Abstract
Long non-coding RNAs (lncRNAs) have been identified to be critical mediators in various tumors associated with cancer progression. Long non-coding RNA activated by TGF-β (lncRNA-ATB) is a stimulator of epithelial-mesenchymal transition (EMT) and serves as a novel prognostic biomarker for hepatocellular carcinoma. However, the biological role and clinical significance of lncRNA-ATB in human prostate cancer have yet to be fully elucidated. The present study was designed to explore the expression of lncRNA-ATB in human prostate cancer patients and the role of lncRNA-ATB in prostate cancer cells. We showed that lncRNA-ATB expression was significantly upregulated in tumor tissues in patients with prostate cancer in comparison with adjacent non-tumor tissues. Further analysis indicted that high lncRNA-ATB expression may be an independent prognostic factor for biochemical recurrence (BCR)-free survival in prostate cancer patients. Overexpression of lncRNA-ATB promoted, and knockdown of lncRNA-ATB inhibited the growth of prostate cancer cells via regulations of cell cycle regulatory protein expression levels. In addition, lncRNA-ATB stimulated epithelial-mesenchymal transition (EMT) associated with ZEB1 and ZNF217 expression levels via ERK and PI3K/AKT signaling pathways. These results indicated that lncRNA-ATB may be considered as a new predictor in the clinical prognosis of patients with prostate cancer. Overexpression of lncRNA-ATB exerts mitogenic and EMT effects of prostate cancer via activation of ERK and PI3K/AKT signaling pathways.
Keywords: lncRNA-ATB, prostate cancer, prognosis, proliferation, cell cycle regulatory protein
Introduction
Prostate cancer is the most frequently diagnosed malignancy in males worldwide and is the second most common cause of cancer mortality in the United States (1). It accounts for 15% of all cancer diagnoses (2). The incidence of prostate cancer is robustly increasing due to increase in the life expectancy, modified diagnostic techniques and increased awareness of prostate cancer screening (3). It is reported that the 5-year survival rate of prostate cancer patients was only 29%, exerting a big burden on public health a worldwide (4). The pathogenesis of tumorigenesis and progression of prostate cancer has not been fully elucidated although intensive studies of prostate cancer have been performed. Despite the therapeutic strategies developed in prostate cancer, the annual morbidity rate has explosively increased by 14% since 1990 (5). The management of prostate cancer is recognized to be one of the most serious medical challenges (6). It is unfortunate that no implementation of a population screening program is available for early detection of prostate cancer (7).
The prostate-specific antigen (PSA) is indicated to be employed as a biomarker for early detection of prostate cancer and digital rectal examination (DRE) is also conventionally used for diagnosis of prostate cancer (8). Prostate biopsy is considered as a gold standard in patients with raised suspicion from digital rectal examination or increased PSA levels (9). However, the rate of negative biopsies is substantially high even in cases with increased PSA levels, thus greatly affecting the patient quality of life (10,11). The low specificity of PSA has been demonstrated in aggressive and indolent prostate cancer (12). The U.S. Preventive Services Task Force has proposed to quit the conventional screening by PSA testing for prostate cancer (13). Therefore, it is required to identify novel prognostic biomarkers for improvement of patient outcomes and clinical management in prostate cancer patients.
The long non-coding RNAs (lncRNAs) are defined as <200 nucleotide non-protein-coding RNA molecules and play critical roles in the biological and pathological processes of many cancers associated with regulation of cell proliferation, migration and invasion (14,15). A number of studies have clarified that lncRNAs may function as oncogenes or tumor suppressors in various types of cancers (16). It is evidenced that lncRNA DANCR is upregulated in human colorectal cancer tissues and may serve as an independent negative prognostic factor for both overall survival and disease-free survival in colorectal cancer (17). The lncRNA HOTTIP overexpression is found to be associated with increased metastasis of hepatocellular carcinoma and is suggested to be a negative prognostic factor in hepatocellular carcinoma (18). Mounting evidence highlights that the lncRNAs may be emerged as novel and improved markers or therapeutic targets in the diagnosis and treatment of cancer (19). The lncRNA-ATB is observed to be higher in hepatocellular carcinoma tissues and have a poor prognosis in hepatocellular carcinoma patients (20). To date, however, little is known about the clinical significance and role of lncRNA-ATB in human prostate cancer.
In the present study, we aimed to determine the lncRNA-ATB expression in human prostate cancer tissue and the association between lncRNA-ATB and clinicopathological factors and prognosis the prostate cancer. The role of lncRNA-ATB in proliferation and EMT of prostate cancer cells was also investigated.
Materials and methods
Ethics statement
The approval of this study protocol was obtained from the Ethics Committee of Jinling Hospital and the written informed consent was provided from all subjects. All experiments were performed in accordance with relevant guidelines and regulations.
Subjects
A total of 57 prostate cancer patients who underwent radical prostatectomy were included from Department of Urology, Jinling Hospital, School of Medicine, Nanjing University ranged from September 2001 to August 2005. All patients were diagnosed with H&E-stained tissue section and histopathological grading for each subject was confirmed by experienced pathologists. The biochemical and clinicopathological parameters pre- and post-operation were recorded and summarized. The date of prostatectomy was considered as the beginning of the follow-up period. The endpoint time of biochemical recurrence was set as the period from surgical treatment to the assay of two successive values of serum PSA level ≥0.2 ng/ml. The patients who received any treatment including chemotherapy, radiation therapy, androgen deprivation treatment before radical prostatectomy or died of unexpected events or other diseases were excluded from this study. The primary prostate cancer and paired noncancerous prostate tissues from 57 cases were collected and were frozen in liquid nitrogen and stored at −80°C prior to use.
RNA isolation and quantitative real-time PCR
The TRIzol reagent (Life Technologies, Gaithersburg, MD, USA) was used to extract the total RNA according to the manufacturer's instructions. The mRNA of lncRNA-ATB, ZEB1 and ZNF217 were analyzed by real-time PCR (SYBR Premix Ex Taq™, Takara, Otsu, Shiga, Japan) in triplicates by reacting with sequence-specific PCR primers in ABI PRISM 7500 sequence detection PCR system (Applied Biosystems, Foster City, CA, USA). GAPDH was used as an internal control for each sample. The relative quantification of gene expression was expressed as fold changes via normalization against GAPDH by using the 2−ΔΔCT method. The sequences of primers were listed as follows: lncRNA-ATB: forward: 5′-CTTCACCAGCACCCAGAGA-3′, reverse: 5′-AAGACAGAAAAACAGTTCCGAGTC-3′; ZEB1: forward: 5′-GACAGTGTTACCAGGGAGGAGCA-3′, reverse: 5′-TTCAGGTGCCTCAGGAAAAATGA-3′; ZNF21: forward: 5′-CTGAAACGGGGAAGAAGCCT-3′, reverse: 5′-CTTGCCCCGATTCCTTCACT-3′; GAPDH: forward: 5′-CAGGAGGCATTGCTGATGAT-3′, reverse: 5′-GAAGGCTGGGGCTCATTT-3′. E-cadherin: forward: 5′-GCCCCATCAGGCCTCCGTTT-3′, reverse: 5′-ACCTTGCCTTCTTTGTCTTTGTTGGA-3′; ZO-1: forward: 5′-CACGCAGTTACGAGCAAG-3′, reverse: 5′-TGAAGGTATCAGCGGAGG-3′; N-cadherin forward: 5′-TGGACCATCACTCGGCTTA-3′, reverse: 5′-ACACTGGC AAACCTTCACG-3′; Vimentin: forward: 5′-CCTGAACCTG AGGGAAACTAA-3′, reverse: 5′-GCAGAAAGGCACTTGAAAGC-3′.
