Abstract
Current evidence suggests that older adults have reduced suppression of, and greater implicit memory for, distracting stimuli, due to age-related declines in frontal-based control mechanisms. In this study, we used fMRI to examine age differences in the neural underpinnings of attentional control and their relationship to differences in distractibility and subsequent memory for distraction. Older and younger adults were shown a rapid stream of words or nonwords superimposed on objects and performed a 1-back task on either the letters or the objects, while ignoring the other modality. Older adults were more distracted than younger adults by the overlapping words during the 1-back task, and they subsequently showed more priming for these words on an implicit memory task. A multivariate analysis of the imaging data revealed a set of regions, including the rostral PFC and inferior parietal cortex, that younger adults activated to a greater extent than older adults during the ignore-words condition, and activity in this set of regions was negatively correlated with priming for the distracting words. Functional connectivity analyses using right and left rostral PFC seeds revealed a network of putative control regions, including bilateral parietal cortex, connected to the frontal seeds at rest. Older adults showed reduced functional connectivity within this frontoparietal network, suggesting that their greater distractibility may be due to decreased activity and coherence within a cognitive control network that normally acts to reduce interference from distraction.
Keywords: aging, attention, cognitive control, distraction, implicit memory, frontoparietal network, functional connectivity
A long line of behavioral work suggests that older adults are less able to ignore distracting information than younger adults (e.g., Rabbit, 1965; Hasher & Zacks, 1988). Mirroring this effect, several neuroimaging and electrophysiological studies have shown an age-related increase in activity associated with the processing of irrelevant distraction (Chao & Knight, 1997; Alain & Woods, 1999; Gazzaley, Cooney, Rissman, & D’Esposito, 2005; Fabiani, Low, Wee, Sable, & Gratton, 2006; Stevens et al., 2008). While older adults’ greater distractibility usually interferes with their ability to attend to and subsequently remember target information (e.g., Lustig, Hasher, & Tonev, 2006; Hamm & Hasher, 1992), recent work has begun testing memory for the distracting information itself and has shown that older adults actually encode and remember more task-irrelevant information than younger adults (Rowe, Valderrama, Hasher, & Lenartowicz, 2006; Kim, Hasher, & Zacks, 2007; Campbell, Hasher, & Thomas, 2010; Schmitz, Cheng, & De Rosa, 2010; for a review, see Healey, Campbell, & Hasher, 2008). For instance, Rowe et al. (2006) asked younger and older adults to perform a 1-back task on pictures that were superimposed with irrelevant words they were told to ignore. After a 10-minute filled interval, participants were given a word-fragment completion task that included some fragments that could be solved with previously distracting words. Older adults showed more priming for the distraction and, as a result, solved more fragments overall than younger adults, suggesting that one advantage of impaired attentional control is better memory for previously irrelevant information.
Greater processing of distraction on the part of older adults is thought to be due to age differences in attentional control (Hasher, Zacks, & May, 1999), which normally allows for the selection of goal-relevant information and the suppression of goal-irrelevant information (Desimone & Duncan, 1995; Vogel, McCollough, Machizawa, 2005; Lustig, Hasher, & Zacks, 2007; Gazzaley, 2011). In younger adults, attentional control is associated with a widespread network of frontal and parietal regions (e.g., Kastner & Ungerleider, 2000; Corbetta & Shulman, 2002; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008; Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010). Older adults often show greater activity relative to younger adults within this frontoparietal network during task performance and, when accompanied by minimal age differences in behavior, this over-recruitment is thought to be compensatory (Grady et al., 1994; Cabeza et al., 1997). However, a growing number of studies suggest that as attentional demands increase, older adults are less able to rally additional resources to meet the increasing task load, and in these cases, they show less activity within frontoparietal regions and tend to underperform younger adults (Reuter-Lorenz & Cappell, 2008). These studies have tended to manipulate task demands by increasing the number of items to be maintained in working memory (e.g., Mattay et al., 2006; Nagel et al., 2009, 2011; Carp, Gmeindl, & Reuter-Lorenz, 2010) or by increasing the need for task-set shifting or maintenance (e.g., Madden et al., 2010; Hedden, Van Dijk, Shire, Sperling, Johnson, & Buckner, 2011). An intriguing question is whether increasing task demands by making distracting information more salient, and thus more difficult to ignore, will also lead to age differences in frontoparietal recruitment.
Thus, in the present study, we used functional magnetic resonance imaging (fMRI) to examine age differences in the neural underpinnings of attentional control and their relationship to concomitant differences in distractibility and subsequent memory for distraction. To this end, we adapted a paradigm previously used to show that younger adults, with their superior attentional control abilities, are capable of ignoring centrally presented distracting words (Rees, Russell, Frith, & Driver, 1999). In our study, older and younger adults were shown a rapid stream of letter stimuli (words and nonwords) superimposed on line drawings of objects, and were asked to perform a 1-back task on either the letters or the objects, with the attended modality manipulated across runs (see Figure 1). Implicit memory for the superimposed words (i.e., priming) was later tested using two word fragment completion tasks – one after each 1-back run. Older adults were expected to show more priming than younger adults for the distracting (unattended) words on the first fragment task (Rowe et al., 2006). The second fragment task was mainly included as a manipulation check, given after the attend-letters 1-back task, to ensure that participants showed priming for words that were directly attended. In line with previous work (e.g., La Voie & Light, 1994), we expected little or no age differences in priming for previously attended-to words.
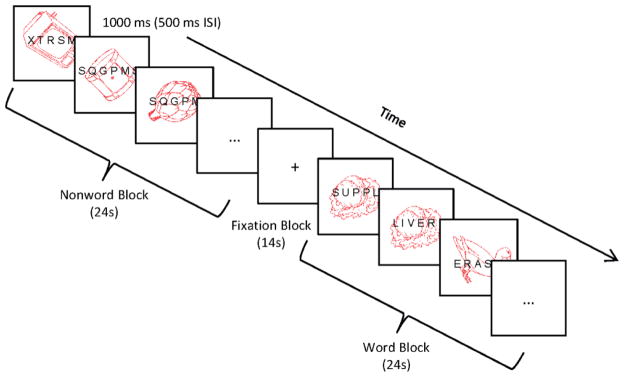
Figure 1.
Example of 1-back procedure. Participants attended to either the pictures (Run 1) or the letter stimuli (Run 2) and made a speeded response to immediate repetitions in the attended modality. Letter stimuli were divided into word and nonword blocks, interleaved with blocks of fixation. ISI = interstimulus interval.
In our analysis of the neuroimaging data, we first performed region of interest (ROI) analyses to examine age differences in activity associated with processing of the to-be-ignored words. We expected younger adults to show greater activity in word-processing areas when performing the 1-back task on words relative to nonwords, and to show little difference between these conditions when performing the 1-back task on pictures (i.e., when ignoring the letter stimuli; as found in Rees et al., 1999). Older adults, on the other hand, were expected to show greater activity in word-processing areas for word blocks relative to nonword blocks, regardless of whether they were explicitly attending to the letter stimuli or not. This finding would be consistent with previous work showing an age-related increase in neural activity associated with the processing of irrelevant distraction (e.g., Gazzaley et al., 2005; Fabiani et al., 2006; Stevens et al., 2008; Schmitz et al., 2010).
Further, in order to assess age differences in the recruitment of frontal and parietal attentional control regions, we used partial least squares (PLS), a data-driven multivariate analysis technique that identifies whole-brain patterns of activity associated with the individual task conditions. This method can be used to identify groups of regions (e.g., the frontoparietal control network, in this case) that are active during the task and shows the degree to which these areas are activated by each condition. Here, we expected younger adults to show greater activity within regions consistent with the frontoparietal control network when attempting to ignore the relatively salient letter stimuli (particularly when those letter stimuli formed words and thus, required a dampening down of a prepotent word-reading response [Kahneman & Chajczyk, 1983]), relative to when attempting to ignore the background pictures. Older adults were expected to show poorer recruitment of frontoparietal control regions relative to younger adults, especially during the ignore-words condition which required the greatest degree of attentional control, consistent with evidence that older adults’ activation of cognitive control regions asymptotes much earlier with increasing task demands (Reuter-Lorenz & Cappell, 2008).
