Abstract
Background
Anorexia nervosa (AN) and body dysmorphic disorder (BDD) are characterized by distorted body image and are frequently comorbid with each other, although their relationship remains little studied. While there is evidence of abnormalities in visual and visuospatial processing in both disorders, no study has directly compared the two. We used two complementary modalities – event-related potentials (ERP) and fMRI – to test for abnormal activity associated with early visual signaling.
Methods
We acquired fMRI and ERP data in separate sessions from 15 unmedicated individuals in each of three groups (weight-restored AN, BDD, and healthy controls) while they viewed images of faces and houses of different spatial frequencies. We used joint independent component analyses to compare activity in visual systems.
Results
AN and BDD groups demonstrated similar hypoactivity in early secondary visual processing regions and the dorsal visual stream when viewing low spatial frequency faces, linked to the N170 component, as well as in early secondary visual processing regions when viewing low spatial frequency houses, linked to the P100 component. Additionally, the BDD group exhibited hyperactivity in fusiform cortex when viewing high spatial frequency houses, linked to the N170 component. Greater activity in this component was associated with lower attractiveness ratings of faces.
Conclusions
Results provide preliminary evidence of similar abnormal spatio-temporal activation in AN and BDD for configural/holistic information for appearance- and nonappearance-related stimuli. This suggests a common phenotype of abnormal early visual system functioning, which may contribute to perceptual distortions.
Keywords: face processing, house processing, joint ICA, electroencephalography, event-related potential, dorsal/ventral visual streams
Introduction
Individuals with body dysmorphic disorder (BDD) and those with anorexia nervosa (AN) express distortions in how the body is perceived, which suggests underlying abnormalities in visual information processing. In BDD, the cardinal diagnostic psychopathology is preoccupation with perceived defects in appearance, which are unnoticeable, or slight, to others (American Psychiatric Association 2013). Sufferers experience significant distress, disability, and functional impairment, often accompanied by depression and, in many, suicidality (Phillips 2005). The pathophysiology is likely complex (Monzani et al. 2012), including frontostriatal dysfunction (Rauch et al. 2003; Feusner et al. 2010a), emotion recognition (Buhlmann et al. 2002, 2004; Feusner et al. 2010c), and atypical visual processing (Feusner et al. 2007, 2010c). Regarding the latter, functional magnetic resonance imaging (fMRI) studies using own-face (Feusner et al. 2010a), other-face (Feusner et al. 2007), and house stimuli (Feusner et al. 2011) point to abnormalities in primary and secondary visual processing systems, particularly when images are filtered to selectively convey configural and holistic information. In accord with these results, one preliminary EEG study found increased N170 latencies in BDD subjects, suggesting increased use of detailed visual processing (Li et al. 2013).
Several behavioral studies in BDD suggest imbalances in global (configural and/or holistic) and local (detailed) visual processing, although the results are not entirely consistent. Several of these studies have tested the inversion effect, which is the phenomenon that recognition of inverted faces (or other naturalistic stimuli) is normally slower and less accurate compared to upright faces, due to the absence of a holistic template for inverted faces. One study found reduced face inversion effects in BDD compared to controls for long but not short duration stimuli, suggesting a greater propensity for detailed and piecemeal processing of faces, whether upright or inverted (Feusner et al. 2010b). Another study found that individuals with BDD had superior recognition of inverted famous faces relative to controls; this reduced inversion effect may also be an indication of greater focus on single facial features (Jefferies et al. 2012). A study using inverted faces, scenes, and bodies found that individuals with high degree of body dysmorphic concerns also had reduced inversion effects (Mundy & Sadusky 2014). Individuals with BDD were found to be slower and less accurate on the Embedded Figures Test (EFT) and the Navon task, suggesting abnormal global and local processing (Kerwin et al. 2014). However, a study examining holistic processing using the face inversion effect, composite face effect, and Navon task, found that the BDD and control groups performed similarly on all three tasks (Monzani et al. 2013). Thus, while evidence exists for abnormal global and/or local processing, the findings are still somewhat inconclusive. This may be attributed to differences in experimental conditions (e.g. stimulus duration), insufficient power, or else there may be nuances due to heterogeneity within BDD samples.
In AN, the image distortion is the perception of excess weight and fatness, culminating in a marked restriction of energy intake and lowering of body mass. Several studies (although not all) investigating neurocognition in AN have found enhanced local (detail) processing at the expense of visuospatial processing that is more global and integrated (Lopez et al. 2008; Urgesi et al. 2013). On the Rey-Osterrieth Complex Figures Task, which requires recall and re-creation of a complex figure, AN performed worse (Lopez et al. 2009; Kim et al. 2011) or equal (Sherman et al. 2006; Lopez et al. 2008; Castro-Fornieles et al. 2009; Danner et al. 2012; Stedal et al. 2012) relative to controls. Several of these studies found that those with AN drew detailed aspects of the figure first and showed less continuity in reproduction (Sherman et al. 2006; Lopez et al. 2008; Stedal et al. 2012). Individuals with AN have also been found to be faster and more accurate on the EFT, suggesting superior detail-focused processing (Jolliffe & Baron-Cohen 1997; Booth et al. 2003). Other studies have found superior attention to detail and poor central coherence compared with controls, in active and recovered AN participants and their sisters (Tenconi et al. 2010; Roberts et al. 2013). Several functional and structural imaging studies support these abnormal visual processing findings in AN. An fMRI study using the EFT found greater activation in the fusiform gyrus in AN compared with healthy controls (Fonville et al. 2013), suggesting a strategy marked by enhanced ventral visual stream activity, which is responsible for detailed image elements (Iidaka et al. 2006). Furthermore, an fMRI study by Favaro et al. 2012 found decreased brain connectivity in a ventral visual network in both underweight and weight restored individuals with AN, but hyperconnectivity within the somatosensory network only in the underweight group. Structurally, AN also show decreased gray matter density in the left extrastriate body area, an area in the occipital cortex involved in processing human body parts, compared to controls (Suchan et al. 2010). These neurocognitive and brain imaging studies suggest the possibility of aberrant visual processing in AN, although the body of research is still growing.
Thus, a potentially important clinical phenotype in AN and BDD is perceptual distortion of appearance. Both disorders tend to display a common pattern of abnormalities in visual processing and visuospatial organization, manifesting as over-attention to details with less global perception and feature integration (Madsen et al. 2013). However, no study has directly compared visual processing in these disorders.
