mapping of the deletion regions has linked BDNF haploinsufficiency to the accompanied obesity disorder24. These genetic studies clearly show that impairment in TrkB signalling does lead to obesity in humans; however, these mutations are rare and would not be significant contributors to the high prevalence of obesity.
Single nucleotide polymorphisms (SNPs) in the human BDNF gene could have a larger impact on human obesity prevalence than mutations in the BDNF and NTRK2 genes. Genome-wide association studies found several common SNPs in or near the BDNF gene to be associated with increased body mass index147–154. One SNP (rs6265) is particularly interesting. This polymorphism is in the sequence encoding the pro-domain of the BDNF precursor, changes the Val residue at position 66 of preproBDNF to a Met residue (Val66Met), and impairs BDNF secretion via the regulated secretory pathway155,156. A mouse strain that mimics the rs6265 polymorphism has been generated, and mice homozygous for the Val66Met substitution have significantly higher body weight than normal mice on a regular diet157, further supporting a role for rs6265 in human obesity.
Key energy balance circuits
Several factors have been demonstrated to play an important role in the central control of energy balance, and they include leptin, melanocortin, melanin-concentrating hormone, orexin, and dopamine26–32. Due to space limitations, we will only discuss leptin and melanocortin in this review.
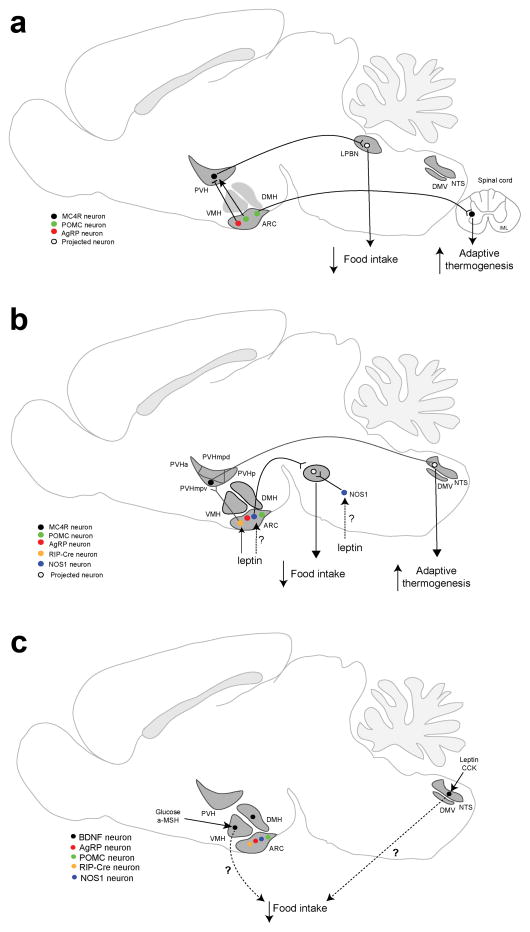
Remarkable progress has been made over the past two decades in understanding how leptin and melanocortin regulate energy balance. In the arcuate nucleus of the hypothalamus (ARH), ARHPOMC neurons that express proopiomelanocortin (POMC) produce αMSH, which as already mentioned activates MC4R (Fig. 1a). ARHAgRP neurons co-express neuropeptide Y (NPY) and agouti-related peptide (AgRP), an inverse agonist for MC4R17,33. These two distinct neuronal populations project to MC4R-expressing neurons in multiple CNS regions34–36. Studies have shown that ARHPOMC and ARHAgRP neurons project to glutamatergic paraventricular hypothalamic MC4R-expressing neurons (PVHMC4R), which in turn project to the lateral parabrachial nucleus (LPBN) to mediate the effect of MC4R on appetite35,37,38. By contrast, the projection of ARHPOMC neurons to the MC4R-expressing sympathetic preganglionic neurons in the intermediolateral column (IML) of the spinal cord mediates the stimulatory effect of MC4R on energy expenditure39.
Figure 1. Key central neural circuits in the regulation of energy balance.
a. Neural circuits mediating the effect of melanocortin on food intake and energy expenditure. POMC neurons and AgRP neurons in the ARH regulate food intake through stimulating or inhibiting LPBN-projecting MC4R-expressing neurons in the PVH, respectively. POMC neurons that project to the spinal cord stimulate adaptive thermogenesis in brown adipose tissues through the MC4R in sympathetic preganglionic neurons located in the IML.
b. Neural circuits mediating the effect of leptin on food intake and energy expenditure. In addition to POMC neurons and AgRP neurons, the ARH contains RIP-Cre neurons and NOS1 neurons. NOS1 neurons are also present in other brain areas. Leptin stimulates adaptive thermogenesis in brown adipose tissues by increasing GABA release of ARH Rip-Cre neurons onto NTS-projecting neurons in the ventral medial parvicellular part of the PVH (PVHmpv). Leptin simultaneously suppresses food intake by acting on NOS1 neurons in an undefined location.
c. Locations of BDNF neurons that sense nutritional state. Glucose and anorexigenic factors, such as leptin, CCK, and αMSH, stimulate Bdnf gene expression in the VMH and dorsal vagal complex that is comprised of the DMV and NTS, suggesting that BDNF neurons in these two brain areas are involved in the control of food intake. It remains to be elucidated how BDNF produced in these neurons regulates food intake.
Abbreviations: ARH, arcuate nucleus of the hypothalamus; DMH, dorsomedial hypothalamus; DMV, dorsal motor nucleus of the vagus; IML, intermediolateral column of the spinal cord; LPBN, lateral parabrachial nucleus; NTS, nucleus of the solitary tract; PVH, paraventricular hypothalamus; PVHa: anterior part of the PVH; PVHmpd, dorsal medial parvicellular part of the PVH; PVHmpv, ventral medial parvicellular part of the PVH; PVHp, posterior part of the PVH; VMH, ventromedial hypothalamus.
Although leptin has been shown to regulate the gene expression and firing rate of both ARHPOMC neurons and ARHAgRP neurons40–42, genetic evidence indicates that the effects of leptin to suppress appetite and to promote energy expenditure are mainly mediated through action on other neuronal types43,44. Recent studies show that GABAergic Rip-Cre neurons in the ARH and hypothalamic neurons expressing nitric oxide synthase-1 (NOS1) mediate the effect of leptin on energy balance45,46 (Fig. 1b). In the ARH, NOS1 neurons and RIP-Cre neurons are distinct from POMC neurons and AgRP neurons45,46. ARHRip-Cre neurons project to glutamatergic neurons in the ventral medial parvicellular part of the PVH (PVHmpv), which make connections with GABAergic neurons in the nucleus of the solitary tract (NTS). Leptin stimulates GABA release from ARHRip-Cre neurons into the PVHmpv to promote thermogenesis in brown adipose tissues45. NOS1 neurons are distributed in several brain areas, including the ARH and dorsomedial hypothalamus (DMH)46. Because leptin promotes satiety through GABAergic neurons47, it would be expected that the NOS1 neurons that afford leptin the anorexigenic activity would be GABAergic; however, their exact location remains to be determined.
BDNF: a key regulator of energy balance
As discussed earlier, induction of satiety and increased energy expenditure are two key regulators of overall energy balance, and BDNF appears to play a crucial role in both. The first clue that BDNF may be involved in the control of energy balance came from a study in which chronic intracerebroventricular infusion of recombinant human BDNF was intended to examine the effect of BDNF on the function of cholinergic neurons but instead was found to reduce body weight of adult rats48. The weight loss resulted from appetite suppression and consequent decreased food intake49. This pharmacological discovery was corroborated by genetic evidence collected from several mouse mutants. Bdnf heterozygous mice display increased body weight and mild hyperphagia50,51. Furthermore, severe obesity was observed in hypomorphic mice expressing TrkB at ~25% of normal levels52 and in mice where the Bdnf gene is deleted using a Cre transgene driven by the promoter for the alpha isoform of Ca2+/calmodulin-dependent protein kinase II (CaMKIIα)53. Because CaMKIIα is a brain-specific protein54 and the Camk2α-Cre transgene has not been reported to be active outside the brain although transcripts derived from the Camk2α gene are present in peripheral tissues such as muscle55, the last two observations suggest that BDNF produced in the brain acts on the TrkB receptor to regulate energy balance.
If BDNF acts as a signal regulating energy balance, its expression levels would be expected to change to reflect nutritional state (Fig. 1c). Two-day food deprivation was found to drastically and selectively reduce levels of Bdnf mRNA in the mouse ventromedial hypothalamus (VMH)52,56,57. Furthermore, food deprivation was also found to reduce BDNF protein levels in the dorsal vagal complex (DVC) that contains the NTS and dorsal motor nucleus of the vagus (DMV), whereas refeeding or peripheral injection of the anorexigenic hormones leptin or CCK increased BDNF protein levels in this area58, although it was not clear whether the regulation is at the level of transcription, translation, or protein release. Melanocortin and glucose are likely key mediators linking energy status to Bdnf gene expression in the VMH, as administration of either a melanocortin analog or glucose into fasted mice increased levels of Bdnf mRNA in the VMH52,56. These gene expression data strongly support the notion that BDNF actively participates in the regulation of energy balance.