Cell culture and transfection
The prostate cell lines PC-3 and DU145 were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI-1640 medium supplemented with 10% FBS coupled with 1% penicillin/streptomycin and 1% non-essential amino acids and 1% (mg/ml) sodium pyruvate at 37°C in a humidified incubator with 5% CO2. The day before transfection, PC-3 and DU145 cells were seeded in 6-well plates, and the transient transfection of lncRNA-ATB siRNA (5′-CCAUGAGGAGUACUGCCAATT-3′) and non-targeting Control (5′-UUCUCCGAACGUGUCACGUtt-3′) were conducted with Lipofectamine 2000 Transfection Reagent (Invitrogen) following manufacturer's instructions.
Construction of lentivirus-mediated overexpression of lncRNA-ATB
The construction of lentiviral vector expressing human lncRNA-ATB gene was performed by Genomeditech (Shanghai, China). The primers used for lentivirus production sequences were as follows: forward: 5′-CCTCTCTCCCCAGGGGGATCCTGGCTAGTTAAGCTTGGCCCT-3′, reverse: 5′-ATCCAGAGGTTGATTGTCGACTATCTGCAGAATTCTGGTAAATg-3′ (20).
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8, Dojindo, Japan) was employed to determine the proliferation of PC-3 and DU145 cells accordance with the manufacturer's instructions (21).
Western blotting
Equal amounts of protein in each sample were loaded onto an SDS gel and transferred to immobilon polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membrane was incubated with designed primary antibodies overnight at 4°C. The positive signals from HRP-coupled secondary antibodies (Santa Cruz Biotechnology; Santa Cruz, CA, USA) were visualized by enhanced chemiluminescence detection kit (Thermo Scientific, Rockford, IL, USA). The densitometric analysis of the band intensities was measured and normalized to the band intensities of GAPDH using the ImageJ softwere (NIH, Bethesda, MD, USA).
Chemicals
The primary antibodies against proliferating cell nuclear antigen (PCNA), phosphorylated histone H3 (P-H3) and GAPDH were obtained from Santa Cruz Biotechnology. The antibodies of phosphorylated Akt, phosphorylated ERK, Akt, ERK cyclin E, cyclin D1, p21 and p27 were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). The ZEB1 and ZNF217 antibodies were purchased from Abcam (Cambridge, UK). LY294002 were obtained from Selleck Chemicals (Houston, TX, USA). U0126 was purchased from Beyotime Biotechnology (Shanghai, China). The siRNA specifically targeting lncRNA-ATB and non-targeting control were synthesized by GenePharma Co. (Shanghai, China).
Statistical analysis
SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. All data on continuous variables were expressed as mean ± SD. The test for categorical variables was made by Chi-square test, and the small cell variables were compared by Fisher's exact test. Comparisons between two groups were made by Student's t-test. One-way or two-way ANOVA followed by post hoc Bonferroni test was used when multiple comparisons were made. The receiver operating characteristic (ROC) curve was applied to calculate the area under the ROC curve (AUC) for evaluating the diagnostic efficacy of lncRNA-ATB. Survival analysis was conducted with the Kaplan-Meier method. Multivariate analysis was carried out with the Cox proportional hazards model. P-values <0.05 was considered statistically significant.
Results
Upregulation of lncRNA-ATB expression in prostate cancer tissues
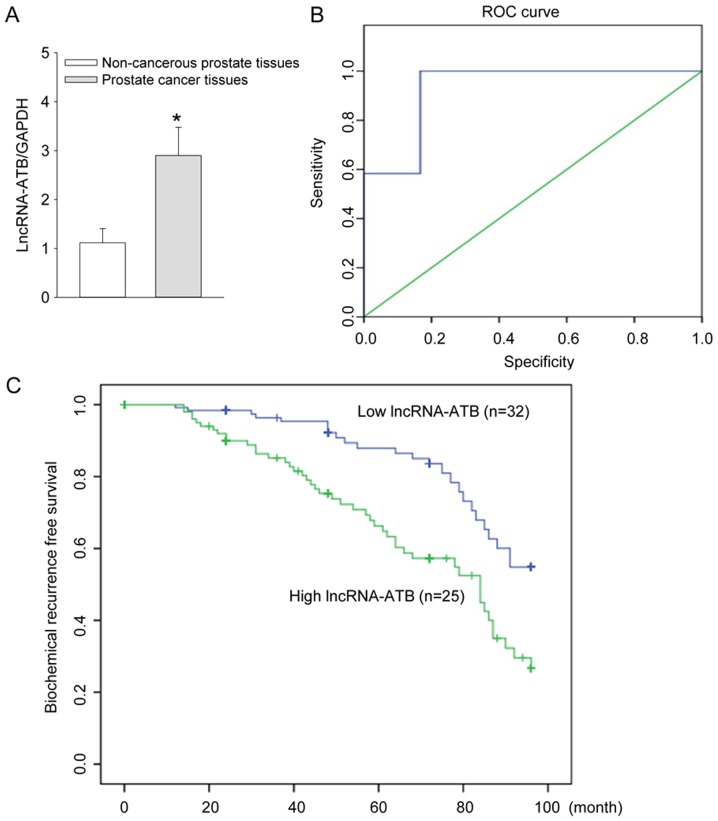
The lncRNA-ATB expression in 57 pairs of prostate cancer and adjacent non-cancerous prostate tissues was measured by qRT-PCR analysis. The results showed that the expression of lncRNA-ATB was significantly increased in the prostate cancer patients in comparison with the adjacent noncancerous prostate tissues (P<0.05, Fig. 1A).
Figure 1.
Expression of lncRNA-ATB, diagnostic efficacy of lncRNA-ATB and the biochemical recurrence (BCR)-free survival curves in patients with prostate cancer. (A) The expression level of lncRNA-ATB were detected in 57 pairs of prostate cancer and adjacent non-cancerous prostate tissues by qRT-PCR analysis; (B) assessment of the diagnostic efficacy of lncRNA-ATB in prostate cancer patients by calculating the area under the receiver operating characteristic curve. (AUC=0.931, P<0.001); (C) biochemical recurrence (BCR)-free survival curves for two groups defined by low and high expression of lncRNA-ATB in patients with prostate cancer (P<0.001). *P<0.05 vs. non-cancerous prostate tissues.
Diagnostic efficacy of lncRNA-ATB in prostate cancer patients
The diagnostic efficacy of lncRNA-ATB in prostate cancer tissues of prostate cancer patients was evaluated by calculating the area under the receiver operating characteristic curve. The ROC curve analysis revealed that AUC was 0.931 (95% CI=0.000–1.000, P<0.001). When the cutoff value=1.30, the diagnostic sensitivity (100%) and specificity (83.3%) reached their peak values (P<0.05, Fig. 1B). Thus the lncRNA-ATB expression was further classified into the low expression group (n = lncRNA-ATB expression <1.30, n=32) and high expression group (lncRNA-ATB expression ≥1.30, n=25) as the threshold ROC curve value of 1.30.
Relationship between lncRNA-ATB expression and biochemical recurrence-free survival
The Kaplan-Meier curves plotted between high or low lncRNA-ATB expression and biochemical recurrence (BCR)-free survival suggested that prostate cancer patients with a high lncRNA-ATB expression had a shorter BCR-free survival compared with low lncRNA-ATB expression (P<0.001, Fig. 1C).
