Abstract
The junctional adhesion molecule B (JAM-B) is a multifunctional transmembrane protein, which belongs to the immunoglobulin superfamily (IgSF). JAM-B is localized to cell-cell contacts and enriched at cell junctions in epithelial and endothelial cells, as well as on the surface of erythrocytes, leukocytes, and platelets. Recent research in this field has shown that JAM-B plays an important role in numerous cellular processes, such as tight junction assembly, spermatogenesis, regulation of paracellular permeability, leukocytic transmigration, angiogenesis, tumor metastasis and cell proliferation. This study provides a new research direction for the diagnosis and treatment of relevant diseases. In this review, we briefly focus on what is currently known about the structure, function, and mechanism of JAM-B, with particular emphasis on cancer.
Keywords: tight junction, junctional adhesion molecule-B, tumor progression, JAM family proteins
1. Introduction
Tight junctions (TJs) are the apical junctional complex in epithelial and endothelial cells (1). TJs play a key role in maintaining distinct tissue spaces and regulating paracellular flux of small molecules and inflammatory cells, as well as promoting bi-directional signaling between the intracellular and extracellular compartments (2–4). Furthermore, tumor differentiation is related to the reduction of TJs, and tumor cells must overcome the frontline structures of TJs in order to successfully metastasize (5–7).
The JAM family belongs to the immunoglobulin subfamily involved in the formation of TJ in most cell types, including leukocytes, platelets, endothelial and epithelial cells (2,8–10). JAMs have been reported to regulate epithelial TJ assembly, and interact in both a homo- and heterotypic fashion, as well as with integrins (3,11,12). The JAM family comprises five members, namely JAM-A, JAM-B, JAM-C, JAM-4, and JAM-like (13–16). There are many differences between JAM-B and the other JAM family members. This review will highlight that which is currently known about the structure, function, and mechanisms of action of JAM-B and potential areas for future study.
2. Structure of the JAM family
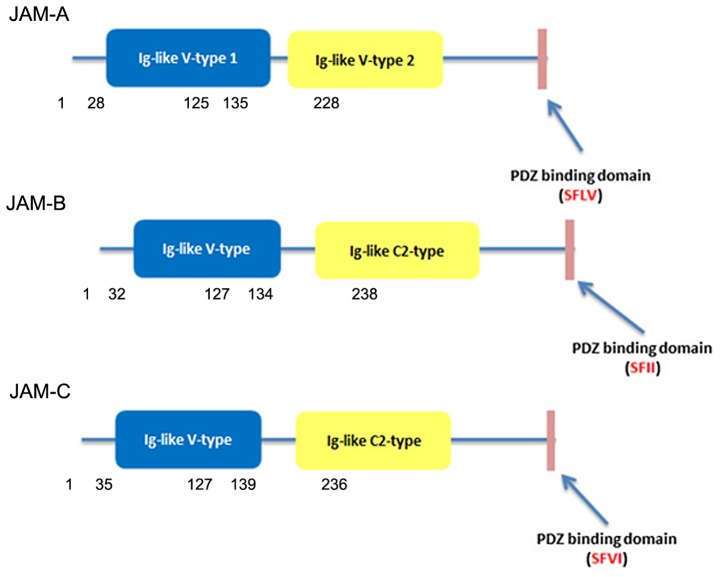
Members of the JAM family are type I transmembrane glycoproteins consisting of a short N-terminal signal peptide, two immunoglobulin-like domains, one transmembrane domain and one cytoplasmic tail of variable length containing a C-terminal PDZ-domain domain (4,17–20). In the case of JAM-A, the sequence was described by multiple investigators as two C2-type domains in mouse (10) and two V-type domains in human (2). However, the Ig-like domains of JAM-B and JAM-C are composed of V type and C2 type (18) (Fig. 1). A J-like sequence is contained in the V domain and an extra disulfide bridge between cysteines involved incorporated in the C2-type domain (19).
Figure 1.
The structure of JAM-A, -B and -C.
Some studies have found that JAM-A has a three amino acid sequence (Val-Leu-Val) in the connection between the two Ig-like domains which causes the distal Ig-like domains to bend and bind to another three amino acid sequence (Arg-Leu-Glu) in the V type Ig-like domains and dimerise (20,21). A JAM-A dimer at the surface of cells may interact in a trans orientation with another dimer of JAM2 expressed by the adjacent cell, forming a tetramer. The successive JAM tetramer would be able to structure a zipper-like structure surrounding the cell at the apical cells border. It was found that JAM-B (Arg-Leu-Glu) and JAM-C (Arg-Ile-Glu) could form the same type of dimerisation motifs as seen in JAM-A (19).
3. Expression profiles and subcellular localization
The JAM family exhibits different expression profiles and transcript level in various tissues, organs and tumors (Table I). Overall, as an important member of the TJ complex, JAMs are generally expressed in subsets of leukocytes, as well as in epithelial and endothelial cells (22,23). However, compared with JAM-A and JAM-C, JAM-B displays a restricted distribution. It is expressed in the junctions of endothelial cells of different vessels, but mostly localizes in high endothelial venules (13,25).
Table I.
