Abstract
Background/Aims
Corneal impression cytology is usually performed with mixed cellulose ester membranes and a limited array of stains. A method using polycarbonate membrane air dried preparations led to the discoveries of fluorescein staining in cells from patients with and without dry eye disease and a membrane induced defect that is not due to cell removal.
Methods
Impressions were performed using polycarbonate and mixed cellulose ester membranes with rapid staining protocols for Diff-Quick as well as hematoxylin and eosin stains. Prior to staining the air dried material was examined for fluorescence.
Results
Epithelium of both normal and dry eye corneas retained fluorescence from clinical instillation of fluorescein. Corneal defects created by the polycarbonate membrane could not be explained by membrane induced cell removal. After rapid staining, polycarbonate membranes revealed less background, dissolved easily prior to coverslip application, but showed lower cellular yield compared to the mixed cellulose membranes.
Conclusion
Polycarbonate membrane impression cytology enables immediate assessment with rapid stains. Topically applied fluorescein penetrates corneal epithelial cells in both normal and dry eye patients. Cells fluoresce on the cytology membranes. The impression induced defect on the cornea is not due to cell stripping and may represent removal of mucins.
Keywords: impression cytology, polycarbonate, Diff-Quick, fluorescein, dry eye
In impression cytology, air dried or ethanol fixed membranes are usually stained with Giemsa, Periodic Acid Schiff (PAS), or Papanicolaou stains for assessment of goblet cell density or squamous metaplasia in dry eye.[1–3] Immediate assessment of cellular adequacy is precluded because cells are invisible on the cytology membranes until dyes are applied. Rapid staining dyes bind avidly to mixed cellulose ester membranes and require destaining. Their opaque fibrous nature led to the need to dissolve the membranes or to remove cells from the membranes for immunohistochemistry.[4, 5] A modification is needed for immediate assessment of cellular yield, use of rapid stains and complete dissolution of the membrane.
INNOVATION
A polycarbonate membrane permitted immediate assessment with rapid staining dyes. The membrane dissolves completely with non-polar organic solvents, eliminating the background seen in mixed fiber membranes. If the air dried membrane preparation is impressed after instillation of topical fluorescein, cellular adequacy can be instantly assessed by fluorescence. This technique led to the discovery of fluorescein penetration in normal cornea surface epithelium, a phenomenon that has not been observed at the slit lamp. A membrane derived defect that produces clinical fluorescein staining was also discovered.
METHODS
Impression Cytology
Trimmed polycarbonate membranes (Millipore-Isopore, 0.1 μm) were pressed on the cornea for 20 seconds and air dried. The membranes were photographed directly under a fluorescent microscope (Nikon SMZ 1500 excitation 450–490nm; emission 500–550nm).
Comparison of Membranes for Cytologic Staining and Cellular Yield
Polycarbonate (Millipore-Isopore, 0.1 μm) mixed cellulose ester membranes (Millipore-MF, 0.22 μm and 0.45 μm) were impressed on buccal mucosa, air dried, ethanol fixed, and stained with Diff-Quick (Richard Allen Company) or hematoxylin and eosin. The membranes were dissolved with chloroform and coverslipped.
CASES
Patient 1 Dry Eye Disease
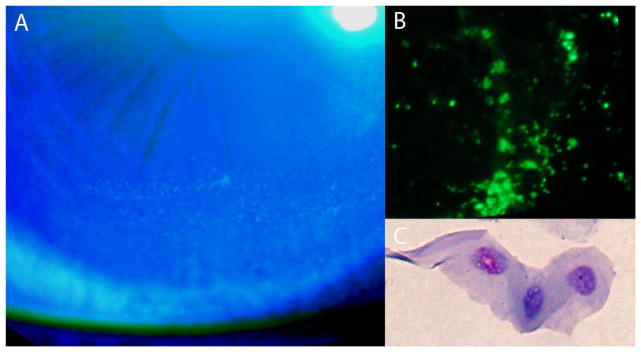
A 24-year-old female had symptoms of dryness, soreness, stinging, itching, and redness of the right eye. The OSDI score was 12.5.[6] Visual acuity was 20/15 in both eyes. Schirmer’s values were 2 mm and 7 mm for the right and left eye, respectively. The mean fluorescein tear breakup time (using Bio Glo, Rose Stone Enterprises) was 10.6 and 11.6 seconds for the right and left eye, respectively.[7] Superficial punctate staining matched a grade II staining pattern in the Oxford classification (fig 1A).[8]
Figure 1.