A limitation of fMRI studies is their relatively poor temporal resolution, typically on the order of 2–3 seconds. Because of this it remains unclear if abnormal neural activation or connectivity patterns in visual systems result primarily from aberrant early visual cortex activity, or are due primarily to modulation from prefrontal and/or limbic systems. This question, however, is well suited for electroencephalography (EEG), which, unlike fMRI, allows characterization of fast changing neuronal dynamics. EEG has a time resolution on the order of milliseconds, but has limited spatial resolution, while fMRI has the capability to localize, with higher spatial resolution, changes in blood oxygenation in the brain on the order of millimeters. Therefore, a combination of fMRI and EEG can be used to generate a joint spatiotemporal profile that leverages spatial and temporal resolution advantages of the respective modalities.
Herein we report what is, to our knowledge, the first use of this dual modality approach to study visual processing in BDD and AN. We analyzed the P100 component of the ERP, which indexes first-order processing localized to V1 and V2 and early dorsal visual stream (Itier & Taylor 2004), and the N170, which indexes higher level visual processing and structural encoding, and has sources including the ventral visual stream and posterior fusiform gyrus (Pascual-Marqui 1999). (As our principal interest was in early visual processing, we did not study P300 or N400.) We used independent component analysis of task-related fMRI data in combination with ERP data from a separate session, using the same stimuli and paradigm to perform joint independent component analysis (jICA). jICA combines data from these two modalities by joint estimation of the temporal ERP components and spatial fMRI components (Calhoun et al. 2006) (see Suppl. Information). For task stimuli, we used faces and houses. Faces are appearance-related stimuli that have been extensively studied using fMRI and EEG (Bentin et al. 1996; Costen et al. 1996; Bartlett et al. 2003). We chose to use face rather than body images, as visual EEG profiles in response to body images have not been well characterized. Houses have a visuospatial complexity similar to faces, but they are of neutral salience, and have been previously studied using fMRI and EEG (Epstein & Kanwisher 1998; Iidaka et al. 2006; Desjardins & Segalowitz 2013). We hypothesized that AN and BDD will differ from healthy controls (HC) in configural processing of unaltered face and house stimuli (normal spatial frequency – NSF), and in processing the same stimuli when they are filtered to contain only low spatial frequency (LSF) information. Although our overarching hypothesis was that AN and BDD may express similar abnormal visual processing phenotypes, we did not expect to prove phenotypic or endophenotypic equivalence, both due to the preliminary nature of this study and the complexity inherent within psychiatric diagnostic categories.
We tested three specific primary hypotheses:
AN and BDD would demonstrate hypoactivity compared with HC: a) in early dorsal stream regions for the P100 component; and b), ventral stream regions for the N170 component. We predicted that this would be observed for LSF faces and houses, as well as NSF images because they contain LSF information.
Symptom severity in both groups will correlate negatively with activity in early dorsal and ventral stream regions for NSF and LSF joint P100 and N170 components.
Neither BDD nor AN will differ from HC in visual system response to high spatial frequency (HSF) images, as these were not detected in previous fMRI studies using HSF faces and houses (Feusner et al. 2007, 2011).
Methods and Materials
We recruited 15 AN, 15 BDD, and 15 HC participants, of equivalent age and sex, from UCLA and local treatment centers, as well as from the community through print and online advertisements. Diagnoses of clinical participants were confirmed through detailed interviews conducted by three of the authors (JDF, MS and CB), which included the Mini International Neuropsychiatric Interview (MINI) to determine a primary diagnosis of AN and/or comorbid diagnoses (Sheehan et al. 1998). The BDD Diagnostic Module (Phillips et al. 1995), a 6-item clinician-administered structured interview based on DSM-IV criteria for BDD, was used to make a primary diagnosis of BDD. Severity of concurrent psychiatric symptoms was measured using validated clinical scales: (Hamilton Anxiety Rating Scale (HAMA; Hamilton 1960); Brown Assessment of Beliefs Scale (BABS; Eisen et al. 1998); Montgomery-Asberg Depression Rating Scale (MADRS; Williams & Kobak 2008). BDD participants also received the BDD version of the Yale–Brown Obsessive–Compulsive Scale (BDD-YBOCS; (Phillips et al. 1997), and AN participants received the Eating Disorder Examination V16.0D (EDE; Fairburn et al. 2008). We did not administer the EDE to BDD participants, nor did we administer the BDD-YBOCS to AN participants, as these scales have not been validated in these populations.
Inclusion/Exclusion criteria
Participants were free from psychoactive medications for at least 8 weeks prior to entering the study. Individuals who met criteria for BDD by the BDD Diagnostic Module (Phillips et al. 1997) modeled after the DSM-IV were eligible. AN participants were weight-restored (BMI of ≥18.5), but they must have previously met full DSM-IV criteria for AN at some point in their lifetime, as determined by the MINI. We chose to study weight-restored AN individuals to avoid confounds of starvation on brain activity. For individuals with comorbid diagnoses, they must have had a primary diagnosis of AN or BDD based on symptom severity to be eligible.
(See Suppl. Information for additional inclusion/exclusion criteria; and Table 1 and S8 for categories and specific areas of BDD appearance concerns, respectively.)
Table 1. Demographics and Psychometrics.
Demographics and Psychometrics for AN, BDD, and HC participants. Letter superscripts that are different indicate significant pairwise differences from post hoc t-tests at p<.05.
