Abstract
A mesonephric adenocarcinoma of the cervix is a very rare tumor deriving from remnants of the mesonephric duct. Differential diagnosis from other cervical carcinomas is difficult and little is known regarding its biological behavior, prognosis, and the optimal management strategy. We present a case of a mesonephric adenocarcinoma of the cervix with a comprehensive review of the existing literature. In this case a 66-year-old woman presented with postmenopausal vaginal bleeding. She was diagnosed with a FIGO stage IIB mesonephric adenocarcinoma of the cervix and treated with neoadjuvant chemoradiotherapy and a Wertheim hysterectomy. The recovery from surgery was uneventful and the patient remains with no evidence of disease with 2 years of follow-up.
Keywords: Cervical cancer, Mesonephric carcinoma, Mesonephric adenocarcinoma, Adenocarcinoma cervix
Highlights
-
•
A mesonephric adenocarcinoma is a rare tumor deriving from remnants of the Wolffian duct.
-
•
Challenging diagnosis since this tumor can mimic more common adenocarcinomas.
-
•
Immunohistochemistry can be helpful in questionable cases.
-
•
Rational to base treatment on current guidelines for adenocarcinoma until more data.
-
•
It seems mesonephric carcinomas carry a worse prognosis.
1. Case
1.1. Clinical presentation
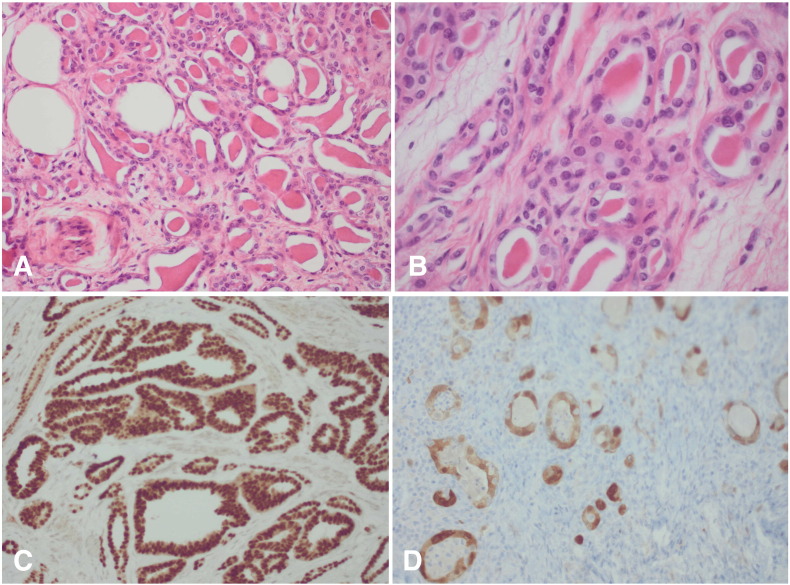
A 66-year-old woman with no medical history presented with postmenopausal vaginal bleeding. In speculo a small punctiform orifice on the right side of the cervical ostium was observed. Transvaginal ultrasound revealed a mass of 2 by 2 cm in de right lateral cervical wall, palpable as a hard nodule without extension to the pelvic wall. Taking into consideration there was a mass in a suspicious-looking cervix an immediate conization was performed. The patient had a normal PAP smear 2 years before. The pathological examination of the conus revealed the presence of an invasive mesonephric adenocarcinoma of the cervix, characterized by infiltrating tubular structures containing eosinophilic, hyaline secretions in their lumens (Fig. 1A). The tubular structures were lined by cuboidal epithelium exhibiting mild to moderate nuclear atypia. Immunohistochemical stainings for PAX8, p16 and CD10 were positive (Fig. 1C).
Fig. 1.