Classic JAM family: Tissue distribution and main functions.
| Cell expression | Tissue expression | References | |
|---|---|---|---|
| JAM-A | Epithelial cells, endothelial cells, leukocytes, platelets, spermatozoa/Sertoli cells, HSC | Brain, heart, blood, bone marrow, intestine, kidney, liver, lung, pancreas, spleen, lymph node, skin, placenta, testis | (2,24,81–87) |
| JAM-B | HEV, lymphatics, Sertoli cells, HSC, NSC vascular and lymphatic endothelial cells | Heart, placenta, testis, kidney, lymph nodes, intestine, brain, lymph node (HEV), skin, heart, lung | (18,36,38,46,78,88,89) |
| JAM-C | Vascular and lymphatic endothelial cells, DC, Pl, NK, HEV, lymphatics, platelets, spermatid, HSC, Schwann cells, fibroblast, leukocytes (human) | Brain, bone marrow, heart, smooth muscle, uterus, lung, liver, kidney, spleen, testis, nerve, lymph node (HEV), skin, cornea, placenta, testis | (18,36,86,88,89) |
Amongst different types of tumors, it is difficult to quantitatively analyze differences in the expression of the JAM family. Therefore, the differential expression of JAM-B in cancer and normal tissues must be compared in a similar tumor type. Multiple studies have shown that the expression of JAM-B shows significant differences when comparing normal and cancerous tissues with the data presented in Table II.
Table II.
Regulation of CCNs in cancer.
| Tumor type | JAM-B expression level |
|---|---|
| Gastric carcinoma | ↑ |
| Colorectal cancer | ↓ |
| Glioma | ↑ |
| ESCC | ↓ |
| Breast Cancer | ↑/↓ |
| NSCLC | ↓ |
| OSCC | ↑ |
| RA | ↓ |
ESCC, esophageal squamous cell carcinoma; NSCLC, non-small cell lung cancer; OSCC, oral squamous cell carcinoma; RA, rheumatoid arthritis. ↓: JAM-B is lower expressed in tumor tissue than in corresponding normal tissue; ↑: JAM-B is higher expressed in tumor tissue than in corresponding normal tissue; ↑/↓: JAM-B expression in tumor is controversial.
4. Related signaling pathways
JAM receptor
As reported, classical JAMs, containing an intracellular PDZ binding domain which may act as an anchor for scaffolding adaptor proteins, can mediate cellular functions, embryonic development (26), junction assembly, spermatogenesis, paracellular permeability, leukocytic transmigration, angiogenesis, tumor metastasis and cell proliferation through binding and interacting with receptors such as ligand and integrin (17,27–33). The heterodimeric transmembrane receptors, the integrins, contain α- and β-subunits, which mediate cell-to-cell adhesive interactions.
Role of the receptor in JAM-B functions
There are several studies which report JAM-B interacting with JAM-C and the integrin α4β1, forming multimer interactions with integrin counter-receptors. JAM-B can interact with the integrin α4β1, but this is dependent on the presence of JAM-C. In 1996, Butcher and Picker described integrin-mediated T-lymphocyte adhesion inflamed endothelium through VCAM-1 (34). However, whether the integrin could engage JAM-B and VCAM-1 simultaneously through different binding sites is still unclear. However, JAM-B and VCAM-1 may interact with one another laterally and reinforce integrin through conformational changes (35). Other reports claim that on human circulating platelets, dendritic cells, NK cells, and subsets of T and B cells, JAM-C plays an extended role as the leukocyte counter receptor for JAM-B expressed on endothelial cells (11,36–40). In 2009, Ludwig et al found that using a model of DNFB-induced contact hypersensitivity, JAM-B played a role in rolling and firm adhesion of T lymphocytes by interacting with α4β1 (41). However, a recent study proposed JAM-B to play an independent role in leukocyte transmigration.
Functional epitopes in JAM-B proteins
JAM-B has been reported involved in many kinds of biological processes, for instance, leukocyte migration (39,42–44), spermatogenesis (45) and angiogenesis (46) through its interaction with JAM-C. Previous study has shown that blocking the interactions of JAM-B and -C can reduce monocyte numbers in the ablumenal compartment by increasing reverse transmigration rather than through reducing transmigration (47).
The effect of JAM-B and JAM-C in leukocyte accumulation during inflammation has also been monitored (39,43,48). For example, a study suggested anti-JAM-B and anti-JAM-C antibodies played a role of addition agent in blocking leukocytes infiltration in the model of allergic contact dermatitis, indicating that the actions of JAM-B and -C are indispensable for development of cutaneous inflammation (50). Previous study has shown that blocking the interactions of JAM-B and -C can reduce monocyte numbers in the ablumenal compartment by increasing reverse transmigration rather than through reducing transmigration. Overall, the results indictate that the blocking of JAM-B/-C interactions decreased the number of monocytes in inflamed tissues owing to considerably increased level of reverse-transmigrated monocytes in the peripheral blood.
Signaling pathways
The cellular localization of JAMs is related to specific signals of the extracellular environment. Phosphorylation of the cytoplasmic tail of JAMs plays a key role in the mechanism of regulating the localization of JAMs. The classical JAM proteins all contain C-terminal PDZ-binding domains which can be modified by kinases and may facilitate the interaction with PDZ-containing proteins, such as TJ-associated cytoplasmic proteins AF6, PAR3, ZO1, CASK, MAGI and MUPP1 (15,50–54). In 2003, Ebnet et al showed that JAM-B and JAM-C could directly associate with PAR-3 through its first PDZ domain, and could also associate with protein ZO-1 in a PDZ domain-dependent manner (55). Recently, antibodies against murine JAM-B inhibited microvessel endothelial cell proliferation were demonstrated through in vitro endothelial network formation assays and ex vivo aortic rings. This research also showed that JAM-B can negatively regulate pro-angiogenic pathways and inhibit VEGF-induced ERK1/2 activation through integrin activation in MAPK pathway (56).