(A) Photograph of the fluorescein stained right eye shows punctate staining pattern inferiorly on the cornea of a dry eye patient. (B) Fluorescent microscopy of polycarbonate filter shows singly stained cells throughout the filter (x40). (C) Polycarbonate filter stained with Diff-Quick and dissolved using a solution of chloroform showing a stained epithelial cell (x500).
Patient 2 Normal Subject
A 25-year-old male had no symptoms of dry eye. The OSDI score was 4.2.[6] Visual acuity was 20/20 in both eyes. Schirmer’s values were 15 mm and 16 mm for the right and left eye, respectively. The tear breakup time was 12.3 and 12.9 seconds for the right and left eye, respectively.[7] Fluorescein staining was grade 0 bilaterally, Oxford classification.[8]
In both cases, the inferior cul de sac was gently rinsed with normal saline over a 20 minute interval to clear residual fluorescein and impression cytology performed. The corneas were examined immediately after impression cytology, then after fluorescein re-instillation, and once more after 3 hours.
RESULTS
Patients 1 and 2
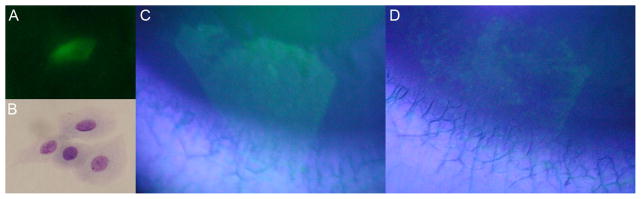
Fluorescent cells were observed on the membrane when viewed directly under the fluorescent stereomicroscope but not when viewed under slit lamp illumination with a cobalt blue filter (fig 1B and 2A). Squamous epithelium was seen in the Diff-Quick stained cytology preparations (fig 1C and 2B). Cellular yield was greater in the dry eye than the normal cornea.
Figure 2.
(A) Fluorescent microscopy of polycarbonate filter shows stained cell of a normal subject (x400). (B) The polycarbonate filter, stained with Diff-Quick, shows epithelial cells (x400). (C) Photograph of the fluorescein stained cornea immediately post impression cytology. The pattern matches the size and shape of the polycarbonate membrane. (D) Photograph of the fluorescein stained impression defect 3 hours later.
Induced Non-epithelial Defect
The cornea of patient 2 showed no residual fluorescein staining immediately after removal of the membrane. With installation of additional fluorescein a defect that matched the shape of the impression membrane appeared (fig 2C). Cells on the membrane were too few to account for the defect and localized to one area (fig 2A). After 3 hours the defect was smaller and patchy (fig 2D).
Comparison of Membrane Staining
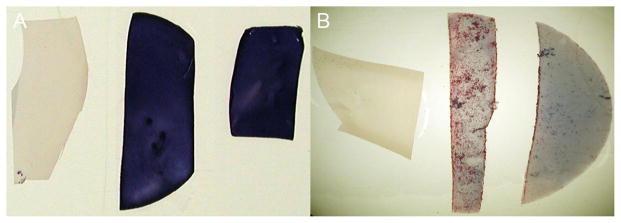
Stains of polycarbonate membranes were compared to mixed cellulose ester. The polycarbonate membrane showed less background stain but lower cellular yield than did the mixed cellulose membranes (fig 3).
Figure 3.
(A) Buccal mucosa samples stained with Diff-Quick; membranes from left to right: Millipore Isopore 0.1 μm, Millipore-MF 0.45 μm, Millipore-MF 0.22 μm. (B) Buccal mucosa samples stained with hematoxylin and eosin; membranes from left to right: Millipore-Isopore 0.1 μm, Millipore-MF 0.45 μm, Millipore-MF 0.22 μm.
DISCUSSION
Impression cytology of the cornea is usually performed with Millipore mixed cellulose ester, Biopore, or nitrocellulose membranes in concert with PAS and/or hematoxylin and eosin stains (table 1). The fibers, debris and intense background staining of mixed cellulose ester membranes may obfuscate cells.[5] Hematoxylin binds strongly to mixed cellulose ester membranes so that cells must be viewed with a very bright light source. Rapid stains, such as Diff-Quick, bind even more avidly to mixed cellulose ester membranes and are reported only in conjunction with Thermanox plastic discs for conjunctival cytology.[9]
Table 1.
