Abstract
Tert-butylhydroquinone (tBHQ), an inducer of nuclear factor erythroid 2-related factor 2 (Nrf2), has been demonstrated to attenuate oxidative stress-induced injury and the apoptosis of human neural stem cells and other cell types. However, whether tBHQ is able to exert a protective effect against oxidative stress and the apoptosis of cardiomyocytes has not yet been determined. Thus, the objective of the present study was to determine whether tBHQ protects H9c2 cardiomyocytes against ethanol-induced apoptosis. For this purpose, four sets of experiments were performed under standard culture conditions as follows: i) untreated control cells; ii) cell treatment with 200 mM ethanol; iii) cell treatment with 5 µM tBHQ; and iv) cell pre-treatment with 5 µM tBHQ for 24 h, followed by medium change and co-culture with 200 mM ethanol containing 5 µM tBHQ for a further 24 h. The viability of the cardiomyocytes was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The levels of intracellular reactive oxygen species (ROS) and apoptosis were assessed by flow cytometry. Protein expression was measured by western blot analysis, and Nrf2 nuclear localization was observed by immunofluorescence. Exposure to ethanol led to a decrease in the protein expression of Nrf2 and its downstream antioxidant enzymes, accompanied by an increase in ROS generation and in the apoptosis of H9c2 cells. Pre-treatment with tBHQ significantly prevented the H9c2 cells from undergoing ethanol-induced apoptosis. tBHQ also increased the expression of B-cell lymphoma-2 (Bcl-2), whereas Bcl-2-associated X protein (Bax) expression was decreased. tBHQ promoted Nrf2 nuclear localization and increased the expression of Nrf2, superoxide dismutase (SOD), catalase (CAT) and heme oxygenase-1 (HO-1), and simultaneously inhibited the ethanol-induced overproduction of intracellular ROS. Therefore, tBHQ confers protection against the ethanol-induced apoptosis of and activates the Nrf2 antioxidant pathway in H9c2 cardiomyocytes.
Keywords: tert-butylhydroquinone, cardioprotection, ethanol, nuclear factor erythroid 2-related factor 2, antioxidant, Bcl-2, Bax, caspase-3, oxidative stress, apoptosis
Introduction
Excessive alcohol consumption increases the risk of heart disease, which continues to be one of the major causes of mortality and morbidity in many countries. High doses of ethanol can induce or exacerbate a series of pathophysiological disorders, leading to cardiomyocyte apoptosis, myocardial fibrosis, cardiomyopathy and congestive heart failure, all associated with alcoholic cardiomyopathy (ACM) (1).
Several mechanisms are involved in mediating the adverse effects of ethanol, including the induction of oxidative stress and apoptotic cell death (1). There is abundant evidence indicating that ethanol promotes cellular apoptosis and this in turn causes the loss of cardiomyocytes (2–5). Myocyte loss or cell death may be an important mechanism of organ dysfunction and pathology. Several early studies on animal models of ACM and patients with ACM support a role for myocyte loss as an underlying mechanism of ethanol-induced cardiac dysfunction (2,6,7). These observations collectively imply that high-dose ethanol can diminish the cardiomyocyte population by the induction of apoptosis, which appears to result in subsequent abnormalities.
Compelling evidence indicates that oxidative stress, excessive intracellular reactive oxygen species (ROS) production, exceeding the antioxidant capacity of cells, plays a critical role in ethanol-induced apoptosis (8–11). ROS generation has been observed in ethanol-exposed cultured cells, including cardiac cells (8–10), as well as in the pathologies of several other types of cardiovascular insults, such as ischemia-reperfusion injury and cardiomyopathies (12). Others have suggested that ethanol-induced excessive ROS generation and oxidative stress may result from several processes or mechanisms involving mitochondrial cytochrome p450, xanthine oxidase, and NADPH oxidase (13,14). Once produced, ROS may not only cause oxidative damage to biomolecules, such as DNA, protein and lipids, but can also regulate the expression of genes related to growth and cell death (15,16). Of note, a number of events typical of oxidative stress are observed in cardiomyocytes following exposure to ethanol, which include myocyte loss and disarray (2), as well as changes in intracellular organelles. In addition, studies have also revealed that not only antioxidants, such as vitamin E and vitamin C (9), but also antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT) and heme oxygenase-1 (HO-1), all can inhibit ethanol-induced oxidative stress and apoptosis (5,14,17). Therefore, it is important to find effective antioxidants or means with which to improve myocardial cell oxidative stress states caused by ethanol.
Nuclear factor erythroid 2-related factor 2 (Nrf2), a member of the cap 'n' collar family of basic region-leucine zipper (bZIP) transcription factors, which is expressed in a variety of tissues, is considered as one of the major intracellular defense systems with which to combat oxidative stress (18,19). Under basal conditions, Nrf2 is located mainly in the cytoplasm bound to the Kelch-like ECH-associated protein 1 (Keap1), an adaptor protein for the Cul3-dependent ubiquitination and degradation of Nrf2. When activated, Nrf2 stabilizes and translocates to the nucleus, leading to the induction of the expression of phase II detoxifying and antioxidant genes, such as SOD, HO-1 and CAT (18,19). Nrf2 has been shown to provide protection against a number of oxidative stress-related cardiovascular diseases (19–22), and may be a target for the treatment of cardiomyocyte injury (23). Preliminary data from a recent review revealed that low doses (5 mM ethanol) of alcohol increased the expression of Nrf2 and its homolog Nrf1 to provide cardioprotection (24). Recently, studies have also evidenced that the upregulation of Nrf2 by inducers, such as 3H-1,2-dithiole-3-thione (D3T) or sulforaphane (SFN), significantly decrease the ethanol-induced ROS generation in and the apoptosis of cells i the nervous system (25,26). However, an inducer of Nrf2 successfully employed to prevent ACM has not yet been identified.