| Demographics | Anorexia Nervosa (AN) | Body Dysmorphic Disorder (BDD) | Healthy Control (HC) | Statistical values |
|---|---|---|---|---|
| Total Subjects (N) | 15 | 15 | 15 | |
| Gender (F/M) | 13/2 | 13/2 | 13/2 | |
| Age | 23.6±3.46 | 24.93±5.15 | 22.07±3.85 | F=1.7, p=.18 |
| Years of Education | 14.79±2.22 | 16.36±3.86 | 14.3±2.45 | F=2.0, p=.15 |
| EDE Score | 2.96±1.42 | N/A | N/A | |
| BDD-YBOCS Score | N/A | 29.07±4.79 | N/A | |
| BABS Score | 12.31±6.51 | 14.6±3.31 | N/A | t=1.19, p=1.20 |
| HAMA Score | 6.67±5.98a | 9.2±6.91a | 2.27±1.94b | F=6.3, p=.003 |
| MADRS Score | 8.93±9.14a | 14±6.78a | 1.07±1.22b | F=13.6, p<.0001 |
| BMI | 20.36±1.61a | 22.92±3.68b | 21.43±1.85 | F=3.8, p=.03 |
| BDD appearance concerns (number of individuals in each category) | Face only: 7 Non-face only: 5 Face and non-face: 3 |
Face and house matching task
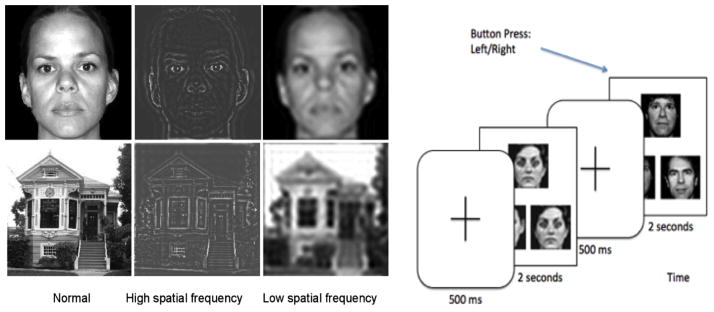
All participants performed a visual matching task in the fMRI scanner, and then performed the same tasks during a separate EEG session on a separate day. Participants were brought back for the EEG session after a mean of 191.8±305.7 days (range: 1–1006). The mean interval between sessions was not significantly different among groups (F2,42=2.08, p=.14). The stimuli (Fig. 1) were face and house photographs that were NSF, or filtered to LSF or HSF, and circles/ovals or squares/rectangles as control images (see Feusner et al. 2007b). During the presentation, participants pressed a button corresponding to which of two images matched the target image in the top half of the screen. The face and house orders, for NSF, LSF, and HSF, were counterbalanced across participants. There were 72 trials for each category; each trial consisted of a 500 ms crosshair, then 4 seconds of face or house presentation (2 seconds for the EEG session, given constraints on total time) during which button responses were recorded.
Fig. 1.
Left: Example stimuli used in the experiment, consisting of digital images filtered to normal, high, and low spatial frequencies. Right: Task paradigm, consisting of a face- or house-matching task.
EEG Acquisition and Processing
All participants were seated 1 m from the screen. EEG data were recorded using a high-density 256-channel Geodesic Hydrocel Sensor Net (Electrical Geodesics, Inc.) with a sampling rate of 250 Hz, in a dimly lit, copper-shielded room. Between experiments, electrode impedances below 50kΩ were ensured by reapplying saline solution to high impedance electrodes. Data preprocessing included bandpass filtering from 0.1 to 30 Hz for visual ERPs.
Artifact Detection
(See Suppl. Information)
Segmentation
Data were segmented 200 msec before and 500 msec after stimuli presentation. Segments were averaged across each stimuli condition, and grand averaged over all participants. ERP were baseline-corrected using the 200 msec baseline prior to the stimulus onset. All channels were referenced to an average reference of all electrodes except electrooculography.
fMRI Acquisition and Processing
Blood oxygen level dependent (BOLD) contrast images were acquired using a 3-T Trio MRI system (Siemens AG, Munich, Germany). We used a T2*-weighted echoplanar imaging gradient-echo pulse sequence (repetition time, 2.5 seconds; echo time, 25 milliseconds; flip angle, 80; acquisition matrix, 64×64 pixels; field of view, 192×192×120 mm; in-plane voxel size, 3×3 mm; section thickness, 3 mm; .75-mm intervening spaces; and 28 total sections). There were 133 whole-brain images per subject per run. We also obtained matched-bandwidth and high-resolution Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) T1-weighted images for each subject to provide detailed brain anatomy during structural image acquisition.
For processing and analysis of the fMRI data, we used Oxford Centre for Functional Magnetic Resonance Imaging of the Brain Software Library (FSL) (http://www.fmrib.ox.ac.uk/fsl). Preprocessing steps are described in Suppl. Information.
We used FEAT (fMRI Expert Analysis Tool version 5.4) for general linear model analyses. At the individual subject level, we modeled the hemodynamic response function using a convolution of the experimental paradigms of each stimulus type versus crosshair baseline with the canonical hemodynamic response function and its temporal derivative (Aguirre & Farah 1998). Motion correction was performed by adding 24 motion parameters, modeling the 6 rigid body motion parameters, their temporal derivatives, and their squares (Friston et al. 1996). We thresholded the z-score images using clusters determined by z>2.3 and a corrected cluster significance threshold of p<.05.
JICA
Inputs to jICA were BOLD responses to HSF, NSF, and LSF stimuli, contrasted to crosshair baseline, registered to the MNI brain. These contrasts were submitted to jICA using the Fusion ICA Toolbox (FIT) (http://icatb.sourceforge.net) in Matlab, and were combined with the ERP waveforms as described previously (Calhoun et al. 2006). We performed ICA using the Infomax algorithm, a gradient descent algorithm to maximize entropy of the output of single layer neural network (Lee & Sejnowski 1997), to generate the shared unmixing matrix and the fused ERP and fMRI sources (Calhoun et al. 2006). We used principal components analysis to reduce dimensionality from subjects to components. Fifteen joint components were estimated from ERP time courses and fMRI activation maps. The jICA mixing matrix’s rows consist of each participant’s concatenated data; thus, the maximum number of components extracted from each group is fifteen since this was the number of participants in each group. Fifteen ICs thus maximizes the extent of separation between sources for this sample size.
Within-Group jICA Components
From the IC decomposition, we extracted P100 and N170 ICs using a template-matching algorithm that calculated similarity scores for each component. These scores were calculated by comparing each IC against the average ERP at 100 ms windows taken symmetrically around timepoints 100 and 170 ms. We then submitted the IC with the largest similarity score for each component (P100 or N170) for analysis with dual regression.
To reduce the number of multiple comparisons, we restricted between-group comparisons by using masks constructed from each respective spatial frequency contrast using a group GLM analysis for each group. We created masks from thresholded images (z-scores >1.7), from which we combined the two compared groups’ masks and then binarized the results.
Dual Regression
We used Matlab’s GIFT Toolbox’s Spatiotemporal Reconstruction tool to create subject level Ics from each group spatial map, by adopting a dual regression procedure (as previously described in: (Filippini et al. 2009), see Suppl. Information).
Statistical Analyses
We performed permutation tests between groups to test for statistical differences using Statistical nonparametric Mapping (SnPM, http://warwick.ac.uk/snpm), with 10000 iterations. We used a Bonferroni corrected significance threshold of α=.016 to account for pairwise comparisons for each of our specific hypotheses; although our hypotheses only included two group pairwise comparisons (AN vs. controls and BDD vs. controls) we used a more conservative threshold of α=.05/3=.016 to additionally account for the post hoc AN vs. BDD comparison.
Correlations with Clinical Variables
We calculated correlations between individual subject spatial map average intensity values and global scores on the EDE (for AN) and BDD-YBOCS (BDD), for NSF and LSF joint P100 and N170 components. We Bonferroni-corrected for multiple comparisons (α=.05/8=.00625).