Finally, we also used PLS to examine age differences in functional connectivity within the frontoparietal network at rest. Functional connectivity, or the degree to which activity in different regions covaries together, can be used to identify large-scale brain networks and is thought to be an important marker of the structural integrity of connections between those areas (Greicius, Supekar, Menon, & Dougherty, 2009; Ghosh, Rho, McIntosh, Kotter, & Jirsa, 2008). Previous work examining age differences in functional connectivity has shown reduced and/or altered patterns of connectivity in older adults within the default mode network (e.g., Andrews-Hanna et al., 2007; Esposito et al., 2008; Grady et al., 2010), memory network (e.g., Grady et al., 2003; Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006), and attentional control network (Madden et al., 2010; Nagel et al., 2011). Thus, we looked at the functional connectivity of two frontal regions previously identified as nodes of the frontoparietal control network (one from each hemisphere), and identified by our initial task analysis, to determine which regions they covaried with at rest. We expected older adults to show reduced functional connectivity within this frontoparietal network, as well as reduced activity, consistent with the expected increase in misguided attention towards distracting words.
Methods
Participants
Participants were 12 younger (18 – 28; M = 21.67, SD = 3.14; 6 males) and 12 older adults (60 – 78; M = 67.83, SD = 5.80; 3 males). None of the participants had any history of psychiatric or neurological disorder, or of drug or alcohol abuse, which might compromise cognitive function. All participants were right handed, had normal or corrected to normal vision, and older adults scored in the normal range on the Mini Mental Status Exam (> 26) (Folstein, Folstein, & McHugh, 1975). Younger adults had an average of 14.00 (SD = 1.60) years of education and a mean score of 32.84 (SD = 3.84) on the Shipley Vocabulary Test (Shipley, 1946). Older adults had more years of education (M = 15.83, SD = 2.41) than younger adults, t (22) = 2.20, p < .05, and they performed similarly on the vocabulary test (M = 34.98, SD = 3.77), t (22) = 1.35, p = .19.
Experimental Design
Participants performed a series of tasks within the fMRI scanner. The precise order of tasks was as follows: (1) 1-back task on pictures with irrelevant words/nonwords (Run 1; see Figure 1), (2) 7-minute nonverbal filler task (arrow flanker task; unscanned), (3) implicit word-fragment completion task (to test memory for the unattended words from the first 1-back task; unscanned), (4) 1-back task on letter stimuli with irrelevant pictures (Run 2), (5) 7-minute resting state scan with eyes closed (Run 3), and finally, (6) a second word-fragment completion task (to test memory for the attended words from the second 1-back task; unscanned).
The picture 1-back task was always given first, as we wanted to test participants’ implicit memory for previously “irrelevant” words and testing memory for the attended words first could have alerted them to a connection between successive tasks. As an additional precaution, we also asked participants after the first fragment task if they noticed a connection between the tasks thus far and if so, what they thought it was. Only one younger adult was aware of the connection, as will be discussed in the results section.
For the 1-back tasks, participants viewed a series of superimposed picture and letter stimuli (words and nonwords), with the pictures shown in red and the letters shown in black (see Figure 1). The approximate visual angles subtended by these stimuli were 5.5° for the pictures and 3° for the letters. Each stimulus pair was shown for 1000 ms, followed by a blank screen for 500 ms. Across two separate runs, participants were asked to attend to either the pictures or the letters and to press a response key whenever the same stimulus (from the attended modality) appeared twice in a row. They were told to ignore stimuli from the unattended modality, as attention towards these stimuli would only worsen target task performance. One-back targets occurred every 6 trials on average and never coincided with targets for the word-fragment task. A block design was used, with the letter stimuli divided into word and nonword blocks. Thus, there were five different block types in total: fixation, ignore-words (attend pictures), ignore-nonwords (attend pictures), attend-words (ignore pictures), and attend-nonwords (ignore pictures). Each run began with 10 seconds of fixation, followed by 8 task blocks of 24 seconds each, interleaved with 8 fixation blocks of 14 seconds each. Each task block contained 16 trials of the same condition and thus, there were 64 trials per condition. Pictures were taken from Snodgrass and Vanderwart (1980) and superimposed with either random consonant strings or words (including 10 words in each task that would later serve as targets on a word-fragment completion task). All picture and word lists were counterbalanced, such that the pictures and words used on the attend-pictures 1-back (and corresponding fragment task) for one person would be used on the attend-letters 1-back for another person, and vice versa.
Immediately after the first 1-back task, participants performed an arrow flanker task (Eriksen & Eriksen, 1974) for approximately 7 minutes, which was not scanned. Participants responded with a button press to the direction of a centrally presented arrow that either appeared alone or was flanked on either side by other arrows. The flanking arrows could either point in the same (congruent trials) or opposite (incongruent trials) direction as the central arrow. This task was included to distract participants during the interval and obscure the connection between the 1-back and word fragment tasks, as has been done previously (e.g., Rowe et al., 2006; Campbell et al., 2010).
Memory for the superimposed words was tested with two word-fragment completion tasks. Each task included 30 word fragments: 10 were target fragments that could be solved with words seen on the preceding 1-back task, 10 were control fragments which participants from another counterbalance condition would have seen, and 10 were easily solved fragments that served to maintain morale and to obscure the connection between the test and input task. Each fragment was shown in the centre of the screen for 3000 ms and participants were told to respond aloud with the first solution that came to mind.
All fragments had multiple solutions in the language, but only one in the experiment. Separate baseline word-fragment completion rates (the proportion of solved fragments from the unseen list) were determined for each age group and fragment task. These did not differ either by age (Ms = 11% & 9% for young and older adults, respectively) or by fragment task (Ms = 10% & 10% for the first and second tasks, respectively), F’s < 1. For each participant on each task, priming scores were calculated, as is typical in the priming literature, as the difference between the proportion of target-word fragments correctly solved and their age group’s baseline for that task. Because we expected older adults to show more priming than younger adults on the first fragment task (i.e., that tested priming for previously distracting words; Rowe et al., 2006), we used a 1-tailed test for this comparison.
fMRI Data Acquisition and Analysis
Participants were scanned using a Siemens Trio 3T scanner. Anatomical scans were acquired with a 3D MP-RAGE sequence (TR = 2 sec, TE = 2.63 ms, FOV = 25.6 cm2, 256×256 matrix, 160 slices of 1 mm thickness). Functional runs were acquired with an EPI sequence, with 157 volumes for each 1-back run and 210 volumes for the resting state run (TR = 2 sec, TE = 30 ms, flip angle = 70°, FOV = 20 cm2, 64×64 matrix, 30 axial slices of 5 mm thickness, no gap). Measures of pulse and respiration were obtained during the scan.
Preprocessing of the image data was performed with Analysis of Functional Neuroimages (AFNI; Cox, 1996). This included physiological motion correction, rigid motion correction, spatial normalization to Montreal Neurological Institute (MNI) space, and smoothing with an 8 mm Gaussian filter (the final voxel size was 4 × 4 × 4 mm). We also regressed out the white matter time series from each voxel time series (Grady et al., 2010). This involved using a probablistic white matter mask to define a region consisting only of white matter in each subject, determining the signal in this region, and then regressing out this signal from each individual’s data for each run. Finally, we discarded the first 2 TRs from each block in order to have crisper transitions between conditions.
ROI analyses
ROI analyses were used to examine age differences in activity associated with processing of the to-be-ignored words. A left inferior frontal region (MNI coordinates x = −40, y = 4, z = 32) was taken from the study by Rees et al. (1999). This was a region that, in their study, showed greater activity for words than nonwords during the letter 1-back task, but no difference between words and nonwords during the picture 1-back task.1 A 10-voxel ROI was centred on the voxel with these coordinates and the mean voxel intensity response for each condition in each participant was extracted. The voxel values used for the ROI analysis (and for the PLS analysis described below) used data that were normalized to the first time point in each block on a voxel-wise basis, and then averaged across the block. These mean responses were then submitted to a repeated measures ANOVA with age (young, old) as a between-subject factor and attended modality (pictures, letters) and letter stimuli (words, nonwords) as within-subject factors.
