Abstract
Current evidence suggests that two spatially distinct neuroanatomical networks, the dorsal attention network (DAN) and the default mode network (DMN), support externally and internally oriented cognition, respectively, and are functionally regulated by a third, frontoparietal control network (FPC). Interactions among these networks contribute to normal variations in cognitive functioning and to the aberrant affective profiles present in certain clinical conditions, such as major depression. Nevertheless, their links to non-clinical variations in affective functioning are still poorly understood. To address this issue, we used fMRI to measure the intrinsic functional interactions among these networks in a sample of predominantly younger women (N = 162) from the Human Connectome Project. Consistent with the previously documented dichotomous motivational orientations (i.e., withdrawal versus approach) associated with sadness versus anger, we hypothesized that greater sadness would predict greater DMN (rather than DAN) functional dominance, whereas greater anger would predict the opposite. Overall, there was evidence of greater DAN (rather than DMN) functional dominance, but this pattern was modulated by current experience of specific negative emotions, as well as subclinical depressive and anxiety symptoms. Thus, greater levels of currently experienced sadness and subclinical depression independently predicted weaker DAN functional dominance (i.e., weaker DAN-FPC functional connectivity), likely reflecting reduced goal-directed attention towards the external perceptual environment. Complementarily, greater levels of currently experienced anger and subclinical anxiety predicted greater DAN functional dominance (i.e., greater DAN-FPC functional connectivity and, for anxiety only, also weaker DMN-FPC coupling). Our findings suggest that distinct affective states and subclinical mood symptoms have dissociable neural signatures, reflective of the symbiotic relationship between cognitive processes and emotional states.
Keywords: sadness, anger, functional networks
Negative mood states foster internally oriented attention and perceptual decoupling from the here-and-now (Smallwood, O’Connor, Sudbery, & Obonsawin, 2007; Smallwood, Fitzgerald, Miles, & Phillips, 2009). Nevertheless, the neural signature and unique contribution of distinct negative emotions to this effect of mood on engagement with the external world have not been identified despite their significance to both normal and pathological variations in emotional functioning. To address this issue, the present research capitalized on existing evidence that the human brain is organized into dissociable anatomical networks (Fox & Raichle, 2007), which provide a latent functional architecture that is readily recruited during goal-directed cognition (Laird et al., 2011; Smith et al., 2009). Importantly, recent investigations have documented the key role that these intrinsic functional networks play in supporting not only cognitive, but also affective processes (i.e., emotion experience and perception, cf. Touroutoglou, Lindquist, Dickerson, & Barrett, in press), including those observed during experimentally induced variations in mood states (e.g., sadness, Harrison et al., 2008) and those underlying individual differences in emotion-relevant traits (e.g., emotional reactivity, Touroutoglou, Bickart, Barrett, & Dickerson, 2014; trait anger, Fulwiler, King, & Zhang, 2012).
Of the intrinsic functional networks identified to date (e.g., van den Heuvel and Sporns, 2013), most relevant to the present investigation are two networks with activity that tends to be anti-correlated both at rest and during task. These two networks, the dorsal attention network (DAN) and the default mode network (DMN), have garnered considerable attention in the literature, and are thought support externally and internally oriented cognition, respectively (Andrews-Hanna, Smallwood, & Spreng, 2014; Corbetta & Shulman, 2002; Fox et al., 2005). The two are spatially distinct, with the DAN encompassing the dorsolateral prefrontal cortex (PFC), frontal eye fields, middle temporal motion complex, and superior parietal lobule, and the DMN incorporating the medial PFC, posterior cingulate cortex, superior frontal gyri, medial temporal lobes, and the angular gyri (Spreng et al., 2013). A third network, the frontoparietal control network (FPC), encompassing the lateral PFC, the anterior part of the inferior parietal lobule (IPL), medial superior PFC, and the anterior insula, has been recently identified as the “adjudicator” of the DAN-DMN functional competition, based on its flexible, task-driven coupling with either the DMN or the DAN (Cole et al., 2013; Spreng et al., 2010).
Individual differences in the functional architecture underlying the DMN, DAN and FPC carry significant implications for both normal and pathological variations in cognitive functioning across the lifespan (e.g., Andrews-Hanna et al., 2007; Spreng & Schacter, 2012). Moreover, they also play a role in the maladaptive affective profiles that characterize certain clinical conditions (e.g., major depression, Andrews-Hanna et al., 2014; Belleau, Taubitz, Larson, 2014; Hyett, Breakspear, Friston, Guo, Parker, 2015; Kaiser, Andrews-Hanna, Wager, Pizzagalli, 2015; Sambataro, Wolf, Pennuto, Vasic, & Wolf, 2013; van Wingen et al., 2013). For example, in a recent meta-analysis of resting state functional connectivity studies with major depression disorder (MDD) patients and healthy controls (Kaiser et al., 2015), MDD was reportedly linked to a pattern of hypoconnectivity between the DAN and the FPC and a complementary pattern of hyperconnectivity between the DMN and the FPC. In light of behavioral studies, linking negative, predominantly sad, mood states to greater internally oriented attention and perceptual decoupling from the here-and-now (Smallwood et al., 2007, 2009), Kaiser et al.’s findings raise the intriguing possibility that individual differences in DAN-FPC versus DMN-FPC connectivity patterns, suggestive of reduced attentional allocation to the external environment, relative to the inner milieu, would be related to affect in the general population and also may be a premorbid neural marker of depression.
To test this hypothesis, we assessed resting state functional interactions among the DAN, DMN, and FPC in a large sample of younger women who were part of the Human Connectome project (HCP). To shed light on the unique link between sadness and its associated functional connectivity patterns, we used participants’ reports regarding their current experience of three negative emotions (i.e., sadness, fear, and anger), which had been identified as basic constituents of affective experience (Shaver, Schwartz, Kirson, & O’Connor, 1987) and pivotal determinants of variations in optimal emotional functioning (Schimmack, 2003).
Our main goal was to elucidate whether participants who were currently experiencing greater levels of sadness would demonstrate greater DMN-FPC and weaker DAN-FPC coupling, suggestive of greater attention to the internal milieu and reduced goal-directed engagement with the external environment (cf. Spreng et al., 2010). To disentangle the effects of normal versus more pathological variations in sad mood states, we also assessed whether the aforementioned internetwork connectivity patterns would be more strongly expressed among participants who were experiencing subclinical depressive symptoms. Such a constellation of results would be broadly consistent with our proposal that variations in DAN-FPC versus DMN-FPC connectivity may be a premorbid marker of depression.
As a secondary goal, we tested whether participants who were currently experiencing greater levels of anger would evidence a pattern of DAN-FPC connectivity opposite to the one predicted for sadness. This hypothesis was based on previous findings that anger is linked to greater approach motivation (for reviews, see Carver & Harmon-Jones, 2009; Harmon-Jones et al., 2010), and, thus, arguably, associated with greater goal-directed attention towards the external perceptual environment, which, in turn, is manifest neurally as greater DAN-FPC coupling (cf. Spreng et al., 2010). We did not have any specific hypotheses regarding the effect of anger on DMN-FPC connectivity because, although rumination helps maintain angry mood states (Ray, Wilhelm, & Gross, 2008), the extent to which it makes a unique contribution to anger experiences beyond its broad contribution to negative affect is unclear. Thus, it is possible that, as predicted, anger would exert a strong positive effect on DAN-FPC coupling and a weaker, but still a positive effect on DMN-FPC coupling (cf. Ray et al., 2008).
The extant literature suggested opposing hypotheses regarding the link between individual differences in fear/anxiety and functional brain architecture. Specifically, there is evidence that fear/anxiety is associated with a motivation to withdraw (from potential environmental threats, cf. Carver & Harmon-Jones, 2009), as well as greater attentional engagement with the external environment (to scrutinize for potential threats, e.g., Baas et al., 2006; Cornwall et al., 2006). Thus, although we could not formulate any specific hypotheses regarding their associated internetwork connectivity patterns, we reasoned that it would be important to also include measures of normal and subclinical variations in fear/anxiety. Not only is fear foundational to emotional experience (Shaver et al., 1987), but, in the clinical domain, anxiety and depression often co-occur (Brown, Campbell, Lehman, Grisham, & Mancill, 2001; Joorman, Kosfelder, & Schulte, 2005; Kessler, Dupont, Berglund, & Wittchen, 1999; Sanderson, DiNardo, Rapee, & Barlow, 1990) and the severity of co-occurring anxiety has been found to influence brain function in depression (Engels et al., 2007; Heller, 1993; Heller & Nitschke, 1998; Heller, Nitschke, Etienne, & Miller, 1997; Keller et al., 2000; Nitschke, Heller, Palmieri, & Miller, 1999).
