Abstract
Background
Little is known about structural brain abnormalities associated with methamphetamine (METH) abuse; therefore, we aimed: 1) to evaluate possible morphometric changes, especially in the striatum of recently abstinent METH-dependent subjects; 2) to evaluate whether morphometric changes are related to cognitive performance; and 3) to determine whether there are sex-by-METH interactions on morphometry.
Methods
Structural MRI was performed in 50 METH and 50 comparison subjects with the same age range and sex proportion; quantitative morphometric analyses were performed in the subcortical gray matter, cerebellum and corpus callosum. Neuropsychological tests were also performed in 44 METH and 28 comparison subjects.
Results
METH users showed enlarged putamen (left: + 10.3%, p = .0007; right: + 9.6%, p = .001) and globus pallidus (left: + 9.3%, p = .002; right: + 6.6%, p = .01). Female METH subjects additionally showed larger mid-posterior corpus callosum (+ 9.7%, p = .05). Although METH users had normal cognitive function, those with smaller striatal structures had poorer cognitive performance and greater cumulative METH usage.
Conclusions
Since METH subjects with larger striatal structures had relatively normal cognitive performance and lesser cumulative METH usage, the enlarged putamen and globus pallidus might represent a compensatory response to maintain function. Possible mechanisms for the striatal enlargement include glial activation and inflammatory changes associated with METH-induced injury.
Keywords: Methamphetamine, morphometry, putamen, globus pallidus, cognitive, MRI
Methamphetamine (METH) is an addictive stimulant drug that primarily affects the dopaminergic and serotonergic systems in the brain. The neurotoxic effects of METH have been demonstrated in numerous animal models. Similarly, potential METH-induced neurotoxicity in humans has been suggested by neuropsychological tests and several functional neuroimaging studies (Chang et al 2002; Ernst et al 2000; Paulus et al 2002; Volkow et al 2001a, 2001b, 2001c, 2001d), and further evaluated by postmortem analyses (Moszczynska et al 2004). However, little is known about the possible morphometric brain abnormalities in METH abusers. A recent study found that METH users had smaller hippocampal volumes and significant white matter hypertrophy (Thompson et al 2004); however, possible volume changes in the striatum, where the dopaminergic terminals and major site of action for METH are located, were not measured. Evaluation of the striatal structures (putamen, caudate and the globus pallidus) might yield insights into brain injury associated with methamphetamine.
PET studies of METH users found both lower dopamine (DA) D2 receptor (Volkow et al 2001a) and lower DA transporter densities (Volkow et al 2001c) in the striatum. Fluorodeoxyglucose (18FDG) PET further found decreased metabolism in the subcortical brain structures but increased metabolism in the parietal cortices (Volkow et al 2001b). This pattern of abnormal brain metabolism parallels the regional cerebral blood flow (rCBF) abnormalities observed in a perfusion MR study of abstinent METH-dependent subjects (Chang et al 2002). These neuroimaging abnormalities correlated with poorer cognitive performance; for example, decreased DA transporters correlated with slower motor speed and poorer verbal memory (Volkow et al 2001c) while rCBF abnormalities correlated with poorer performance and psychomotor speed on working memory tasks (Chang et al 2002). On proton MR spectroscopy (1H MRS), abstinent METH-dependent subjects showed decreased neuronal metabolite and elevated glial markers, especially in those with high levels of cumulative lifetime doses (Ernst et al 2000). Finally, a functional MRI study of METH users reported decreased prefrontal activation and elevated parietal activation during tasks that involved decision-making (Paulus et al 2002). Few of these studies, however, evaluated potential sex differences in the long-term effects of METH on the brain despite the fact that more than half of METH users are female (Substance Abuse and Mental Health Services Administration 2002).
The aims of this study were: 1) to evaluate possible morphometric changes on structural MRI in a group of recently abstinent METH-dependent subjects; 2) to evaluate whether morphometric changes might be related to cognitive performance; and 3) to determine whether there are sex-by-drug (METH) interactions on morphometry. Based on prior studies, we hypothesized that compared to nondrug-using healthy volunteers, abstinent METH-dependent subjects would have smaller striatal brain volumes, and that decreased volumes would be associated with poorer performance on cognitive tasks, especially those related to psychomotor speed and reaction times. Since preclinical studies have shown sex-differences on behavior and striatal injury in response to METH (Dluzen et al 2003), we also hypothesized that compared to women, METH-dependent men would suffer greater neurotoxic effects of METH, as measured by brain volume and cognitive performance.
Methods and Materials
Subjects
Fifty adult subjects with a history of METH-dependence (24 men, 26 women, 32.1 ± 7.1 years old, range 19–49 years) were studied. Subjects were evaluated and diagnosed using a structured clinical interview (SCID-I, Clinician Version) (First et al 1997) by a board-certified psychiatrist, and the cumulative amount of METH usage was estimated by detailed interviews that questioned the subjects regarding average amount of METH use/day, number of days used per week, and duration of use. Subjects were additionally screened with physical, neurological and psychiatric examinations, and laboratory screening blood and urine tests. They fulfilled the following inclusion criteria: 1) men or women of any ethnicity, 18–50 years of age; 2) DSM-IV criteria for history of METH dependence; average METH use .25 g /day, at least 4 days per week, for at least 2 years; abstinent from METH, or any other drug use, for more than one week; 3) negative urine toxicology screen (except for METH) and no current or history of other drug dependence (including cocaine, alcohol, opiates, inhalants, and barbiturates); 4) on no medications, except for oral contraceptives or vitamins; and 5) ability to give informed consent. All METH subjects also fulfilled these exclusion criteria: 1) co-morbid or history of chronic medical or psychiatric illnesses which might confound the analysis of the study (e.g., schizophrenia, major depression, multiple sclerosis, Parkinson’s disease, degenerative brain diseases, any brain infections or neoplasms); 2) any major structural brain abnormalities (e.g. strokes, vascular malformations); 3) severe hepatic or renal dysfunction; 4) head trauma with loss of consciousness for more than 30 min; 5) metallic or electronic implants (e.g., pacemaker, surgical clips, pumps, etc.); 6) inability to read English at 8th grade level; 7) seropositive for HIV-1; and 8) for women, pregnant or breast feeding.