Correlation of lncRNA-ATB expression with clinical features of prostate cancer patients
As seen in Table I, the high lncRNA-ATB expression in prostate cancer tissues of prostate cancer patients is closely related with aggressive clinical pathological parameters including histological grade (P=0.017), high preoperative PSA level (P=0.001), pathological stage (P=0.003), high Gleason score (P=0.045), lymph node metastasis (P=0.007), angiolymphatic invasion (P=0.008), biochemical recurrence (P=0.003). However, there was no correlation between lncRNA-ATB with age (P=0.952) and surgical margin status (P=0.257). The Cox multivariate analysis was conducted to assess the relationship of lncRNA-ATB expression with the BCR-free survival of patients with prostate cancer. The results established the significance of lncRNA-ATB expression (P<0.001), and other clinicopathological parameters including histological grade (P=0.020), preoperative PSA level (P=0.002), Gleason score (P=0.049), pathological stage (P<0.001), lymph node metastasis (P=0.025) and angiolymphatic invasion (P=0.011) for independent prognostic predictors of BCR-free survival of prostate cancer patients (Table II).
Table I.
Prostate cancer patient clinicopathological characteristics and clinical features.
| Variable | All Cases (%) | lncRNA-ATB expression
|
χ2 | P-value | |
|---|---|---|---|---|---|
| Low (%) | High (%) | ||||
| Age | |||||
| ≤70 | 39 (68.4) | 22 (56.4) | 17 (43.6) | 0.004 | 0.952 |
| >70 | 18 (31.6) | 10 (55.6) | 8 (44.4) | ||
| Histological grade | |||||
| G1 + G2 | 35 (61.4) | 24 (68.6) | 11 (31.4) | 5.691 | 0.017 |
| G3 | 22 (38.6) | 8 (36.4) | 14 (63.6) | ||
| Preoperative PSA | |||||
| <10 ng/ml | 21 (36.8) | 18 (85.7) | 3 (14.3) | 11.811 | 0.001 |
| ≥10 ng/ml | 36 (63.2) | 14 (38.9) | 22 (61.1) | ||
| Pathological stage | |||||
| I + II | 37 (64.9) | 26 (70.2) | 11 (29.7) | 8.550 | 0.003 |
| III + IV | 20 (35.1) | 6 (30.0) | 14 (70.0) | ||
| Gleason score | |||||
| 4–6 | 22 (38.6) | 16 (72.7) | 6 (27.3) | 4.003 | 0.045 |
| 7–10 | 35 (61.4) | 16 (45.7) | 19 (54.3) | ||
| Lymph node metastasis | |||||
| Negative | 49 (86.0) | 31 (63.3) | 18 (36.7) | 7.198 | 0.007 |
| Positive | 8 (14.0) | 1 (12.5) | 7 (87.5) | ||
| Surgical margin status | |||||
| Negative | 47 (82.5) | 28 (59.6) | 19 (40.4) | 1.283 | 0.257 |
| Positive | 10 (17.5) | 4 (40.0) | 6 (60.0) | ||
| Angiolymphatic invasion | |||||
| Negative | 40 (70.2) | 27 (67.5) | 13 (32.5) | 7.029 | 0.008 |
| Positive | 17 (29.8) | 5 (29.4) | 12 (70.6) | ||
| Biochemical recurrence | |||||
| Negative | 43 (75.4) | 29 (67.4) | 14 (32.6) | 9.081 | 0.003 |
| Positive | 14 (24.6) | 3 (21.4) | 11 (78.6) | ||
Table II.
Multivariate analysis by Cox regression model on factors related to biochemical recurrence (BCR)-free survival in 57 prostate cancer patients.
| Variables | BCR-free survival
|
||
|---|---|---|---|
| Exp (B) | 95% CI | P-value | |
| Age (≤70 vs. >70) | 1.035 | 0.336–3.188 | 0.952 |
| Histological grade (G1+ G2 vs. G3) | 3.818 | 1.241–11.752 | 0.020 |
| Preoperative PSA (<10 ng/ml vs. ≥10 ng/ml) | 9.429 | 2.339–38.001 | 0.002 |
| Pathological stage (I + II vs. III + IV) | 5.515 | 1.681–18.059 | <0.001 |
| Gleason score (4–6 vs. 7–10) | 3.167 | 1.003–10.001 | 0.049 |
| Lymph node metastasis (Negative vs. Positive) | 12.056 | 1.371–106.041 | 0.025 |
| Surgical margin status (Negative vs. Positive) | 2.211 | 0.549–8.900 | 0.264 |
| Angiolymphatic invasion (Negative vs. Positive) | 4.985 | 1.449–17.146 | 0.011 |
| lncRNA-ATB expression (High vs. Low) | 1.749 | 2.313–14.249 | <0.001 |
Effect of lncRNA-ATB on prostate cell growth
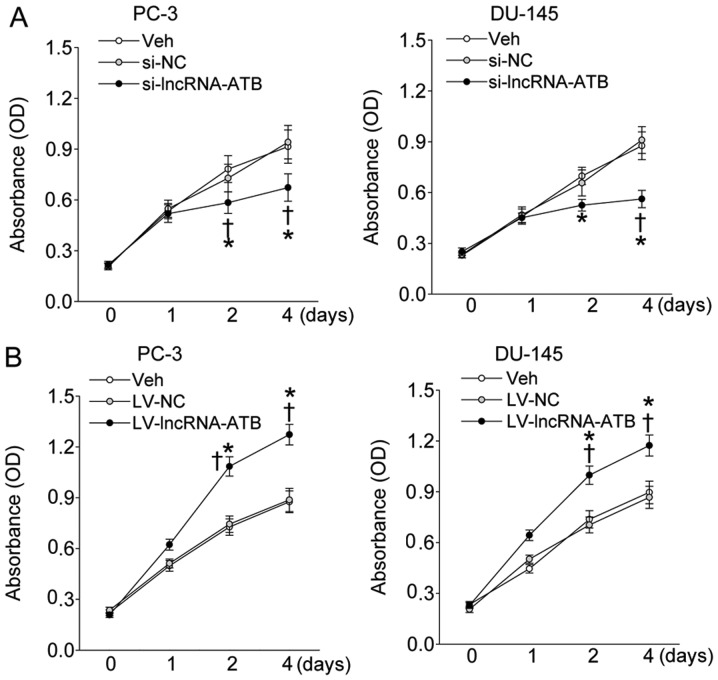
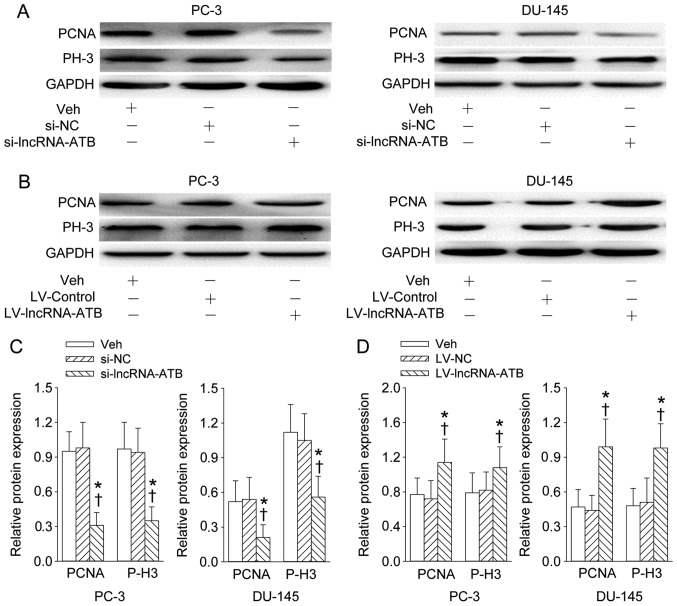
The lncRNA-ATB-specific siRNA or lentiviral vector expressing human lncRNA-ATB gene was transfected to PC-3 and DU-145 prostate cell lines to determine its effect on the proliferation of prostate cell in vitro. The depletion of lncRNA-ATB obviously attenuated the proliferation rate of PC-3 and DU-145 prostate cell lines (Fig. 2A), but the enforced overexpression of lncRNA-ATB effectively affected the proliferation rate of PC-3 and DU-145 prostate cell lines at day 2 and 4 post-transfection (Fig. 2B). Furthermore, the proliferating markers including PCNA and phosphorylated histone H3 (P-H3) was also obviously inhibited by reduction of lncRNA-ATB (Fig. 3A and C), but further enhanced by lncRNA-ATB overexpression (Fig. 3B and D).
