Summary
Haplodeficiency of the microglia gene TREM2 increases risk for late-onset Alzheimer’s disease (AD) but the mechanisms remain uncertain. To investigate this, we used high-resolution confocal and super-resolution (STORM) microscopy in AD-like mice and human AD tissue. We found that microglia processes, rich in TREM2, tightly surround early amyloid fibrils and plaques promoting their compaction and insulation. In Trem2 or DAP12 haplodeficient mice and in humans with R47H TREM2 mutations, microglia had a markedly reduced ability to envelop amyloid deposits. This led to an increase in less compact plaques with longer and branched amyloid fibrils resulting in greater surface exposure to adjacent neurites. This was associated with more severe neuritic tau hyperphosphorylation and axonal dystrophy around amyloid deposits. Thus, TREM2 deficiency may disrupt the formation of a neuroprotective microglia barrier that regulates amyloid compaction and insulation. Pharmacological modulation of this barrier could be a novel therapeutic strategy for AD.
Introduction
Microglia are the resident immune cells in the central nervous system (CNS) where they constantly survey their microenvironment and become activated in various neurological disorders (Davalos et al., 2005; Hanisch and Kettenmann, 2007). In Alzheimer’s disease (AD), a striking feature of microglia is their universal clustering around amyloid-β (Aβ) deposits, one of the major pathological hallmarks of this condition. It has traditionally been thought that activated microglia remove Aβ deposits via phagocytosis (Lee and Landreth, 2010). However multiple recent lines of evidence indicate that microglia are ineffective at removing fibrillar Aβ in vivo (Condello et al., 2011; Liu et al., 2010; Stalder et al., 2001). At the same time, given their ability to secrete cytokines and reactive oxygen species, microglia have the potential to be neurotoxic (Block et al., 2007). Thus, it remains unknown whether certain aspects of microglial function play beneficial or detrimental roles that could be important for disease pathogenesis.
TREM2 (Triggering Receptor Expressed on Myeloid cells 2) gene is specifically expressed by microglia in the CNS (Schmid et al., 2002). A loss-of-function R47H (rs75932628, Arginine-47-Histidine) mutation in TREM2 constitutes one of the strongest single allele genetic risk factors for AD (Guerreiro et al., 2013; Jin et al., 2014; Jonsson et al., 2013; Korvatska et al., 2015; Ruiz et al., 2013) providing the clearest link between microglia dysfunction and AD pathogenesis. While loss of Trem2 in vitro disrupts Aβ phagocytosis by microglia (Kleinberger et al., 2014), Trem2 haplodeficient AD-like mice have shown inconsistent results with respect to the amounts of cerebral Aβ deposition (Jay et al., 2015; Ulrich et al., 2014; Wang et al., 2015). Thus it remains unclear to what extent Trem2 regulates microglia phagocytosis in vivo, suggesting that the increased risk of AD in Trem2 haplodeficiency may not be due to differences in amyloid plaque load. Interestingly, most studies in Trem2 deficient AD-like mice have shown reduced number of microglia around amyloid plaques (Jay et al., 2015; Wang et al., 2015). However, it remains unknown how subtle changes in microglia around plaques could profoundly increase the risk of AD. Furthermore, it remains unclear how these mouse studies correlate with the human pathology.
Recently, we identified a protective function of microglia, whereby their processes tightly wrap around plaques and act as a physical barrier that prevents the outward extension of amyloid fibrils (Condello et al., 2015). This barrier function promotes formation of highly compact plaque microregions that have minimal affinity for soluble Aβ42 (Figure S1). Conversely, areas not covered by microglia processes display hotspots with very high soluble Aβ42 affinity leading to markedly concentrated protofibrillar Aβ plaque regions. These Aβ hotspots are neurotoxic given that adjacent axons develop a greater extent of dystrophy compared to those next to non-hotspot regions covered by microglia (Condello et al., 2015). Despite these intriguing observations in mice, the importance of microglia in the pathogenesis of AD and more specifically the relevance of this newly discovered microglial barrier function in humans has not been demonstrated.
We thus hypothesized that disruption of the microglia barrier function is a cellular mechanism underlying association between loss of function TREM2 mutations and increased risk of AD. To test this, we used quantitative high-resolution confocal microscopy and super-resolution Stochastic Optical Reconstruction Microscopy (STORM) in both human brains of patients with R47H TREM2 mutations and in AD-like mice lacking either Trem2 or its adaptor protein DNAX-binding protein of 12 kDa (DAP12). This revealed unprecedented structural detail of the landscape of amyloid fibrils, axonal dystrophy and microglia heterogeneity around plaques in TREM2 haplodeficiency.
Our data in mice and humans indicates that microglia processes closely interact with individual amyloid fibrils in early non-compact filamentous plaques and later form specialized protrusions rich in TREM2 and DAP12 that tightly wrap around the surface of compact plaques. We then demonstrate in both TREM2 and DAP12 haplodeficiency a striking microglia phenotype in which these cells fail to polarize and engage nascent amyloid fibrils and mature compact plaques. However, this was not associated with differences in amyloid phagocytosis or plaque numbers. Instead, we observed a marked reduction in amyloid plaque compaction and a subsequent increase in plaque surface area contacting neuropil, which was associated with a significant increase in the degree of axonal dystrophy. A strikingly similar phenotype was also observed in human TREM2 R47H mutants, demonstrating that this neuroprotective microglia barrier is conserved in humans. Our data indicate that TREM2 deficiency disrupts this specialized barrier function, and may thus constitute a previously unknown cellular mechanism linking TREM2 R47H variant with increased risk of dementia. Future studies may determine whether similar mechanisms play a role in the pathogenesis of late onset AD associated with other microglia-related genetic risk alleles.
Results
Microglia processes contacting plaques are highly enriched with Trem2
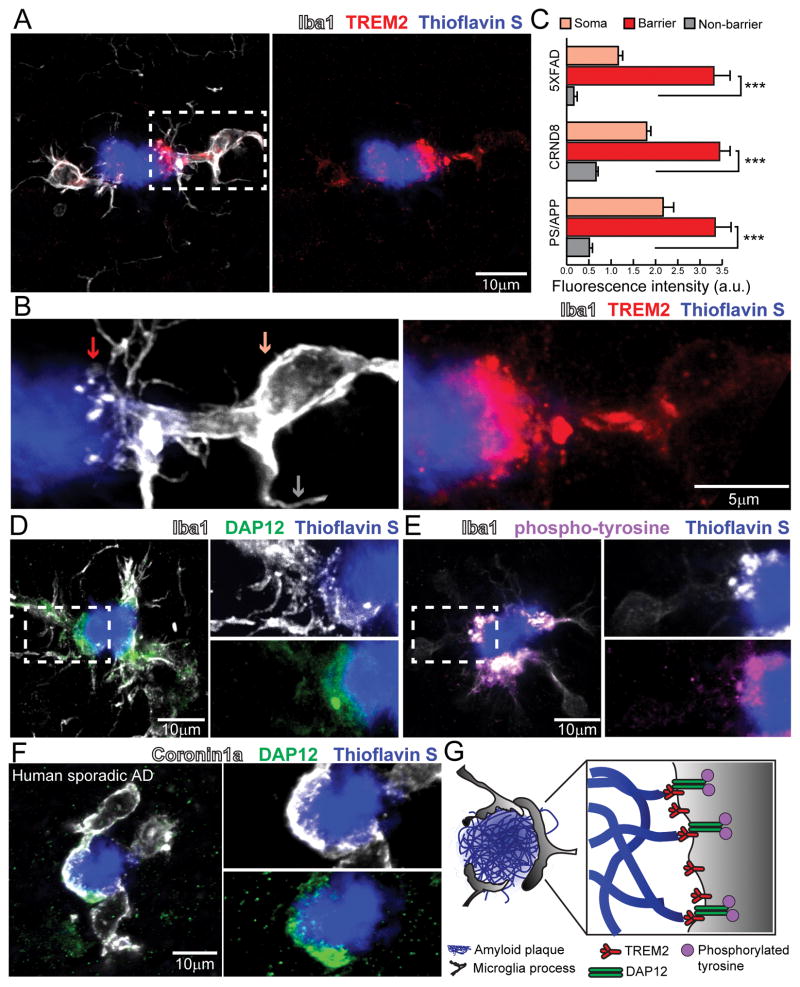
We first examined the expression pattern of Trem2 in the brains of 3 transgenic AD mouse models. Building upon previous reports (Frank et al., 2008; Lue et al., 2015; Matarin et al., 2015), high-resolution confocal microscopy revealed that Trem2 is enriched within plaque-associated microglia, but not in microglia away from plaques or other neural cells (Figure S2). Interestingly, we found that Trem2 expression was exceptionally high at the leading edges of microglia processes directly contacting amyloid plaques (Figure 1A–C). We also detected polarized expression of its signaling adaptor protein DAP12 (Figure 1D), and a marker of phosphorylated tyrosine (Figure 1E) consistent with downstream kinase-mediated signal transduction (Colonna, 2003). Similar to mice, in postmortem human brain, we found enhanced DAP12 expression specifically polarized to microglia processes contacting amyloid plaques (Figure 1F and Table S1). The robust enrichment of Trem2, DAP12 and a marker of downstream tyrosine phosphorylation, suggests that these signaling proteins could play a key role in the envelopment of the plaque surface by microglia processes and the establishment of the microglia barrier (Figure 1G).