Whole-brain task analyses
Whole-brain analyses were conducted using PLS (McIntosh et al., 1996; McIntosh et al., 2004), a multivariate analysis technique that identifies whole-brain patterns of activity related to the experimental design (task-PLS) or to activity in a predefined region (seed-PLS). This method is similar to principal component analysis, in that it identifies a set of principal components or ‘latent variables’ (LVs) that optimally capture the covariance between two sets of measurements. In task-PLS, each LV indentifies a pattern of brain regions that, as a whole, maximally relate to a certain profile of task conditions also identified by that LV. Each brain voxel has a weight, known as a salience, which indicates how strongly that voxel contributes to the LV overall. The significance of each LV as a whole was determined using a permutation test (McIntosh et al., 1996), using 500 permutations. In addition, the reliability of each voxel’s contribution to a particular LV was tested by submitting all saliences to a bootstrap estimation of the standard errors (SEs; Efron, 1981), using 100 bootstraps. Peak voxels with a salience/SE ratio ≥ 3.0 (p <.001) were considered to be reliable. Clusters containing at least 10 reliable contiguous voxels were extracted, with a local maximum defined as the voxel with a salience/SE ratio higher than any other voxel in a 2 cm cube centered on that voxel (the minimum distance between peaks was 10 mm). We routinely use a 10-voxel cluster-size threshold because it is a relatively conservative size, yet not so large that we lose activity in small brain regions (e.g., Garrett, Kovacevic, McIntosh, & Grady, 2011; Grady et al., 2010; Protzner & McIntosh, 2007). Coordinates of these locations are reported in MNI space. Finally, to obtain summary measures of each participant’s expression of each LV pattern, we calculated ‘brain scores’ by multiplying each voxel’s salience by the BOLD signal in the voxel, and summing over all brain voxels for each participant in each condition. These brain scores were then mean-centered (using the grand mean across all participants) and confidence intervals (95%) for the mean brain scores in each condition were calculated from the bootstrap. Differences in activity between conditions (within a group) and between groups (within a given condition) were determined via the lack of overlap of these confidence intervals.
To examine the effects of varying forms of distraction on brain activity during the 1-back task, PLS was performed on the four task conditions (ignore-words, ignore-nonwords, attend-words, attend-nonwords) and fixation baseline for both age groups simultaneously. This analysis identified a pattern of brain regions (LV2, see results), similar to the frontoparietal control network outlined by Vincent and colleagues (2008), that younger adults activated more than older adults during the ignore-words condition. We also examined the degree to which activity in this frontoparietal network during the ignore-words condition related to priming for the distracting words. To do so, we correlated priming with individual brain scores from the ignore-words condition for LV2 from the task-PLS analysis. Brain scores provide a measure of the degree to which an individual expresses a given pattern of brain activity (specified by that LV) during a particular condition. Thus, we expected higher brain scores during the ignore words condition to be related to less priming for the distracting words.
Functional connectivity analyses
In order to examine age differences in the intrinsic functional connectivity of this network at rest, we subsequently submitted the data from our resting state run to two separate seed-PLS analyses using two frontal regions identified by the task-PLS analysis: one in the right (X: 24, Y: 48, Z: 24) and one in the left (X: −28, Y: 44, Z: 16) rostral PFC. In seed-PLS, each LV represents the pattern of correlation, or functional connectivity, between activity in a predefined region and all other voxels in the brain. For this analysis, we first averaged each consecutive 5 volumes from the resting run, to produce 40 volumes of 10 seconds each (after dropping the first 10 seconds). This averaging process effectively produced a low-pass filter of 0.1 Hz and reduced temporal noise. Then for each time point, we extracted the mean signal from the seed voxel and correlated it to the signal in all other brain voxels, across participants. To provide an assessment of functional connectivity in seed-PLS, brain scores were correlated with the seed activity in each “block” and the bootstrapping technique was used to calculate 95% confidence intervals around these correlations. Further, for each analysis we ran two contrasts: one assessing those areas which showed similar correlations to the seed in both young and older adults, and the other assessing age differences in connectivity with the seed. That is, contrasts were specified for each group, testing for similar connectivity (a series of 1’s were entered for both groups) and different connectivity (a series of 1’s and −1’s were entered for young and old, respectively). Each contrast produced a single brain pattern (and corresponding LV) that showed the specified pattern of correlation, or functional connectivity, between activity in the seed region and all other voxels in the brain. These procedures for using PLS to analyze functional connectivity during resting state fMRI runs have been shown to produce robust patterns of connectivity (Grigg & Grady, 2010a,b) similar to those obtained with methods of within-subject voxelwise correlations (e.g., Buckner et al., 2009).
Results
Behavioral Results
1-back tasks
False alarms on the 1-back tasks were very rare and did not differ either by age (Ms = 0 & 0.06 for young and old, respectively) or by condition (largest F (1, 21) = 1.77, p = .20, for the interaction between age and attended modality). Percent correct (hits) on the 1-back tasks was submitted to a mixed analysis of variance (ANOVA), with age (young, old) as a between-subject factor and attended modality (pictures, letters) and letter stimuli (words, nonwords) as within-subject factors. One younger adult was excluded from this analysis (and the corresponding task-PLS analysis) because he scored more than 3 standard deviations below the younger group mean (this person was included in the resting state analysis, however, for which there was no behavioral measure2). As can be seen in Table 1, younger adults detected more targets than older adults overall, F (1, 21) = 8.05, p < .01, and this effect was qualified by a significant interaction between age and letter stimuli, F (1, 21) = 10.99, p < .01, as well as a significant interaction between attended modality and letter stimuli, F (1, 21) = 9.84, p < .01. Considering the two age groups separately, younger adults were close to perfect across all conditions and they were no more distracted by words than by nonwords on the attend-pictures 1-back task, t (10) = 1.00, p = .34. Older adults, on the other hand, were significantly more distracted by words than by nonwords on the attend-pictures 1-back task, t (11) = 3.41, p < .01. These results suggest that older adults are particularly poor at suppressing obligatory responses, such as reading, and that this greater distractibility can have a negative effect on concurrent task performance.
Table 1.
Mean (SD) proportion of hits on the 1-back tasks by condition
| Attend Pictures
|
Attend Letters
|
|||
|---|---|---|---|---|
| Words | Nonwords | Words | Nonwords | |
|
| ||||
| Younger adults | 0.99 (.04) | 1.00 (.00) | 1.00 (.00) | 0.97 (.06) |
| Older adults | 0.83 (.14) | 0.98 (.04) | 0.87 (.21) | 0.89 (.17) |
Word-fragment tasks
One younger adult was aware of the connection between the first 1-back task and the first fragment task and thus, her priming score for this task was not included in the analysis. Means and standard errors for the remaining participants are shown in Figure 2.
Figure 2.
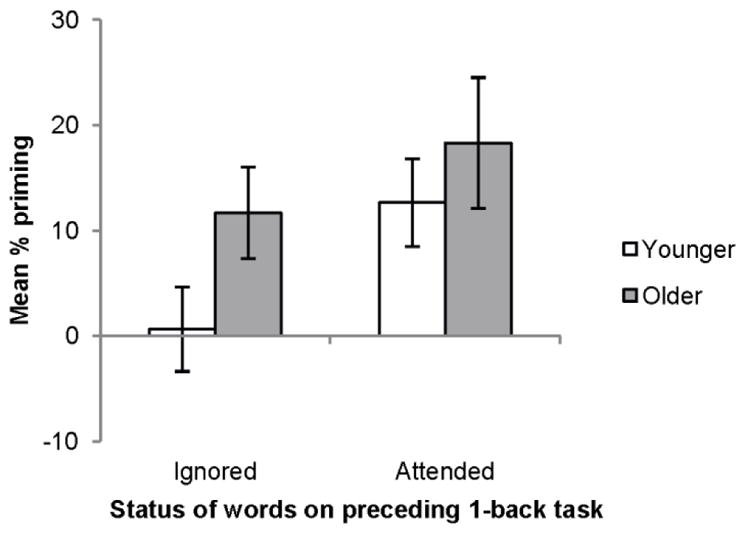
Mean percent priming as a function of age and attention. Error bars represent standard errors of the means.