Neurons that produce BDNF to suppress appetite
As discussed above, food deprivation selectively and drastically reduces levels of Bdnf mRNA in the mouse VMH52,56,57. This observation raises the possibility that VMH neurons produce BDNF to suppress appetite (Fig. 2). Indeed, selective deletion of the Bdnf gene in the VMH and DMH of adult mice with adeno-associated virus (AAV) expressing Cre recombinase caused modest hyperphagic obesity56. Because severe obesity was observed in mice where the Bdnf gene was deleted in CaMKIIα-expressing neurons59 or where TrkB was down-regulated in the whole body throughout the life52, this modest phenotype could be due to either incomplete AAV-mediated Bdnf deletion in the VMH or Bdnf deletion in adulthood, which would bypass the impact of BDNF deficiency on neuronal development. However, no body weight phenotype was detected in mice where the Bdnf gene in the VMH was deleted during development using the Sf1-Cre transgene, which is driven by the promoter for steroidogenic factor 1 that is required for differentiation of VMH neurons59. These conflicting results remained inexplicable until a study was untaken in the PVH60.
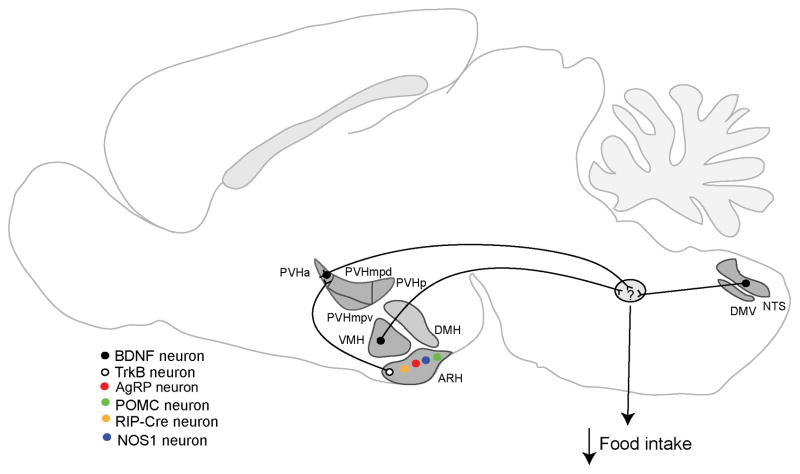
Figure 2. Neural circuits mediating the effect of BDNF on energy intake.
BDNF neurons in the anterior PVH (PVHa), VMH, and DVC (including the NTS and DMV) have been implicated in the control of energy intake. The majority of TrkB neurons in the ARH are distinct from AgRP neurons, POMC neurons, and RIP-Cre neurons. BDNF-expressing PVH neurons likely receive inputs from TrkB neurons in the ARH; however, it is unknown which neurons provide inputs to the BDNF neurons in the VMH and DVC. It is also unknown where these BDNF neurons project to suppress food intake (indicated by question mark in the green oval). Abbreviations: ARH, arcuate nucleus of the hypothalamus; DMH, dorsomedial hypothalamus; DMV, dorsal motor nucleus of the vagus; DVC, dorsal vagal complex; NTS, nucleus of the solitary tract; PVH, paraventricular hypothalamus; PVHa: anterior part of the PVH; PVHmpd, dorsal medial parvicellular part of the PVH; PVHmpv, ventral medial parvicellular part of the PVH; PVHp, posterior part of the PVH; VMH, ventromedial hypothalamus.
The PVH is a heterogeneous brain structure with many different cell types61. BDNF-expressing neurons (PVHBDNF) are scattered throughout the rostrocaudal extent of the PVH60. Approximately 30% of PVHBDNF neurons express tyrosine hydroxylase or thyrotropin-releasing hormone, and the rest express a little of other known PVH markers, including oxytocin, somatostatin, growth hormone releasing hormone, corticotropin-releasing hormone, vasopressin, and MC4R60. Thus, the majority of PVHBDNF neurons are distinct from previously defined cell types. Deletion of the Bdnf gene in the adult PVH using AAV-Cre led to marked hyperphagia and severe obesity, and this phenotype was strongly associated with ablation of Bdnf gene expression in the anterior part of PVH60. This exciting finding indicates that anterior PVHBDNF neurons are key cells that produce BDNF to suppress appetite (Fig. 2). Identification of these neurons makes it possible to determine where BDNF interacts with TrkB to regulate food intake in future studies.
Deletion of the Bdnf gene in the PVH during embryogenesis using the Sim1-Cre transgene38 still caused hyperphagia and obesity; however, the phenotype was much less robust than that observed in mice where the Bdnf gene was deleted in the adult PVH60. This phenomenon has been proposed to result from developmental compensation60. Because TrkB hypomorphic mutant mice still develop marked hyperphagia and severe obesity even though TrkB expression is down regulated throughout the entire lifespan52, this compensation should come from up-regulation of BDNF in brain regions outside the PVH, rather than increased activity of other anorexigenic signalling pathways. A similar compensation mechanism may explain why deleting the Bdnf gene in the adult VMH leads to modest obesity56, whereas deleting the Bdnf gene in the embryonic VMH using the Sf1-Cre transgene does not alter body weight59. These observations suggest that BDNF neurons in some brain structures (including the VMH) other than the PVH also play a role in the regulation of energy intake.
As BDNF is expressed in the DVC62,63, one population of such BDNF neurons could be in the DVC. It has been shown that nutritional state, leptin, and CCK regulate levels of BDNF protein in the rat DVC58. Genetic studies are needed to determine the role of DVC BDNF in the control of food intake and energy balance.
BDNF and the regulation of energy expenditure
Energy expenditure has several components, including basal metabolism rate, spontaneous physical activity, thermoregulation, and exercise. Rodents produce heat through β-oxidation of fatty acid in brown adipose tissues (BAT) to maintain body temperature, and the BAT thermogenesis is a main component of energy expenditure in mice1,64. Although it was initially thought that adult humans did not possess BAT, recent studies using new technologies such as positron emission tomography indicate a significant amount of metabolically active BAT65. The distribution of BAT deposits is similar between adult humans and rodents: a large BAT pad in the vicinity of the upper thorax, which is called interscapular BAT (iBAT) in rodents, and small BAT pads atop each of the sympathetic ganglia and near the adrenal gland66. This stereotypical localization of BAT depots indicates that regulation of thermogenesis is conserved from rodents to humans.
Sympathetic outflow increases the expression of uncoupling protein 1 (UCP1) in iBAT, which allows the energy generated from β oxidation of fatty acids to dissipate as heat in response to physiological and environmental stimuli such as overeating and cold by uncoupling the proton gradient from ATP synthesis in mitochondria67,68. Both peripheral and central administration of BDNF were found to increase turnover of norepinephrine, a neurotransmitter of sympathetic neurons, and Ucp1 gene expression in iBAT and to restore thermogenesis in food-deprived mice with a defective leptin receptor69,70. Furthermore, direct injection of BDNF into the hypothalamus in rats resulted in an increase in energy expenditure71,72. These pharmacological studies suggest that BDNF is involved in the regulation of energy expenditure.
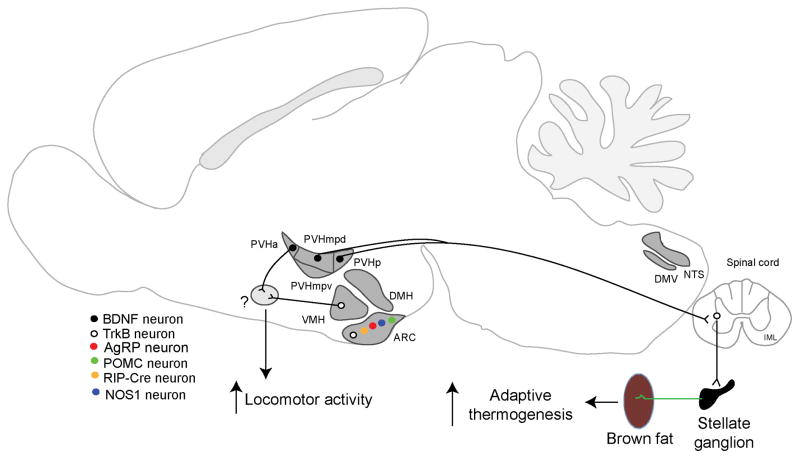
The genetic evidence for an important role for BDNF in regulating energy expenditure comes from a study of the PVH (Fig. 3). Deletion of the Bdnf gene in the PVH with either the Sim1-Cre transgene or stereotaxic AAV-Cre injection led to reduced energy expenditure in mice by decreasing locomotor activity and impairing iBAT thermogenesis60. Trans-neuronal tract tracing studies using pseudorabies virus have identified neurons in three PVH divisions that form polysynaptic connection to iBAT in rats: dorsal medial parvicellular part (PVHmpd), ventral medial parvicellular part (PVHmpv) and posterior part (PVHp)73,74. Impressively, all neurons in the PVHmpd and PVHp that are connected to iBAT express BDNF in mice60. Furthermore, the vast majority of BDNF neurons in these two PVH divisions send axons to the spinal cord60. These observations suggest that BDNF neurons in the PVHmpd and PVHp release BDNF at their axonal terminals to support sympathetic preganglionic neurons in the IML of the spinal cord, which control iBAT metabolism through sympathetic postganglionic neurons in the stellate ganglion. In support of this notion, sympathetic preganglionic neurons in the IML were found to express TrkB and become atrophic when the Bdnf gene was deleted in the PVH60. Thus, BDNF neurons in the anterior PVH promote locomotor activity, whereas those in the medial and posterior PVH drive iBAT thermogenesis through the PVH–IML–stellate ganglion– iBAT neural circuit.
