Abstract
Fibroblast growth factor receptor (FGFR)-like protein 1 (FGFRL1) is the most recently discovered member of the FGFR family. Owing to the fact that it interacts with FGF ligands, but lacks the intracellular tyrosine kinase domain, several researchers have speculated that it may function as a decoy receptor and exert a negative effect on cell proliferation. In this study, we performed overexpression experiments with TetOn-inducible cell clones and downregulation experiments with siRNA oligonucleotides, and found that FGFRL1 had absolutely no effect on cell growth and proliferation. Likewise, we did not observe any influence of FGFRL1 on ERK1/2 activation and on the phosphorylation of 250 other signaling proteins analyzed by the Kinexus antibody microarray. On the other hand, with bacterial petri dishes, we observed a clear effect of FGFRL1 on cell adhesion during the initial hours after cell seeding. Our results suggest that FGFRL1 is a cell adhesion protein similar to the nectins rather than a signaling receptor similar to FGFR1-FGFR4.
Keywords: fibroblast growth factor, fibroblast growth factor receptor, fibroblast growth factor receptor-like protein 1, cell proliferation, cell adhesion
Introduction
The family of the fibroblast growth factor receptors (FGFRs) comprises five transmembrane receptors that control the proliferation, differentiation, migration and apoptosis of most cell types (1,2). The classical four receptors, FGFR1 to FGFR4, function by binding to FGFs and heparin. This interaction triggers the phosphorylation of selected residues in the intracellular part of the polypeptides, followed by the activation of various signaling cascades, such as the RAS-MAP kinase pathway, the phospholipase Cγ pathway and the PI3-kinase pathway.
FGFR-like protein 1 (FGFRL1) is the fifth member of the FGFR family (3). Similar to the classical receptors, FGFRL1 contains three extracellular Ig-like domains and a single transmembrane domain. It also interacts with FGF ligands and heparin. However, in contrast to the classical receptors, it does not possess any tyrosine kinase activity in the intracellular domain and consequently, it cannot signal by transphosphorylation. It has therefore been speculated that FGFRL1 may function as a negatively acting receptor (decoy receptor) that binds and neutralizes FGFs (4,5). The intracellular domain of FGFRL1 harbors a tandem tyrosine motif (YXXΦYXXΦ) and a peculiar histidine-rich sequence. Both motifs act as sorting signals, which target the receptor to endosomes and lysosomes, and control its retention time at the cell membrane (6). It has also been suggested that the tyrosine motif may interact with SHP-1 phosphatase and induce the activation of ERK1/2 protein in β cells (7).
FGFRL1 must fulfill a crucial function during embryonic development, as shown by the fact that FgfrL1 knockout mice die during birth with a penetrance of 100% (8,9). When compared to their wild-type littermates, the FgfrL1-deficient mice reveal a striking phenotype: they lack metanephric kidneys (10), they show a malformed diaphragm (8) and they have a dome-shaped skull (9). In a recent study, we showed that the alterations of the diaphragm are caused by the absence of slow muscle fibers (11). Without slow muscle fibers, the diaphragm is too weak to inflate the lungs and the mice will die by suffocation within minutes after birth.
We have recently compared the expression profiles of tissues from wild-type and FgfrL1 knockout mice by gene arrays and RT-PCR (12). Unexpectedly, we found that neither of the typical target genes of FGF signaling was expressed at higher levels in FgfrL1-deficient mice as would be expected after deletion of a negatively acting regulator. It is therefore unlikely that FgfrL1 acts as a decoy receptor, which attenuates FGF signaling. We have also produced mice with a targeted disruption of the intracellular domain of FgfrL1 (13). To our surprise, such mice are fully viable and do not display any obvious alterations when compared to their wild-type littermates. Thus, the intracellular domain is dispensable for normal life and most, if not all, of the vital functions of FgfrL1 must be conducted by the extracellular domain.
In this study, we examined the effects of FgfrL1 on cell proliferation and FGF signaling. We found that FgfrL1 did not affect cell proliferation, but that it promoted cell adhesion. It is therefore likely that FGFRL1 is a cell adhesion protein, rather than a signaling receptor.
Materials and methods
Cell culture
HEK-TetOn cells were purchased along with the advanced TetOn expression system from Clontech Laboratories (Takara Bio Europe/Clontech, Saint-Germain-en-Laye, France). All other cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA): 293 cells (CRL-1573), A204 rhabdomyosarcoma cells (HTB82), MG63 osteosarcoma cells (CRL-1427), FaDu squamous carcinoma cells (HTB-43) and Detroit 562 pharyngeal carcinoma cells (CCL-138). The cells were grown under standard conditions in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, penicillin (100 U/ml) and streptomycin (100 µg/ml). For cell adhesion assays, the HEK-TetOn cells were cultivated on bacterial (non-tissue culture) petri dishes obtained from Sarstedt AG (Nümbrecht, Germany) (35 mm, 82.1135) or Falcon (60 mm, 1007; Thermo Fisher Scientific AG, Reinach, Switzerland). In some experiments, FGF signaling was stimulated with 15–100 ng/ml of human recombinant FGF2 (PeproTech, London, UK and Sino Biological, Inc., Beijing, China). The growth and shape of the cells were examined under a Nikon E800 eclipse microscope (Nikon AG, Elgg, Switzerland)
Generation of tetracycline-inducible cell clones
The HEK-TetOn cells were co-transfected with two cDNA plasmids. One plasmid contained human full-length FGFRL1 or human FGFRL1ΔC (encoding amino acids 1–417) in the pTRE expression vector, which harbors a tetracycline responsive promoter element. The other was a linearized hygromycin selection marker (Clontech). Following transfection, the cells were selected with 100 µg/ml hygromycin until resistant colonies could be observed (2–4 weeks). Individual colonies were isolated with the help of cloning cylinders and expanded. Approximately 40 individual cell clones were isolated from each transfection experiment. The clones were pre-screened for inducible FGFRL1 expression by immunostaining with a monoclonal antibody against human FGFRL1 as described elsewhere (6). For further cultivation of the stable cell clones, hygromycin was used at 60 µg/ml to maintain the selection pressure.
Northern blot analysis
Total RNA was isolated from the confluent cell cultures with the GenElute miniprep kit from Sigma-Aldrich (St. Louis, MO, USA). The RNA was resolved on 1% agarose gels in the presence of formaldehyde. The gels were stained with ethidium bromide to visualize the 18S and 28S ribosomal RNAs. The RNAs were quantitatively transferred to a nylon membrane by vacuum blotting. The blot was hybridized under standard conditions with probes that contained full-length cDNAs for FGFRL1 and GAPDH, respectively. Beforehand, these probes had been radiolabeled by random primed oligolabeling with 32P-dCTP (New England Nuclear/PerkinElmer, Waltham, MA, USA). The blot was extensively washed with 1X SSC and exposed to X-ray film (Carestream Kodak BioMax MS; Sigma). For quantification of the bands, the blot was scanned with a phosphorimager (Storm 840; Molecular Dynamics, Sunnyvale, CA, USA).