Tert-butylhydroquinone (tBHQ), a potent inducer of Nrf2, has received increasing attention due to its ability to activate many cytoprotective and detoxifying enzymes (27–31). Of note, tBHQ has been approved for human use as a synthetic food antioxidant to protect oils and fats from oxidative deterioration and rancidity (32). tBHQ can enhance Nrf2-mediated transcriptional activation depending on the increase of Nrf2 protein stability through the inhibition of the Keap1-mediated ubiquitination (27,28). There is substantial evidence to support the finding that tBHQ can aid in protecting various cells and organs against oxidative insults through the activation of Nrf2 signaling (29–31). A recent study revealed that tBHQ prevented ethanol-induced apoptosis by the induction of the Nrf2-driven antioxidant response in cranial neural crest cells (33). Nevertheless, to date, in spite of being a well-recognized and effective strategy for the protection of a variety of different cell types and organs under oxidative stress conditions, the cardioprotective effects of tBHQ have not been investigated, at least to the best of our knowledge.
This study aimed to examine the effects of tBHQ on ethanol-induced oxidative stress and the apoptosis of a cultured H9c2 cell line, a cloned heart muscle cell line originating from embryonic rat hearts that presents many cardiomyocyte phenotypes.
Materials and methods
Reagents
Cell culture and treatment
The H9c2 cells (from the Shanghai Institutes for Biological Sciences, Shanghai, China) were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), penicillin G (100 U/ml) and streptomycin (100 mg/ml) (Gibco-BRL, Grand Island, NY, USA). The cells were maintained in a tissue culture incubator at 37°C in a 5% CO2 atmosphere. The medium was changed every 2–3 days, and the cells were subcultured when the cell population density reached 70–80% confluence. In order to determine the optimal concentrations of ethanol (EtOH; Beijing Chemical Reagent Co., Ltd., Beijing, China) and tBHQ, the cells were exposed to various concentrations of both agents [EtOH: 0, 25, 50, 100, 200, 400 and 800 mM for 24 h; and tBHQ (Sigma Chemical Co., St. Louis, MO, USA): 0, 0.625, 1.25, 2.5, 5, 10, 20, 50 and 100 µM for 48 h]. Four sets of experiments were then performed under standard culture conditions: i) untreated control cells; ii) cell treatment with 200 mM ethanol; iii) cell treatment with 5 µM tBHQ; and iv) cell pre-treatment with 5 µM tBHQ for 24 h, followed by medium change and co-culture with 200 mM ethanol containing 5 µM tBHQ for a further 24 h.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Cell viability was examined by MTT assay. The H9c2 cells were seeded on 96-well plates at a density of 1.5×104 cells/well and were maintained in regular growth medium for 24 h. The cells were then subjected to the ethanol and tBHQ treatments, as described above. After the treatments, the H9c2 cells were incubated with 20 µl MTT solution (5 mg/ml phosphate buffer; obtained from Sigma Chemical Co.) at 37°C for 4 h, and the purple formazan crystals were then solubilized with 150 µl DMSO (Sigma Chemical Co.) at room temperature for 10 min. The absorbance at 490 nm was measured using a microplate reader (Sunrise RC; Tecan Group, Ltd., Mannedorf, Switzerland), and cell viability was expressed as a percentage of the control culture value.
Detection of apoptosis using Annexin V-FITC/propidium iodide (PI)
The apoptotic cells were detected by using Annexin V-FITC apoptosis detection kits (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). The cells were collected, washed twice with PBS, resuspended in binding buffer (10 mM HEPES/NaOH pH 7.4, 140 mM NaCl and 2.5 mM CaCl2) and incubated with Annexin V at room temperature in the dark for 15 min. The cells were then centrifuged, resuspended in binding buffer, and incubated with PI. Subsequently, binding buffer (500 µl) was added, and the apoptotic cells were assessed using a flow cytometer (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA).
Measurement of ROS production
ROS generation was measured using a 5(6)-carboxy-2′,7′-dichlorofluorescein diacetate (cDCFH-DA) detection kit (Beyotime Biotechnology, Jiangsu, China). After harvest, the cells were washed with PBS and then incubated with 10 µM of cDCFH-DA diluted in serum-free medium at 37°C for 30 min. The fluorescence intensity was monitored for 30 min after excitation at 488 nm and emission at 525 nm using a FACSort cell sorter (Becton-Dickinson, San Diego, CA, USA).
Western blot analysis
The cells were lysed in ice-cold RIPA buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton, 0.5% deoxycholate, 0.1% SDS, 1 mM EDTA, 10 mM NaF, and 0.1 mM phenylmethylsulfonyl fluoride (PMSF)] to obtain total proteins. Nuclear protein was extracted using a nuclear and cytoplasmic protein extraction kit following the manufacturer's instructions. The protein concentration was detected via the BCA protein assay (Beyotime Biotechnology). Equal amounts of protein samples (50 µg protein/lane) were separated by 10–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes. The non-specific antibodies were blocked with 5% non-fat dried milk in PBS for 2 h at room temperature. Membranes were then incubated overnight at 4°C with primary antibodies directed against the Nrf2 (ab31163) at 1:200 dilution, and HO-1 (ab13248) (both from Abcam, Cambridge, UK), SOD (sc-101523) and CAT (A-4; sc-271358) (both from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), caspase-3 (3CSP03; ab2171; Abcam), Bcl-2-associated X protein (Bax; P-19; sc-526) and B-cell lymphoma-2 (Bcl-2; N-19; sc-492) (Santa Cruz Biotechnology, Inc.) at 1:500 dilution. The membranes were then washed with TBST 3 times and further incubated with horseradish peroxidase-conjugated secondary antibody (1:2,000) for 1 h at room temperature. After washing, the membranes were processed using an electroche-miluminescence (ECL) reagent (Pierce Biotechnology, Inc., Rockford, IL, USA) and the light emission was captured on X-ray film. The signals were visualized by chemiluminescent horseradish peroxidase substrate and then subjected to a densi-tometric analysis and normalized to tubulin or lamin-B1.