Figure 3. Neural circuits mediating the effect of BDNF on energy expenditure.
BDNF neurons in the PVHmpd and PVHp have been shown to drive adaptive thermogenesis in brown adipose tissues through a polysynaptic circuit that includes TrkB-expressing sympathetic preganglionic neurons in the IML of the spinal cord and sympathetic postganglionic neurons in the stellate ganglion. In addition, there is evidence to support that BDNF neurons in the PVHa and TrkB neurons in the VMH stimulate energy expenditure by promoting locomotor activity through undefined neural circuits (indicated by question mark in the green oval). Abbreviations: ARH, arcuate nucleus of the hypothalamus; DMH, dorsomedial hypothalamus; DMV, dorsal motor nucleus of the vagus; IML, intermediolateral column of the spinal cord; NTS, nucleus of the solitary tract; PVH, paraventricular hypothalamus; PVHa: anterior part of the PVH; PVHmpd, dorsal medial parvicellular part of the PVH; PVHmpv, ventral medial parvicellular part of the PVH; PVHp, posterior part of the PVH; VMH, ventromedial hypothalamus.
The observation that PVHBDNF neurons play a role in regulating energy expenditure does not contradict with the reports that hyperphagia is the cause of obesity in several Bdnf mouse mutants. Pair feeding, which restricts mutant mice to consume the same amount of food as wild-type mice, was found to correct excessive weight gain in Bdnf heterozygous mice75, in conditional knockout mice where the Bdnf gene was selectively deleted in the DMH and VMH56, and in mice lacking the long isoform of Bdnf mRNA76. However, Bdnf heterozygous mice show elevated locomotor activity50, which may compensate for reduced BAT thermogenesis; the DMH/VMH-BDNF conditional knockout mice should still have normal BDNF expression in the PVH to drive iBAT thermogenesis and promote locomotor activity; neurons also produce a short form of Bdnf mRNA77, and BDNF derived from the short form of Bdnf mRNA in the PVH may be sufficient to regulate BAT thermogenesis and locomotor activity. In support of this argument, young mice that lack the long form of Bdnf mRNA have normal expression of thermogenic genes in iBAT and normal size of sympathetic preganglionic neurons in the IML60.
The PVHmpv neurons have been shown to receive innervation from GABAergic RIP-Cre neurons in the ARH and project to the NTS to stimulate BAT thermogenesis45. This ARHRIP-Cre neuron–PVHmpv–NTS neural circuit and the PVHBDNF neuron–IML neural circuit may play distinct roles in the control of BAT thermogenesis. Disruption of the former circuit impairs BAT thermogenesis in response to high-fat diet ingestion45, while the latter circuit is not required for BAT thermogenesis in response to high-fat diet ingestion, although it is essential for BAT thermogenesis in response to cold exposure60,78. Studies are needed to address how these two neural circuits interact with each other to regulate adaptive thermogenesis.
In addition to the PVH, BDNF expressed in other brain regions may also have a role in the control of energy expenditure. For example, BDNF injection to the VMH has been found to increase energy expenditure by promoting locomotor activity in rats71; environmental enrichment has been thought to induce browning of white adipocytes by stimulating Bdnf gene expression in the hypothalamus79. It remains to be determined which neurons produce BDNF to mediate these effects.
TrkB neurons in energy balance regulation
BDNF exerts its biological effects through two receptors, p75NTR and TrkB80. Mutations in the gene for TrkB, but not p75NTR, lead to obesity in humans and mice21,52,81, suggesting that the effects of BDNF on energy balance are mediated via the TrkB receptor. There are four modes by which BDNF produced in a neuron can reach TrkB-expressing neurons82–84: being released and binding to TrkB on the same neuron (autocrine) or neighbour neurons (paracrine); being anterogradely transported to the axonal terminals of the neuron and released to bind TrkB on targeted neurons (anterograde); and being retrogradely transported to cell bodies of innervating neurons following release and binding to TrkB at axonal terminals (retrograde). Therefore, BDNF may act on TrkB neurons near or far away from the cell bodies of its producing neurons to regulate energy balance. The binding of BDNF to TrkB activates several intracellular signalling cascades (Box 2), and no studies have been conducted to determine which signalling cascades are required for BDNF regulation of energy balance.
Box 2. TrkB signalling and energy balance regulation.
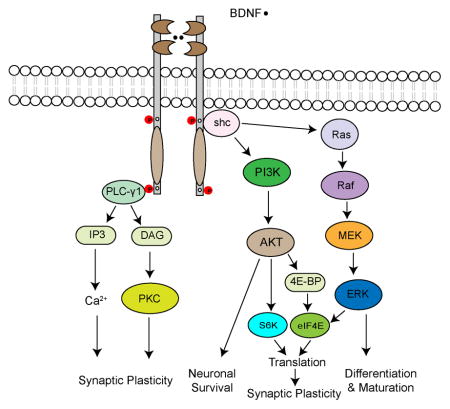
The binding of BDNF to TrkB receptors causes receptor dimerization, which, leads to activation of their tyrosine kinase and phosphorylation of tyrosine residues located in their intracellular domains (see figure). Some of the phosphorylated tyrosine residues serve as the docking sites of signalling molecules such as Shc and phospholipase C-γ1 (PLC-γ1). Recruitment of these signalling molecules triggers activation of a variety of intracellular signalling pathways, such as those mediated by mitogen-activated protein kinase (MAPK, also called ERK for extracellular signal-regulated kinase), phosphatidylinositol 3-kinase (PI3K) and PLC-γ1. Activation of the RAS-RAF-ERK kinase (MEK)-MAPK signalling cascade promotes neuronal differentiation and maturation, whereas activation of the PI3K-AKT signalling cascade is essential for neuronal survival80. In addition, the MAPK and PI3K signalling cascades increase translation by phosphorylating eukaryotic initiation factor 4E (eIF4E), 4E-binding protein (4E-BP) and ribosomal protein S6 kinase (S6K), which play an important role in the regulation of protein synthesis-dependent synaptic plasticity158. Activated PLC-γ1 hydrolyses phosphatidylinositol 4,5-bisphosphate to produce diacylglycerol (DAG), which can activate protein kinase C (PKC), and inositol 1,4,5-triphosphate (IP3), which leads to the Ca2+ release from intracellular stores. Activation of PKC- and Ca2+-regulated pathways can enhance synaptic plasticity80.
It is unknown which signalling cascade is required for BDNF regulation of energy balance. Because synaptic plasticity is important to the regulation of energy balance133,134 and BDNF is a potent regulator of synaptic plasticity in multiple brain regions159,160, it is plausible to speculate that BDNF regulates energy balance by modulating synaptic plasticity of neuronal circuits in the hypothalamus and brainstem.
TrkB neurons in several brain structures have been implicated in the control of energy balance. As expected, these structures include several hypothalamic nuclei such as ARH, VMH, and PVH72,85–91. In addition, some evidence indicates that TrkB neurons in nucleus accumbens and NTS are also involved in regulating energy balance92,93.
The ARH is situated in close vicinity to the bottom of the third ventricle and is an important brain region that senses circulating factors reflecting the state of feeding and energy store17. It contains at least three neuronal populations on the basis of the expression of three markers: POMC, AgRP, and RIP-Cre. The ARH also expresses TrkB, and the majority of ARHTrkB neurons are distinct from these three neuronal populations88. Some ARHTrkB neurons are activated in response to either fasting or refeeding after fasting88, indicating that these neurons may play a role in the control of food intake. It is necessary to further evaluate the role of ARHTrkB neurons in the control of energy balance by deleting the Ntrk2 gene or altering the activity of these neurons.
If ARHTrkB neurons do regulate food intake, they may do so by projecting to PVHBDNF neurons (Fig. 2). This view is supported by the observation that the axonal number of ARH neurons is significantly reduced in the PVH of mutant mice that lack the long form of Bdnf mRNA and display marked hyperphagia76,88. It has been shown that the long form of Bdnf mRNA is transported to dendrites for local translation94. Thus, the mouse mutant that lacks the long form of Bdnf mRNA would not synthesize BDNF in dendrites. Because dendritically synthesized BDNF is released from dendrites, ARHTrkB neurons should innervate dendrites of PVHBDNF neurons to achieve their potential effect on food intake.