Proliferation assay
Cells grown in tissue culture flasks were collected by trypsinization and seeded into the wells of 24-well plates at a density of approximately 2×104 cells/well. One day after seeding, half of the wells was stimulated with 1 µg/ml doxycycline, while the other half remained untreated. During the following 5 days, the cell number was determined with a colorimetric assay that measures the amount of the endogenous enzyme hexosaminidase (14). To this end, the media were removed from the cell layers by aspiration and 100 µl of a solution containing 3.75 mM p-nitrophenyl-N-acetyl-β-D-glucosaminide, 50 mM sodium citrate, 0.25% Triton X-100, pH 5.0 was added to each well. After 45 min, the reaction was terminated by the addition of 200 µl of 50 mM glycine, 5 mM EDTA, pH 10.4 and the absorbance of the yellow product was determined at 405 nm using an Infinite M200 microplate reader (Tecan AG, Männedorf, Switzerland).
Western blot analysis
Cell cultures were rinsed with cold phosphate-buffered saline (PBS) and directly dissolved in hot SDS sample buffer containing 2% mercaptoethanol. Proteins were separated under standard conditions on 10% SDS polyacrylamide gels containing 5% stacking gels. Resolved polypeptides were transferred from the gels to nitrocellulose membranes by semi-dry blotting (Trans-Blot SD; Bio-Rad Laboratories, Hercules, CA, USA). Unspecific sites of the membranes were blocked with 3% milk powder in PBS. The membranes were incubated with affinity-purified antibodies against phosphorylated human ERK1/2 (E7028, 1:1,000; Sigma). After 12 h, the membranes were rinsed with PBS and incubated for 2 h with phosphatase-conjugated secondary antibodies (711-055-152; Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Bound antibodies were detected by reaction with 5-bromo-4-chloro-3-indolyl-phosphate and nitroblue tetrazolium substrate. After stripping, the blots were stained in a similar way with a monoclonal antibody against total p42/44 MAP kinase (L34F12; 1:2,000; Cell Signaling Technology Inc., Danvers, MA, USA).
Kinexus antibody microarrays
Protein extracts were prepared from HEK-TetOn cells grown on 10 cm tissue culture plates in the presence or absence of the inducer, doxycycline. Special care was taken to ensure that the two samples were always treated in parallel in the same way. The cell layers were rinsed with ice-cold PBS and lysed with 0.2 ml lysis buffer containing 20 mM MOPS, 1% Triton X-100, pH 7.0 and proteinase and phosphatase inhibitors as suggested by Kinexus Bioinformatics Corp. (Vancouver, BC, Canada). To rupture the cell bodies, the suspensions were sonicated twice for 15 min with an ultrasonic processor (Sonics Vibra-Cell; Sonics & Materials, Inc., Newtown, CT, USA). The homogenates were cleared in a Centrikon T-2170 ultracentrifuge at 100,000 x g for 30 min. Subsequently, the clear homogenates were analyzed on Kinex Antibody Microarrays (KAM-1.1; Kinexus Bioinformatics Corp.) that contained >650 different antibodies against signaling proteins. Selected proteins were further verified by the Kinetworks multi-immunoblotting service.
Downregulation by siRNA
The expression of FGFRL1 was downregulated with siRNA oligonucleotides from Dharmacon (ON-TARGETplus SMARTpool siRNA; Thermo Fisher Scientific, Waltham, MA, USA). To target the human FGFRL1 mRNA, a pool of the following four oligonucleotides was utilized: GGCUGAAGCGCGUGGAGUA, GGACACUGAG CCUGAAGAA, GCUCCUACCUCAAUAAGCU and GAA CACGACGGUGGACUUC. As a control, a pool of four non-targe ting oligonucleotides was used (D-001810-10-05). To form lipid complexes, the oligonucleotides were mixed with Lipofectamine 2000 reagent in Opti-MEM medium (Invitrogen Life Technologies, Inc., Carlsbad, CA, USA) and incubated for 5 min at room temperature. The complexes were added dropwise to the cells grown either in 35 mm dishes (for northern blot analysis) or 24-well plates (for proliferation assays) at a final concentration of 25–50 nM.
Results
Effect on cell proliferation
To measure the effects of FGFRL1 on cell growth and proliferation, and to avoid problems of reproducibility that are frequently observed by transient transfection experiments, we used a stable, tetracycline-inducible expression system. Commercially available HEK-TetOn cells, which constitutively express the Tet transactivator protein, were stably transfected with two different tetracycline-inducible FGFRL1 constructs, namely the human wild-type construct FGFRL1 and the human construct FGFRL1ΔC, which lacks the intracellular domain (Fig. 1A). Following selection with antibiotics, colonies were isolated and pre-screened for FGFRL1 expression by immunostaining with an antibody against FGFRL1. Positive clones were further checked for strong inducibility of FGFRL1 expression by northern blot analysis (Fig. 1B and C). Clones with a relatively high induction of FGFRL1 expression and relatively low background expression before the addition of doxycycline were selected for further experiments (full-length clones: K3F, K5F, K9F, K17F, K19F, K24F; truncated clones FGFRL1ΔC: K13ΔC, K14ΔC, K33ΔC, K35ΔC, K41ΔC). In the following, we will describe several experiments performed with these clones and present data for some selected clones. However, it should be emphasized that most experiments were performed with all of the clones and that we never observed any qualitative differences among the clones.
Figure 1.
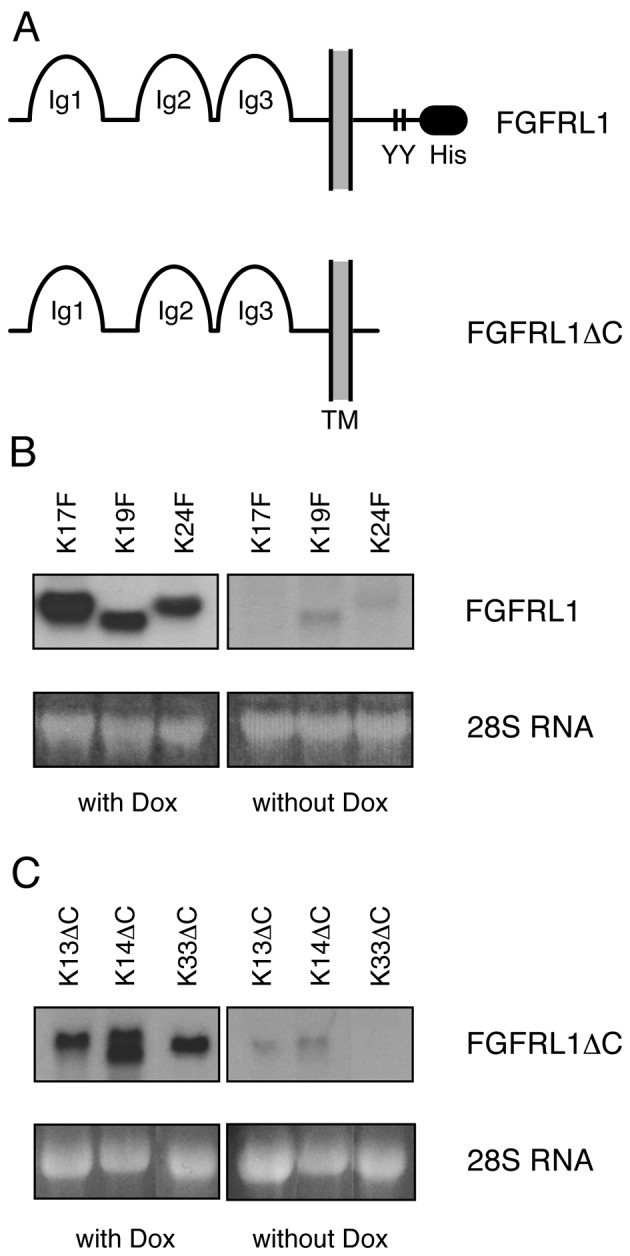
Generation of HEK-TetOn cell lines with tetracycline-inducible fibroblast growth factor receptor-like protein 1 (FGFRL1) expression. (A) Schematic representation of the two FGFRL1 constructs that were stably transfected into HEK-TetOn cells. FGFRL1 represents the full-length, wild-type receptor, while FGFRL1ΔC contains a truncated C-terminal domain lacking the histidine-rich sequence and the tandem tyrosine motif. The two motifs are known to control the retention time of the receptor at the cell membrane. Hygromycin resistant cell clones were analyzed by northern blotting for inducible FGFRL1 expression. (B) Three representative full-length clones and (C) three representative C-terminally truncated clones are shown before and after induction with 1 µg/ml doxycycline. As a loading control, the 28S ribosomal RNA stained with ethidium bromide is included.