5. Multiple roles of JAM-B in cancer
Following the investigation of JAM-B protein involvement in the control of cellular motility and formation of cellular projections in cancer cells, a number of researchers have focused on the roles of JAM-B and regulation of this molecule in cancer cells and tissues. Clinical studies have mentioned different expression profiles and roles of JAM-B in various types of cancers. The inconsistency of the results in different cancers has led to an uncertainty regarding the role of JAM-B in carcinogenesis.
Tumor metastasis is the movement or spreading of tumor cells from the primary site to a distant one. Circulating tumor cells, separated from primary tumor, can interact with vascular endothelial cells during hematogenous metastatic dissemination. Metastatic and invasive potential of a tumor cell depends on the expression of various proteins at each step of the metastatic cascade. Numerous studies showed JAM-B played an important role in the metastasis of tumor cells (57–59).
JAM-B in gastric carcinoma
Gastric cancer, one of the most common malignancies, is the second leading cause of cancer-related death globally. Many patients who had gastric cancer previously could still suffer from tumor recurrence and metastasis following curative gastrectomy. Deregulation of the expression and function of TJ proteins leads to the initiation and progression of cancer by activation of cytoskeleton mechanism. Hajjari et al examined the changes in expression levels of genes encoding tight junction-associated proteins of JAM-B and JAM-C in gastric adenocarcinoma in comparison with their corresponding marginal normal gastric tissues from the same patients. They found that JAM-B was upregulated significantly in tumor samples compared with adjacent normal tissues and was higher in high grade tumors than in the low grade and intermediate grade tumors. Moreover, they showed JAM-B and JAM-C activated the expression of actin filament-associated protein (AFAP) gene as a downstream factor of JAM-B and JAM-C and this resulted in a significantly higher expression of AFAP (57). Recently, whole genome and transcriptome sequencing in primary and peritoneal metastatic gastric carcinoma indicated JAM-B was only expressed in primary tumors (58). Overall, deregulation of JAM-B and JAM-C expression may potentially be involved in progression of gastric adenocarcinoma tumors. However, JAM-A was downregulated in gastric cancer tissues compared to adjacent non-tumor tissues from the same patients, and low JAM-A expression contributed to poor clinical outcome and increased cell invasion and migration in gastric cancer (59).
JAM-B in colorectal cancer
Recently, Kok-Sin et al studied 150 colorectal tissues with the methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA), and they found JAM-B was expressed at very low levels in colorectal cancer due to JAM-B genes being hypermethylated on promoters in CpG islands (60). The hypermethylation of the promoter and the downregulation of JAM-B in colon cancer were also supported by a report from 2011 (61). Another recent study showed the expression changes of cell-cell adhesion-related genes among 26 colorectal cancer, 42 adenoma and 24 normal mucosa samples. Between these tissue types, different expression were observed in the mRNA levels of JAM-B genes encoding adherence junction proteins and JAM-B was expressed at lower levels in adenocarcinoma and adenoma than in normal colonic mucosa. Further, JAM-B has the lowest expression levels in adenoma (62).
JAM-B in glioma
Brain invasion is a biological hallmark of glioma which contributes to its aggressiveness and prognosis. Deregulated expression of JAMs in glioma cells has been implicated in this process. Recently, a study showed that JAM-B was aberrantly expressed in glioma as a high affinity JAM-C ligand. The interaction of JAM-B and JAM-C could activate c-Src proto-oncogene, which is known as a central upstream molecule in the pathways regulating cell invasion and migration. Also it was shown that JAM-B/C blocking antibodies impaired in vivo glioma invasion and proliferation in mice (63). Magnetic nanoparticle mediated JAM-2 silencing also inhibited the growth and migration of glioma in vitro and in vivo (64). A similar study from the same laboratory also found that the silencing of JAM-2 by proton-sponge coated quantum dots inhibited the migration of glioma in vitro through NOTCH pathway blockage (65). In short, targeting of the JAMs family may provide a potential lead for the treatment of human gliomas.
JAM-B in melanoma
Melanoma is the most dangerous type of skin cancer. Many factors have been proposed to influence the metastasis of melanoma in patients. Arcangeli et al indicted JAM-B expressed by endothelial cells was involved in melanoma cell metastasis via its interaction with JAM-C on tumor cells. Furthermore, using a JAM-B-deficient mouse model, it was found that the differential migration disappeared between JAM-C expressing cells and JAM-C silenced cells, revealing that JAM-B acted as a counter-receptor for JAM-C mediated reservation of melanoma cells in the lungs (66).
JAM-B in esophageal squamous cell carcinoma (ESCC)
Esophageal squamous cell carcinoma is an aggressive and malignant tumor for which there is limited treatment option. The reason for this is that most cases are diagnosed late, and patients are already in the advanced stages of the disease. Lymph node metastasis often arises from this type of cancer, due to the rich lymphatic drainage system in the esophagus. Recently, it was demonstrated that the methylation of JAM-B was increased in the region downstream of the gene and its expression was decreased (67). Furthermore, a similar result showed that the expression JAM-B was downregulated and JAM-B was identified as a key player in the signal transduction networks in ESCC (68).