On the first fragment task, only older adults showed above baseline priming for the ignored words, t (11) = 2.83, p < .05, and their priming scores were higher than those of younger adults, t (20) = 1.86, p = .039 (one-tailed), which replicates previous work showing age differences in memory for distraction (e.g., Rowe et al., 2006). Younger adults, on the other hand, did not show significant priming for the ignored words, t (9) = 0.25, p = .81, suggesting that they successfully ignored the letter stimuli while performing the 1-back task on pictures. In contrast, priming performance on the second task, which tested memory for the attended words, did not differ between older and younger adults, t (21) = 0.70, p = .49.
fMRI Results
ROI analyses
In order to assess age differences in neural activity associated with the processing of distracting words, we examined activation within a left inferior frontal ROI previously shown to be selectively activated by attended word stimuli in younger adults (Rees et al., 1999). Although the omnibus ANOVA assessing the effects of age, modality, and letter stimuli did not reveal any significant effects (largest F (1, 21) = 2.25, p = .15, for the interaction between age and modality), the pattern of means shown in Figure 3 is similar to the predicted pattern, at least for younger adults. That is, replicating the findings of Rees et al. (1999), younger adults showed greater activation within this left inferior frontal region when attending to words versus nonwords, t (10) = 2.59, p < .05, but no difference between these conditions when the letter stimuli were being ignored, t (10) = 0.36, p = .73. Older adults, on the other hand, did not show greater activity for words than nonwords in this ROI, regardless of whether they were attending to the letter stimuli or not, p’s > .50.
Figure 3.
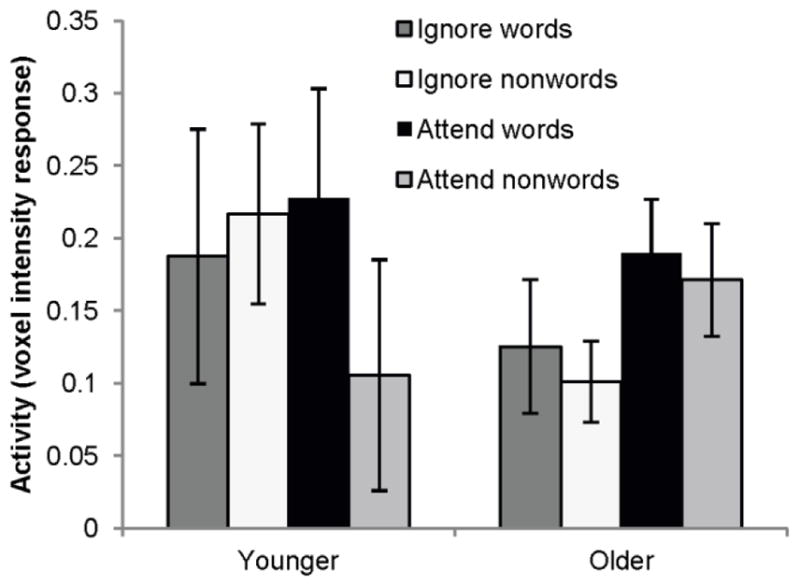
Mean activation in the left inferior frontal ROI by younger and older adults across conditions. Error bars represent standard errors of the mean voxel intensity responses in each condition.
Whole-brain task analyses
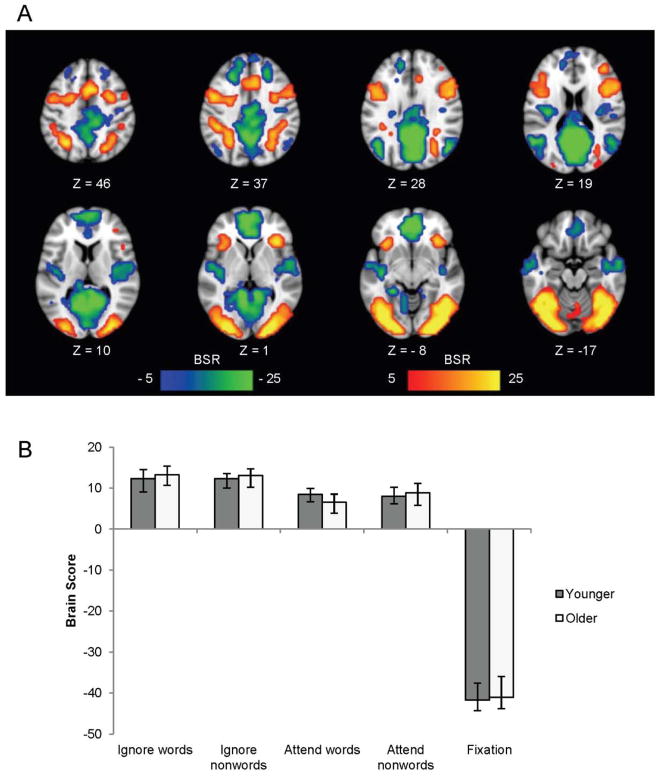
The first LV from the task-PLS analysis (p < .001) accounted for 77.89% of the covariance in the data and differentiated the four task conditions from fixation in both young and older adults (see Figure 4). This LV showed the classic pattern of increased activity in the default mode network during baseline fixation (Raichle et al., 2001; Buckner, Andrews-Hanna, & Schacter, 2008) and increased activity in the task-positive network (Fox et al., 2005; Toro, Fox, & Paus, 2008) during the four task conditions, and did not differ between older and younger adults (see supplemental Table S1).
Figure 4.
LV1 from the task-PLS analysis contrasting modulations of activity across all conditions in younger and older adults, shown on axial slices from the MNI152 average brain. The pattern identified by LV1 in (a) shows areas with greater activity during all 4 tasks (shown in red and associated with positive brain scores) contrasting with those showing more activity during fixation (blue areas and associated with negative brain scores in both age groups. The graph in (b) shows the mean-centered mean brain scores for both groups on this LV (error bars represent the 95% confidence intervals). A bootstrap ratio threshold of 5.0 was used to form the brain image in (a).
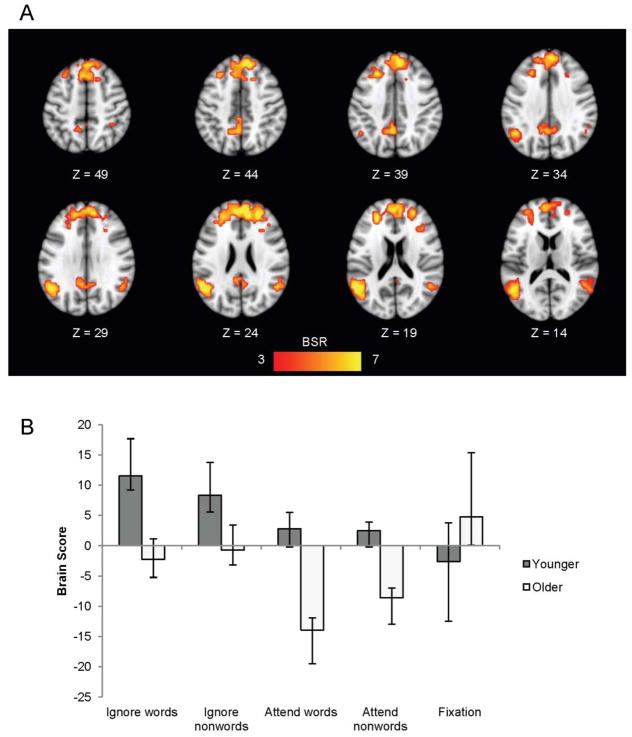
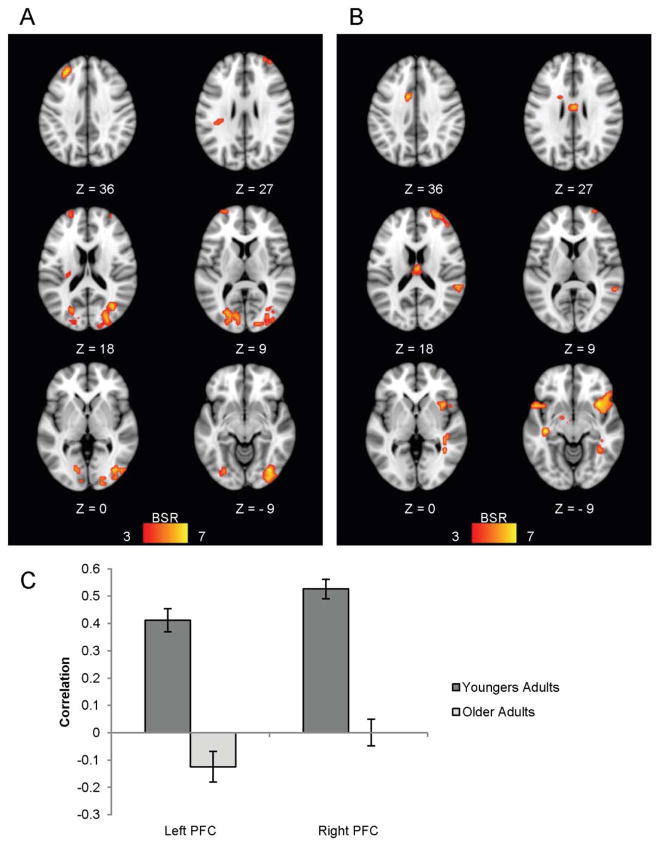
The second LV from this analysis was also significant (p < .05) and accounted for 5.97% of the covariance in the data. This LV showed a group difference in activation across the different task conditions (Figure 5b). As can be seen in Figure 5, younger adults activated a group of regions (shown in warm colours) during the ignore words/nonwords conditions (indicated by positive mean brain scores) relative to the other conditions, and they activated this network to the greatest extent during the ignore-words condition. Older adults, on the other hand, did not show reliably increased activity in this network during the ignore words/nonwords conditions, but did show decreased activity in these regions during the attend words/nonwords conditions (indicated by the negative brain scores). This pattern of regions included the rostral PFC bilaterally and the left inferior parietal cortex (Figure 5a, Table 2). This constellation of areas is similar to the frontoparietal control network outlined by Vincent and colleagues (2008) and may reflect younger adults’ greater recruitment of cognitive control regions during the attention-demanding ignore-words condition.