On a northern blot analysis, our clones showed a relatively tight repression before, but a relatively strong expression of FGFRL1 after the addition of the inducer, doxycycline (Fig. 1B and C). Nevertheless, when the blot was overexposed, bands corresponding to the FGFRL1 mRNA could also be detected in the lanes without doxycycline, suggesting that our expression system showed some leakiness. The extent of inducibility was determined by quantification of the northern blotting with the help of a phosphorimager. Clone K17F could be induced >180-fold, and clone K24F >30-fold.
A slight heterogeneity was noted between the individual clones with respect to the length of the mRNAs. This heterogeneity may originate from different sizes of the 3′UTR and the poly(A) tract due to integration of the cDNA constructs at different sites in the genome. In two selected cases (K17F, K13ΔC) we subcloned the expressed cDNA by RT-PCR and fully confirmed the open reading frame of the FGFRL1 sequence.
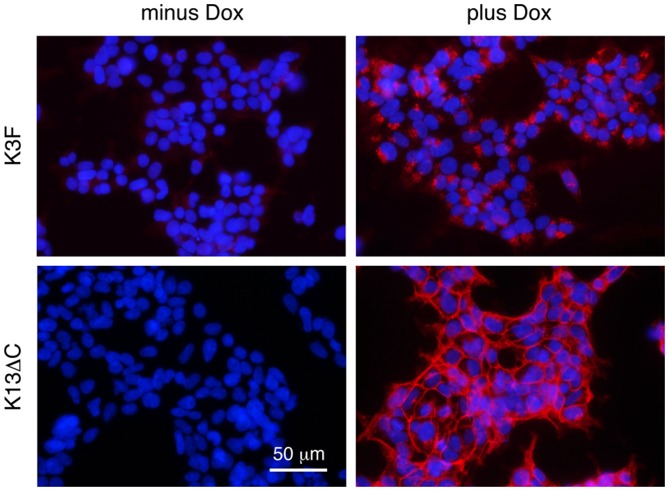
Fig. 2 shows two examples of FGFRL1 expression in cell culture by immunofluorescence with a monoclonal antibody against human FGFRL1. In the absence of the inducer doxycycline, no expression was observed. In the presence of doxycycline, a strong fluorescent signal was observed, indicating strong inducibility of FGFRL1 expression. As expected, the fluorescent signal was predominantly found at the cell membrane in the case of the FGFRL1ΔC construct (K13ΔC), but mainly in intracellular compartments in the case of the full-length construct (K3F). As previously reported, this difference in the distribution of the expressed protein is explained by the presence or absence of two sorting signals in the transfected FGFRL1 constructs (6). Thus, we have successfully generated cell lines, in which we can overexpress FGFRL1 by 30 to 180-fold.
Figure 2.
Inducible expression of fibroblast growth factor receptor-like protein 1 (FGFRL1) demonstrated by immunofluorescence. A representative full-length clone (K3F) and a representative truncated clone (K13ΔC) were cultivated in the presence (plus Dox) or absence (minus Dox) of the inducer doxycycline. Expression was visualized with a monoclonal antibody against human FGFRL1. Note that the wild-type receptor resided primarily in intracellular compartments, while the truncated form accumulated at the plasma membrane.
The inducible TetOn clones were utilized to examine the effects of FGFRL1 on cell proliferation. Individual clones were seeded on cell culture plates and grown for up to 5 days in the presence or absence of the inducer, doxycycline. As shown in Fig. 3, the addition of doxycycline at 1 µg/ml had absolutely no effect on cell growth. Albeit we noted a slight difference in the growth curves between individual clones, a given clone always showed the same growth characteristics in the absence or presence of doxycycline. This observation was true for full-length clones (e.g., clones K3F, K19F) as well as for C-terminally truncated clones (e.g., clones K13ΔC, K33ΔC). Thus, the presence of FGFRL1 does not appear to have any effect on cell growth, at least not on the proliferation of our HEK-TetOn cells.
Figure 3.
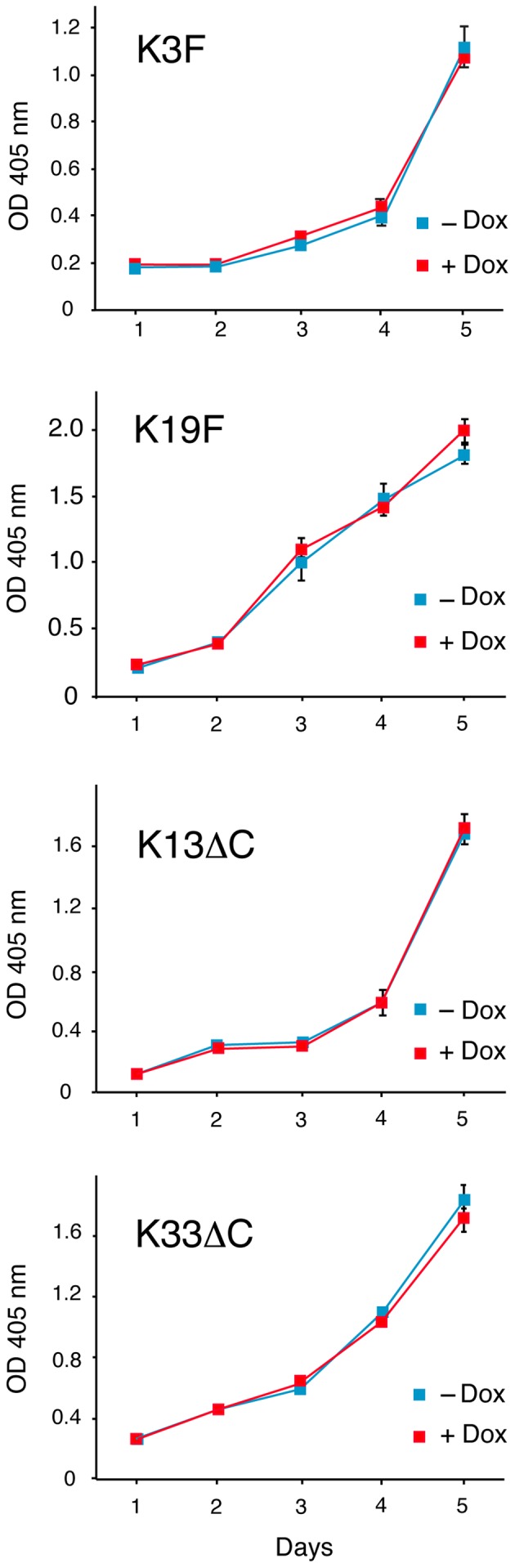
Effect of fibroblast growth factor receptor-like protein 1 (FGFRL1) expression on cell proliferation. Stable HEK-TetOn clones were cultivated in the presence or absence of doxycycline in 24-well plates. Cell proliferation was measured over the course of five days with the hexosaminidase assay. No effect on cell proliferation was observed by the presence of FGFRL1 or FGFRL1ΔC. Standard errors are given at some time-points where meaningful (n=3).
