Abstract
Background and Purpose
Inflammation and compromise in structure and function of cerebral parenchymal microvasculature begins early after subarachnoid hemorrhage (SAH). We recently found greater inflammation and greater vascular compromise in male than in female rats following SAH. In this study we investigated whether this cross-sexual difference in pathology is reflected in expression levels of genes related to vascular inflammation and structural compromise.
Method
Age-matched male and female rats underwent sham surgery or SAH by endovascular perforation. Early physiology (intracranial pressure (ICP), blood pressure (BP), heart rate (HR) and cerebral blood flow (CBF)) was monitored. Cerebral RNA was extracted at sacrifice 3 hours after surgery and assayed for expression of thrombomodulin (Thbd), endothelial nitric oxide synthase (eNos;Nos3), intracellular adhesion molecule-1 (Icam1), vascular endothelial growth factor (Vegf), interleukin-1beta (Il1β) tumor necrosis factor-alpha (Tnf-α) and arginine vasopressin (Avp).
Results
Increases in ICP and BP at SAH appeared slightly greater in males but the difference did not reach statistical difference, indicating that SAH intensity did not differ significantly between the sexes. Of the seven genes studied two; Tnf-α and Vegf, did not change after injury while the remainder showed significant responses to SAH. Response of Nos3 and Thbd was markedly different between the sexes, with expression greater in males.
Conclusion
This study finds that sexual dimorphism is present in the response of some but not all genes to SAH. Since products of genes exhibiting sexual dimorphism have anti-inflammatory activities, our results indicate that previously found sex-based differences in vascular pathology are paralleled by sexually dimorphic changes in gene expression following SAH.
Keywords: Gender, early brain injury, vascular pathology, inflammation, stroke
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is sexually dimorphic in prevalence and age of attack but not in outcome. Women harbor more cerebral aneurysms and have a greater incidence of SAH. Remarkably, although the average age of female victims of SAH is greater than that of male victims the two groups experience similar outcomes.1, 2 We previously hypothesized that this similarity in outcomes reflects sex-based differences in pathophysiology associated with SAH, and examined the influence of sex on acute physiology and early brain injury in an experimental model. We found that the rise in intracranial pressure and peripheral blood pressure at SAH was greater in males than in same-aged females, and that microvessel pathology and neuronal apoptosis were greater in the males than in the females.3 The present study extends these observations by examining sexual dimorphism in gene expression after SAH. The study includes five genes known previously to increase early after SAH, endothelial nitric oxide synthase (Nos3), intracellular adhesion molecule-1 (Icam1), interleukin-1beta (Il1β), tumor necrosis factor-alpha (Tnf-α), and vascular endothelial growth factor (Vegf), and in addition two genes known to increase early after ischemic stroke, thrombomodulin (Thbd), and vasopressin (Avp).4-7
We found that five of those genes respond to SAH, and the response of two was sexually dimorphic. Interestingly, those genes that showed sexually dimorphic expression, with expression greater in males, encode products that oppose vascular inflammation. Taken together our data indicate that previously found sex-based differences in vascular pathology are paralleled by sex-related differences in gene expression following SAH.
Methods
All experimental procedures and protocols used in this study were reviewed and approved by the Animal Care Committee of the Icahn School of Medicine at Mount Sinai.
Surgical preparation, physiological monitoring and SAH production
SAH was induced in three-month-old male (408.6 ± 5.5 g) and female (299 ± 5.0 g) Sprague Dawley rats (N=9 per sex) using the endovascular suture model.8 Briefly, rats were anesthetized with ketamine-xylazine (50mg/Kg+5mg/Kg IP), transorally intubated, and positioned in a stereotactic frame. Thereafter, ventilation and anesthesia were maintained by inspired isoflurane (1-2% in 21% oxygen-supplemented room air) and body temperature was maintained at 37°C by a homeothermic blanket (Harvard Apparatus) and a rectal temperature probe. The right femoral artery was exposed and cannulated for blood gas and blood pressure monitoring (BP). The atlanto-occipital membrane was exposed and cannulated for measurement of intracranial pressure (ICP). A Laser Doppler Flowmetry (LDF) probe (0.8mm diameter, model P-433, Vasamedics Inc.) was placed adjacent to the coronal suture and 5 mm lateral to the right of midline, over the territory of the middle cerebral artery and away from large meningeal vessels for cerebral blood flow (CBF) measurement.