It is worth noting that one study shows that although central administration of TrkB agonists (BDNF, NT-4, or TrkB antibody agonist) is anorexigenic in non-human primates, similar to what has been observed for rodents, peripheral administration is orexigenic, opposite to what is observed for rodents95. Peripherally administered TrkB agonists likely act on ARHTrkB neurons to affect food intake, because the blood-brain barrier is more permeable in the region of the ARH. It is possible that some ARHTrkB neurons may promote feeding when the TrkB is activated. Peripherally administered TrkB agonists could preferentially act on these orexigenic neurons in non-human primates, but mainly acting on anorexigenic ARHTrkB neurons in rodents, owing to anatomical differences in these two species.
Direct injection of BDNF into either the VMH or the PVH of adult rats decreased food intake and increased energy expenditure71,72,86,87. One study suggests that BDNF suppresses feeding in the VMH by increasing cell-surface expression of a thrombospondin receptor, which enhances the function of excitatory synapses90. Delivery of BDNF into the medial NTS of the brainstem also reduced food intake and increased thermogenesis, and these effects could be blocked with a TrkB antagonist96. These results suggest that TrkB in these three brain areas is important for the control of energy balance. However, genetic evidence is still needed to demonstrate that endogenous BDNF-to-TrkB signalling in each of these brain regions is involved in the control of energy balance.
Besides the ARH, VMH, PVH and NTS, BDNF may acts on TrkB neurons in other brain regions to exert homeostatic regulation of feeding and energy expenditure. It has become clear that neuronal compositions in each hypothalamic or hindbrain nucleus, such as the ARH88 and PVH60, are complex. It is reasonable to consider that TrkB neurons in each of these nuclei are connected to multiple neural circuits. Once a brain region is confirmed as a BDNF action site, the next challenge is to map the neural circuits that are the basis for BDNF-regulated energy balance. This information is essential to understand where BDNF interacts with TrkB and how BDNF modulates the activity of the neural circuit in response to changes in feeding state and energy store.
BDNF and hedonic eating
In addition to its role in the homeostatic regulation of energy balance, BDNF may suppress hedonic feeding of palatable food through the reward system, which begins in the ventral tegmental area (VTA) and connects to the nucleus accumbens97. Mice with deficient BDNF-to-TrkB signalling displayed much worse hyperphagia on high-fat diets than on low-fat diets52,92,98, which is attributed to increased meal size98. Mice lacking BDNF synthesis in CaMKIIα-expressing neurons displayed hypersensitivity of the dopamine receptor D1, such that peripheral injection of a D1 receptor agonist normalized the hyperphagia on high-fat diets92. The effect of BDNF deficiency on the dopamine receptor D1 could be a compensatory response to decreased evoked release of dopamine in the nucleus accumbens and the dorsal striatum. As the VTA has been identified as an important source of BDNF contributing to regulation of hedonic eating92, it would be interesting to determine whether BDNF acts on TrkB-expressing neurons in the VTA or the nucleus accumbens to regulate consumption of palatable food. It is critical to understand the changes in the structure and function of the reward circuit when BDNF signalling is impaired, which leads to hyperphagia on high-fat diet, as excessive intake of high-fat diets is likely an important cause of human obesity.
Ciliary neurotrophic factor
CNTF was identified as a survival factor for parasympathetic neurons isolated from the chick ciliary ganglion99,100. It is sequestered within the cytoplasm, probably due to the lack of a signal peptide101. Due to its intracellular localization99,100, CNTF is regarded as a lesion factor rather than a target-derived neurotrophic factor102. Indeed, CNTF is a key player in injury response in the nervous system. Lesions in the brain cause a significant increase in both CNTF mRNA and protein levels103,104. However, application of CNTF prevented degeneration of motor neurons after axotomy102 and promoted survival of hippocampal neurons in culture105. Furthermore, disruption of the Cntf gene led to motor neuron degeneration, demonstrating its physiological role in the maintenance of motor neurons106.
CNTF signalling cascades
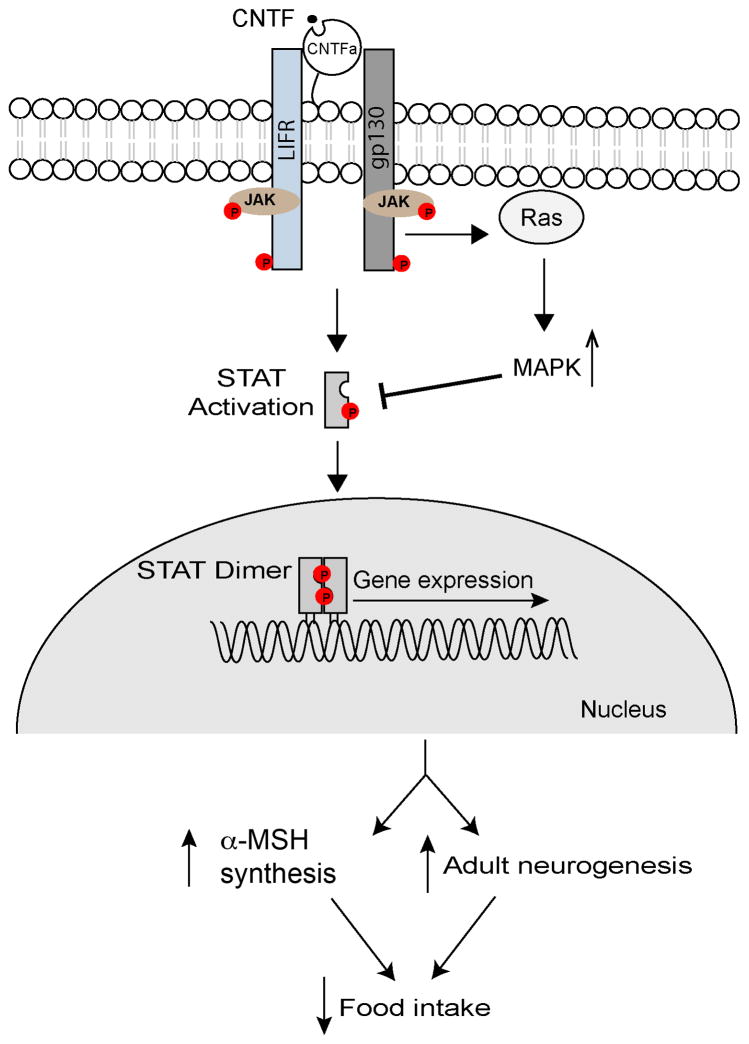
Cell injury will release intracellular CNTF. Upon binding to a receptor complex that is composed of the CNTF receptor α (CNTFRα), the leukemia inhibitory factor receptor β (LIFRβ) and glycoprotein 130 (gp130) on the cell surface, CNTF activates one signalling cascade that involves the Janus tyrosine kinase/signal transducers and activators of transcription (JAK-STAT) and another signalling cascade that involves Ras and MAP kinase107–111 (Fig. 4). CNTFRα lacks a transmembrane domain and is tethered to the plasma membrane via a glycosylphosphatidylinositol linkage107. The activated CNTF receptor complex phosphorylates STAT3 and STAT1, leading to either homo- or heterodimerization of STAT3 and STAT1, and subsequent nuclear translocation to induce gene expression112. The activation of MAPK temporally follows the STAT activation108. It involves recruitment of Src homology 2 phosphatase (SHP2) to phosphorylated gp130, interaction of SHP2 with membrane-associated Ras, and subsequent activation of MAPK by Ras113,114. The activated MAPK can down-regulate STAT transcriptional activity by suppressing its tyrosine phosphorylation115, thus serving as a negative feedback. Additionally, one study found that CNTF could interact with its receptor complex in the nucleus to activate the aforementioned signalling pathways116, which may explain the neuronal survival activity of endogenous CNTF that lacks a signal peptide. However, this interesting observation needs to be confirmed, given that none of CNTF and its receptor components contains a nucleus localization signal.
Figure 4. CNTF signalling pathways.
Binding of CNTF to its receptor complex, which is composed of CNTFRα, LIFR and gp130, activates the associated JAK, a tyrosine kinase, which phosphorylates (round dots with P) STAT, LIFR, and gp130 at tyrosine residues. Phosphorylated STAT dimerizes and enters the nucleus to induce gene expression. The consequent change in gene expression is likely to be essential for CNTF-mediated suppression of food intake by increasing the number of ARHPOMC neurons through adult neurogenesis and boosting synthesis of αMSH, the MC4R agonist. The activated receptor complex also activates MAPK, which can suppress STAT phosphorylation, thus providing a negative feedback.