2. Method
2.1 Participants
The present study included a sample of 162 younger women (27 between 22–25, 77 between 26–30 and 58 between 31–36 years of age, see Van Essen et al., 2013 for the rationale behind this age reporting strategy in HCP data releases) from the Human Connectome Project (HCP). We used the data from these 162 participants because this sample represents the largest number of HCP female participants with available resting state fMRI and emotion data who are unrelated to each other. We opted to focus exclusively on women due to evidence of significant sex differences in both emotional experience and functional brain anatomy (Caeyenberghs & Leemans, 2014; Tomasi & Volkow, 2012; Wager, Phan, Liberzon, & Taylor, 2003), which could have thus interfered with the detection of our predicted effects.
The majority of participants (N =147) were right-handed1. All participants were screened for a history of neurological and psychiatric conditions and use of psychotropic drugs, as well as for physical conditions or bodily implants that may render their participation unsafe. Diagnosis with a mental health disorder and structural abnormalities, as revealed by the MRI structural scans, were also exclusion criteria. Participants provided informed consent in accordance with the HCP research ethics board.
2.2 Measures
Participants completed the measures described below on the day of their Session 1 fMRI appointment. Scores on all the variables were provided in the latest HCP data release.
2.2.1 Current negative emotion experience
Participants completed the NIH Toolbox Negative Affect Survey, which assesses separately sadness, anger, and fear, respectively, by requiring participants to rate their experience on the relevant dimension within the past seven days (see Appendix 1 for the complete scales). Because the NIH Anger and Fear scales each focus on the participants’ affective experience of the two relevant emotions, we used the respective composite scores as an indicator of the extent to which participants currently experience anger and fear, respectively. However, the NIH Sadness scale assesses not only inter-individual variations in sad mood states, but also in feelings of loneliness, a significant predictor of sadness (cf. Cacioppo et al., 2015) that is nonetheless strongly linked to other negative emotions as well (e.g., anger, anxiety, and boredom, cf. Mikulincer & Segal, 1990). Importantly, there is empirical evidence that loneliness and sadness are separable constructs (cf. Caccioppo et al., 2006), which are associated with divergent motivational orientations and perceptual biases. Specifically, sadness is linked to withdrawal motivation and perceptual decoupling from the here-and-now (cf. Harmon-Jones et al., 2010; Smallwood et al., 2007, 2009), whereas loneliness is associated with approach motivation (i.e., motivation to connect with other people) and increased vigilance to the external environment (cf. Cacioppo et al., 2015; Qualter et al., 2015; see Supplemental Note 1 for findings from the present data suggestive of a link between approach motivation and loneliness). Consequently, because our hypotheses regarding internetwork connectivity patterns were specific to the motivational dynamics that typify sad mood states, we created a residual “pure” sadness score by regressing out from the NIH Sadness the composite loneliness score (assessed separately through the NIH Toolbox Social Relationships survey, see Appendix 2 for the specific scale).
2.2.2 Subclinical depressive and anxiety symptoms
To assess the impact of clinically sub-threshold variations in depression and anxiety on patterns of internetwork connectivity, we used participants’ scores on the DSM-oriented depression and anxiety scales. These measures were completed as part of the Achenbach Adult Self-Report (ASR) for ages 18–59 (i.e., the 123 items from Section VIII of this instrument, Achenbach, 2009).
2.3 fMRI Data Acquisition
Images were acquired with a customized Siemens 3T “Connectome Skyra” scanner housed at Washington University in St. Louis (32-channel coil). Pulse and respiration were measured during scanning. T1-weighted anatomical scans were acquired with a 3D MP-RAGE sequence (TR = 2400 ms, TE = 2.14 ms, FOV = 224 mm, 320 x 320 matrix, 256 slices of 0.7 mm isotropic voxels). The high-resolution structural scan preceded the acquisition of functional scans.
Functional images were acquired with a multiband EPI sequence (TR=720 ms, TE=33.1 ms, flip angle=52°, FOV = 208 mm, 104 × 90 matrix, 72 slices of 2 × 2 mm in-plane resolution, 2 mm thick, no gap). Resting state scan acquisition preceded the acquisition of functional task runs. Four resting state runs of 14:33 minutes each were obtained in two distinct sessions (2 runs/session), one acquired with a left to right (L-R), and the other with a right to left (R-L), EPI phase coding sequence. During their resting state scan, participants were instructed to lie with eyes open, with “relaxed” fixation on a white cross (on a dark background), think of nothing in particular, and try not to fall asleep.
Individual L-R and R-L scans exhibit distinct regions of complete signal loss, but it has been verified that the preprocessed datasets are anatomically well-aligned with one another, even in areas of complete signal loss (cf. Smith et al., 2013). Consequently, because it is only the dropout that differs between the two scan types, it has been recommended that connectivity analyses based on resting state HCP data aggregate the respective metrics from the L-R and R-L resting state scans (cf. Smith et al., 2013; for further details, see fMRI Analysis below). For 153 of the 162 participants, we used only their Session 1 resting state scans. The remaining 7 participants had either unusable L-R (4 participants) or R-L (5 participants) Session 1 scans (i.e., either distorted images or excessively high signal values), which we replaced with the corresponding Session 2 scans.
2.4 fMRI Data Preprocessing
The present report used the FIX-denoised fMRI data, acquired during the L-R and R-L resting state runs of the Human Connectome Project (HCP) and preprocessed with the HCP spatial and temporal pipelines (Smith et al., 2013). Spatial preprocessing involved removal of spatial and gradient distortions, correction for participant movement, bias field removal, spatial normalization to the standard Montreal Neurological Institute (MNI)-152 template (2 mm isotropic voxels), intensity normalization to a global mean and masking out of non-brain voxels. Subsequent temporal preprocessing steps involved weak high-pass temporal filtering with the goal of removing linear trends in the data and the use of ICA-based artefact removal with the aim of eliminating non-neural spatiotemporal components from the data (Smith et al., 2013). We applied the following additional preprocessing steps: resampling to 4 mm isotropic voxels and smoothing (using a 3 D Gaussian kernel of FWHM = 4 mm, cf. Barch et al., 2013).
2.5 fMRI Data Analysis
2.5.1 Whole-brain analyses
The first 10 s of data (i.e., first 14 TRs) in each run were discarded to allow the MR signal to reach steady-state equilibrium. The remaining preprocessed functional data were analyzed with partial least squares (i.e., PLS, McIntosh & Lobaugh, 2004), a multivariate technique, similar to principal components analysis, that can identify whole-brain spatiotemporal patterns (latent variables; LVs) linked to correlated neuronal activity (seed PLS). An advantage of PLS over univariate seed-based correlation analyses is that the decomposition of the data matrix is performed in one analytic step, thereby circumventing the need for post-hoc correction of p-values due to multiple comparisons (McIntosh & Lobaugh, 2004). In seed PLS, activity in a reference (“seed”) region is correlated with activity in all the other brain voxels, across participants. The goal is to identify those regions for which activity is significantly correlated with activity in the seed, which, in turn, serves as our measure of functional connectivity (McIntosh & Lobaugh, 2004). Moreover, PLS also provides an estimate of how much of the covariance in the whole-brain data is accounted for by the identified pattern of functional connectivity.
Prior to the analysis, the resting state data, which combined the full L-R and R-L resting state scans, respectively, were broken down into 84 sequential “blocks” of data, each approximately 10 s in length (14 TRs). The function of this averaging process was to further minimize temporal noise (Grigg & Grady, 2010). Previous research has shown that such 10-s long blocks are adequate for performing seed-based connectivity analyses (Campbell et al., 2013; Grigg & Grady, 2010). In all the reported analyses, the significance of each LV was determined using a permutation test with 500 permutations (McIntosh & Lobaugh, 2004). In PLS, each brain voxel is assigned a weight, which reflects the respective voxel’s contribution to a specific LV. The reliability of each voxel’s contribution to a particular LV was tested by submitting all voxel weights to a bootstrap estimation (100 bootstraps, as in McIntosh & Lobaugh, 2004) of the standard errors (SEs, Efron, 1981). Clusters of activity were identified using a cluster size of at least 10 above-threshold adjacent voxels and a weight/SE ratio ≥3 (the bootstrap ratio, or BSR, p <0.005).