Figure 5.
LV2 from the task-PLS analysis contrasting modulations of activity across all conditions in younger and older adults, shown on axial slices from the MNI152 average brain. The pattern identified by LV2 in (a) shows areas where younger adults had more activity during the ignore words/nonwords conditions relative to older adults. The graph in (b) shows the mean-centered mean brain scores for both groups on this LV (error bars represent the 95% confidence intervals). A bootstrap ratio threshold of 3.0 was used to form the brain image in (a).
Table 2.
Brain areas activated more by younger adults than older adults during the ignore words/nonwords conditions
| Region | Hem | X(mm) | Y(mm) | Z(mm) | BSR |
|---|---|---|---|---|---|
| right rostral PFC | R | 24 | 48 | 24 | 6.54 |
| medial frontal gyrus | L | −4 | 52 | 24 | 6.92 |
| medial frontal gyrus | L | −8 | 24 | 52 | 5.33 |
| left rostral PFC | L | −28 | 44 | 16 | 6.25 |
| middle temporal gyrus | R | 60 | −48 | 4 | 6.99 |
| superior temporal gyrus / IPL | L | −56 | −52 | 20 | 6.69 |
Note: MNI coordinates of cluster maxima showing the pattern of activity depicted in Figure 5b (i.e., areas where younger adults showed greater increases in activity than older adults in the ignore words/nonwords conditions); clusters shown in Figure 5a. Hem = hemisphere; R = right; L = left; BSR = bootstrap ratio; IPL = inferior parietal lobe; PFC = prefrontal cortex.
Relationship between frontoparietal activity and priming
In order to determine whether increased frontoparietal activity during the ignore-words condition increased participants’ ability to ignore the distracting words, and to subsequently show less priming for them, we correlated priming with individual brain scores from the ignore-words condition from the second LV indentified by the above task-PLS analysis. These brain scores indicate the degree to which each person expressed the specified pattern of brain activity (seen in Figure 5a) during the ignore-words condition. One younger adult was identified as an overly influential case (brain score > 2.5 SDs above the mean, Cook’s distance > 1, leverage value > 3 times the average leverage) and removed from the model. Across the remaining participants, both young and old, there was a significant correlation between priming and the degree to which participants activated frontoparietal regions during the ignore-words condition (Figure 6), showing the expected relation of less priming as activity increased, r = −.53, p < .05, 95% bootstrap CI = (−.78, −.21)3. Thus, greater activation of these areas when attempting to ignore distracting words was associated with less implicit memory for those words on a subsequent fragment completion test.
Figure 6.
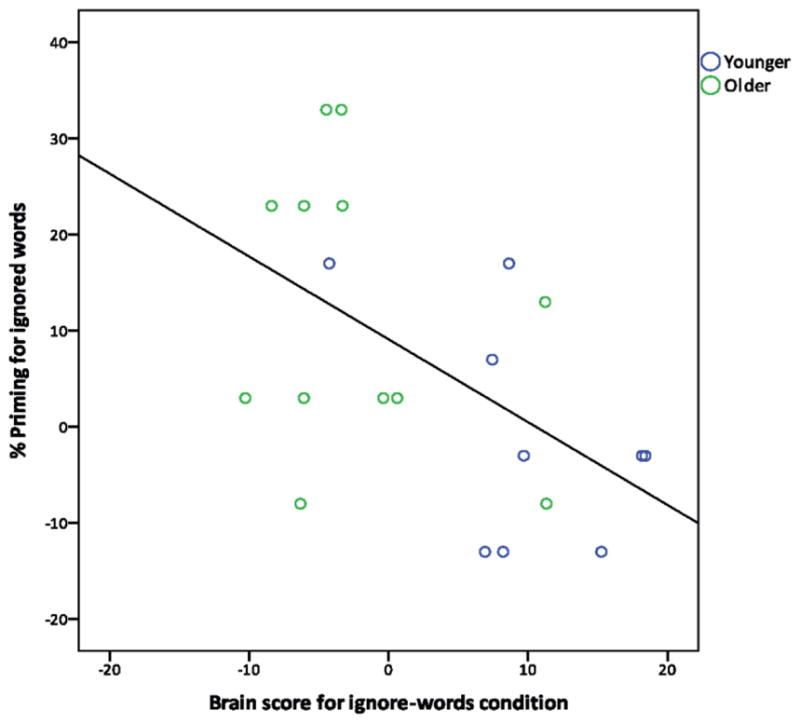
Negative correlation between the degree to which each participant activated the frontoparietal control network during the ignore-words condition (brain score) and the amount of priming shown for the distracting words (r = −.53, p < .05).
Functional connectivity analyses
The task-PLS analysis identified a set of regions that younger adults activated to a greater extent than older adults in the ignore-words condition, including some rostral PFC and parietal areas that have previously been shown to be functionally correlated at rest (Vincent et al., 2008). In order to assess whether these task-related regions are indeed part of the previously defined frontoparietal control network, and to assess age differences in the intrinsic coherence of this network, we submitted our resting-state data to two whole-brain functional connectivity analyses using left and right rostral PFC regions identified by our task analysis as seeds. Furthermore, we used two contrasts for each analysis: one assessing those areas which showed similar correlations to the seed in both older and younger adults, and the other assessing age differences in connectivity with the seed.
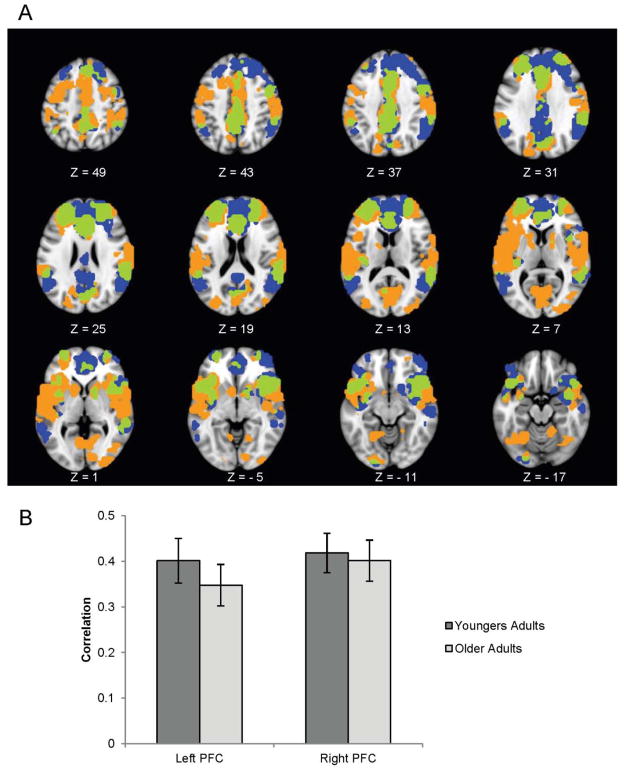
The first analysis using the left PFC seed revealed a significant pattern of regions that were similarly connected to the seed in both groups (p < .001), including other putative control regions, such as the inferior frontal gyri and widespread regions of posterior cortex, extending into the inferior parietal lobes (Figure 7, regions shown in orange and green). In addition, the left PFC seed correlated with several other areas not previously identified as part of the frontoparietal control network, including the cerebellum, temporal pole, fusiform gyrus, and occipital lobes (supplemental Table S2).