Weight loss induced by CNTF administration
Because CNTF can promote survival of motor neurons102,106, it has been tested as a therapeutic agent for amyotrophic lateral sclerosis. In the clinical trials, CNTF treatments induced unexpected and dramatic weight loss in obese human patients, and this weight loss persisted after the cessation of treatment117,118. Similarly, systemic administration of recombinant CNTF suppressed food intake and decreased body weight in leptin-deficient ob/ob mice and in mice with diet-induced obesity (DIO)119. Given that CNTF levels are acutely elevated in response to neuronal injury, CNTF may exert its substantial weight-loss effect in a similar way to cachectic cytokines. Indeed, it was reported that CNTF possessed inflammatory properties120, and its administration triggered inflammatory responses including fever121. A study designed to address this issue found that efficacious doses of recombinant CNTF reduced food intake and body weight in ob/ob mice and DIO mice without inducing cachectic responses such as muscle wasting, proinflammatory gene expression, conditioned taste aversion, or corticosterone release122. Thus, CNTF is mostly likely to induce weight loss by promoting satiety. The ability of CNTF to induce weight loss in obese humans and in leptin-resistant DIO mice gives rise to the hope that CNTF could be developed into a therapeutic agent for obesity treatment, although a clinical trial using Axokine, a modified human CNTF with improved stability and potency, as an obesity therapy failed due to the development of neutralizing antibodies to the drug.
So far, there is no strong evidence to implicate endogenous CNTF signalling in the control of energy balance. Neither obesity nor hyperphagia has been reported in Cntf−/− null mice106, Cntfrα−/− null mice123, and humans with CNTF deficiency124. However, deletion of either the Cntf gene or the Cntfrα gene throughout the body leads to loss of motor neurons106,123, which may impair feeding and thus block the development of hyperphagia and obesity. A conclusive demonstration on the physiological role of CNTF in the control of energy balance may have to come from selective ablation of CNTF signalling in the hypothalamus.
Mechanism underlying the CNTF regulation of body weight
There are some similarities between leptin signalling and CNTF signalling in the brain. First, the leptin receptor is closely related to the LIF receptor and gp130 in the CNTF receptor complex107,125. Second, CNTF and leptin activate overlapping signalling molecules, particularly the STAT3 transcription factor126–128. It has been shown that the STAT3 activation is required for leptin regulation of energy balance129. Third, the CNTF receptors and the leptin receptor have overlapping distributions in some hypothalamic nuclei that are involved in the control of feeding, such as the ARH and PVH130,131. These similarities raise the possibility that CNTF acts on leptin-responsive neurons to regulate satiety and body weight. However, one study found that deleting the Cntfrα gene in cells expressing the leptin receptor did not diminish the anorectic effect of exogenous CNTF treatment132. This genetic finding demonstrates that CNTF and leptin regulate energy balance by activating similar signalling cascades but in distinct neuronal populations.
Neuronal plasticity in the hypothalamus is important to the regulation of energy balance. Synaptic density in ARHPOMC and ARHAgRP neurons in ob/ob mice rapidly changes in response to leptin administration before the anorexigenic effect is detected133. Furthermore, hunger induces synaptogenesis of excitatory synapses in ARHAgRP neurons, as indicated by an increase in the number of dendritic spine in these neurons of fasted mice134. Adult neurogenesis is also a form of neuronal plasticity by changing neuronal density and composition in a brain region. Central administration of CNTF was found to promote cell proliferation in the adult mouse hypothalamus. Many of these newborn cells express neuronal markers and can respond to leptin. Moreover, killing the newborn cells blocks the long-term, but not the short-term, effect of CNTF on body weight135. These observations suggest that CNTF might suppress food intake in part by stimulating adult neurogenesis in the hypothalamus.
Interaction with leptin and MC4R pathways
Leptin-leptin receptor, αMSH-MC4R and BDNF-TrkB are arguably the most important ligand-receptor pairs in the central control of energy balance, because deficits in any of these three signalling pathways lead to severe obesity in both humans21,24,136–138 and mice3,52,53,125,139,140. It is plausible to expect that the BDNF-TrkB pathway interact with the other two pathways in the control of feeding and energy expenditure.
Studies show that BDNF interacts with MC4R signalling in both the hypothalamus and brainstem to regulate energy balance. Activation of MC4R stimulated the expression of BDNF in the VMH52 and BDNF release from rat hypothalamus explants141, suggesting that BDNF regulate energy balance downstream of MC4R. In support of this view, BDNF infusion into the lateral ventricle attenuated hyperphagia and excessive weight gain in mice with decreased MC4R activity52. Similarly, simultaneous administration of BDNF reversed hyperphagia induced by MC4R antagonist infused into the fourth ventricle142. Furthermore, BDNF delivered directly into the medial NTS reduced food intake, which was reversed by pre-treatment with a TrkB antagonist96, whereas acute blockade of TrkB activation abolished the anorexigenic effect of MC4R agonists in the brainstem142. There are several possible reasons why the anorexigenic effect of MC4R in the brainstem is dependent on TrkB activation. First, TrkB activation could be essential to keep a neural circuit functional, thereby providing a permissive condition for the action of MC4R. Second, the signalling cascades activated by the two receptors may interact with each other inside neurons. Third, TrkB and MC4R may act on two distinct but interdependent neural circuits to suppress food intake. Delineation of the neural circuits that mediate the anorexigenic effect of the two receptors in the brainstem will help answer this question.
Much less is known about the interaction between the BDNF-TrkB pathway and the leptin system. Very few BDNF neurons express the leptin receptor in the hypothalamus76, and it is unlikely that leptin would directly regulated BDNF gene expression in this brain area. However, leptin is able to increase neuronal excitability, for example, in ARHPOMC neurons41, which can in turn change BDNF gene expression. In addition, leptin has been shown to increase dendritic BDNF synthesis in cultured hypothalamic neurons through increases in neuronal activity76. Furthermore, some TrkB neurons in the hypothalamus express the leptin receptor88. The co-expression of the two receptors could also be true in the DVC, because co-administration of a selective TrkB antagonist into the medial NTS was found to attenuate the suppressive effect of leptin on food intake in rats93. It would be interesting to investigate if there is a crosstalk between the signalling cascades activated by TrkB and leptin receptor.
Although CNTF treatment causes marked weight loss, its connection with the leptin and melanocortin systems remains to be defined. Some CNTF-induced adult-born neurons in the ARH express POMC and are responsive to leptin135. Furthermore, CNTF was found to stimulate Pomc gene expression in the ARH116,143. Thus, CNTF treatment should increase the activity of the leptin and melanocortin systems.
Perspectives
Evidence from both genetic and pharmacological studies has demonstrated that BDNF plays a vital role in the control of energy balance by promoting satiety and energy expenditure. Case studies have clearly shown that impairment in TrkB signalling or BDNF haploinsufficiency causes severe hyperphagia and obesity in humans. This type of mutations is very rare, however, it is still unclear what role TrkB signalling impairment plays in the obesity of the general population. The current obesity problem we are facing is likely related to consumption of calorie-dense food. It is important to investigate whether alterations in TrkB signalling contribute to diet-induced obesity.
As discussed above, researchers have started to identify the neurons that produce BDNF and the neurons that BDNF acts on to regulate energy balance. Identification of these neurons and their connected neural circuits would make it possible to investigate how BDNF regulates the function of these neural circuits. It would be interesting to know whether BDNF modulates synaptic plasticity in these circuits as it does at hippocampal synapses. Because a deficit in TrkB signalling leads to extreme hyperphagia in both mice and humans, activation of these neural circuits should exert powerful suppression on appetite. Studies on the composition and regulation of these neural circuits will not only increase our understanding of the central control of feeding behaviour but also may reveal a way to manipulate the activity of the circuits. Pharmacological activation of the neural circuits could be an effective way to combat the obesity problem.
In contrast to BDNF, evidence is still lacking to implicate endogenous CNTF in the control of energy balance. The evidence may come from future studies to examine the change of CNTF signalling in DIO and the effect of region-specific ablation of CNTF signalling in the brain on energy balance. In rodent DIO models, exposure to high-fat diets increases the expression of proinflammatory cytokine genes, such as the genes encoding interleukin 1β, interleukin 6, and tumour necrosis factor alpha, in the hypothalamus144,145. It would be interesting to examine whether high-fat diet feeding leads to changes in the gene expression of CNTF and its receptors in the hypothalamus. The region-specific mouse mutants will bypass the complication of motor neuron degeneration caused by general ablation of CNTF signalling. Even if endogenous CNTF signalling is not involved in the control of energy balance, it is still valuable to elucidate the molecular and neural mechanisms by which CNTF treatment induces drastic and lasting weight loss in leptin-resistant obese humans and mice. The mechanisms could be employed to develop novel interventions for obesity.
Online summary (or Key points).
Brain-derived neurotrophic factor (BDNF) plays an important role in the control of energy balance in addition to its roles in neuronal survival and development. Mutations in the gene for BDNF or its receptor TrkB lead to marked overeating and severe obesity in both mice and humans.
Nutritional state, glucose, and anorexigenic hormones, such as leptin and melanocortin, have been found to regulate BDNF gene expression in some known appetite-regulating brain regions such as the ventromedial hypothalamus and dorsal vagal complex, indicating that BDNF actively participates in the control of satiety.