To investigate the functional connectivity within the DMN, FPC, and DAN, we conducted six distinct seed PLS analyses. We opted to run the seed PLS analyses separately for the L-R versus R-L data, because we sought to verify that the three networks of interest are reproduced across these datasets.
2.5.1.1 Seed regions
To identify the DMN, we focused on a region in the anterior MFPC (aMPFC) (MNI coordinates: -2 38 12, cf. Kaiser et al., 2015). We opted to do so because the connectivity pattern of this region with the remaining DMN regions appears to be most sensitive to variations in psychopathology that are linked to increased self-referential thinking. Specifically, this aMPFC region has been found to exhibit hyperconnectivity with the DMN in MDD, a disorder characterized by increased self-referential thinking (for a recent meta-analysis, see Kaiser et al., 2015)2. The seed coordinates for the DAN and FPC were averages derived from published studies (Andrews-Hanna et al., 2007; Campbell, Grady, Ng, & Hasher, 2012; Fox et al., 2006; Grady et al., 2010; Grady, Grigg, & Ng, 2013; Spreng et al., 2010, 2013; Vincent et al., 2008). Specifically, to identify the DAN, we used a seed in the SPL (MNI coordinates: 24 -60 52), because, of all the DAN regions, it reportedly exhibits the most aberrant connectivity pattern in MDD (i.e., reduced connectivity with the FPC, cf. Kaiser et al., 2015)3. Finally, to identify the frontoparietal network, we used an IPL seed (MNI coordinates: 48 -52 44), because, of all the FPC component regions, this region has been found most consistently to show a clearly differentiated functional connectivity pattern (see Spreng et al., 2013; Nelson et al., 2010).
2.5.2 Negative emotion experience and functional connectivity
To test our hypotheses regarding the link between negative emotion experience and variations in functional connectivity between the FPC and the DMN or DAN, we used one-level multivariate HLM models (HLM 7.01, Raudenbush, Bryk, & Congdon, 2013). As indicators of FPC network activity, we used the brain scores from the seed functional connectivity analysis because these scores represent the expression of network activity in each participant (brain scores are the voxel-wise sum of the activity in each voxel multiplied by the weight of that voxel on the LV). Thus, the brain scores in each of the 84 blocks were regarded as indicators of joint recruitment of the FPC network component regions, thereby yielding 84 intrinsic connectivity indicators. Greater brain score values reflected greater coherent recruitment of the FPC components. To probe the functional connectivity between the DMN and the FPC, we conducted a regression analysis, predicting, for each of the 84 blocks, the mean signal change in the aMPFC seed from the FPC brain scores. Likewise, to investigate the functional connectivity between the DAN and the FPC, we regressed the mean percent signal change in the SPL seed on the FPC brain scores in each of the 84 blocks. The FPC brain scores were used as the predictors because of the FPC’s presumed role as the switch that engages/disengages other networks, such as the DMN and DAN. Following recommendations on HCP data analysis (Smith et al., 2013), for the aMPFC and SPL seeds, we used the averaged mean percent signal value from the L-R and R-L scans. Similarly, for the FPC, we used the averaged brain scores obtained from the IPL-based seed PLS analyses involving the L-R and R-L resting state data, respectively. In all analyses, scores on currently experienced sadness, anger, and fear, together with depression and anxiety scores on the DSM-oriented scales, were introduced as level-1 predictors of the association between FPC brain scores and the mean percent signal change in the aMPFC and SPL seeds, respectively.
2.6 Brain and behavior analyses
2.6.1 Standardization of the behavioral variables
Because the emotion measures had different rating ranges, we standardized participants’ scores on these variables. For the “pure” sadness score, we used the standardized residual obtained after regressing out participants’ loneliness scores from their original sadness score. These z-scores are used in all the reported analyses.
3. Results
3.1 Preliminary Analyses
3.1.1 Behavioral variables
In line with extant literature on the high comorbidity of depressive and anxiety symptoms (cf. Clark, 1989; Clark & Watson, 1991; Mineka et al., 1998), depression and anxiety scores from the DSM-oriented scales were highly correlated, r(161) = .71, p < .0001. In turn, scores on both DSM-oriented scales were strongly correlated with scores on all three variables of current emotional experience (rs ranging from .47 to .61, all ps < .0001). Finally, scores on the three emotion experience variables were also positively interrelated (rs ranging from .46 to .66, all ps < .0001).
3.1.2 Brain variables
3.1.2.1 Seed PLS
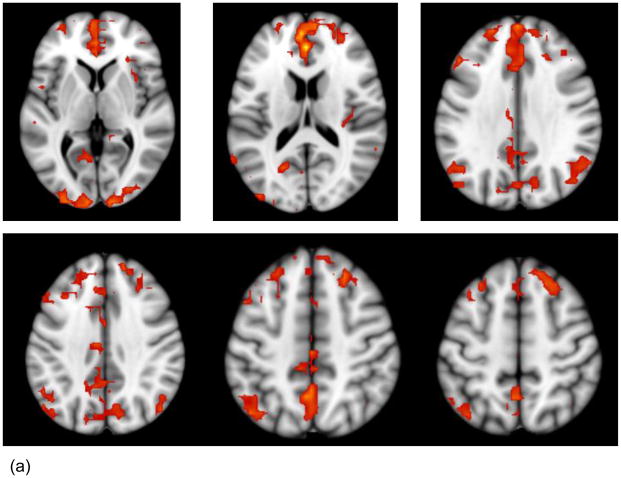
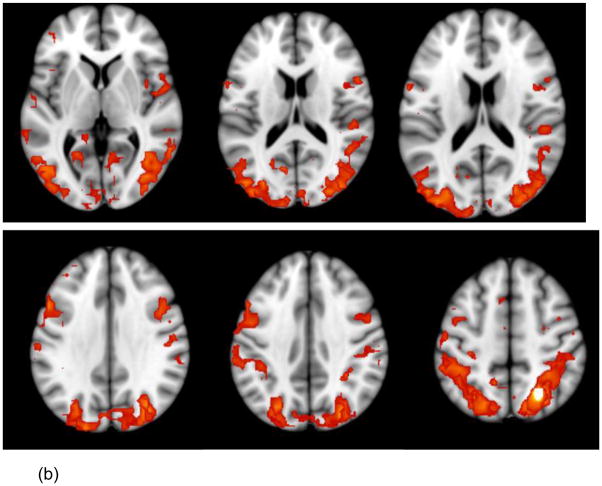
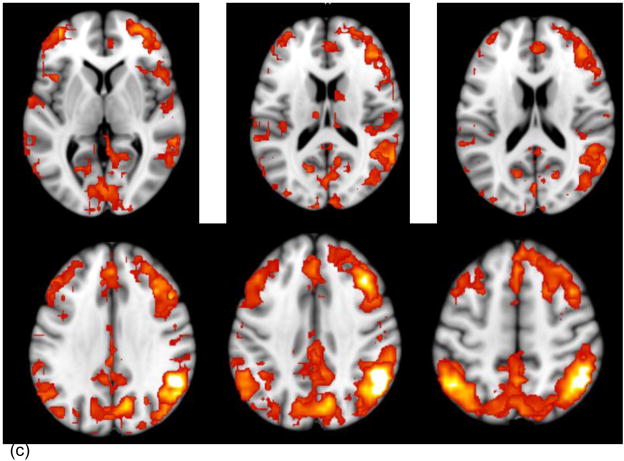
Across both types of resting state data (i.e., L-R and R-L phase encoding), the aMPFC-, SPL-, and IPL-based seed PLS analyses each revealed significant patterns of functional connectivity (p < .002), which were reliable across all scrutinized blocks, accounted for 86.21 to 89.57% of the total variance in the data and were consistent with the DMN, DAN and FPC, respectively (see Table 1 and Figure 1, a–c).
Table 1.