Immunofluorescence staining
The cellular localization of Nrf2 was determined by immunofluorescence staining. The H9c2 cells were seeded at 1.5×104 cells/well in glass chamber slides (glass coverslips) and cultured overnight at 37°C. The cells were then exposed to ethanol or tBHQ alone, or in combination, as described above. At the end of incubation, the cells were washed twice in PBS and fixed in 4% paraformaldehyde for 10 min at room temperature. The cells were then permeabilized with 0.1% Triton X-100, washed and incubated with blocking buffer (10% NGS) for 1 h at room temperature. The cells were then incubated overnight with primary antibodies against AC-tubulin (6-11B-1; ab24610) at 1:200 dilution and Nrf2 (both from Abcam) at a 1:200 dilution in a humidified chamber at 4°C. Subsequently, the cells were washed with PBS and incubated with secondary antibodies conjugated to either FITC or TRITC at a dilution of 1:500 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 1 h at room temperature in the dark, and were then counterstained with DAPI dye to show the nuclear morphology. After the slides were rinsed with PBS, coverslips were mounted on slides, and images of the labeled cells were visualized and photographed using a confocal fluorescence microscope (TCS SP2; Leica, Wetzlar, Germany).
Statistical analysis
The data are presented as the means ± SD. One-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls test was applied to calculate the statistical significance between various groups. The differences were considered to be statistically significant at p<0.05.
Results
Treatment with tBHQ markedly enhances the viability of H9c2 cardiomyocytes exposed to ethanol
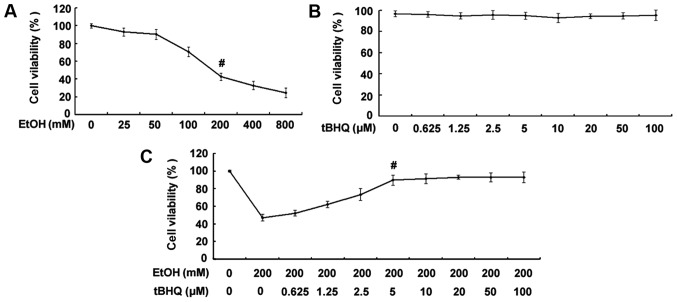
As shown in Fig. 1A, the exposure to ethanol at 200 mM for 24 h significantly decreased the percentage of surviving cells. Thus, the concentration of 200 mM ethanol was used as a standard concentration with which to induce apoptosis in the subsequent experiments. The results of MTT assay demonstrated no decrease in the viability of cells exposed to various concentrations of tBHQ (0, 0.625, 1.25, 2.5, 5, 10, 20, 50 and 100 µM) for 48 h (Fig. 1B). Furthermore, the results revealed that pre-incubation of the H9c2 cardiomyocytes with various concentrations of tBHQ (0, 0.625, 1.25, 2.5, 5, 10, 20, 50 and 100 µM) enhanced cell viability which was decreased due to exposure to ethanol in a dose-dependent manner (Fig. 1C). Maximum viability was apparent at a concentra tion of 5 µM. However, higher concentrations (50 and 100 µM) of tBHQ did not cause any attenuation of the inhibitory effects of ethanol on cell viability. Therefore, we employed 5 µM of tBHQ as a concentration for use in our subsequent experiments.
Figure 1.
(A) Effect of various concentrations of ethanol (EtOH) on cell viability. H9c2 cardiomyocytes were exposed to various concentrations of ethanol (0, 25, 50, 100, 200, 400 and 800 mM) for 24 h. Viable cells were identified by MTT assay. The data are shown as the means ± SD; n=5, #p<0.01 vs. control group. (B) Effect of various concentrations of tert-butylhydroquinone (tBHQ) on cell viability. The H9c2 cardiomyocytes were incubated with various concentrations of tBHQ (0, 0.625, 1.25, 2.5, 5, 10, 20, 50 and 100 µM) for 48 h. Cell viability was measured by MTT assay. No decrease in the viability of cells exposed to various concentrations of tBHQ for 48 h was established. (C) tBHQ protects against EtOH-induced cell death of cultured H9c2 cardiomyocytes in a dose-dependent manner. The cells were treated with various concentrations of tBHQ (0, 0.625, 1.25, 2.5, 5, 10, 20, 50 and 100 µM) for 24 h, followed by incubation for 24 h with 200 mM of ethanol. Cell viability was analyzed by MTT assay. The values are expressed as the means ± SD; n=5 experiments, #p<0.05 vs. EtOH alone.