It is likely that multiple brain regions mediate the effect of BDNF on energy balance. These brain regions include the arcuate nucleus of the hypothalamus, paraventricular hypothalamus, ventromedial hypothalamus, and dorsal vagal complex.
The paraventricular hypothalamus is a key structure that produces BDNF to control energy balance. BDNF neurons in the anterior part of this nucleus inhibit food intake and stimulate locomotor activity, whereas BDNF neurons in the medial and posterior parts of the nucleus drive adaptive thermogenesis by polysynaptically projecting to brown adipose tissues.
Administration of recombinant ciliary neurotrophic factor (CNTF) can overcome leptin resistance to suppress appetite and induce lasting weight loss in obese rodents and humans.
CNTF likely reduces obesity-related phenotypes by regulating gene expression in neurons of the arcuate nucleus and stimulating adult neurogenesis in the hypothalamus.
Acknowledgments
We apologize to all colleagues whose work could not be cited due to space limitations. This work was supported by the grants from U.S. National Institutes of Health to BX (DK103335, NS073930, and NS095425).
Glossary terms
- Intracerebroventricular infusion
Administration of chemicals or other reagents into the ventricular system of the brain.
- Hypomorphic mice
Mutant mice in which the expression of a gene is reduced.
- Adaptive thermogenesis
A process in which brown adipose tissues enhance combustion of fatty acids into heat in response to physiological and environmental stimuli such as cold.
- Hedonic eating
Consuming food to obtain pleasure in the absence of energy deficits.
- Signal peptide
A short peptide of 5–30 amino acids at the N-terminus of a newly synthesized protein that is destined toward to the plasma membrane or to be secreted from the cell.
- Amyotrophic lateral sclerosis
A nervous system disease that is often called Lou Gehrig’s disease, which causes muscle weakness and impacts physical function.
- Cachectic
Having cachexia that is characterized by loss of appetite, loss of weight, muscle atrophy, fatigue, and weakness.
Biographies
Baoji Xu received his Ph.D. in molecular biology at Stanford University in Stanford, California, USA, and postdoctoral training in neuroscience at the University of California, San Francisco, California. He subsequently became Professor at Georgetown University Medical Centre in Washington, DC, USA. In 2013, he moved to the Scripps Research Institute (TSRI) in Jupiter, Florida, USA. His research focuses on the mechanisms by which growth factors such as brain-derived neurotrophic factor regulate the development and function of brain neural circuits that control learning, mood, appetite, and energy expenditure. TSRI homepage; Baoji Xu’s homepage
Xiangyang Xie obtained his Ph.D. in biochemistry and molecular biology at the institute of biophysics, Chinese Academy of Sciences, Beijing, China. He did his postdoctoral training at the Sanford Burnham Medical Research Institute, Orlando, USA, focusing on glucose transport and energy homeostasis. In 2013, he joined Baoji Xu’s laboratory in the Department of Neuroscience at TSRI, Jupiter, Florida, as a research associate. His research interests include understanding how the CNS regulates the whole-body energy balance.
References
- 1.Garland T, Jr, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol. 2011;214:206–29. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall KD, et al. Energy balance and its components: implications for body weight regulation. Am J Clin Nutr. 2012;95:989–94. doi: 10.3945/ajcn.112.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 4.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–5. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 5.Nakazato M, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 6.Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 7.Shintani M, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–32. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 8.Horvath TL, Diano S, Sotonyi P, Heiman M, Tschop M. Minireview: ghrelin and the regulation of energy balance--a hypothalamic perspective. Endocrinology. 2001;142:4163–9. doi: 10.1210/endo.142.10.8490. [DOI] [PubMed] [Google Scholar]
- 9.Tang-Christensen M, et al. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271:R848–56. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 10.Turton MD, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 11.Batterham RL, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 12.Pocai A, et al. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest. 2006;116:1081–91. doi: 10.1172/JCI26640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He W, Lam TK, Obici S, Rossetti L. Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci. 2006;9:227–33. doi: 10.1038/nn1626. [DOI] [PubMed] [Google Scholar]
- 14.Loftus TM, et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–81. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 15.Cota D, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–30. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 16.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–50. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 17.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 18.Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J Endocrinol. 2005;184:291–318. doi: 10.1677/joe.1.05866. [DOI] [PubMed] [Google Scholar]
- 19.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 21.Yeo GS, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7:1187–9. doi: 10.1038/nn1336. This study is the first demonstration that impairment in TrkB signaling can cause severe obesity in humans. [DOI] [PubMed] [Google Scholar]
- 22.Gray J, et al. Hyperphagia, Severe Obesity, Impaired Cognitive Function, and Hyperactivity Associated With Functional Loss of One Copy of the Brain-Derived Neurotrophic Factor (BDNF) Gene. Diabetes. 2006;55:3366–3371. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray J, et al. Functional characterization of human NTRK2 mutations identified in patients with severe early-onset obesity. Int J Obes (Lond) 2007;31:359–64. doi: 10.1038/sj.ijo.0803390. [DOI] [PubMed] [Google Scholar]
- 24.Han JC, et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med. 2008;359:918–27. doi: 10.1056/NEJMoa0801119. This study carefully mapped the location of chromosomal deletion in patients with the WAGR syndrome and discovered that BDNF haploinsufficiency was the cause of obesity associated with the syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller RG, et al. A placebo-controlled trial of recombinant human ciliary neurotrophic (rhCNTF) factor in amyotrophic lateral sclerosis. rhCNTF ALS Study Group. Ann Neurol. 1996;39:256–60. doi: 10.1002/ana.410390215. [DOI] [PubMed] [Google Scholar]
- 26.Friedman J. 20 years of leptin: leptin at 20: an overview. J Endocrinol. 2014;223:T1–8. doi: 10.1530/JOE-14-0405. [DOI] [PubMed] [Google Scholar]
- 27.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–8. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 28.Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012;15:1350–5. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–23. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pissios P, Bradley RL, Maratos-Flier E. Expanding the scales: The multiple roles of MCH in regulating energy balance and other biological functions. Endocr Rev. 2006;27:606–20. doi: 10.1210/er.2006-0021. [DOI] [PubMed] [Google Scholar]
- 31.Gao XB, Horvath T. Function and dysfunction of hypocretin/orexin: an energetics point of view. Annu Rev Neurosci. 2014;37:101–16. doi: 10.1146/annurev-neuro-071013-013855. [DOI] [PubMed] [Google Scholar]
- 32.Richard D. Cognitive and autonomic determinants of energy homeostasis in obesity. Nat Rev Endocrinol. 2015;11:489–501. doi: 10.1038/nrendo.2015.103. [DOI] [PubMed] [Google Scholar]
- 33.Sohn JW, Elmquist JK, Williams KW. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 2013;36:504–12. doi: 10.1016/j.tins.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–50. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garfield AS, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci. 2015;18:863–71. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah BP, et al. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1407843111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, et al. Glutamate mediates the function of melanocortin receptor 4 on Sim1 neurons in body weight regulation. Cell Metab. 2013;18:860–70. doi: 10.1016/j.cmet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balthasar N, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 39.Rossi J, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell metabolism. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet. 2002;3:589–600. doi: 10.1038/nrg862. [DOI] [PubMed] [Google Scholar]
- 41.Cowley MA, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–4. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 42.Williams KW, Scott MM, Elmquist JK. Modulation of the central melanocortin system by leptin, insulin, and serotonin: co-ordinated actions in a dispersed neuronal network. European journal of pharmacology. 2011;660:2–12. doi: 10.1016/j.ejphar.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boston BA, Blaydon KM, Varnerin J, Cone RD. Independent and additive effects of central POMC and leptin pathways on murine obesity. Science. 1997;278:1641–4. doi: 10.1126/science.278.5343.1641. [DOI] [PubMed] [Google Scholar]
- 44.Marsh DJ, et al. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–22. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 45.Kong D, et al. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151:645–57. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leshan RL, Greenwald-Yarnell M, Patterson CM, Gonzalez IE, Myers MG., Jr Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat Med. 2012;18:820–3. doi: 10.1038/nm.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vong L, et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–54. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lapchak PA, Hefti F. BDNF and NGF treatment in lesioned rats: effects on cholinergic function and weight gain. Neuroreport. 1992;3:405–8. doi: 10.1097/00001756-199205000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131:229–38. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 50.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. Embo J. 2000;19:1290–300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyons WE, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–44. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu B, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–42. doi: 10.1038/nn1073. This study provided the first evidence that TrkB plays a key role in the control of energy balance in mice, showed that nutrition state regulates hypothalamic Bdnf gene expression, and suggested that BDNF regulates food intake downstream of the MC4R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–57. doi: 10.1210/mend.15.10.0706. This study demonstrated that BDNF expressed in the brain is essential for the control of energy balance. [DOI] [PubMed] [Google Scholar]