Figure 2.
Effect of lncRNA-ATB on the proliferation of indicated prostate cancer cell lines that were transfected with lncRNA-ATB siRNA or LV-mediated lncRNA-ATB plasmid and measured at day 1, 2, and 4 post-transfection. (A) The proliferation of prostate cancer cell lines in response to lncRNA-ATB knockdown; (B) the proliferation of prostate cancer cell lines in response to forced expression of lncRNA-ATB. Values are mean ± SD. *P<0.05 vs. Veh; ✝P<0.05 vs. si-NC or LV-NC. n=4 for each group.
Figure 3.
Effect of lncRNA-ATB on the proliferating cell nuclear antigen (PCNA) and phosphorylated histone H3 (P-H3) levels (markers of proliferation) in prostate cell lines. (A and C) Representative images showing the effect of lncRNA-ATB knockdown on the PCNA and P-H3 expression levels; (B and D) representative images showing the effect of lncRNA-ATB overexpression on the PCNA and P-H3 expression levels. After 4 days of transfection of lncRNA-ATB siRNA or lentivirus (LV)-mediated lncRNA-ATB plasmid in PC-3 and DU-145 prostate cell lines, the PCNA and P-H3 were determined with western blotting. Values are mean ± SD. *P<0.05 vs. Veh; ✝P<0.05 vs. si-NC or LV-NC. n=4 for each group.
Effects of lncRNA-ATB on cell cycle regulatory protein expression
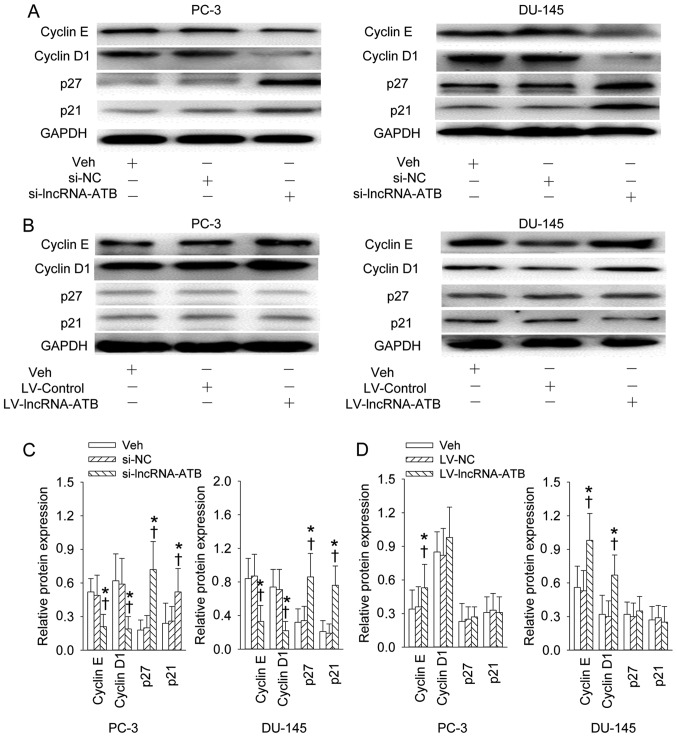
The increased expression levels of cyclin E and cyclin D1 and decreased expression levels of p27 and p21 in PC-3 and DU-145 prostate cell lines were significantly rescued by gene silencing of lncRNA-ATB (Fig. 4A and C). Overexpression of lncRNA-ATB further promoted the cyclin E expression in PC-3 cell line and cyclin E and cyclin D1 in DU-145 prostate cell line, but had no effect on p27 or p21 expression levels (Fig. 4B and D).
Figure 4.
Effect of lncRNA-ATB on the cell cycle related-proteins. (A and C) Representative images showing the effect of lncRNA-ATB knockdown on the cyclin E, cyclin D1, p27 and p21 expression levels; (B and D) representative images showing the effect of lncRNA-ATB overexpression on the cyclin E, cyclin D1, p27 and p21 expression levels. After 4 days of transfection of lncRNA-ATB siRNA or lentivirus (LV)-mediated lncRNA-ATB plasmid in PC-3 and DU-145 prostate cell lines, the cells were lysed, and proteins from cell lysates were resolved by SDS-PAgE, transferred to a PVDF membrane blotted with anti-cyclin E, -cyclin D1, -p27, -p21 antibodies. Values are mean ± SD. *P<0.05 vs. Veh; ✝P<0.05 vs. si-NC or LV-NC. n=4 for each group.
Effect of lncRNA-ATB on the mRNA expression levels of EMT markers
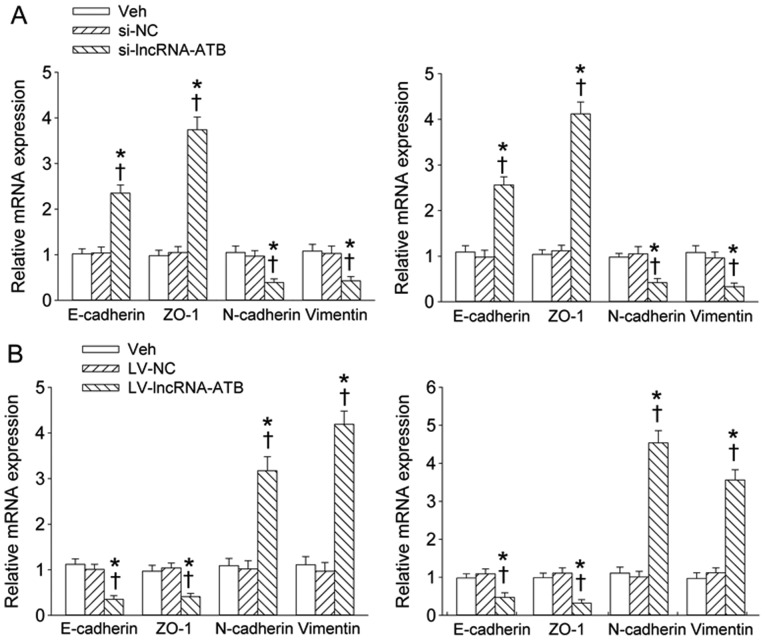
Knockdown of lncRNA-ATB significantly rescued the increased expression levels of E-cadherin and ZO-1 and decreased expression levels of N-cadherin and Vimentin in PC-3 and DU-145 prostate cell lines (Fig. 5A). Overexpression of lncRNA-ATB further inbibited the E-cadherin and ZO-1 expression levels, but further promoted expression levels of N-cadherin and Vimentin in PC-3 and DU-145 prostate cell lines (Fig. 5B).