A, The lesion shows infiltrating tubular structures containing eosinophilic, hyaline secretions (original magnification, × 200). B, The tubular structures are lined by cuboidal tumor cells demonstrating mild to moderate nuclear atypia (original magnification, × 400). C, The tumor cells show strong nuclear expression of the transcription factor PAX8 (immunohistochemistry for PAX8, original magnification × 200). D, There is nuclear and cytoplasmic expression of the tumor suppressor p16 (immunohistochemistry for p16, original magnification × 200).
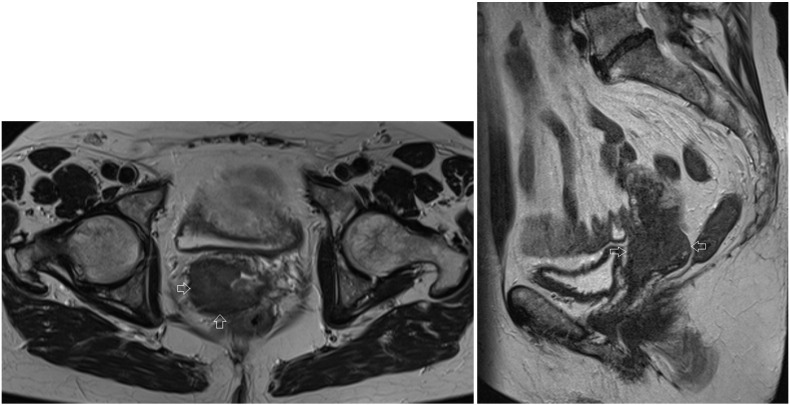
There was extension of tumor cells in all resection margins. Magnetic Resonance Imaging (MRI) performed after the conization showed a mass in the right side of the cervix, measuring 38 × 35 × 38 mm, with extension to the uterus, the right parametrium and the upper part of the vagina (Fig. 2). These clinical findings corresponded with an International Federation of Gynecology and Obstetrics (FIGO) stage IIB.
Fig. 2.
Axial and sagittal T2 MRI image of the patient before treatment. Arrows indicate the cervical tumor on the right side of the cervix, measuring 38 × 35 × 38 mm, with extension to the uterus, the right parametrium and the upper part of the vagina.
A Positron Emission Tomography (PET) scan showed no evidence of pathological lymphadenopathies or distant metastases. The CA-125 blood serum level was normal.
1.2. Treatment
Given the presence of a bulky tumor with parametrial invasion, the patient underwent neoadjuvant chemoradiotherapy: 50 Gy (Gray) of Intensity Modulated Radiation Therapy (IMRT) in 25 fractions of 2 Gy daily and concomitant chemotherapy (Cisplatin) once a week. There was a limited reduction in size of the cervical mass to a volume of 37 × 23 × 30 mm. After the neoadjuvant therapy the patient underwent a type 2 Wertheim hysterectomy without pelvic lymphadenectomy.
Histopathologic examination confirmed the presence of a mesonephric adenocarcinoma, predominantly on the right side, however, with almost complete circumferential extension. The lesion measured 3 cm in greatest dimension. There was extension to the isthmus and the paracervical fat tissue. The resection margins were free of tumor.
The lesion stained for cytokeratin 7, EMA and vimentin. Stainings for calretinin, carcinoembryonic antigen (CEA), estrogen and progesterone receptor (ER/PR) were negative.
The final diagnosis was a mesonephric adenocarcinoma of the cervix, FIGO stage IIB. Tumor cells expressed p16, but chromogenic in situ hybridization did not demonstrate low- or high-risk Human Papillomavirus (HPV).
1.3. Outcome and follow up
The patient did not receive adjuvant therapy and she remains with no evidence of disease with 2 years of follow-up, with control visits every 3 months and MRI- and PET-scans every 12 months.
2. Review of the literature
2.1. Definition
In order to understand the origin of this tumor we recapitulate the embryology in the supplementary material.
2.2. Incidence
To the best of our knowledge there are only 40 cases of this tumor reported in the literature to date, including present case (Table 2). The incidence of this neoplasm is uncertain since it is often confused with more common adenocarcinomas or mistaken for benign florid mesonephric hyperplasia (Hart, 2002 Oct, Ferry and Scully, 1990, Kenny et al., 2012).
