Abstract
Current behavioral evidence suggests that attention regulation in humans varies with a circadian arousal rhythm that is influenced by age. However it is not known whether functional BOLD activation also varies with performance across the day in older adults. We used fMRI to compare activity in the control network in older adults tested in the morning and older and younger adults tested in the afternoon. Using a 1-back task with simultaneously presented stimuli (words superimposed on pictures), we show that older adults tested in the morning are not only more able to ignore the unattended stimulus than older adults in the afternoon, but activate similar cognitive control regions to young adults (rostral prefrontal and superior parietal cortex). We conclude that time of day modulates task-related fMRI signal in older adults and that age differences are reduced when older adults are tested at peak times of day.
Keywords: time of day, circadian arousal, aging, attention, cognitive control, distraction, implicit memory, control network
There are well known circadian fluctuations in cognitive alertness (Hasher et al., 1999; Yoon et al., 1999; Paradee et al., 2005; Blatter and Cajochen, 2007; Murray et al., 2009), fluctuations measurable with paper and pencil inventories highly correlated with physiological arousal (Horne and Ostberg, 1976; Roenneberg et al., 2003; Zavada et al., 2005). Additionally, there are age and individual differences in alertness patterns, such that the majority of older adults are shifted towards morningness, with younger adults falling into neutral and evening type ranges of alertness. Similar effects have also been demonstrated in animal studies which report robust age by synchrony interactions for arousal and memory (Winocur and Hasher, 1999, 2002).
Concerning cognitive functioning, there is a substantial literature showing a synchrony effect (May et al., 1993; May and Hasher, 1998) such that performance, particularly on tasks requiring effortful (top-down) executive control or attention regulation are best performed at one’s better times of day (May and Hasher, 1998; May, 1999; Yoon et al., 1999; Hasher et al., 2005; Ramírez et al., 2006, 2012; Goldstein et al., 2007; Rowe et al., 2009; Hahn et al., 2012; Lehmann et al., 2013).
Despite a rich behavioral literature, the influence of circadian rhythms and time of testing is largely unexplored in the neuroimaging literature and what research there is has focused on young adults (e.g. Marek et al., 2010) showing time of day differences in the ability to regulate strong but incorrect responses in the orienting attentional network – a division of the task-positive network that regulates where and when attention is directed in response to external cues (Schmidt et al., 2012).
These findings are suggestive for young adults but an open and critical question is the impact of different times of testing for older adults – whose behavioral data shows substantially larger fluctuations, including in regulation of distraction, across the day than is true for young adults (May, 1999; e.g. Lustig et al., 2001). We focus here on regulation of distraction because of its well-known time of day effects for older adults and because it has been shown to correlate with other cognitive functions including working memory capacity and fluid intelligence (Hasher and Zacks, 1988; Dempster, 1991, 1992; Lustig et al., 2006).
To address the question of distraction regulation by older adults – and the neural correlates of this ability across the day, we used functional magnetic resonance imaging and tested a group of elderly participants at an optimal time of day (~8AM) and compared their performance on a simple 1-back task with target information (pictures or words) superimposed by distraction to that of younger and older participants tested at ~3pm, some of whose data had been previously collected (Campbell et al., 2012). We report dramatic differences in activity patterns across the day, with older adults tested in the morning having neural activity that is closer to that of young adults than is the case for older adults tested in the afternoon. These data suggest that our understanding of neural differences between young and old adults may be far from complete, particularly because few if any publications indicate the time at which participants were tested.
Materials and Methods
Participants
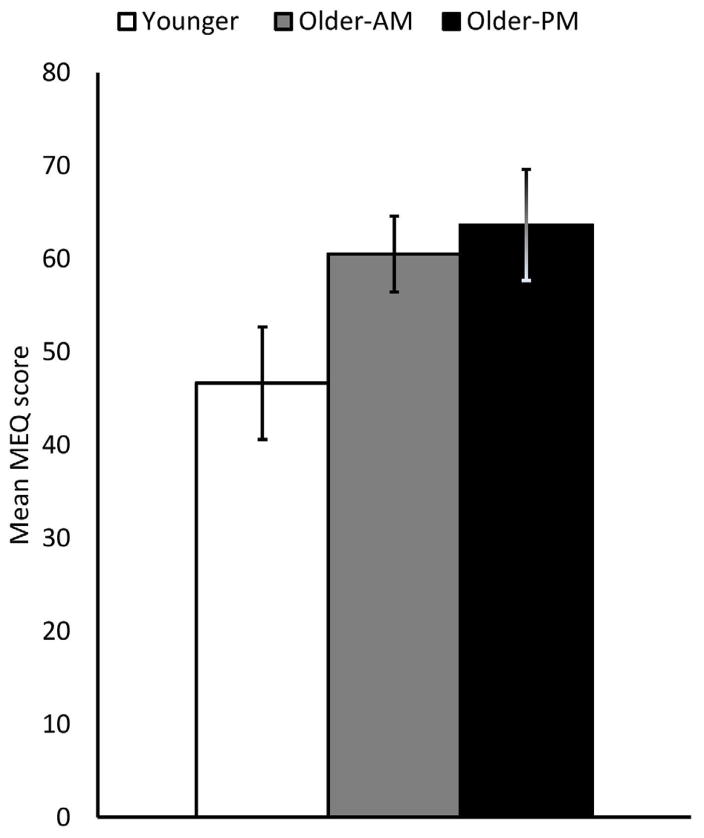
Participants included 17 younger and 16 older adults tested in the afternoon (1:00–5:00 PM, average time = 2:42 PM; data from 24 afternoon participants were previously reported in Campbell et al., 2012), as well as 20 older adults tested in the morning (8:30–10:30 AM, average time = 8:47 AM). One older participant from the morning group was excluded due to an incidental finding, and another due to awareness on the implicit task. One younger adult was excluded from all analyses for scoring more than 3 standard deviations from the mean on the behavioral scores. The final Ns s were therefore: 16 younger adults (19–30; M= 23.94, SD= 4.17; 8 males), 16 afternoon older adults (60–82; M= 71.27, SD= 7.68; 4 males), and 18 morning older adults (60–87; M= 68.83, SD= 7.2; 6 males). Participants were all right handed, had normal or corrected to normal vision, and were cognitively normal according to the Mini Mental State Exam (Folstein et al., 1975). Older adults in the morning (M = 60.53, SD= 8.17) and evening (M= 63.67, SD= 10.81) groups did not differ on the Morningness Eveningness Questionnaire (MEQ), p = .35, but using two independent t-tests, each was significantly different from younger adults (M = 46.66, SD= 11.35), p’s< .001, who tended towards eveningness (See Figure 1) (Horne and Ostberg, 1976).