Treatment with tBHQ prevents the ethanol-induced apoptosis of H9c2 cells
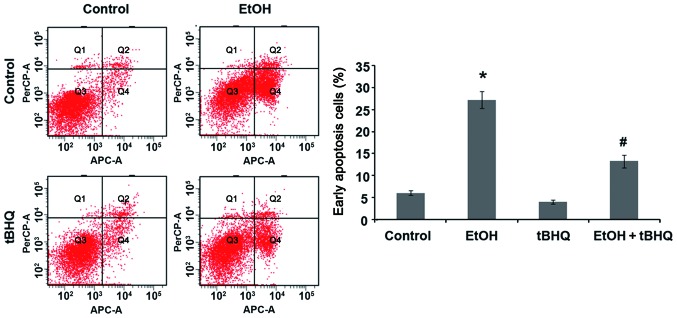
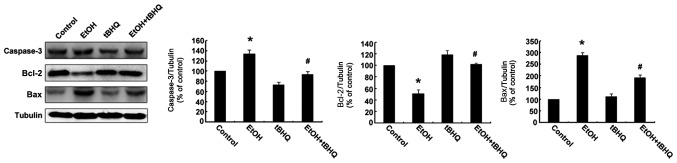
We performed a flow cytometric analysis and found that ethanol caused a substantial increase in the number of apoptotic cells, whereas pre-treatment with tBHQ significantly lowered the amount of apoptotic cells compared with the cells exposed to ethanol alone (Fig. 2). In addition, the results of western blot analysis revealed that the H9c2 cells exposed to ethanol manifested a marked increase in the expression of the apoptotic protein, caspase-3, and the pro-apoptotic protein, Bax, while a significant decrease in the expression of the anti-apoptotic protein, Bcl-2, was observed. However, the pre-treatment with tBHQ markedly inhibited the ethanol-induced increase in caspase-3 and Bax expression, and enhanced Bcl-2 expression (Fig. 3).
Figure 2.
Flow cytometric analysis of the apoptotic of H9c2 cells with Annexin V-FITC indicated that pre-treatment with tert-butylhydroquinone (tBHQ) significantly prevented ethanol (EtOH)-induced apoptosis. Apoptosis was assessed in H9c2 cells cultured in control medium (control), exposed to 200 mM EtOH or 5 µM tBHQ (tBHQ) alone, or exposed to EtOH and pre-treated with tBHQ (EtOH + tBHQ). The values are expressed as the means ± SD; n=3 experiments, *p<0.01 vs. control, #p<0.05 vs. EtOH.
Figure 3.
Representative western blots illustrating protein expression. Apoptosis was measured in the H9c2 cells cultured in control medium (control), as well as those exposed to 200 mM ethanol (EtOH) or 5 µM tert-butylhydroquinone (tBHQ) (tBHQ) alone, or exposed to EtOH and pre-treated with tBHQ (EtOH + tBHQ). The data are expressed as a densitometric ratio. The values are the means ± SD; n=3 experiments, *p<0.05 vs. control, #p<0.05 vs. EtOH.
Treatment with tBHQ significantly prevents the ethanol- induced excessive production of ROS in H9c2 cells
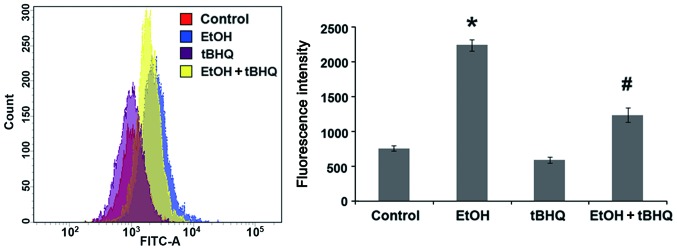
To determine whether tBHQ can prevent the oxidative stress induced by ethanol in cardiomyocytes, a cDCFH-DA assay (Beyotime Biotechnology) was performed to measure ROS production, representing one of the key events in apoptotic cell death. We found that the exposure to ethanol at 200 mM induced considerable ROS production, which was attenuated significantly by pre-treatment with BHQ (Fig. 4).
Figure 4.
Pre-treatment with tert-butylhydroquinone (tBHQ) significantly diminishes reactive oxygen species (ROS) generation in ethanol (EtOH)-exposed H9c2 cells. ROS generation was detected in H9c2 cells cultured in control medium (control), exposed to 200 mM EtOH or 5 µM tBHQ (tBHQ) alone, or exposed to EtOH and pre-treated with tBHQ (EtOH + tBHQ). The data are shown as relative fluorescence intensity units and represent the means ± SD of 3 separate experiments; *p<0.05 vs. control, #p<0.05 vs. EtOH.
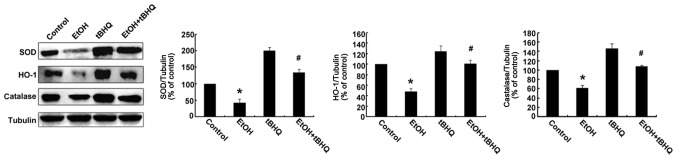
Treatment with tBHQ restores the expression of antioxidant proteins, including SOD, CAT and HO-1 in ethanol-exposed H9c2 cells
Western blot analysis was performed to determine whether tBHQ affects the expression of Nrf2 downstream antioxidant proteins (SOD, HO-1, and CAT) in H9c2 cells exposed to ethanol. As illustrated in Fig. 5, the protein expression of SOD, HO-1 and CAT was decreased in the ethanol-exposed cells compared with that in the ethanol-untreated cells, whereas it was restored by tBHQ in the ethanol-exposed H9c2 cells.
Figure 5.
Tert-butylhydroquinone (tBHQ) prevents the ethanol (EtOH)-induced inhibition of antioxidant protein expression in H9c2 cells. Cell lysates were prepared from H9c2 cells cultured in control medium (control), exposed to 200 mM EtOH or tBHQ at 5 µM without EtOH, or exposed to EtOH and pre-treated with tBHQ (EtOH + tBHQ). The data are expressed as the means ± SD of 3 separate experiments; *p<0.05 vs. control, #p<0.05 vs. EtOH.
