Abstract
Purpose
Berberine is a plant alkaloid that is widely used to treat gastrointestinal infections, diabetes, hypertension, and hypercholesterolemia. Many studies have reported interactions between berberine-containing products and cytochromes P450 (CYPs), but little is known about whether berberine alters CYP activities in humans, especially after repeated doses.
Methods
A two-phase randomized-crossover clinical study in healthy male subjects was performed. After 2 weeks of berberine (300 mg, t.i.d., p.o.) administration, midazolam, omeprazole, dextromethorphan, losartan, and caffeine were used to evaluate enzyme activities of CYP3A4, 2C19, 2D6, 2C9, and CYP1A2, respectively.
Results
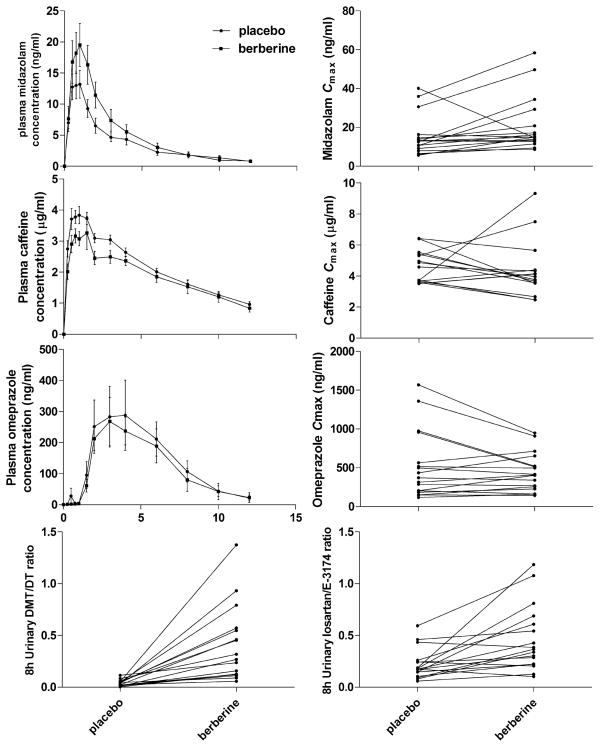
A decrease in CYP2D6 activity was observed as the 0–8 h urinary dextromethorphan/dextrorphan increased ninefold (P<0.01). In addition, losartan/E-3174 ratio doubled (P<0.01) after BBR administration, indicating a decrease in CYP2C9 activity. CYP3A4 activity was also inhibited, as the Cmax, AUC0–∞, and AUC0–12 of midazolam were increased 38% (P<0.05), 40% (P<0.01), and 37% (P<0.05) after BBR treatment, respectively. Compared with the placebo period, the Tmax and T1/2 of midazolam during BBR administration were prolonged from 3.03±0.27 to 3.66±0.37 h and 0.66±0.08 to 0.99±0.09 h, respectively; the oral clearance of midazolam was decreased 27% (P< 0.05); and the phenotypic indices of 1 h midazolam/1′-hydroxymidazolam increased 59% (P<0.01). There were no statistically significant differences in the pharmacokinetic parameters of the other probe drugs between placebo and the BBR-treated group.
Conclusions
Repeated administration of berberine (300 mg, t.i.d., p.o.) decreased CYP2D6, 2C9, and CYP3A4 activities. Drug-drug interactions should be considered when berberine is administered.
Keywords: Berberine, CYP2D6, CYP2C9, CYP3A4, Humans
Introduction
Berberine is a major isoquinoline alkaloid in herbs such as goldenseal, berberis, and Coptis chinensis, and has been used for about 1,000 years to treat diarrhea. Presently, it is widely used in Oriental countries for diabetes, hypercholesterolemia, and other medical conditions [1]. Goldenseal, a main source of berberine, ranked as the sixth most commonly used herb supplement in the United States for children [2]. In renal-transplant recipients, berberine was reported to markedly elevate the blood concentration of cyclosporine A, a drug metabolized by CYP3A4 [3]. In human liver microsomes, berberine inhibited CYP2D6 (IC50 =45 μM) and CYP3A4 (IC50 ~400 μM) activities, but a CYP metabolic-intermediate complex formation was not demonstrated for berberine [4]. In the current clinical study, berberine (300 mg, t.i.d., p.o.) was given to healthy male volunteers for 2 weeks, and the effect of repeated doses of berberine on the activity of major CYPs was quantified.