Figure 1.
Morningness/eveningness score by group.
Procedure
The experimental design, materials and procedure were all identical to those reported in Campbell et al.(2012), except that the new group of older adults was tested in the morning, and additional participants were added to the other groups. Briefly, participants performed a series of tasks within the fMRI scanner. The precise order of tasks was as follows: (1) 1-back task on pictures with irrelevant words/ nonwords (Run 1), (2) 7-min nonverbal Flanker-task which served as a filler task (unscanned), (3) implicit word-fragment completion task (to test memory for the unattended words from the first 1-back task; unscanned), (4) 1-back task on letter stimuli with irrelevant pictures (Run 2), (5) 7-min resting state scan with eyes closed (Run 3), and finally, (6) a second word-fragment completion task (to test memory for the attended words from the second 1-back task; unscanned). The picture 1-back task was always given first, as we wanted to test participants’ implicit memory for previously “irrelevant” words and testing memory for the attended words first could have alerted them to a connection between successive tasks. As an additional precaution, we also asked participants after the first fragment task if they noticed a connection between the tasks thus far and if so, what they thought it was. Only one younger and one older adult were aware of the connection.
For the 1-back tasks, participants viewed a series of superimposed picture and letter stimuli (words and nonwords), with the pictures shown in red and the letters shown in black. Each stimulus pair was shown for 1000 ms, followed by a blank screen for 500 ms. Across two separate runs, participants were asked to attend to either the pictures or the letters and to press a response key whenever the same stimulus (from the attended modality) appeared twice in a row. They were told to ignore stimuli from the unattended modality, as attention towards these stimuli would only worsen target task performance. One-back targets occurred every 6 trials on average and never coincided with targets for the word-fragment task. A block design was used, with the letter stimuli divided into word and nonword blocks. Thus, there were five different block types in total: fixation, ignore-words (attend pictures), ignore-nonwords (attend pictures), attend-words (ignore pictures), and attend-nonwords (ignore pictures). Each run began with 10 s of fixation, followed by 8 task blocks of 24 s each, interleaved with 8 fixation blocks of 14 s each. Each task block contained 16 trials of the same condition and thus, there were 64 trials per condition. Pictures were taken from Snodgrass and Vanderwart (1980) and superimposed with either random consonant strings or words (including 10 words in each task that would later serve as targets on a word-fragment completion task).
All picture and word lists were counterbalanced, such that the pictures and words used on the attend-pictures 1-back (and corresponding fragment task) for one person would be used on the attend-letters 1-back for another person, and vice versa.
Memory for both the attended and unattended superimposed words was tested with two word-fragment completion tasks. Each task included 30 word fragments: 10 were target fragments that could be solved with words seen on the preceding 1-back task, 10 were control fragments which participants from another counterbalance condition would have seen, and 10 were easily solved fragments that served to maintain morale and to obscure the connection between the test and input task. Each fragment was shown in the center of the screen for 3000 ms and participants were told to respond aloud with the first solution that came to mind.
All fragments had multiple solutions in the language, but only one in the experiment. Separate baseline word-fragment completion rates (the proportion of solved fragments from the unseen list) were determined for each age group and fragment task.
We used the Arrow-Flanker task as our unscanned filler between the initial priming and the word-fragment completion task; briefly, participants are asked to respond only to the central chevron which can be flanked by congruent (facing in the same direction) or incongruent chevrons. The “flanker-effect” is obtained by subtracting reaction times on congruent trials from incongruent trials, and the amount of slowing relative to the congruent trial is a measure of attentional failure (Eriksen and Eriksen, 1974).
fMRI Data Acquisition
Participants were scanned using a Siemens Trio 3T scanner. Anatomical scans were acquired with a 3D MP-RAGE sequence (TR = 2 sec, TE = 2.63 ms, FOV = 25.6 cm2, 256x256 matrix, 160 slices of 1 mm thickness). Functional runs were acquired with an EPI sequence, with 157 volumes for each the 1-back runs (TR = 2 sec, TE = 30 ms, flip angle = 70°, FOV = 20 cm2, 64X64 matrix, 30 slices of 5 mm thickness, no gap). Measures of pulse and respiration were obtained during the scan.