We also tried to prepare other stable cell lines (HT1080, A204) that would express FGFRL1 in an inducible manner. However, we failed to obtain any other stable cell clones. This finding is in keeping with the remarks of the distributor of the TetOn expression system that it is extremely difficult to select cell lines, which stably express the Tet transactivator protein.
ERK1/2 signaling
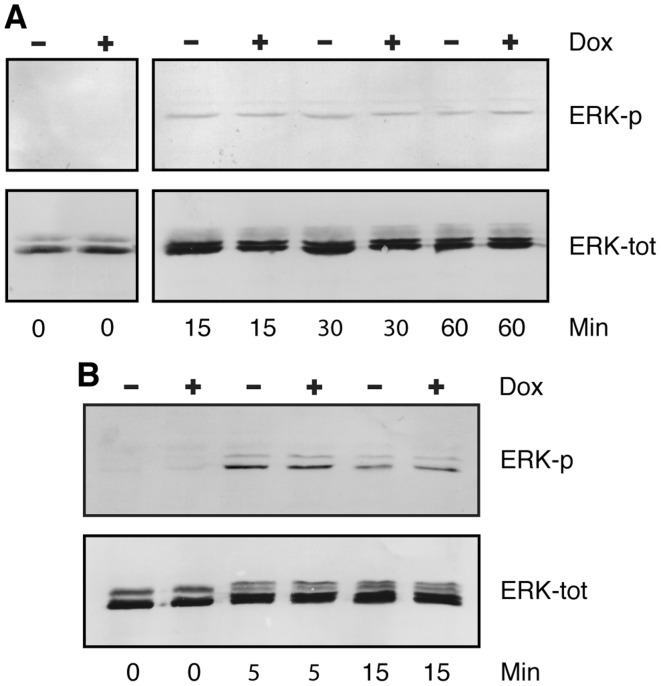
Since we had expected that FGFRL1 would be involved in FGF signaling, we examined the effects of FGFRL1 on the activation of the MAP/ERK signaling pathway. These experiments were conducted only with the full-length clones, K3F, K5F, K17F K19F and K24F, which possess an intact intracellular domain. The clones were grown on cell culture plates for 1–2 days in the presence of fetal bovine serum, starved overnight and then stimulated with FGF2 at 15 ng/ml or 30 ng/ml (a concentration of 15 ng/ml has a maximal effect on ERK phosphorylation according to a previous publication) (15). At the same time, half of the cultures were treated with doxycycline to induce the expression of FGFRL1, while the other half were left untreated. After different time intervals, the cell layers were dissolved in SDS sample buffer and analyzed by western blotting with specific antibodies against phosphorylated ERK (Fig. 4A). Before the addition of FGF2, the level of phosphorylated ERK of our HEK-TetOn cells was below the detection level. After the addition of FGF2, we observed two faint bands that should correspond to phosphorylated ERK1 and ERK2. Most importantly, we did not observe any differences in the levels of phosphorylated ERK between cultures grown in the absence or the presence of the inducer, doxycycline. To verify that our samples contained equal amounts of unphosphorylated ERK, we treated the same western blot after stripping with an antibody to total ERK. Two bands corresponding to ERK1 and ERK2 were observed and these bands had similar intensities in the samples before and after doxycycline treatment. Thus, the expression of FGFRL1 did not appear to have any effect on ERK1/2 signaling.
Figure 4.
Effect of fibroblast growth factor receptor-like protein 1 (FGFRL1) on ERK phosphorylation. FGFRL1 inducible HEK-TetOn cells were cultivated in multi-well plates, starved overnight in medium lacking fetal bovine serum and then stimulated for 0–60 min as indicated with human FGF2 (15 ng/ml). Cells were lysed with hot SDS sample buffer. Cellular proteins were resolved on polyacrylamide gels and processed for western blotting with antibodies against phosphorylated ERK1/2 (ERK-p). The blots were stripped and reprobed with antibodies against total ERK (ERK-tot). An experiment conducted with clone K24F is depicted in panel A. Panel B shows an experiment with clone K17F. However in this case, the cells had been transfected - prior to starvation and FGF2 stimulation - with a full-length clone for human FGFR1 to increase signaling. ERK1/2 phosphorylation was extremely low before stimulation, but clearly visible after stimulation with FGF2. No difference in ERK phosphorylation was observed between doxycycline-induced and uninduced cells.
Since the ERK signal detected in Fig. 4A was rather faint, we transfected a full-length clone for FGFR1 into our cell lines in order to enhance FGF signaling. In fact, we were able to increase the ERK signal significantly by this treatment (Fig. 4B). At the beginning of the experiment (0 min), no ERK phosphorylation was detected, but 5 and 15 min after FGF stimulation, the bands of phosphorylated ERK1/2 became much stronger than in the experiment without FGFR1 transfection. Nevertheless, we could not detect any differences in ERK phosphorylation between cultures grown in the presence or absence of doxycycline.