SAH was induced by advancing a suture retrogradely through the ligated right external carotid artery (ECA), and then distally through the internal carotid artery (ICA), until the suture perforated the intracranial bifurcation of the ICA. This event was confirmed by a rapid rise in ICP and a decrease in CBF.9, 10 Following surgery, each animal was returned to its cage as it regained consciousness and was able to breathe spontaneously. Sex- and time-matched sham operated animals (N=9 per sex) were used as controls in this study. Animals were sacrificed 3 hours after hemorrhage or sham surgery. As described previously, sham surgery included all steps carried for SAH induction, except that perforation of the internal carotid artery was not effected 11. Animals were randomized for gender and type of surgery (SAH or sham).
Data Acquisition and Statistical Analysis
CBF, ICP, and BP data were recorded continuously from 20 minutes before to 60 minutes after SAH using PolyView software (Grass Instruments; MS, USA).8 Heart rate (HR) was computed from the recorded BP data.12 BP, CBF and HR data were normalized to baseline values defined as average values over the 20 minutes prior to SAH, and responses to surgery were analyzed as percentage of baseline. Statistical analysis was performed using two-way ANOVA followed by multiple Fisher's PLSD post-hoc t-tests setting experimental significance at p<0.05.
Reverse transcription polymerase chain reaction (rtPCR)
RNA extraction and rtPCR was performed in the Quantitative PCR (qPCR) CORE at Icahn School of Medicine at Mount Sinai by staff members who were blind to the identity of specimens.
Primers
Primers (Table 1) were designed using the BLAST program and were purchased from Eurofins MWG Operon (Alabama, USA).
Table 1.
Accession numbers and primer sequences
| Gene Name | Abbreviation | Accession Number | Primer sequence |
|---|---|---|---|
| Intracellular adhesion molecule-1 | Icam1 | NM_012967.1 |
S: TGATATCCGGTAGACACAAGCA AS: CTTCACCAGGGTCTAGGCAA |
| Endothelial nitric oxide synthase | eNos or Nos3 | NM_021838.2 |
S: TGAAGCCGACGCTCATGCACA AS: ATTGCCTCGGTTTGTTGCAT |
| Thrombomodulin | Thbd | NM_031771.2 |
S: ACTCTCCACTGTGTTTGCCAT AS: GACTCCTTTCCCAAGTTGCCAT |
| Vascular endothelial growth factor | Vegf | NM_001287107.1 | S: GCTCACTTCCAGAAACACGAC AS: ATCCACAATAGTGCCATGTCC |
| Interleukin-1beta | Il1β | NM_031512.2 |
S: TACATCAGCACCTCTCAAGCAG AS: AAGTCAACTATGTCCCGACCA |
| Tumor necrosis factor-alpha | Tnf-α | NM_012675.3 |
S:CAGTTCCATGGCCCAGACCCTC AS: ACTCCAGCTGCTCCTCCGCTTG |
| Arginine vasopressin | Avp | NM_016992.2 |
S: ACCTCTGCCTGCTACTTCCA AS: GTCTCAGCTCCATGTCGGAT |
Primers were designed using BLAST program and were purchased from Eurofins MWG Operon. S: sense, AS antisense.