Preprocessing of the image data was performed with Analysis of Functional Neuroimages (Cox, 1996). This included physiological motion correction, rigid motion correction, spatial normalization to Montreal Neurological Institute (MNI) space, and smoothing with an 8 mm Gaussian filter (the final voxel size was 4 x 4 x 4 mm). We also regressed out the white matter, cerebral spinal fluid, vasculature, and motion time series from each voxel time series (Grady et al.,2010; Campbell et al., 2013) and did not analyze the first 2 TRs from each block in order to have crisper transitions between conditions. As motion has been demonstrated to affect brain activity measures, even after standard correction procedures (e.g. Power et al., 2012), we followed a motion-scrubbing procedure described in Campbell et al., 2013. Briefly, this procedure uses a multivariate technique to identify outliers in both the motion-parameter estimates and fMRI signal itself. Where such outliers co-occurred (never more than 5% of the total volumes) we removed the fMRI volumes and replaced them with values interpolated with cubic splines. As reported by Campbell et al., (2013), this method has the advantage of suppressing spikes, yet keeping the length of the time-course intact across subjects.
The image data were analyzed with partial least squares (PLS) (McIntosh et al., 1996, 2004; for a detailed tutorial and review of PLS, see Krishnan et al., 2011), a multivariate analysis technique that identifies whole-brain patterns of covariance related to the experimental design (task-PLS) in a single step for multiple groups. This method is similar to principal component analysis, in that it identifies a set of principal components or ‘latent variables’ (LVs) that optimally capture the covariance between two sets of measurements (Friston et al., 1993). PLS uses singular value decomposition in a data driven approach to reduce the complexity of the dataset into orthogonal LVs that attempt to explain the maximum amount of covariance between the experimental design, the groups, and the BOLD signal. The first LV always explains the most covariance, and often identifies networks held in common across groups or conditions – subsequent LVs explaining the remaining covariance start to tease apart differences.
In task-PLS, each brain voxel has a weight, known as a salience, indicating how strongly that voxel contributes to the LV overall. The significance of each LV as a whole was determined using a permutation test (McIntosh et al., 1996), using 500 permutations. In addition, the reliability of each voxel’s contribution to a particular LV was tested by submitting all saliences to a bootstrap estimation of the standard errors (SEs) (Efron, 1981), using 500 bootstraps. Peak voxels with a salience/SE ratio ≥ 3.0 (p <.001) were considered to be reliable (Sampson et al., 1989). Clusters containing at least 10 reliable contiguous voxels were extracted, with a local maximum defined as the voxel with a salience/SE ratio higher than any other voxel in a 2 cm cube centered on that voxel (the minimum distance between peaks was 10 mm). Coordinates of these locations are reported in MNI space. Because the extraction of the LVs and the corresponding brain images is done in a single step, no correction for multiple comparisons is required.
Finally, to obtain summary measures of each participant’s expression of each LV spatio-temporal pattern, we calculated brain scores by multiplying each voxel’s salience by the BOLD signal in the voxel, and summing over all brain voxels for each participant in each condition. These brain scores were then mean-centered (using the grand mean across all subjects and conditions entered into the analysis) and confidence intervals (95%) for the mean brain scores in each condition were calculated from the bootstrap. Conservative estimates of differences in activity between conditions and between groups were determined via a lack of overlap in these bootstrapped confidence intervals. That is, non overlapping intervals between conditions within a group, or between groups within a condition, indicate a significant difference.
Results
Behavioral Results
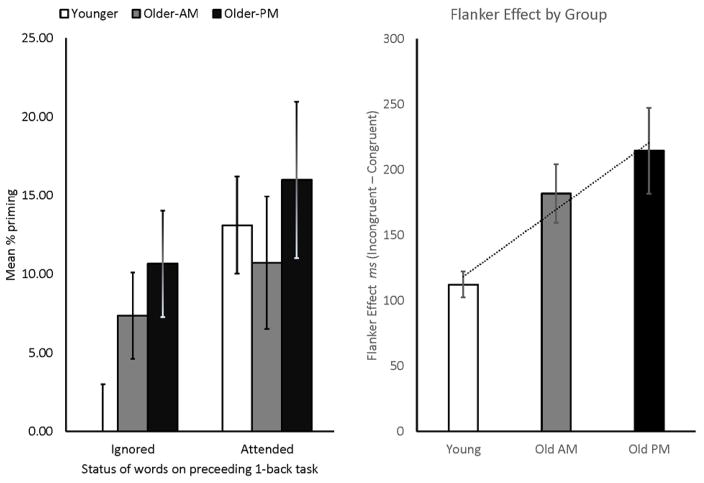
Our primary measure of interest was percent priming on the word-fragment completion tasks. Priming scores were calculated for each participant on each fragment task as the difference between the proportion of target-word fragments correctly solved and the baseline for that list (using the baseline for each group). Mean baseline completion rates did not differ across age or time of testing (Old AM = .10, Old PM = .09, Young PM = .13), p’s > .5. One younger adult was aware of the connection between the first 1-back task and the first fragment task, and one older adult in the afternoon gave only 2 responses during the task, and thus, their priming scores for this task were not included in the analyses. Means and standard errors are shown in Figure 2. On the first fragment task, which tested priming for distraction from the 1-back task on pictures, we initially conducted within-group t-tests to assess whether priming was different from zero. Young adults showed no significant priming, t(15 ) =0, while both groups of older adults did, t (17) = 3.07, p = 0.007, and t(14) = 3.155, p = 0.007 for the morning and afternoon groups respectively. To test group differences, an ANOVA was conducted on the priming scores with a planned linear contrast. The overall ANOVA was significant, F (2, 46) = 3.5, p = .038, the linear contrast was also significant, p = 0.017 and suggested that priming increased from nil in young adults to 7% in the morning group, and finally 11% in old adults tested in the afternoon. Our current results therefore agree with both the age and time of day differences reported previously (Hasher et al., 1999; Rowe et al., 2006).
Figure 2.
Behavioral results. Panel 1 shows mean percent priming as a function of age, time of day and attention. Error bars represent +/−1 standard error of the mean (SEM). The second panel plots the flanker effect (difference in RT to congruent vs. incongruent trials) across group.
