Abstract
Bone water exists in different states with the majority bound to the organic matrix and to mineral, and a smaller fraction in ‘free’ form in the pores of cortical bone. In this study we aimed to develop and evaluate ultrashort echo time (UTE) magnetic resonance imaging (MRI) techniques for assessment of T2*, T1 and concentration of collagen-bound and pore water in cortical bone using a 3T clinical whole-body scanner. UTE MRI together with an isotope study using tritiated and distilled water (THO-H2O) exchange as well as gravimetrical analysis were performed on ten sectioned bovine bone samples. In addition, 32 human cortical bone samples were prepared for comparison between pore water concentration measured with UTE MRI and cortical porosity derived from micro computed tomography (μCT). A short T2* of 0.27 ± 0.03 ms and T1 of 116±6 ms were observed for collagen-bound water in bovine bone. A longer T2* of 1.84 ± 0.52 ms and T1 of 527±28 ms were observed for pore water in bovine bone. UTE MRI measurements showed a pore water concentration of 4.7-5.3% by volume and collagen-bound water concentration of 15.7-17.9% in bovine bone. THO-H2O exchange studies showed a pore water concentration of 5.9 ± 0.6% and collagen-bound water concentration of 18.1 ± 2.1% in bovine bone. Gravimetrical analysis showed a pore water concentration of 6.3 ± 0.8% and collagen-bound water concentration of 19.2 ± 3.6% in bovine bone. A mineral water concentration of 9.5 ± 0.6% was derived in bovine bone with the THO-H2O exchange study. UTE measured pore water concentration is highly correlated (R2 = 0.72, P < 0.0001) with μCT porosity in the human cortical bone study. Both bovine and human bone studies suggest that UTE sequences could reliably measure collagen-bound and pore water concentration in cortical bone using a clinical scanner.
Keywords: bone, bound water, pore water, organic matrix, UTE
Introduction
Cortical bone is a composite material with three major components: mineral (principally hydroxyapatite, ~40% of bone by volume), collagen (~35% by volume) and water (~25% by volume) 1. Dual-energy X-ray absorptiometry (DXA) and computed tomography (CT) have been used for quantitative analysis of bone mineral density (BMD) 2. However, the organic matrix and water, which together represent ~60% of bone by volume, make little contribution to the signal obtained with these techniques. BMD measurement alone predicts fractures with only a 30-50% success rate 3,4. From ages 60 to 80 years fracture risk increases about 13-fold, however the decrease in BMD alone only explains a doubling in this fracture risk 5. A recent study of over 7800 patients reported that only 44% of all non-vertebral fractures occurred in women with a T-score below −2.5 (the World Health Organization definition of osteoporosis based on BMD), and this percentage dropped to 21% in men 6. The limitations of BMD measurement have sparked research into the use of other imaging modalities to assess bone quality 7, focusing on architectural deterioration as well as the contribution of organic matrix and water to the biomechanical properties of cortical bone.
Water in cortical bone is present at various locations and in different states 8. In normal bone a small fraction of water exists in ‘free’ form (pore water or PW) in Haversian canals (typical diameters ~50-200 μm) as well as in lacunae (~5 μm) and canaliculi (~0.1 μm) 9,10. A larger portion of bone water exists in ‘bound’ form, either loosely bound to the organic matrix (collagen-bound water or CW) 11 or tightly bound to mineral (mineral-bound water or MW) 12,13. Recent nuclear magnetic resonance (NMR) spectroscopy studies suggest that the loosely bound water concentration reflects organic matrix density of bone 14,15, while the “free” pore water concentration can potentially provide a surrogate measure of cortical porosity 16. This provides an opportunity to develop noninvasive techniques to measure bound and pore water concentration in cortical bone and potentially provides a more accurate assessment of bone quality.
As a complementary tool to DXA and CT, magnetic resonance imaging (MRI) detects signal from protons (mostly water) in bone rather than from mineral. However, water in cortical bone has a very short apparent transverse relaxation time (or T2*) and it provides no detectable signal when examined with conventional clinical MRI sequences 17. Recently ultrashort echo time (UTE) sequences, with reduced nominal echo times (TEs) as short as 8 μs, which is about 100-1000 times shorter than the TEs of conventional clinical pulse sequences, have been used to detect signal from cortical bone 18. More recently, magnetization prepared UTE techniques including adiabatic inversion recovery (AIR) and double adiabatic full passage (DAFP) sequences have been developed for mapping of bound and pore water in cortical bone in vivo 19. This is important considering that bound and pore water make different contributions to the mechanical properties of cortical bone 20.
In this study we aimed to assess the value of two-dimensional (2D) and 3D UTE techniques for determining bound and pore water in bovine and human cortical bone samples. An isotope study, namely tritiated and distilled water (THO-H2O) exchange in sectioned bovine bone samples, together with gravimetrical analysis were used to validate UTE-based cortical bone water components quantification. A comparison study between UTE MRI measured pore water concentration and micro computed tomography (μCT) measured cortical porosity of cadaveric human cortical bone samples was also performed to further assess the accuracy of UTE MRI measurements of bone water components.
Materials and Methods
Sample Preparation
Fourteen bovine cortical bone samples of approximate dimensions 10×10×10 mm3 were prepared from mature bovine femoral midshafts obtained from a local slaughterhouse. These samples were initially sectioned using a low-speed diamond saw (Isomet 1000, Buehler) with constant saline irrigation, and stored in phosphate buffered saline (PBS) solution for 24 hours prior to study. Four of the samples were used to determine the time required for THO and H2O exchange to reach equilibrium. Ten samples were used for bound and pore water measurement with UTE and THO-H2O isotope exchange studies (details below).
Cadaveric human cortical bone samples (n = 32) from 12 donors (7 females, 5 males, 30-92 years old) were obtained from tissue banks, as approved by Institutional Review Board. Using the precision circular diamond-edge saw and saline irrigation, larger bone blocks were sectioned into rectangular slabs of cortical bone with dimensions of ~1×1×10 mm3. Individual samples were wrapped in saline-wet gauze and frozen at −70 °C in an ultralow freezer (Bio-Freezer; Forma Scientific, Marietta, OH, USA). The samples were allowed to thaw for 12 hours at 4 °C prior to UTE MR imaging and micro computed tomography (μCT).