RNA extraction
Rats were transcardially perfused with chilled saline and brains were rapidly removed and frozen (2 methylbutane on dry ice). Coronal brain slices spanning bregma −8.0 to +1.2 13 were cut and immediately placed in RNeasy (Qiagen). RNA was isolated from brain tissue using RNeasy Lipid mini kit following the manufacturer's protocol (QIAGEN) and its quantity, quality and purity but not integrity were assessed (optical density (OD) 260, and OD ratios 260/280 and 260/230, NanoDrop).
rtPCR
cDNA was synthesized from total RNA with AffinityScript™ Multi-Temp RT (Agilent Technologies) with oligo dT as primer. rtPCR was performed using PlatinumTaq DNA polymerase (Invitrogen) and SYBR® green (Molecular Probes) with an Applied Biosystems thermocycler (ABI7900HT). The PCR conditions used were: 95° for 2 min, 40 cycles of 95° for 15s, 55° for 15s, 72° for 30s. RNA levels for the housekeeping gene tubulin was also assayed in all samples and was used as internal control. Relative fold mRNA levels were determined by the 2− ΔΔCT (cycle threshold) method (ABI) and the results (“copy number”) are the ratios of test gene to housekeeping gene mRNA.
Statistical analysis was performed by multivariate (StatView v. 5.0.1, SAS Institute Inc. USA) using a design crossed in sex and treatment (sham, SAH) with interaction and nested in animal. Results showed significant effects in hemorrhage (F(1,16)= 64.1, p= 0.0001) and interaction terms (Sex*Hemorrhage: F(1,16)=6.34, p=0.022). Consequently, univariate ANOVAs were performed on individual genes, with pairwise comparisons performed by Fisher's PLSD post-hoc t-tests.
Results
SAH Physiology
ICP and BP increased and CBF and CPP decreased at SAH (Figure-1). Post-hemorrhage increases in ICP and in BP appeared slightly greater in males than in females but this difference did not reach statistical significance (ICP males: 67 ± 7mmHg, females: 52 ± 4 mmHg; BP rise % baseline males: 123 ± 8 %, females 108± 4 %). CBF fell to similar levels in males and females, while HR remained unchanged at SAH. Taken together these data indicate that the intensity of SAH in male and females was similar and is graded moderate.8
Figure-1. SAH physiology.

Intracranial pressure (ICP) and blood pressure (BP) increased, and cerebral blood flow (CBF) fall and heart rate (HR) remained unchanged at SAH induction. The rise in ICP and BP appeared slightly greater in males than in female, but these differences did not reach statistical significance. Data are mean ± sem from 9 male and 9 female animals.
Our results are in contrast to our previous findings in 6 month old Wister rats where identical arterial perforation created greater ICP peak and BP rises in the males than in females.3 We note that the present study used animals of a different age and of a different strain from the previous study and an effect of age and strain on intensity of brain injury is established.14-16
Gene Expression
Three different patterns of response are evident among the genes analyzed. A sexually dimorphic response to SAH occurred in Nos3 and Thbd gene expression (Fig. 2). In both cases, expression levels in sham operated animals were similar in the two sexes; however, in both cases SAH caused a 3-5 fold increase in males but no significant change in females. By contrast, Icam1, Il1β and Avp showed significant changes in expression after hemorrhage in both males and females, and the magnitude of the response was equal in the two sexes (Fig. 3). Vegf and Tnf-α, showed no significant response to SAH and no significant sex difference (Fig. 4).
Figure-2. Genes with sexually dimorphic response after SAH.
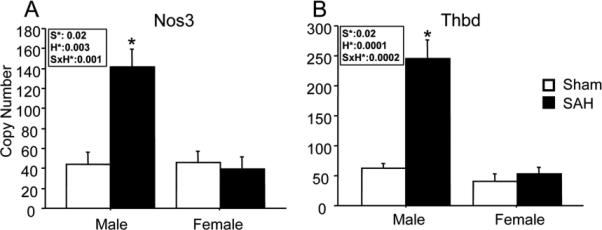
Expression of Nos3 and Thbd genes increased in males and not females after SAH. Open bars: sham; filled bars, SAH. Bars show mean relative mRNA copy number (9 animals per group). Inset boxes show ANOVA effect of sex (S), hemorrhage (H) and interaction between the two (SxH) for each gene. Effects which reach significance are in bold. 2 way ANOVA results: A; Nos3: sex effect (S): F=6, p=0.02; hemorrhage effect (H): F=11, p=0.003; sex by hemorrhage interaction (S*H): F=15, p=0.001. B; Thbd: S: F=7, p=0.02; H: F; 30, p<0.0001; S*H: F=23, p=0.0002. Data are mean ± sem. * significantly difference at P<0.05 in comparison of hemorrhage vs sham within sex.