On the second fragment task, which tested priming for attended words from the 1-back task on letters, all groups showed significant priming, p’s < .05, and an ANOVA did not reveal priming differences across groups, F (2, 45) = 0.23, p = 0.79.
We also tested for age and time-of-day effects on our filler task (the arrow flanker task, another measure of inhibitory control). Accuracy for this task was close to ceiling (all groups scored above 80% for every condition), and only reaction times associated with a correct response were analyzed (i.e. the participant correctly identified the direction of the middle arrow). An ANOVA testing the difference in the “flanker effect” (where RT on congruent trials is subtracted from RT on incongruent trials) across groups was significant; F (2, 47) = 4.62, p = 0.015. As can be seen in Figure 2, young adults showed the smallest flanker effect, followed by the morning old and finally the afternoon old groups, and this was confirmed by a planned linear contrast, p = 0.005. Post-hoc Bonferroni tests confirm that once again, only the young and afternoon old groups differ, p = 0.014, showing that age differences in inhibitory control are largest when older adults are tested at a non-optimal time of day.
fMRI Results
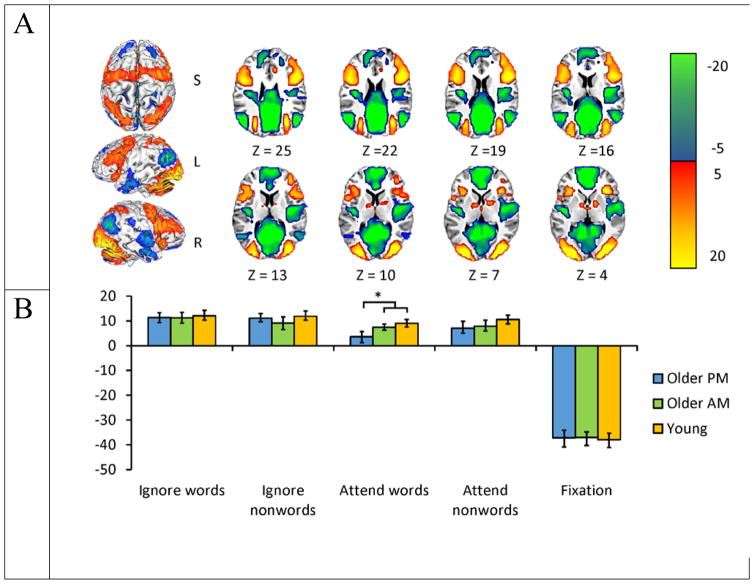
The first latent variable (LV) from the task-PLS analysis (p < .0001) accounted for 81.88% of the covariance in the data and differentiated the four task conditions from fixation across all groups (see Figure 3). This LV showed classic activations in the task-positive and default networks that corresponded to task and fixation, respectively (Raichle et al., 2001; Fox et al., 2005; Buckner et al., 2008; Toro et al., 2008). The task positive network generally activates when participants engage in cognitively demanding tasks and includes such regions as the middle frontal gyri and parietal lobes. The default network on the other hand is activated by rest or self-reflection and includes medial prefrontal and posterior cingulate regions. This initial LV largely revealed no age or time of testing differences between groups across condition, as indexed by the overlapping confidence intervals in the graph. One exception is that older adults tested in the afternoon activated the task-positive network less during the attend-words condition than both young adults and old adults tested in the morning, supporting the interpretation that testing older adults in the morning may alleviate age differences in brain activity.
Figure 3.
Results from the task-PLS shown on a high resolution MNI 152 axial image. The pattern identified by this LV in (a) shows areas that all participants activated relative to fixation (warm colors) or where there was more activity during fixation (cool colors). The graph in (b) shows the mean-centered mean brain scores for each group on this LV (error bars represent the 95% confidence intervals). Positive brain scores during the tasks correspond to more activity in warm colored areas relative to overall mean activity (0 in the graph) and negative brain scores are associated with more activity during fixation (cool colors). A bootstrap ratio threshold of 5.0 was used to form the brain image in (a).
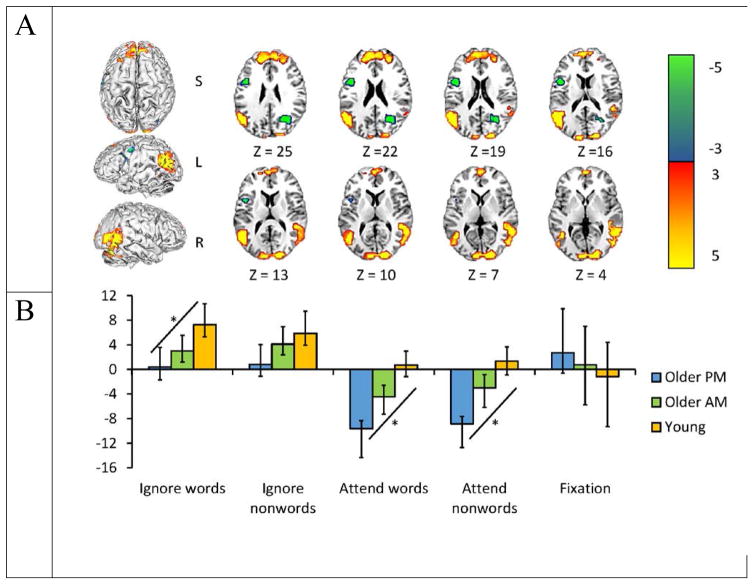
The second LV was also significant (p < .022), and explained 3.78% of the covariance in the data. This LV differentiated between groups on the tasks and neatly parallels the pattern of behavioral findings in the ignore-words condition, with a large difference between young and old adults tested in the afternoon, and the older adults tested in the morning falling in-between (Figure 4). Importantly, older adults tested in the morning showed no statistical difference relative to younger adults in any of the conditions on this LV. Younger adults and older adults tested in the morning activated a set of regions (shown in warm colors in Figure 4) during the ignore words/nonwords conditions (indicated by the positive brain scores on the graph), whereas these same regions were not activated by older adults tested in the afternoon (their confidence limits cross zero). These regions included the middle frontal gyri and parietal regions bilaterally (Figure 4A). These regions are part of the control network delineated by Vincent et al., (2008), which is thought to underlie cognitive control during attentionally demanding tasks. The pattern of activity seen here suggests that young adults are most able to access these control regions, and the age effect is partially mediated by time of testing. To test this pattern of brain activity for linear contrasts, we extracted the brain-scores for each individual by group and condition for the ignore-words and ignore-nonword conditions. In the ignore words condition, the linear trend was significant at p = 0.003, indicating an increasing engagement of cognitive control areas from afternoon older adults to morning older adults, and with the largest increase in young adults, to support inhibition of the distracting material. The linear effect was not significant in the ignore-nonword condition.