Pulse Sequences
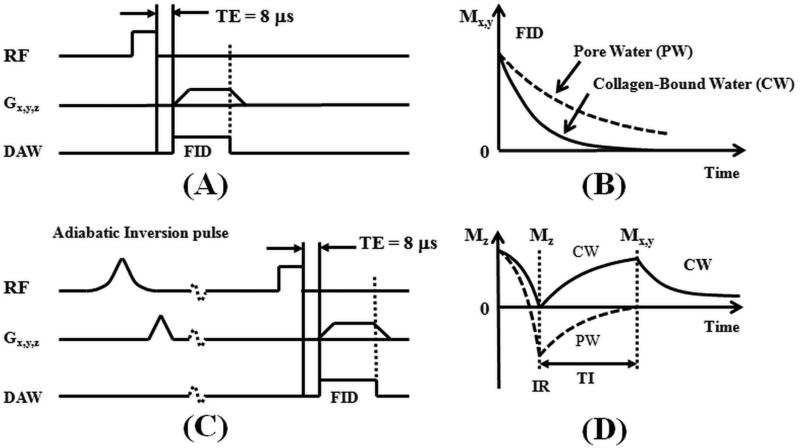
3D UTE sequences (Figure 1) were implemented on a 3T Signa TwinSpeed scanner (GE Healthcare Technologies, Milwaukee, WI). The basic 3D UTE sequence employed a short radio frequency (RF) rectangular pulse (duration = 26-52 μs) for signal excitation 21. The z-gradient could be turned off to allow non-selective 2D UTE imaging for fast imaging of cortical bone. Both collagen-bound and pore water are detectable with the basic UTE sequences (Figure 1B). Furthermore, adiabatic inversion recovery prepared UTE (IR-UTE) sequences were developed for selective imaging of collagen bound water (Figure 1C). In the IR-UTE sequence, a Silver-Hoult adiabatic inversion pulse (duration = 8.64 ms, bandwidth = 1.5 kHz) was used to invert the longitudinal magnetization of pore water 18,22. The longitudinal magnetization of collagen-bound water which has a very short T2* was not inverted but largely saturated by the adiabatic IR pulse 22. After an inversion time (TI) during which the inverted pore water magnetization approached the null point, the UTE acquisition was initiated to selectively detect signal from collagen-bound water (Figure 1D). 2D UTE and IR-UTE sequences were used for fast T1 and T2* quantification, while 3D UTE and IR-UTE sequences were used for bound and pore water concentration quantification (details below).
Figure 1.
The 3D UTE (A) and IR-UTE (C) sequences, as well as the contrast mechanisms for imaging of total water (B) and collagen-bound water (D). The UTE sequence employs a short rectangular pulse for signal excitation followed by 3D radial ramp sampling with a minimal nominal TE of 8 μs, which is short enough to detect signal from both collagen-bound water (CW) with very short T2* (solid lines in B and D) and pore water (PW) with slightly longer T2* (dashed lines in B and D). The IR-UTE sequence employs an adiabatic inversion pulse to invert and null the pore water magnetization. The collagen-bound water magnetization is not inverted and is detected by a subsequent UTE data acquisition (D). The z gradient can be turned off for fast non-selective 2D UTE and IR-UTE imaging.
Quantitative MR Imaging
Collagen-Bound and Pore Water T2*s
Each sample was placed in perfluorooctyl bromide (PFOB) solution. This helped maintain the hydration of cortical bone and minimize susceptibility effects at tissue-air interfaces. A birdcage coil (diameter = 2.5 cm, length = 12.2 cm) was used for signal excitation and reception. 2D UTE and IR-UTE sequences were performed for fast T2* measurement with the following parameters: field of view (FOV) = 4 cm, sampling bandwidth (BW) = 62.5 kHz, flip angle = 10°, pulse duration = 26 μs, TR = 100 ms for UTE and 300 ms for IR-UTE, TI = 110 ms (for IR-UTE only), reconstruction matrix size = 128×128, in-plane pixel size = 0.31×0.31 mm2, 211 projections, number of excitation (NEX) = 1, scan time = 21 sec for UTE and 1 min for IR-UTE. UTE and IR-UTE images were acquired at a series of TE delays (TE = 8 μs, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.8, 1, 1.2, 1.4, 1.6, 2, 2.5, 3, 3.5, 4, 5, 6, 7 ms) to separate collagen-bound and pore water using the following bi-component analysis model 23:
| [1] |
where SZ0,cw and SZ0,pw are the magnetization of the collagen-bound and pore water components, and T2,cw* and T2,pw* are their T2* relaxation times.
Collagen-Bound and Pore Water T1s
Collagen-bound and pore water may have different T1 values. Both contribute to the UTE signal when a minimal TE of 8 μs is used. When a longer TE (e.g., 2.4 ms) is used, the UTE signal mainly comes from pore water since the signal from collagen-bound water with a T2* ~0.3 ms very largely decays to zero. In this study we proposed to use a saturation recovery dual echo UTE imaging technique to measure the T1s of collagen-bound and pore water components.
In the saturation recovery UTE sequence a 900 rectangular pulse is followed by a crusher gradient to dephase signals from both long and short T2 species. Dual echo 2D UTE acquisitions with progressively increasing saturation recovery times (TSRs) are used to detect the recovery of the longitudinal magnetization of cortical bone. For simplification, we assume an effective T1 (T1eff) of collagen-bound and pore water is measured using 2D saturation recovery UTE acquisitions with a minimal TE of 8 μs, and pore water T1 (T1pw) is measured using saturation recovery UTE acquisitions with a longer TE of 2.4 ms. T1eff and T1pw were calculated using the following two equations:
| [2] |
| [3] |
where k accounts for the residual fraction of the longitudinal magnetization of cortical bone after a nominal 90° pulse. Similar imaging parameters were used except for a long TR of 3500 ms (which is long enough to ensure almost complete recovery of both bound and pore water longitudinal magnetizations after each 90° pulse), TE = 8 μs and 2.4 ms, a series of TSRs (7, 25, 50, 100, 200, 400, 600, 800, 1000, 1200, 1600, 2000, 2400, 2800, 3400 ms) with a total scan time of 110 min.
The collagen-bound water signal obtained with IR-UTE imaging can be described by the following equation 24:
| [4] |
where is the T1 of collagen-bound water, and Q is the inversion efficiency of the adiabatic IR pulse. For collagen-bound water with a T2* of ~0.3 ms, Q approximates 0 (i.e., < 0.04) according to Bloch equation simulation 18. As a result, the IR-UTE signal can be simplified as follows 24:
| [5] |
here can be measured by fitting the IR-UTE signal acquired with a series of TR and TI combinations, under the condition that each TR/TI combination satisfies the inversion and nulling condition necessary to suppress the signal from pore water. We used imaging parameters similar to the 2D IR-UTE T2* analysis above, but with a series of TR/TI combinations (e.g., TR = 50, 100, 200, 300, 400, 500 ms, TI was chosen for each sample based on the criteria to null pore water with a measured T1pw). The total scan time was about 6 min.
Collagen-Bound and Pore Water Concentration Measurement
Total water concentration (WCTotal) was measured by comparing the 3D UTE signal intensity of cortical bone with that from a doped water phantom 25. Accurate estimation of bone water requires consideration of relaxation times and coil sensitivity. In our experiments the external reference was a mixture of distilled water (20%) and D2O (80%) doped with 26 millimolar MnCl2, resulting in a short T2* of ~300 μs and a T1 of ~ 5 ms (measured with standard UTE acquisitions wit variable TEs or TSRs, as shown in Ref 18). With the use of a short excitation pulse (14 μs), ultrashort TE (8 μs), relatively long TR (50 ms) and a small flip angle (5°), T1 and T2* relaxation effects can be ignored, and can be simplified as follows:
| [6] |
where and are the 3D UTE signal intensities of cortical bone and H2O-D2O water phantom, respectively, and η is a coil sensitivity correction. η was corrected by dividing the 3D UTE signal from cortical bone or doped water phantom by the 3D UTE signal obtained from a separate scan of a bottle of water, which was large enough to cover the region occupied by both the cortical bone sample and doped water phantom. Other imaging parameters were similar to those used for 2D UTE T2* measurements except for 3D UTE acquisitions with 20,000 projections and a voxel size of 0.31×0.31×0.31 mm3. The total scan time was 16.7 min.