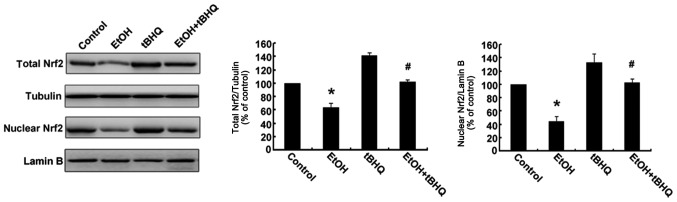
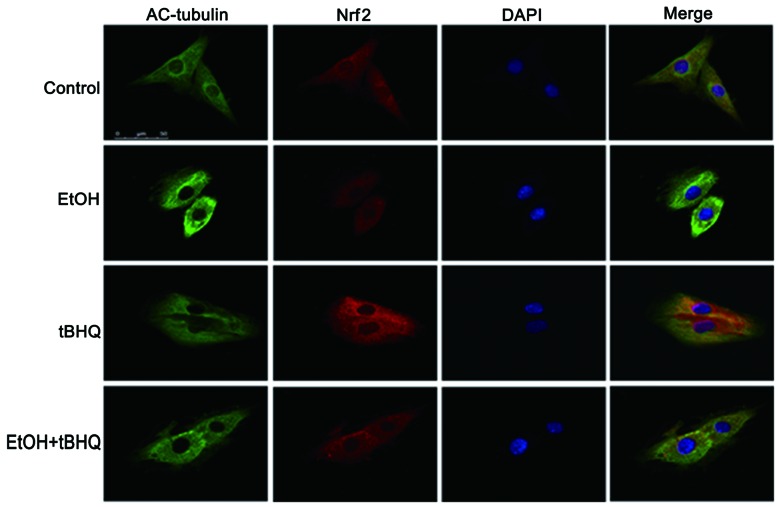
Treatment with tBHQ increases the protein expression and promotes the nuclear translocation of Nrf2 which is inhibited by exposure to ethanol in H9c2 cells
As depicted in Figs. 6 and 7, ethanol inhibited not only Nrf2 protein expression, but also Nrf2 nuclear translocation, which may have made the cells more sensitive to ethanol-induced toxicity. tBHQ alone partially, but significantly increased the Nrf2 protein level in the H9c2 cells. Pre-treatment wit htBHQ restored the level of Nrf2 protein and promoted its nuclear accumulation in the H9c2 cells, which were inhibited by exposure to ethanol, which may have elicited the cytoprotective response, resulting in the increased resistance of the cells to the ethanol challenge. The above-mentioned results were confirmed by western blot analysis and immunofluorescence staining.
Figure 6.
Pre-treatment with tert-butylhydroquinone (tBHQ) restores the total nuclear factor erythroid 2-related factor 2 (Nrf2) protein level and nuclear accumulation which are inhibited by ethanol (EtOH) in H9c2 cells. Western blot analysis was performed to analyze the level of Nrf2 protein. Cell lysates were prepared from H9c2 cells cultured in control medium (control), exposed to 200 mM EtOH or 5 µM tBHQ (tBHQ) alone, or exposed to EtOH and pre-treated with tBHQ (EtOH + tBHQ). The data are expressed as fold change over control and represent the means ± SD of 3 separate experiments; *p<0.05 vs. control, #p<0.05 vs. EtOH.
Figure 7.
The immunofluorescence assay evidenced that tert-butylhydroquinone (tBHQ) promotes the nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) in H9C2 cells. Nrf2 protein was visualized with a TRITC-labeled antibody, AC-tubulin was visualized with an FITC-labeled antibody, and the nuclear morphology was visualized with DAPI dye.
Discussion
Previous studies have demonstrated that apoptosis is the major factor responsible for ethanol-induced myocardial cell death, which is closely associated with ACM (1). For this reason, for preventing ACM, it is crucial to find an effective anti-apoptotic agent. In this study, treatment with tBHQ significantly reduced the occurrence of ethanol-induced cardiomyocyte apoptosis and promoted cell survival, as confirmed by the results of MTT assay, flow cytometry and the expression of caspase-3. Simultaneously, treatment withy tBHQ significantly reduced the ethanol-induced generation of ROS (as shown in Fig. 4). Furthermore, treatment with tBHQ not only promoted the nuclear translocation of Nrf2 (as depicted in Fig. 7), but also increased Nrf2 expression which was decreased by the exposure of the H9c2 cells to ethanol, which in turn led to the enhancement of the levels of antioxidant proteins, including SOD, HO-1 and CAT. In addition, the results also revealed that tBHQ protected the H9c2 cells from ethanol-induced apoptosis by participating in the upregulation of Bcl-2 expression and the downregulation of Bax expression. Taken together, these results provide substantial evidence that tBHQ can protect H9c2 cardiomyocytes against ethanol-induced oxidative stress and apoptosis, which may be associated with the activation of the Nrf2 antioxidant response pathway. To the best of our knowledge, this study is the first to demonstrate the cardioprotec tive effects of tBHQ on the apoptosis of H9c2 cardiomyocytes induced by ethanol.