Figure 1. Trem2-mediated signaling is activated within microglia processes directly contacting amyloid plaques.
(A) Confocal images showing the subcellular localization of immunostained Trem2 (red) within Iba1-positive microglia (white) clustered around an amyloid plaque (blue) in a 5XFAD mouse. (B) Zoomed images of Trem2 in plaque-associated microglia processes from the dashed box in A. Microglia processes were divided into 3 subcellular compartments: 1) soma (pink arrow), 2) barrier (processes contacting plaque, red arrow) and 3) non-barrier (processes not engaged with plaque, gray arrow). (C) Quantification of Trem2 fluorescence intensity in microglia associated with plaques in AD mouse models: 5XFAD, CRND8 and APPPS1-21. N=30 microglia from each model. Student t-tests were used for statistical comparisons, ***: p<0.001; a.u.: arbitrary unit. (D and E) Confocal images showing elevated levels of immunostained-DAP12 (green) and phospho-tyrosine (magenta) at the microglia-plaque interface (white). Right panels show zoomed split channels from the dashed-box. (F) Confocal images of DAP12 (green) and microglia (white) in human sporadic AD postmortem brain showing strong polarization to processes contacting plaques. (G) Diagram of specialized microglial processes wrapping around amyloid plaque microregions, with enriched TREM2 and downstream signaling proteins. All analyses were performed in mouse somatosensory cortex human middle frontal gyrus.
Trem2 haplodeficiency abolishes the microglia barrier but does not impair Aβ phagocytosis
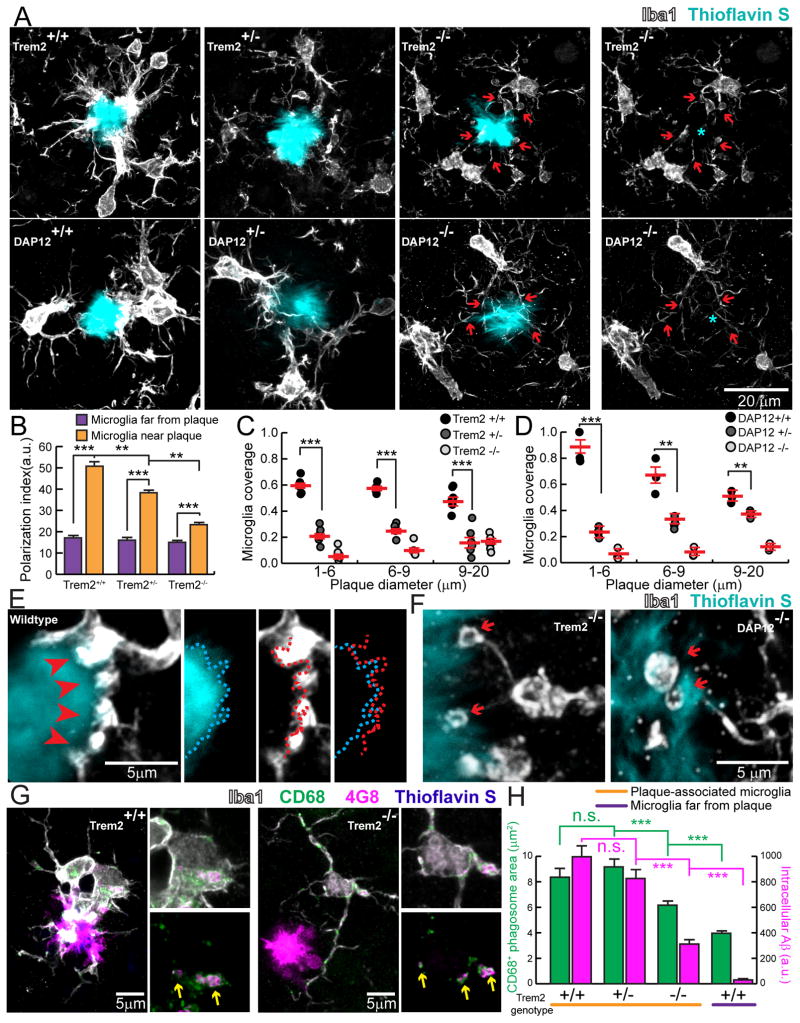
We next examined structural and functional features of microglia in AD-like transgenic mice carrying different copy numbers of the Trem2 or DAP12 genes (Figures 2, S2 and S4). High resolution confocal microscopy revealed that Trem2 or DAP12 deletion markedly reduced the degree of plaque surface coverage by microglia processes (Figure 2A–D). The dramatic change of plaque coverage in Trem2 and DAP12 deficiency cannot be explained alone by the modest ~20% reduction in the number of plaque-associated microglia (Figure S3 and S4). Instead, it appears that microglia in Trem2 or DAP12 deficiency have an impairment in the ability to polarize their processes towards the plaque surface and thus are unable to create an effective barrier around them (Figure 2A–D). Interestingly, in Trem2 deficiency, microglia processes contacting plaques did not show enrichment of phosphorylated tyrosine (Figure S3), a marker of downstream tyrosine kinase activation which is potentially necessary for triggering actin reorganization during process polarization (Peng et al., 2010). Indeed, contrary to the robust microglia processes seen in wild type mice (Figure 2E), processes contacting plaques in either Trem2 or DAP12 deficient mice displayed characteristic dysmorphic features with fine processes and terminal loops that failed to closely track the boundaries of the plaque (Figure 2F). Thus, Trem2 likely plays a critical role in signaling microglia process specialization underlying the formation of the microglia barrier around plaques.
Figure 2. Defective microglia barrier but normal Aβ phagocytosis in Trem2 or DAP12 haplodeficiency.
(A) Confocal images of Iba1-immunostained microglia (white) around thioflavin S+ amyloid plaques (cyan) in 5XFAD mice with different Trem2 genotypes or in APPPS1-21 mice with different DAP12 genotypes. Cyan asterisks indicate locations of the plaque. Red arrows point to the polarized microglia processes. (B) Quantification of microglia process polarization away from or near plaques in 5XFAD mice with different Trem2 genotypes. N=3 mice for each group; 540 microglia analyzed. (C and D) Quantification of microglia plaque coverage (“barrier”) around plaques in 5xFAD mice with different Trem2 genotypes and APPPS1-21 mice with different DAP12 genotypes. N=6 mice for each group, 2887 plaques for 5XFAD:Trem2 and 598 plaques for APPPS1-21:DAP12. (E) Microglia barrier (white) interdigitates with the surface of an amyloid plaque (cyan) in wildtype 5XFAD mice. Dotted cyan and red lines indicate thresholded borders of plaque and microglia, respectively. (F) Dysmorphic microglia processes (white) with looping structures (red arrows) in mice with Trem2 or DAP12 deficiency. (G) Confocal images of Aβ immunolabeling (4G8) within microglial phagosomes (CD68+; green) in Trem2+/+ (left panels) and Trem2−/− (right panels) from 5xFAD brain slices. Yellow arrows indicate colocalized 4G8+ Aβ (magenta) and CD68+ vesicles (green). (H) Quantification of CD68+ vesicle area and 4G8 fluorescence per microglia. N=3 mice for each group and 321 cells. Student t-tests were used for all statistical comparisons, **: p<0.01, ***: p<0.001; n.s.: p>0.05; a.u.: arbitrary unit. All analyses were performed with mouse somatosensory cortex.
To assess whether Trem2 deficiency also affected Aβ fibril engulfment by microglia, we performed immunohistochemistry and quantitative confocal image analysis to measure the Aβ content within CD68-immunolabeled microglial phagosomes, a correlate of Aβ phagocytosis efficiency. While in Trem2−/− mice, there was a significant reduction in Aβ phagocytosis, we were surprised to see that in Trem2+/− mice, a more relevant model of human TREM2 haplodeficiency leading to AD, there was no significant difference in either the number of phagosomes or amount of Aβ within phagosomes (Figure 2G–H). This suggests that a defect in microglia phagocytosis of Aβ is not the main cause of neuropathology in Trem2 deficiency. Instead it suggests that inability of microglia processes to polarize and establish an effective barrier may be a neuropathological mechanism in TREM2 R47H variant carriers.