Collagen-bound water concentration (WCCollagen) was measured by comparing the 3D IR-UTE signal intensity of cortical bone with that from the water phantom. Based on Eq.4, can be measured as follows:
| [7] |
where and are the IR-UTE signal intensities of cortical bone and H2O-D2O water phantom, respectively. After each IR pulse the longitudinal magnetization of H2O-D2O is almost fully recovered since its T1 is much shorter than TI. Imaging parameters were similar to those used for 2D IR-UTE T2* measurements, except for 3D IR-UTE with 20,000 projections and a voxel size of 0.31×0.31×0.31 mm3. The total scan time was 100 min.
With total water and collagen-bound water known, pore water concentration (WCPore) can be calculated as their difference:
| [8] |
μCT Imaging
The human cortical bone samples were imaged using a μCT scanner (1076, Skyscan, Kontich, Belgium) with the following parameters: 0.5 mm Aluminum filter, 72 kV, 140 μA, 720 views collected at 0.5° increments corresponding to one full rotation of each bone specimen, FOV 25 mm, isotropic 9 μm voxels. The total scan time was ~ 3 hours.
THO-H2O exchange study
THO is radioactive, with a half life of 12.3 years. The relatively long half life makes it ideal for isotope study. Four bovine cortical samples were used to determine the time required for THO-H2O exchange to reach equilibrium. Each bone sample was blotted dry and put in a glass bottle filled with 2 ml of THO for times of 1, 2, 4, 7 and 10 days and maintained at room temperature. After each exchange stage, 0.1 ml of the mixed solution (a mixture of THO and H2O) was mixed by vortex with 5 ml of scintillation fluid (Ecoscint, National Diagnostics, Atlanta, GA) in scintillation vials and placed into a liquid scintillation counter (LS6000SC, Beckman Coulter Inc, Brea, CA) for beta radiation measurement in counts per minute (CPM). CPM is defined by the total number of photons counted divided by the count time. CPM was plotted against isotope exchange time to determine the time required to reach equilibrium.
Each glass vial containing an individual bone sample was filled with 2 ml of THO. The glass vial was tightly sealed for THO-H2O exchange. After equilibrium (~7 days) 0.1 ml of the mixed solution was prepared for beta radiation detection. Meanwhile a calibration curve was generated by measuring the radiation of solutions with eight different ratios (1:0, 1:0.05, 1:0.1, 1:0.15, 1:0.2, 1:0.3, 1:0.5 and 1:1) of THO and H2O. CPM was plotted against the THO dilution factor and then used as the calibration curve. Based on the radiation calibration curve a dilution factor for the THO-H2O exchange was calculated, and used for accurate measurement of the volume of H2O in each bovine cortical bone. Bone water concentration was calculated by dividing H2O volume by bone volume from μCT (more details are given in the Gravimetrical Analysis section below).
Gravimetrical Analysis
Previous literatures suggest that air-drying at room temperature mainly results in loss of pore water, while subsequent oven-drying at 60-100 °C mainly results in loss of water bound to the organic matrix 26-30. In this study we employed air-drying (in an oven with controlled humidity at room temperature) of blot-dried bovine cortical bone for 48 hours to remove pore water, followed by oven-drying at 100 °C for 72 hours to remove collagen-bound water, after which water remaining in the bone is assumed to be tightly bound to mineral. The air-drying and oven-drying times were empirically determined based on weight change. About two days were needed for air-drying and three days were needed for oven-drying to reach equilibrium mass (weight change of less than 0.1% in the last five hours of drying).
Mankin et al. used an approach combining THO-H2O exchange and drying course to measure bound and free water in articular cartilage 31. We adopted a similar approach in this study. First, all bovine cortical bone samples (n=10) were blotted dry and subjected to UTE and IR-UTE imaging to quantify T1pw, T1cw, T2,pw*, T2,cw*, WCCollagen and WCPore, and μCT imaging to measure bone volume (), as well as use of a digital precision balance (Mettler Toledo AL104) to measure bone weight (). Then half of the bone samples (n=5) were subjected to air-drying to remove pore water, followed by measurement of bone weight (). The volume of H2O from each blot-dried () and air-dried () bovine cortical bone sample was measured via THO-H2O exchange. Each bone sample was then immersed in saline (50 ml), changed daily until the CPM was reduced to background level (<40), indicating that all THO had been replaced by H2O. Oven-drying for 72 hours at 100 °C was used to remove collagen-bound and pore water. Each bovine bone sample was cooled down in a sealed glass tube to minimize reabsorbtion of water. The oven-dry weight was measured as . Finally each bone sample was placed into THO-H2O for a second THO-H2O exchange to measure the volume of mineral-bound water () which had not been removed by the oven-drying process. Total bone water concentration from the THO-H2O exchange study () was calculated as follows:
| [9] |
The concentration of water bound to collagen () was calculated as follows:
| [10] |
The concentration of water bound to mineral () was calculated as follows:
| [11] |
Pore water concentration () was calculated as follows:
| [12] |
Both pore water () and collagen-bound water () can also be estimated from the gravimetric measurements using the following equations 29:
| [13] |
| [14] |
Mineral-bound water is inaccessible with gravimetric analysis due to the difficulty in separating it from collagen gravimetrically.
Data Analysis
A semi-automated MATLAB (The Mathworks Inc. Natick, MA, USA) program was developed for single- and bi-component T2* and T1 analysis. Regions of interest (ROIs) were drawn in the central part of cortical bone for data analysis. Goodness of fit statistics including the R-squared value and standard error or fitting confidence level were computed. Fit curves along with their 95% confidence intervals (CI) and residual signal curves were created. Total, collagen-bound, mineral-bound and pore water concentrations were calculated using the equations shown in [6-14].
For each human cortical bone sample, 12 μCT images every 2.5 mm along the sample long axis were selected and imported into Matlab. A custom program was used to determine the global histogram for all the images and this was used to determine a local minimum value used for thresholding. Binarized images were despeckled to remove noise, and regions of interest corresponding to the outer boundary of the sample as well as to bone were automatically generated. Cortical porosity was determined as one minus the ratio of the area of the bone to that of the outer boundary of the sample.