Results
Demographics and Psychometrics (Table 1)
All participants were unmedicated. All AN participants met DSM-IV criteria for restricting type; 1 had comorbid generalized anxiety disorder (GAD). Of the BDD participants, 2 had comorbid major depressive disorder (MDD), 3 had dysthymic disorder, 1 had panic disorder, 1 had MDD and GAD, and 1 had MDD, GAD, and social anxiety disorder. In the BDD group, 3 had facial concerns, 1 had non-facial concerns, and 11 had facial and non-facial concerns.
Behavioral Results
There were no significant differences among groups for accuracy or response times for the EEG or fMRI tasks (see Suppl. Information).
Within-Groups jICA Results
For AN, BDD, and HC groups the P100 component has associated activations in lingual gyrus and middle occipital cortex, while the N170 component has activations in fusiform gyrus, lingual gyrus, and inferior/middle occipital cortex. This is consistent with previous findings using EEG source localization that localized the P100 (Itier & Taylor 2004) to early visual cortices and the N170 (Pascual-Marqui 1999) to mainly the posterior fusiform gyrus. Figs. S2 and S3 show example components for the P100 and N170 components, respectively, in AN, BDD and HC derived from jICA (thresholded at |Z|>3.5 for display purposes).
Between-Groups jICA Results: Faces Task
P100 Component
There were no statistically significant differences among groups for any spatial frequencies for the joint P100 component.
N170 Component
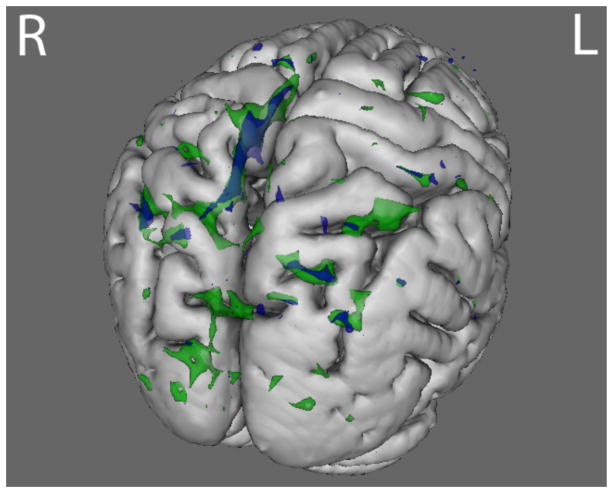
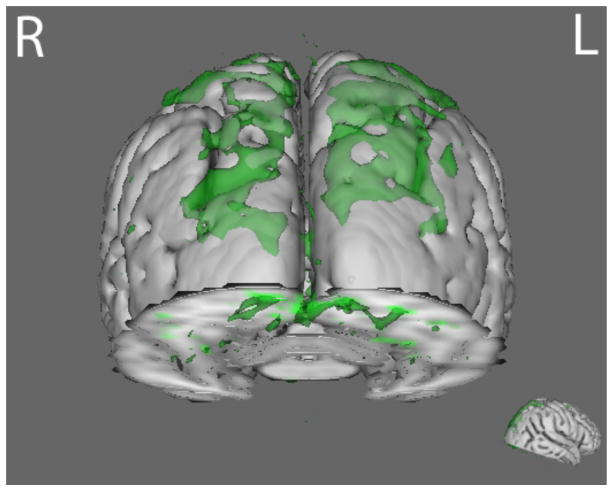
Compared to HC, AN and BDD groups each showed statistically significant hypoactivity in similar dorsal visual stream systems (precuneus, lateral occipital cortex) for LSF faces in the joint N170 component (p<.016) (Fig. 2 and Table S3).
Fig. 2.
N170 joint component for LSF face stimuli. The BDD group (green) demonstrated hypoactivity (p<.016) compared with healthy controls in regions including lateral occipital cortex, occipital pole, and precuneus. The AN group (blue) demonstrated hypoactivity (p<.016) compared with healthy controls in the precuneus and the lateral occipital cortex. See table S4 for local max coordinates.
Between-Groups jICA Results: Houses Task
P100 Component
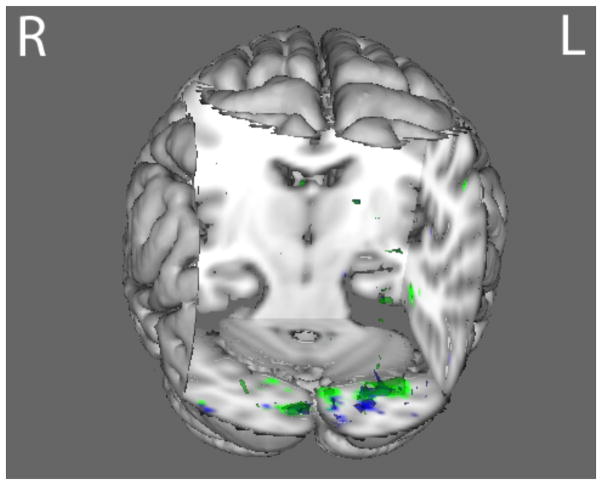
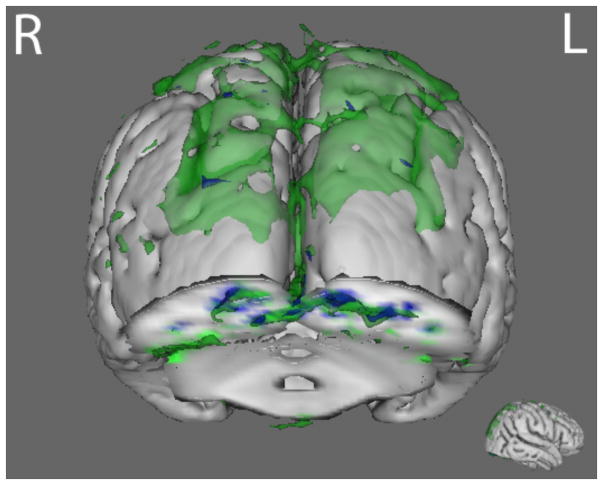
Compared with HC, AN and BDD groups each showed statistically significant hypoactivity in similar early visual regions (occipital fusiform gyrus) for LSF houses in the joint P100 component (p<.016) (Fig. 3 and Table S4).
Fig. 3.
P100 joint component for LSF house stimuli. The BDD group (green) and the AN group (blue) both demonstrated hypoactivity (p<.016) compared with healthy controls in regions including the middle temporal gyrus, the occipital fusiform gyrus, and the inferior temporal gyrus. See table S5 for local max coordinates.