Clusters of Activity Observed in the Canonical DMN, FPC and DAN regions in the aMPFC-based, IPL-based, and SPL-based Seed PLS Analyses of the Resting State Data
| X | Y | Z | BSR | Cluster size | |
|---|---|---|---|---|---|
| DMN | |||||
|
|
|||||
| R/M Anterior medial prefrontal cortex | 0 | 40 | 12 | 1105.20 | 531 |
| R Medial frontal gyrus | 24 | 32 | 32 | 4.57 | 24 |
| L Inferior frontal gyrus | −48 | 28 | 32 | 6.13 | 41 |
| R Inferior frontal gyrus | 28 | 16 | −12 | 4.88 | 28 |
| L Angular gyrus | −44 | −68 | 48 | 5.96 | 142 |
| R Angular gyrus | 48 | −72 | 36 | 5.44 | 76 |
| L Superior temporal gyrus | −48 | 4 | 0 | 4.84 | 16 |
| R Superior temporal gyrus | 40 | 12 | −20 | 5.48 | 70 |
| L Superior frontal gyrus | −36 | 20 | 52 | 4.9 | 24 |
| L Posterior cingulate cortex | −8 | −60 | 4 | 4.48 | 17 |
| R Parahippocampal gyrus | 16 | −36 | 8 | 4.02 | 10 |
|
| |||||
| FPC | |||||
|
|
|||||
| L Middle frontal gyrus | −40 | 52 | 4 | 9.12 | 436 |
| R Middle frontal gyrus | 44 | 24 | 40 | 11.05 | 1100 |
| L Inferior parietal lobule | −52 | −64 | 44 | 11.61 | 428 |
| R Inferior parietal lobule/Supramarginal gyrus | 52 | −20 | 36 | 4.95 | 39 |
| L Insula | −40 | 16 | 4 | 5.31 | 21 |
| R Insula | 36 | −32 | 16 | 4.56 | 51 |
|
| |||||
| DAN | |||||
|
|
|||||
| L MT+ | −52 | −48 | 12 | 5.39 | 32 |
| R MT+ | 56 | −64 | 0 | 6.53 | 114 |
| L Precentral gyrus | −56 | −16 | 8 | 4.60 | 28 |
| R Precentral gyrus | 44 | 8 | 28 | 6.04 | 94 |
| 28 | −8 | 52 | 5.22 | 45 | |
| L Superior parietal lobe | −20 | −40 | 64 | 5.55 | 12 |
| L Superior occipital gyrus | −12 | −100 | 16 | 5.36 | 24 |
Note. L = left. R = right. BSR = bootstrap ratio. Listed are the largest spatial extent clusters of activity in the canonical DMN, FPC and DAN regions. All the DMN, FPC, and DAN regions, respectively, that contained activity clusters ≥10 voxels at a BSR ≥3 are pictured in Figure 1.
Figure 1.
Regions showing significant positive activations (BSR [Bootstrap ratio] > 3, p < .0027 with the network seeds are shown on axial slices from the MNI152 average structural brain. The images combine the areas obtained from the relevant seed PLS analyses conducted on the L-R and R-L datasets. The panels show the regions whose activity is significantly positively correlated with the aMPFC (a) SPL (b) and IPL (c) seed, respectively.
3.1.2.1.1 Within network connectivity
For control purposes, we conducted three multivariate HLM analyses, predicting DMN, DAN and FPC brain scores from scores on our emotion variables. Results of these analyses revealed that higher levels of currently experienced fear predicted greater intrinsic connectivity in both the DAN, b = .18, SE = .08, t(156) = 2.32, p = .02, and the FPC, b = .20, SE = .08, t(156) = 2.43, p = .02. No other effects emerged as significant (all other ps > .10).
3.1.2.2 Standardization
Because the FPC brain scores and mean percent signal values for the aMPFC and SPL seeds, respectively, were derived from different analyses, we first standardized these variables within each dataset (i.e., R-L versus L-R). Subsequently, to create an index of FPC recruitment, as well as of mean activity in the aMPFC and SPL seeds across the two datasets, we averaged the R-L and L-R values for the FPC brain scores, as well as for the percent signal change in the aMPFC and SPL, respectively. Subsequently, we added a constant to render positive the value of all the scrutinized brain variables. Finally, in order to test our hypotheses regarding DAN versus DMN functional dominance as a function of emotion variables, we created a ratio of SPL to aMPFC activity, where higher positive values indicate relatively greater SPL, rather than aMPFC, activity.
3.2 Negative Emotion Experience and DAN versus DMN Functional Dominance
A regression analysis predicting the relative activity of the SPL versus the aMPFC seed (i.e., the ratio of SPL to aMPFC activity, with higher values indicating greater SPL, rather than aMPFC recruitment) as a function of FPC engagement and scores on negative emotion experience, as well as the DSM-oriented scales, revealed that, overall, the FPC was more strongly coupled with the SPL, rather than the aMPFC. Thus, greater FPC recruitment predicted greater SPL, rather than aMPFC, activity (there was a significantly positive value of the coefficient for β10 in regression 1, Table 2). This finding suggests that overall, participants showed neural markers indicative of greater DAN, rather than DMN, functional dominance in FPC interactions.
Table 2.
Multivariate HLM Regression Analyses Predicting Mean Activity Level in the aMPFC and SPL Seed, respectively, from FPC Brain Scores and Individual Differences in Negative Emotion Experience
| Fixed Effect | Coefficient | Standard Error | t-value (dfs) |
|---|---|---|---|
| 1. Outcome: SPL/aMPFC relative activity | |||
|
|
|||
| For overall INTERCEPT, β0 | |||
| INTERCEPT, β00 | 1.032 | .020 | 52.79 (156)*** |
| Sadness SLOPE, β01 | .001 | .024 | .02 (156) |
| Anger SLOPE, β02 | −.009 | .027 | −.35 (156) |
| Fear SLOPE, β03 | −.017 | .030 | −.54 (156) |
| DSM Depression SLOPE, β04 | .017 | .029 | .58 (156) |
| DSM Anxiety SLOPE, β05 | .040 | .030 | 1.34 (156) |
| For FPC Brain Scores SLOPE, β1 | |||
| INTERCEPT, β10 | .028 | .003 | 10.10 (13596)*** |
| Sadness SLOPE, β11 | −.013 | .004 | −3.67 (13596)*** |
| Anger SLOPE, β12 | .014 | .004 | 3.84 (13596)*** |
| Fear SLOPE, β13 | −.003 | .004 | −.63 (13596) |
| DSM Depression SLOPE, β14 | −.017 | .004 | −3.98 (13596)*** |
| DSM Anxiety SLOPE, β15 | .016 | .005 | 3.52 (13596)*** |
|
|
|||
| 2. Outcome: SPL seed mean activity | |||
|
|
|||
| For overall INTERCEPT, β0 | |||
| INTERCEPT, β00 | 4.503 | .047 | 94.13 (156)*** |
| Sadness SLOPE, β01 | .058 | .059 | 1.00 (156) |
| Anger SLOPE, β02 | −.065 | .065 | −1.00 (156) |
| Fear SLOPE, β03 | −.067 | .076 | −.89 (156) |
| DSM Depression SLOPE, β04 | .042 | .071 | .59 (156) |
| DSM Anxiety SLOPE, β05 | .070 | .073 | .96 (156) |
| For FPC Brain Scores SLOPE, β1 | |||
| INTERCEPT, β10 | .352 | .008 | 46.54 (13596)*** |
| Sadness SLOPE, β11 | −.036 | .010 | −3.76 (13596)*** |
| Anger SLOPE, β12 | .057 | .010 | 5.62 (13596)*** |
| Fear SLOPE, β13 | .007 | .011 | .62 (13596) |
| DSM Depression SLOPE, β14 | −.075 | .012 | −6.42 (13596)*** |
| DSM Anxiety SLOPE, β15 | .027 | .012 | 2.20 (13596)* |
|
|
|||
| 3. Outcome: aMPFC seed mean activity | |||
|
|
|||
| For overall INTERCEPT, β0 | |||
| INTERCEPT, β00 | 4.499 | .052 | 86.86 (156)*** |
| Sadness SLOPE, β01 | .018 | .063 | .29 (156) |
| Anger SLOPE, β02 | .040 | .071 | .56 (156) |
| Fear SLOPE, β03 | −.018 | .082 | −.21 (156) |
| DSM Depression SLOPE, β04 | −.078 | .077 | −1.02 (156) |
| DSM Anxiety SLOPE, β05 | −.045 | .79 | −.57 (156) |
| For FPC Brain Scores SLOPE, β1 | |||
| INTERCEPT, β10 | .209 | .007 | 30.28 (13596)*** |
| Sadness SLOPE, β11 | .003 | .009 | .39 (13596) |
| Anger SLOPE, β12 | .012 | .009 | 1.29 (13596) |
| Fear SLOPE, β13 | .027 | .010 | 2.65 (13596)** |
| DSM Depression SLOPE, β14 | −.003 | .011 | −.27 (13596) |
| DSM Anxiety SLOPE, β15 | −.047 | .011 | −4.24 (13596)*** |
Note.
p < .05,
p < .01,
p < .001.