Results
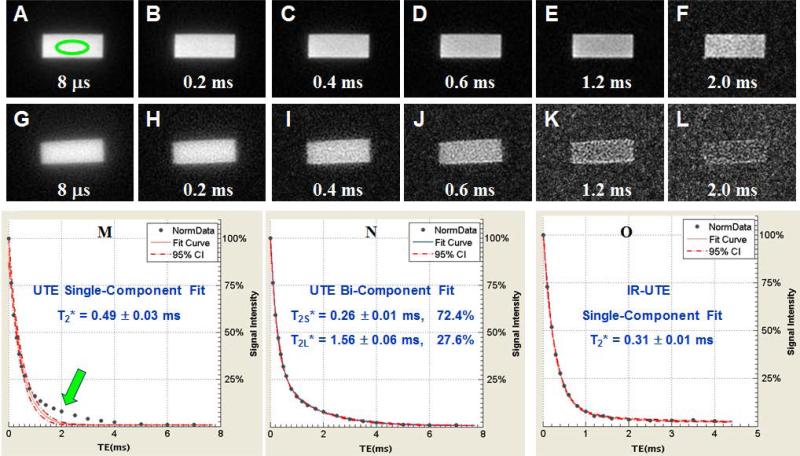
UTE and IR-UTE sequences were used to access different water components in cortical bone using a clinical MR scanner. Figure 2 shows selected UTE and IR-UTE images of a bovine cortical bone sample with different TEs. Systematic residual signal is seen with single-component fitting of UTE signal decay, suggesting the existence of another water component. Excellent curve fitting is achieved with a bi-component model which shows two distinct water components: one with a short T2* of 0.26 ms accounting for 72.4% of the total UTE signal, and the other with a longer T2* of 1.56 ms accounting for 27.6% of the signal decay. The shorter and longer T2* values are consistent with those of collagen-bound and pore water respectively based on recent NMR spectroscopic studies by Ni et al., who reported a short T2* of 0.21 ms and a longer T2* of 2.70 ms 16, suggesting that UTE bi-component analysis can access bound and pore water using a whole-body clinical MR scanner. The IR-UTE images show a single component T2* decay of 0.31 ms which is close to the short T2* value of 0.26 ms obtained from the bi-component fitting of UTE T2* signal decay, suggesting that water bound to the organic matrix is selectively detected in IR-UTE imaging. The pore water component with a longer T2* is largely suppressed by the IR preparation pulse through adiabatic inversion and signal nulling.
Figure 2.
Selected 2D UTE imaging of a bovine cortical bone sample with TEs of 8 μs (A), 0.2 ms (B), 0.4 ms (C), 0.6 ms (D), 0.8 ms (E), 1.6 ms (F), 2 ms (G), 3 ms (H), 4 ms (I), 5 ms (J), 6 ms (K) and 7 ms (L) as well as single component (M) and bi-component (N) fitting of the UTE images. Single component fitting of the corresponding IR-UTE images with a TR of 300 ms and TI of 90 ms was also shown (O). An ROI was drawn in (A). Single component fitting of the UTE images shows significant residual signal (> 10%) (M), which is reduced to less than 0.5% by bi-component fitting (N), which shows a shorter T2* of 0.25 ms and a longer T2* of 2.20 ms with respective fractions of 81.2% and 18.8% by volume. Excellent single component fitting of the IR-UTE images is achieved with residual signal less than 0.3% (O), and a T2* of 0.30 ms which is close to that of the short T2* component in UTE images, suggesting that bound water was detected by the IR-UTE sequence with pore water largely suppressed by the long adiabatic inversion pulse.
Table 1 shows the mean and standard deviation of T2* values of 10 bovine bone samples. An effective T2* of 0.41 ± 0.05 ms was demonstrated from single-component fitting of UTE signal decays with contribution from both collagen-bound and pore water components. Bi-component fitting shows a mean pore water T2*pw of 0.28 ± 0.03 ms and collagen-bound water T2*cw of 1.84 ± 0.52 ms, with a fraction of 25.4 ± 4.2% and 74.6 ± 4.2%, respectively. The IR-UTE signal shows an excellent single-component decay behavior, with a mean T2*cw of 0.32 ± 0.02 ms, which is comparable to that derived from bi-component fitting of UTE signal decay.
Table 1.
Measurement of pore water T2* (T2*pw), collagen-bound water T2* (T2*cw) and total water effective T2* (T2*eff) using single-component and bi-component fitting of UTE images, and single-component fitting of IR-UTE images, respectively.
| T2* Measurements | T2*PW | T2*CW | PW Fraction | CW Fraction | T2*eff |
|---|---|---|---|---|---|
| UTE Single-Component Analysis | - | - | - | - | 0.41 ± 0.05 ms |
| UTE Bi-Component Analysis | 1.84 ± 0.52 ms | 0.28 ± 0.03 ms | 25.4 ± 4.2% | 74.6 ± 4.2% | - |
| IR-UTE Bi-Component Analysis | - | 0.32 ± 0.02 ms | - | - | - |
Figure 3 shows T1 measurements using saturation recovery dual echo UTE acquisitions as well as IR-UTE acquisitions with variable TR/TI combinations. Single-component fitting of the saturation recovery curve shows a T1eff of 268 ms, which is the effective T1 of collagen-bound and pore water since both water components contribute to the UTE signal with a minimal TE of 8 μs, and a T1pw of 509 ms, which is the T1 of pore water only since signal from collagen-bound water decays to near zero with a TE of 2.4 ms. Exponential fitting of the IR-UTE curve shows a T1cw of 122 ms, which is likely the T1 of collagen-bound water since signal from pore water is selectively suppressed with each of IR-UTE acquisitions with variable TR/TI combinations.
Figure 3.
T1 measurement using saturation recovery dual echo UTE acquisitions with a TE of 8 μs (A) and 2.4 ms (B), and IR-UTE acquisitions with variable TR/TI combinations (C). A T1eff of 268 ms and fitting error of ±22 ms was measured with saturation recovery UTE acquisitions with a TE of 8 μs (A). A T1pw of 509 ms and fitting error of ±22 ms were measured with saturation recovery UTE acquisitions with a TE of 2.4 ms (B). A T1CW of 122 ms and fitting error of ±12 ms were measured with IR-UTE acquisitions with variable TR/TI combinations (C).
Table 2 shows the mean and standard deviation of T1 values of 10 bovine bone samples. A mean effective T1eff of 243 ± 37 ms was demonstrated from single-component fitting of saturation recovery UTE signal recovery with a TE of 8 μs, where both collagen-bound and pore water components contributed to the UTE signal recovery. A mean pore water T1pw of 527 ± 28 ms was demonstrated from single-component fitting of saturation recovery UTE signal recovery with a TE of 2.4 ms, where only pore water contributed to the UTE signal recovery. A mean collagen-bound water T1cw of 116 ± 6 ms was demonstrated from single-component fitting of IR-UTE signal recovery with different TR/TI combinations. T1cw is significantly shorter than T1pw.
Table 2.