Table 2.
summary of the 40 cases of mesonephric carcinoma of the cervix reported in the literature, including present case.
| Author | Year | Case | Age | Symptoms | Tumor type | Stage | Treatment | Clinical course | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| McGee | 1962 | 1 | 36 | PC VBV | AC | IB | HRT | Pelvic R (6 yr): NFT | DOD 7 yr |
| Buntine | 1979 | 2 | 48 | ‘fibroids’ | AC | / | HRT + BSO | Vaginal R (7 yr): NFT | DOD 9 yr |
| Valente & Susin | 1987 | 3 | 58 | PMP VBV | AC | IB1 | HRT + BSO + LA | Pelvis, sacral R (2 yr): RT | DOD 2.8 yr |
| Lang | 1990 | 4 | 46 | Cervical polyp | AC | / | HRT | RT | NED 10mo |
| 5 | 55 | / | AC | IB | HRT | / | / | ||
| Ferry & Scully | 1990 | 6 | 55 | Pelvic relaxation | AC | / | HRT + BSO | RT | NED 5 yr |
| Stewart | 1993 | 7 | 37 | Constipation, menorrhagia | AC | I | HRT + BSO + LA | NFT | NED 10 yr |
| Clement | 1995 | 8 | 46 | Menorrhagia, “fibroid uterus” | AC | IB | HRT + BSO + LA | NFT | NED 2 yr |
| 9 | 34 | Menometrorrhagia | AC | IB | HRT + BSO + LA | Abd R (1 yr): CT | NED 2 yr | ||
| 10 | 71 | AC on PAP smear, pelvic mass | AC | IB | HRT + BSO + LA | RT, abd R (4 mo): CT | DOD 8,5mo | ||
| 11 | 67 | PMP VB | AC | IB | HRT + BSO + LA | / | / | ||
| 12 | 37 | PC VB | MMMT | IB | HRT + BSO + LA | Adj CT, abd R (9 & 11 yr): CT | AWD 13 yr | ||
| 13 | 73 | PMP VB | MMMT | IB | HRT + BSO | RT | NED 3 yr | ||
| 14 | 40 | Menorrhagia, cervical polyp | MMMT | IB | HRT + BSO | RT | NED 2.3 yr | ||
| Silver | 2001 | 15 | 62 | PMP VB | AC | IB | HRT + USO | NFT | NED 1.5 yr |
| 16 | 47 | Uterine prolapse | AC | IB | HRT + BSO | NFT | NED 2.1 yr | ||
| 17 | 40 | SIL on PAP smear | AC | IB | HRT + BSO | NFT | NED 3.2 yr | ||
| 18 | 54 | PMP VB | AC | IB | HRT + BSO + LA | NFT | NED 6.1 yr | ||
| 19 | 67 | PMP VB | AC | IB | HRT + BSO | NFT | NED 8.3 yr | ||
| 20 | 72 | AGC on PAP smear | AC | IB | HRT + BSO + LA | RT, rectovaginal R (1.7 yr): CT | NED 2.5 yr | ||
| 21 | 39 | Menorrhagia, cervical “fibroid” | MMMT | IB | HRT + BSO | Mediastinal metastasis (5.6 yr): CT | DOD 6.2 yr | ||
| 22 | 62 | PMP VB | AC | IB | HRT + BSO + LA | NFT | / | ||
| 23 | 56 | Right ovarian cystadenoma | AC | / | HRT + BSO | NFT | NED 7.4 yr | ||
| 24 | 35 | VB, pelvic pain | AC | IIB | RT | Pelvic R (2.2 yr):CT | DOD 3.2 yr | ||
| 25 | 43 | Metrorrhagia, anemia | MMMT | IVB | HRT + BSO + LA + O | CT, bladder & pelvic R (8 mo): RT | DOD 10 mo | ||
| Angeles | 2004 | 26 | 47 | Menometrorrhagia, pelvic pain | AC | IIA | HRT + BSO | RT | NED 1 yr |
| Bagué | 2004 | 27 | 41 | Abnormal VB | AC | IB | HRT + BSO + LA + A | RT | NED 11.4 yr |
| 28 | 24 | / | AC | IIA | / | / | / | ||
| 29 | 45 | PC VB | AC | IB1 | HRT + USO | NFT | NED 3.1 yr | ||
| 30 | 54 | PMP VB | MMMT | IIA | HRT + BSO + LA + O | NFT | DOD 7 mo | ||