Nevertheless, this effect was modulated by emotion experience (see regression 1, Table 2). Specifically, as predicted, the global neural pattern of DAN functional dominance (as per the significantly positive value of the coefficient for β10 in regression 1, Table 2) was more weakly expressed among participants who were currently experiencing greater levels of sadness, b = −.013, SE = .004, t(13596) = −3.67, p < .01, as well as among participants who scored higher on the DSM-oriented depression scale, b = −.017, SE = .004, t(13596) = −3.98, p < .001. In contrast, but also consistent with our hypotheses, participants who reported greater levels of currently experienced anger demonstrated a stronger pattern of DAN functional dominance, b = .014, SE = .004, t(13596) = 3.84, p < .001. Beyond these predicted effects, we also found evidence of stronger DAN functional dominance among participants scoring higher on the DSM-oriented anxiety scale, b = .016, SE = .005, t(13594) = 3.52, p < .001 (see Supplemental Note 2 for the replication of DAN versus DMN functional dominance patterns as a function of emotion in the R-L versus L-R datasets). Subsequently, to elucidate the mechanisms underlying the DAN versus DMN functional dominance effects, we conducted two separate multivariate HLM analyses predicting mean activity levels in the SPL and aMPFC seed, respectively, from FPC brain scores and scores on the emotion-related variables (see regressions 2 and 3, respectively, Table 2). We present these results separately for each emotion variable (see Supplemental Note 3 for results of the analyses incorporating general cognitive function, positive affect and Big Five personality factors as additional control variables).
3.2.1 Sadness
The neural pattern of weaker DAN functional dominance, demonstrated by participants currently experiencing greater levels of sadness was due to the weaker SPL-FPC connectivity observed in these participants, b = −.036, SE = .010, t(13596) = −3.76, p < .001.
3.2.2 Anger
The neural pattern of greater DAN functional dominance, showed by participants who reported greater levels of currently experienced anger was due to the stronger SPL-FPC coupling observed among higher anger scorers, b = .057, SE = .010, t(13596) = 5.62, p < .001.
3.2.2 Subclinical depressive symptoms
The neural pattern of weaker DAN functional dominance, demonstrated by participants who reported more depressive symptoms was due to the weaker SPL-FPC connectivity observed among higher scorers, b = −.075, SE = .012, t(13596) = −6.42, p < .001.
3.2.4 Subclinical anxiety symptoms
The neural pattern of greater DAN functional dominance, showed by participants who reported greater anxiety symptoms, was due to the weaker aMPFC-FPC connectivity, b = −.047, SE = .011, t(13596) = −4.24, p < .001, and stronger SPL-FPC coupling, b = .027, SE = .012, t(13596) = 2.20, p = .027, observed in these participants.
3.3 Post-hoc Findings
Results of the multivariate HLM analysis predicting mean activity levels in the aMPFC seed from FPC brain scores and scores on the emotion-related variables revealed an unexpected positive relationship between greater levels of currently experienced fear and greater aMPFC-FPC connectivity, b = .027, SE = .010, t(13596) = 2.65, p < .01 (see regression 3, Table 2). Of note, though, there was no evidence that greater levels of currently experienced fear were linked to overall greater DMN functional dominance (see regression 1, Table 1). We will elaborate on the potential significance of this finding in the Discussion.
4. Discussion
To the best of our knowledge, the current research is the first to document a significant link between naturally occurring normal and subclinical variations in negative emotion experience and resting state functional neural architecture. Overall, the FPC exhibited positive coupling with both the DMN and the DAN seeds, although the latter link appeared to be stronger, thus implying a bias towards externally oriented attention among healthy individuals observed at rest. Most likely, this global pattern of DAN functional dominance is, at least partly, accounted for by the fact that during the acquisition of the resting state scans in the HCP, participants were required to focus on an external visual stimulus (i.e., keep their eyes open with a relaxed focus on a fixation cross).
Importantly, though, the expression of the aforementioned global pattern of DAN functional dominance was moderated by variations in the experience of sadness and anger, as well as subclinical variations in depressive and anxiety symptoms. Thus, in line with their dichotomous motivational orientations (cf. Harmon-Jones et al., 2010), greater levels of currently experienced sadness versus anger predicted weaker versus stronger patterns of DAN functional dominance (i.e., weaker versus stronger patterns of SPL-FPC intrinsic connectivity), reflective of less versus greater goal-directed attention towards the external perceptual environment. Of note, SPL-FPC connectivity patterns in particular appeared to be sensitive to the distinction between normal versus subclinical variations in sad mood states, since the pattern of weaker SPL-FPC coupling, linked to greater levels of currently experienced sadness, appeared to be more strongly expressed by individuals who showed subclinical depressive symptoms. Finally, although not predicted, but nonetheless consistent with previous empirical accounts on the role of anxiety in facilitating sensory processing of environmental threats and swift activation of defense mechanisms (Baas et al., 2006; Cornwall et al., 2006), we found that individuals showing subclinical anxiety symptoms demonstrated a pattern of greater DAN functional dominance (i.e., weaker aMPFC-FPC and stronger SPL-FPC connectivity) suggestive of increased vigilance to the external environment.
Interestingly, the hypothesized link between greater self-reported sadness and stronger aMPFC-FPC connectivity, assumed to be indicative of heightened attention to the internal milieu, did not reach conventional levels of statistical significance. One interpretation is that the increased attention to the internal environment, which typifies depressive rumination and is associated with increased DMN intrinsic connectivity (Berman et al., 2011; Sheline et al., 2009) and stronger DMN-FPC functional coupling during internally-directed tasks (cf. Spreng et al., 2010), is primarily triggered by exposure to emotionally evocative stimuli and is more weakly expressed under emotionally unarousing circumstances, such as the ones encompassed by the resting state environment. Indeed, consistent with this proposal, Berman and colleagues (2014) reported significantly increased DMN intrinsic connectivity among depressed individuals during an induced ruminative period, relative to rest. In light of these findings, it seems plausible that among healthy individuals experiencing sad mood states, the hypothesized DMN-FPC connectivity pattern would emerge as reliably significant only with exposure to emotionally relevant stimuli or circumstances triggering a ruminative episode. Future studies are needed to test this hypothesis.
On a related note, although behavioral research suggests a significant role for rumination in maintaining angry mood states (Ray et al., 2008), we found no evidence of a significant link between greater levels of currently experienced anger and aMPFC-FPC coupling. Like sadness, it may be that exposure to emotionally charged situations is necessary to trigger the ruminative processing, supported by the DMN, which has been linked to angry mood episodes. Under such circumstances, ruminative processing may either have the function to amplify angry mood states or be triggered in an attempt to inhibit, at least temporarily, more destructive behavioral expressions of anger. Indeed, with respect to the latter proposal, it has been suggested that rumination may be particularly likely to accompany anger under circumstances in which behavioral expression of aggression (i.e., the approach behavior that is traditionally linked to angry mood states, cf. Hortensius, Schutter, & Harmon-Jones, 2012) is either dangerous or socially inappropriate and thus needs to be inhibited (Zinner, Brodish, Devine, & Harmon-Jones, 2008). Under such circumstances, when engagement in rumination may be the safe alternative to aggression, there is the possibility that the pattern of DAN functional dominance, linked to anger in our study, may be weakened because of an increase in DMN-FPC connectivity. Future studies are needed to test these hypotheses.
Interestingly, of all three negative emotions, it was only fear that was significantly linked to variations in aMPFC-FPC coupling. Although not predicted (which is why we are hesitant to interpret it extensively), such an effect is nonetheless consistent with the well-documented role of worry in anxious/fearful mood states (e.g., anxious apprehension, cf. Engels et al., 2010). The link between currently experienced levels of fear and aMPFC-FPC coupling becomes even more intriguing in light of the negative association between subclinical anxiety symptoms and aMPFC-FPC coupling. Thus, one may speculate that, while normal variations in the experience of fear are linked to greater worry-related ruminative processing and thus greater aMPFC-FPC coupling, more clinically relevant variations in fear/anxiety are linked to hyper-vigilance to the external environment and reduced self-referential thinking. Whether patterns of DMN-FPC coupling may be an adequate index of the distinction between normal versus clinical variations in fear/anxiety remains a question for future research. Similarly, future investigations are needed to shed light on whether the pattern of greater within-network connectivity in the DAN and FPC, respectively, which was observed among individuals experiencing greater levels of fear may represent a neural precursor of the pattern of heightened SPL-FPC connectivity documented among individuals who reported subclinical anxiety symptoms.