Figure 7.
Brain regions correlated with the right and left rostral PFC seeds in older and younger adults. Orange represents the left PFC connectivity pattern; blue represents the right PFC connectivity pattern; and green represents the overlap. The graph in (b) shows the average correlation between each seed and the pattern of regions shown in (a) across the resting state scan. A bootstrap ratio threshold of 6.0 was used to form the brain image in (a). The error bars in (b) represent standard errors of the mean correlations.
The contrast assessing age differences in connectivity to the left rostral PFC seed was also significant (p < .001). This analysis revealed several regions that were correlated to the left rostral PFC seed more strongly in younger adults than in older adults, including superior and middle frontal areas, the left insula, and the occipital lobes bilaterally (Figure 8a; Table 3). Older adults showed slightly stronger connectivity between the seed and the middle frontal gyrus and caudate (see Table 3). Thus, younger adults not only recruit these control regions to a greater extent than older adults during an attention-demanding task, but they also show greater functional connectivity within some parts of this network while at rest.
Figure 8.
Brain regions showing an age difference in connectivity. Brain regions in (a) correlated with the left rostral PFC seed more strongly in younger adults than in older adults. Brain regions in (b) correlated with the right rostral PFC seed more strongly in younger adults than in older adults. The graph in (c) shows the average correlation between each seed and the pattern of regions shown in (a) and (b) across the resting state scan. A bootstrap ratio threshold of 3.0 was used to form the brain image. The error bars in (c) represent standard errors of the mean correlations.
Table 3.
Brain areas showing an age difference in connectivity with the left rostral PFC seed.
| Region | Hem | X(mm) | Y(mm) | Z(mm) | BSR |
|---|---|---|---|---|---|
| Younger > older | |||||
| middle frontal gyrus | L | −24 | 60 | 24 | 3.8099 |
| middle frontal gyrus | R | 32 | 56 | 24 | 5.4541 |
| superior frontal gyrus | L | −28 | 40 | 36 | 5.0527 |
| temporal pole | L | −24 | 8 | −24 | 3.8866 |
| medial temporal pole | R | 28 | 8 | −32 | 3.8386 |
| insula | L | −28 | −24 | 20 | 4.2528 |
| middle temporal gyrus | R | 36 | −68 | 16 | 5.3472 |
| cerebellum | R | 12 | −72 | −24 | 4.0513 |
| inferior occipital gyrus | R | 36 | −76 | −8 | 5.4363 |
| middle occipital gyrus | L | −20 | −80 | 12 | 4.455 |
| inferior occipital gyrus | L | −28 | −80 | −12 | 4.301 |
| Older > younger | |||||
| caudate | L | −16 | 20 | 0 | −3.742 |
| caudate | R | 20 | 16 | 8 | −4.1508 |
| middle frontal gyrus | L | −28 | 12 | 44 | −4.7634 |
Note: Clusters shown in Figure 8a.
The second analysis using the right PFC seed also revealed a significant pattern of regions that were similarly connected to the seed in both groups (p < .001). This pattern included several regions that overlapped with those connected to the left PFC seed, including the left rostral PFC itself, as well as other frontal and parietal regions (Figure 7, overlap regions shown in green; supplemental Table S3). The right rostral PFC seed also correlated with several areas not connected to the right PFC seed, including more posterior portions of the inferior parietal lobes, and more extensive regions of the posterior cingulate and medial frontal cortex (Figure 7, regions shown in blue).
The contrast assessing age differences in resting connectivity to the right PFC seed was also significant (p < .05). This analysis revealed several regions that correlated more strongly with the right rostral PFC seed in younger adults than in older adults, including inferior frontal/anterior insula areas, middle cingulate cortex, and within the right rostral PFC itself (Figure 8b; Table 4). There were no areas where older adults had stronger connectivity relative to younger adults. Thus, once again, younger adults showed stronger functional connectivity between the rostral PFC and other parts of the frontoparietal network.
Table 4.
Brain areas functionally connected to the right rostral PFC seed more strongly in younger than in older adults at rest
| Region | Hem | X(mm) | Y(mm) | Z(mm) | BSR |
|---|---|---|---|---|---|
| superior frontal gyrus | R | 24 | 60 | 16 | 4.26 |
| insula | R | 36 | 12 | −4 | 6.06 |
| middle cingulate cortex | L | −12 | 4 | 36 | 4.80 |
| superior temporal gyrus | L | −56 | 12 | −12 | 5.05 |
| superior temporal gyrus | R | 60 | −44 | 12 | 4.34 |
| middle temporal gyrus | R | 40 | −44 | −4 | 4.25 |
| hippocampus | L | −36 | −24 | −8 | 5.61 |
| thalamus | midline | 0 | −16 | 20 | 5.05 |
| hypothalamus | midline | 0 | −4 | −16 | 4.75 |
| cerebellum | R | 4 | −44 | −44 | 3.99 |
Note: Clusters shown in Figure 8b.
Discussion
In this study, we used a multivariate approach to investigate the neural underpinnings of older adults’ greater implicit memory for previously viewed distraction. Older adults were more distracted than younger adults by overlapping word stimuli during a picture 1-back task, as indicated by their lower hit rate in the ignore-words condition, as well as by their greater implicit memory for the distracting words on a subsequent word fragment completion task. Task-PLS revealed a set of regions, including the rostral PFC and inferior parietal cortex, that younger adults activated to the greatest extent during the ignore-words condition, and which older adults did not activate at all relative to fixation. Further, the degree to which participants activated this set of regions when attempting to ignore the distracting words predicted the amount of priming they subsequently showed for those words, suggesting that engagement of this frontoparietal network allows for the down-regulation of distraction. Subsequent functional connectivity analyses using right and left rostral PFC seeds revealed a set of regions that were functionally connected to the rostral PFC in both groups at rest. These included other putative control regions, such as the inferior frontal and inferior parietal cortex. Direct comparisons between older and younger adults revealed several regions that correlated more strongly with the rostral PFC seeds in younger adults, suggesting that functional connectivity among some nodes within the frontoparietal control network is reduced with age.
Recent behavioral work (Rowe et al., 2006; Kim et al., 2007; Campbell et al., 2010) has suggested that older adults’ lessened inhibitory control leads to excessive encoding of distraction, which can subsequently interfere with or facilitate their future performance, at least at an implicit level. The present results are in accordance with this work, in that only older adults showed above baseline priming for the unattended words. Younger adults seemed able to ignore the distracting words, as their performance on the picture 1-back task was unaffected by whether words or nonwords served as distraction, and they ultimately showed no priming for the unattended words. The ignore-words condition was arguably the most challenging condition on the 1-back task, as it required participants to overcome a relatively automatic word-reading response (Kahneman & Chajczyk, 1983) in favour of attending to the pictures. It was in this condition that younger adults showed greater activation than older adults in regions previously associated with cognitive control (Nelson et al., 2003; Badre, 2008; Koechlin, Basso, Pietrini, Panzer, & Grafman, 1999; Christoff & Gabrieli, 2000; Burgess, Dumontheil, & Gilbert, 2007; Cabeza, Ciaramelli, Olson, Moscovitch, 2008), including bilateral activation in the rostral PFC and inferior parietal cortex. Furthermore, greater activity in these regions during the ignore-words condition was associated with less priming for those words later on, suggesting that greater recruitment of cognitive control regions helped to prevent distracting words from being encoded.