Trem2 haplodeficiency decreases amyloid plaque compaction and increases total fibril surface area
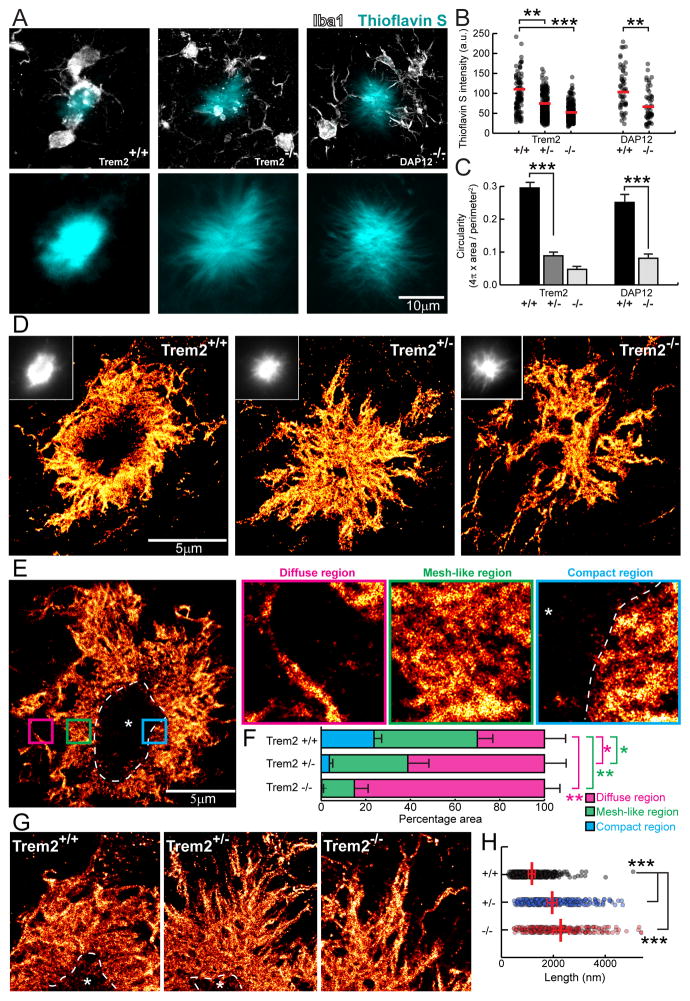
We next asked how deficiency in the microglia barrier may impact the structural organization of individual plaques. Quantitative confocal analyses of individual plaques labeled by the β-sheet binding dye thioflavin S, in Trem2 and DAP12 deficient mice, showed a reduction in the fluorescence and a diffuse plaque morphology with amyloid fibrils projecting outwards radially (Figures 3A–C). To directly observe Aβ fibrils, we next performed super-resolution nanoscopy using stochastic optical reconstruction microscopy (STORM) in brain slices fluorescently immunolabeled with an N-terminus anti-Aβ antibody. While plaques from Trem2 +/+ mice exhibited circumscribed borders, in Trem2+/− and −/− mice, plaques displayed spike-like fibrils extending radially (Figure 3D). We then defined three different plaque regions based on Aβ fibril organization: 1) diffuse region, 2) mesh-like region (likely formed by interweaving of individual Aβ fibrils); and 3) compact region (where densely packed fibrils limit antibody penetration, (Figures 3E and S5). Trem2 deficiency markedly increased the proportion of diffuse fibrils and decreased the proportion of mesh-like and compact fibrils (Figure 3F). Furthermore, loss of Trem2 increased the average length of diffuse fibrils radiating outwards from the mesh-like compact core (Figure 3G and H) (average 231nm in Trem2 +/+, 396nm in Trem2 +/− and 442nm in Trem2 −/−). This suggest that the microglia barrier prevents the radial extension of β-amyloid fibrils, promoting their intermingling and formation of mesh-like structures with various degrees of compaction. The longer amyloid fibers, coupled with their increased branching at the nano scale (Figure 4G) leads to a marked increase in fibril surface area in Trem2 deficiency (see Supplementary Information).
Figure 3. Super-resolution STORM imaging reveals a marked reduction in plaque compaction in Trem2 haplodeficiency.
(A) Confocal images of thioflavin S-labeled amyloid plaques in Trem2+/+ and Trem2−/− 5XFAD mice and DAP12−/− APPPS1-21 mice. (B and C) Quantification of mean thioflavin S fluorescence intensity and circularity (roundness) in plaques from 5XFAD mice with different Trem2 genotypes and APPPS1-21 mice with different DAP12 genotypes. N=3 mice for each group, 747 plaques for 5XFAD:Trem2 and 198 plaques for APPPS1-21:DAP12. (D and E) Immunolabeled Aβ plaque fibrils reconstructed by super-resolution STORM imaging. Images were pseudo-colored according to the reconstructed intensities. Insets in D show the same plaque imaged with wild-field illumination. Dashed line and asterisk in E indicate locations of the compact plaque core which is not labeled due to poor antibody penetration in 5XFAD Trem2+/+ mice (Figure S6). Three insets in E show high zoom examples of different types of Aβ fibril organization located in plaque regions with different degree of compaction. (F) Quantification of the proportion of diffuse, mesh-like and compact regions in amyloid plaques from 5XFAD mice with different Trem2 copy numbers. N=8 plaques from each genotype. Multiple t-tests were used to compare different regions between genotypes. Multiple comparisons were corrected with Holm-Sidak method. *: p<0.0005, **: p<0.0001. (G) Example images of fibrils within the diffuse plaque region extending radially from the plaque center in 5XFAD mice with different Trem2 genotypes. Dashed lines and asterisks indicate locations of the unlabeled compact plaque core. (H) Quantification of lengths of the diffuse fibrils in plaques of 5XFAD mice with different Trem2 copy numbers. Scatter plot shows individual data point. Red bars indicate group averages by genotypes. Except for comparison in F, Student t-tests were used for all statistical comparisons, **: p<0.01, ***: p<0.001; a.u.: arbitrary unit.
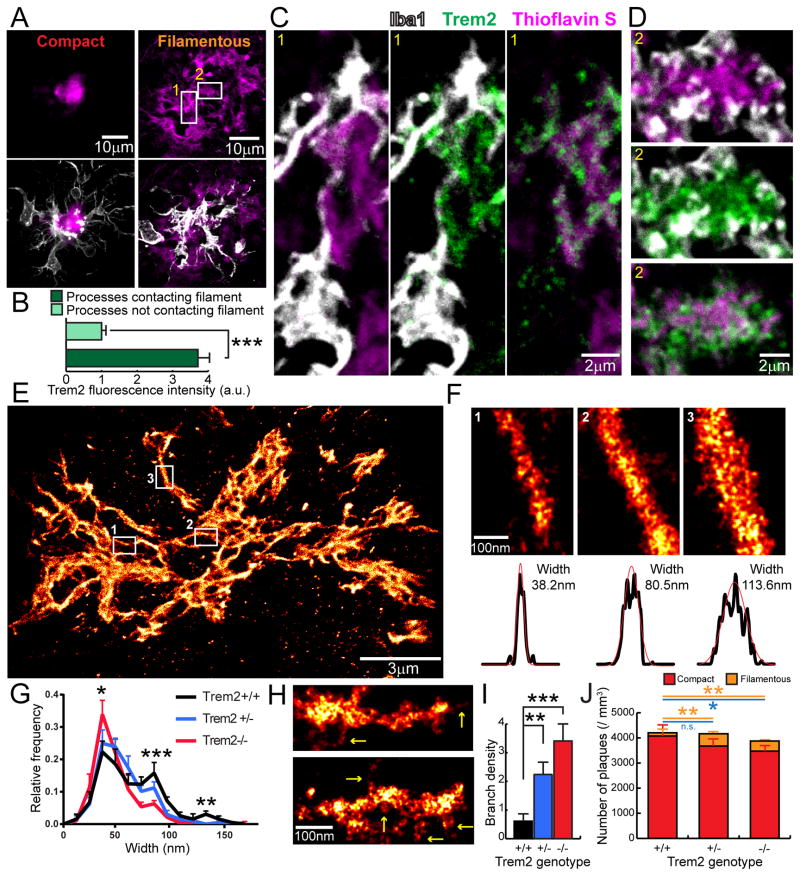
Figure 4. Trem2 deficiency impairs early-stage amyloid fibril envelopment by microglia leading to increased fibril branching.
(A) Confocal images of filamentous and compact plaques using thioflavin S labeling (magenta) in 5XFAD mice showing close interaction with surrounding microglia (anti-Iba1 immunostaining, white). (B) Quantification of Trem2 fluorescence intensity in microglia processes that do and do not contact with amyloid fibrils. N=30 plaques, student t-tests were used for statistical comparisons. (C and D) Zoomed images from insets in A, showing Trem2-enriched microglia processes wrapping around individual amyloid fibrils in a filamentous plaque. (E and F) Examples of Aβ fibril nanostructures revealed by STORM imaging. Images were pseudo-colored according to the reconstructed intensities. Three insets in E show examples of individual fibrils. By fitting the cross-section fluorescence intensity to a Gaussian distribution, the widths of the fibril were derived as 2.35 times the standard deviation of the fitted curve. (G) Distribution of the Aβ fibril widths in plaques of 5XFAD mice with different copy numbers of the Trem2 gene. N=8 plaques from each genotype, more than 200 fibrils were measured per plaque. (H) Examples of individual Aβ fibril nanostructures branching orthogonally from the primary fiber bundle revealed by STORM imaging (Yellow arrows). (I) Quantification of the frequency of branched Aβ nanostructures per 500nm in 5XFAD mice with different copy numbers of the Trem2 gene. (J) Quantification of compact (thioflavin S+ core with 4G8+ halo, red) and filamentous (diffuse filamentous thioflavin S labeling with 4G8+ halo, orange) plaque densities in 5XFAD mice with different copy numbers of the Trem2 gene. Blue lines refer to statistical analyses for total plaques. N=6 mice for each group; total 2887 plaques analyzed. Student t-tests were used for all statistical comparisons, *: p<0.05, **: p<0.01, ***: p<0.001; a.u.: arbitrary unit; n.s.: p>0.05.