Methods
The experimental protocol was approved by the Ethics Committee of Central South University Xiangya School of Medicine, Hunan, China, and written informed consent was obtained prior to participation in this trial. This study was a two-phase, randomized, cross-over design with a 4-week washout period. Eighteen healthy Chinese male volunteers [(mean±SD) age, 21.6 ±1.5 years; weight, 61.35±3.89 kg] entered and 17 of them completed the study, as 1 volunteer quit because of a schedule conflict. All subjects were nonsmokers and determined to be healthy. All of the subjects were free of other medications, caffeine- and alcohol-containing products as well as grapefruit prior to and during the study. The subjects received orally either placebo or berberine capsules (Yunnan Baiyao Group Dali Pharmaceutical, Yunnan, China) at a dose of 300 mg three times daily (8 am, 4 pm, and 11 pm) for 14 days. Midazolam (Shanghai Roche, Shanghai, China; probe drug for CYP3A4) 2 mg, omeprazole (Northeast Pharmaceutical Group, Shenyang, China; probe drug for CYP2C19) 20 mg, dextromethorphan (the United Laboratories International Holding, Zhuhai, China; probe drug for CYP2D6) 30 mg, losartan (Merck, West Point, PN; probe drug for CYP2C19) 30 mg, and caffeine 93 mg (contained in a standard cup of coffee; probe drug for CYP1A2) [5] were administered before and at the end of each phase, as reported previously [6]. All probe drugs were given at 8:00 am. Meals were allowed 3 h after administration of the probe drugs.
A series of 10 ml venous blood samples were collected into heparinized tubes from the antecubital vein immediately before (0 min) and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, and 12 h after probe drug administration. Urine samples of 0–4, 4–8, 8–12 h were collected in plastic jars. The total volume of the 0–8 h urine samples was determined. The harvested plasma and urine aliquots (10 ml) were stored at −40°C until analysis. The 12 h pharmacokinetics of omeprazole, midazolam, caffeine, and 0–8 h losartan/E-3174 as well as dextromethorphan/dextrorphan urinary ratio were measured. Midazolam/1′-hydroxymidazolam ratio at 1 h, caffeine/paraxanthine ratio at 6 h, and omeprazole/5′-hydroxyomeprazole ratio at 4 h in plasma were used as extra phenotypic indices [6–8] for quantifying corresponding enzyme activity change. Blood and urine samples were prepared as previously reported [6]. The analytic strategies for midazolam, losartan, caffeine, omeprazole, and their metabolites were developed previously [9–12]. The parameters were calculated as follows: AUC was calculated by the linear trapezoidal rule, and ke is the slope of the linear terminal part of the plasma concentration vs. time curve after semilogarithmic transformation. T1/2=0.693/ke and AUC(0–n)=AUC(0–12)+Cp(12 h)/ke, where Cp(12 h) is the plasma concentration at 12 h. Oral clearance CL/F=dose/AUC(0–t) (ng·h/ml). Cmax and Tmax were assigned by visual inspection. All data were analyzed by Wilcoxon signed-rank test with a P value of less than 0.05 considered significant.
Results
After 2 weeks of berberine (300 mg, t.i.d., p.o.) administration to healthy male volunteers, the major pharmacokinetic parameters of the probe drugs for CYP3A4, CYP1A2, CYP2C19, CYP2D6, and CYP2C9 were determined, as shown in Table 1, and the individual data are presented in Fig. 1. A decrease in CYP2D6 activity was observed as the 0–8 h urinary dextromethorphan/dextrorphan ratio increased ninefold (P<0.01). In addition, losartan/E-3174 ratio doubled (P<0.01) after BBR administration, indicating a decrease in CYP2C9 activity. CYP3A4 activity was also inhibited, as the Cmax, AUC0–∞, and AUC0–12 of mid-azolam were increased 38% (P<0.05), 40% (P<0.01), and 37% (P<0.05) after BBR treatment. Compared with the placebo period, the Tmax and T1/2 of midazolam during BBR administration were prolonged from 3.03±0.27 to 3.66± 0.37 h and 0.66±0.08 to 0.99±0.09 h, respectively; the oral clearance of midazolam was decreased 27% (P<0.05); and the phenotypic indices of 1 h midazolam/1′-hydroxymidazolam increased 59% (P<0.01). There were no statistically significant differences in the pharmacokinetic parameters of the other probe drugs between placebo and the BBR-treated group.