JAM-B in breast cancer
In females, breast cancer is the leading cancer with high incidence and frequently diagnosed malignancy, with lower survival rates in patients. JAM-B is a necessary component of apical junctional components. Recently, Coradini et al reported that mutant p53 tumors of breast cancer were characterized by a dramatic under-expression of several genes coding for apical junctional components (69). Further, a report from Bhan et al stated that JAM-B was associated with breast cancer progression (70). In 2010, our laboratory showed JAM-B had a significantly lower level in breast tumors from patients who developed metastasis than those who were disease-free. We concluded that JAM-B decreased migration and metastasis of breast cancer cells. In contrast, JAM-A expression was upregulated in breast cancer tissues compared to the normal specimen and was correlated to reduced survival of breast tumor patients, thus JAM-A overexpression could be a possible mechanism promoting breast cancer cell migration (71).
JAM-B in lung cancer
Lung cancer is a malignant lung tumor characterized by uncontrolled cell proliferation in tissues of the lung. JAM-2 was negatively expressed in normal mouse lung, but positively expressed in spleen and lymph nodes surrounding the lung (72). An interesting discovery showed that overexpression of JAM-B in a trisomy-21 mouse model of Down's syndrome was responsible for inhibiting VEGF-induced angiogenesis and, thus, restraining tumor effects in a lung carcinoma mouse model (73). Conversely, the expression level of JAM-A in lung tumor tissues was higher than corresponding normal lung tissues and served as a negative predictor of survival in lung cancer patients. Thus, JAM-A contributes to lung cell growth through cell cycle regulation (74).
JAM-B in oral squamous cell carcinoma
Oral squamous cell carcinoma (OSCC) characteristically metastasizes from the primary organ to regional lymph nodes in the early stages of the cancer. It was demonstrated that JAM-B correlated with the metastatic potential, as it was upregulated in human oral cancer LNM Tca8113 cells which metastasized to lymph nodes at an higher rate than its parental cell line Tca8113 (75). Therefore it was suggested JAM-B acts as a potential factor for the metastasis of OSCC.
Role of JAM-B in other diseases
Zhang et al examined the relationship between JAM-B polymorphisms and the risk of rheumatoid arthritis (RA) among the Chinese population and excluded the affiliation between JAM-B polymorphisms and a decreased risk of RA (76). A study on inflammation indicated JAM-B expression was restricted to the endothelium of arterioles in and adjacent to lymphocytic inflammation sites but was not discovered in liver specimens, signifying JAM-B may only be inducible in some inflammatory tissue types (11). Another similar study showed that JAM-B was involved in leukocyte extravasation to inflammation sites (49). Tumor angiogenesis is a fundamental step of cancer progression. Moreover, Meguenani et al reported that JAM-B interfered with angiogenic VEGF/VEGFR2 signaling which is a major pathway necessary for tumor angiogenesis, suggesting JAM-B could provide a useful possibility for the diagnosis and treatment of relevant diseases (56).
6. Conclusion
Recent studies focusing on the interaction of JAMs have determined multiple homophilic and heterophilic cis/trans-interactions. Further, regulation of JAM functions arises by establishing intracellular/extracellular multiprotein complexes. The family of JAMs shows stability in expression levels under various conditions, indicating a vital role under pathological and homeostatic conditions (42,77). JAMs also play an important role in decreasing epithelial paracellular permeability. An increasing number of studies has concentrated on the role of JAMs in tumors. It is clear that JAMs regulate cell growth, invasion and migration. Perturbed expression of JAMs has been observed in various malignancy types. The abnormal expression of certain JAMs is related to disease progression and poor prognosis. Different JAMs may play contrasting roles in the same cancer, while the same JAM may undertake converse roles in different tumor types.
7. Future perspectives
JAM-B interact with various receptors and cytokines to modulate downstream signal transduction. Most studies concerning JAM-B showed it does not assume a single role in regulating tumors, but always with JAM-C-dependence. JAM-B/-C is an important complex in cell-cell signaling. Its molecular mechanism of action is in need of further investigation. Many researchers have shown that JAM-B is an important regulator in various diseases. According to the above cited studies, the expression level of JAM-B is different in various tumors compared with normal tissues from the same patients. Thus, JAM-B may have different potentiality in the regulation of cancer metastasis. Further, insight into the detailed mechanisms involved in JAM-B-mediated regulation would be useful in the understanding its function and role in tumorigenesis and tumor metastasis. Further investigations will highlight its clinical significance and application for predicting prognosis. In the future JAM-B may well provide preoperative diagnosis and a new avenue for targeted therapy in certain malignancies.
Acknowledgments
This study was supported by Beijing Municipal Science and Technology Commission (no. Z151100001615039). HSC was a recipient of Cardiff university China Medical Scholarship. The authors wish to than Cancer Research Wales and the Welsh Network of Life Science.