Kinexus antibody microarray
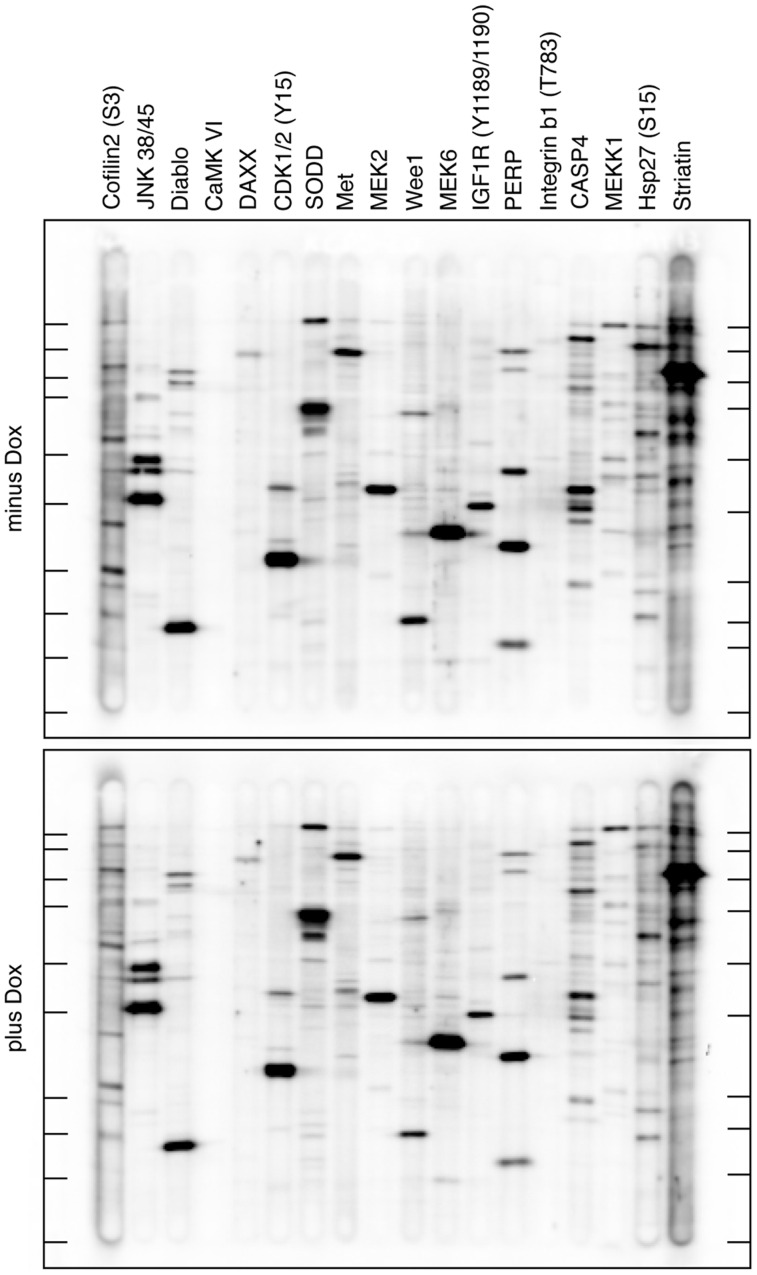
Since FGFRL1 may also affect a pathway other than ERK signaling, we subjected two selected clones (K3F, K17F) to a global analysis of signaling proteins. As above, the cells were grown for 32 h in the presence or absence of the inducer doxycycline and protein extracts were prepared according to an established protocol. The extracts were analyzed by the Kinexus antibody microarray service, which utilizes >650 verified antibodies against most well-studied signaling proteins, including phospholipase Cγ, PI3-kinase, STAT and Akt. Differences in the absolute amount of the signaling proteins (using 380 pan-specific antibodies), as well as differences in their phosphorylation status (using 270 phospho-site specific antibodies) were investigated. To our surprise, we did not find any significant differences between the doxycycline-treated and untreated cell cultures. Nevertheless, 18 proteins that had shown slight (but not significant) changes between the two culture conditions were selected for further investigation. To this end, the two protein extracts were subjected to western blotting with a set of specific, verified antibodies against the 18 signaling proteins. However, as demonstrated by the immunoblot of Fig. 5, we could not detect any significant differences in the absolute amount or in the phosphorylation status of the selected signaling proteins. Thus, the expression of FGFRL1 in our cell clones did not appear to have any major effect on the signaling pathways that are covered by the Kinexus antibody microarray.
Figure 5.
Kinexus antibody microarray. Fibroblast growth factor receptor-like protein 1 (FGFRL1) inducible cells (clone K3F) were cultivated for 32 h in the presence or absence of the inducer doxycycline. Protein extracts were prepared and analyzed on Kinexus antibody microarrays containing more than 650 different antibodies against various signaling proteins. No significant differences were observed between doxycycline-treated and untreated cells (not shown). To verify some of the results, the two protein extracts were separated on SDS polyacrylamide gels, transferred to an immunoblot membrane and probed with antibodies against 18 selected proteins as indicated using a multiscreen apparatus from BioRad. The immunoblot was analyzed with a chemiluminescence imager. No significant differences were observed between doxycycline-induced (lower panel) and uninduced (top panel) cells. The migration positions of molecular weight markers are shown in the left and right margins by short horizontal lines. These lines correspond (from top to bottom) to 250 K, 150 K, 100 K, 75 K, 50 K, 37 K, 25 K, 20 K and 15 K.
Downregulation of FGFRL1 expression by siRNA
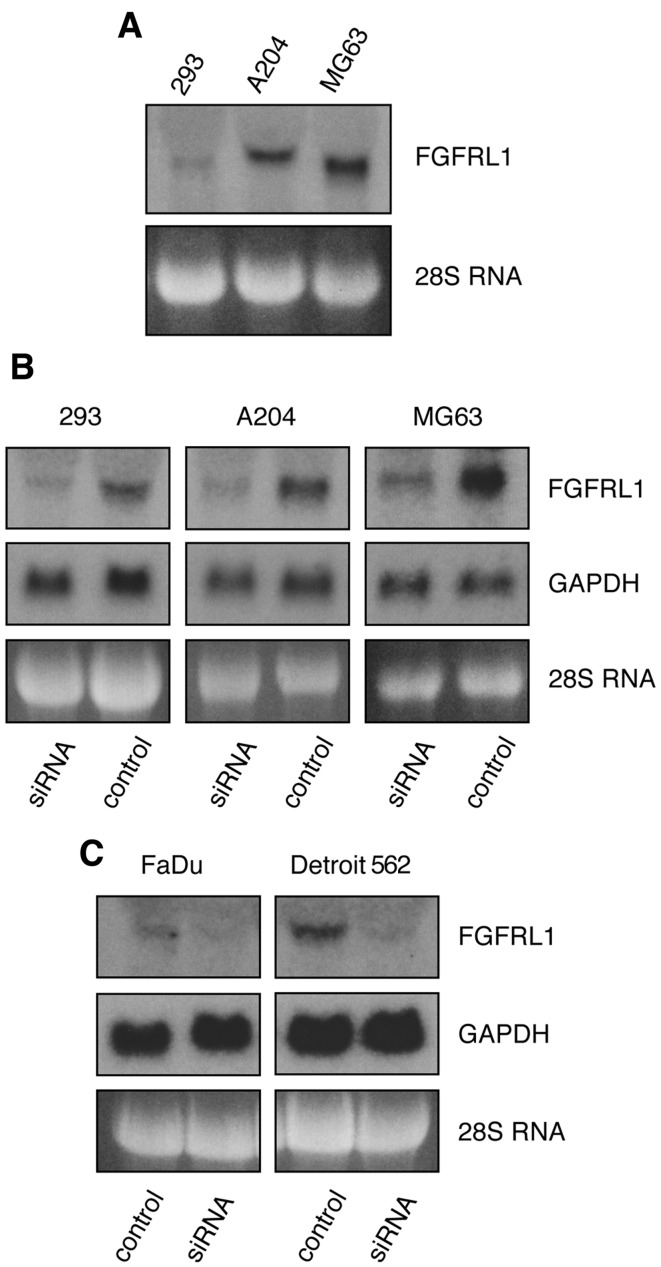
As mentioned before, our doxycycline-inducible FGFRL1 TetOn clones show, in addition to their strong inducibility, a low level of leakiness. If a low level of FGFRL1 expression would already elicit a full response, we would not see any additional effect upon the overexpression of FGFRL1. To tackle this problem, we performed a set of converse experiments, in which we tried to downregulate FGFRL1 expression. For this purpose, we selected three different cell lines that express either low levels of FGFRL1 (293 kidney cells), moderate levels of FGFRL1 (A204 rhabdomyosarcoma cells) or relatively high levels of FGFRL1 (MG63 osteosarcoma cells), as demonstrated by northern blotting with a probe for human FGFRL1 (Fig. 6A). A pool of siRNA oligonucleotides was obtained from Dharmacon, which should effectively downregulate FGFRL1 mRNA levels following transfection into cells. In fact, when used at 25 nM, these oligonucleotides decreased FGFRL1 levels in the 293, A204 and MG63 cells by 75–84%, as demonstrated by northern blotting and quantification with a phosphorimager (Fig. 6B). However, the same siRNA oligonucleotides had no effect on the levels of the GAPDH mRNA or 28S RNA. Likewise, a pool of control RNA oligonucleotides had no effect on the level of the FGFRL1 mRNA, demonstrating the specificity of the FGFRL1 siRNA pool.