| 31 | 62 | / | MMMT | IVB | HRT + BSO | CT & RT, bone R | AWD 3.3 yr | ||
| 32 | 54 | / | MMMT | IB1 | HRT + BSO + LA + O | NFT | NED 1.1 yr | ||
| Yap | 2006 | 33 | 54 | PMP VB | AC | IB1 | HRT + BSO + LA | RT | NED 3.1 yr |
| Fukunaga | 2008 | 34 | 46 | Abnormal VB | AC | IB | HRT + BSO + LA + O | NFT | NED 4 mo |
| Anagnostopoulos | 2012 | 35 | 64 | Suspicious looking cervix | AC | IB1 | HRT + BSO + LA | NFT | NED 6 mo |
| Nomoto | 2012 | 36 | 64 | PMP VB | AC | IB1 | HRT + BSO + LA | Multifocal lung metastases (1 yr): CT | / |
| 37 | 64 | PMP VB | AC | IB1 | HRT + BSO + LA | NFT | / | ||
| Meguro | 2013 | 38 | 63 | PMP VB | MMMT | IIA | HRT + BSO + LA | Local R (7 mo): CRT | NED 7 mo |
| Menon | 2013 | 39 | 65 | PMP VB | AC | IB1 | HRT + BSO + LA | RT | NED 6 mo |
| Present case | 2013 | 40 | 66 | PMP VB | AC | IIB | CRT + HRT + BSO | NFT | NED 2 yr |
PMP VB: postmenopausal vaginal bleeding. PC VB: postcoital vaginal bleeding. HRT: hysterectomy. BSO: bilateral salpingo-oophorectomy. USO: unilateral salpingo-oophorectomy LA: lymphadenectomy. O: omentectomy. A: appendectomy. AC: adenocarcinoma. MMMT: malignant mixed mesonephric tumor. CT: chemotherapy. RT: radiotherapy. CRT: chemo- and radiotherapy. NFT: no further treatment. R: recurrence. Yr: year. Mo: month. NED: no evidence of disease. AWD: alive with disease. DOD: dead of disease.
2.3. Diagnosis
The mean age at the time of diagnosis was 52 years in this literature review. Unlike the more common squamous epithelial carcinoma, this type of cervical cancer is rarely discovered by PAP smear (Anagnostopoulos et al., 2012). Most patients present with abnormal vaginal bleeding, often with a visible cervical lesion (Hart, 2002). The diagnosis is usually made on biopsy specimens, endometrial curettings or hysterectomy specimens. A common finding on endometrial biopsy is a coexisting endometroid adenocarcinoma (Anagnostopoulos et al., 2012).
Most tumors exhibit a widely infiltrative and confluent pattern of growth and extension into the lower uterine segment is common (Nomoto et al., 2013). Because of the widespread distribution within the cervix at the time of diagnosis the initial site of origin in the lateral part of the cervix is often no longer apparent (Kenny et al., 2012), as was in this case.
2.4. Pathology
One of the most characteristic features of a mesonephric adenocarcinoma is that it exhibits a mixture of morphologic patterns. Therefore they are often confused with serous, clear cell or endometroid adenocarcinomas (Anagnostopoulos et al., 2012, Nomoto et al., 2013).
In this literature review 23% of the reported mesonephric carcinomas were associated with a spindled cell component (malignant mixed mesonephric tumor, MMMT). This is a biphasic variant of a mesonephric carcinoma with sarcomatoid features (Clement et al., 1995, Yap et al., 2006).