Figure 5.
Effect of lncRNA-ATB on the mRNA expression levels of EMT markers. (A) The effect of lncRNA-ATB knockdown on the E-cadherin, ZO-1, N-cadherin and vimentin mRNA expression levels; (B) the effect of lncRNA-ATB overexpression on the E-cadherin, ZO-1, N-cadherin and vimentin mRNA expression levels. Values are mean ± SD. *P<0.05 vs. Veh; ✝P<0.05 vs. si-NC or LV-NC. n=4 for each group.
Effects of lncRNA-ATB on ZEB1 and ZNF217 expression levels
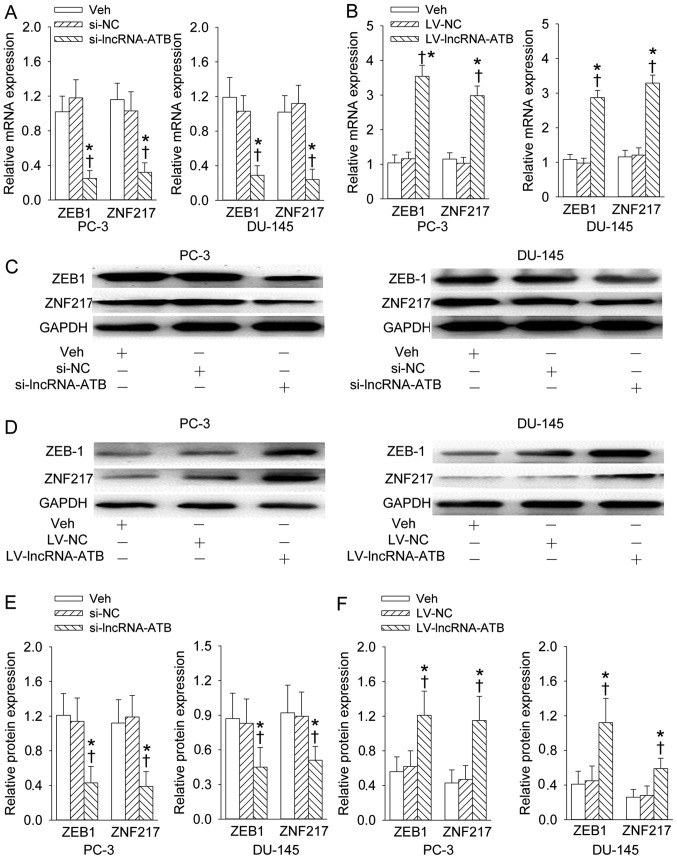
We found that downregulation of lncRNA-ATB could inhibit the expression levels of ZEB1 and ZNF217 at mRNA and protein levels in PC-3 and DU-145 prostate cell lines (Fig. 6A, C and E). In contrast, upregulation of lncRNA-ATB further augmented the mRNA and protein levels of ZEB1 and ZNF217 in PC-3 and DU-145 prostate cell lines (Fig. 6B, D and F).
Figure 6.
Effects of lncRNA-ATB on ZEB1 and ZNF217 expression levels. (A) The effect of lncRNA-ATB knockdown on the ZEB1 and ZNF217 mRNA expression levels; (B) the effect of lncRNA-ATB overexpression on the ZEB1 and ZNF217 mRNA expression levels; (C and E) representative images showing the effect of lncRNA-ATB knockdown on the ZEB1 and ZNF217 protein expression levels; (D and F) representative images showing the effect of lncRNA-ATB overexpression on the ZEB1 and ZNF217 protein expression levels. Values are mean ± SD. *P<0.05 vs. Veh; ✝P<0.05 vs. si-NC or LV-NC. n=4 for each group.
Effects of lncRNA-ATB on PI3K/Akt and ERK signaling pathways
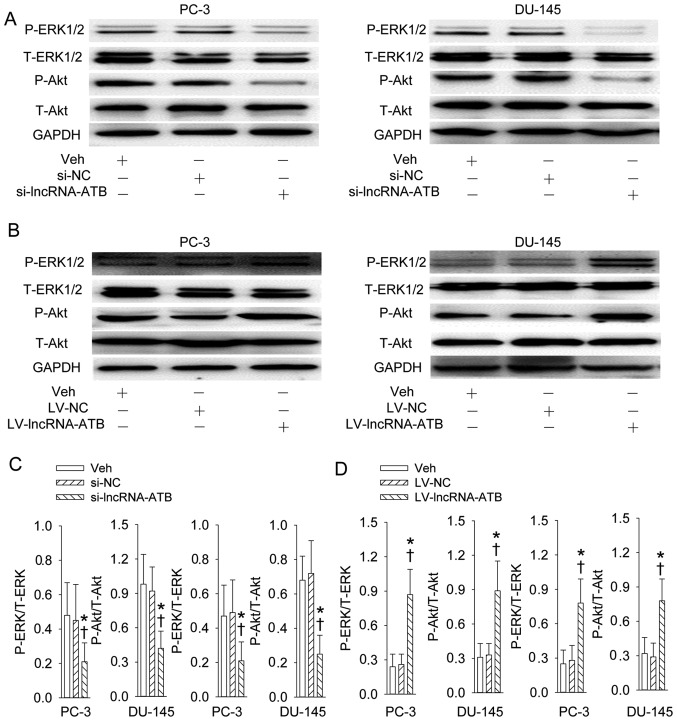
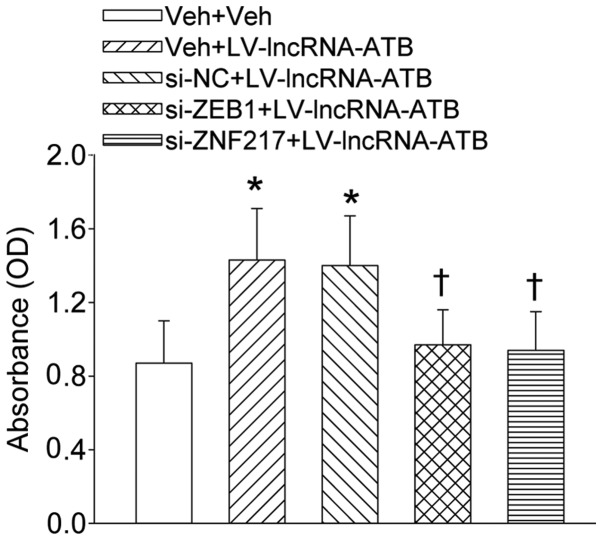
The activation of PI3K/Akt and ERK signaling pathways reflected the increase in phosphorylation levels of Akt and ERK was suppressed by knockdown of lncRNA-ATB (Fig. 7A and C). The continuous lncRNA-ATB expression further stimulated the phosphorylation levels of Akt and ERK in both PC-3 and DU-145 prostate cell lines (Fig. 7B and D). Additionally, inhibition of PI3K/Akt and ERK signaling pathways prevented the proliferation of PC-3 and DU-145 prostate cell line responses to overexpression of lncRNA-ATB (Fig. 9).
Figure 7.
Effects of lncRNA-ATB on PI3K/Akt and ERK signaling pathways. (A and C) Representative images showing the effect of lncRNA-ATB knockdown on the phosphorylation of Akt and ERK; (B and D) representative images showing the effect of lncRNA-ATB overexpression on the phosphorylation of Akt and ERK. Values are mean ± SD. *P<0.05 vs. Veh; ✝P<0.05 vs. si-NC or LV-NC. n=4 for each group.