Oxidative stress is well known as a major mechanism leading to apoptosis induced by ethanol (8–10). ROS are the major cause of cellular oxidative stress, which can both oxidize biomolecules and regulate the expression of genes related to apoptosis, and can serve as a signaling link between ethanol-induced oxidative stress and apoptosis, leading to cell apoptosis (16,34). In the present study, we confirmed that the exposure of H9c2 cells to 200 mM ethanol increased ROS generation excessively (Fig. 4). In our experiments, 200 mM ethanol was used as an inducing concentration according to the results of MTT assay and our pilot trial. It is a high-dose concentration which has been confirmed to effectively induce the ROS-mediated apoptosis of cardiomyocytes (34). The results from this study also demonstrated that ethanol markedly induced the apoptosis of H9c2 cells, as indicated by the results of flow cytometric analysis, which revealed a significant increase in the apoptotic cell number in the ethanol-exposed H9c2 cells as compared to the controls (Fig. 2). In parallel, ethanol activated caspase-3 and Bax, but inhibited Bcl-2, as shown in Fig. 3. These results further confirmed that the oxidative stress induced by ethanol played a crucial role in eliciting cardiomyocyte apoptosis. Thus, anti-oxidative stress processes may indeed be critically beneficial to prevent apoptosis and protect cardiac function.
tBHQ is a potent antioxidant compound which exerts protective effects on multiple cells and organs, including neuroprotective (35–37), hepa toprotective (38) and renal protective effects (30,39). It has been shown that tBHQ diminishes apoptosis by reducing oxidative stress (37). More importantly, tBHQ inhibits ethanol-induced oxidative stress and the apoptosis of cranial neural crest cells (33). Through examination, we further evidenced that tBHQ markedly inhibited the apoptosis of cardiomyocytes exposed to ethanol (as confirmed by MTT assay, Annexin V-FITC staining and the protein expression of caspase-3) accompanied by a decrease of ROS (as illustrated in Fig. 4). As a central regulator of oxidative stress, Nrf2 can effectively inhibit ROS generation and prevent apoptosis (40,41). Following activation, Nrf2 can translocate to the nucleus to promote the expression of antioxidant enzymes to reduce ROS and prevent apoptosis (42). Moreover, other researchers have found that Nrf2 increases the levels of the anti-apoptotic proteins, Bcl-2 and Bcl-xL, which inhibits apoptosis (43). Nrf2 can be activated by a chemical inducer. tBHQ has long been known as an effective inducer of Nrf2 (36). Substantial evidence has indicated that activating the Nrf2 pathway by tBHQ can prevent apoptosis (27,29). Independently of Nrf2 activation, other protective mechanisms of tBHQ are also involved in the induction of autophagy (44,45). In addition, a recent study demonstrated that tBHQ activated Akt rather than Nrf2 to suppress apoptosis (46). In our study, pre-treatment with tBHQ significantly restored the level of Nrf2 protein (Fig. 6) and promoted nuclear translocation (as shown in Fig. 7) previously inhibited by ethanol exposure in H9c2 cells, with an increase in the levels of the antioxidant proteins, SOD, HO-1 and CAT. All the findings mentioned above provide substantial evidence that tBHQ enhances the resistance against ethanol-induced cytotoxicity in H9c2 cells, and activates the Nrf2 cytoprotective response pathway, thus reducing apoptosis.
There are many studies demonstrating that the anti-apoptotic Bcl-2 and proapoptotic Bax genes play a major role in maintaining the balance of cell death and survival, and the upregulation of the anti-apoptotic Bcl-2 protein can antagonize the pro-apoptotic activities of Bax (47). Moreover, Niture and Jaiswal found that tBHQ antagonized Bcl-2, INrf2 (inhibitor of Nrf2) interaction, led to the release and stabilization of Bcl-2, increased Bcl-2, Bax heterodimers, and reduced the apoptosis of mouse Hepa-1 cells (48). Further examination indicated that pre-treatment with tBHQ destabilized Nrf2-Keapl/PGAM5 (phosphog1ycerate mutase 5)-Bcl-xL, increased the release of Bcl-xL and inhibited apoptosis (49). In the present study, we found that ethanol markedly downregulated Bcl-2 expression, while it upregulated Bax expression. Conversely, pre-treatment with tBHQ reversed these changes in Bax and Bcl-2 protein expression in the H9c2 cardiomyocytes exposed to ethanol, as confirmed by the resutls of western blot analysis. Consistently, pre-treatment with tBHQ markedly inhibited the ethanol-induced increase in the expression of caspase-3 in H9c2 cells.
In conclusion, these new experimental findings indicate that pre-treatment with tBHQ protects H9c2 cardiomyocytes against ethanol-induced oxidative stress and apoptosis. Our results also suggest that the anti-apoptotic effects of tBHQ are at least partly mediated by the inhibition of ROS generation, the activation of Nrf2 signaling pathway, the reduction of caspase-3 expres sion and the modulation of Bcl-2 and Bax expres sion. tBHQ may thus provide a valuable therapeutic intervention for the treatment of ACM.