Figure 4.
Results from the second LV shown on a high resolution MNI 152 axial image. The pattern identified by this LV in (a) shows areas with increased activity primarily during the ignore-words and ignore-nonwords conditions (warm colors). The graph in (b) shows the mean-centered mean brain scores for both groups on this LV (error bars represent the 95% confidence intervals). Group differences are indicated by a lack of overlap in the confidence intervals; for example during the ignore-words condition, younger adults showed more activity than older adults tested in the afternoon, but activity did not differ between younger adults and older adults tested in the morning. A bootstrap ratio threshold of 3.0 was used to form the brain image in (a).
In the ignore-picture conditions, where attention was directed to the words or nonwords, brain activity in the older adults tested in the afternoon differed from both of the other groups, showing robust increases of activity in a number of regions, including left inferior frontal cortex (shown in cool colors in Figure 4). Older adults tested in the morning also showed this activity, but to a lesser extent. The left inferior frontal region is commonly reported in language processing including premotor speech, subvocalization, learning new words and encoding during speech production (e.g. Veroude et al., 2010; Szenkovits et al., 2012), suggesting that old adults tested in the afternoon, and to a lesser extent old adults tested in the morning were attending far more to the language component of the task than young adults. More activity in this region suggests that old adults, particularly when tested in the afternoon, tend to preferentially attend to highly salient word or word-like stimuli. More activity in the afternoon older group during the ignore-picture conditions also was seen in right posterior cingulate cortex (PCC), left Rolandic operculum, and the middle occipital gyrus (cool colored regions in Figure 4). While the PCC is commonly associated with default mode activity, it also has been implicated in relating encoded information to prior knowledge (e.g. Maguire et al., 1999), suggesting that older adults in the afternoon were less able to regulate DMN activity. We tested for a linear effect in this activity, as described above. This linear effect was significant, such that there was increased activation in the older adults tested in the afternoon during the attend-letter conditions, with less activation in the morning older adults, and no significant activation in the younger adults (both p’s < 0.001, Figure 4).
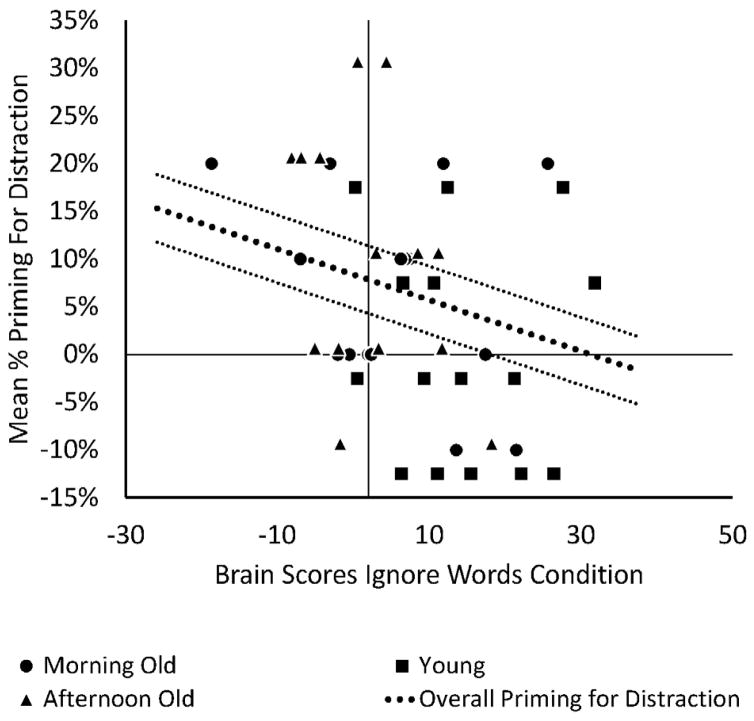
Finally, to test for brain-behavior relationships, we correlated the extracted brain scores from the ignore-words condition with priming for distraction (see Figure 5). Under the assumption that engagement of fronto-parietal control regions during the ignore-words condition would lead to less priming regardless of age or time of testing, we collapsed across groups and tested the overall correlation. This correlation was significant, r= −.29, p = 0.047, suggesting that greater engagement of the regions that regulate cognitive control was associated with less priming for the distracting words.
Figure 5.
Brain-behavior correlation between priming for distraction and Brain Scores on the “ignore-words” condition. The correlation is −.29, p = .047, and is collapsed across groups.