References
- 1.Runkle EA, Mu D. Tight junction proteins: From barrier to tumorigenesis. Cancer Lett. 2013;337:41–48. doi: 10.1016/j.canlet.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martìn-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, Parkos CA. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113:2363–2374. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- 4.Mandell KJ, Parkos CA. The JAM family of proteins. Adv Drug Deliv Rev. 2005;57:857–867. doi: 10.1016/j.addr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Martin TA, Watkins G, Mansel RE, Jiang WG. Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer. Eur J Cancer. 2004;40:2717–2725. doi: 10.1016/j.ejca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Martin TA, Mansel RE, Jiang WG. Antagonistic effect of NK4 on HGF/SF induced changes in the transendothelial resistance (TER) and paracellular permeability of human vascular endothelial cells. J Cell Physiol. 2002;192:268–275. doi: 10.1002/jcp.10133. [DOI] [PubMed] [Google Scholar]
- 7.Hoevel T, Macek R, Mundigl O, Swisshelm K, Kubbies M. Expression and targeting of the tight junction protein CLDN1 in CLDN1-negative human breast tumor cells. J Cell Physiol. 2002;191:60–68. doi: 10.1002/jcp.10076. [DOI] [PubMed] [Google Scholar]
- 8.Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: A possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 2001;154:491–497. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tajima M, Hirabayashi S, Yao I, Shirasawa M, Osuga J, Ishibashi S, Fujita T, Hata Y. Roles of immunoglobulin-like loops of junctional cell adhesion molecule 4; involvement in the subcellular localization and the cell adhesion. Genes Cells. 2003;8:759–768. doi: 10.1046/j.1365-2443.2003.00673.x. [DOI] [PubMed] [Google Scholar]
- 10.Liang TW, DeMarco RA, Mrsny RJ, Gurney A, Gray A, Hooley J, Aaron HL, Huang A, Klassen T, Tumas DB, et al. Characterization of huJAM: Evidence for involvement in cell-cell contact and tight junction regulation. Am J Physiol Cell Physiol. 2000;279:C1733–C1743. doi: 10.1152/ajpcell.2000.279.6.C1733. [DOI] [PubMed] [Google Scholar]
- 11.Liang TW, Chiu HH, Gurney A, Sidle A, Tumas DB, Schow P, Foster J, Klassen T, Dennis K, DeMarco RA, et al. Vascular endothelial-junctional adhesion molecule (VE-JAM)/JAM 2 interacts with T, NK, and dendritic cells through JAM 3. J Immunol. 2002;168:1618–1626. doi: 10.4049/jimmunol.168.4.1618. [DOI] [PubMed] [Google Scholar]
- 12.Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 2009;1788:872–891. doi: 10.1016/j.bbamem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Bazzoni G. The JAM family of junctional adhesion molecules. Curr Opin Cell Biol. 2003;15:525–530. doi: 10.1016/S0955-0674(03)00104-2. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham SA, Arrate MP, Rodriguez JM, Bjercke RJ, Vanderslice P, Morris AP, Brock TA. A novel protein with homology to the junctional adhesion molecule. Characterization of leukocyte interactions. J Biol Chem. 2000;275:34750–34756. doi: 10.1074/jbc.M002718200. [DOI] [PubMed] [Google Scholar]
- 15.Hirabayashi S, Tajima M, Yao I, Nishimura W, Mori H, Hata Y. JAM4, a junctional cell adhesion molecule interacting with a tight junction protein, MAGI-1. Mol Cell Biol. 2003;23:4267–4282. doi: 10.1128/MCB.23.12.4267-4282.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 17.Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): More molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 18.Aurrand-Lions M, Johnson-Leger C, Wong C, Du Pasquier L, Imhof BA. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98:3699–3707. doi: 10.1182/blood.V98.13.3699. [DOI] [PubMed] [Google Scholar]
- 19.Garrido-Urbani S, Bradfield PF, Imhof BA. Tight junction dynamics: The role of junctional adhesion molecules (JAMs) Cell Tissue Res. 2014;355:701–715. doi: 10.1007/s00441-014-1820-1. [DOI] [PubMed] [Google Scholar]
- 20.Prota AE, Campbell JA, Schelling P, Forrest JC, Watson MJ, Peters TR, Aurrand-Lions M, Imhof BA, Dermody TS, Stehle T. Crystal structure of human junctional adhesion molecule 1: Implications for reovirus binding. Proc Natl Acad Sci USA. 2003;100:5366–5371. doi: 10.1073/pnas.0937718100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kostrewa D, Brockhaus M, D'Arcy A, Dale GE, Nelboeck P, Schmid G, Mueller F, Bazzoni G, Dejana E, Bartfai T, et al. X-ray structure of junctional adhesion molecule: Structural basis for homophilic adhesion via a novel dimerization motif. EMBO J. 2001;20:4391–4398. doi: 10.1093/emboj/20.16.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naik UP, Ehrlich YH, Kornecki E. Mechanisms of platelet activation by a stimulatory antibody: Cross-linking of a novel platelet receptor for monoclonal antibody F11 with the Fc gamma RII receptor. Biochem J. 1995;310:155–162. doi: 10.1042/bj3100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malergue F, Galland F, Martin F, Mansuelle P, Aurrand-Lions M, Naquet P. A novel immunoglobulin superfamily junctional molecule expressed by antigen presenting cells, endothelial cells and platelets. Mol Immunol. 1998;35:1111–1119. doi: 10.1016/S0161-5890(98)00102-3. [DOI] [PubMed] [Google Scholar]