References
- 1.Piano MR, Phillips SA. Alcoholic cardiomyopathy: pathophysiologic insights. Cardiovasc Toxicol. 2014;14:291–308. doi: 10.1007/s12012-014-9252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capasso JM, Li P, Guideri G, Malhotra A, Cortese R, Anversa P. Myocardial mechanical, biochemical, and structural alterations induced by chronic ethanol ingestion in rats. Circ Res. 1992;71:346–356. doi: 10.1161/01.RES.71.2.346. [DOI] [PubMed] [Google Scholar]
- 3.Li SY, Li Q, Shen JJ, Dong F, Sigmon VK, Liu Y, Ren J. Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: role of MAP kinase signaling. J Mol Cell Cardiol. 2006;40:283–294. doi: 10.1016/j.yjmcc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Chen DB, Wang L, Wang PH. Insulin-like growth factor I retards apoptotic signaling induced by ethanol in cardiomyocytes. Life Sci. 2000;67:1683–1693. doi: 10.1016/S0024-3205(00)00759-1. [DOI] [PubMed] [Google Scholar]
- 5.Tan Y, Li X, Prabhu SD, Brittian KR, Chen Q, Yin X, McClain CJ, Zhou Z, Cai L. Angiotensin II plays a critical role in alcohol- induced cardiac nitrative damage, cell death, remodeling, and cardiomyopathy in a protein kinase C/nicotinamide adenine dinucleotide phosphate oxidase-dependent manner. J Am Coll Cardiol. 2012;59:1477–1486. doi: 10.1016/j.jacc.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández-Solà J, Fatjó F, Sacanella E, Estruch R, Bosch X, Urbano-Márquez A, Nicolás JM. Evidence of apoptosis in alcoholic cardiomyopathy. Hum Pathol. 2006;37:1100–1110. doi: 10.1016/j.humpath.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Solà J, Lluis M, Sacanella E, Estruch R, Antúnez E, Urbano-Márquez A. Increased myostatin activity and decreased myocyte proliferation in chronic alcoholic cardiomyopathy. Alcohol Clin Exp Res. 2011;35:1220–1229. doi: 10.1111/j.1530-0277.2011.01456.x. [DOI] [PubMed] [Google Scholar]
- 8.Kannan M, Wang L, Kang YJ. Myocardial oxidative stress and toxicity induced by acute ethanol exposure in mice. Exp Biol Med (Maywood) 2004;229:553–559. doi: 10.1177/153537020422900614. [DOI] [PubMed] [Google Scholar]
- 9.Guan Z, Lui CY, Morkin E, Bahl JJ. Oxidative stress and apoptosis in cardiomyocyte induced by high-dose alcohol. J Cardiovasc Pharmacol. 2004;44:696–702. doi: 10.1097/00005344-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Jing L, Jin CM, Li SS, Zhang FM, Yuan L, Li WM, Sang Y, Li S, Zhou LJ. Chronic alcohol intake-induced oxidative stress and apoptosis: role of CYP2E1 and calpain-1 in alcoholic cardiomyopathy. Mol Cell Biochem. 2012;359:283–292. doi: 10.1007/s11010-011-1022-z. [DOI] [PubMed] [Google Scholar]
- 11.Umoh NA, Walker RK, Al-Rubaiee M, Jeffress MA, Haddad GE. Acute alcohol modulates cardiac function as PI3K/Akt regulates oxidative stress. Alcohol Clin Exp Res. 2014;38:1847–1864. doi: 10.1111/acer.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho E, Karimi Galougahi K, Liu CC, Bhindi R, Figtree GA. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1:483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas DL, Brown RA, Wassef M, Giles TD. Alcohol and the cardiovascular system: research challenges and opportunities. J Am Coll Cardiol. 2005;45:1916–1924. doi: 10.1016/j.jacc.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 14.Zhang RH, Gao JY, Guo HT, Scott GI, Eason AR, Wang XM, Ren J. Inhibition of CYP2E1 attenuates chronic alcohol intake-induced myocardial contractile dysfunction and apoptosis. Biochim Biophys Acta. 2013;1832:128–141. doi: 10.1016/j.bbadis.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7:153–163. doi: 10.1016/S0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 16.Zu L, Zheng X, Wang B, Parajuli N, Steenbergen C, Becker LC, Cai ZP. Ischemic preconditioning attenuates mitochondrial localization of PTEN induced by ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2011;300:H2177–H2186. doi: 10.1152/ajpheart.01138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kino M. Chronic effects of ethanol under partial inhibition of catalase activity in the rat heart: light and electron microscopic observations. J Mol Cell Cardiol. 1981;13:5–21. doi: 10.1016/0022-2828(81)90225-X. [DOI] [PubMed] [Google Scholar]
- 18.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, Cui T. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29:1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 20.Calvert JW, Elston M, Nicholson CK, Gundewar S, Jha S, Elrod JW, Ramachandran A, Lefer DJ. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation. 2010;122:11–19. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Zhang C, Xing Y, Janicki JS, Yamamoto M, Wang XL, Tang DQ, Cui T. Up-regulation of p27(kip1) contributes to Nrf2-mediated protection against angiotensin II-induced cardiac hypertrophy. Cardiovasc Res. 2011;90:315–324. doi: 10.1093/cvr/cvr010. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Wang W, Niu T, Wang H, Li B, Shao L, Lai Y, Li H, Janicki JS, Wang XL, et al. Nrf2 deficiency exaggerates doxorubicin-induced cardiotoxicity and cardiac dysfunction. Oxid Med Cell Longev. 2014;2014:748524. doi: 10.1155/2014/748524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Ichikawa T, Janicki JS, Cui T. Targeting the Nrf2 pathway against cardiovascular disease. Expert Opin Ther Targets. 2009;13:785–794. doi: 10.1517/14728220903025762. [DOI] [PubMed] [Google Scholar]