Figure 9.
Effect of ZEB1 and ZNF217 on the proliferation of indicated prostate cancer cell lines that were transfected with LV-mediated lncRNA-ATB plasmid and measured at day 4 post-transfection. Values are mean ± SD. *P<0.05 vs. Veh + Veh; ✝P<0.05 vs. si-NC+LV-lncRNA-ATB. n=4 for each group.
PI3K/Akt and ERK signaling pathways contribute to ZEB1 and ZNF217 protein expression levels
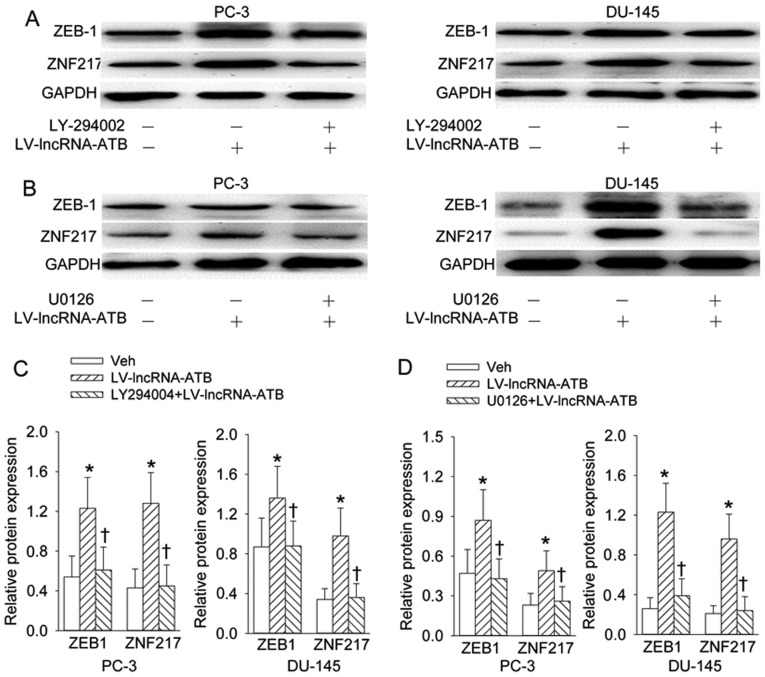
Pretreatment with PI3K inhibitor LY294002 (Fig. 8A and C) or ERK inhibitor U0126 (Fig. 8B and D) almost abolished the effects of lncRNA-ATB overexpression on the ZEB1 and ZNF217 protein expression levels.
Figure 8.
PI3K/Akt and ERK signaling pathways contribute to ZEB1 and ZNF217 protein expression. (A and C) Effects of inhibition of PI3K with LY-294002 (10 µM) on the ZEB1 and ZNF217 protein expression response to lncRNA-ATB overexpression; (B and D) effects of inhibition of ERK with U0126 (10 µM) on the ZEB1 and ZNF217 protein expression levels to lncRNA-ATB overexpression. Values are mean ± SD. *P<0.05 vs. Veh; ✝P<0.05 vs. LV-lncRNA-ATB. n=4 for each group.
Discussion
Our results for the first time established that the expression of lncRNA-ATB was remarkably increased in prostate cancer tissues, and might have prognostic significance in human prostate cancer. We also showed that lncRNA-ATB may be a potential stimulator in growth and EMT of prostate cancer cells.
Prostate cancer is a common cause of malignant mortality among males all over the world and brings a huge and increasing healthy burden in China (22). The clinical outcome of patients with prostate cancer is still poor even with the aid of advanced diagnosis and adjuvant therapy (23). It is also challenging to identify the pathogenesis, and develop novel diagnosis and medical treatments of prostate cancer (24). It is recommended that the early and accurate diagnosis is helpful for improvement of therapeutic efficiency of prostate cancer patients (25). Therefore, it is urgent to identify new molecular targets of prostate cancer in order to understand the pathogenesis and prognosis of prostate cancer (26). It is estimated that 15,000 lncRNAs are identified in human genome (27) and lncRNAs have multiple roles in the carcinogenesis and cancer metastasis (28).
Several studies have disclosed the lncRNAs are possible diagnostic or prognostic biomarkers in human cancers (29). For example, it is reported that the expression of lncRNA MALAT-1 was increased in colorectal cancer tissues and is negatively correlated with the progression of colorectal cancer (30). In addition, the lncRNA HOTAIR is also progressively upregulated in colorectal cancer patients and is employed as a biomarker for clinical prognosis of human colorectal cancer (31). A recent study indicates that lncRNA SNURF-1 is upregulated in prostate cancer (32). LncRNA PCA3 is demonstrated to be a dominant-negative oncogene in human prostate cancer (33). LncRNA BX647187 is characterized to be the important mediator of mitotic regulator Hec1 in human prostate cancer (22). These results suggested that lncRNAs are emerging as potential prognostic biomarkers or useful therapeutic target in prostate cancer. LncRNA-ATB was recently identified to play a critical role in invasion of human hepatocellular carcinoma and positively associated with poor prognosis of human hepatocellular carcinoma (20).
Gastric cancer tissues exhibit overexpressed lncRNA-ATB and the gastric cancer patients with low lncRNA-ATB expression have a longer overall survival rate, and lncRNA-ATB may be an independent prognostic factor in human gastric cancer (34). However, to our knowledge, the clinical significance and biological function of lncRNAs, especially lncRNA-ATB in prostate cancer remains largely unknown. Our data in the present study showed obvious increase in lncRNA-ATB expression in prostate cancer tissues in comparison with adjacent non-cancerous prostate tissues. Further analysis showed that the upregulation of lncRNA-ATB was positively associated with the histological grade, high preoperative PSA level, pathological stage, high Gleason score, lymph node metastasis, angiolymphatic invasion and biochemical recurrence in prostate cancer patients. Moreover, it is proven that prostate cancer patients with high lncRNA-ATB expression had poor biochemical recurrence (BCR)-free survival. The multivariate analyses clarified that the loss of lncRNA-ATB expression was a potential independent prognostic factor for BCR-free survival of prostate cancer patients. These results suggested that the lncRNA-ATB may be a novel and important prognostic biomarker for prostate cancer.
The abnormal growth is closely related with tumorigenesis and tumor progression. Recent studies indicate that lncRNAs are essential modulators in tumor growth (35). Overexpression of lncRNA MVIH stimulates the proliferation and cell cycle in human breast cancer (35). Knockdown of lncRNA H9 obviously prevented the proliferation of non-small cell lung cancer cells (36). In the present study, we showed that lncRNA-ATB overexpression promoted cell proliferation of PC-3 and DU-145 prostate cancer cell lines reflected by CCK8 assay and the protein downregulations of PCNA and phosphorylated histone H3 (markers of proliferation) in response to transfection with LV-mediated lncRNA-ATB plasmid, while reduced lncRNA-ATB expression had the opposite effect. These results imply that lncRNA-ATB functions as oncogene in prostate cancer and lncRNA-ATB may be defined as a potential treatment target for prostate cancer.