- 24.Williams LA, Martin-Padura I, Dejana E, Hogg N, Simmons DL. Identification and characterisation of human junctional adhesion molecule (JAM) Mol Immunol. 1999;36:1175–1188. doi: 10.1016/S0161-5890(99)00122-4. [DOI] [PubMed] [Google Scholar]
- 25.Palmeri D, van Zante A, Huang CC, Hemmerich S, Rosen SD. Vascular endothelial junction-associated molecule, a novel member of the immunoglobulin superfamily, is localized to intercellular boundaries of endothelial cells. J Biol Chem. 2000;275:19139–19145. doi: 10.1074/jbc.M003189200. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi I, Kobayashi-Sun J, Kim AD, Pouget C, Fujita N, Suda T, Traver D. JAM1a-JAM2a interactions regulate haematopoietic stem cell fate through Notch signalling. Nature. 2014;512:319–323. doi: 10.1038/nature13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 28.Wegmann F, Ebnet K, Du Pasquier L, Vestweber D, Butz S. Endothelial adhesion molecule ESAM binds directly to the multidomain adaptor MAGI-1 and recruits it to cell contacts. Exp Cell Res. 2004;300:121–133. doi: 10.1016/j.yexcr.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Naik MU, Naik UP. Junctional adhesion molecule-A-induced endothelial cell migration on vitronectin is integrin alpha v beta 3 specific. J Cell Sci. 2006;119:490–499. doi: 10.1242/jcs.02771. [DOI] [PubMed] [Google Scholar]
- 30.Mirza M, Raschperger E, Philipson L, Pettersson RF, Sollerbrant K. The cell surface protein coxsackie- and adenovirus receptor (CAR) directly associates with the Ligand-of-Numb Protein-X2 (LNX2) Exp Cell Res. 2005;309:110–120. doi: 10.1016/j.yexcr.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 31.Sobocka MB, Sobocki T, Babinska A, Hartwig JH, Li M, Ehrlich YH, Kornecki E. Signaling pathways of the F11 receptor (F11R; a.k.a. JAM-1, JAM-A) in human platelets: F11R dimerization, phosphorylation and complex formation with the integrin GPIIIa. J Recept Signal Transduct Res. 2004;24:85–105. doi: 10.1081/RRS-120034252. [DOI] [PubMed] [Google Scholar]
- 32.Reymond N, Garrido-urbani S, Borg JP, Dubreuil P, Lopez M. PICK-1: A scaffold protein that interacts with Nectins and JAMs at cell junctions. FEBS Lett. 2005;579:2243–2249. doi: 10.1016/j.febslet.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Kansaku A, Hirabayashi S, Mori H, Fujiwara N, Kawata A, Ikeda M, Rokukawa C, Kurihara H, Hata Y. Ligand-of-Numb protein X is an endocytic scaffold for junctional adhesion molecule 4. Oncogene. 2006;25:5071–5084. doi: 10.1038/sj.onc.1209468. [DOI] [PubMed] [Google Scholar]
- 34.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 35.Santoso S, Sachs UJH, Kroll H, Linder M, Ruf A, Preissner KT, Chavakis T. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196:679–691. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moog-Lutz C, Cavé-Riant F, Guibal FC, Breau MA, Di Gioia Y, Couraud PO, Cayre YE, Bourdoulous S, Lutz PG. JAML, a novel protein with characteristics of a junctional adhesion molecule, is induced during differentiation of myeloid leukemia cells. Blood. 2003;102:3371–3378. doi: 10.1182/blood-2002-11-3462. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham SA, Rodriguez JM, Arrate MP, Tran TM, Brock TA. JAM2 interacts with alpha4beta1. Facilitation by JAM3. J Biol Chem. 2002;277:27589–27592. doi: 10.1074/jbc.C200331200. [DOI] [PubMed] [Google Scholar]
- 38.Arrate MP, Rodriguez JM, Tran TM, Brock TA, Cunningham SA. Cloning of human junctional adhesion molecule 3 (JAM3) and its identification as the JAM2 counter-receptor. J Biol Chem. 2001;276:45826–45832. doi: 10.1074/jbc.M105972200. [DOI] [PubMed] [Google Scholar]
- 39.Johnson-Léger CA, Aurrand-Lions M, Beltraminelli N, Fasel N, Imhof BA. Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood. 2002;100:2479–2486. doi: 10.1182/blood-2001-11-0098. [DOI] [PubMed] [Google Scholar]
- 40.Langer HF, Daub K, Braun G, Schönberger T, May AE, Schaller M, Stein GM, Stellos K, Bueltmann A, Siegel-Axel D, et al. Platelets recruit human dendritic cells via Mac-1/JAM-C interaction and modulate dendritic cell function in vitro. Arterioscler Thromb Vasc Biol. 2007;27:1463–1470. doi: 10.1161/ATVBAHA.107.141515. [DOI] [PubMed] [Google Scholar]