N170 Component
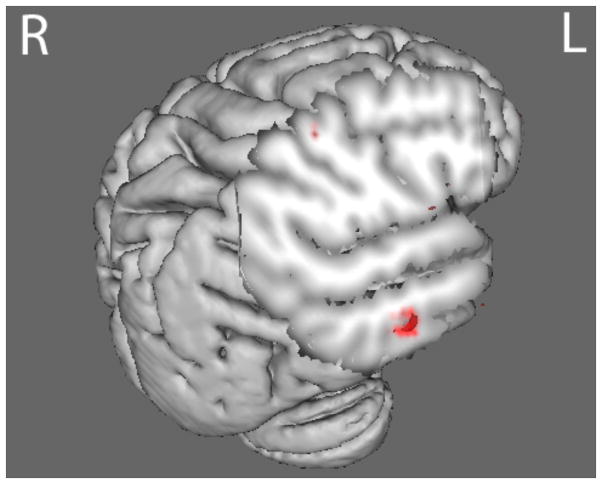
Compared with HC, the BDD group demonstrated statistically significant hyperactivity in regions including the posterior temporal fusiform cortex (part of the ventral visual stream) for HSF houses in the joint N170 component (p<.016) (Fig. 4 and Table S5). The BDD group demonstrated statistically significant hypoactivity compared with HC in early ventral visual stream areas (occipital fusiform, temporal occipital fusiform, and lateral occipital cortices), and dorsal visual stream areas (superior parietal lobule) for LSF houses in the joint N170 component stimuli (Fig. 5 and Table S6). By contrast, the AN group showed hypoactivity compared with HC in early ventral visual stream areas as well (occipital fusiform cortex, occipital pole, and precuneus), but only at a lower p-threshold of p<.05 (Fig. 6).
Fig. 4.
N170 joint component for HSF house stimuli. The BDD group (red) demonstrated hyperactivity (p<.016) in regions including the temporal fusiform cortex compared with healthy controls. See table S6 for local max coordinates.
Fig. 5.
N170 joint component for LSF house stimuli. The BDD group (green) demonstrated hypoactivity (p<.016) compared with healthy controls in regions including the occipital fusiform cortex, lateral occipital cortex, and frontal pole. See table S7 for local max coordinates.
Fig. 6.
N170 joint component for LSF house stimuli using a lower statistical threshold (p<.05). The BDD group (green) demonstrated hypoactivity compared with healthy controls in regions including the occipital fusiform cortex, lateral occipital cortex, and frontal pole. The AN group (blue) demonstrated hypoactivity in occipital fusiform cortex, occipital pole, and precuneus.
Post hoc AN vs. BDD comparisons
Although not among our primary hypotheses, we conducted post hoc comparisons directly between AN and BDD. The BDD group demonstrated statistically significant hypoactivity compared with AN in left lateral occipital cortex for the LSF houses in the joint N170 component (p<.016) (see Fig. S6 and Table S7). There were no other statistically significant differences for the other spatial frequencies and joint ERP components.
Correlational Analyses
Activity for LSF faces in the joint N170 component in the AN group correlated negatively with global EDE scores: r=−.55, p=.04, suggesting that lower activity for faces that only contain global and configural information was associated with worse eating disorder symptoms. However, this did not survive correction for multiple comparisons.
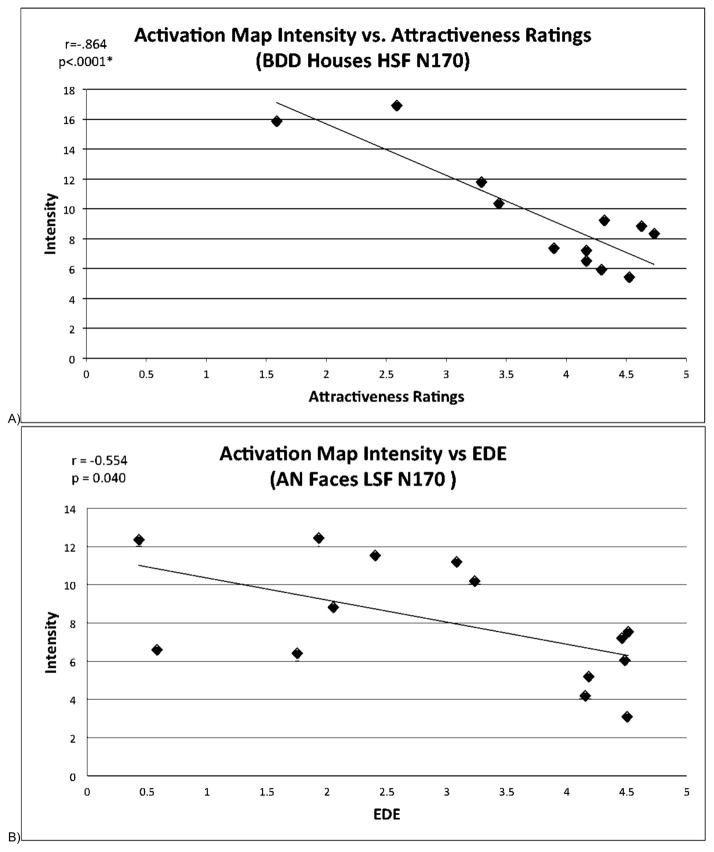
As exploratory post hoc analyses, we calculated correlations between mean scores on ratings of face attractiveness, aversiveness, and degree to which the face triggered thoughts of their own appearance (obtained immediately after the fMRI session) and individual subject spatial map averages for all joint components that were significantly different among groups. There was a significant correlation between face attractiveness ratings for the BDD group and activation for HSF houses in the joint N170 component. (q=.035, corrected for multiple comparisons using false discovery rate (FDR)) (Fig. 7).
Figure 7.
A) Scatterplot showing significant negative correlation (q=.035 after FDR correction) between mean activation map intensities and attractiveness ratings for the BDD group for HSF houses in the joint N170 component.
B) Scatterplot showing negative correlation (p=.04) between mean activation map intensities and EDE ratings for the AN group for LSF faces in the joint N170 component. The correlation was not significant after multiple comparison correction.
Because the BDD and AN groups differed significantly on depression, anxiety, and BMI (see Table 1), we conducted post hoc analyses investigating the relationship between MADRS, HAMA, and BMI (and BABS scores, although not significantly different), and all joint components that were significantly different among groups. There were no significant correlational findings, using FDR correction with a q threshold of .05. (See Table S2 for full correlation results with psychometric scores.)