Figure-3. Genes with sex-equal response.
Expression of Icam1, Il1β and Avp genes increased in both SAH males and females as compared to shams. Open bars: sham; filled bars, SAH. Bars show mean relative mRNA copy number (9 animals per group). Inset boxes show ANOVA effect of sex (S), hemorrhage (H) and interaction between the two (SxH) for each gene. Effects which reach significance are in bold. 2 way ANOVA results: A; Icam1: sex effect (S): F=2, p=0. 2; hemorrhage effect (H): F=24, p=0.0001; sex by hemorrhage interaction (S*H): F=1.2, p=0.3. B; Il1β: S: F=0.6, p=0.6; H: F=25, p=0.0001; S*H: F=1, p=0.3. C; Avp: S: F=0.05, p=0.8; H: F-5, p=0.045; S*H: F=0.2, p=0.7. Data are mean ± sem. *significantly difference at P<0.05 in comparison of hemorrhage vs sham within sex.
Figure-4. Genes unaffected by sex or hemorrhage.
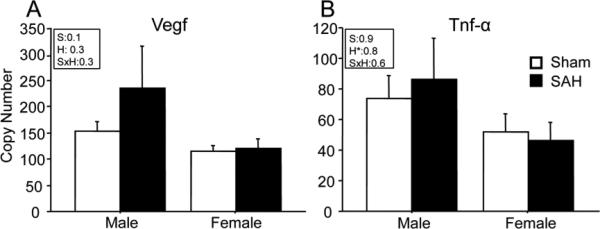
No sex -or injury- specific differences were present in Vegf and Tnf-α gene expression at 3 hours after SAH. Bars show mean relative mRNA copy number (9 animals per group). Inset boxes show ANOVA effect of sex (S), hemorrhage (H) and interaction between the two (SxH) for each gene. Effects which reach significance are in bold. 2 way ANOVA results: A; Vegf: sex effect (S): F=0.1, p=0.7; hemorrhage effect (H): F=1, p=0.3; sex by hemorrhage interaction (S*H): F=1, p=0.3. B; Tnf-α: S: F=0.007, p=0.9; H: F=0.05, p=0.8; S*H: F=0.3, p=0.6. Data are mean ± sem. *significantly difference at P<0.05 in comparison of hemorrhage vs sham within sex.
Discussion
A key finding of this study is that sexual dimorphism in gene expression after SAH exists. Expression of five of the seven genes studied increased in response to SAH, and two of those showed an mRNA response significantly greater in males than in females. It is interesting to note that the products of those genes which exhibited similar responses to SAH in males and females promote inflammation, while those genes which exhibited the sexually dimorphic response inhibit inflammation. Furthermore, the greater expression of anti-inflammatory genes in males functionally counters the known sexually dimorphic pattern of vessel inflammation, which is also greater in males than in females 3. This pattern could reflect the operation of a feedback loop for which we have no data.