In addition to expecting to see age differences in the recruitment of cognitive control regions, we also expected age differences in activity associated with processing of the to-be-ignored words, as is predicted by other work showing an age-related deficit in the down regulation of activity in regions that are involved selectively in processing these distractors (e.g., Gazzaley et al., 2005; Fabiani et al., 2006; Stevens et al., 2008; Schmitz et al., 2010). In line with previous work (Rees et al., 1999), younger adults showed greater activation within a left inferior frontal region (Brodmann area 44 or Broca’s area), typically associated with phonological processing or rehearsal (e.g., Fiez & Petersen, 1998; Smith, Jonides, Marshuetz, & Koeppe, 1998), when attending to words versus nonwords, but no difference between these conditions when attending to pictures. Thus, when asked to ignore the letter stimuli, younger adults’ brain activity did not distinguish between words and nonwords, suggesting that they were able to prevent or quickly down-regulate a prepotent word-reading response. In contrast, older adults showed little modulation within the left inferior frontal ROI across conditions, raising the possibility that this region did not distinguish between word and nonword stimuli in older adults. Thus, we did not find evidence of an age-related increase in distractor-related activity, an outcome that may be due to the materials used here. Word reading and picture naming tend to activate similar language processing regions (for a review, see Martin, 2003) and thus, these stimuli may not have been optimally suited for distinguishing between target- and distractor-related activity. In line with this view, younger adults showed similar activation in this region when attending to pictures as they did when attending to words (a similar effect to that shown by Rees et al., 1999, see their Figure 3). Nonetheless, older adults showed more priming for the distracting words than younger adults, suggesting that, at least at behavioral level, older adults were processing irrelevant distraction to a greater extent than younger adults.
Younger adults activated frontal and parietal control regions to a greater extent than older adults during the ignore-words condition, and activation within these regions related to priming for the distracting words. In order to determine if this group of regions is indeed similar to the frontoparietal control network detailed by Vincent and colleagues (2008), and to assess age differences in the coherence of this network at rest, we also assessed the functional connectivity of two peak rostral PFC seeds taken from the initial task analysis. Two of these analyses revealed a network of areas that were similarly correlated to the rostral PFC in both groups during rest. Although there are some differences between the frontoparietal network outlined here and that of Vincent et al. (2008; see our Figures 7a, their Figure 7), there is also a large degree of overlap, suggesting that the frontoparietal network is both robust to changes in precise methodology, as well as relatively stable across the lifespan. Recent work also suggests that this network is flexibly coactivated with either the default or dorsal attention network depending on the type of goal-directed cognition necessitated by a task (Spreng et al., 2010), and that individual differences in resting-state frontoparietal connectivity correlate with measures of executive control (Seeley et al., 2007). Taken together with the current results, these findings suggest that the frontoparietal network plays an important role in cognitive control, particularly when, as here, the down-regulation of distraction is required.
Although older and younger adults showed a similar pattern of functional connectivity with the two rostral PFC seeds, a direct contrast between the groups revealed a set of areas that were more strongly correlated to the seed in younger adults. Across the two analyses, age differences in functional connectivity were most consistently seen within the frontal lobes bilaterally (Figure 8). Thus, in addition to being less able to recruit the frontoparietal control network when it is most needed to overcome distraction, older adults also appear to show weaker functional connectivity within this network at rest. Similar age-related reductions in functional connectivity have also been found in the default mode network during rest (Andrews-Hanna et al., 2007; Esposito et al., 2008; Grady et al., 2010). Thus, functional connectivity appears to decline with age within different neural systems, suggesting the possibility that the effect may be driven by a generalized age-related change across the brain, rather than degradation in any one particular region. Reduced functional connectivity may be a byproduct of decreased neural specificity (Park et al., 2004) and/or white matter integrity with age (Andrews-Hanna et al., 2007; Chen, Chou, Song, & Madden, 2009). These age-related changes may lead to less efficient communication between brain regions and ultimately, to older adults’ lessened ability to flexibly recruit or down-regulate large-scale neural networks in service of a particular goal, such as inhibiting irrelevant distraction.
This study was not without limitations. First, the order of the 1-back tasks was fixed rather than counterbalanced. This was done to minimize the chance that participants would notice a connection between the distracting words on the attend-pictures 1-back task and the following word-fragment task. However, we therefore cannot rule out the influence of order effects in our fMRI results. Second, our sample size was relatively small, but this is unlikely to be a problem for the multivariate analyses used here, which are known to be more robust than univariate analyses (e.g., Fletcher et al., 1996; Lukic, Wernick, & Strother, 2002; Nichols & Holmes, 2002). Nevertheless, the ROI analysis may have been underpowered, as well as the within-group correlations between priming and brain scores (reported in footnote #3 above). Finally, although we cannot rule out the possibility that age differences in functional connectivity during the resting state run is not due to carry-over effects from the preceding attend-letters 1-back task (e.g., reflecting differential rehearsal of the words), we think this unlikely. The resting state run was relatively long (7 minutes) and participants were unaware that their memory for the words would be tested and thus, it seems unlikely that they would have spent a significant portion of that time rehearsing the words. Moreover, the regions that differentiated young and old adults in our study were not those that have elsewhere been associated with verbal rehearsal (e.g., left inferior PFC and superior parietal lobes; Davachi, Maril, Wagner, 2001; Shivde & Thompson-Schill, 2004).
In summary, we found age differences in both the recruitment and functional connectivity of a frontoparietal network previously implicated in cognitive control (Vincent et al., 2008; Spreng et al., 2010). Relative to younger adults, older adults showed less activation of this network when it was most needed and poorer functional connectivity within this network during rest. Despite these age-related reductions in neural functioning, older adults actually demonstrated greater implicit memory for the distraction on a later task, suggesting that one benefit of older adults’ lessened cognitive control is greater knowledge of previously extraneous information. Although this excessive encoding on the part of older adults may often lead to interference on traditional tests of explicit memory, and hence, contribute to the memory declines normally associated with aging, it may also put older adults at an advantage whenever past information is relevant to the present, and the current situation permits tacit use of that information (e.g., Campbell et al., 2010; Thomas & Hasher, 2011).
Acknowledgments
The authors would like to thank Annette Weeks-Holder and Wendy Elson for technical assistance. This work was supported by the Canadian Institutes of Health Research (MOP89769 to LH and MOP14036 to CG), the Natural Sciences and Engineering Research Council of Canada (PGS to KLC), and the Canada Research Chairs program, the Ontario Research Fund, the Canadian Foundation for Innovation (all to CG), and the Heart and Stroke Foundation Centre for Stroke Recovery.
Footnotes
Two other word-processing regions (located in the left inferior temporal lobe, MNI coordinates x = − 52, y = − 52, z = − 24, and x = − 42, y = − 39, z = − 31) that showed this pattern in the Rees et al. (1999) study were outside the scanned area for some of our participants and thus, could not be examined within this dataset.
Removing this individual does not change either the significance or pattern of any of the functional connectivity results.
We note that there is a fair degree of overlap between the groups in Figure 6, suggesting that this correlation was not simply reflective of the group differences reported earlier. However, when looking at the correlation separately within each group, there is only a trend in the younger group (r = −.51, p = .17) and no effect in the older group (r = −.27, p = .39).