- 41.Ludwig RJ, Hardt K, Hatting M, Bistrian R, Diehl S, Radeke HH, Podda M, Schön MP, Kaufmann R, Henschler R, et al. Junctional adhesion molecule (JAM)-B supports lymphocyte rolling and adhesion through interaction with alpha4beta1 integrin. Immunol. 1990;128:196–205. doi: 10.1111/j.1365-2567.2009.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keiper T, Al-Fakhri N, Chavakis E, Athanasopoulos AN, Isermann B, Herzog S, Saffrich R, Hersemeyer K, Bohle RM, Haendeler J, et al. The role of junctional adhesion molecule-C (JAM-C) in oxidized LDL-mediated leukocyte recruitment. FASEB J. 2005;19:2078–2080. doi: 10.1096/fj.05-4196fje. [DOI] [PubMed] [Google Scholar]
- 43.Aurrand-Lions M, Lamagna C, Dangerfield JP, Wang S, Herrera P, Nourshargh S, Imhof BA. Junctional adhesion molecule-C regulates the early influx of leukocytes into tissues during inflammation. J Immunol. 2005;174:6406–6415. doi: 10.4049/jimmunol.174.10.6406. [DOI] [PubMed] [Google Scholar]
- 44.Lamagna C, Meda P, Mandicourt G, Brown J, Gilbert RJ, Jones EY, Kiefer F, Ruga P, Imhof BA, Aurrand-Lions M. Dual interaction of JAM-C with JAM-B and alpha(M)beta2 integrin: Function in junctional complexes and leukocyte adhesion. Mol Biol Cell. 2005;16:4992–5003. doi: 10.1091/mbc.E05-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–324. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- 46.Lamagna C, Hodivala-Dilke KM, Imhof BA, Aurrand-Lions M. Antibody against junctional adhesion molecule-C inhibits angiogenesis and tumor growth. Cancer Res. 2005;65:5703–5710. doi: 10.1158/0008-5472.CAN-04-4012. [DOI] [PubMed] [Google Scholar]
- 47.Bradfield P, Scheiermann C, Nourshargh S, Ody C, Luscinskas F, Rainger G, et al. JAM-C, a turnstile for monocyte transendothelial migration in inflammation. Swiss Med Wkly. 2007;137:12s. doi: 10.1182/blood-2007-03-078733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vonlaufen A, Aurrand-Lions M, Pastor CM, Lamagna C, Hadengue A, Imhof BA, Frossard JL. The role of junctional adhesion molecule C (JAM-C) in acute pancreatitis. J Pathol. 2006;209:540–548. doi: 10.1002/path.2007. [DOI] [PubMed] [Google Scholar]
- 49.Ludwig RJ, Zollner TM, Santoso S, Hardt K, Gille J, Baatz H, Johann PS, Pfeffer J, Radeke HH, Schön MP, et al. Junctional adhesion molecules (JAM)-B and -C contribute to leukocyte extravasation to the skin and mediate cutaneous inflammation. J Invest Dermatol. 2005;125:969–976. doi: 10.1111/j.0022-202X.2005.23912.x. [DOI] [PubMed] [Google Scholar]
- 50.Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem. 2002;277:455–461. doi: 10.1074/jbc.M109005200. [DOI] [PubMed] [Google Scholar]
- 51.Martinez-Estrada OM, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G. Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial caco-2 cells. J Biol Chem. 2001;276:9291–9296. doi: 10.1074/jbc.M006991200. [DOI] [PubMed] [Google Scholar]
- 52.Ebnet K, Schulz Cu, Meyer Zu, Brickwedde MK, Pendl GG, Vestweber D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem. 2000;275:27979–27988. doi: 10.1074/jbc.M002363200. [DOI] [PubMed] [Google Scholar]
- 53.Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem. 2000;275:20520–20526. doi: 10.1074/jbc.M905251199. [DOI] [PubMed] [Google Scholar]
- 54.Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu, Brickwedde MK, Ohno S, Vestweber D. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM) EMBO J. 2001;20:3738–3748. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ebnet K, Aurrand-Lions M, Kuhn A, Kiefer F, Butz S, Zander K, Meyer zu Brickwedde MK, Suzuki A, Imhof BA, Vestweber D. The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: A possible role for JAMs in endothelial cell polarity. J Cell Sci. 2003;116:3879–3891. doi: 10.1242/jcs.00704. [DOI] [PubMed] [Google Scholar]
- 56.Meguenani M, Miljkovic-Licina M, Fagiani E, Ropraz P, Hammel P, Aurrand-Lions M, Adams RH, Christofori G, Imhof BA, Garrido-Urbani S. Junctional adhesion molecule B interferes with angiogenic VEGF/VEGFR2 signaling. FASEB J. 2015;29:3411–3425. doi: 10.1096/fj.15-270223. [DOI] [PubMed] [Google Scholar]
- 57.Hajjari M, Behmanesh M, Sadeghizadeh M, Zeinoddini M. Junctional adhesion molecules 2 and 3 may potentially be involved in progression of gastric adenocarcinoma tumors. Med Oncol. 2013;30:380. doi: 10.1007/s12032-012-0380-z. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Huang JY, Chen YN, Yuan F, Zhang H, Yan FH, Wang MJ, Wang G, Su M, Lu G, et al. Whole genome and transcriptome sequencing of matched primary and peritoneal metastatic gastric carcinoma. Sci Rep. 2015;5:13750. doi: 10.1038/srep13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang JY, Xu YY, Sun Z, Wang ZN, Zhu Z, Song YX, Luo Y, Zhang X, Xu HM. Low junctional adhesion molecule A expression correlates with poor prognosis in gastric cancer. J Surg Res. 2014;192:494–502. doi: 10.1016/j.jss.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 60.Kok-Sin T, Mokhtar NM, Ali Hassan NZ, Sagap I, Mohamed Rose I, Harun R, Jamal R. Identification of diagnostic markers in colorectal cancer via integrative epigenomics and genomics data. Oncol Rep. 2015;34:22–32. doi: 10.3892/or.2015.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oster B, Thorsen K, Lamy P, Wojdacz TK, Hansen LL, Birkenkamp-Demtröder K, Sørensen KD, Laurberg S, Orntoft TF, Andersen CL. Identification and validation of highly frequent CpG island hypermethylation in colorectal adenomas and carcinomas. Int J Cancer. 2011;129:2855–2866. doi: 10.1002/ijc.25951. [DOI] [PubMed] [Google Scholar]