Discussion
The current research represents both a behavioral confirmation and a neuroscientific advance in the understanding of the synchrony effect, and also stands as a reminder to neuroimagers to take time of testing into account – particularly when testing older adults. We confirm previously established behavioral effects showing that at synchrony, older adults are able to resist distraction (Hasher et al., 2005; Rowe et al., 2006; Schmidt et al., 2007), and crucially for the first time, demonstrate that to do so they activate a set of attentional control regions recruited by younger adults. Older adults tested in the afternoon during their off-peak time of day showed both a behavioral and neural decrement, as they are not as able to resist distraction nor draw on the appropriate brain regions as their young peers or age mates tested in the morning.
The cognitive control network, which includes lateral and rostral prefrontal cortices and the inferior parietal lobules, has recently risen to prominence as a possible master “switch,” controlling access to the default and the dorsal attention network s. Recent work has suggested that not only is the control network interposed between the dorsal attention and default networks (Vincent et al., 2008), but it functionally modulates the relationship between them during tasks (Spreng et al., 2010), and at rest is affected by endogenous levels of dopamine (Dang et al., 2012).
The idea that older adults have difficulty regulating activity in control regions, and by implication in modulating a putative network “switch”, agrees with other literature suggesting that this group also has difficulty modulating functional connections between various regions comprising both task-positive and default networks (Lustig et al., 2003; Persson et al., 2007; Grady et al., 2010). There is also evidence that older adults may have difficulty suppressing the default network when on task, suggesting that they may be more prone to interference from mind-wandering, or reflecting on autobiographical memories during an experiment (Grady et al., 2006; Park et al., 2010). The current work suggests that time of testing may impact older adults’ ability to effectively modulate these networks.
Our results should also be interpreted in light of “CRUNCH” (compensation-related utilization of neural circuits hypothesis) – a theory suggesting that at higher cognitive load, older adults recruit broadly from distributed neural networks to bolster performance (Reuter-Lorenz and Cappell, 2008). We also note that the load (or difficulty of a task) may vary with general fatigue and circadian fluctuations in alertness – older adults tested in the morning may therefore experience lighter load than those in the afternoon. The observation that older adults generally recruit more resources, is not new (e.g. Grady et al., 1994; Cabeza, 2002), however the integration of load by Reuter-Lorenz is an attempt to unify arguments about whether this represents dedifferentiation (i.e. greater activity represents a loss of specificity that does not help task), or compensation. Broadly, and in line with CRUNCH we do show greater activation in older adults collectively (i.e. if we were to collapse across the times of testing). The question of whether this activity is compensatory is debatable. Previously, our group has argued that older adults can make beneficial use of distraction, and that implicit rehearsal can actually selectively benefit older adults (e.g. Healey et al., 2008; Biss et al., 2013)– from this perspective, the CRUNCH hypothesis is certainly plausible. Within the current experiment however, participants were instructed to ignore distractors – a goal that was better maintained by the young and old AM groups. While it is possible that older adults tested in the afternoon (presumably under higher load) were implicitly attempting to draw upon additional regions in a compensatory manner, two arguments rebut this theory. First, old adults in the afternoon do not activate task-positive regions – their activity is not additional recruitment, and secondly, at least within this particular context, their activity is not compensatory– rather we would suggest that the pattern observed in the afternoon is more akin to dedifferentiation.
Hasher et al., (2005) observe that failing to take time of testing and chronotype (i.e. preferred time of day as measured by the MEQ or similar) into account when studying age differences might bias result s. In short, some of the variance in age differences could in fact reflect time-of-day differences, particularly in cases where all participants are tested in the PM. The current results also indicate that there are differences in brain activity across the day for older adults that match behavioral differences, at least in the paradigm used here. Since few neuroimaging studies report time of testing, it is difficult to know to what degree age differences in such studies are associated with time of testing effects, and to what degree these differences influence the conclusions from such studies. Given the accumulation of evidence that time of testing can modulate age related results this information should at the least be routinely reported or controlled for.
While we acknowledge that one limitation of the present study is our lack of a younger group tested in the morning, there are reasons why we did not fully cross our design. First, our primary interest was in the behavior and functional organization of the aging brain, hence we focused our attention on this group and used a fixed baseline for the young. Furthermore we note that despite according to Yoon et al. (1999), young adults may not show as consistently strong a time-of-day preference as older adults do, because young adults were tested in the afternoon at either a neutral or even optimal time, this biases our results against finding the lack of difference we observe between them and the older adults tested in the morning–especially given that it was more likely for the data-driven nature of PLS to collapse across the two older groups in a comparison with the young. Finally, although we did not include measures of circadian variation beyond the MEQ, the MEQ nevertheless correlates strongly with circadian fluctuations in core body temperature and alertness and is therefore an excellent proxy measure of circadian type in humans (Horne and Ostberg, 1976; Bailey and Heitkemper, 2001). That said, future work may need to include measures of sleep activity, including sleep diaries and actigraphy.
Neuroimaging results from this study suggest that brain activity for older adults varies substantially across the day, with greater engagement of attentional control areas in the AM than in the PM. Further, these differences in brain recruitment accompany behavior al differences seen across the day. While it is currently unknown what is true for young adults, these data at least suggest the importance of reporting or controlling for the time at which testing occurs, since conclusions about brain activity may be partially tied to time, rather than to age. In summary, our results lend further credence to the extant behavioral work demonstrating that age-related decrements in cognitive control can be modulated by time of testing and chronotype, and establish for the first time that these effects extend to BOLD activity in fMRI. Finally, we emphasize that ignoring the time of day when testing older adults on some tasks may create an inaccurate picture of age differences in brain function.
Table 1.