Studies published in English of human corneal impression cytology that detail membrane and processing procedures, [3, 18–25] references therein. Studies that did not detail the type of membrane or procedure have been excluded.
| Number of Studies Publishing Specific Membranes Usage and Procedures | ||||
|---|---|---|---|---|
| Histochemical Stain | Immunohistochemistry | PCR | DNA fingerprinting | |
| Millipore mixed cellulose ester | PAS* 9 PAP 3 H&E 1 | 0 | 4 | 2 |
| Millipore Biopore | PAS* 1 | 6 | 0 | 0 |
| Millipore nitrocellulose | PAS* 3 | 4 | 0 | 0 |
| Glass slide | 0 | 2 | 0 | 0 |
| FTA paper | 0 | 0 | 0 | 1 |
| Polycarbonate | Diff Quick** 1 | 0 | 0 | 0 |
Includes all modifications of the PAS stain.
Data from the current study.
The absence of background stain with polycarbonate membranes is ideal for rapid stains and air dried material, but the sample collection appears reduced. A lower yield from the polycarbonate membranes may be limiting in certain applications. This decreased yield may be related to the smaller pore size of the polycarbonate membranes.[3] The cellular yield was greater from the dry eye patient than the normal subject. Apical cells in dry eye patients may be less cohesive than normal.[10] A quantitative study is needed for an accurate assessment.
One observation accrued by air drying cells to the membrane was visualization of fluorescein in cells during routine examination. This fact has escaped clinical detection. Many studies state that fluorescein neither stains vital tissues nor penetrates intact human corneal epithelium.[8, 11, 12] In our study, a powerful fluorescence light source with specific excitation and emission filter sets supplanted the insensitive cobalt blue filter at the slit lamp. Studies in lapin corneas and cultured cell lines also show fluorescein penetration into epithelium.[13]
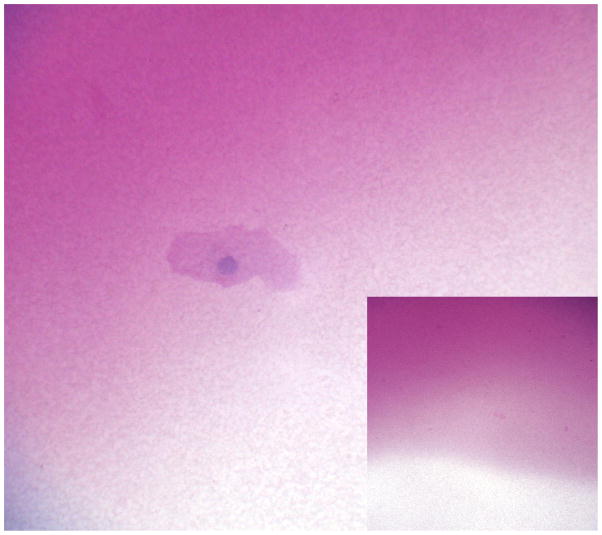
The nature of superficial punctate staining in dry eye syndrome is uncertain. The punctate fluorescence may reflect a greater concentration of fluorescein in unprotected cells or an environment of altered fluorescence quenching.[12, 14, 15] The observation of a fluorescein staining defect left by impression cytology may hint at a possible explanation. Because the defect matches the size and shape of the membrane but not the focal location of cells, a true epithelial cell defect is excluded. Membrane disruption of the mucin derived glyocalyx might alter permeability to fluorescein.[16, 17] Acellular PAS positive material on membranes indicates the capacity to adhere mucins (fig 4).
Figure 4.
Acellular PAS positive material is evident on this impression cytology membrane (x1300) and (inset x130).
Acknowledgments
FUNDING
NIH R01-EY 11224 and the Edith and Lew Wasserman Professorship in Ophthalmology.
Footnotes
COMPETING INTERESTS
The authors declare that they have no competing interests.
COPYRIGHT LICENCE STATEMENT
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd, and its Licensees to permit this article (if accepted) to be published in BJO and any other BMJPGL products and to exploit all subsidiary rights, as set out in our licence.