The typical background lesion of a mesonephric carcinoma is florid mesonephric hyperplasia, characterized by a densely eosinophilic luminal secretion (Menon et al., 2013). In contrast to mesonephric hyperplasia, a mesonephric carcinoma does not have a lobular architecture and the nuclei appear cytological malignant. The Ki-67 proliferation index is less than 1% in mesonephric hyperplasia compared to 15–20% in mesonephric carcinoma (Silver et al., 2001).
2.5. Immunohistochemistry
Given its potential mimicry of other neoplasms, immunohistochemistry can be helpful in the differential diagnosis (Table 1). Positive immunostaining for CD10, CK7 and calretinin along with a negative immunostaining for CEA is suggestive for a mesonephric origin (Silver et al., 2001). Mesonephric adenocarcinoma is also usually positive for epithelial membrane antigen (EMA) (Silver et al., 2001) and vimentin (Clement et al., 1995, Silver et al., 2001, Lang and Dallenbach-Hellweg, 1990) whereas ER/PR are usually absent (Silver et al., 2001). Mesonephric adenocarcinoma is one of the few subtypes of cervical cancer that is not related to HPV (Kenny et al., 2012).
Table 1.
Immunohistochemical staining profile of mesonephric, endocervical and endometroid adenocarcinoma of the cervix and the present case.
| Present case | Mesonephric adenocarcinoma | Endocervical adenocarcinoma | Endometroid adenocarcinoma | |
|---|---|---|---|---|
| PAX8 | + | + | + | + |
| Calretinin | − | + | − | − |
| CD10 | + | + | − | − |
| CK7 | + | + | + | + |
| Vimentin | + | + | − | + |
| EMA | + | + | + | + |
| CEA | − | − | + | − |
| ER/PR | − | − | − | + |
| HPV | − | − | + | − |
PAX8 staining is usually positive in mesonephric carcinomas (Kenny et al., 2012, Fregnani et al., 2008). CA125 is also usually positive in mesonephric carcinoma but negative in benign mesonephric structures (Kenny et al., 2012).
2.6. Treatment
In the 39 cases described in the literature, treatment depended on the stage of the disease and consisted of hysterectomy (HRT) with or without bilateral salpingo-oophorectomy (BSO), pelvic lymphadenectomy (LA) and (neo-) adjuvant chemo- or radiotherapy.
In the present literature review the vast majority of patients (70%) were diagnosed at a stage I. All patients with stage I carcinoma, except for two, were treated with HRT and BSO, and LA was performed in eighteen patients (64%). No adjuvant treatment was given in patients with stage I disease except for radiotherapy in five patients with adenocarcinoma (AC) and chemotherapy in one patient with a mesonephric adenocarcinoma with spindled cell component (MMMT). There seems to be no difference in disease recurrence and mean recurrence interval between the patients with stage I mesonephric AC of the cervix treated merely surgically and those who received adjuvant therapy afterwards (Table 2). However, the biological behavior of this unusual tumor remains unclear and until there are sufficient data to recommend a particular course of therapy it seems reasonable to manage patients with mesonephric adenocarcinoma of the cervix according to current guidelines for cervical adenocarcinoma of similar stage.
2.7. Prognosis
Owing to the small number of cases with adequate follow-up, prognosis cannot be accurately predicted but it seems that mesonephric carcinomas carry a worse prognosis. Patients with a stage I mesonephric AC had a recurrence rate of 32% and a mean recurrence interval of 24 months (Table 2), compared to a reported recurrence rate of 11% for squamous cell carcinomas and 16% for adenocarcinomas in early stage cervical cancer (Fregnani et al., 2008). However, these results should be interpreted with caution given the small number of patients.
Four out of 24 patients diagnosed at stage I with adequate follow up available died of the disease, with a mean survival of 50 months. All of the patients diagnosed at stage IV had a spindled cell component.