Brain areas showing an age and time-of-day difference across conditions from LV2. BSR (Bootstrapped Ratios) can be used to estimate the contribution of each cluster to the LV.
| Region | Hem | X(mm) | Y(mm) | Z(mm) | BSR |
|---|---|---|---|---|---|
| Ignore nonwords/words > Attend nonwords/words | |||||
| Middle Temporal Gyrus | L | −52 | −60 | 16 | 7.08 |
| Fusiform Gyrus | R | 32 | −60 | −16 | 6.36 |
| Superior Frontal Gyrus | L | −4 | 40 | 44 | 6.06 |
| Superior Occipital Gyrus | R | 20 | −92 | 16 | 6.01 |
| Medial Frontal Gyrus | L | −8 | 52 | 24 | 5.49 |
| Inferior Temporal Gyrus | R | 56 | −52 | 0 | 5.48 |
| SMA | R | 8 | 8 | 60 | 4.80 |
| Middle Frontal Gyrus | R | 24 | 44 | 24 | 4.62 |
| Fusiform Gyrus | L | −24 | −68 | −16 | 4.49 |
| Cerebellum | L | −20 | −52 | −24 | 4.38 |
| Superior Parietal Lobule | R | 32 | −48 | 56 | 4.37 |
| Cerebellum | R | 28 | −76 | −36 | 4.30 |
| Posterior Cingulate Cortex | L | −4 | −52 | 24 | 4.18 |
| Precuneus | L | −4 | −52 | 40 | 4.16 |
| SMA | R | 12 | −12 | 64 | 4.11 |
| Inferior Temporal Gyrus | L | −52 | −8 | −24 | 3.68 |
| Mid Frontal Gyrus | L | −16 | 8 | 64 | 3.55 |
| Ignore nonwords/words < Attend nonwords/words | |||||
| Rolandic Operculum | L | −44 | 4 | 16 | −5.70 |
| Posterior Cingulate Cortex | R | 28 | −60 | 20 | −5.65 |
| Middle Occipital Gyrus | L | −28 | −60 | 32 | −4.45 |
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (MOP89769 to LH and MOP14036 to CG) and the Canada Research Chairs program, the Ontario Research Fund, the Canadian Foundation for Innovation, and the Heart and Stroke Foundation Centre for Stroke Recovery. Dr. Campbell’s current address is the Department of Psychology, University of Cambridge, Downing Street, Cambridge CB2 3EB UK. The authors would like to thank Annette Weeks-Holder and Wendy Elson for technical assistance.
Footnotes
The authors declare no competing financial interests.
References
- Bailey SL, Heitkemper MM. Circadian rhythmicity of cortisol and body temperature: morningness-eveningness effects. Chronobiol Int. 2001;18:249–261. doi: 10.1081/cbi-100103189. [DOI] [PubMed] [Google Scholar]
- Biss RK, Ngo KWJ, Hasher L, Campbell KL, Rowe G. Distraction can reduce age-related forgetting. Psychol Sci. 2013;24:448–455. doi: 10.1177/0956797612457386. [DOI] [PubMed] [Google Scholar]
- Blatter K, Cajochen C. Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings. Physiol Behav. 2007;90:196–208. doi: 10.1016/j.physbeh.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Grady CL, Ng C, Hasher L. Age differences in the frontoparietal cognitive control network: Implications for distractibility. Neuropsychologia. 2012:1–12. doi: 10.1016/j.neuropsychologia.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Grigg O, Saverino C, Churchill N, Grady CL. Age differences in the intrinsic functional connectivity of default network subsystems. Front Aging Neurosci. 2013;5 doi: 10.3389/fnagi.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dang LC, O’Neil JP, Jagust WJ. Dopamine supports coupling of attention-related networks. J Neurosci. 2012;32:9582–9587. doi: 10.1523/JNEUROSCI.0909-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster F. Inhibitory processes: a negleted dimension of intelligence. Intelligence. 1991;173:157–173. [Google Scholar]
- Dempster F. The rise and fall of the inhibitory mechanism: Toward a unified theory of cognitive development and aging. Dev Rev. 1992;75:45–75. [Google Scholar]
- Efron B. Nonparametric estimates of standard error: the jackknife, the bootstrap and other methods. Biometrika. 1981;68:589–599. [Google Scholar]
- Eriksen Ba, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS. Principal component analysis learning algorithms: a neurobiological analysis. Proc Biol Sci. 1993;254:47–54. doi: 10.1098/rspb.1993.0125. [DOI] [PubMed] [Google Scholar]
- Goldstein D, Hahn CS, Hasher L, Wiprzycka UJ, Zelazo PD. Time of day, Intellectual Performance, and Behavioral Problems in Morning Versus Evening type Adolescents: Is there a Synchrony Effect? Pers Individ Dif. 2007;42:431–440. doi: 10.1016/j.paid.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JaE, Churchill N, McIntosh AR. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Hahn C, Cowell JM, Wiprzycka UJ, Goldstein D, Ralph M, Hasher L, Zelazo PD. Circadian rhythms in executive function during the transition to adolescence: the effect of synchrony between chronotype and time of day. Dev Sci. 2012:1–9. doi: 10.1111/j.1467-7687.2012.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Goldstein D, May CP. It’s about time: Circadian rhythms, memory, and aging. In: Izawa C, Ohta N, editors. Human learning and memory: Advances in theory and application: The 4th Tsukuba International Conference on Memory. Kansas: Lawrence Erlbaum Associates; 2005. pp. 199–217. [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The Psychology of Learning and Motivation. New York, NY: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hasher L, Zacks RT, May CP. Inhibitory control, circadian arousal, and age. In: Gopher D, Koriat A, editors. Attention and performance XVII: Cognitive regulation of performance: Interaction of theory and application. Cambridge, MA: MIT Press; 1999. pp. 653–675. [Google Scholar]
- Healey M, Campbell K, Hasher L. Cognitive aging and increased distractibility: Costs and potential benefits. Prog Brain Res. 2008 doi: 10.1016/S0079-6123(07)00022-2. [DOI] [PubMed] [Google Scholar]