We collected mRNA samples three hours after hemorrhage. At this time, a substantial response to SAH is underway both in the microvasculature and in the brain parenchyma. This response includes adhesion of intravascular neutrophils and platelet aggregates to adluminal endothelial surfaces; entry of substantial numbers of neutrophils and platelets into brain parenchyma; activation of collagenases and destruction of microvascular basal lamina and of blood-brain barrier, and apoptosis of both neurons and non-neuronal cells.3, 17
The sexually dimorphic response
Expression of Nos3 and Thbd genes increased in males and not females after SAH. Nos3 encodes endothelial nitric oxide synthase, a main source of vascular nitric oxide (NO) which is a vasodilator and inhibits platelet aggregation. In male rats, the cerebral NO level falls acutely after SAH and restoration of NO reduces microvessel injury.18, 19 The effect of SAH on NO level in female rats has not been determined. In humans, Nos3 expression increases early after SAH but it is not currently known if this increase is sexually dimorphic.20 In contrast, in ischemic stroke patients Nos3 expression has been found to be not sexually dimorphic.21
Thbd encodes thrombomodulin (TM), a endothelial membrane surface protein that plays an important role in protecting vasculature, maintaining vessel patency and blood fluidity of vessels.22 The anti-inflammatory and anti-coagulant effects of thrombomodulin derive from its presence on the adluminal endothelial plasmalemma, and its shedding from the endothelial surface favors thrombosis and associates with vascular disorders. Since circulating soluble TM (sTM) retains only a small fraction of its anti-inflammatory activity, its level is used as a biomarker for endothelial injury and dysfunction.23 In general, sTM levels are lower in women than in age-matched men.24, 25 Moreover, in a number of diseases and disorders sexual dimorphism is reported in levels of circulating thrombomodulin. For example, thrombomodulin levels are lower in women with pulmonary thromboembolism or with metastatic carcinoma as compared to their male counterparts.24, 25 Plasma sTM levels was shown to increase after ischemic stroke, but sexual differences were not addressed.23 The effect of SAH on cerebral Thbd gene expression has not previously been described. Our data show that Thbd gene expression is upregulated in males following SAH, but not in females.
The sex-equal response
Expression of Icam1, Il1β, and Avp genes increased in both SAH males and females as compared to shams. It is interesting to note that the products of Icam1 and Il1β promote inflammation. Icam1, a cell adhesion molecule, aids platelets and neutrophil adhesion to the vessel wall and Il1β, a pro inflammatory cytokine regulates the activity of MMP9, a collagenase implicated in the degradation of vascular basement membrane.26 Expression of both of these genes has been shown to increase at 24 hours after experimental SAH.27-29. The new findings here are that the increase in Icam1 and Il1β gene expression is present substantially earlier, at 3 hours after experimental SAH, and that the response is not sexually dimorphic.
As noted above, we have previously found substantial platelet, neutrophil adhesion to vessel wall and damage to collagen IV lining of basement membrane in both males and females at 3 hours after SAH. The increases we observe in Icam1 and Il1β gene expression in SAH male and female rats could initiate or contribute to those phenomena. Others have shown that Icam1 and Il1β proteins are increased and contribute to both early and delayed brain injury after SAH.28 Moreover, neutralization of Icam1 reduces the severity of delayed vasospasm, and neutralization of Il1β reduces activation of peripheral leukocytes after experimental SAH.30, 31 In SAH patients, high cerebrospinal fluid (CSF) Il1β and blood Icam1 levels associate with poor neurological status.32, 33 These data suggest that increased expression of these genes after SAH might be a key mechanism driving the post-hemorrhage early inflammatory response.
Avp is a potent vasoconstrictor and contributes to the pathogenesis of vasogenic edema and cellular swelling after stroke and during hyponatremia. We included Avp gene expression in this study since a surge in Avp protein levels occurs at SAH and contributes to the observed rise in BP.3 The effect of SAH on Avp gene expression has not previously been studied; however, increased in Avp gene expression within hours after ischemic and traumatic brain injury is well established.6, 7 Hence the increase we observe in Avp gene expression after SAH parallels that in other cerebral injuries. Indirect evidence suggests that the Avp surge at SAH contributes to early brain injury after SAH: inhibition of vasopressin V (1a) receptor prior of SAH blunts BP elevation and reduces the severity of brain damage and mortality in male rats.34
Genes unaffected by sex or hemorrhage
No sex- or injury-specific change was present in Vegf and Tnf-α gene expression at 3 hours after SAH. Vegf, the protein product of vegf gene is a cytokine involved in increased microvascular permeability. In ischemic stroke Vegf increases in CSF and in plasma and has angiogenic and direct neurotrophic effects.35 In SAH Vegf gene and protein expression increase at 24 to 72 hours after artery rupture.4, 5 The lack of change in Vegf gene expression in our study may indicate that this gene upregulates later and not early after SAH.