Table 1.
Pharmacokinetic parameters of probe drugs in test and reference periods of a two phase cross-over study
| Parameter | Placebo group | Berberine group | Ratio (90% CI)a |
|---|---|---|---|
| Midazolam (CYP3A4)b | |||
| AUC0–∞ (ng·h/ml) | 45.08 (34.84) | 65.18 (55.13)** | 140% (117%, 168%) |
| AUC0–12 h (ng·h/ml) | 39.79 (27.87) | 57.71 (46.96)* | 137% (112%, 167%) |
| Cmax (ng/ml) | 15.30 (10.31) | 20.88 (14.18)* | 138% (107%, 177%) |
| tmax (h) | 0.66 (0.33) | 0.99 (0.37)* | 152% (120%, 192%) |
| t1/2 (h) | 3.03 (1.11) | 3.66 (1.53)* | 119% (101%, 139%) |
| CL/F (l/h) | 70.25 (40.32) | 53.35 (32.70)* | 73% (60%, 89%) |
| Midazolam/1′-hydroxymidazolam (1 h, in plasma) | 6.91 (3.50) | 10.34 (3.50)** | 159% (130%, 194%) |
| Caffeine (CYP1A2)b | |||
| AUC0–∞ (μg·h/ml) | 33.98 (9.40) | 29.80 (11.30) | 85% (76%, 95%) |
| Cmax (μg/ml) | 4.48 (1.03) | 4.16 (1.86) | 88% (75%, 103%) |
| Tmax (h) | 0.96 (0.45) | 1.00 (0.41) | 108% (86%, 135%) |
| T1/2 (h) | 5.41 (1.81) | 4.75 (1.15) | 89% (78%, 102%) |
| Caffeine/paraxanthine (6 h, in plasma) | 1.51 (2.06) | 0.94 (0.45) | 80% (59%, 108%) |
| Omeprazole (CYP2C19)b | |||
| AUC0–∞ (μg·h/ml) | 1.73 (1.86) | 1.47 (1.48) | 98% (81%, 120%) |
| Cmax (μg/ml) | 0.52 (0.45) | 0.44 (0.25) | 99% (84%, 117%) |
| Tmax (h) | 1.47 (0.82) | 1.49 (0.54) | 107% (90%, 128%) |
| T1/2 (h) | 4.00 (1.69) | 3.41 (1.61) | 85% (70%, 103%) |
| CL/F (l/h) | 24.13 (17.40) | 21.70 (12.29) | 102% (84%, 124%) |
| Omeprazole/5-hydroxyomeprazole (4 h, in plasma) | 6.83 (9.94) | 7.34 (9.61) | 121% (82%, 179%) |
| Dextromethorphan (CYP2D6)c | |||
| 0–8 h Urinary DTM/DT ratio | 0.04 (0.04) | 0.40 (0.37)** | 979% (650%, 1,474%) |
| Losartan (CYP2C9)c | |||
| 0–8 h Urinary losartan/E-3174 ratio | 0.22 (0.16) | 0.46 (0.33)** | 202% (152%, 270%) |
Values are mean ± SD;
P<0.05,
P<0.01
AUC0–12 Area under concentration-time curve from 0–12 h, AUC0–∞ area under concentration-time curve extrapolated to infinity, CL/F oral clearance, T1/2 elimination half-life, Cmax maximum concentration, Tmax time to reach maximum concentration, DTM/DT dextromethorphan to dextrorphan, CI confidence interval
Geometric mean ratio of berberine/control and 90% confidence interval of ratio
Values for 17 individuals
The urinary metabolic ratio represents the mean of 17 individuals
Fig. 1.