- Horne J, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56:455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Lehmann CA, Marks ADG, Hanstock TL. Age and synchrony effects in performance on the Rey Auditory Verbal Learning Test. Int Psychogeriatr. 2013;25:657–665. doi: 10.1017/S1041610212002013. [DOI] [PubMed] [Google Scholar]
- Lustig C, Hasher L, Tonev ST. Inhibitory control over the present and the past. Eur J Cogn Psychol. 2001;13:107–122. [Google Scholar]
- Lustig C, Hasher L, Tonev ST. Distraction as a determinant of processing speed. Psychon Bull Rev. 2006;13:619–625. doi: 10.3758/bf03193972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire Ea, Frith CD, Morris RG. The functional neuroanatomy of comprehension and memory: the importance of prior knowledge. Brain. 1999;122(Pt 1):1839–1850. doi: 10.1093/brain/122.10.1839. [DOI] [PubMed] [Google Scholar]
- Marek T, Fafrowicz M, Golonka K, Mojsa-Kaja J, Oginska H, Tucholska K, Urbanik A, Beldzik E, Domagalik A. Diurnal patterns of activity of the orienting and executive attention neuronal networks in subjects performing a Stroop-like task: a functional magnetic resonance imaging study. Chronobiol Int. 2010;27:945–958. doi: 10.3109/07420528.2010.489400. [DOI] [PubMed] [Google Scholar]
- May CP. Synchrony effects in cognition: the costs and a benefit. Psychon Bull Rev. 1999;6:142. doi: 10.3758/bf03210822. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L. Synchrony effects in inhibitory control over thought and action. J Exp Psychol Hum Percept Perform. 1998;24:363–379. doi: 10.1037//0096-1523.24.2.363. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L, Stoltzfuz ER, Stoltzfus ERE. Optimal time of day and the magnitude of age differences in memory. Psychol Sci. 1993;4:326–330. [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Chau WK, Protzner AB. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 2004;23:764–775. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, Trinder J. Nature’s clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009;9:705–716. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- Paradee CV, Rapport LJ, Hanks Ra, Levy Ja. Circadian preference and cognitive functioning among rehabilitation inpatients. Clin Neuropsychol. 2005;19:55–72. doi: 10.1080/13854040490524173. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk Ta, Hebrank AC, Jenkins LJ. Age differences in default mode activity on easy and difficult spatial judgment tasks. Front Hum Neurosci. 2010;3:75. doi: 10.3389/neuro.09.075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz Pa. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes Ka, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez C, García A, Valdez P. Identification of circadian rhythms in cognitive inhibition and flexibility using a Stroop task. Sleep Biol Rhythms. 2012;10:136–144. [Google Scholar]
- Ramírez C, Talamantes J, García A, Morales M, Valdez P, Menna-Barreto L. Circadian rhythms in phonological and visuospatial storage components of working memory. Biol Rhythm Res. 2006;37:433–441. [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between Clocks: Daily Temporal Patterns of Human Chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Rowe G, Hasher L, Turcotte J. Age and synchrony effects in visuospatial working memory. Q J Exp Psychol (Hove) 2009;62:1873–1880. doi: 10.1080/17470210902834852. [DOI] [PubMed] [Google Scholar]
- Rowe G, Valderrama S, Hasher L, Lenartowicz A. Attentional disregulation: a benefit for implicit memory. Psychol Aging. 2006;21:826–830. doi: 10.1037/0882-7974.21.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson PD, Streissguth P, Barr HM, Bookstein FL. Neurobehavioral effects of prenatal alcohol: Part II. Partial least squares analysis. Neurotoxicol Teratol. 1989;11:477–491. doi: 10.1016/0892-0362(89)90025-1. [DOI] [PubMed] [Google Scholar]
- Schmidt C, et al. Circadian preference modulates the neural substrate of conflict processing across the day. PLoS One. 2012;7:e29658. doi: 10.1371/journal.pone.0029658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Collette F, Cajochen C, Peigneux P. A time to think: circadian rhythms in human cognition. Cogn Neuropsychol. 2007;24:755–789. doi: 10.1080/02643290701754158. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JJP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szenkovits G, Peelle JE, Norris D, Davis MH. Individual differences in premotor and motor recruitment during speech perception. Neuropsychologia. 2012;50:1380–1392. doi: 10.1016/j.neuropsychologia.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veroude K, Norris DG, Shumskaya E, Gullberg M, Indefrey P. Functional connectivity between brain regions involved in learning words of a new language. Brain Lang. 2010;113:21–27. doi: 10.1016/j.bandl.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Hasher L. Aging and time-of-day effects on cognition in rats. Behav Neurosci. 1999;113:991. doi: 10.1037//0735-7044.113.5.991. [DOI] [PubMed] [Google Scholar]
- Winocur G, Hasher L. Circadian rhythms and memory in aged humans and animals. Neuropsychol Mem 2002 [Google Scholar]
- Yoon C, May CP, Hasher L. Aging, circadian arousal patterns, and cognition. Cogn aging, self-reports. 1999:117–143. [Google Scholar]
- Zavada A, Gordijn M, Beersma D, Daan S, Roenneberg T. Comparison of the Munich Chronotype Questionnaire with the Horne-Östberg’s Morningness-Eveningness score. Chronobiol Int. 2005;22:267–278. doi: 10.1081/cbi-200053536. [DOI] [PubMed] [Google Scholar]