Figure 6.
Downregulation of fibroblast growth factor receptor-like protein 1 (FGFRL1) by siRNA oligonucleotides. (A) A northern blot demonstrates the levels of FGFRL1 mRNA in three different cell lines. Osteosarcoma cells (MG63) express relatively high levels of FGFRL1, A204 rhabdomyosarcoma cells moderate levels and 293 cells very low levels. (B) The cells were transfected with a pool of siRNA oligos against FGFRL1 or with control RNA oligos. In each of the three cell lines, the FGFRL1 mRNA levels were downregulated to near zero, while the levels of GAPDH and 28S RNA remained unaffected. (C) An analogous experiment was performed with two cell lines from esophageal cell carcinomas. Detroit 562 cells express moderate levels of FGFRL1, FaDu cells extremely low levels. In both cases, the FGFRL1 levels could be downregulated to near zero with siRNA oligos.
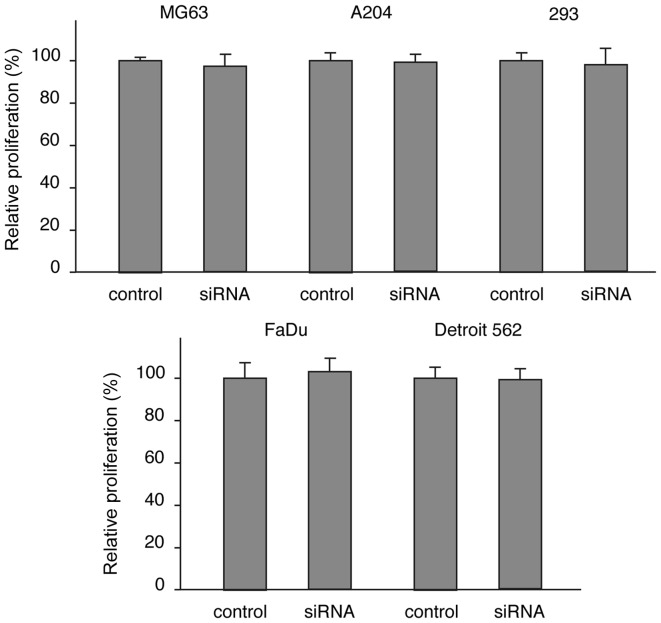
Cell proliferation was determined by the hexosaminidase assay two days following transfection of the siRNAs or control RNAs into the three cell types. Although the siRNA pool had downregulated FGFRL1 mRNA levels to near zero, we did not detect any changes in the proliferation rate of the 293, A204 and MG63 cells (Fig. 7A). Obviously, the downregulation of the FGFRL1 mRNA had no significant effect on cell proliferation.
Figure 7.
Effect of fibroblast growth factor receptor-like protein 1 (FGFRL1) downregulation on cell proliferation. Expression of FGFRL1 was downregulated with siRNA oligos in different cells lines as indicated and cell proliferation was measured with the hexosaminidase assay after two days. The results are expressed relative to a control treated with unrelated RNA oligos (100%). The bars indicate standard errors from four independent samples. No differences in cell proliferation were noted between control and FGFRL1 downregulated cells.
Recently, another research group reported that FGFRL1 would have a stimulatory effect on the proliferation of certain tumor cells (16,17). These authors studied primary and established cell lines derived from human esophageal squamous cell carcinoma (ESCC). We therefore extended our siRNA experiments on esophageal squamous carcinoma cells. As before, we used a cell line that expressed low levels of FGFRL1 (FaDu cells) and another cell line that expressed relatively high levels of FGFRL1 (Detroit 562 cells) as assessed by northern blotting (Fig. 6C). When our pool of siRNA oligonucleotides was transfected into these cells, we observed a downregulation of FGFRL1 mRNA levels to nearly zero. However, cell proliferation, measured by the hexosaminidase assay, did not significantly differ between doxycycline-treated and untreated cells after two days in culture (Fig. 7B). Thus, it is very unlikely that FGFRL1 has any stimulatory effect on the proliferation of our esophageal tumor cells.
Cell adhesion
If FGFRL1 does not have any effect on cell growth and proliferation, it would be of interest to determine what function it has, if any. We have previously observed that recombinant FGFRL1 induced cell adhesion when coated on bacterial plastic dishes (18).