Only five of the nine reported cases of MMMT were diagnosed at stage I, two were stage II and two were stage IV. It seems that mesonephric carcinomas with a spindled cell component are diagnosed at a more advanced stage, implying a worse prognosis.
3. Conclusion
A mesonephric adenocarcinoma of the cervix arises from remnants of the mesonephric (Wolffian) duct in the lateral wall of the cervix. It is a rare type of cervical cancer, to the best of our knowledge only 40 cases have been reported in literature.
The most common symptom is postmenopausal bleeding. Because of its localization, this type of cervical cancer is rarely discovered on PAP smear. Diagnosis is made for the most part on biopsy or hysterectomy specimens. Because of its widely infiltrative growth potential a barrel-shaped cervix can occur. If a barrel-shaped cervix is palpated during pelvic examination, an adenocarcinoma of the cervix should be considered.
It is a pathologically challenging diagnosis since a mesonephric adenocarcinoma typically exhibits a mixture of morphologic patterns. Therefore they are often confused with more common adenocarcinomas. The incidence of mesonephric carcinoma is probably underestimated because of this frequent misclassification. The possibility of a mesonephric carcinoma of the cervix should be considered when encountering a histological unusual-appearing carcinoma or MMMT. In this case a targeted search for associated mesonephric hyperplasia is required, characterized by small glands or tubules with eosinophilic luminal secretions. In questionable cases, immunohistochemical profiling can be helpful.
Prognosis cannot be accurately predicted owing to the small number of cases with sufficient follow up but it seems that mesonephric carcinomas carry a worse prognosis. In this review the majority of patients (70%) were diagnosed at stage IB with a mean age of 52 years.
Treatment consisted of hysterectomy, bilateral salpingo-oophorectomy and pelvic lymphadenectomy in the majority of these patients. The recurrence rate in these patients was 32% and the mean recurrence interval 24 months.
Conflicts of interest
None.
Acknowledgments
I would like to thank Kathleen Lambein and Jo Van Dorpe for reviewing the pathology content and providing the pathology slides. I would like to thank Julie Vercauteren for providing the illustrations.
Footnotes
The authors declare that there are no conflicts of interest. No financial support.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.gore.2016.05.002.
Appendix A. Supplementary data
Supplementary material.
References
- Hart W.R. Symposium part II: special types of adenocarcinoma of the uterine cervix. Int. J. Gynecol. Pathol. 2002 Oct;21(4):327–346. doi: 10.1097/00004347-200210000-00003. [DOI] [PubMed] [Google Scholar]
- Ferry J.A., Scully R.E. Mesonephric remnants, hyperplasia, and neoplasia in the uterine cervix. A study of 49 cases. Am. J. Surg. Pathol. 1990;14(12) doi: 10.1097/00000478-199012000-00002. [DOI] [PubMed] [Google Scholar]
- Kenny S.L., McBride H.A., Jamison J., McCluggage W.G. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-β. Am. J. Surg. Pathol. 2012;36(6):799–807. doi: 10.1097/PAS.0b013e31824a72c6. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos A., Ruthven S., Kingston R. Mesonephric adenocarcinoma of the uterine cervix and literature review. BMJ Case Rep. 2012 doi: 10.1136/bcr.01.2012.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto K., Hayashi S., Tsuneyama K., Hori T., Ishizawa S. Cytopathology of cervical mesonephric adenocarcinoma: a report of two cases. Cytopathology. 2013;24(2):129–131. doi: 10.1111/j.1365-2303.2012.00959.x. [DOI] [PubMed] [Google Scholar]