References
- 1.Egbert PR, Lauber S, Maurice DM. A simple conjunctival biopsy. Am J Ophthalmol. 1977;84:798–801. doi: 10.1016/0002-9394(77)90499-8. [DOI] [PubMed] [Google Scholar]
- 2.Tseng SC. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology. 1985;92:728–33. doi: 10.1016/s0161-6420(85)33967-2. [DOI] [PubMed] [Google Scholar]
- 3.Calonge M, Diebold Y, Saez V, et al. Impression cytology of the ocular surface: a review. Exp Eye Res. 2004;78:457–72. doi: 10.1016/j.exer.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Baudouin C, Haouat N, Brignole F, et al. Immunopathological findings in conjunctival cells using immunofluorescence staining of impression cytology specimens. Br J Ophthalmol. 1992;76:545–9. doi: 10.1136/bjo.76.9.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anshu, Munshi MM, Sathe V, et al. Conjunctival impression cytology in contact lens wearers. Cytopathology. 2001;12:314–20. doi: 10.1046/j.1365-2303.2001.00349.x. [DOI] [PubMed] [Google Scholar]
- 6.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 7.Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221–32. [PubMed] [Google Scholar]
- 8.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–50. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hershenfeld S, Kazdan JJ, Mancer K, et al. Impression cytology in conjunctivitis. Can J Ophthalmol. 1981;16:76–8. [PubMed] [Google Scholar]
- 10.De Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47:2847–56. doi: 10.1167/iovs.05-1281. [DOI] [PubMed] [Google Scholar]
- 11.Maurice DM. The use of fluorescein in ophthalmological research. Invest Ophthalmol. 1967;6:464–77. [PubMed] [Google Scholar]
- 12.Romanchuk KG. Fluorescein. Physiochemical factors affecting its fluorescence. Surv Ophthalmol. 1982;26:269–83. doi: 10.1016/0039-6257(82)90163-1. [DOI] [PubMed] [Google Scholar]
- 13.Feenstra RP, Tseng SC. Comparison of fluorescein and rose bengal staining. Ophthalmology. 1992;99:605–17. doi: 10.1016/s0161-6420(92)31947-5. [DOI] [PubMed] [Google Scholar]
- 14.Williams RT, Bridges JW. Fluorescence of Solutions: A Review. J Clin Pathol. 1964;17:371–94. doi: 10.1136/jcp.17.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward KW. Superficial punctate fluorescein staining of the ocular surface. Optom Vis Sci. 2008;85:8–16. doi: 10.1097/OPX.0b013e31815ed756. [DOI] [PubMed] [Google Scholar]
- 16.Gipson IK, Hori Y, Argueso P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004;2:131–48. doi: 10.1016/s1542-0124(12)70149-0. [DOI] [PubMed] [Google Scholar]
- 17.Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008;8:477–83. doi: 10.1097/ACI.0b013e32830e6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacchetti M, Lambiase A, Cortes M, et al. Clinical and cytological findings in limbal stem cell deficiency. Graefes Arch Clin Exp Ophthalmol. 2005;243:870–6. doi: 10.1007/s00417-005-1159-0. [DOI] [PubMed] [Google Scholar]
- 19.Okada N, Fukagawa K, Takano Y, et al. The implications of the upregulation of ICAM-1/VCAM-1 expression of corneal fibroblasts on the pathogenesis of allergic keratopathy. Invest Ophthalmol Vis Sci. 2005;46:4512–8. doi: 10.1167/iovs.04-1494. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi Y, Kao WW, Kohno N, et al. Expression patterns of sialylated epitope recognized by KL-6 monoclonal antibody in ocular surface epithelium of normals and dry eye patients. Invest Ophthalmol Vis Sci. 2004;45:2212–7. doi: 10.1167/iovs.03-0988. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia RP, Lipika R, Garbyal RS, et al. Corneal and conjunctival impression cytology in soft contact lens wearers. Ann Ophthalmol (Skokie) 2006;38:117–20. doi: 10.1385/ao:38:2:117. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Garcia JS, Rivas L, Garcia-Lozano I, et al. Analysis of corneal surface evolution after moderate alkaline burns by using impression cytology. Cornea. 2006;25:908–13. doi: 10.1097/01.ico.0000225711.54888.e8. [DOI] [PubMed] [Google Scholar]
- 23.Satake Y, Dogru M, Yamane GY, et al. Barrier function and cytologic features of the ocular surface epithelium after autologous cultivated oral mucosal epithelial transplantation. Arch Ophthalmol. 2008;126:23–8. doi: 10.1001/archopht.126.1.23. [DOI] [PubMed] [Google Scholar]
- 24.Shortt AJ, Secker GA, Rajan MS, et al. Ex vivo expansion and transplantation of limbal epithelial stem cells. Ophthalmology. 2008;115:1989–97. doi: 10.1016/j.ophtha.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 25.Abedin A, Mohammed I, Hopkinson A, et al. A novel antimicrobial peptide on the ocular surface shows decreased expression in inflammation and infection. Invest Ophthalmol Vis Sci. 2008;49:28–33. doi: 10.1167/iovs.07-0645. [DOI] [PubMed] [Google Scholar]