- 54.Lin CR, et al. Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1987;84:5962–6. doi: 10.1073/pnas.84.16.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bayer KU, Lohler J, Harbers K. An alternative, nonkinase product of the brain-specifically expressed Ca2+/calmodulin-dependent kinase II alpha isoform gene in skeletal muscle. Mol Cell Biol. 1996;16:29–36. doi: 10.1128/mcb.16.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27:14265–74. doi: 10.1523/JNEUROSCI.3308-07.2007. This study showed that glucose regulates Bdnf gene expression in the VMH and found that BDNF expressed in the DMH and VMH plays a role in regulating food intake. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tran PV, et al. Diminished hypothalamic bdnf expression and impaired VMH function are associated with reduced SF-1 gene dosage. J Comp Neurol. 2006;498:637–48. doi: 10.1002/cne.21070. [DOI] [PubMed] [Google Scholar]
- 58.Bariohay B, Lebrun B, Moyse E, Jean A. Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology. 2005;146:5612–20. doi: 10.1210/en.2005-0419. This paper reports that nutritional state and anorexigenic hormones leptin and CCK regulate levels of BDNF in the dorsal vagal complex. [DOI] [PubMed] [Google Scholar]
- 59.Dhillon H, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 60.An JJ, Liao GY, Kinney CE, Sahibzada N, Xu B. Discrete BDNF Neurons in the Paraventricular Hypothalamus Control Feeding and Energy Expenditure. Cell Metab. 2015;22:175–88. doi: 10.1016/j.cmet.2015.05.008. This study elegantly demonstrated that BDNF produced in the anterior PVH inhibits food intake and stimulates locomotor activity, while neurons in the medial and posterior PVH promote adaptive thermogenesis by releasing BDNF into spinal cord to boost sympathetic outflow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biag J, et al. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol. 2012;520:6–33. doi: 10.1002/cne.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Charrier C, et al. BDNF regulation in the rat dorsal vagal complex during stress-induced anorexia. Brain Res. 2006;1107:52–7. doi: 10.1016/j.brainres.2006.05.099. [DOI] [PubMed] [Google Scholar]
- 63.Moyse E, et al. Effects of aging and caloric restriction on brainstem satiety center signals in rats. Mechanisms of ageing and development. 2012;133:83–91. doi: 10.1016/j.mad.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 64.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes & development. 2013;27:234–50. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whittle A, Relat-Pardo J, Vidal-Puig A. Pharmacological strategies for targeting BAT thermogenesis. Trends in pharmacological sciences. 2013;34:347–55. doi: 10.1016/j.tips.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 66.Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Frontiers in endocrinology. 2012;3:5. doi: 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clapham JC. Central control of thermogenesis. Neuropharmacology. 2012;63:111–23. doi: 10.1016/j.neuropharm.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 68.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 69.Nonomura T, et al. Brain-derived neurotrophic factor regulates energy expenditure through the central nervous system in obese diabetic mice. Int J Exp Diabetes Res. 2001;2:201–9. doi: 10.1155/EDR.2001.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsuchida A, et al. The effects of brain-derived neurotrophic factor on insulin signal transduction in the liver of diabetic mice. Diabetologia. 2001;44:555–66. doi: 10.1007/s001250051661. [DOI] [PubMed] [Google Scholar]
- 71.Wang C, Bomberg E, Billington CJ, Levine AS, Kotz CM. Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure. Brain research. 2010;1336:66–77. doi: 10.1016/j.brainres.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am J Physiol Regul Integr Comp Physiol. 2007;293:R992–1002. doi: 10.1152/ajpregu.00516.2006. [DOI] [PubMed] [Google Scholar]
- 73.Oldfield BJ, et al. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110:515–26. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- 74.Cano G, et al. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. The Journal of comparative neurology. 2003;460:303–26. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 75.Coppola V, Tessarollo L. Control of hyperphagia prevents obesity in BDNF heterozygous mice. Neuroreport. 2004;15:2665–8. doi: 10.1097/00001756-200412030-00022. [DOI] [PubMed] [Google Scholar]
- 76.Liao GY, et al. Dendritically targeted Bdnf mRNA is essential for energy balance and response to leptin. Nat Med. 2012;18:564–71. doi: 10.1038/nm.2687. This study suggests that local BDNF synthesis in dendrites is important for the control of food intake. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Timmusk T, et al. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–89. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- 78.Godar R, et al. Reduction of high-fat diet-induced obesity after chronic administration of brain-derived neurotrophic factor in the hypothalamic ventromedial nucleus. Neuroscience. 2011;194:36–52. doi: 10.1016/j.neuroscience.2011.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao L, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell metabolism. 2011;14:324–38. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–64. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee KF, et al. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–49. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- 82.DiStefano PS, et al. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–93. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- 83.von Bartheld CS, Byers MR, Williams R, Bothwell M. Anterograde transport of neurotrophins and axodendritic transfer in the developing visual system. Nature. 1996;379:830–3. doi: 10.1038/379830a0. [DOI] [PubMed] [Google Scholar]
- 84.Altar CA, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–60. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 85.Wang C, Bomberg E, Billington CJ, Levine AS, Kotz CM. Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure. Brain Res. 2010;1336:66–77. doi: 10.1016/j.brainres.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang C, Bomberg E, Levine A, Billington C, Kotz CM. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1037–45. doi: 10.1152/ajpregu.00125.2007. [DOI] [PubMed] [Google Scholar]
- 87.Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1003–12. doi: 10.1152/ajpregu.00011.2007. [DOI] [PubMed] [Google Scholar]
- 88.Liao GY, et al. Brain-derived neurotrophic factor is required for axonal growth of selective groups of neurons in the arcuate nucleus. Mol Metab. 2015;4:471–82. doi: 10.1016/j.molmet.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liao GY, Li Y, Xu B. Ablation of TrkB expression in RGS9–2 cells leads to hyperphagic obesity. Mol Metab. 2013;2:491–7. doi: 10.1016/j.molmet.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cordeira JW, et al. Hypothalamic dysfunction of the thrombospondin receptor alpha2delta-1 underlies the overeating and obesity triggered by brain-derived neurotrophic factor deficiency. J Neurosci. 2014;34:554–65. doi: 10.1523/JNEUROSCI.1572-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ozek C, Zimmer DJ, De Jonghe BC, Kalb RG, Bence KK. Ablation of intact hypothalamic and/or hindbrain TrkB signaling leads to perturbations in energy balance. Mol Metab. 2015;4:867–80. doi: 10.1016/j.molmet.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2533–41. doi: 10.1523/JNEUROSCI.5768-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spaeth AM, Kanoski SE, Hayes MR, Grill HJ. TrkB receptor signaling in the nucleus tractus solitarius mediates the food intake-suppressive effects of hindbrain BDNF and leptin. Am J Physiol Endocrinol Metab. 2012;302:E1252–60. doi: 10.1152/ajpendo.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.An JJ, et al. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–87. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin JC, et al. Appetite enhancement and weight gain by peripheral administration of TrkB agonists in non-human primates. PLoS ONE. 2008;3:e1900. doi: 10.1371/journal.pone.0001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spaeth AM, Kanoski SE, Hayes MR, Grill HJ. TrkB receptor signaling in the nucleus tractus solitarius mediates the food intake-suppressive effects of hindbrain BDNF and leptin. American journal of physiology. Endocrinology and metabolism. 2012;302:E1252–60. doi: 10.1152/ajpendo.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–37. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fox EA, Byerly MS. A mechanism underlying mature-onset obesity: evidence from the hyperphagic phenotype of brain-derived neurotrophic factor mutants. Am J Physiol Regul Integr Comp Physiol. 2004;286:R994–1004. doi: 10.1152/ajpregu.00727.2003. [DOI] [PubMed] [Google Scholar]
- 99.Stockli KA, et al. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature. 1989;342:920–3. doi: 10.1038/342920a0. [DOI] [PubMed] [Google Scholar]
- 100.Lin LF, et al. Purification, cloning, and expression of ciliary neurotrophic factor (CNTF) Science. 1989;246:1023–5. doi: 10.1126/science.2587985. [DOI] [PubMed] [Google Scholar]
- 101.Ip NY, Yancopoulos GD. The neurotrophins and CNTF: two families of collaborative neurotrophic factors. Annu Rev Neurosci. 1996;19:491–515. doi: 10.1146/annurev.ne.19.030196.002423. [DOI] [PubMed] [Google Scholar]
- 102.Sendtner M, Kreutzberg GW, Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990;345:440–1. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- 103.Ip NY, Wiegand SJ, Morse J, Rudge JS. Injury-induced regulation of ciliary neurotrophic factor mRNA in the adult rat brain. Eur J Neurosci. 1993;5:25–33. doi: 10.1111/j.1460-9568.1993.tb00201.x. [DOI] [PubMed] [Google Scholar]