Microglia processes envelop individual fibrils in early filamentous plaques and promote their compaction
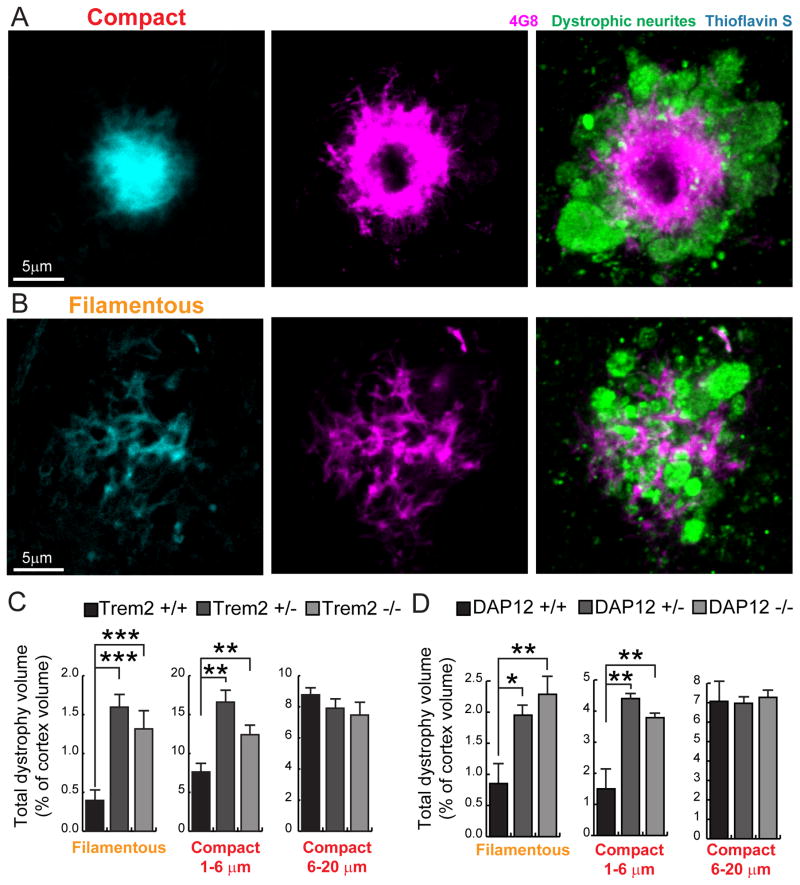
To explore the role of microglia and TREM2 deficiency in the earliest stages of amyloid deposition in mice, we obtained high-resolution confocal images of clusters of thioflavinS positive fibrils that lacked a dense core, which we termed filamentous plaques (Figure 4A). Interestingly, we observed that these plaques were highly intermingled with microglia processes (Figure 4A). Processes that directly contacted thioflavin S positive amyloid filaments were highly enriched in Trem2 (Figure 4B). These processes appeared to closely wrap around individual amyloid filaments as evidenced by the fact that the membrane-bound Trem2 was highly colocalized with the filaments, while the parent microglia branches filled with cytoplasmic Iba1 were immediately adjacent (Figure 4C). We also observed instances in which more mesh-like clusters of fibrils were fully wrapped by fine Trem2 positive microglia processes that appeared to cap individual fibril endings. (Figure 4D). This suggests that these early interactions between microglia processes and amyloid filaments could be of importance in determining the degree of fibril compaction.
To explore this possibility, we examined the nanostructures within diffuse fibrils revealed by super-resolution STORM imaging. Within individual plaques, we observed fibrils with variable widths ranging from 30nm (about the resolution limit of our STORM imaging) to 160nm (Figure 4E and F). Consistent with atomic-force microscopy data in vitro (Stine et al., 1996), this suggests that Aβ fibrils coil together to form bundle-like structures of different widths in vivo. Interestingly, Trem2 deficiency led to an increased proportion of thin fibril bundles (~40nm) and decreased proportion of thicker ones (average 68nm in Trem2 +/+, 57nm in Trem2 +/− and 48nm in Trem2 −/−) (Figure 4G). Furthermore, we observed branched structures extending orthogonally from the main Aβ fibril bundle (Figure 4H), which were markedly increased in plaques from Trem2 deficient mice (Figure 4I). Together, the increased numbers of diffuse thin fibers projecting outwards for greater lengths and the increased orthogonal branches, implies that the surface to volume ratio of amyloid fibers in Trem2 deficient mice is significantly greater than in wild type mice (We estimate a ~300–400% increase. See supplemental information). This phenomenon would be predicted to dramatically increase the contact area between amyloid fibrils and the adjacent exposed cellular structures. Importantly, while the total number of amyloid plaques was not significantly different between Trem2 deficient and wild type mice (Figure 4J and Figure S3), we observed a significant increase in the proportion of filamentous plaques in both Trem2 +/− and −/− mice (Figure 4J). The increase in filamentous plaques coupled with the greater surface area of individual plaques in Trem2+/− mice may lead to worsened neurotoxicity.
Decreased amyloid plaque compaction in Trem2 or DAP12 deficiency leads to severe axonal dystrophy
To directly test the degree of neurotoxicity, we measured the volume of axonal dystrophy around individual amyloid plaques using immunohistochemistry for Lamp1, a lysosomal protein highly enriched in dystrophic neurites (DN) that serves as a reliable marker for quantitative DN measurements (Condello et al., 2015, 2011; Gowrishankar et al., 2015). We classified plaques by morphology and size with specific attention to early filamentous plaques which were surprisingly associated with a massive amount of dystrophic neurites (Figure 5A and B). We found that Trem2 or DAP12 +/− and −/− mice had a much greater degree of axonal dystrophy than wild type mice (Figure 5C and D). Interestingly, the main difference in dystrophy occurred in non-compact filamentous and small compact plaques (up to 6μm in diameter), suggesting that the protective effect of microglia is most effective at early stages of plaque evolution. Given that filamentous plaques are associated with a surprisingly large degree of axonal dystrophy (Figure 5B), a shift of the distribution towards filamentous plaques could have critical effects on the overall degree of neural circuit disruption at early stages of amyloid accumulation.
Figure 5. Trem2 or DAP12 deficiency leads to more severe plaque-associated axonal dystrophy.
(A and B) Visualization of Lamp1-immunolabeled axonal dystrophy (green) around compact (A) and filamentous (B) plaques in AD-like mouse brains. (C–D) Quantification of total dystrophy volume in cortex of AD-like mice with different Trem2 or DAP12 genotypes. N=6 mice for each group of 5XFAD:Trem2 mice; total 2887 plaques analyzed. N=3 mice for each group of APPPS1-21:DAP12 mice; total 1076 plaques analyzed. Student t-tests were used for all statistical comparisons, *: p<0.05, **: p<0.01, ***: p<0.001; a.u.: arbitrary unit. All analyses were performed with somatosensory cortex in mice.
TREM2 R47H mutations disrupt the microglia barrier function in late-onset human AD
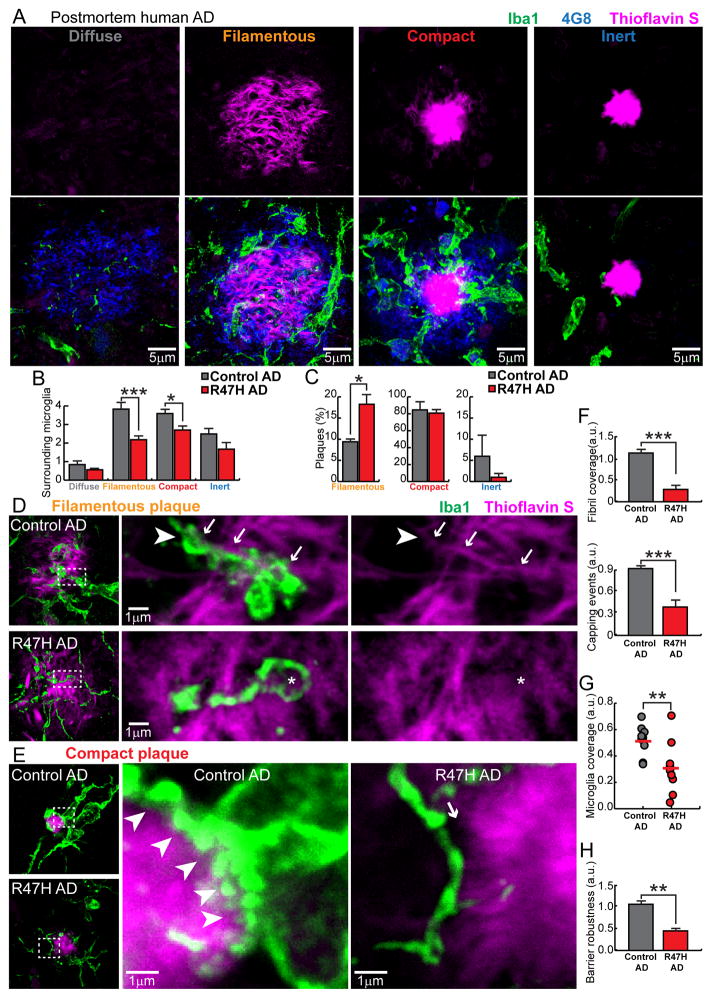
To determine whether our findings in mice are relevant for human AD pathogenesis, we next characterized the microglia and plaque phenotypes in postmortem human brains from sporadic late onset AD cases and patients with R47H TREM2 variant. Consistent with previous reports on late onset AD, we observed different types of plaque morphologies using anti-Aβ immunolabeling (4G8) and thioflavin S (TS) staining (Figure 6A). We classified them into four categories: 1) Diffuse plaques: diffuse 4G8+ fibrils lacking TS labeling. 2) Filamentous plaques: 4G8+ halo and filamentous TS labeling with no plaque core. 3) Compact plaques: 4G8+ halo and TS-labeled core. 4) Inert: TS-labeled core with no 4G8 halo. Interestingly, microglia processes did not display any polarization towards diffuse plaques but showed enlarged processes that robustly intermingled with both filamentous and compact plaques (Figure 6A and B). Moreover, plaques that had a TS-labeled core with no 4G8 halo had very few microglia processes (Figure 6A) or dystrophic neurites (Figure 7A) around them, which motivated us to call them inert.