- Clement P.B., Young R.H., Keh P., Ostör A.G., Scully R.E. Malignant mesonephric neoplasms of the uterine cervix. A report of eight cases, including four with a malignant spindle cell component. Am. J. Surg. Pathol. 1995;19(10):1158–1171. doi: 10.1097/00000478-199510000-00006. [DOI] [PubMed] [Google Scholar]
- Yap O.W., Hendrickson M.R., Teng N.N., Kapp D.S. Mesonephric adenocarcinoma of the cervix: a case report and review of the literature. Gynecol. Oncol. 2006;103(3):1155–1158. doi: 10.1016/j.ygyno.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Menon S., Kathuria K., Deodhar K., Kerkar R. Mesonephric adenocarcinoma (endometrioid type) of endocervix with diffuse mesonephric hyperplasia involving cervical wall and myometrium: an unusual case report. Indian J. Pathol. Microbiol. 2013;56(1):51–53. doi: 10.4103/0377-4929.116150. [DOI] [PubMed] [Google Scholar]
- Silver S.A., Devouassoux-Shisheboran M., Mezzetti T.P., Tavassoli F.A. Mesonephric adenocarcinomas of the uterine cervix. A study of 11 cases with immunohistochemical findings. Am. J. Surg. Pathol. 2001;25:379–387. doi: 10.1097/00000478-200103000-00013. [DOI] [PubMed] [Google Scholar]
- Lang G., Dallenbach-Hellweg G. The histogenetic origin of cervical mesonephric hyperplasia and mesonephric adenocarcinoma of the uterine cervix studied with immunohistochemical methods. Int. J. Gynecol. Pathol. 1990;9:145–147. doi: 10.1097/00004347-199004000-00006. [DOI] [PubMed] [Google Scholar]
- Fregnani J.H., Soares F.A., Novik P.R., Lopes A., Latorre M.R. Comparison of biological behavior between early-stage adenocarcinoma and squamous cell carcinoma of the uterine cervix. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008;136(2):215–223. doi: 10.1016/j.ejogrb.2006.10.021. [DOI] [PubMed] [Google Scholar]
- McGee C.T., Cromer D.W., Greene R.R. Mesonephric carcinoma of the cervix-differentiation from endocervical adenocarcinoma. Am. J. Obstet. Gynecol. 1962;84:358–366. [Google Scholar]
- Buntine D.W. Adenocarcinoma of the uterine cervix of probable Wolffian origin. Pathology. 1979;11:713–718. doi: 10.3109/00313027909059053. [DOI] [PubMed] [Google Scholar]
- Valente P.T., Susin M. Cervical adenocarcinoma arising in florid mesonephric hyperplasia: report of a case with immunocytochemical studies. Gynecol. Oncol. 1987;27:58–68. doi: 10.1016/0090-8258(87)90230-7. [DOI] [PubMed] [Google Scholar]
- Stewart C.J.R., Taggart C.R., Brett F., Mutch A.F. Mesonephric adenocarcinoma of the uterine cervix with focal endocrine cell differentiation. Int. J. Gynecol. Pathol. 1993;12:264–269. doi: 10.1097/00004347-199307000-00011. [DOI] [PubMed] [Google Scholar]
- Angeles R., August C., Weisenberg E. Pathologic quiz case. An incidentally detected mass of the uterine cervix. Arch. Pathol. Lab. Med. 2004;128(10):1179–1180. doi: 10.5858/2004-128-1179-PQCAID. [DOI] [PubMed] [Google Scholar]
- Bague S., Rodriguez I.M., Prat J. Malignant mesonephric tumors of the female genital tract. A clinicopathologic study of 9 cases. Am. J. Surg. Pathol. 2004;28:601–607. doi: 10.1097/00000478-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Fukunaga M., Takahashi H., Yasuda M. Mesonephric adenocarcinoma of the uterine cervix: a case report with immunohistochemical and ultrastructural studies. Pathol. Res. Pract. 2008;204(9):671–676. doi: 10.1016/j.prp.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Meguro S., Yasuda M., Shimizu M., Kurosaki A., Fujiwara K. Mesonephric adenocarcinoma with a sarcomatous component, a notable subtype of cervical carcinosarcoma: a case report and review of the literature. Diagn. Pathol. 2013;8:74. doi: 10.1186/1746-1596-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.