Dysregulated cell cycle is considered as a crucial event in the cellular processes during cancer development (37). Emerging evidence indicates that dysregulated lncRNAs are of great importance in tumor-associated cell cycle defects (38). Herein, we found that decrease of lncRNA-ATB retarded the cell cycle-related proteins, including downregulation of cyclin E and cyclin D1, but upregulation of p21 and p27 in prostate cancer cell lines of PC-3 and DU-145, but overexpression of lncRNA-ATB had the contrary effect. These results indicated that lncRNA-ATB exerts promotion of growth of prostate cancer cells via direct stimulating effect on the cell cycle progression, suggesting a unifying mechanism whereby lncRNA-ATB overexpression might contribute to the growth in malignant prostate cancer.
In various cancers EMT has been intimately related with enhanced cell motility and invasiveness in tumor development (39). New emerging evidence indicates that EMT plays an important role in development and metastasis of prostate cancer and EMT may be a key therapeutic target in prostate cancer (40). ZEB1 is demonstrated to be a direct target of miR-200 during EMT in breast cancer cell lines (41). The reduction of miR-652 induced EMT by stimulating ZEB1 expression levels in pancreatic cancer cells (42). ZNF217 is also indicated to be an accelerator of EMT in breast cancer (42). Both ZEB1 and ZNF217 are negatively regulated in the inhibition of EMT in response to miR-200 overexpression (43). In the present study, we found that overexpression of lncRNA-ATB induced EMT reflected by increases in E-cadherin and ZO-1 and decreases in N-cadherin and Vimentin in PC-3 and DU-145 prostate cell lines, knockdown of lncRNA-ATB exerted contrary effect. In addition, downregulation of lncRNA-ATB inhibited, while upregulation of lncRNA-ATB promoted the expression levels of ZEB1 and ZNF217 at mRNA and protein levels in PC-3 and DU-145 prostate cell lines. These results indicated that lncRNA-ATB may drive EMT in prostate cancer cells via positive modulation of ZEB1 and ZNF217 expression levels.
Both MAPK/ERK pathways and PI3K-Akt pathways are essential intracellular signaling pathways involving in the proliferation and EMT during the processes of cancers (44). Herein, our results showed that the signaling pathways of PI3K/Akt and ERK were suppressed by knockdown of lncRNA-ATB, but the continuous lncRNA-ATB expression further stimulated the phosphorylation levels of Akt and ERK in both PC-3 and DU-145 prostate cell lines. It is interesting that inhibition of PI3K/Akt and ERK signaling pathways prevented the proliferation of PC-3 and DU-145 prostate cell line responses to overexpression of lncRNA-ATB. Furthermore, pretreatment with PI3K inhibitor LY294002 or ERK inhibitor U0126 almost diminished the effects of lncRNA-ATB overexpression on the ZEB1 and ZNF217 protein expression levels. These results indicated both PI3K/Akt and ERK signaling pathways involved lncRNA-ATB-mediated growth and EMT of human prostate cancer. However, studies on the role of lncRNA-ATB in the migration, invasion and tumor growth in nude mice as well as their molecular mechanisms are necessary to be further elucidated.
In conclusion, our results demonstrated that high lncRNA-ATB expression is significantly associated with a poor prognosis independently of other factors in prostate cancer. LncRNA-ATB might be considered as a novel molecular target for the diagnosis and treatment of prostate cancer.
Acknowledgments
We appreciate the co-operation of all the people, and patients who participated in this study.
References
- 1.Scher HI, Solo K, Valant J, Todd MB, Mehra M. Prevalence of Prostate Cancer Clinical States and Mortality in the United States: Estimates Using a Dynamic Progression Model. PLoS One. 2015;10:e0139440. doi: 10.1371/journal.pone.0139440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, Lee KH. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47:127–141. doi: 10.4143/crt.2015.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll PR. Early stage prostate cancer - do we have a problem with over-detection, overtreatment or both? J Urol. 2005;173:1061–1062. doi: 10.1097/01.ju.0000156838.67623.10. [DOI] [PubMed] [Google Scholar]
- 5.Ishizaki F, Hara N, Koike H, Kawaguchi M, Tadokoro A, Takizawa I, Nishiyama T, Takahashi K, Hohenfellner R. Prediction of pathological and oncological outcomes based on extended prostate biopsy results in patients with prostate cancer receiving radical prostatectomy: A single institution study. Diagn Pathol. 2012;7:68. doi: 10.1186/1746-1596-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopes PM, Sepúlveda L, Ramos R, Sousa P. The role of transrectal ultrasound in the diagnosis of prostate cancer: New contributions. Radiol Bras. 2015;48:7–11. doi: 10.1590/0100-3984.2013.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilic D, O'Connor D, Green S, Wilt T. Screening for prostate cancer: A Cochrane systematic review. Cancer Causes Control. 2007;18:279–285. doi: 10.1007/s10552-006-0087-6. [DOI] [PubMed] [Google Scholar]
- 8.Pritchard CC, Morrissey C, Kumar A, Zhang X, Smith C, Coleman I, Salipante SJ, Milbank J, Yu M, Grady WM, et al. Complex MSH2 and MSH6 mutations in hypermutated micro-satellite unstable advanced prostate cancer. Nat Commun. 2014;5:4988. doi: 10.1038/ncomms5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merdan S, Womble PR, Miller DC, Barnett C, Ye Z, Linsell SM, Montie JE, Denton BT. Toward better use of bone scans among men with early-stage prostate cancer. Urology. 2014;84:793–798. doi: 10.1016/j.urology.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgia G, Russo GI, Tubaro A, Bortolus R, Randone D, Gabriele P, Trippa F, Zattoni F, Porena M, Mirone V, et al. Members of the LUNA Foundation. Società Italiana d'Urologia Patterns of prescription and adherence to European Association of Urology guidelines on androgen deprivation therapy in prostate cancer: An Italian multicentre cross-sectional analysis from the Choosing Treatment for Prostate Cancer (CHOICE) study. BJU Int. 2015 Aug 31; doi: 10.1111/bju.13307. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Albertsen PC. The unintended burden of increased prostate cancer detection associated with prostate cancer screening and diagnosis. Urology. 2010;75:399–405. doi: 10.1016/j.urology.2009.08.078. [DOI] [PubMed] [Google Scholar]
- 12.Martin NE, D'Amico AV. Progress and controversies: Radiation therapy for prostate cancer. CA Cancer J Clin. 2014;64:389–407. doi: 10.3322/caac.21250. [DOI] [PubMed] [Google Scholar]
- 13.Moyer VA, U.S. Preventive Services Task Force Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 14.Shi X, Sun M, Liu H, Yao Y, Song Y. Long non-coding RNAs: A new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Li CH, Chen Y. Targeting long non-coding RNAs in cancers: Progress and prospects. Int J Biochem Cell Biol. 2013;45:1895–1910. doi: 10.1016/j.biocel.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta RA, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Zhang M, Liang L, Li J, Chen YX. Over-expression of lncRNA DANCR is associated with advanced tumor progression and poor prognosis in patients with colorectal cancer. Int J Clin Exp Pathol. 2015;8:11480–11484. [PMC free article] [PubMed] [Google Scholar]