Measurement of pore water T1 (T1pw), collagen-bound water T1 (T1cw) and total water effective T1 (T1eff) using single-component fitting of saturation recovery dual echo UTE acquisitions with TEs of 8 μs and 2.4 ms, as well as IR-UTE acquisitions with variable TR/TI combinations and a TE of 8 μs.
| T1 Measurements | T1PW | T1CW | T1Effective |
|---|---|---|---|
| Saturation Recovery UTE (TE1 = 8 μs) | - | - | 243 ± 37 ms |
| Saturation Recovery UTE (TE2 = 2.4 ms) | 527 ± 28 ms | - | - |
| Inversion Recovery UTE, variable TR/TI | - | 116 ± 6 ms | - |
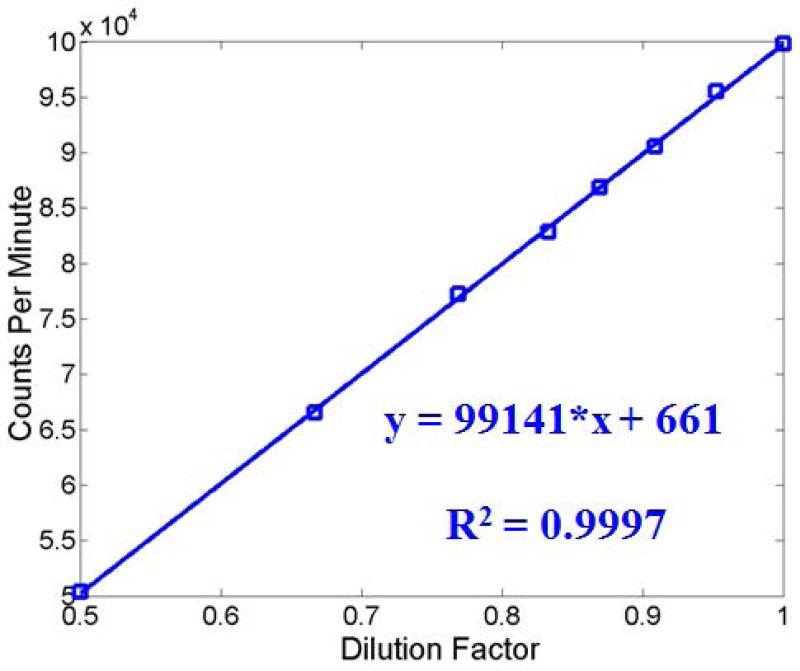
Measurement of bone water concentration using THO-H2O isotope exchange requires calibration. Figure 4 shows the calibration curves generated for water concentration measurement in the THO-H2O experiment. An excellent linear relationship (R2 = 0.9997) between CPM and THO fractions in THO-H2O solutions was observed, suggesting that dilution factor, and thus water concentration from bovine cortical bone can be accurately measured by linearly interpolating CPM of the THO-H2O mixed solution.
Figure 4.
Calibration curve used for THO dilution factor measurement was generated by measuring CPM as a function of THO fraction in THO/H2O solutions. The excellent linear behavior suggests that THO dilution factors can be accurately measured via linear interpolation.
Total, bound and pore water concentration in bovine cortical bone measured with UTE MRI, THO-H2O isotope exchange and gravimetrical analysis are shown in Table 1. UTE measured collagen-bound and pore water concentration approximates that determined by THO-H2O isotope exchange and gravimetrical analysis, suggesting that UTE sequences can reliably measure collagen-bound and pore water in cortical bone. Furthermore, THO-H2O study of oven-dried bone suggests the existence of a significant portion of bone water (~9.5% by volume), which is likely to be water tightly bound to mineral that has not been identified previously.
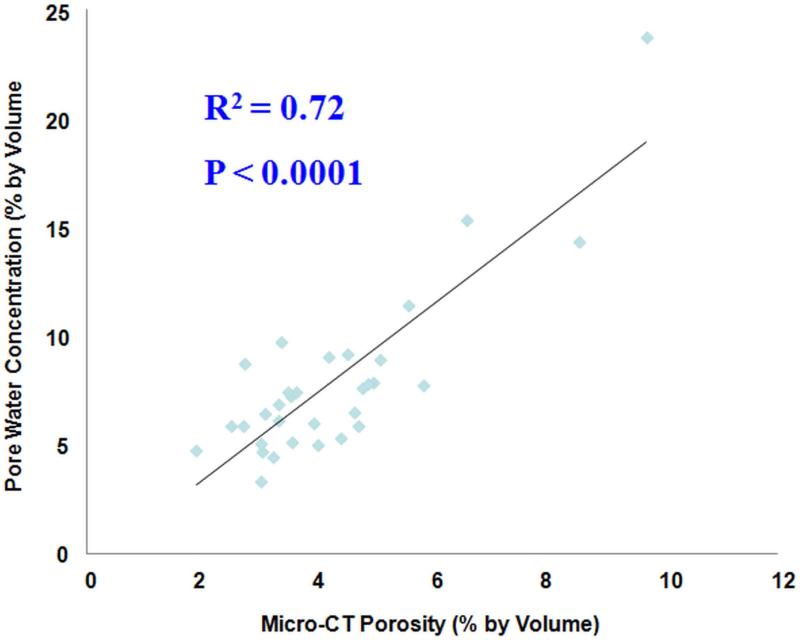
A high correlation (R2 = 0.72; P < 0.0001) was observed between μCT porosity and pore water concentration in 32 cadaveric human cortical bone samples, as shown in Figure 5. Water residing in the microscopic pores of cortical bone is expected to behave more like ‘free’ water. The high correlation between μCT porosity and ‘free’ water concentration further confirmed the accuracy of UTE techniques in assessing bound and ‘free’ water in cortical bone. μCT porosity is consistently lower that ‘free’ water content assessed by UTE MRI, likely due to “free” water in smaller pores being detected by UTE MRI but not μCT imaging.
Figure 5.
Correlation between UTE measured pore water concentration and μCT porosity in cadaveric human cortical bone samples (n=32). A high correlation (R2 = 0.72; P < 0.0001) was observed between pore water concentration and μCT porosity, suggesting that UTE sequences can reliably access water in cortical bone using a clinical MR scanner.
Discussion
UTE imaging has the potential to assess different water components in cortical bone in vivo. Our data indicates that UTE sequences detect both collagen-bound and pore water in cortical bone when using a clinical whole-body 3T scanner. The excellent bi-component fitting suggests that no more than two components are needed to explain the UTE signal decay behavior. The majority of the bone water (70-80%) exists in the form of collagen-bound water in cortical bone. The IR-UTE signal decay shows excellent single-component decay behavior with an untrashort T2* of around 0.3 ms, suggesting that only collagen-bound water is detected by the IR-UTE sequence with pore water effectively suppressed by the adiabatic IR pulse. UTE measured pore water is about 10~20% lower than that measured with THO-H2O isotope exchange, and 15~25% lower than that measured with gravimetric analysis, partly because of the loss of loosely bound water during air-drying at room temperature 26-29. Furthermore, air-drying may not fully remove all pore water, further complicating the comparisons 26. The high correlation (R2 = 0.72) between UTE measured pore water concentration and μCT cortical porosity further suggests that UTE and IR-UTE sequences can reliably access total and collagen-bound water components in cortical bone.