The most significant contribution of the present research is the finding of a robust link between greater levels of currently experienced sadness, as well as current subclinical depressive symptoms and weaker DAN functional dominance, specifically, weaker SPL-FPC coupling, suggestive of chronically reduced goal-directed attention to the external environment. We regard this finding as particularly important because of its potential relevance for research on the social functioning deficits associated with depression. Specifically, there is extensive evidence that depressed individuals show reduced social competence (Levendosky, Okun, & Parker, 1995), have fewer social interactions (Gotlib & Lee, 1989), and find these encounters less rewarding and less enjoyable than do non-depressed individuals (Nezlek Hampton, & Shean, 2000). It has been suggested that core to these social difficulties are reduced sensitivity and impaired decoding of both static and dynamic nonverbal emotional cues (Bylsma et al., 2008; Lee, Harkness, Sabbagh, & Jacobson, 2005; Nesse, 2000; Rottenberg, 2005; Rottenberg & Gross, 2007; Schneider et al., 2012), which further lead to deficits in empathizing with other social actors (Donges et al., 2005; Fujino et al., 2014; Schneider et al., 2012). Interestingly, in the context of this literature, the present data raise the possibility that the reduced SPL-FPC functional connectivity, observed not only among healthy women experiencing greater levels of sadness, but also among those evidencing subclinical depressive symptoms may constitute the neural mechanism underlying the aforementioned depression-related inattention to nonverbal social-affective cues (Bylsma et al., 2008; Lee et al., 2005; Nesse, 2000; Rottenberg, 2005; Rottenberg & Gross, 2007; Schneider et al., 2012). Tests of this hypothesis entail investigation of several intermediary assumptions. At minimum, the following would need to be established: 1.The sadness- and subclinical depressive symptoms-associated pattern of weaker SPL-FPC connectivity is indeed predictive of nonverbal cue decoding performance among clinical and nonclinical populations, and 2. Variations in intrinsic SPL-FPC connectivity constitute a neural marker of vulnerability to clinical depression. Interestingly, preliminary supportive evidence for the latter hypothesis can be derived from our findings that weaker SPL-FPC connectivity, although demonstrated both by women currently experiencing greater levels of sadness and those evidencing subclinical depressive symptoms, it was much strongly expressed among the latter. Alternatively, it is possible that the weaker SPL-FPC coupling, linked in our study to greater sadness and greater depressive symptoms, represents a satellite neural mechanism, whose expression tracks with the progression from nonclinical to clinical sadness and is predictive of the associated functional deficits, but is nonetheless distinct from the core mechanisms that typify clinical depression and predict progression from transient sad mood states to psychopathology.
The divergent DAN functional dominance (i.e., SPL-FPC connectivity) patterns, identified for sadness and anger, respectively, also carry significant implications for behavioral research on self-regulatory strategies aimed at down-regulating these emotions. Specifically, our findings suggest that the experience of anger versus sadness may be linked to differential motivational salience of the external perceptual environment (i.e., heightened for anger, but impoverished for sadness). As such, self-regulatory strategies that mute the sensorial vividness and self-relevance of the external environment may prove successful in stifling angry mood states and their associated behavioral outbursts of aggression (cf. Hortensius et al., 2012). In contrast, self-regulatory strategies that enhance the salience of the external perceptual environment and trigger purposeful engagement with it may be relieve sad mood state episodes. For example, to the extent that physical activities necessitate externally oriented attention in order to facilitate harmonious integration with the outer environment (e.g., for navigation, movement coordination), our latter proposal is compatible with recent evidence that engagement in physical exercise is successful in ameliorating the affective symptoms of depression (Booij, Bos, de Jonge, & Oldehinkel, 2015; Schuch et al., 2015).
The finding of a common pattern of increased DAN functional dominance (i.e., greater SPL-FPC connectivity), linked to both anger and subclinical anxiety, resonates well with previous research on the structure of self-reported mood and emotions which documented a link between anger and fear. Specifically, in factor analyses of mood and emotion data, anxiety and anger items have been found to load on the same factor (for a discussion, see Carver & Harmon-Jones, 2009), which led some researchers to propose that negative emotions have common determinants (e.g., Watson, Wise, Vaidya, & Tellegen, 1999). Interestingly, our present study identifies one such potential source of common variance in anxiety and anger, respectively, in the observed pattern of increased SPL-FPC connectivity, implying greater goal-directed attentional engagement with the external environment, that was linked to both in our study. Future research is needed to test the viability of this hypothesis.
Intriguing as they may be, the present findings are worthy of additional empirical probing. First, because our sample included only female participants, future studies need to verify that our findings generalize to males. Second, our research has a correlational design, which precludes any strong causal claims regarding the reported effects. Consequently, future studies, using in-scanner experimental mood manipulations and assessment of the resulting changes in inter-network connectivity patterns (e.g., by employing dynamic causal modeling methods) would be important in elucidating the mechanisms and functionality of the effects described in the present report. Third, in the present study, participants were not asked to report on their habitual emotional experience, but rather on their emotional experience within a clearly delineated time interval (i.e., seven days prior to the fMRI session), which, importantly, extends beyond the traditional temporal windows linked to mood states (of several hours up to a day – e.g., Crocker et al., 2012; Simmons, Wills, & Neal, 2014). As such, these indicators assess the participants’ current experience of specific emotions, using a time frame that renders it difficult to disentangle emotional state from trait effects in the strictest sense. Consequently, a valuable contribution to the literature would be a direct comparison of state versus trait effects on internetwork connectivity patterns by investigating individuals predisposed towards experiencing sadness or anger when current experience of the scrutinized emotions is held constant across the examined sample (e.g., in the clinical domain, by comparing remitted versus “in episode” depressed patients).
Capitalizing on a well-documented dissociation between intrinsic functional neural networks, supporting internally versus externally oriented cognition, our study provided evidence that variations in such latent brain architecture are robustly linked to variations in the experience of negative emotions, specifically, sadness and anger, as well as subclinical depression and anxiety. Particularly encouraging is the finding that our reported sadness-related neural signature, specifically the SPL-FPC functional link, appeared to be sensitive to the distinction between normal versus subclinical variations in sad mood states, thereby giving us confidence in its potential value for future clinical work as an assay of the efficacy of mood-directed intervention techniques.
Supplementary Material
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (MOP14036), the Canada Research Chairs program, the Ontario Research Fund, the Canadian Foundation for Innovation, and the Heart and Stroke Foundation Centre for Stroke Recovery. The authors would like to thank the Baycrest MRI technologists for technical assistance. The authors also would like to thank the following people for their generosity in support of the imaging centre at Baycrest: Jack & Anne Weinbaum, Sam & Ida Ross, Joseph & Sandra Rotman.
Footnotes
The HCP uses a continuous measure of handedness, with scores ranging from −100 (completely left-handed) to 100 (completely right-handed). Negative scores indicate that the participant is more left-handed than right-handed. Positive scores indicate the participant is more right-handed than left-handed. In our sample, 147 of the 162 participants had handedness scores of 10 or greater, indicating that they were more right- than left-handed. Eliminating the 15 mostly left-handed participants or introducing handedness as a covariate in our analyses did not change any of the reported results. Consequently, for the sake of simplicity, we report the analyses in which handedness scores are not introduced as a covariate.
Although for the reasons outlined in the text, we used Kaiser et al.’s (2015) aMPFC seed in our hypothesis testing analyses, we ran three additional tests, using the coordinates of the MPFC regions, found by Andrews-Hanna et al. (2010) to participate in the DMN core subsystem (MNI -6 52 -2), the dMPFC subsystem (MNI 0 52 26) and the MTL subsystem (MNI 0 26 -18). The pattern of results, reported in the main text, was replicated in each of the analyses using these MFPC seeds (although the results tended to be stronger with the aMPFC seed used in the main analyses). Thus, consistent with Andrews-Hanna et al.’s results that all the DMN subsystems contribute to internally oriented cognition, these results would suggest that along the axis of internally versus externally oriented cognitions, the different DMN subsystems (versus the DAN) play similar roles with respect to the emotion variables used in our study.
The posterior parietal area, found by Kaiser et al. (2015) to show the greatest connectivity abnormalities in MDD, was quite extensive, spanning across both FPC and DAN (e.g., SPL) regions. Consequently, to identify the DAN, we used a smaller seed region, encompassing only the SPL.