Tnf-α, the protein product of the Tnf-α gene, is a proinflammatory cytokine that is implicated in endothelial cell apoptosis and BBB permeability after SAH.36, 37 Increased Tnf-α protein has been reported in Wister males at 3 after SAH 38 and in Sprague Dawley males at 7 days after SAH 39 In our Sprague-Dawley rats we found no difference in Tnf-α gene expression between sham and SAH operated animals at 3 hours after SAH, and no difference in Tnf-α gene expression between males and females. Clinically, an early increase in cerebral Tnf-α correlates with the size of the ruptured aneurysm and with poor outcome in SAH patients.40, 41 However, since Tnf-α plays an causative role in cerebral aneurysm formation, the early increase in its level in SAH patients may not be the consequence of aneurysmal rupture but a cause of it.41 Our data indicate that Tnf-α gene expression does not increase in the first hours after SAH; however, further work is clearly needed in this area.
Our present findings, in agreement with our previous findings, show that the early response to experimental SAH differs between males and females. We find this difference in the extent of vessel pathology and in the expression of genes whose products inhibit inflammation. Our results emphasize the importance of including sex as a variable in experimental studies of SAH and the urgency of considering sex as a factor in current and future clinical studies and in the clinical management of SAH cases.
References
- 1.Vaartjes I, Reitsma JB, Berger-van Sijl M, et al. Gender differences in mortality after hospital admission for stroke. Cerebrovasc Dis. 2009;28:564–571. doi: 10.1159/000247600. [DOI] [PubMed] [Google Scholar]
- 2.Hamdan A, Barnes J, Mitchell P. Subarachnoid hemorrhage and the female sex: analysis of risk factors, aneurysm characteristics, and outcomes. J Neurosurg. 2014:1–7. doi: 10.3171/2014.7.JNS132318. [DOI] [PubMed] [Google Scholar]
- 3.Friedrich V, Bederson JB, Sehba FA. Gender influences the initial impact of subarachnoid hemorrhage; an experimental study. PLoS One. 2013;8:e80101. doi: 10.1371/journal.pone.0080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Josko J, Gwozdz B, Jedrzejowska-Szypulka H, et al. Vascular endothelial growth factor (VEGF) and its effect on angiogenesis. Med Sci Monit. 2000;6:1047–1052. [PubMed] [Google Scholar]
- 5.Sun BL, Hu DM, Yuan H, et al. Extract of Ginkgo biloba promotes the expression of VEGF following subarachnoid hemorrhage in rats. The International journal of neuroscience. 2009;119:995–1005. doi: 10.1080/00207450902815842. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Jin Y, Zheng H, et al. Arginine vasopressin gene expression in supraoptic nucleus and paraventricular nucleus of hypothalamous following cerebral ischemia and reperfusion. Chinese medical sciences journal = Chung-kuo i hsueh k'o hsueh tsa chih / Chinese Academy of Medical Sciences. 2000;15:157–161. [PubMed] [Google Scholar]
- 7.Pascale CL, Szmydynger-Chodobska J, Sarri JE, et al. Traumatic brain injury results in a concomitant increase in neocortical expression of vasopressin and its V1a receptor. J Physiol Pharmacol. 2006;57(Suppl 11):161–167. [PubMed] [Google Scholar]
- 8.Sehba FA. Rat Endovascular Perforation Model. Translational stroke research. 2014;5:660–668. doi: 10.1007/s12975-014-0368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26:1086–1091. doi: 10.1161/01.str.26.6.1086. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz AY, Masago A, Sehba FA, et al. Experimental models of subarachnoid hemorrhage in the rat: A refinement of the endovascular filament model. J Neurosci Methods. 2000;96:161–167. doi: 10.1016/s0165-0270(00)00156-4. [DOI] [PubMed] [Google Scholar]