Figure 6. TREM2 R47H mutations in human AD lead to abnormal microglia coverage around filamentous and compact plaques.
(A) Confocal images of thioflavin S-stained amyloid deposits (magenta), 4G8-labeled Aβ (blue) and Iba1-labeled surrounding microglia (green) in human postmortem AD brains, showing four different subtypes of plaques. (B) Quantification of the number of microglia within a 25 μm radius from the plaque perimeter for each plaque subtype in AD brains with and without TREM2 R47H mutation. Total 1966 plaques analyzed. (C) Quantification of the proportion of plaque subtypes that demonstrated robust microglia interactions (Diffuse plaques do not attract microglia processes and were excluded in this analysis) in AD brains with and without TREM2 R47H mutation. Total 1474 plaques analyzed. (D and E) Confocal images of microglia coverage of individual amyloid filaments (D) and the surface of compact plaques (E). Right panels show zoomed images from dashed boxes on the left panels. Arrows indicate microglia processes closely wrapping around an amyloid fibril with a “capping” structure (arrow head). Asterisk shows a dysmorphic loop structure formed by microglia in AD brains with TREM2 R47H mutation. In E, white arrow heads outline the robust microglia coverage of the plaque surface. Arrow points to the gap between a dysmorphic microglia process and the plaque border in R47H mutant. (F) Quantification of the amyloid filaments wrapped by microglia and the capping events in individual filamentous plaques. Total 226 plaques analyzed. (G and H) Quantification of compact plaque surface coverage and barrier robustness (see Figure S7) by microglia in AD with and without R47H mutations. Total 1157 plaques analyzed. For all quantifications, N=9 control AD patients and N=10 R47H AD patients. Student t-tests were used for all statistical comparisons, *: p<0.05, **: p<0.01, ***: p<0.001; a.u.: arbitrary unit. All analyses were performed on plaques from middle frontal gyrus.
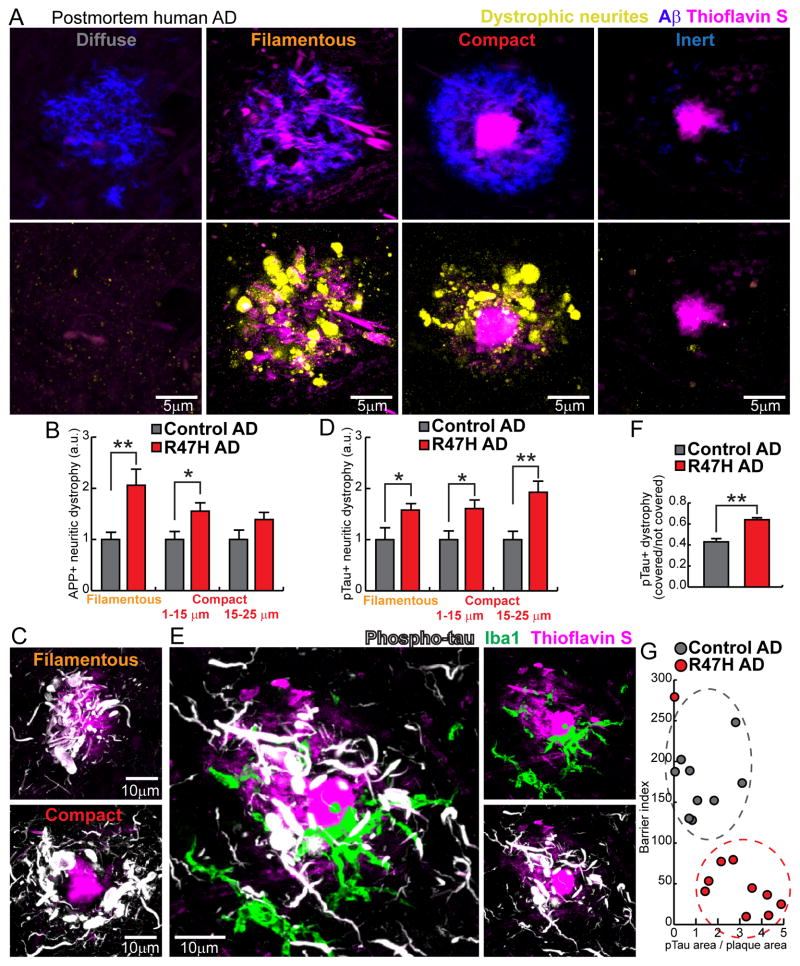
Figure 7. R47H mutation in humans is associated with severe neuritic tau hyperphosphorylation and axonal dystrophy.
(A) 4 subtypes of plaques in human brain. Diffuse plaques (anti Aβ+, thioflavin S−); Filamentous plaques (anti Aβ+, filamentous thioflavin S+); Compact plaques (anti Aβ+, thioflavin S+ compact core); Inert plaques (anti Aβ−, thioflavin S+ compact core). Lower panel: images of dystrophic neurites (labeled by anti-APP immunolabeling, yellow) around different subtypes of amyloid plaques in postmortem human brains. Notice the marked degree of dystrophy within the otherwise inconspicuous filamentous plaques. (B) Quantification of APP+ dystrophic neurites around individual plaques in AD patients with and without R47H mutations. Total 1406 plaques analyzed. (C) Confocal images of neurites with hyper-phosphorylated tau (white) around filamentous and compact plaques (magenta). Notice the marked degree of hyperphosphorylation in filamentous plaques. (D) Quantification of area neurites with phosphorylated tau around individual plaques in AD patients with and without R47H mutations. Total 1369 plaques analyzed. (E) Microglia (green) in close contact with a plaque (magenta) but anti-colocalized with hyper-phosphorylated dystrophic neurites (white). (F) Quantification of the degree of neuritic tau hyper-phosphorylation as a ratio between areas covered and not covered by microglia processes. Total 468 plaques analyzed. (G) Quantification of the degree of microglia barrier (radial coverage multiply robustness; see methods) as a function of the degree of phosphorylated tau (normalized for plaque size). Total 1008 plaques analyzed. For all quantifications, N=9 control humans and N=10 R47H humans. Student t-tests was used for all statistical comparisons, *: p<0.05, **: p<0.01; a.u.: arbitrary unit. All analyses were performed on plaques from middle frontal gyrus.
We next quantified the relative proportions of these plaque categories in AD cases with and without the R47H TREM2 mutation (Table S2). While we did not find a significant difference in the overall microglia density or amyloid plaque burden between genotypes (Figure S6), we did observe a robust increase in the proportion of plaques with filamentous morphology (Figure 6C). Remarkably, we observed a marked reduction in the number of surrounding microglia in both filamentous and compact plaques but we saw no significant microglia differences around diffuse and inert plaques (Figure 6C) or in non-plaque regions (Figure S6). Thus the R47H mutation in humans leads to a specific defect of microglia plaque engagement which is strikingly similar to the phenotype in Trem2 and DAP12 haplodeficient mice.
We then sought to determine how the observed decrease in microglia numbers in R47H mutants impacts their capacity for envelopment of individual filaments and plaque cores. Like in mice, we observed that microglia processes infiltrated filamentous plaques and had a tendency to surround individual filaments (Figure 6D). In contrast, microglia around compact plaques projected robust processes that closely enveloped the plaque surface (Figure 6E). Interestingly, while microglia in R47H mutants were able to send processes towards amyloid deposits, these appeared to be less robust and did not tightly engage with individual fibrils or the plaque surface, leading to a markedly diminished microglia coverage of amyloid aggregates (Figure 6F–H). Thus TREM2 in humans likely regulates the microglia barrier function and disruption of this function in R47H mutants is associated with inability of microglia for enveloping fibrils or amyloid deposits.
Increased tau hyperphosphorylation and axonal dystrophy around filamentous and compact plaques in human R47H mutants
To determine the consequences of a deficient microglia barrier in human AD with R47H TREM2 variant, we quantified the extent of axonal dystrophy around individual plaques. Similar to mice, we found that filamentous plaques lacking a core had a surprisingly large number of dystrophic axons, as visualized by anti-APP immunohistochemistry (Figure 7A). Interestingly, we found a dramatic increase in axonal dystrophy around both filamentous and small compact plaques in R47H mutants compared to sporadic non-mutant AD cases (Figure 7B).
These dystrophic swellings were associated with marked cytoskeletal abnormalities as evidenced by the extensive neuritic labeling with anti-phosphotau antibody around both filamentous and compact amyloid deposits (Figure 7C). Similar to our results quantifying APP positive dystrophic neurites, phosphotau labeling was markedly increased in R47H AD brains compared to sporadic AD cases (Figure 7D). Consistent with our previous findings in mice (Condello et al., 2015) (Figure S1), we observed a strong anti-colocalization between microglia processes and hyperphosphorylated tau-positive dystrophic neurites (Figure 7E and F) suggesting that microglia normally exert a protective insulating effect. This function is disrupted in R47H mutants as demonstrated by a significantly reduced degree and robustness of microglia plaque coverage associated with increased neuritic abnormalities (Figure 7G).