- 24.Walker RK, Cousins VM, Umoh NA, Jeffress MA, Taghipour D, Al-Rubaiee M, Haddad GE. The good, the bad, and the ugly with alcohol use and abuse on the heart. Alcohol Clin Exp Res. 2013;37:1253–1260. doi: 10.1111/acer.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong J, Yan D, Chen SY. Stabilization of Nrf2 protein by D3T provides protection against ethanol-induced apoptosis in PC12 cells. PLoS One. 2011;6:e16845. doi: 10.1371/journal.pone.0016845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Liu J, Chen SY. Sulforaphane protects against ethanol-induced oxidative stress and apoptosis in neural crest cells by the induction of Nrf2-mediated antioxidant response. Br J Pharmacol. 2013;169:437–448. doi: 10.1111/bph.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Johnson D, Calkins M, Wright L, Svendsen C, Johnson J. Stabilization of Nrf2 by tBHQ confers protection against oxidative stress-induced cell death in human neural stem cells. Toxicol Sci. 2005;83:313–328. doi: 10.1093/toxsci/kfi027. [DOI] [PubMed] [Google Scholar]
- 28.Kaspar JW, Jaiswal AK. An autoregulatory loop between Nrf2 and Cul3-Rbx1 controls their cellular abundance. J Biol Chem. 2010;285:21349–21358. doi: 10.1074/jbc.M110.121863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Cheng M, Liu Q, Yang J, Wu S, Lu X, Jin C, Ma H, Cai Y. Protective role of tert-butylhydroquinone against sodium fluoride-induced oxidative stress and apoptosis in PC12 cells. Cell Mol Neurobiol. 2015;35:1017–1025. doi: 10.1007/s10571-015-0196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Zhang L, Wang F, Shi Y, Ren Y, Liu Q, Cao Y, Duan H. Attenuation of glomerular injury in diabetic mice with tert-butylhydroquinone through nuclear factor erythroid 2-related factor 2-dependent antioxidant gene activation. Am J Nephrol. 2011;33:289–297. doi: 10.1159/000324694. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Ji C, Wu L, Qiu J, Li Q, Shao Z, Chen G. Tert-butylhydroquinone alleviates early brain injury and cognitive dysfunction after experimental subarachnoid hemorrhage: role of Keap1/Nrf2/ARE pathway. PLoS One. 2014;9:e97685. doi: 10.1371/journal.pone.0097685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gharavi N, Haggarty S, El-Kadi AO. Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Curr Drug Metab. 2007;8:1–7. doi: 10.2174/138920007779315035. [DOI] [PubMed] [Google Scholar]
- 33.Yan D, Dong J, Sulik KK, Chen SY. Induction of the Nrf2-driven antioxidant response by tert-butylhydroquinone prevents ethanol-induced apoptosis in cranial neural crest cells. Biochem Pharmacol. 2010;80:144–149. doi: 10.1016/j.bcp.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Zhao J, Yang W, Bi Y, Chi J, Tian J, Li W. High-dose alcohol induces reactive oxygen species-mediated apoptosis via PKC-β/p66Shc in mouse primary cardiomyocytes. Biochem Biophys Res Commun. 2015;456:656–661. doi: 10.1016/j.bbrc.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Jin W, Ni H, Dai Y, Wang H, Lu T, Wu J, Jiang J, Liang W. Effects of tert-butylhydroquinone on intestinal inflammatory response and apoptosis following traumatic brain injury in mice. Mediators Inflamm. 2010;2010:502564. doi: 10.1155/2010/502564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J, Ren X, Simpkins JW. Sequential upregulation of superoxide dismutase 2 and heme oxygenase 1 by tert-butylhy-droquinone protects mitochondria during oxidative stress. Mol Pharmacol. 2015;88:437–449. doi: 10.1124/mol.115.098269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan X, Liu D, Xing X, Li J, Zhao S, Nie H, Zhang Y, Sun G, Li B. Tert-butylhydroquinone as a phenolic activator of Nrf2 antagonizes arsenic-induced oxidative cytotoxicity but promotes arsenic methylation and detoxication in human hepatocyte cell line. Biol Trace Elem Res. 2014;160:294–302. doi: 10.1007/s12011-014-0042-4. [DOI] [PubMed] [Google Scholar]
- 39.Wang C, Li C, Peng H, Ye Z, Zhang J, Liu X, Lou T. Activation of the Nrf2-ARE pathway attenuates hyperglycemia-mediated injuries in mouse podocytes. Cell Physiol Biochem. 2014;34:891–902. doi: 10.1159/000366307. [DOI] [PubMed] [Google Scholar]
- 40.He X, Kan H, Cai L, Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol. 2009;46:47–58. doi: 10.1016/j.yjmcc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Liu J, Chen SY. Over-expression of Nrf2 diminishes ethanol-induced oxidative stress and apoptosis in neural crest cells by inducing an antioxidant response. Reprod Toxicol. 2013;42:102–109. doi: 10.1016/j.reprotox.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang XS, Chen HP, Yu HH, Yan YF, Liao ZP, Huang QR. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for hypoxic preconditioning-mediated delayed cardioprotection. Mol Cell Biochem. 2014;385:33–41. doi: 10.1007/s11010-013-1812-6. [DOI] [PubMed] [Google Scholar]
- 43.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S, Li J, Shen C, Zhang X, Sun S, Cho M, Sun C, Song Z. Tert-butylhydroquinone (tBHQ) protects hepatocytes against lipotoxicity via inducing autophagy independently of Nrf2 activation. Biochim Biophys Acta. 2014;1841:22–33. doi: 10.1016/j.bbalip.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li T, Sun KJ, Wang HD, Zhou ML, Ding K, Lu XY, Wei WT, Wang CX, Zhou XM. Tert-butylhydroquinone ameliorates early brain injury after experimental subarachnoid hemorrhage in mice by enhancing Nrf2-independent autophagy. Neurochem Res. 2015;40:1829–1838. doi: 10.1007/s11064-015-1672-4. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Liu FF, Bi X, Wang S, Wu X, Jiang F. The antioxidant compound tert-butylhydroquinone activates Akt in myocardium, suppresses apoptosis and ameliorates pressure overload-induced cardiac dysfunction. Sci Rep. 2015;5:13005. doi: 10.1038/srep13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 48.Niture SK, Jaiswal AK. INrf2 (Keap1) targets Bcl-2 degradation and controls cellular apoptosis. Cell Death Differ. 2011;18:439–451. doi: 10.1038/cdd.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Niture SK, Jaiswal AK. Inhibitor of Nrf2 (INrf2 or Keap1) protein degrades Bcl-xL via phosphoglycerate mutase 5 and controls cellular apoptosis. J Biol Chem. 2011;286:44542–44556. doi: 10.1074/jbc.M111.275073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]