Discussion
To our knowledge, this is the first study to investigate visual processing concurrently in individuals with AN and in those with BDD for appearance- and nonappearance-related images, and the first to use joint analysis of fMRI and EEG data to study brain activation patterns in these clinical populations. We found that both AN and BDD showed hypoactivity in dorsal visual stream systems for low spatial frequencies, suggesting that a common deficiency in holistic processing is operating in primary visual structures as early as 100 ms post-exposure, which extends later in time (170 ms) into dorsal higher-order processing regions. However, the patterns of hypoactivity are not identical; BDD demonstrated hyperactivity when compared with controls in ventral visual stream systems for high spatial frequency houses, but they showed hypoactivity in dorsal visual regions for low spatial frequency faces in comparison to participants with AN.
In line with our hypothesis, individuals with BDD and those with AN demonstrate hypoactivity in early secondary visual processing regions when viewing faces that contain only LSF information, corresponding to electrical activity in the N170 component. But contrary to our hypotheses, we did not find hypoactivity in the P100 component profile for faces, which suggests that abnormalities in face visual processing may not manifest until the structural encoding that occurs approximately 170 ms after stimulus presentation.
Also in line with our hypotheses, BDD individuals demonstrate hypoactivity in primary and secondary visual regions for the P100 and N170 component, respectively, in response to low spatial frequency houses. This suggests deficient activation for configural/holistic information in the dorsal visual stream within the first 200 ms of visual processing, even for stimuli that are appearance neutral. Contrary to prediction, in response to HSF house images, individuals with BDD demonstrate hyperactivity in the fusiform gyrus (part of the ventral visual stream) in the N170 component, suggesting greater engagement of detailed processing. As we did not find this in our previous fMRI studies of BDD (Feusner et al. 2007, 2010c, 2011), the general linear modeling we used in those studies may not have had the sensitivity necessary for its detection, as jICA specifically isolates fMRI activations linked to electrophysiological activity at predefined time-points. The results herein suggest a general imbalance for detailed versus configural/holistic processing of visual information.
We observed similar hypoactivity in visual systems for LSF house images in participants with AN, but with some exceptions. In the P100 profile, they show hypoactivity in early visual areas, although more limited relative to that observed in participants with BDD. In the N170 profile, participants with AN did not show significant differences in activation at the (corrected) threshold of p<.016, however, they did show hypoactivity in primary visual areas at a lower threshold of p<.05. These support previous findings of poor central coherence and diminished holistic and configural processing in individuals with AN, for bodies ((Lopez et al. 2008; Urgesi et al. 2013) and complex figures (Lopez et al. 2009; Kim et al. 2011). We did not, however, find evidence of increased detailed processing in AN, from our HSF condition.
The post hoc direct comparison of AN to BDD did not reveal significant differences in these primary visual areas, but there was lower activity in a superior portion of the left lateral occipital cortex in BDD compared to AN. One possibility is that AN is characterized by similar deficient activity in primary visual regions as seen BDD, although of slightly lower magnitude and extent. Yet in later, dorsal stream structures, BDD may have reduced activity compared to AN, possibly signifying a greater deficiency in configural processing. Finally, although the AN group did not demonstrate the same ventral visual stream hyperactivity compared with controls as observed for BDD, the direct AN to BDD comparison was not statistically significant. Thus, an imbalance in detailed versus configural/holistic processing for non appearance-related stimuli may characterize both disorders, but the defect appears to be more pronounced in BDD.
These findings may indicate a possible deficit in magnocellular pathway systems that normally construct a low-resolution holistic template of the visual field, which in turn may contribute to the distorted perceptions underlying appearance concerns in both disorders. However, similarities in abnormal neural activity as elicited by this experiment does not prove that AN and BDD have identical pathophysiology contributing to their respective clinical phenotypes. This is a preliminary study in these disorders, so further behavioral, connectivity, and neuropsychological research should be conducted to fully elucidate their differences.
The observation that case-control differences were more pronounced for the house than the face images is somewhat unexpected, given how often those with BDD perceive defects in their own faces. One possible explanation is that faces on average have more emotional and social saliency than houses, and this may also be more variable person-to-person. Such variability could introduce noise into the ERP responses from top-down modulation, resulting in a more heterogeneous signal. (The specific emotional and cognitive reactions to faces would not likely be directly detected in this study, as we chose to focus solely on early visual joint components.) The house stimuli, in contradistinction, may have allowed for a more consistent, less modulated response in visual systems and thus be more sensitive to underlying abnormalities.
The study has several limitations. First, the small sample size may have hindered full independent components decomposition. Second, although we collected the fMRI and EEG data using the same stimuli, task, and participants, they were not collected concurrently. This invites the possibility of subjective and experimental differences between sessions, although the normalization step when performing the joint ICA accounts for linear effects of habituation. However, concurrent fMRI-EEG studies have their own limitations, such as significant artifacts due to gradient and heartbeat noise. Third, although we chose face images for appearance-related stimuli as the N170 and P100 are well characterized, faces are more likely to be salient for participants with BDD. It is possible that this may have differentially affected attention. The N170 and P100 joint components are less likely than later components to be affected by top-down modulation, although it remains a possibility. Moreover, the BDD group may have misinterpreted neutral face expressions as being angry or contemptuous, as has been observed in other BDD studies (Buhlmann et al. 2006), which, in turn, may have resulted in between-group differences in image salience or emotion. The BDD group had higher average anxiety and depression scores than both AN and HC participants, although this is unlikely to account for differences in activation patterns given a lack of significant correlations between component activation and these ratings. Finally, it remains unknown due to the cross-sectional design of the study if the differences found reflect intrinsic variations that enhance risk of illness, or epiphenomena of illness or correlates of other neural processes.
The results of this study suggest the possibility that a general visual processing phenotype may operate in psychiatric disorders that involve distortion of one’s appearance and are accompanied by emotional distress and related self-esteem concerns (Madsen et al. 2013). The phenotype may explain the peculiar, increased attention given to miniscule defects seen on the skin (in BDD), or areas of perceived cellulite, or “fat,” on thighs and stomach (in AN), and the inability to process these perceptions contextually, i.e., to see them as inconsequential relative to the body as a whole (Madsen et al. 2013). The finding in the BDD group that greater degree of activation for high detail stimuli is associated with lower face attractiveness ratings supports this interpretation; enhanced detail processing may lead to a greater likelihood of flaw detection, and hence lower perception of attractiveness. The fact that this relationship was significant for house stimuli and not face stimuli is somewhat unexpected, but may be due to the aforementioned possibility of greater neural signal variability related to differences in salience of the faces. For the AN group, lower activation for LSF faces is associated with worse eating disorder symptoms, suggesting a possible link between deficiencies in global and configural processing and clinical symptomatology, although this correlation did not survive correction for multiple comparisons. As the neural differences observed are linked to ERP components as early as 100 ms post-stimulus, we speculate that abnormalities exist in BDD and AN in primary and secondary visual brain regions before this information is transferred forward to areas that form and regulate memory, emotion, and cognitive schemas.