- 62.Bujko M, Kober P, Mikula M, Ligaj M, Ostrowski J, Siedlecki JA. Expression changes of cell-cell adhesion-related genes in colorectal tumors. Oncol Lett. 2015;9:2463–2470. doi: 10.3892/ol.2015.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tenan M, Aurrand-Lions M, Widmer V, Alimenti A, Burkhardt K, Lazeyras F, Belkouch MC, Hammel P, Walker PR, Duchosal MA, et al. Cooperative expression of junctional adhesion molecule-C and -B supports growth and invasion of glioma. Glia. 2010;58:524–537. doi: 10.1002/glia.20941. [DOI] [PubMed] [Google Scholar]
- 64.Qi LF, Liu J, Zhu HY, Li ZQ, Lu K, Li T, Shi D. Inhibition of glioma proliferation and migration by magnetic nanoparticle mediated JAM-2 silencing. J Mater Chem B Mater Biol Med. 2014;2:7168–7175. doi: 10.1039/C4TB00954A. [DOI] [PubMed] [Google Scholar]
- 65.Qi LF, Shao WJ, Shi DL. JAM-2 siRNA intracellular delivery and real-time imaging by proton-sponge coated quantum dots. J Mater Chem B Mater Biol Med. 2013;1:654–660. doi: 10.1039/C2TB00027J. [DOI] [PubMed] [Google Scholar]
- 66.Arcangeli ML, Frontera V, Bardin F, Thomassin J, Chetaille B, Adams S, Adams RH, Aurrand-Lions M. The junctional adhesion molecule-B regulates JAM-C-dependent melanoma cell metastasis. FEBS Lett. 2012;586:4046–4051. doi: 10.1016/j.febslet.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 67.Li X, Wu Z, Mei Q, Li X, Guo M, Fu X, Han W. Long non-coding RNA HOTAIR, a driver of malignancy, predicts negative prognosis and exhibits oncogenic activity in oesophageal squamous cell carcinoma. Br J Cancer. 2013;109:2266–2278. doi: 10.1038/bjc.2013.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu CQ, Zhu ST, Wang M, Guo SL, Sun XJ, Cheng R, Xing J, Wang WH, Shao LL, Zhang ST. Pathway analysis of differentially expressed genes in human esophageal squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 2015;19:1652–1661. [PubMed] [Google Scholar]
- 69.Coradini D, Fornili M, Ambrogi F, Boracchi P, Biganzoli E. TP53 mutation, epithelial-mesenchymal transition, and stemlike features in breast cancer subtypes. J Biomed Biotechnol. 2012;2012:254085. doi: 10.1155/2012/254085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhan A, Hussain I, Ansari KI, Kasiri S, Bashyal A, Mandal SS. Antisense transcript long noncoding RNA (lncRNA) HOTAIR is transcriptionally induced by estradiol. J Mol Biol. 2013;425:3707–3722. doi: 10.1016/j.jmb.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McSherry EA, McGee SF, Jirstrom K, Doyle EM, Brennan DJ, Landberg G, Dervan PA, Hopkins AM, Gallagher WM. JAM-A expression positively correlates with poor prognosis in breast cancer patients. Int J Cancer. 2009;125:1343–1351. doi: 10.1002/ijc.24498. [DOI] [PubMed] [Google Scholar]
- 72.Singh B, Tschernig T, van Griensven M, Fieguth A, Pabst R. Expression of vascular adhesion protein-1 in normal and inflamed mice lungs and normal human lungs. Virchows Arch. 2003;442:491–495. doi: 10.1007/s00428-003-0802-6. [DOI] [PubMed] [Google Scholar]
- 73.Reynolds LE, Watson AR, Baker M, Jones TA, D'Amico G, Robinson SD, Joffre C, Garrido-Urbani S, Rodriguez-Manzaneque JC, Martino-Echarri E, et al. Tumour angiogenesis is reduced in the Tc1 mouse model of Down's syndrome. Nature. 2010;465:813–817. doi: 10.1038/nature09106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang M, Luo W, Huang B, Liu Z, Sun L, Zhang Q, Qiu X, Xu K, Wang E. Overexpression of JAM-A in non-small cell lung cancer correlates with tumor progression. PLoS One. 2013;8:e79173. doi: 10.1371/journal.pone.0079173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhuang Z, Jian P, Longjiang L, Bo H, Wenlin X. Oral cancer cells with different potential of lymphatic metastasis displayed distinct biologic behaviors and gene expression profiles. J Oral Pathol Med. 2010;39:168–175. doi: 10.1111/j.1600-0714.2009.00817.x. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Zhang H, Huang Y, Sun R, Liu R, Wei J. Human leukocyte antigen (HLA)-C polymorphisms are associated with a decreased risk of rheumatoid arthritis. Mol Biol Rep. 2014;41:4103–4108. doi: 10.1007/s11033-014-3280-9. [DOI] [PubMed] [Google Scholar]
- 77.Harita Y, Miyauchi N, Karasawa T, Suzuki K, Han GD, Koike H, Igarashi T, Shimizu F, Kawachi H. Altered expression of junctional adhesion molecule 4 in injured podocytes. Am J Physiol Renal Physiol. 2006;290:F335–F344. doi: 10.1152/ajprenal.00253.2005. [DOI] [PubMed] [Google Scholar]