Discussion
Our study has uncovered previously unrecognized microglia functions that may participate in the pathogenesis of Alzheimer’s disease. We examined microglia-plaque interactions in AD-like mice lacking Trem2 and DAP12 genes as well as in postmortem brains from human AD patients with R47H mutations. This enabled us to demonstrate that the tight envelopment of fibrils and early stage plaques by microglia processes (“microglia barrier”) may play a role in inducing amyloid fibril compaction, and in reducing the surface area of potentially neurotoxic fibrils exposed to surrounding neural structures (Figure S7). This potentially neuroprotective microglial function provides a novel cellular mechanism that may explain the increased risk of Alzheimer’s disease with TREM2 genetic variants. It may also provide important insights for the more common forms of sporadic AD in which microglia senescence may drive similar functional deficits in barrier formation (Condello et al., 2015).
Microglia function in AD remains controversial with some evidence suggesting that they play a neurotoxic role by secreting reactive oxygen species and cytokines (Block et al., 2007). In advance neuropathological stages in mice, it has been shown that eliminating microglia with CSF1R antagonists leads to beneficial behavioral effects without changes in plaque numbers (Dagher et al., 2015; Olmos-Alonso et al., 2016), consistent with a toxic effect of microglia. In contrast, our results demonstrate a potentially neuroprotective role of microglia in early stages of plaque evolution, evidenced by the fact that areas covered by microglia processes develop fewer dystrophic neurites (Condello et al., 2015) and by the finding that inability of microglia to engage with plaques in R47H mutations is associated with increased axonal dystrophy in humans. Furthermore, we find it unlikely that microglia are actively involved in the formation or pruning of dystrophic axons given that we never find their processes directly surrounding or engulfing individual dystrophic neurites (Figure S7) (Condello et al., 2015).
While the function of TREM2 in peripheral immune cell responses to pathogens has been extensively studied (Colonna, 2003), its role in AD remains poorly understood. Particularly, the precise mechanisms underlying the association between increased risk for AD and R47H TREM2 variant is not known. The present study describes a novel role for Trem2 in the polarization of microglial processes to envelope Aβ deposits and form a potentially neuroprotective barrier around them. Trem2 or DAP12 haplodeficiency in AD mice dramatically reduces plaque-associated microglia and disrupts the barrier formation. Strikingly, in humans with R47H mutations, we observed a nearly identical phenotype with a marked reduction in microglia coverage of filamentous and small compact plaques that was associated with more severe axonal dystrophy and hyper-phosphorylated tau in neurites.
Surprisingly, despite in vitro studies suggesting otherwise (Kleinberger et al., 2014), we found that Aβ phagocytosis by microglia in Trem2 haplodeficiency appears to remain normal in vivo. This result suggests that rather than defective phagocytosis, disruption of the microglia barrier could be a driving mechanism underlying increased risk for AD with single allele TREM2 mutations in humans. In contrast, when complete protein loss occurred in Trem2 −/− mice, we did observe a significant reduction in phagocytosis (Figure 2G–H). Defective phagocytosis may be more relevant for explaining the much less common Nasu-Hakola disease in which homozygous TREM2 mutations cause a multi-organ condition associated with aggressive neurodegeneration but with very different neuropathological features from AD (Paloneva et al., 2000).
Interestingly, while in Trem2 and DAP12 deficient mice as well as R47H human mutants, microglia processes are unable to form a robust barrier, they are still moderately polarized, sending fine processes towards plaques (Figure 2A and B). This suggests that separate molecular mechanisms (El Khoury et al., 2003; Reed-Geaghan et al., 2009; Song et al., 2011) mediate the chemotactic plaque attraction and projection of microglia processes versus the formation of a tight and robust envelopment of amyloid fibrils and deposits, which seems to be highly dependent on Trem2 signaling. Given the recent findings that Trem2 recognizes various types of lipids that are present in amyloid plaques (Wang et al., 2015), we postulate that the extent and types of lipids decorating the plaque surface may regulate the microglia barrier through Trem2 signaling. Consistent with this hypothesis, microglia around diffuse plaques do not have a very activated and polarized morphology (Figure 6), and spectrochemical analysis shows that diffuse plaques do not have prominent lipid coating on their surface (Liao et al., 2013; Rak et al., 2007). In contrast, as amyloid deposits become filamentous and thioflavin S positive, which likely makes them more lipophilic (Hilbich et al., 1992), they robustly attract microglia processes, consistent with recent in vivo imaging studies (Jung et al., 2015). It is therefore conceivable that conditions affecting brain lipid metabolism, such as ApoE genetic polymorphisms (Kim et al., 2009) or other mechanisms that alter brain lipids (Dietschy and Turley, 2004), may affect amyloid pathology by differentially modulating the formation of a microglia barrier.
Loss of microglia barrier function leads to decreased Aβ fibril compaction as evidenced by our direct visualization of amyloid plaque architecture with super-resolution STORM imaging (Figure 3 and 4) and the observed reduced thioflavin S fluorescence intensity (Figure 3). STORM imaging revealed that plaques in Trem2 +/+ mice have a more compact organization with more mesh-like structures and fibrils exhibiting greater widths and shorter lengths than those in Trem2 +/− and −/− mice. It also showed that in Trem2 deficiency, fibrils have more nano-scale orthogonal branches originating from the primary bundle (Figure 3H and 3I). These is consistent with our previous observations that plaque regions not covered by microglia processes have a markedly higher density of Aβ protofibrils than covered regions (Condello et al., 2015).
The absolute widths measured by STORM imaging likely do not reflect the precise physical dimensions of single Aβ fibrils, given resolution limits (~30nm) and the fact that antibody labeling itself changes the size of the imaged object. Interestingly, however, the range of different widths that we observed demonstrate 3 main size peaks (~40nm, 80nm and 120nm), suggesting that STORM might be capable of differentiating unitary Aβ fibrils (with attached primary and secondary antibodies) coiling together into larger bundles, consistent with in vitro studies using atomic-force microscopy (Stine et al., 1996). Together these structural differences resulting from the lack of a microglia barrier are predicted to dramatically increase the surface area of an individual plaque (Supplemental information). Differences in the degree of plaque compaction and fibril surface area may be key factors determining the degree of plaque neurotoxicity. Plaque regions not covered by microglia processes have lower degree of amyloid compaction but greater affinity for soluble Aβ42 (Figure S1) and form hotspots of potentially neurotoxic protofibrillar Aβ42 (Condello et al., 2015). Higher protofibrillar Aβ42, together with an overall increased contact area with surrounding neurites in TREM2 deficiency, could synergistically contribute to the increased plaque neurotoxicity we observed.
One intriguing aspect of our data is that the total number of thioflavin S compact plaques was modestly reduced in Trem2 deficient mice, while filamentous amyloid plaques without a compact core increased significantly. This is consistent with our model suggesting that the microglia barrier increases plaque compaction and failure of the barrier such as in Trem2 deficiency shifts the balance of plaques towards a more diffuse phenotype without significantly altering total plaque number. This finding which is dependent on the type of labels used for amyloid plaque quantification, may partly explain the initial contradicting results reported with regards to plaque density in Trem2 deficiency (Jay et al., 2015; Wang et al., 2015).
Our surprising observation that small filamentous plaques cause a marked degree of axonal dystrophy around them in both mice and humans (Figure 5B and 7A), is consistent with the idea that less compact fibrils are more toxic than the compact ones in the plaque core. Thus the observed shift towards a less compact plaque phenotype, coupled with the greater numbers of filamentous plaques, could significantly increase the overall degree of neural circuit damage despite a stable number of total plaques. Interestingly, our finding that diffuse plaques do not cause axonal dystrophy suggest that the transition from diffuse to β-sheet rich filamentous conformation (evidenced by thioflavin S labeling) is the critical neurotoxic event that leads to dystrophy consistent with recent in vivo imaging data (Bittner et al., 2012). While filamentous plaques represent a modest proportion of all plaques in AD-like mice (Figure 4J), we found them to be more prevalent in human sporadic AD brains (Figure 6C), which is likely due to the fact that plaque growth is protracted over decades compared to months in mice (Burgold et al., 2014; Condello et al., 2011). In addition, a plaque phenotype we called inert, because its core was intensely labeled by thioflavin S but was virtually devoid of dystrophic neurites, suggests that highly compact plaques may be minimally neurotoxic. These findings urge caution against using positron emission tomography (PET) amyloid tracers with predominant affinity to dense plaques and could partly explain the modest predictive value of current PET tracers such as PIB (Kepe et al., 2013) and the weak correlation between plaque load and cognitive dysfunction in postmortem studies (Morris et al., 2014).