Mean plasma concentration-time profiles and the main pharmacokinetic parameters of midazolam, caffeine, omeprazole, dextromethorphan, and losartan after 2 weeks of placebo and berberine administration. Data are expressed as mean ± SE. Circles represent the placebo administration phase, and squares represent the berberine administration phase
Discussion
In this clinical study, a high-throughput method for evaluation of the five major human CYPs isoforms in vivo was used [6, 13]. Although a single-point assay has been recommended using a 4 h blood sample and an 8 h urine collection, 0–12 h blood samples as well as 0–8 h urine collections were analyzed to obtain the pharmacokinetics alteration in a period of time.
The individual variability was relatively large in the current study, which suggests that the inhibitory effects of berberine might be genotype-dependent. Further studies with genotypes for relevant CYPs might be helpful to clarify the results.
The 0–8 h urinary ratio of dextromethorphan to dextrorphan is a phenotyping marker for CYP2D6 activity [14], and a dramatic increase in the ratio of parent drug to the metabolite indicates that enzyme activity decreases markedly. Our previous data showed that CYP2D6 plays a key role in the metabolism of berberine [18]. Therefore, the inhibitory effect of berberine on CYP2D6 activity may be caused by substrate competition. CYP2D6 has highly polymorphic enzyme activity which may result in a dramatic difference in the elimination of berberine for ultra-rapid metabolizers, extensive metabolizers, and poor metabolizers [15], thus the risk of treatment failure, dose-dependent drug toxicity, and drug-drug interactions is of concern when berberine is administered. The 0–8 h urinary ratio of losartan to its metabolite E-3174 increased about twofold, which implied decreased CYP2C9 enzyme activity. Since the previous data showed that recombinant human CYP2C9 did not metabolize berberine, further study about berberine and CYP2C9 gene regulation may provide evidence to clarify this alteration.
CYP3A is important in metabolizing drugs both in liver and intestine. The inhibition of CYP3A4 activity by berberine was demonstrated by changes in midazolam pharmacokinetics. The Tmax of midazolam was prolonged somewhat after berberine administration, which might be caused by berberine slowing the motility of the digestive system [16]. Although CYP3A4 is a minor enzyme that metabolizes berberine in humans [18], the mRNA expression was suppressed at higher doses of berberine as indicated in our previous study. According to the criteria established by the Food and Drug Administration in the industry guidance concerning in vivo drug interaction studies, when there is a bioequivalence, the geometric mean and the 90% CIs for AUC, oral clearance, and Cmax fall into the default no-effect boundaries of 80–125%. The observed changes for midazolam (90% CIs of geometric means for the ratio of berberine to control were either less than 80% or more than 125%) suggest a non-bioequivalence between the control and berberine treatment phases; moreover, these differences were statistically significant and might also be clinically important [17].
CYP1A2 is another major enzyme metabolizing berberine [18]. Although AUC0–∞ and Cmax of caffeine did not fall into the bioequivalence criteria of 80–125%, which implies a difference between berberine and controls, the changes in caffeine pharmacokinetics were not statistically significant. This discrepancy might be due to the small sample size of the study. Further studies in larger populations and multiple dosage administrations will be helpful to confirm and expand our results, which will guide the usage of berberine as a supplementary or protective prescription for people.
Conclusion
Inhibitory effects of berberine to CYP2D6, 3A4, and CYP2C9 were observed in the current study, and drug-drug interactions as well as interethnic variability in berberine metabolism need to be considered when berberine or berberine-containing products are administered.
Acknowledgments
We would like to thank all members of Dr. Klaassen′s laboratory for reviewing this short communication. This work was supported by the following grants: National Institute of Health (ES-009649, ES-019487, DK-081461, and RR021940); National Scientific Foundation of China (No. 30801421); Hunan Provincial Innovation Foundation For Postgraduates (No. 2009bsxt020); Huge Project to Boost Chinese Drug Development (No. 2009ZX09501-032); 863 Projects (No. 2009AA022710, 2009AA022703, 2009AA022704)
Footnotes
Conflict of interest The authors report no conflict of interest.