- 18.Quagliata L, Matter MS, Piscuoglio S, Arabi L, Ruiz C, Procino A, Kovac M, Moretti F, Makowska Z, Boldanova T, et al. Long noncoding RNA HOTTIP/HOXA13 expression is associated with disease progression and predicts outcome in hepatocellular carcinoma patients. Hepatology. 2014;59:911–923. doi: 10.1002/hep.26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji J, Tang J, Deng L, Xie Y, Jiang R, Li G, Sun B. LINC00152 promotes proliferation in hepatocellular carcinoma by targeting EpCAM via the mTOR signaling pathway. Oncotarget. 2015;6:42813–42824. doi: 10.18632/oncotarget.5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–681. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Sun HJ, Liu TY, Zhang F, Xiong XQ, Wang JJ, Chen Q, Li YH, Kang YM, Zhou YB, Han Y, et al. Salusin-β contributes to vascular remodeling associated with hypertension via promoting vascular smooth muscle cell proliferation and vascular fibrosis. Biochim Biophys Acta. 2015;1852:1709–1718. doi: 10.1016/j.bbadis.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Gao X, Lu X, Wang Y, Ma C, Shi Z, Zhu F, He B, Xu C, Sun Y. The mitotic regulator Hec1 is a critical modulator of prostate cancer through the long non-coding RNA BX647187 in vitro. Biosci Rep. 2015;35:35. doi: 10.1042/BSR20150003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Liu Z, Wang L, Qiao B, Du E, Li L, Xu Y, Zhang Z. Prognostic significance of GPC5 expression in patients with prostate cancer. Tumour Biol. 2015 Dec 2; doi: 10.1007/s13277-015-4499-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Ma C, Shi X, Zhu Q, Li Q, Liu Y, Yao Y, Song Y. The growth arrest-specific transcript 5 (gAS5): a pivotal tumor suppressor long noncoding RNA in human cancers. Tumour Biol. 2015 Dec 3; doi: 10.1007/s13277-015-4521-9. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Mapelli P, Picchio M. Initial prostate cancer diagnosis and disease staging - the role of choline-PET-CT. Nat Rev Urol. 2015;12:510–518. doi: 10.1038/nrurol.2015.191. [DOI] [PubMed] [Google Scholar]
- 26.Degirmenci M, Erdogan AP, Bulut G, Atmaca H, Uzunoglu S, Karaca B, Karabulut B, Uslu R. Octreotide in combination with AT-101 induces cytotoxicity and apoptosis through up-regulation of somatostatin receptors 2 and 5 in DU-145 prostate cancer cells. Tumour Biol. 2015 Nov 3; doi: 10.1007/s13277-015-4331-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Walsh AL, Tuzova AV, Bolton EM, Lynch TH, Perry AS. Long noncoding RNAs and prostate carcinogenesis: The missing 'linc'? Trends Mol Med. 2014;20:428–436. doi: 10.1016/j.molmed.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enfield KS, Pikor LA, Martinez VD, Lam WL. Mechanistic roles of noncoding RNAs in lung cancer biology and their clinical implications. Genet Res Int. 2012;2012:737416. doi: 10.1155/2012/737416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng HT, Shi DB, Wang YW, Li XX, Xu Y, Tripathi P, Gu WL, Cai GX, Cai SJ. High expression of lncRNA MALAT1 suggests a biomarker of poor prognosis in colorectal cancer. Int J Clin Exp Pathol. 2014;7:3174–3181. [PMC free article] [PubMed] [Google Scholar]
- 31.Svoboda M, Slyskova J, Schneiderova M, Makovicky P, Bielik L, Levy M, Lipska L, Hemmelova B, Kala Z, Protivankova M, et al. HOTAIR long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis. 2014;35:1510–1515. doi: 10.1093/carcin/bgu055. [DOI] [PubMed] [Google Scholar]
- 32.Zhao T, Xu J, Liu L, Bai J, Xu C, Xiao Y, Li X, Zhang L. Identification of cancer-related lncRNAs through integrating genome, regulome and transcriptome features. Mol Biosyst. 2015;11:126–136. doi: 10.1039/C4MB00478G. [DOI] [PubMed] [Google Scholar]
- 33.Salameh A, Lee AK, Cardó-Vila M, Nunes DN, Efstathiou E, Staquicini FI, Dobroff AS, Marchiò S, Navone NM, Hosoya H, et al. PRUNE2 is a human prostate cancer suppressor regulated by the intronic long noncoding RNA PCA3. Proc Natl Acad Sci USA. 2015;112:8403–8408. doi: 10.1073/pnas.1507882112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito T, Kurashige J, Nambara S, Komatsu H, Hirata H, Ueda M, Sakimura S, Uchi R, Takano Y, Shinden Y, et al. A long non-coding RNA activated by transforming growth factor-β is an independent prognostic marker of gastric cancer. Ann Surg Oncol. 2015;22(Suppl 3):S915–S922. doi: 10.1245/s10434-015-4554-8. [DOI] [PubMed] [Google Scholar]
- 35.Lei B, Xu SP, Liang XS, Li YW, Zhang JF, Zhang GQ, Pang D. Long non-coding RNA MVIH is associated with poor prognosis and malignant biological behavior in breast cancer. Tumour Biol. 2015 Nov 10; doi: 10.1007/s13277-015-4360-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Zhang E, Li W, Yin D, De W, Sun S, Han L. c-Myc-regulated long non-coding RNA H19 indicates a poor prognosis and affects cell proliferation in non-small-cell lung cancer. Tumour Biol. 2015 Oct 20; doi: 10.1007/s13277-015-4185-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Zeng XX, Chu TJ, Yuan JY, Zhang W, Du YM, Ban ZY, Lei DM, Cao J, Zhang Z. Transmembrane 7 superfamily member 4 regulates cell cycle progression in breast cancer cells. Eur Rev Med Pharmacol Sci. 2015;19:4353–4361. [PubMed] [Google Scholar]
- 38.Yang L, Qiu M, Xu Y, Wang J, Zheng Y, Li M, Xu L, Yin R. Upregulation of long non-coding RNA PRNCR1 in colorectal cancer promotes cell proliferation and cell cycle progression. Oncol Rep. 2016;35:318–324. doi: 10.3892/or.2015.4364. [DOI] [PubMed] [Google Scholar]
- 39.Emadi Baygi M, Soheili ZS, Essmann F, Deezagi A, Engers R, Goering W, Schulz WA. Slug/SNAI2 regulates cell proliferation and invasiveness of metastatic prostate cancer cell lines. Tumour Biol. 2010;31:297–307. doi: 10.1007/s13277-010-0037-5. [DOI] [PubMed] [Google Scholar]
- 40.Li P, Yang R, Gao WQ. Contributions of epithelial-mesenchymal transition and cancer stem cells to the development of castration resistance of prostate cancer. Mol Cancer. 2014;13:55. doi: 10.1186/1476-4598-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 42.Vendrell JA, Thollet A, Nguyen NT, Ghayad SE, Vinot S, Bièche I, Grisard E, Josserand V, Coll JL, Roux P, et al. ZNF217 is a marker of poor prognosis in breast cancer that drives epithelial-mesenchymal transition and invasion. Cancer Res. 2012;72:3593–3606. doi: 10.1158/0008-5472.CAN-11-3095. [DOI] [PubMed] [Google Scholar]
- 43.Bai WD, Ye XM, Zhang MY, Zhu HY, Xi WJ, Huang X, Zhao J, Gu B, Zheng GX, Yang AG, et al. MiR-200c suppresses TGF-β signaling and counteracts trastuzumab resistance and metastasis by targeting ZNF217 and ZEB1 in breast cancer. Int J Cancer. 2014;135:1356–1368. doi: 10.1002/ijc.28782. [DOI] [PubMed] [Google Scholar]
- 44.Lui GY, Kovacevic Z, Richardson V, Merlot AM, Kalinowski DS, Richardson DR. Targeting cancer by binding iron: Dissecting cellular signaling pathways. Oncotarget. 2015;6:18748–18779. doi: 10.18632/oncotarget.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]