References
- Alain C, Woods DL. Age-related changes in processing auditory stimuli during visual attention: Evidence for deficits in inhibitory control and sensory memory. Psychology and Aging. 1999;14:507–519. doi: 10.1037//0882-7974.14.3.507. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends in Cognitive Sciences. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. Journal of Neuroscience. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends in Cognitive Sciences. 2007;11:290–298. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: An attentional account. Nature Reviews Neuroscience. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle Sl, Craik FIM. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. Journal of Neuroscience. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Hasher L, Thomas RC. Hyper-binding: A unique age effect. Psychological Science. 2010;21:399–405. doi: 10.1177/0956797609359910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, Gmeindl L, Reuter-Lorenz PA. Age differences in the neural representations of working memory revealed by multi-voxel pattern analysis. Frontiers in Human Neuroscience. 2010;4:217. doi: 10.3389/fnhum.2010.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cerebral Cortex. 1997;7:63–69. doi: 10.1093/cercor/7.1.63. [DOI] [PubMed] [Google Scholar]
- Chen N, Chou Y, Song AW, Madden DJ. Measurement of spontaneous signal fluctuations in fMRI: Adult age differences in intrinsic functional connectivity. Brain Structure and Function. 2009;213:571–585. doi: 10.1007/s00429-009-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computational Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. Cerebral Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Maril A, Wagner AD. When keeping in mind supports later bringing to mind: Neural markers of phonological rehearsal predict subsequent remembering. Journal of Cognitive Neuroscience. 2001;13:1059–1070. doi: 10.1162/089892901753294356. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Efron B. Nonparametric estimates of standard error: The jackknife, the bootstrap, and other methods. Biometrika. 1981;68:589–599. [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Esposito F, Aragri A, Pesaresi I, Cirillo S, Tedeschi G, Marciano E, Goebel R, Di Salle F. Independent component model of the default-mode brain function: combining individual-level and population-level analyses in resting-state fMRI. Magnetic Resonance Imaging. 2008;26:905–13. doi: 10.1016/j.mri.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Low KA, Wee E, Sable JJ, Gratton G. Reduced suppression or labile memory? Mechanisms of inefficient filtering of irrelevant information in older adults. Journal of Cognitive Neuroscience. 2006;18:637–650. doi: 10.1162/jocn.2006.18.4.637. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proceedings of the National Academy of Science U S A. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Dolan RJ, Shallice Frith CD, Frackowiak RSJ, Friston KJ. Is multivariate analysis of PET data more revealing than the univariate approach? Evidence from a study of episodic memory retrieval. Neuroimage. 1996;3:209–215. doi: 10.1006/nimg.1996.0023. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini Mental State” – a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Science U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL. The importance of being variable. Journal of Neuroscience. 2011;31:4496–4503. doi: 10.1523/JNEUROSCI.5641-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A. Influence of early attentional modulation on working memory. Neuropsycholgia. 2011;49:1410–1424. doi: 10.1016/j.neuropsychologia.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Rho Y, McIntosh AR, Kotter R, Jirsa VK. Cortical network dynamics with time delays reveals functional connectivity in the resting brain. Cognitive Neurodynamics. 2008;2:115–120. doi: 10.1007/s11571-008-9044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. Journal of Neuroscience. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Age-Related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13:572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz MA, Anderson JAE, Churchill N, McIntosh AR. A multivariate analysis of age-related differences in default mode and task positive networks across multiple cognitive domains. Cereb Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg O, Grady CL. Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PLoS One. 2010a;5(10):e13311. doi: 10.1371/journal.pone.0013311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg O, Grady CL. The default network and processing of personally relevant information: Converging evidence from task-related modulations and functional connectivity. Neuropsychologia. 2010b;48:3815–3823. doi: 10.1016/j.neuropsychologia.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M, Supekar KS, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm VP, Hasher L. Age and the availability of inferences. Psychology and Aging. 1992;7:56–64. doi: 10.1037//0882-7974.7.1.56. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The Psychology of Learning and Motivation. Vol. 22. New York, NY: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hasher L, Zacks RT, May CP. Inhibitory control, circadian arousal, and age. In: Gopher D, Koriat A, editors. Attention and performance. XVII. Cambridge, MA: MIT Press; 1999. pp. 653–675. [Google Scholar]
- Healey MK, Campbell KL, Hasher L. Cognitive aging and increased distractibility: Costs and potential benefits. In: Sossin WS, Lacaille JC, Castellucci VF, Belleville S, editors. Progress in brain research. Vol. 169. Amsterdam: Elsevier; 2008. pp. 353–363. [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KRA, Shire EH, Sperling RA, Johnson KA, Buckner RL. Failure to modulate attentional control in advanced aging linked to white matter pathology. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Chajczyk D. Tests of the automaticity of reading: Dilution of Stroop effects by color-irrelevant stimuli. Journal of Experimental Psychology: Human Perception and Performance. 1983;94:497–509. doi: 10.1037//0096-1523.9.4.497. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kim S, Hasher L, Zacks RT. Aging and a benefit of distractibility. Psychonomic Bulletin & Review. 2007;14:301–305. doi: 10.3758/bf03194068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- La Voie D, Light LL. Adult age differences in repetition priming: A meta-analysis. Psychology and Aging. 1994;9:539–553. doi: 10.1037//0882-7974.9.4.539. [DOI] [PubMed] [Google Scholar]
- Lukic AS, Wernick MN, Strother SC. An evaluation of methods for detecting brain activations from functional neuroimages. Artificial Intelligence in Medicine. 2002;25:69–88. doi: 10.1016/s0933-3657(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Lustig C, Hasher L, Tonev ST. Distraction as a determinant of processing speed. Psychonomic Bulletin and Review. 2006;13:619–625. doi: 10.3758/bf03193972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Hasher L, Zacks RT. Inhibitory deficit theory: Recent developments in a “new view”. In: Gorfein DS, MacLeod CM, editors. The place of inhibition in cognition. Washington, DC: American Psychological Association; 2007. pp. 145–162. [Google Scholar]
- Madden DJ, Costello MC, Dennis NA, Davis SW, Shepler AM, Spaniol J, Bucur B, Cabeza R. Adult age differences in functional connectivity during executive control. Neuroimage. 2010;52:643–657. doi: 10.1016/j.neuroimage.2010.04.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC. Language processing: Functional organization and neuroanatomical basis. Annual Review of Psychology. 2003;54:55–89. doi: 10.1146/annurev.psych.54.101601.145201. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neuroscience Letters. 2006;392:32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using Partial Least Squares. NeuroImage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Chau WK, Protzner AB. Spatiotemporal analysis of event-related fMRI data using partial least squares. NeuroImage. 2004;23:764–75. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li SC, Nyberg L, Backman L, Lindenberger U, Heekeren HR. Performance level modulates adult age differences in brain activation during spatial working memory. Proceedings of the National Academy of Sciences U S A. 2009;106:22552–22557. doi: 10.1073/pnas.0908238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li SC, Nyberg L, Backman L, Lindenberger U, Heekeren HR. Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. Journal of Cognitive Neuroscience. 2011;23:2030–2045. doi: 10.1162/jocn.2010.21560. [DOI] [PubMed] [Google Scholar]
- Nelson JK, Reuter-Lorenz PA, Sylvester CC, Jonides J, Smith EE. Dissociable neural mechanisms underlying response-based and familiarity-based conflict in working memory. Proceedings of the National Academy of Sciences U S A. 2003;100:11171–11175. doi: 10.1073/pnas.1334125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduced neural specialization in ventral visual cortex. Proceedings of the National Academy of Science U S A. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protzner AB, McIntosh AR. The interplay of stimulus modality and response latency in neural network organization for simple working memory tasks. Journal of Neuroscience. 2007;27:3187–3197. doi: 10.1523/JNEUROSCI.4963-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbit PMA. An age deficit in the ability to ignore irrelevant information. Journal of Gerontology. 1965;20:233–238. doi: 10.1093/geronj/20.2.233. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Russell C, Frith CD, Driver J. Inattentional blindness versus inattentional amnesia for fixated but ignored words. Science. 1999;286:2504–2507. doi: 10.1126/science.286.5449.2504. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;17:177–182. [Google Scholar]
- Rowe G, Valderrama S, Hasher L, Lenartowicz A. Attentional disregulation: A benefit for implicit memory. Psychology and Aging. 2006;21:826–830. doi: 10.1037/0882-7974.21.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Cheng FHT, De Rosa E. Failing to ignore: Paradoxical neural effects of perceptual load on early attentional selection in normal aging. Journal of Neuroscience. 2010;30:14750–14758. doi: 10.1523/JNEUROSCI.2687-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivde G, Thompson-Schill SL. Dissociating semantic and phonological maintenance using fMRI. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:10–19. doi: 10.3758/cabn.4.1.10. [DOI] [PubMed] [Google Scholar]
- Shipley WC. Institute of Living Scale. Los Angeles: Western Psychological Services; 1946. [Google Scholar]
- Smith EE, Jonides J, Marshuetz C, Koeppe RA. Components of verbal working memory: Evidence from neuroimaging. Proceedings of the National Academy of Science U S A. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. Norms for picture stimuli. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:205–210. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Hasher L, Chiew KS, Grady CL. A neural mechanism underlying memory failure in older adults. Journal of Neuroscience. 2008;28:12820–12824. doi: 10.1523/JNEUROSCI.2622-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RC, Hasher L. Reflections of distraction in memory: Transfer of previous distraction improves recall in younger and older adults. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2011 doi: 10.1037/a0024882. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cerebral Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]