Contributor Information
Ying Guo, Email: yguo2@kumc.edu, Pharmacogenetics Research Institute, Institute of Clinical Pharmacology, Central South University, XiangYa School of Medicine, 110 Xiang-Ya Road, Changsha, Hunan 410078, People’s Republic of China. Department of Pharmacology, Toxicology and Therapeutics, University of Kansas Medical Center, 4099 HLSIC; MS1018; 3901 Rainbow Boulevard, Kansas City, KS 66160, USA.
Yao Chen, Pharmacogenetics Research Institute, Institute of Clinical Pharmacology, Central South University, XiangYa School of Medicine, 110 Xiang-Ya Road, Changsha, Hunan 410078, People’s Republic of China.
Zhi-rong Tan, Pharmacogenetics Research Institute, Institute of Clinical Pharmacology, Central South University, XiangYa School of Medicine, 110 Xiang-Ya Road, Changsha, Hunan 410078, People’s Republic of China.
Curtis D. Klaassen, Email: cklaasse@kumc.edu, Department of Pharmacology, Toxicology and Therapeutics, University of Kansas Medical Center, 4099 HLSIC; MS1018; 3901 Rainbow Boulevard, Kansas City, KS 66160, USA
Hong-hao Zhou, Email: hhzhou2003@163.com, Pharmacogenetics Research Institute, Institute of Clinical Pharmacology, Central South University, XiangYa School of Medicine, 110 Xiang-Ya Road, Changsha, Hunan 410078, People’s Republic of China.
References
- 1.Vuddanda PR, Chakraborty S, Singh S. Berberine: a potential phytochemical with multispectrum therapeutic activities. Expert Opin Investig Drugs. 2010;19(10):1297–1307. doi: 10.1517/13543784.2010.517745. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1–23. [PubMed] [Google Scholar]
- 3.Wu X, Li Q, Xin H, Yu A, Zhong M. Effects of berberine on the blood concentration of cyclosporin A in renal transplanted recipients: clinical and pharmacokinetic study. Eur J Clin Pharmacol. 2005;61(8):567–572. doi: 10.1007/s00228-005-0952-3. [DOI] [PubMed] [Google Scholar]
- 4.Etheridge AS, Black SR, Patel PR, So J, Mathews JM. An in vitro evaluation of cytochrome P450 inhibition and P-glycoprotein interaction with goldenseal, Ginkgo biloba, grape seed, milk thistle, and ginseng extracts and their constituents. Planta Med. 2007;73(8):731–741. doi: 10.1055/s-2007-981550. [DOI] [PubMed] [Google Scholar]
- 5.Gunes A, Ozbey G, Vural EH, Uluoglu C, Scordo MG, Zengil H, Dahl ML. Influence of genetic polymorphisms, smoking, gender and age on CYP1A2 activity in a Turkish population. Pharmacogn. 2009;10(5):769–778. doi: 10.2217/pgs.09.22. [DOI] [PubMed] [Google Scholar]
- 6.Ryu JY, Song IS, Sunwoo YE, Shon JH, Liu KH, Cha IJ, Shin JG. Development of the “Inje cocktail” for high-throughput evaluation of five human cytochrome P450 isoforms in vivo. Clin Pharmacol Ther. 2007;82(5):531–540. doi: 10.1038/sj.clpt.6100187. [DOI] [PubMed] [Google Scholar]
- 7.Chen XP, Tan ZR, Huang SL, Huang Z, Ou-Yang DS, Zhou HH. Isozyme-specific induction of low-dose aspirin on cytochrome P450 in healthy subjects. Clin Pharmacol Ther. 2003;73(3):264–271. doi: 10.1067/mcp.2003.14. [DOI] [PubMed] [Google Scholar]