Previous NMR spectroscopy studies have confirmed the existence of multiple water components in cortical bone. Proton NMR spin grouping and exchange in dentin, a bone-like hard tissue, shows that 30% of the water is strongly bound to mineral with a T2* of ~ 12 μs, 52% of the water is loosely bound to the organic matrix with a T2* of ~200 μs, and 18% of the water is trapped in the dentinal tubules with a T2* of ~1000 μs 12. Considering that mineral-bound water has a too short T2* to be detected by clinical MR scanners, loosely bound water accounts for ~75% of the total UTE detectable signal. Inversion relaxation analysis of FID signal of bone samples also showed three distinct signal components with T2*s of ~11μs, ~210 μs, and ~2.7 ms, respectively 16. Multiple component analysis of FID and CPMG signals of cortical bone samples identified five signal sources, including collagen-bound water, pore water, mineral-bound water, collagen methylene, and lipid methylene 11. Mineral-bound water has an extremely short T2* of ~11.8 μs, accounting for ~8% of total water in bone. Collagen-bound water accounts for ~73% of total water, with the other ~18% for pore water. Furthermore, our measured mean T1pw of 527 ± 28 ms for bovine cortical bone samples at 3T are comparable with the recent published results by Horch, et al 22, who reported a mean T1pw of 551 ± 120 ms for human cortical bone samples at 4.7 T. Our measured mean T1cw of 116 ± 6 ms at 3 T is significantly shorter than the mean T1cw of 357 ± 10 ms at 4.7 T reported by Horch et al 22. This might be partly due to the field dependence of T1. More recently Seifert et al reported a mean T1pw of 880 ± 281 ms and a mean T1cw of 145 ± 25 ms at 3 T28. T1cw value from our study is very close to that reported by Seifert et al 28. Meanwhile, the mean T1pw values of 880 ± 281 ms at 3T and 1790 ± 470 ms at 7T from the Seifert study are significantly higher than the mean T1pw values of 527 ± 28 ms at 3T from our study and 551 ± 120 ms at 4.7T from the Horch study 22, 28. The difference might be due to multiple factors, including different field strengths (T1 is field strength dependent) and type of specimen (T1pw in human cortical bone with bigger pores is expected to be longer than in bovine cortical bone with smaller pores).
Bulk water in the pores has a long T2 (> 100 ms) but a short T2* (< 10 ms), and can be detected with conventional clinical fast spin echo (FSE) sequences with TEs of 10 ms or longer 32. FSE-determined porosity is highly correlated (R2 = 0.83) with μCT porosity 33. However, it should be noted that not all “free” water in pores has T2* long enough to be detectable by conventional FSE sequences. There is a broad distribution of T2 and T2* values in pore water 11, 30. Water loosely bound to the organic matrix has very short T2 and T2*, and remains invisible to conventional clinical MR sequences. Wu et al developed water- and fat-suppressed proton projection MRI (WASPI) for bone imaging in vitro 15. Gravimetric and amino analyses showed that the WASPI signal is highly correlated (R2 = 0.98) with collagen content 14. Water tightly bound to mineral has an extremely short T2*. Bone mineral crystals contain hydroxyl (OH−) ions which can be observed with proton magic angle spinning (MAS) NMR spectroscopy 34. More recently, using the 2D Lee-Goldberg cross-polarization under magic-angle spinning (2D LG-CPMSA) pulse sequence, Wilson et al observed a structured water layer at the surface of the mineral in cortical bone 35. In a later study, they identified three types of structurally-bound water 36, which serves to stabilize these defect-containing crystals or mediating mineral-organic matrix interactions. Tightly-bound water was also observed by Ivanova et al. using proton MAS NMR spectroscopy 37. However, water bound to mineral is largely neglected in commonly used drying techniques 26-29. The difference between oven-dried weight and ash weight is typically considered to be the weight of the organic phase 29. This simplification is expected to overestimate the organic matrix content, and underestimate the total water content in cortical bone. As our study indicated, approximately 28% of bone water (i.e., mineral-bound water) would be misclassified as organic matrix content according to the conventional gravimetric techniques.
The strength of bone is determined by its composition and structure. Recent work by Zebaze et al. suggested that accurate assessment of bone structure, especially cortical porosity, could improve identification of individuals at high risk of fracture and assist treatment 38. Age related adverse changes in the collagen network may lead to the decreased toughness of bone 39. A recent work done by Gallant et al. 40 found that collagen-bound water, rather than pore water, was highly correlated with canine bone biomechanics following raloxifene treatment, while cortical porosity, BMD and bone mineral concentration (BMC) show no significant correlation. Those results suggest the importance of simultaneous assessment of bound and pore water in cortical bone. More recently, two other UTE-derived indices, the porosity index 41 and suppression ratio 42, were introduced. However, these indices only indirectly measure bone quality without direct information on cortical porosity and collagen content. The 3D UTE and IR-UTE techniques introduced in this study have the potential to assess organic matrix via bound water measurement and cortical porosity via pore water measurement, thus allowing direct comparison of cortical porosity and collagen matrix across different groups of people (e.g., aging and disease related changes in cortical bone). UTE together with magnetization transfer (MT) imaging may provide further information on mineral-bound water, and potentially bone mineral content 43. More importantly, these techniques allow accurate volumetric mapping of different water components in cortical bone in vivo in a time-efficient way using clinical whole-body scanners. This may significantly advance the study of bone diseases including OP, osteomalacia, osteopenia, Paget disease, renal osteodystrophy and insufficiency fractures in the setting of biophosphonate therapy or raloxifene treatment 40.
There are several limitations in this study. First, the 2D and 3D UTE sequences are time consuming, limiting their clinical applications. However, more advanced UTE sequences employing a 3D Cones trajectory have shown promise in measuring bound and pore water in cortical bone in vivo 44. Second, the 2D and 3D UTE sequences are based on the use of T2* than T2 to separate bound water from pore water. Susceptibility and surface relaxation shortening of the pore water components, making it challenging to achieve robust separation of bound water and pore water especially at higher field strengths 28. Third, reference techniques including THO-H2O isotope exchange and gravimetrical method have limitations 26-29. During air-drying bound water is partly removed. Oven-drying cannot completely remove all the bound water, especially water tightly bound to bone mineral. T2 spectral analysis is a more accurate technique in separating bound and pore water 28. However, it is technically difficult or impossible to acquire multi-echo spin echo data for T2 analysis in vivo, due to the limitations in RF power and specific absorption ratio (SAR) associated with clinical MR scanners. Fourth, no comparison has been done between our 2D and 3D UTE techniques and several other short T2 imaging techniques, such as zero echo time (ZTE) imaging 45, pointwise encoding time reduction with radial acquisition (PETRA) 46, double adiabatic full passage (DAFP) 19, and sweep imaging with Fourier transformation (SWIFT) 47. Imaging techniques such as ZTE and SWIFT have potential advantages over 2D and 3D radial UTE sequences when imaging extremely short T2 species due to their shorter effective TEs. However, the contrast mechanisms associated with ZTE and SWIFT, and their applications in measuring bound and pore water T1s, T2*s and water concentrations remain to be established. Fifth, computed tomography (CT) is an established imaging technique for studying bone mineral density and content. Comparison between UTE MRI and CT techniques would be of considerable interest and will be performed in future studies.