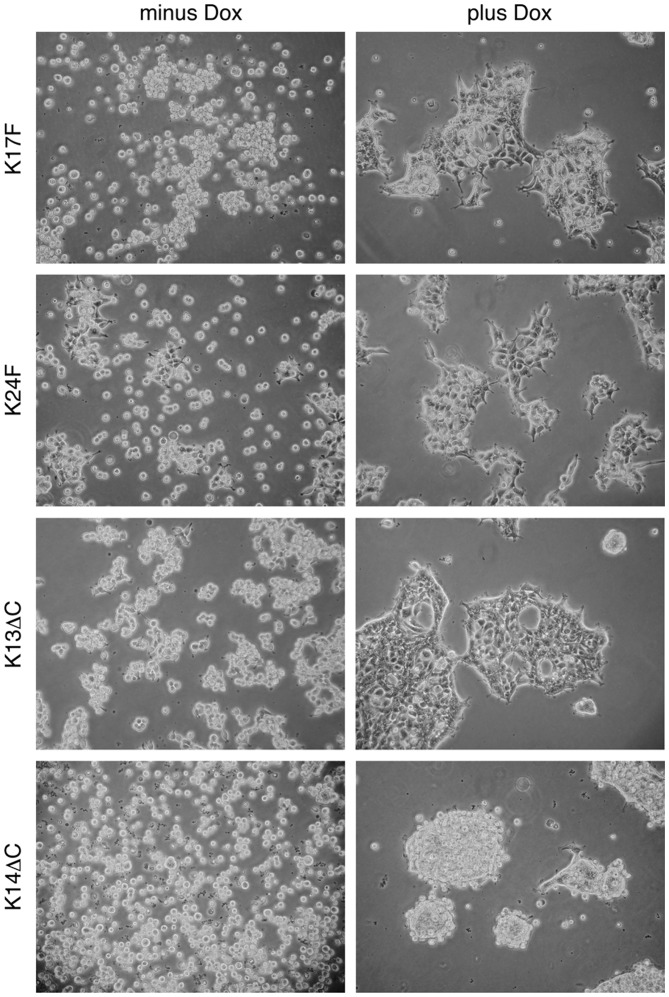
We therefore investigated whether the inducible expression of FGFRL1 in our HEK-TetOn cells would have any effect on cell adhesion. To this end, our cell clones were seeded into untreated (bacterial) petri dishes and grown in the absence or presence of the inducer, doxycycline. Cell adhesion was inspected under a microscope 10–15 h after seeding. As demonstrated in Fig. 8, neither of the cell lines was able to attach to the bacterial plastic plates in the absence of doxycycline within this short period of time. However in its presence, the cells began to attach to the plastic surface and to spread. Moreover, they aggregated and began to form large clusters, probably by cell-cell adhesion. This adhesion and clustering phenomenon was observed with the full-length clones as well as with the C-terminally truncated clones. A slight difference was noted between individual clones in the extent of clustering. This difference may reflect different amounts of FGFRL1 protein synthesized by individual cell clones within a given period of time. After 24 h, the differences between doxycycline-treated and untreated cells were no longer dramatic and the cells began to attach and cluster also in the absence of doxycycline. This may be explained by the leakiness of inducible FGFRL1 expression and/or by the synthesis of adhesive proteins other than FGFRL1 (fibronectin, collagen, NCAM). Nevertheless, our experiments show that FGFRL1 has a clear effect on cell adhesion during the initial hours after seeding.
Figure 8.
Effect of fibroblast growth factor receptor-like protein 1 (FGFRL1) on cell adhesion. FGFRL1 inducible HEK-TetOn cells were seeded into bacterial (non-tissue culture) petri dishes and cultivated for 10–15 h in the absence (minus) or presence (plus) of the inducer doxycycline. Note that expression of FGRL1, in its full-length form as well as its truncated form, promoted cell-cell adhesion and cell clustering. In sharp contrast, most cells remained in their single-cell state in the absence of FGFRL1 within this short period of time. A control with original HEK-TetOn cells (prior to transfection with FGFRL1 constructs) did not show any differences between the two conditions.
Discussion
In the present study, we presented compelling evidence that the novel receptor, FGFRL1, does not control cell growth and proliferation, but that it rather exerts a positive effect on cell adhesion. This conclusion was drawn from various experimental appro aches, including: i) the inducible overexpression of FGFRL1 in HEK-TetOn cells; ii) the downregulation of FGFRL1 by siRNA oligonucleotides in established cell lines; iii) the determination of ERK phosphorylation in the presence and absence of FGFRL1; iv) the analysis of 650 signaling molecules by Kinexus antibody microarrays; and v) cell adhesion on bacterial petri dishes upon induction of FGFRL1 synthesis. Originally, we as well as other researchers had expected that FGFRL1 would have a negative effect on cell growth as it interacts with growth factors of the FGF family but cannot signal by phosphorylation as it lacks the intracellular tyrosine kinase domain (5,19). The interaction with FGF ligands has been confirmed in different laboratories using various approaches, such as coprecipitation, dot blot assays, cell-based ligand binding assays and surface plasmon resonance, and it was found that FGFRL1 mainly interacted with FGF3 and FGF8 (3). Obviously, we must face the fact that this interaction does not trigger cell proliferation. Nevertheless, the new findings are compatible with the domain structure of FGFRL1 as this receptor is lacking the intracellular tyrosin kinase domain that would be required for phosphorylation. The new findings are also in accordance with the observation that genetically manipulated mice with a deletion of the intracellular domain of FgfrL1 are viable and do not exhibit any abnormal phenotype when compared with wild-type littermates (13). Obviously, the intracellular domain of FGFRL1 is dispensable for survival.
The only definite effect of FGFRL1 that we could document in our study was a positive influence on cell adhesion. Upon expression of FGFRL1 in our inducible HEK-TetOn clones, the cells started to aggregate and to form large clusters. Without FGFRL1 induction, no similar clustering was observed. However, the effect was detected only during the initial hours after cell seeding and after two days, we did not notice any differences between FGFRL1 induced and uninduced cell cultures. It is possible that after two days, other cell adhesion molecules, such as fibronectin, collagen and NCAM, have taken over the adhesive function of FGFRL1. Furthermore, the effect could not be demonstrated with surface-treated culture dishes that are normally utilized for cell culture experiments. A critical function of FGFRL1 in cell adhesion is compatible with our previous observations that FGFRL1 can bind to heparin and to heparan sulfate proteoglycans on cell surfaces (18). At that time, we showed that the second Ig domain was responsible for the heparin-binding activity and that it could be blocked with soluble heparin as well as with heparin-binding peptides.
An important function of FGFRL1 in cell-cell adhesion had already been suggested by our studies with knockout mice. FgfrL1-null mice lack metanephric kidneys because the metanephric mesenchyme is not able to undergo the essential conversion into the nephrogenic epithelium (10). Obviously, FgfrL1 must be expressed at the cell surface of mesenchymal cells in order to induce proper alignment and tight cell clustering as required for differentiation into renal epithelium. A similar effect was also noticed in the diaphragm. FgfrL1 knockout mice die because their diaphragm is too weak to inflate the lungs after birth (8,11). During development of the diaphragm muscle, individual muscle precursor cells must align with each other in order to fuse into myotubes and myofibers. Obviously, FGFRL1 is required at the cell surface of muscle precursor cells to initiate such an alignment.
A critical function in cell adhesion is also in keeping with our observations that FGFRL1 can induce cell-cell fusion in culture. With CHO cells and similar cell types, the mere over-expression of FGFRL1 leads to fusion of the cells into large syncitia containing several dozens of nuclei (20). It is unlikely that this fusogenic activity is a normal, physiological function of FGFRL1, as it is not observed with other cell types. However, it demonstrates that FGFRL1 can pull together two cell surfaces with high tensile strength. In a recent publication, we have found evidence that FGFRL1 binds to a target molecule on neighboring cells to induce cell-cell fusion (21). A hydrophobic pocket in the third Ig domain of FGFRL1 appeared to interact with this target protein. However, the identity of the target protein remained elusive.