The authors declare no competing financial interests.
Contributor Information
Raluca Petrican, Rotman Research Institute, Toronto, Ontario, M6A 2E1.
Cristina Saverino, Toronto Rehabilitation Institute, University of Toronto, Toronto, Ontario, M5G 2A2.
R. Shayna Rosenbaum, Department of Psychology, York University and Rotman Research Institute, Toronto, Ontario, M6A 2E1.
Cheryl Grady, Rotman Research Institute and Department of Psychology and Psychiatry, University of Toronto, Toronto, Ontario, M6A 2E1.
References
- Achenbach TM. The Achenbach System of Empirically Based Assessment (ASEBA): Development, Findings, Theory and Applications. University of Vermont Research Center for Children, Youth and Families; Burlington, VT: 2009. [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood JS, Spreng RN. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Annals of New York Academy of Sciences - Year in Cognitive Neuroscience Special Issue. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas JMP, Milstein J, Donlevy M, Grillon C. Brainstem correlates of defensive states in humans. Biological Psychiatry. 2006;59:588–593. doi: 10.1016/j.biopsych.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, et al. Function in the human connectome: Task-fMRI and individual differences in behavior. NeuroImage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleau EL, Taubitz LE, Larson CL. Imbalance of default mode and regulatory networks during externally focused processing in depression. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu117. Published online before print October 1, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Misic B, Buschkuehl M, Kross, … Jonides J. Does resting-state connectivity reflect depressive rumination? A tale of two analyses. NeuroImage. 2014;103:267–279. doi: 10.1016/j.neuroimage.2014.09.027. [DOI] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Social Cognitive and Affective Neuroscience. 2011;6:548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, … Prabhakaran V. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. NeuroImage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij SH, Bos EH, de Jonge P, Oldehinkel AJ. Markers of stress and inflammation as potential mediators of the relationship between exercise and depressive symptoms: Findings from the TRAILS study. Psychophysiology. 2015;52:352–358. doi: 10.1111/psyp.12329. [DOI] [PubMed] [Google Scholar]
- Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM–IV anxiety and mood disorders in a large clinical sample. Journal of Abnormal Psychology. 2001;110:585–599. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, Cole SW, Capitanio JP, Goossens L, Boomsma DI. Loneliness across phylogeny and a call for animal models. Perspectives on Psychological Science. 2015;10:202–212. doi: 10.1177/1745691614564876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Ernst JM, Burleson M, Berntson GG, Nouriani B, Spiegel D. Loneliness within a nomological net: An evolutionary perspective. Journal of Research in Personality. 2006;40:1054–1085. [Google Scholar]
- Caeyenberghs K, Leemans A. Hemispheric Lateralization of Topological Organization in Structural Brain Networks. Human Brain Mapping. 2014;35:4944–4957. doi: 10.1002/hbm.22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Grady CL, Ng C, Hasher L. Age differences in the frontoparietal cognitive control network: Implications for distractibility. Neuropsychologia. 2012;50:2212–2223. doi: 10.1016/j.neuropsychologia.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Grigg O, Saverino C, Churchill N, Grady CL. Age differences in the intrinsic functional connectivity of default network subsystems. Frontiers in Aging Neuroscience. 2013;5:73. doi: 10.3389/fnagi.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Harmon-Jones E. Anger is an approach-related affect: Evidence and implications. Psychological Bulletin. 2009;135:183–204. doi: 10.1037/a0013965. [DOI] [PubMed] [Google Scholar]
- Clark LA. Temperament as a unifying basis for personality and psychopathology. Journal of Abnormal Psychology. 2005;11:505–521. doi: 10.1037/0021-843X.114.4.505. [DOI] [PubMed] [Google Scholar]
- Clark LA. The anxiety and depressive disorders: Descriptive psychopathology and differential diagnosis. In: Kendall PC, Watson D, editors. Anxiety and depression: Distinctive and overlapping features. San Diego, CA: Academic Press; 1989. pp. 83–129. [Google Scholar]
- Clark LA, Watson D. Theoretical and empirical issues in differentiating depression from anxiety. In: Becker J, Kleinman A, editors. Psychosocial aspects of depression. Hillsdale, NJ: Erlbaum; 1991. pp. 39–65. [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology. 1994;103:103–116. [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience. 2013;16:1348–1355. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Influence of extraversion and neuroticism on subjective wellbeing: Happy and unhappy people. Journal of Personality and Social Psychology. 1980;38:668–678. doi: 10.1037//0022-3514.38.4.668. [DOI] [PubMed] [Google Scholar]
- Costa PT, Somerfield MR, McCrae RR. Personality and coping: A reconceptualization. In: Zeidner M, Endler NS, editors. Handbook of Coping: Theory, Research, Applications. Oxford: Wiley; 1986. pp. 44–61. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers & Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crocker LD, Heller W, Spielberg JM, Warren SL, Bredemeir K, … Miller GA. Neural mechanisms of attentional control differentiate trait and state negative affect. Frontiers in Psychology. 2012;3:298. doi: 10.3389/fpsyg.2012.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Baas JMP, Johnson L, Holroyd T, Carver FW, Lissek S, Grillon C. Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography. Neuroimage. 2007;37:282–289. doi: 10.1016/j.neuroimage.2007.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donges US, Kersting A, Dannlowski U, Lalee-Mentzel J, Arolt V, Suslow T. Reduced awareness of others? Emotions in unipolar depressed patients. Journal of Nervous Mental Disorders. 2005;193:331–337. doi: 10.1097/01.nmd.0000161683.02482.19. [DOI] [PubMed] [Google Scholar]
- Efron B. Nonparametric estimates of standard error: The jackknife, the bootstrap, and other methods. Biometrika. 1981;68:589–599. [Google Scholar]
- Engels AS, Heller W, Spielberg JM, Warren SL, Sutton BP, Banich MT, Miller GA. Co-occurring anxiety influences patterns of brain activity in depression. Cognitive Affective and Behavioral Neuroscience. 2010;10:141–156. doi: 10.3758/CABN.10.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels AS, Heller W, Mohanty A, Herrington JD, Banich MT, Webb AG, Miller GA. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology. 2007;44:352–363. doi: 10.1111/j.1469-8986.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Human Brain Mapping. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino J, Yamasaki N, Miyata J, Kawada R, Sasaki H, Matsukawa N, … Murai T. Altered brain response to others’ pain in major depressive disorder. Journal of Affective Disorders. 2014;165:170–175. doi: 10.1016/j.jad.2014.04.058. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, King JA, Zhang N. Amygdala–orbitofrontal resting-state functional connectivity is associated with trait anger. NeuroReport. 2012;23:606–610. doi: 10.1097/WNR.0b013e3283551cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic Resonance in Medicine. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Lee CM. The social functioning of depressed patients: A longitudinal assessment. Journal of Social and Clinical Psychology. 1989;8:223–237. [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, et al. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cerebral Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EK, Watson D. Emotion, mood, and temperament: Similarities, differences, and a synthesis. In: Payneand RL, Cooper CL, editors. Emotions at Work: Theory, Research, and Applications in the Management. Chichester: JohnWiley & Sons, Ltd; 2001. pp. 21–43. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg O, Grady CL. Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PLoS ONE. 2010;5:e13311. doi: 10.1371/journal.pone.0013311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biological Psychiatry. 2010;84:451–462. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yucel M. Modulation of Brain Resting-State Networks by Sad Mood Induction. PLoS ONE. 2008;3:e1794. doi: 10.1371/journal.pone.0001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W. Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology. 1993;7:476–489. [Google Scholar]
- Heller W, Nitschke JB. The puzzle of regional brain activity in depression and anxiety: The importance of subtypes and comorbidity. Cognition & Emotion. 1998;12:421–447. [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, Miller GA. Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology. 1997;106:376–385. doi: 10.1037//0021-843x.106.3.376. [DOI] [PubMed] [Google Scholar]