In conclusion, we have demonstrated that T1, T2* and concentration of collagen-bound and pore water components can be measured with UTE and IR-UTE sequences. Further clinical studies will be necessary to evaluate the diagnostic power of this method.
Table 3.
Mean and standard deviation of pore water concentration (WCPore), collagen-bound water concentration (WCCollagen) and mineral-bound water concentration (WCMineral) using UTE imaging techniques, THO-H2O isotope exchange study and gravimetric analysis, respectively.
| Pore Water (WCPore) |
Collagen-Bound Water (WCCollagen) |
Mineral-Bound Water (WCMineral) |
WCPore + WCCollagen | WCPore + WCCollagen + WCMineral |
|
|---|---|---|---|---|---|
| UTE Measured WCTotal | - | - | - | 21.0 ± 2.8% | - |
| IR-UTE Measured WCCollagen | - | 17.9 ± 1.6% | - | - | - |
| Pore Water (WCTotal – WCCollagen) | 4.7 ± 0.5% | - | - | - | - |
| UTE + Bi-Component Analysis | 5.3 ± 0.4% | 15.7 ± 2.3% | - | - | - |
| THO-H2O Exchange | 5.9 ± 0.6% | 18.1 ± 2.1% | 9.5 ± 0.6% | - | 33.5 ± 0.6% |
| Gravimetric Analysis | 6.3 ± 0.8% | 19.2 ± 3.6% | - | 25.5 ± 0.6% | - |
Acknowledgement
The authors acknowledge grant support from GE Healthcare, Donald and Darlene Shiley, and NIH (1R01 AR062581-01A1 and 1R21 AR063894-01A1).
Abbreviations used
- 2D
two-dimensional
- BMC
bone mineral concentration
- BMD
bone mineral density
- CPM
counts per minute
- CW
collagen-bound water
- DXA
Dual-energy X-ray absorptiometry
- FSE
fast spin echo
- FID
free induction decay
- FOV
field of view
- IR
inversion recovery
- LG-CPMSA
Lee-Goldberg cross-polarization under magic-angle spinning
- MAS
magic angle spinning
- MRI
magnetic resonance imaging
- MT
magnetization transfer
- NEX
number of excitations
- NMR
nuclear magnetic resonance
- PFOB
perfluorooctyl bromide
- PBS
phosphate buffered saline
- PW
pore water
- RF
radio frequency
- ROI
region of interest
- SNR
signal to noise ratio
- TE
echo time
- TI
inversion time
- TSR
saturation recovery time
- μCT
micro computed tomography
- UCSD
University of California, San Diego
- UTE
ultrashort echo time
- WASP
water- and fat-suppressed proton projection MRI
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
References
- 1.American Society for Bone and Mineral Research ASBMR Bone Curriculum. 2004 http://depts.washington.edu/bonebio/ASBMRed/ASBMRed.html.
- 2.Kalpakcioglu BB, Morshed S, Engelke K, Genant HK. Advanced imaging of bone macrostructure and microstructure in bone fragility and fracture repair. J Bone Joint Surg Am. 2008;90:68–78. doi: 10.2106/JBJS.G.01506. [DOI] [PubMed] [Google Scholar]
- 3.Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–995. doi: 10.1007/s001980170006. [DOI] [PubMed] [Google Scholar]
- 4.Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183–187. doi: 10.1359/jbmr.2000.15.2.183. [DOI] [PubMed] [Google Scholar]
- 5.De Lact C, van Hout B, Burger H, Hofman A, Pols H. Bone density and the risk of hip fracture in men and women: cross sectional analysis. Br Med J. 1997;315:221–225. doi: 10.1136/bmj.315.7102.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuit SCE, Klift M, Weel AEAM, Laet CEDH de, Burger H, Seeman E, Hofman A, Uitterlinden AG, Leeuwen JPTM van, Pols HAP. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Link TM. The Founder's Lecture 2009: advances in imaging of osteoporosis and osteoarthritis. Skeletal Radiol. 2010;39:943–955. doi: 10.1007/s00256-010-0987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wehrli FW, Song HK, Saha PK, Wright AC. Quantitative MRI for the assessment of bone structure and function. NMR in Biomed. 2006;19:731–764. doi: 10.1002/nbm.1066. [DOI] [PubMed] [Google Scholar]
- 9.Seeman E, Delmas PD. Bone quality – the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 10.Cowin SC. Bone poroelasticity. J Biomechanics. 1999;32:217–238. doi: 10.1016/s0021-9290(98)00161-4. [DOI] [PubMed] [Google Scholar]
- 11.Horch RA, Nyman JS, Gochberg DF, Dortch RD, Does MD. Characterization of 1H NMR signal in human cortical bone for magnetization resonance imaging. Magn Reson Med. 2010;64:680–687. doi: 10.1002/mrm.22459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreiner LJ, Cameron IG, Funduk N, Miljkovic L, Pintar NN, Kydon DN. Proton NMR spin grouping and exchange in dentin. Biophys J. 1991;59:629–639. doi: 10.1016/S0006-3495(91)82278-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho G, Wu Y, Ackerman JL. Detection of hydroxyl ions in bone mineral by solid-state NMR spectroscopy. Science. 2003;300:1123–1127. doi: 10.1126/science.1078470. [DOI] [PubMed] [Google Scholar]
- 14.Cao H, Ackerman JL, Hrovat MI, Graham L, Glimcher MJ, Wu Y. Quantitative bone matrix density measurement by water- and fat-suppressed proton projection MRI (WASPI) with polymer calibration phantoms. Magn Reson Med. 2008;60:1433–1443. doi: 10.1002/mrm.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Chesler DA, Glimcher MJ, Garrido L, Wang J, Jiang HJ, Ackerman JA. Multinuclear solid-state three-dimensional MRI of bone and synthetic calcium phosphates. Proc Natl Acad Sci. 1999;96:1574–1578. doi: 10.1073/pnas.96.4.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni Q, Nyman JS, Wang X, Santos ADL, Nicolella DP. Assessment of water distribution changes in human corticalbone by nuclear magnetic resonance. Meas Sci Technol. 2007;18:715–723. [Google Scholar]
- 17.Robson MD, Gatehouse PD, Bydder M, Bydder GM. Magnetic resonance: an introduction to Ultrashort TE (UTE) imaging. J Comput Assist Tomogr. 2003;27:825–846. doi: 10.1097/00004728-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Du J, Carl M, Bydder M, Takahashi A, Chung CB, Bydder GM. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. J Magn Reson. 2010;207:304–311. doi: 10.1016/j.jmr.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Manhard MK, Horch RA, Gochberg DF, Nyman JS, Does MD. In vivo quantitative MR imaging of bound and pore water in cortical bone. Radiology. 2015 May;25:140336. doi: 10.1148/radiol.2015154032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horch RA, Gochberg DF, Nyman JS, Does MD. Non-invasive Predictors of Human Cortical Bone Mechanical Properties: T-2-Discriminated H-1 NMR Compared with High Resolution X-ray. PLoS ONE. 2011;6(1):e16359. doi: 10.1371/journal.pone.0016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du J, Bydder M, Takahashi AM, Carl M, Chung CB, Bydder GM. Short T2 contrast with three-dimensional ultrashort echo time imaging. Magn Reson Imaging. 2011;29:470–82. doi: 10.1016/j.mri.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horch R, Gochberg D, Nyman J, Does M. Clinically-compatible MRI strategies for discriminating bound and pore water in cortical bone. Magn Reson Med. 2012;68:1774–1784. doi: 10.1002/mrm.24186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz E, Chung CB, Bae WC, Statum S, Znamirowski R, Bydder GM, Du J. Ultrashort echo time spectroscopic imaging (UTESI): an efficient method for quantifying bound and free water. NMR in Biomed. 2012;25:161–8. doi: 10.1002/nbm.1728. [DOI] [PubMed] [Google Scholar]