Our data shows that loss of the microglia barrier in mice with Trem2 deficiency has the greatest impact on axonal dystrophy in filamentous and small compact plaques, but as plaques get bigger the difference in the degree of axonal dystrophy diminishes (Figure 5). A similar effect restricted to small plaques was previously observed when we enhanced the protective effects of the microglia barrier by genetic deletion of the chemokine receptor CX3CR1 in mice (Condello et al., 2015). We hypothesize that the reason for this phenomenon is that in mice, the relatively rapid plaque growth may overwhelm the overall capacity of microglia neuroprotection and eventually most axons in their vicinity will be affected, thus causing the degree of measurable axonal dystrophy to plateau in large plaques. In human AD, we found that the effect of R47H mutations on the degree of axonal dystrophy was present over a broader range of plaque sizes than in mice (Figure 7). This is likely due to the fact that plaques are likely to grow much slower in humans. Thus, it is conceivable that the neuroprotective role of the microglia barrier in humans could be effective over more protracted time intervals than in transgenic mice.
While our study provides novel cellular phenomenology potentially relevant for understanding microglia function in both mice and humans, there remain uncertainties as to how the observed defective microglia barrier acts, at a more functional level, to cause pathological defects. Furthermore, while our findings demonstrate marked neuritic changes around plaques in TREM2 deficiency, the relative functional contribution of dystrophic neurites compared to other pathologies such as synaptic loss, cell death and neurofibrillary tangles is unclear. Thus the overall relevance of the microglia barrier function in the context of such complex neurodegenerative disorder remains to be determined.
Nevertheless, our results suggest that boosting the microglia barrier function or increasing plaque compactness by other means could constitute a novel strategy for AD therapeutics. Given the recent findings that APOE and CD33 (whose genetic variants are common risk factors for AD) interact with the TREM2 pathway (Atagi et al., 2015; Chan et al., 2015), our findings could have implications for the more prevalent forms of AD in which no mutations have been identified. Our current understanding of the cellular, molecular and biophysical mechanisms by which microglia exert this potentially neuroprotective barrier function is limited. However, future studies elucidating such mechanisms may uncover targetable pathways that could specifically enhance the barrier function in microglia and reduce risk for AD.
Experimental Procedures
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at Yale, Weill Cornell College of Medicine and Washington University. 5XFAD mice (Oakley et al., 2006) were cross-bred with Trem2 knockout mice (Ulrich et al., 2014) as previously described (Wang et al., 2015). APPPS1-21 (Radde et al., 2006) mice were cross-bred with DAP12 knockout mice to obtain DAP12 deficient mice (Bakker et al., 2000). Unless specifically indicated, 4-month-old mice were used. CRND8 mice (Courtesy of Dr. David Westaway, University of Alberta) were also used.
Human postmortem brain tissue
Formalin-fixed human postmortem brain tissue blocks were acquired from various brain banks. Detailed demographic and clinical information can be found in Table S2, including 10 AD cases with the R47H mutation (Birdsill et al., 2011; Korvatska et al., 2015) and 9 cases with comparable AD pathology but not carrying the mutation. Cases were matched for age, gender and ApoE genotype.
Immunohistochemistry
Brain slices were obtained from the mouse posterior somatosensory cortex and human middle frontal gyrus. Mouse coronal sections were cut (40μm thick) with a cryostat and human tissue was sectioned with a vibratome. Primary antibodies incubation were two days at 4-degrees (PBS with 0.2% Triton X-100 and 5% goat serum) and secondary antibodies for 6 hours, before mounting on slides with PermaFluor (Thermo Scientific, TA-030-FM). Heat-induced sodium citrate antigen retrieval was performed for TREM2, DAP12 and all human tissue staining with the following protocol: tissue was boiled in 50mM sodium citrate with 0.05% tween-20 at 95 degrees for 45 minutes and then washed with PBS. The complete list of antibodies and reagents used in this study is included in the supplemental information.
Confocal microscopy
A Leica SP5 confocal microscope was used to generate all images. Laser and detector (the GaAsP hybrid detection system, photon counting mode) settings were maintained constant. For all analyses, tiled imaging using a motorized stage was used to image across one cerebral hemisphere in mice. A 3mm2 human brain region per brain slice was imaged using a 63x oil immersion objective (N.A. 1.4) at 1024 × 1024 pixel resolution, z-step size of 3μm, and identity was blinded for analyses (Yuan and Grutzendler, 2016). Images were processed with FIJI (ImageJ) software. A customized macro was used to segment individual amyloid plaque. The number of plaques analyzed ranged from 150 to 300 in each brain (see supplemental information).
STORM imaging
Samples were mounted in a buffer consisting of 90% glycerol and 10% 10xPBS with an overall concentration of 10mM cysteamine and 50mM sodium sulfite (J.T. Baker 3922–01). Images were recorded on a modified Nikon Ti-E inverted microscope using a 60× 1.49 N.A. oil immersion objective using an EMCCD camera (Andor IXon Ultra DU-897U-CS0-#BV). The objective was mounted on a custom mount to suppress thermal and mechanical drift and focusing was provided by a piezoelectric objective positioner (Physik Instrumente P-725.4CA). For illumination a 642nm solid-state laser (Omicron Lux, 20 kW/cm2 at the sample) was used with a dichroic (Semrock, Di02-R635-25×36) and a band-pass filter (Semrock, BLP01-647R-25) for separation of excitation and emission. Field of view were selected using metal halide lamp (Prior Lumen 200) illumination before switching to laser illumination and were then imaged for approximately 20,000 frames using an exposure time of 25 ms and an EM-multiplication setting corresponding to a gain of 35.5. Events were detected and localized (Baddeley et al., 2009) and reconstructions were rendered using jittered triangulation (Baddeley et al., 2010) (see supplemental information).
Statistics
Data represented as mean ± s.e.m. Two-tail unpaired Student’s t-tests were employed for comparisons between two groups. A probability of p<0.05 was considered indicative of significant differences between groups.
Supplementary Material
Acknowledgments
We thank Lingling Ji (Yale University) for consultation on statistics and Katie N. Murray (Yale University) for critical reading of the manuscript. We thank the University of Washington Alzheimer’s Disease Research Center Neuropathology Core for provision of human brain tissues (supported by NIA P50 AG05136). We thank Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona for the provision of human brain tissues, supported by the NINDS (U24 NS072026), the NIA (P30 AG19610), the Arizona Department of Health Services (contract 211002), and the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001). We thank Dennis W. Dickson and Michael Deture at Mayo Clinic (Jacksonville, Florida) for providing additional human brain tissues. We thank the Helen and Robert Appel Alzheimer’s Disease Research Institute for providing funding; Xiaoyu Hu for providing DAP12 knockout mice and Mathias Jucker for providing APPPS1-21 mice. This project was supported by R01HL106815 and R21AG048181 (J.G.).
Footnotes
Conflict of Interest
The authors claim no conflict of interest
Author Contributions
P.Y., C.C. and J.G. designed the study. P.Y., C.C. performed experiments. D.B. and P.Y. performed STORM microscopy and analyzed the images. P.Y., C.C. and J.G. analyzed data. P.Y., C.C. and J.G. prepared the manuscript. C.D.K., T.D.B., S.M.P., W.L., Y.W. and M.C. provided critical reagents. J.G. supervised the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atagi Y, Liu CC, Painter MM, Chen XF, Verbeeck C, Zheng H, Li X, Rademakers R, Kang SS, Xu H, Younkin S, Das P, Fryer JD, Bu G. Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) J Biol Chem. 2015;290:26043–50. doi: 10.1074/jbc.M115.679043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley D, Cannell MB, Soeller C. Visualization of localization microscopy data. Microsc Microanal. 2010;16:64–72. doi: 10.1017/S143192760999122X. [DOI] [PubMed] [Google Scholar]
- Baddeley D, Jayasinghe ID, Cremer C, Cannell MB, Soeller C. Light-induced dark states of organic fluochromes enable 30 nm resolution imaging in standard media. Biophys J. 2009;96:L22–4. doi: 10.1016/j.bpj.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker AB, Hoek RM, Cerwenka A, Blom B, Lucian L, McNeil T, Murray R, Phillips LH, Sedgwick JD, Lanier LL. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity. 2000;13:345–53. doi: 10.1016/s1074-7613(00)00034-0. [DOI] [PubMed] [Google Scholar]