- 8.Zheng J, Chen B, Jiang B, Zeng L, Tang ZR, Fan L, Zhou HH. The effects of puerarin on CYP2D6 and CYP1A2 activities in vivo. Arch Pharm Res. 2010;33(2):243–246. doi: 10.1007/s12272-010-0209-2. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Wang G, Wang LS, Zhang W, Tan ZR, Fan L, Chen BL, Li Q, Liu J, Tu JH, Hu DL, Liu ZQ, Zhou HH. Effects of the CYP2C9*13 allele on the pharmacokinetics of losartan in healthy male subjects. Xenobiotica. 2009;39(10):788–793. doi: 10.1080/00498250903134435. [DOI] [PubMed] [Google Scholar]
- 10.Wang LS, Zhou G, Zhu B, Wu J, Wang JG, Abd El-Aty AM, Li T, Liu J, Yang TL, Wang D, Zhong XY, Zhou HH. St John’s wort induces both cytochrome P450 3A4-catalyzed sulfoxidation and 2C19-dependent hydroxylation of omeprazole. Clin Pharmacol Ther. 2004;75(3):191–197. doi: 10.1016/j.clpt.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Xiao P, Ou-Yang DS, Fan L, Guo D, Wang YN, Han Y, Tu JH, Zhou G, Huang YF, Zhou HH. Simultaneous action of the flavonoid quercetin on cytochrome P450 (CYP) 1A2, CYP2A6, N-acetyltransferase and xanthine oxidase activity in healthy volunteers. Clin Exp Pharmacol Physiol. 2009;36(8):828–833. doi: 10.1111/j.1440-1681.2009.05158.x. [DOI] [PubMed] [Google Scholar]
- 12.Tu JH, He YJ, Chen Y, Fan L, Zhang W, Tan ZR, Huang YF, Guo D, Hu DL, Wang D, Hong-Hao Z. Effect of glycyrrhizin on the activity of CYP3A enzyme in humans. Eur J Clin Pharmacol. 2010;66(8):805–810. doi: 10.1007/s00228-010-0814-5. [DOI] [PubMed] [Google Scholar]
- 13.Ghassabian S, Chetty M, Tattam BN, Chem MC, Glen J, Rahme J, Stankovic Z, Ramzan I, Murray M, McLachlan AJ. A high-throughput assay using liquid chromatography-tandem mass spectrometry for simultaneous in vivo phenotyping of 5 major cytochrome p450 enzymes in patients. Ther Drug Monit. 2009;31 (2):239–246. doi: 10.1097/FTD.0b013e318197e1bf. [DOI] [PubMed] [Google Scholar]
- 14.Yasar U, Forslund-Bergengren C, Tybring G, Dorado P, Llerena A, Sjoqvist F, Eliasson E, Dahl ML. Pharmacokinetics of losartan and its metabolite E-3174 in relation to the CYP2C9 genotype. Clin Pharmacol Ther. 2002;71(1):89–98. doi: 10.1067/mcp.2002.121216. [DOI] [PubMed] [Google Scholar]
- 15.Alfaro CL, Lam YW, Simpson J, Ereshefsky L. CYP2D6 status of extensive metabolizers after multiple-dose fluoxetine, fluvoxamine, paroxetine, or sertraline. J Clin Psychopharmacol. 1999;19(2):155–163. doi: 10.1097/00004714-199904000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Yang LH, Lu FE, Dong H, Xu LJ, Wang KF. Effect of emodin and berberine on gastrointestinal motility in type 2 diabetic rats. World Chin J Digestol. 2005;13(5):608–611. [Google Scholar]
- 17.Huang SM, Strong JM, Zhang L, Reynolds KS, Nallani S, Temple R, Abraham S, Habet SA, Baweja RK, Burckart GJ, Chung S, Colangelo P, Frucht D, Green MD, Hepp P, Karnaukhova E, Ko HS, Lee JI, Marroum PJ, Norden JM, Qiu W, Rahman A, Sobel S, Stifano T, Thummel K, Wei XX, Yasuda S, Zheng JH, Zhao H, Lesko LJ. New era in drug interaction evaluation: US Food and Drug Administration update on CYP enzymes, transporters, and the guidance process. J Clin Pharmacol. 2008;48(6):662–670. doi: 10.1177/0091270007312153. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y, Li F, Ma XC, Cheng XG, Zhou HH, Klaassen CD. CYP2D plays a major role in berberine metabolism in liver of mice and humans. Xenobiotica. 2011 doi: 10.3109/00498254.2011.597456. [DOI] [PMC free article] [PubMed] [Google Scholar]