- 104.Rudge JS, et al. Expression of Ciliary Neurotrophic Factor and the Neurotrophins-Nerve Growth Factor, Brain-Derived Neurotrophic Factor and Neurotrophin 3-in Cultured Rat Hippocampal Astrocytes. Eur J Neurosci. 1992;4:459–471. doi: 10.1111/j.1460-9568.1992.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 105.Ip NY, et al. Ciliary neurotrophic factor enhances neuronal survival in embryonic rat hippocampal cultures. J Neurosci. 1991;11:3124–34. doi: 10.1523/JNEUROSCI.11-10-03124.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Masu Y, et al. Disruption of the CNTF gene results in motor neuron degeneration. Nature. 1993;365:27–32. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- 107.Ip NY, et al. CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130. Cell. 1992;69:1121–32. doi: 10.1016/0092-8674(92)90634-o. [DOI] [PubMed] [Google Scholar]
- 108.Peterson WM, Wang Q, Tzekova R, Wiegand SJ. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J Neurosci. 2000;20:4081–90. doi: 10.1523/JNEUROSCI.20-11-04081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stahl N, Yancopoulos GD. The tripartite CNTF receptor complex: activation and signaling involves components shared with other cytokines. J Neurobiol. 1994;25:1454–66. doi: 10.1002/neu.480251111. [DOI] [PubMed] [Google Scholar]
- 110.Stahl N, et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263:92–5. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 111.Davis S, et al. The receptor for ciliary neurotrophic factor. Science. 1991;253:59–63. doi: 10.1126/science.1648265. [DOI] [PubMed] [Google Scholar]
- 112.Bonni A, Frank DA, Schindler C, Greenberg ME. Characterization of a pathway for ciliary neurotrophic factor signaling to the nucleus. Science. 1993;262:1575–9. doi: 10.1126/science.7504325. [DOI] [PubMed] [Google Scholar]
- 113.Chin YE, Kitagawa M, Kuida K, Flavell RA, Fu XY. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol. 1997;17:5328–37. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim H, Baumann H. Dual signaling role of the protein tyrosine phosphatase SHP-2 in regulating expression of acute-phase plasma proteins by interleukin-6 cytokine receptors in hepatic cells. Mol Cell Biol. 1999;19:5326–38. doi: 10.1128/mcb.19.8.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jain N, Zhang T, Fong SL, Lim CP, Cao X. Repression of Stat3 activity by activation of mitogen-activated protein kinase (MAPK) Oncogene. 1998;17:3157–67. doi: 10.1038/sj.onc.1202238. [DOI] [PubMed] [Google Scholar]
- 116.Couvreur O, et al. The anorexigenic cytokine ciliary neurotrophic factor stimulates POMC gene expression via receptors localized in the nucleus of arcuate neurons. Am J Physiol Endocrinol Metab. 2012;302:E458–67. doi: 10.1152/ajpendo.00388.2011. [DOI] [PubMed] [Google Scholar]
- 117.A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis; ALS CNTF Treatment Study Group. Neurology. 1996;46:1244–9. doi: 10.1212/wnl.46.5.1244. [DOI] [PubMed] [Google Scholar]
- 118.Miller RG, et al. Toxicity and tolerability of recombinant human ciliary neurotrophic factor in patients with amyotrophic lateral sclerosis. Neurology. 1996;47:1329–31. doi: 10.1212/wnl.47.5.1329. [DOI] [PubMed] [Google Scholar]
- 119.Gloaguen I, et al. Ciliary neurotrophic factor corrects obesity and diabetes associated with leptin deficiency and resistance. Proc Natl Acad Sci U S A. 1997;94:6456–61. doi: 10.1073/pnas.94.12.6456. This study showed that CNTF could overcome leptin resistance to reduce obesity-related phenotypes in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kelly JF, et al. Ciliary neurotrophic factor and leptin induce distinct patterns of immediate early gene expression in the brain. Diabetes. 2004;53:911–20. doi: 10.2337/diabetes.53.4.911. [DOI] [PubMed] [Google Scholar]
- 121.Shapiro L, Zhang XX, Rupp RG, Wolff SM, Dinarello CA. Ciliary neurotrophic factor is an endogenous pyrogen. Proc Natl Acad Sci U S A. 1993;90:8614–8. doi: 10.1073/pnas.90.18.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lambert PD, et al. Ciliary neurotrophic factor activates leptin-like pathways and reduces body fat, without cachexia or rebound weight gain, even in leptin-resistant obesity. Proc Natl Acad Sci U S A. 2001;98:4652–7. doi: 10.1073/pnas.061034298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.DeChiara TM, et al. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell. 1995;83:313–22. doi: 10.1016/0092-8674(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 124.Takahashi R, et al. A null mutation in the human CNTF gene is not causally related to neurological diseases. Nat Genet. 1994;7:79–84. doi: 10.1038/ng0594-79. [DOI] [PubMed] [Google Scholar]
- 125.Tartaglia LA, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 126.Stahl N, et al. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–53. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 127.Vaisse C, et al. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–7. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 128.Carpenter LR, et al. Enhancing leptin response by preventing SH2-containing phosphatase 2 interaction with Ob receptor. Proc Natl Acad Sci U S A. 1998;95:6061–6. doi: 10.1073/pnas.95.11.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bates SH, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–9. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 130.Kordower JH, Yaping C, Maclennan AJ. Ciliary neurotrophic factor receptor alpha-immunoreactivity in the monkey central nervous system. J Comp Neurol. 1997;377:365–80. doi: 10.1002/(sici)1096-9861(19970120)377:3<365::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 131.Lee MY, Hofmann HD, Kirsch M. Expression of ciliary neurotrophic factor receptor-alpha messenger RNA in neonatal and adult rat brain: an in situ hybridization study. Neuroscience. 1997;77:233–46. doi: 10.1016/s0306-4522(96)00476-9. [DOI] [PubMed] [Google Scholar]
- 132.Stefater MA, et al. The anorectic effect of CNTF does not require action in leptin-responsive neurons. Endocrinology. 2012;153:2647–54. doi: 10.1210/en.2012-1024. This study, using a genetic approach, showed that CNTF and leptin acted on distinct neuronal populations to suppress food intake. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pinto S, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–5. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 134.Liu T, et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–22. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–83. doi: 10.1126/science.1115360. This study shows that CNTF might induce long-term weight loss by stimulating adult neurogenesis in the hypothalamus. [DOI] [PubMed] [Google Scholar]
- 136.Montague CT, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 137.Yeo GS, et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111–2. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 138.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20:113–4. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 139.Huszar D, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–41. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 140.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–70. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 141.Nicholson JR, Peter JC, Lecourt AC, Barde YA, Hofbauer KG. Melanocortin-4 receptor activation stimulates hypothalamic brain-derived neurotrophic factor release to regulate food intake, body temperature and cardiovascular function. J Neuroendocrinol. 2007;19:974–82. doi: 10.1111/j.1365-2826.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 142.Bariohay B, et al. Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system in food intake control. Endocrinology. 2009;150:2646–53. doi: 10.1210/en.2008-1184. [DOI] [PubMed] [Google Scholar]
- 143.Ambati S, et al. Gene expression in arcuate nucleus-median eminence of rats treated with leptin or ciliary neurotrophic factor. Biofactors. 2007;31:133–44. doi: 10.1002/biof.5520310204. [DOI] [PubMed] [Google Scholar]
- 144.De Souza CT, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–9. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 145.Thaler JP, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–62. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fischbach BV, Trout KL, Lewis J, Luis CA, Sika M. WAGR syndrome: a clinical review of 54 cases. Pediatrics. 2005;116:984–8. doi: 10.1542/peds.2004-0467. [DOI] [PubMed] [Google Scholar]
- 147.Thorleifsson G, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 148.Speliotes EK, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nature genetics. 2010;42:937–48. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Okada Y, et al. Common variants at CDKAL1 and KLF9 are associated with body mass index in east Asian populations. Nature genetics. 2012;44:302–6. doi: 10.1038/ng.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wen W, et al. Meta-analysis identifies common variants associated with body mass index in east Asians. Nature genetics. 2012;44:307–11. doi: 10.1038/ng.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hotta K, et al. Association between obesity and polymorphisms in SEC16B, TMEM18, GNPDA2, BDNF, FAIM2 and MC4R in a Japanese population. Journal of human genetics. 2009;54:727–31. doi: 10.1038/jhg.2009.106. [DOI] [PubMed] [Google Scholar]
- 152.Zhao J, et al. The role of obesity-associated loci identified in genome-wide association studies in the determination of pediatric BMI. Obesity. 2009;17:2254–7. doi: 10.1038/oby.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu L, et al. Associations of six single nucleotide polymorphisms in obesity-related genes with BMI and risk of obesity in Chinese children. Diabetes. 2010;59:3085–9. doi: 10.2337/db10-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Elks CE, et al. Adult obesity susceptibility variants are associated with greater childhood weight gain and a faster tempo of growth: the 1946 British Birth Cohort Study. The American journal of clinical nutrition. 2012;95:1150–6. doi: 10.3945/ajcn.111.027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Egan MF, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 156.Chen ZY, et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–11. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen ZY, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–3. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bramham CR, Wells DG. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci. 2007;8:776–89. doi: 10.1038/nrn2150. [DOI] [PubMed] [Google Scholar]
- 159.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nature reviews. Neuroscience. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]