- 24.Du J, Sheth V, He Q, Carl M, Chen J, Corey-Bloom J, Bydder GM. Measurement of T1 of the ultrashort T2* components in white matter of the brain at 3T. PLOS One. 2014;9(8):e103296. doi: 10.1371/journal.pone.0103296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Techawiboonwong A, Song HK, Leonard MB, Wehrli FW. Cortical bone water: in vivo quantification with ultrashort echo-time MR imaging. Radiology. 2008;248:824–833. doi: 10.1148/radiol.2482071995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyman JS, Roy A, Shen X, Rae LA, Tyler JH, Wang X. The influence of water removal on the strength and toughness of cortical bone. J Biochem. 2006;39:931–938. doi: 10.1016/j.jbiomech.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas R, Bae CW, Diaz E, Masuda K, Chung CB, Bydder GM, Du J. Ultrashort echo time (UTE) imaging with bi-component analysis: bound and free water evaluation of bovine cortical bone subject to sequential drying. Bone. 2012;50:749–755. doi: 10.1016/j.bone.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seifert AC, Wehrli SL, Wehrli FW. Bi-component T2* analysis of bound and pore bone water fractions fails at high field strengths. NMR Biomed. 2015;28:861–872. doi: 10.1002/nbm.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeni YN, Brown CU, Norman TL. Influence of bone composition and apparent density on fracture toughness of the human femur and tibia. Bone. 1998;22:79–84. doi: 10.1016/s8756-3282(97)00227-5. [DOI] [PubMed] [Google Scholar]
- 30.Nyman JS, Ni Q, Nicolella DP, Wang X. Measurements of mobile and bound water by nuclear magnetic resonance correlate with mechanical properties of bone. Bone. 2008;42:193–199. doi: 10.1016/j.bone.2007.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mankin HJ, Thrasher AZ. Water content and binding in normal and osteoarthritic human cartilage. J Bone Joint Surg. 1975;57:76–80. [PubMed] [Google Scholar]
- 32.Du J, Hermida JC, Diaz E, Corbeil J, Znamirowski R, D'Lima DD, Bydder GM. Assessment of cortical bone with clinical and ultrashort echo time sequences. Magn Reson Med. 2013;70:697–704. doi: 10.1002/mrm.24497. [DOI] [PubMed] [Google Scholar]
- 33.Bae WC, Patil S, Biswas R, Li S, Chang EY, Statum S, Dlima DD, Chung CB, Du J. Magnetic resonance imaging assessed cortical porosity is highly correlated with μCT porosity. Bone. 2014;66:56–61. doi: 10.1016/j.bone.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yesinowski JP, Eckert H. Hydrogen environments in calcium phosphates: proton MAS NMR at high spinning speeds. J Am Chem Soc. 1987;109:6274–6282. [Google Scholar]
- 35.Wilson EE, Awonusi A, Morris MD, Kohn DH, Tecklenburg M, Beck LW. Highly ordered interstitial water observed in bone by nuclear magnetic resonance. J Bone Miner Res. 2005;20:625–634. doi: 10.1359/JBMR.041217. [DOI] [PubMed] [Google Scholar]
- 36.Wilson EE, Awonusi A, Morris MD, Kohn DH, Tecklenburg M, Beck LW. Three structural roles for water in bone observed by solid-state NMR. Biophys J. 2006;90:3722–3731. doi: 10.1529/biophysj.105.070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivanova TI, Frank-Kamenetskaya OV, Kol'tsov AB, Ugolkov VL. Crystal structure of calcium-deficient carbonate hydroxyapatite. Thermal decomposition. J Solid State Chem. 2001;160:340–349. [Google Scholar]
- 38.Zabaze RMD, Ghasem-Zadeh A, Bohte A, Luliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E. Intracortical remodeling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Li SX, Mauli Agrawal C. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 40.Gallant MA, Brown DM, Hammond M, Wallace JM, Du J, Deymier-Black AC, Almer JD, Stock SR, Allen MR, Burr DB. Bone cell-independent benefits of raloxifene on the skeleton: a novel mechanism for improving bone material properties. Bone. 2014;61:191–200. doi: 10.1016/j.bone.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajapakse CS, Bashoor-Zadeh M, Li C, Sun W, Wright AC, Wehrli FW. Volumetric cortical bone porosity assessment with MR imaging: validation and clinical feasibility. Radiology. 2015 May;19:141850. doi: 10.1148/radiol.15141850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li C, Seifert AC, Rad HS, Bhagat Y, Rajapakse CS, Sun W, Benny Lam SC, Wehrli FW. Cortical Bone Water Concentration: Dependence of MR Imaging Measures on Age and Pore Volume Fraction. Radiology. 2014;272:796–806. doi: 10.1148/radiol.14132585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Springer F, Martirosian P, Machann J, Schwenzer NF, Claussen CD, Schick F. Magnetization transfer contrast imaging in bovine and human cortical bone applying an ultrashort echo time sequence at 3 Tesla. Magn Reson Med. 2009;61:1040–8. doi: 10.1002/mrm.21866. [DOI] [PubMed] [Google Scholar]
- 44.Carl M, Bydder GM, Du J. UTE imaging with simultaneous water and fat signal suppression using a time-efficient multi-spoke inversion recovery pulse sequence. Magn Reson Med. 2015 doi: 10.1002/mrm.25823. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiger M, Pruessmann KP, Hennel F. MRI with zero echo time: hard versus sweep pulse excitation. Magn Reson Med. 2011;66:379–389. doi: 10.1002/mrm.22799. [DOI] [PubMed] [Google Scholar]
- 46.Grodzki DM, Jakob PM, Heismann B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn Reson Med. 2012;67:510–518. doi: 10.1002/mrm.23017. [DOI] [PubMed] [Google Scholar]
- 47.Idiyatullin D, Corum C, Park JY, Garwood M. Fast and quiet MRI using a swept radiofrequency. J Magn Reson. 2006;181:342–349. doi: 10.1016/j.jmr.2006.05.014. [DOI] [PubMed] [Google Scholar]