- 11.Friedrich V, Flores R, Muller A, et al. Luminal platelet aggregates in functional deficits in parenchymal vessels after subarachnoid hemorrhage. Brain Res. 2010;1354:179–187. doi: 10.1016/j.brainres.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen B, Martinelli GP, Ogorodnikov D, et al. Sinusoidal galvanic vestibular stimulation (sGVS) induces a vasovagal response in the rat. Exp Brain Res. 2011;210:45–55. doi: 10.1007/s00221-011-2604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press Inc.; San Diego, California: 1986. [Google Scholar]
- 14.Duverger D, MacKenzie ET. The quantification of cerebral infarction following focal ischemia in the rat: influence of strain, arterial pressure, blood glucose concentration, and age. J Cereb Blood Flow Metab. 1988;8:449–461. doi: 10.1038/jcbfm.1988.86. [DOI] [PubMed] [Google Scholar]
- 15.Fuzik J, Gellert L, Olah G, et al. Fundamental interstrain differences in cortical activity between Wistar and Sprague-Dawley rats during global ischemia. Neuroscience. 2013;228:371–381. doi: 10.1016/j.neuroscience.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Walberer M, Stolz E, Muller C, et al. Experimental stroke: ischaemic lesion volume and oedema formation differ among rat strains (a comparison between Wistar and Sprague-Dawley rats using MRI). Laboratory animals. 2006;40:1–8. doi: 10.1258/002367706775404426. [DOI] [PubMed] [Google Scholar]
- 17.Sehba FA, Friedrich V. Cerebral microvasculature is an early target of subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:199–205. doi: 10.1007/978-3-7091-1192-5_37. [DOI] [PubMed] [Google Scholar]
- 18.Sehba FA, Schwartz AY, Chereshnev I, et al. Acute decrease in cerebral nitric oxide levels after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2000;20:604–611. doi: 10.1097/00004647-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Sehba FA, Makonnen G, Friedrich V, et al. Acute cerebral vascular injury occurs after subarachnoid hemorrhage and can be prevented by administration of a Nitric Oxide donor. J Neurosurg. 2007;106:321–329. doi: 10.3171/jns.2007.106.2.321. [DOI] [PubMed] [Google Scholar]
- 20.Berra LV, Carcereri De Prati A, Suzuki H, et al. The role of constitutive and inducible nitric oxide synthase in the human brain after subarachnoid hemorrhage. J Neurosurg Sci. 2007;51:1–9. [PubMed] [Google Scholar]
- 21.McCullough LD, Zeng Z, Blizzard KK, et al. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 22.Ito T, Maruyama I. Thrombomodulin: protectorate God of the vasculature in thrombosis and inflammation. J Thromb Haemost. 2011;9(Suppl 1):168–173. doi: 10.1111/j.1538-7836.2011.04319.x. [DOI] [PubMed] [Google Scholar]
- 23.Olivot JM, Labreuche J, Aiach M, et al. Soluble thrombomodulin and brain infarction: case-control and prospective study. Stroke. 2004;35:1946–1951. doi: 10.1161/01.STR.0000133340.37712.9b. [DOI] [PubMed] [Google Scholar]
- 24.Strijbos MH, Rao C, Schmitz PI, et al. Correlation between circulating endothelial cell counts and plasma thrombomodulin levels as markers for endothelial damage. Thromb Haemost. 2008;100:642–647. [PubMed] [Google Scholar]
- 25.Yin YD, Wang C, Zhai ZG, et al. Decreased plasma soluble thrombomodulin levels as a risk factor for pulmonary thromboembolism. J Thromb Thrombolysis. 2009;27:274–279. doi: 10.1007/s11239-008-0218-x. [DOI] [PubMed] [Google Scholar]