- Hortensius R, Schutter D, Harmon-Jones E. When anger leads to aggression: Induction of relative left frontal cortical activity with transcranial direct current stimulation increases the anger-aggression relationship. Social Cognitive and Affective Neuroscience. 2012;7:342–347. doi: 10.1093/scan/nsr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyett MP, Breakspear MJ, Friston KJ, Guo CC, Parker GB. Disrupted effective connectivity of cortical systems supporting attention and interoception in melancholia. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2014.2490. Published online before print February 18 2015. [DOI] [PubMed] [Google Scholar]
- Joorman J, Kosfelder J, Schulte D. The impact of comorbidity of depression on the course of anxiety treatments. Cognitive Therapy & Research. 2005;29:569–591. [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0071. Published online before print March 18, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J, Nitschke JB, Bhargava T, Deldin PJ, Gergen JA, Miller GA, Heller W. Neuropsychological differentiation of depression and anxiety. Journal of Abnormal Psychology. 2000;109:3–10. [PubMed] [Google Scholar]
- Kessler RC, Dupont RL, Berglund P, Wittchen HU. Impairment in pure and comorbid generalized anxiety disorder and major depression at 12 months in two national surveys. American Journal of Psychiatry. 1999;156:1915–1923. doi: 10.1176/ajp.156.12.1915. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Nelson CB, McGonagle KA, Liu J, Swartz M, Blazer DG. Comorbidity of DSM-III-R major depressive disorder in the general population: Results from the U.S. National Comorbidity Survey. British Journal of Psychiatry. 1996;6S(Suppl 30):17–30. [PubMed] [Google Scholar]
- Kokkonen M, Pulkkinen L. Extraversion and neuroticism as antecedents of emotion regulation and dysregulation in adulthood. European Journal of Personality. 2001;15:407–424. [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, et al. Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Harkness KL, Sabbagh MA, Jacobsen JA. Mental state decoding abilities in clinical depression. Journal of Affective Disorders. 2005;86:247–258. doi: 10.1016/j.jad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Luten AG, Ralph JA, Mineka S. Pessimistic attributional style:Is it specific to depression versus anxiety versus negative affect? Behavior Research Therapy. 1997;35:703–719. doi: 10.1016/s0005-7967(97)00027-2. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr A contemplated revision of the NEO Five Factor Inventory. Personality and Individual Differences. 2004;36:587–596. [Google Scholar]
- McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: Applications and advances. NeuroImage. 2004;23:S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Mikulincer M, Segal J. A multidimensional analysis of the experience of loneliness. Journal of Social and Personal Relationships. 1990;7:209–230. [Google Scholar]
- Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Moras K, Clark LA, Katon W, Roy-Byrne R, Watson D, Barlow D. Mixed anxiety-depression. In: Widiger TA, Frances AJ, Pincus HA, Ross R, First MB, Davis WW, editors. DSM-1V sourcebook. Washington, DC: American Psychiatric Association Press; 1996. pp. 623–643. [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, et al. A parcellation scheme for human left lateral parietal cortex. Neuron. 2010;67:156–170. doi: 10.1016/j.neuron.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezlek JB, Hampton CP, Shean GD. Clinical depression and day-to-day social interaction in a community sample. Journal of Abnormal Psychology. 2000;109:11–19. doi: 10.1037//0021-843x.109.1.11. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W. Distinguishing neural substrates of heterogeneity among anxiety disorders. International Review of Neurobiology. 2005;67:1–42. doi: 10.1016/S0074-7742(05)67001-8. [DOI] [PubMed] [Google Scholar]
- Ormel J, Rosmalen J, Farmer A. Neuroticism: A non-informative marker of vulnerability to psychopathology. Social Psychology and Psychiatric Epidemiology. 2004;39:906–912. doi: 10.1007/s00127-004-0873-y. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualter P, Vanhalst J, Harris R, Van Roekel E, Lodder G, Bangee M, … Verhagen M. Loneliness across ontogeny. Perspective on Psychological Science. 2015;10:250–264. doi: 10.1177/1745691615568999. [DOI] [PubMed] [Google Scholar]
- Rameson LT, Lieberman MD. Empathy: A social cognitive neuroscience approach. Social and Personality Psychology Compass. 2009;3:94–110. [Google Scholar]
- Raudenbush S, Bryk A, Congdon R. HLM 7.01 for Windows [Hierarchical linear and nonlinear modeling software] Multivariate Software, Inc; 2013. [Google Scholar]
- Ray RD, Wilhelm FH, Gross JJ. All in the mind’s eye? Anger rumination and reappraisal. Journal of Personality and Social Psychology. 2008;94:133–145. doi: 10.1037/0022-3514.94.1.133. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Wolf ND, Pennuto M, Vasic N, Wolf RC. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychological Medicine. 2013;44:2041–2051. doi: 10.1017/S0033291713002596. [DOI] [PubMed] [Google Scholar]
- Sanderson WC, DiNardo PA, Rapee RM, Barlow DH. Syndrome comorbidity in patients diagnosed with a DSM–III–R anxiety disorder. Journal of Abnormal Psychology. 1990;99:308–312. doi: 10.1037//0021-843x.99.3.308. [DOI] [PubMed] [Google Scholar]
- Schimmack U. Affect measurement in experience sampling research. Journal of Happiness Studies. 2003;4:79–106. [Google Scholar]
- Schneider D, Regenbogen C, Kellermann T, Finkelmeyer A, Kohn N, Derntl B, Schneider F, Habel U. Empathic behavioural and physiological responses to dynamic stimuli in depression. Psychiatry Research. 2012;200:294–305. doi: 10.1016/j.psychres.2012.03.054. [DOI] [PubMed] [Google Scholar]
- Schuch FB, Vasconcelos-Moreno MP, Borowsky C, Zimmermann AB, Rocha NS, Fleck MP. Exercise and severe major depression: Effect on symptom severity and quality of life at discharge in an inpatient cohort. Journal of Psychiatric Research. 2015;61:25–32. doi: 10.1016/j.jpsychires.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Shaver P, Schwartz J, Kirson D, O’Connor C. Emotion knowledge: Further exploration of a prototype approach. Journal of Personality and Social Psychology. 1987;52:1061–1086. doi: 10.1037//0022-3514.52.6.1061. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Wills TA, Neal DJ. The Many Faces of Affect: A Multilevel Model of Drinking Frequency/Quantity and Alcohol Dependence Symptoms Among Young Adults. Journal of Abnormal Psychology. 2014;123:676–694. doi: 10.1037/a0036926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Fitzgerald A, Miles LK, Phillips LH. Shifting moods, wandering minds: Negative moods lead the mind to wander. Emotion. 2009;9:271–276. doi: 10.1037/a0014855. [DOI] [PubMed] [Google Scholar]
- Smallwood J, O’Connor RC, Sudberry MV, Obonsawin MC. Mind wandering and dysphoria. Cognition & Emotion. 2007;21:816–842. [Google Scholar]
- Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, et al. Resting-state fMRI in the Human Connectome Project. NeuroImage. 2013;80:144–168. doi: 10.1016/j.neuroimage.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Schacter DL. Default network modulation and large-scale network interactivity in healthy young and old adults. Cerebral Cortex. 2012;22:2610–2621. doi: 10.1093/cercor/bhr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. Journal of Cognitive Neuroscience. 2013;25:74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Gender differences in brain functional connectivity density. Human Brain Mapping. 2012;33:849–860. doi: 10.1002/hbm.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Bickart KC, Barrett LF, Dickerson BC. Amygdala task-evoked activity and task-free connectivity independently contribute to feelings of arousal. Human Brain Mapping. 2014;35:5316–5327. doi: 10.1002/hbm.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Lindquist KA, Dickerson BC, Barrett LF. Intrinsic connectivity in the human brain does not reveal networks for “basic” emotions. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsv013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. An anatomical substrate for integration among functional networks in human cortex. Journal of Neuroscience. 2013;33:14489–500. doi: 10.1523/JNEUROSCI.2128-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. Journal of Neurophysiology. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TE, Bucholz R, et al. The Human Connectome Project: A data acquisition perspective. NeuroImage. 2012;62:2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen GA, Tendolkar I, Urner M, van Marle HJ, Denys D, Verkes RJ, Fernandez G. Short-term antidepressant administration reduces default mode and task-positive network connectivity in healthy individuals during rest. Neuroimage. 2013;88:47–53. doi: 10.1016/j.neuroimage.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. Negative affectivity: The disposition to experience aversive emotional states. Psychological Bulletin. 1984;6:465–490. [PubMed] [Google Scholar]
- Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology. 1999;76:820–838. [Google Scholar]
- Zinner LR, Brodish AB, Devine PG, Harmon-Jones E. Anger and asymmetrical frontal cortical activity: Evidence for an anger-withdrawal relationship. Cognition & Emotion. 2008;22:1081–1093. doi: 10.1080/02699930701622961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.