With its domain structure and its function during cell adhesion, FGFRL1 resembles another family of proteins, the nectins (22,23). Four different nectins (nectin-1 to nectin-4) have been identified that possess a domain structure similar to FGFRL1 with three extracellular Ig domains, a single trans-membrane domain and a relatively short intracellular domain. The nectins are involved in cell-cell adhesion and immune modulation and play an important role during host-pathogen interactions. The adhesive function is accomplished by homophilic as well as heterophilic interactions of the nectins. During the adhesive process, the C-terminal cytoplasmic domain is required to remodel the actin cytoskeleton. It interacts with the PDZ domain of the adapter molecule afadin, which in turn recruits filamentous actin. So far, we do not have any evidence that FGFRL1 would interact with afadin or with any of the four nectins. Furthermore, the C-terminal domain of FGFRL1 appears to be dispensable for its function (13). Thus, the molecular mechanism of inducing cell adhesion must be quite different between FGFRL1 and the nectins, although the proteins share structural and functional homology.
Acknowledgments
This study was supported by grants from the Swiss National Science Foundation (no. 31003A-143350) and the Novartis Foundation for Medical-Biological Research (15A006).
Abbreviations
- FGF
fibroblast growth factor (human)
- Fgf
fibroblast growth factor (mouse)
- FGFR
fibroblast growth factor receptor (human)
- Fgfr
fibroblast growth factor receptor (mouse)
- FGFRL1
fibroblast growth factor receptor-like protein 1
References
- 1.Goetz R, Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol. 2013;14:166–180. doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trueb B. Biology of FGFRL1, the fifth fibroblast growth factor receptor. Cell Mol Life Sci. 2011;68:951–964. doi: 10.1007/s00018-010-0576-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiedemann M, Trueb B. Characterization of a novel protein (FGFRL1) from human cartilage related to FGF receptors. Genomics. 2000;69:275–279. doi: 10.1006/geno.2000.6332. [DOI] [PubMed] [Google Scholar]
- 5.Sleeman M, Fraser J, McDonald M, Yuan S, White D, Grandison P, Kumble K, Watson JD, Murison JG. Identification of a new fibroblast growth factor receptor, FGFR5. Gene. 2001;271:171–182. doi: 10.1016/S0378-1119(01)00518-2. [DOI] [PubMed] [Google Scholar]
- 6.Rieckmann T, Zhuang L, Flück CE, Trueb B. Characterization of the first FGFRL1 mutation identified in a craniosynostosis patient. Biochim Biophys Acta. 2009;1792:112–121. doi: 10.1016/j.bbadis.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Silva PN, Altamentova SM, Kilkenny DM, Rocheleau JV. Fibroblast growth factor receptor like-1 (FGFRL1) interacts with SHP-1 phosphatase at insulin secretory granules and induces beta-cell ERK1/2 protein activation. J Biol Chem. 2013;288:17859–17870. doi: 10.1074/jbc.M112.440677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baertschi S, Zhuang L, Trueb B. Mice with a targeted disruption of the Fgfrl1 gene die at birth due to alterations in the diaphragm. FEBS J. 2007;274:6241–6253. doi: 10.1111/j.1742-4658.2007.06143.x. [DOI] [PubMed] [Google Scholar]
- 9.Catela C, Bilbao-Cortes D, Slonimsky E, Kratsios P, Rosenthal N, Te Welscher P. Multiple congenital malformations of Wolf-Hirschhorn syndrome are recapitulated in Fgfrl1 null mice. Dis Model Mech. 2009;2:283–294. doi: 10.1242/dmm.002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber SD, Steinberg F, Beyeler M, Villiger PM, Trueb B. The murine Fgfrl1 receptor is essential for the development of the metanephric kidney. Dev Biol. 2009;335:106–119. doi: 10.1016/j.ydbio.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Amann R, Wyder S, Slavotinek AM, Trueb B. The FgfrL1 receptor is required for development of slow muscle fibers. Dev Biol. 2014;394:228–241. doi: 10.1016/j.ydbio.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Gerber SD, Amann R, Wyder S, Trueb B. Comparison of the gene expression profiles from normal and Fgfrl1 deficient mouse kidneys reveals downstream targets of Fgfrl1 signaling. PLoS One. 2012;7:e33457. doi: 10.1371/journal.pone.0033457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bluteau G, Zhuang L, Amann R, Trueb B. Targeted disruption of the intracellular domain of receptor FgfrL1 in mice. PLoS One. 2014;9:e105210. doi: 10.1371/journal.pone.0105210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibata H, Yagi T. Rate assay of N-acetyl-beta-D-hexosaminidase with 4-nitrophenyl N-acetyl-beta-D-glucosaminide as an artificial substrate. Clin Chim Acta. 1996;251:53–64. doi: 10.1016/0009-8981(96)06292-4. [DOI] [PubMed] [Google Scholar]
- 15.Zhu H, Duchesne L, Rudland PS, Fernig DG. The heparan sulfate co-receptor and the concentration of fibroblast growth factor-2 independently elicit different signalling patterns from the fibroblast growth factor receptor. Cell Commun Signal. 2010;8:14. doi: 10.1186/1478-811X-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuchiya S, Fujiwara T, Sato F, Shimada Y, Tanaka E, Sakai Y, Shimizu K, Tsujimoto G. MicroRNA-210 regulates cancer cell proliferation through targeting fibroblast growth factor receptor-like 1 (FGFRL1) J Biol Chem. 2011;286:420–428. doi: 10.1074/jbc.M110.170852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimada Y, Okumura T, Nagata T, Hashimoto I, Sawada S, Yoshida T, Fukuoka J, Shimizu K, Tsukada K. Expression analysis of fibroblast growth factor receptor-like 1 (FGFRL1) in esophageal squamous cell carcinoma. Esophagus. 2014;11:48–53. doi: 10.1007/s10388-013-0394-4. [DOI] [Google Scholar]
- 18.Rieckmann T, Kotevic I, Trueb B. The cell surface receptor FGFRL1 forms constitutive dimers that promote cell adhesion. Exp Cell Res. 2008;314:1071–1081. doi: 10.1016/j.yexcr.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Trueb B, Zhuang L, Taeschler S, Wiedemann M. Characterization of FGFRL1, a novel fibroblast growth factor (FGF) receptor preferentially expressed in skeletal tissues. J Biol Chem. 2003;278:33857–33865. doi: 10.1074/jbc.M300281200. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg F, Gerber SD, Rieckmann T, Trueb B. Rapid fusion and syncytium formation of heterologous cells upon expression of the FGFRL1 receptor. J Biol Chem. 2010;285:37704–37715. doi: 10.1074/jbc.M110.140517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang L, Pandey AV, Villiger PM, Trueb B. Cell-cell fusion induced by the Ig3 domain of receptor FGFRL1 in CHO cells. Biochim Biophys Acta. 2015;1853(10 Pt A):2273–2285. doi: 10.1016/j.bbamcr.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Mandai K, Rikitake Y, Mori M, Takai Y. Nectins and nectin-like molecules in development and disease. Curr Top Dev Biol. 2015;112:197–231. doi: 10.1016/bs.ctdb.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Samanta D, Almo SC. Nectin family of cell-adhesion molecules: structural and molecular aspects of function and specificity. Cell Mol Life Sci. 2015;72:645–658. doi: 10.1007/s00018-014-1763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]