- 26.Sozen T, Tsuchiyama R, Hasegawa Y, et al. Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2009;40:2519–2525. doi: 10.1161/STROKEAHA.109.549592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aihara Y, Kasuya H, Onda H, et al. Quantitative analysis of gene expressions related to inflammation in canine spastic artery after subarachnoid hemorrhage. Stroke. 2001;32:212–217. doi: 10.1161/01.str.32.1.212. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XS, Zhang X, Wu Q, et al. Astaxanthin offers neuroprotection and reduces neuroinflammation in experimental subarachnoid hemorrhage. J Surg Res. 2014;192:206–213. doi: 10.1016/j.jss.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 29.Vikman P, Beg S, Khurana TS, et al. Gene expression and molecular changes in cerebral arteries following subarachnoid hemorrhage in the rat. J Neurosurg. 2006;105:438–444. doi: 10.3171/jns.2006.105.3.438. [DOI] [PubMed] [Google Scholar]
- 30.Bavbek M, Polin R, Kwan AL, et al. Monoclonal antibodies against ICAM-1 and CD18 attenuate cerebral vasospasm after experimental subarachnoid hemorrhage in rabbits. Stroke. 1998;29:1930–1935. doi: 10.1161/01.str.29.9.1930. discussion 1935-1936. [DOI] [PubMed] [Google Scholar]
- 31.Larysz-Brysz M, Lewin-Kowalik J, Czuba Z, et al. Interleukin-1beta increases release of endothelin-1 and tumor necrosis factor as well as reactive oxygen species by peripheral leukocytes during experimental subarachnoid hemorrhage. Curr Neurovasc Res. 2012;9:159–166. doi: 10.2174/156720212801619045. [DOI] [PubMed] [Google Scholar]
- 32.Kwon KY, Jeon BC. Cytokine levels in cerebrospinal fluid and delayed ischemic deficits in patients with aneurysmal subarachnoid hemorrhage. J Korean Med Sci. 2001;16:774–780. doi: 10.3346/jkms.2001.16.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mack WJ, Mocco J, Hoh DJ, et al. Outcome prediction with serum intercellular adhesion molecule-1 levels after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002;96:71–75. doi: 10.3171/jns.2002.96.1.0071. [DOI] [PubMed] [Google Scholar]
- 34.Hockel K, Scholler K, Trabold R, et al. Vasopressin V(1a) receptors mediate posthemorrhagic systemic hypertension thereby determining rebleeding rate and outcome after experimental subarachnoid hemorrhage. Stroke. 2012;43:227–232. doi: 10.1161/STROKEAHA.111.626168. [DOI] [PubMed] [Google Scholar]
- 35.Slevin M, Krupinski J, Slowik A, et al. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke. 2000;31:1863–1870. doi: 10.1161/01.str.31.8.1863. [DOI] [PubMed] [Google Scholar]
- 36.Polunovsky VA, Wendt CH, Ingbar DH, et al. Induction of endothelial cell apoptosis by TNF alpha: modulation by inhibitors of protein synthesis. Exp Cell Res. 1994;214:584–594. doi: 10.1006/excr.1994.1296. [DOI] [PubMed] [Google Scholar]
- 37.Zhang C, Xu X, Potter BJ, et al. TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:475–480. doi: 10.1161/01.ATV.0000201932.32678.7e. [DOI] [PubMed] [Google Scholar]
- 38.Simard JM, Geng Z, Woo SK, et al. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2009;29:317–330. doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prunell GF, Svendgaard NA, Alkass K, et al. Inflammation in the brain after experimental subarachnoid hemorrhage. Neurosurgery. 2005;56:1082–1092. discussion 1082-1092. [PubMed] [Google Scholar]
- 40.Hanafy KA, Grobelny B, Fernandez L, et al. Brain interstitial fluid TNF-alpha after subarachnoid hemorrhage. J Neurol Sci. 2010;291:69–73. doi: 10.1016/j.jns.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Starke RM, Chalouhi N, Jabbour PM, et al. Critical role of TNF-alpha in cerebral aneurysm formation and progression to rupture. J Neuroinflammation. 2014;11:77. doi: 10.1186/1742-2094-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]