The results, though preliminary given the small sample and need for replication, suggest potential translational implications. The results may have promise in informing study of these phenotypes as biomarkers of risk, persistence of symptom-conferring pathophysiology, or endophenotypes. Finally, they may inform the development of novel, adjunctive perceptual remediation therapies targeting impairment in early dorsal versus ventral visual streams.
Supplementary Material
Acknowledgments
This work was funded by NIH MH093535-02S1 (Feusner). Dr. Strober received support from the Resnick Endowed Chair in Eating Disorders.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
Contributor Information
Wei Li, University of California, Los Angeles.
Tsz Man Lai, University of California, Los Angeles.
Cara Bohon, Stanford University, Palo Alto
Sandra K Loo, University of California, Los Angeles
Danyale McCurdy, University of California, Los Angeles
Michael Strober, University of California, Los Angeles
Susan Bookheimer, University of California, Los Angeles
Jamie Feusner, University of California, Los Angeles
References
- Aguirre GK, Farah MJ. Human visual object recognition: What have we learned from neuroimaging? Psychobiology. 1998;26:322–332. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-5. 5. Arlington: American Psychiatric Publishing; 2013. p. 991. [Google Scholar]
- Bartlett J, Searcy J, Abdi H. Perception of Faces, Objects, and Scenes: Analytic and Holistic Processes. Oxford: Oxford University Press; 2003. What are the routes to face recognition? pp. 21–52. [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological Studies of Face Perception in Humans. Journal of Cognitive Neuroscience. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R, Charlton R, Hughes C, Happé F, Happe F. Disentangling weak coherence and executive dysfunction: planning drawing in autism and attention 3 deficit / hyperactivity disorder. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2003;358:387–392. doi: 10.1098/rstb.2002.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhlmann U, Etcoff NL, Wilhelm S. Emotion recognition bias for contempt and anger in body dysmorphic disorder. Journal of Psychiatric Research. 2006;40:105–11. doi: 10.1016/j.jpsychires.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, McNally RJ, Etcoff NL, Tuschen-Caffier B, Wilhelm S. Emotion recognition deficits in body dysmorphic disorder. Journal of Psychiatric Research. 2004;38:201–206. doi: 10.1016/s0022-3956(03)00107-9. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, McNally RJ, Wilhelm S, Florin I. Selective processing of emotional information in body dysmorphic disorder. Journal of Anxiety Disorders. 2002;16:289–98. doi: 10.1016/s0887-6185(02)00100-7. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Kiehl Ka. Neuronal chronometry of target detection: fusion of hemodynamic and event-related potential data. NeuroImage. 2006;30:544–53. doi: 10.1016/j.neuroimage.2005.08.060. [DOI] [PubMed] [Google Scholar]
- Castro-Fornieles J, Bargalló N, Lázaro L, Andrés S, Falcon C, Plana MT, Junqué C. A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. Journal of Psychiatric Research. 2009;43:331–40. doi: 10.1016/j.jpsychires.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Costen NP, Parker DM, Craw I. Effects of high-pass and low-pass spatial filtering on face identification. Perception & Psychophysics. 1996;58:602–12. doi: 10.3758/bf03213093. [DOI] [PubMed] [Google Scholar]
- Danner UN, Sanders N, Smeets PaM, van Meer F, Adan RaH, Hoek HW, van Elburg Aa. Neuropsychological weaknesses in anorexia nervosa: set-shifting, central coherence, and decision making in currently ill and recovered women. The International Journal of Eating Disorders. 2012;45:685–94. doi: 10.1002/eat.22007. [DOI] [PubMed] [Google Scholar]
- Desjardins JA, Segalowitz SJ. Deconstructing the early visual electrocortical responses to face and house stimuli. Journal of Vision. 2013;13:1–18. doi: 10.1167/13.5.22. [DOI] [PubMed] [Google Scholar]
- Eisen JL, Phillips Ka, Baer L, Beer Da, Atala KD, Rasmussen Sa. The Brown Assessment of Beliefs Scale: reliability and validity. The American Journal of Psychiatry. 1998;155:102–8. doi: 10.1176/ajp.155.1.102. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, Connor MO. Eating Disorder Examination (Edition 16.0D) Cognitive Behavior Therapy and Eating Disorders. 2008:1–49. [Google Scholar]
- Favaro A, Santonastaso P, Manara R, Bosello R, Bommarito G, Tenconi E, Di Salle F. Disruption of visuospatial and somatosensory functional connectivity in anorexia nervosa. Biological Psychiatry. 2012;72:864–70. doi: 10.1016/j.biopsych.2012.04.025. [DOI] [PubMed] [Google Scholar]
- Feusner J, Hembacher E, Moller H, Moody TD. Abnormalities of object visual processing in body dysmorphic disorder. Psychol Med. 2011:18. doi: 10.1017/S0033291711000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Bystritsky A, Hellemann G, Bookheimer S. Impaired identity recognition of faces with emotional expressions in body dysmorphic disorder. Psychiatry Research. 2010a;179:318–23. doi: 10.1016/j.psychres.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Moller H, Altstein L, Sugar C, Bookheimer S, Yoon J, Hembacher E. Inverted face processing in body dysmorphic disorder. Journal of Psychiatric Research. 2010b;44:1088–94. doi: 10.1016/j.jpsychires.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Moody T, Hembacher E, Townsend J, McKinley M, Moller H, Bookheimer S. Abnormalities of visual processing and frontostriatal systems in body dysmorphic disorder. Archives of General Psychiatry. 2010c;67:197–205. doi: 10.1001/archgenpsychiatry.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, Bookheimer S. Visual information processing of faces in body dysmorphic disorder. Archives of General Psychiatry. 2007;64:1417–25. doi: 10.1001/archpsyc.64.12.1417. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7209–14. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonville L, Lao-Kaim NP, Giampietro V, Van den Eynde F, Davies H, Lounes N, Andrew C, Dalton J, Simmons A, Williams SCR, Baron-Cohen S, Tchanturia K. Evaluation of enhanced attention to local detail in anorexia nervosa using the embedded figures test; an FMRI study. PloS one. 2013;8:e63964. doi: 10.1371/journal.pone.0063964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35:346–55. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A Rating Scale for Depression. J Neurol Neurosurg Psychiatry. 1960;23:56–63. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Matsumoto A, Haneda K, Okada T, Sadato N. Hemodynamic and electrophysiological relationship involved in human face processing: evidence from a combined fMRI-ERP study. Brain and Cognition. 2006;60:176–86. doi: 10.1016/j.bandc.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Taylor MJ. Source analysis of the N170 to faces and objects. Neuroreport. 2004;15:1–5. doi: 10.1097/01.wnr.0000127827.73576.d8. [DOI] [PubMed] [Google Scholar]