- Birdsill AC, Walker DG, Lue L, Sue LI, Beach TG. Postmortem interval effect on RNA and gene expression in human brain tissue. Cell Tissue Bank. 2011;12:311–8. doi: 10.1007/s10561-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner T, Burgold S, Dorostkar MM, Fuhrmann M, Wegenast-Braun BM, Schmidt B, Kretzschmar H, Herms J. Amyloid plaque formation precedes dendritic spine loss. Acta Neuropathol. 2012;124:797–807. doi: 10.1007/s00401-012-1047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Burgold S, Filser S, Dorostkar MM, Schmidt B, Herms J. In vivo imaging reveals sigmoidal growth kinetic of β-amyloid plaques. Acta Neuropathol Commun. 2014;2:30. doi: 10.1186/2051-5960-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, White CC, Winn PA, Cimpean M, Replogle JM, Glick LR, Cuerdon NE, Ryan KJ, Johnson KA, Schneider JA, Bennett DA, Chibnik LB, Sperling RA, Bradshaw EM, De Jager PL. CD33 modulates TREM2: convergence of Alzheimer loci. Nat Neurosci. 2015;18:1556–8. doi: 10.1038/nn.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3:445–53. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- Condello C, Schain A, Grutzendler J. Multicolor time-stamp reveals the dynamics and toxicity of amyloid deposition. Sci Rep. 2011 doi: 10.1038/srep00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condello C, Yuan P, Schain A, Grutzendler J. Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat Commun. 2015;6:6176. doi: 10.1038/ncomms7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher NN, Najafi AR, Kayala KMN, Elmore MRP, White TE, Medeiros R, West BL, Green KN. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J Neuroinflammation. 2015;12:139. doi: 10.1186/s12974-015-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WBB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–97. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197:1657–66. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Burbach GJ, Bonin M, Walter M, Streit W, Bechmann I, Deller T. TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia. 2008;56:1438–47. doi: 10.1002/glia.20710. [DOI] [PubMed] [Google Scholar]
- Gowrishankar S, Yuan P, Wu Y, Schrag M, Paradise S, Grutzendler J, De Camilli P, Ferguson SM. Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer’s disease amyloid plaques. Proc Natl Acad Sci U S A. 2015;112:E3699–708. doi: 10.1073/pnas.1510329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JSK, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368:117–27. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UKK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hilbich C, Kisters-Woike B, Reed J, Masters CL, Beyreuther K. Substitutions of hydrophobic amino acids reduce the amyloidogenicity of Alzheimer’s disease beta A4 peptides. J Mol Biol. 1992;228:460–73. doi: 10.1016/0022-2836(92)90835-8. [DOI] [PubMed] [Google Scholar]
- Jay TR, Miller CM, Cheng PJ, Graham LC, Bemiller S, Broihier ML, Xu G, Margevicius D, Karlo JC, Sousa GL, Cotleur AC, Butovsky O, Bekris L, Staugaitis SM, Leverenz JB, Pimplikar SW, Landreth GE, Howell GR, Ransohoff RM, Lamb BT. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J Exp Med. 2015;212:287–295. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SC, Benitez BA, Karch CM, Cooper B, Skorupa T, Carrell D, Norton JB, Hsu S, Harari O, Cai Y, Bertelsen S, Goate AM, Cruchaga C. Coding variants in TREM2 increase risk for Alzheimer’s disease. Hum Mol Genet. 2014;23:5838–46. doi: 10.1093/hmg/ddu277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368:107–16. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CKE, Keppler K, Steinbach S, Blazquez-Llorca L, Herms J. Fibrillar amyloid plaque formation precedes microglial activation. PLoS One. 2015;10:e0119768. doi: 10.1371/journal.pone.0119768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepe V, Moghbel MC, Långström B, Zaidi H, Vinters HV, Huang SC, Satyamurthy N, Doudet D, Mishani E, Cohen RM, Høilund-Carlsen PF, Alavi A, Barrio JR. Amyloid-β positron emission tomography imaging probes: a critical review. J Alzheimers Dis. 2013;36:613–31. doi: 10.3233/JAD-130485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger G, Yamanishi Y, Suárez-Calvet M, Czirr E, Lohmann E, Cuyvers E, Struyfs H, Pettkus N, Wenninger-Weinzierl A, Mazaheri F, Tahirovic S, Lleó A, Alcolea D, Fortea J, Willem M, Lammich S, Molinuevo JL, Sánchez-Valle R, Antonell A, Ramirez A, Heneka MT, Sleegers K, van der Zee J, Martin JJ, Engelborghs S, Demirtas-Tatlidede A, Zetterberg H, Van Broeckhoven C, Gurvit H, Wyss-Coray T, Hardy J, Colonna M, Haass C. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med. 2014;6:243ra86. doi: 10.1126/scitranslmed.3009093. [DOI] [PubMed] [Google Scholar]
- Korvatska O, Leverenz JB, Jayadev S, McMillan P, Kurtz I, Guo X, Rumbaugh M, Matsushita M, Girirajan S, Dorschner MO, Kiianitsa K, Yu CE, Brkanac Z, Garden GA, Raskind WH, Bird TD. R47H Variant of TREM2 Associated With Alzheimer Disease in a Large Late-Onset Family: Clinical, Genetic, and Neuropathological Study. JAMA Neurol. 2015;72:920–7. doi: 10.1001/jamaneurol.2015.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CYD, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J Neural Transm. 2010;117:949–60. doi: 10.1007/s00702-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CR, Rak M, Lund J, Unger M, Platt E, Albensi BC, Hirschmugl CJ, Gough KM. Synchrotron FTIR reveals lipid around and within amyloid plaques in transgenic mice and Alzheimer’s disease brain. Analyst. 2013;138:3991–7. doi: 10.1039/c3an00295k. [DOI] [PubMed] [Google Scholar]
- Liu Z, Condello C, Schain A, Harb R, Grutzendler J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. J Neurosci. 2010;30:17091–101. doi: 10.1523/JNEUROSCI.4403-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Schmitz CT, Serrano G, Sue LI, Beach TG, Walker DG. TREM2 Protein Expression Changes Correlate with Alzheimer’s Disease Neurodegenerative Pathologies in Post-Mortem Temporal Cortices. Brain Pathol. 2015;25:469–80. doi: 10.1111/bpa.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarin M, Salih DA, Yasvoina M, Cummings DM, Guelfi S, Liu W, Nahaboo Solim MA, Moens TG, Paublete RM, Ali SS, Perona M, Desai R, Smith KJ, Latcham J, Fulleylove M, Richardson JC, Hardy J, Edwards FA. A genome-wide gene-expression analysis and database in transgenic mice during development of amyloid or tau pathology. Cell Rep. 2015;10:633–44. doi: 10.1016/j.celrep.2014.12.041. [DOI] [PubMed] [Google Scholar]
- Morris GP, Clark IA, Vissel B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s disease. Acta Neuropathol Commun. 2014;2:135. doi: 10.1186/s40478-014-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–40. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos-Alonso A, Schetters STT, Sri S, Askew K, Mancuso R, Vargas-Caballero M, Holscher C, Perry VH, Gomez-Nicola D. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain. 2016 doi: 10.1093/brain/awv379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloneva J, Kestilä M, Wu J, Salminen A, Böhling T, Ruotsalainen V, Hakola P, Bakker AB, Phillips JH, Pekkarinen P, Lanier LL, Timonen T, Peltonen L. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000;25:357–61. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, Humphrey MB. TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci Signal. 2010;3:ra38. doi: 10.1126/scisignal.2000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jäggi F, Wolburg H, Gengler S, Haass C, Ghetti B, Czech C, Hölscher C, Mathews PM, Jucker M. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006;7:940–6. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak M, Del Bigio MR, Mai S, Westaway D, Gough K. Dense-core and diffuse Abeta plaques in TgCRND8 mice studied with synchrotron FTIR microspectroscopy. Biopolymers. 2007;87:207–17. doi: 10.1002/bip.20820. [DOI] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29:11982–92. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Dols-Icardo O, Bullido MJ, Pastor P, Rodríguez-Rodríguez E, López de Munain A, de Pancorbo MM, Pérez-Tur J, Alvarez V, Antonell A, López-Arrieta J, Hernández I, Tárraga L, Boada M, Lleó A, Blesa R, Frank-García A, Sastre I, Razquin C, Ortega-Cubero S, Lorenzo E, Sánchez-Juan P, Combarros O, Moreno F, Gorostidi A, Elcoroaristizabal X, Baquero M, Coto E, Sánchez-Valle R, Clarimón J. Assessing the role of the TREM2 p.R47H variant as a risk factor for Alzheimer’s disease and frontotemporal dementia. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Schmid CD, Sautkulis LN, Danielson PE, Cooper J, Hasel KW, Hilbush BS, Sutcliffe JG, Carson MJ. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J Neurochem. 2002;83:1309–20. doi: 10.1046/j.1471-4159.2002.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Jin J, Lim JE, Kou J, Pattanayak A, Rehman JA, Kim HD, Tahara K, Lalonde R, Fukuchi KI. TLR4 mutation reduces microglial activation, increases Abeta deposits and exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. J Neuroinflammation. 2011;8:92. doi: 10.1186/1742-2094-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder M, Deller T, Staufenbiel M, Jucker M. 3D-Reconstruction of microglia and amyloid in APP23 transgenic mice: no evidence of intracellular amyloid. Neurobiol Aging. 2001;22:427–34. doi: 10.1016/s0197-4580(01)00209-3. [DOI] [PubMed] [Google Scholar]
- Stine WB, Snyder SW, Ladror US, Wade WS, Miller MF, Perun TJ, Holzman TF, Krafft GA. The nanometer-scale structure of amyloid-beta visualized by atomic force microscopy. J Protein Chem. 1996;15:193–203. doi: 10.1007/BF01887400. [DOI] [PubMed] [Google Scholar]
- Ulrich JD, Finn MB, Wang Y, Shen A, Mahan TE, Jiang H, Stewart FR, Piccio L, Colonna M, Holtzman DM. Altered microglial response to Aβ plaques in APPPS1-21-21 mice heterozygous for TREM2. Mol Neurodegener. 2014;9:20. doi: 10.1186/1750-1326-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR, Colonna M. TREM2 Lipid Sensing Sustains the Microglial Response in an Alzheimer’s Disease Model. Cell. 2015;160:1061–71. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Grutzendler J. Attenuation of -Amyloid Deposition and Neurotoxicity by Chemogenetic Modulation of Neural Activity. J Neurosci. 2016;36:632–641. doi: 10.1523/JNEUROSCI.2531-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.