- Jefferies K, Laws KR, Fineberg Na. Superior face recognition in Body Dysmorphic Disorder, . Elsevier. Journal of Obsessive-Compulsive and Related Disorders. 2012;1:175–179. [Google Scholar]
- Jolliffe T, Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1997;38:527–34. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Kerwin L, Hovav S, Hellemann G, Feusner JD. Impairment in local and global processing and set-shifting in body dysmorphic disorder., Elsevier Ltd. Journal of psychiatric research. 2014;57:41–50. doi: 10.1016/j.jpsychires.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-R, Lim S-J, Treasure J. Different Patterns of Emotional Eating and Visuospatial Deficits Whereas Shared Risk Factors Related with Social Support between Anorexia Nervosa and Bulimia Nervosa. Psychiatry Investigation. 2011;8:9–14. doi: 10.4306/pi.2011.8.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Sejnowski TJ. Independent Component Analysis for Mixed Sub- Gaussian and Super- Gaussian Sources. 4th Joint Symposium on Neural Computation Proceedings; 1997. pp. 132–139. [Google Scholar]
- Li W, Lai TM, Moody T, Loo S, Strober M, Bookheimer SY, Feusner JD. Study of Visual Processing Deficits in BDD using EEG. Society for Neuroscience; 2013. [Google Scholar]
- Lopez C, Tchanturia K, Stahl D, Treasure J. Central coherence in eating disorders: a systematic review. Psychological Medicine. 2008;38:1393–404. doi: 10.1017/S0033291708003486. [DOI] [PubMed] [Google Scholar]
- Lopez C, Tchanturia K, Stahl D, Treasure J. Weak central coherence in eating disorders: a step towards looking for an endophenotype of eating disorders. Journal of Clinical and Experimental Neuropsychology. 2009;31:117–25. doi: 10.1080/13803390802036092. [DOI] [PubMed] [Google Scholar]
- Madsen SK, Bohon C, Feusner JD. Visual processing in anorexia nervosa and body dysmorphic disorder: Similarities, differences, and future research directions. Journal of Psychiatric Research. 2013:1–9. doi: 10.1016/j.jpsychires.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzani B, Krebs G, Anson M, Veale D, Mataix-Cols D. Holistic versus detailed visual processing in body dysmorphic disorder: testing the inversion, composite and global precedence effects., Elsevier. Psychiatry research. 2013;210:994–9. doi: 10.1016/j.psychres.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Monzani B, Rijsdijk F, Iervolino AC, Anson M, Cherkas L, Mataix-Cols D. Evidence for a genetic overlap between body dysmorphic concerns and obsessive-compulsive symptoms in an adult female community twin sample. American Journal of Medical Genetics. 2012;159B:376–82. doi: 10.1002/ajmg.b.32040. [DOI] [PubMed] [Google Scholar]
- Mundy ME, Sadusky A. Abnormalities in visual processing amongst students with body image concerns. 2014;10:39–48. doi: 10.5709/acp-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Review of Methods for Solving the EEG Inverse Problem. International Journal of Bioelectromagnetism. 1999;1:75–86. [Google Scholar]
- Phillips KA. Clinical Features and Treatment of Body Dysmorphic Disorder. The Journal of Lifelong Learning in Psychiatry. 2005;3:179–183. [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, Decaria C, Goodman WK. A Severity Rating Scale for Body Dysmorphic Disorder: Development, Reliability, and Validity of a Modified Version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacology Bulletin. 1997;33:17–22. [PubMed] [Google Scholar]
- Rauch SL, Phillips Ka, Segal E, Makris N, Shin LM, Whalen PJ, Jenike Ma, Caviness VS, Kennedy DN. A preliminary morphometric magnetic resonance imaging study of regional brain volumes in body dysmorphic disorder. Psychiatry Research. 2003;122:13–9. doi: 10.1016/s0925-4927(02)00117-8. [DOI] [PubMed] [Google Scholar]
- Roberts ME, Tchanturia K, Treasure JL. Is attention to detail a similarly strong candidate endophenotype for anorexia nervosa and bulimia nervosa? The World Journal of Biological Psychiatry. 2013;14:452–63. doi: 10.3109/15622975.2011.639804. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y, Sheehan H, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar G. The Mini-International Neuropsychiatric Interview (M.I.N.I): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Sherman BJ, Savage CR, Eddy KT, Blais Ma, Deckersbach T, Jackson SC, Franko DL, Rauch SL, Herzog DB. Strategic memory in adults with anorexia nervosa: are there similarities to obsessive compulsive spectrum disorders? The International Journal of Eating Disorders. 2006;39:468–76. doi: 10.1002/eat.20300. [DOI] [PubMed] [Google Scholar]
- Stedal K, Rose M, Frampton I, Landrø NI, Lask B. The neuropsychological profile of children, adolescents, and young adults with anorexia nervosa. Archives of Clinical Neuropsychology. 2012;27:329–37. doi: 10.1093/arclin/acs032. [DOI] [PubMed] [Google Scholar]
- Suchan B, Busch M, Schulte D, Grönemeyer D, Grönermeyer D, Herpertz S, Vocks S. Reduction of gray matter density in the extrastriate body area in women with anorexia nervosa. Behavioural Brain Research. 2010;206:63–7. doi: 10.1016/j.bbr.2009.08.035. [DOI] [PubMed] [Google Scholar]
- Tenconi E, Santonastaso P, Degortes D, Bosello R, Titton F, Mapelli D, Favaro A. Set-shifting abilities, central coherence, and handedness in anorexia nervosa patients, their unaffected siblings and healthy controls: exploring putative endophenotypes. The World Journal of Biological Psychiatry. 2010;11:813–23. doi: 10.3109/15622975.2010.483250. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Fornasari L, Canalaz F, Perini L, Cremaschi S, Faleschini L, Thyrion EZ, Zuliani M, Balestrieri M, Fabbro F, Brambilla P. Impaired configural body processing in anorexia nervosa: Evidence from the body inversion effect. British Journal of Psychology. 2013:1–23. doi: 10.1111/bjop.12057. [DOI] [PubMed] [Google Scholar]
- Williams JBW, Kobak Ka. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA) British Journal of Psychiatry. 2008;192:52–8. doi: 10.1192/bjp.bp.106.032532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.