Fifty comparison subjects with the same age range and sex proportion (31.7 ± 7.4 years old, range 19–48 years, 24 men, 26 women) were also studied. These subjects fulfilled the same inclusion and exclusion criteria as the METH users, but had no current or history of drug abuse or dependence (including cocaine, methamphetamine, alcohol, opiates, inhalants, and barbiturates). All subjects signed an informed consent approved by the Institutional Review Board at Harbor-UCLA Medical Center.
Structural MRI
All 100 subjects completed structural MR scans on a 1.5 T scanner (General Electric Signa, Milwaukee, Wisconsin). The MRI protocol included: 1) sagittal Tl weighted localizer (Echo Time/Relaxation Time = 11/500 msec, 4 mm slices, 1 mm gap, 24 cm field of view); 2) coronal fast double spin echo scan (Echo Time1/Echo Time2/Relaxation Time = 17/102/4000 msec, 5 mm slices, no gap, 24 cm field of view); and 3) axial fast inversion recovery scan (Echo Time/Inversion Time/Relaxation Time = 32/120/4000 msec, 3.5 mm slices, no gap, 24 cm field of view). The sagittal and coronal images were used to screen for possible silent infarcts or other unknown brain abnormalities. Because the inversion recovery sequence yielded contiguous brain slices and excellent contrast between white matter, gray matter and cerebrospinal fluid, it was used for morphometric analyses.
Image Analyses
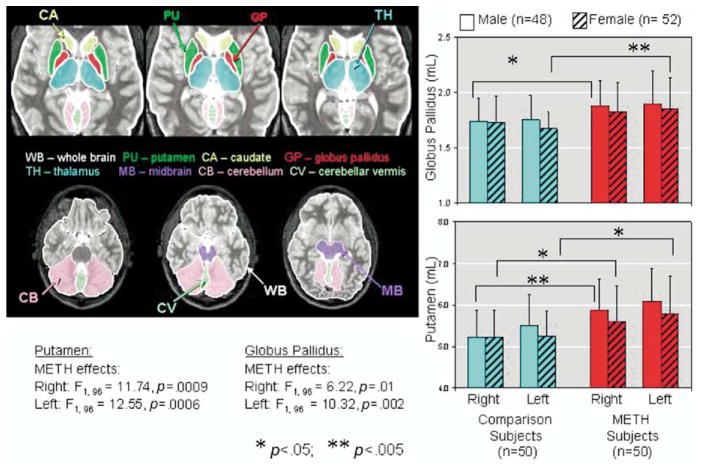
The images were analyzed using a customized program written in C language within the AVS (Advanced Visual Systems, Inc., Waltham, Massachusetts) system. The program allowed volumetric measurements within an outlined region of interest (ROI) and was calibrated (Itti et al 1997) with a Hoffman phantom, which contained multiple compartments that mimic the gray and white matter, and cerebrospinal fluid, within an axial slice of the human brain (Hoffman et al 1983). ROIs were drawn manually on each slice that showed the brain structure (Figure 1), and the volumes were summed automatically for each region. The ROIs were drawn by investigators blinded to the subjects’ status, and were highly reproducible with inter-rater reliability (intraclass r-values) ranged between .92 to .96 and intra-rater reliability (intraclass r-values) ranged between .96 to .98 for 10 different brain regions drawn by two raters 2 to 4 weeks apart.
Figure 1.
Left: Axial MRI slices showing brain regions measured in the current study. Right: Bar graphs showing larger lentiform nuclei (putamen and globus pallidus) in the METH users compared to non-drug users. MRI, magnetic resonance imaging; METH, methamphetamine.
Based on our a priori hypothesis, we specifically evaluated striatal brain regions (caudate, putamen and globus pallidus), where prior imaging studies showed decreased dopamine transporters, brain metabolism and perfusion in METH users (Chang et al 2002; Ernst et al 2000; Volkow et al 2001c). We additionally explored other brain regions that might be affected by METH, such as regions with relatively high density of dopamine receptors (cerebellar vermis) and serotonergic neurons (midbrain). We also evaluated the thalami, because they are important relay centers to other brain regions, and four segments of the corpus callosum (CC), which reflect the commissural fibers radiating to the cortices from the anterior to the posterior portions of the brain.
A semiautomatic program was used to segment the CC into four sectors (CC1-genu, CC2-anterior-middle, CC3-middle-posterior, CC4-splenium) (Kaufer et al 1997); see Figure 1. Briefly, segmentation was performed on the best mid-sagittal slice that showed a distinct border of the entire CC and the mammillary nucleus. A line was drawn across the inferior most points of the rostral and caudal portions of the CC, and a perpendicular line from the mammillary nucleus was automatically plotted to bisect the CC. The entire CC was then automatically divided into ten 18-degree radial sectors; CC1 and CC4 each contained 2 sectors while CC2 and CC3 each contained 3 sectors (Figure 2).
Figure 2.
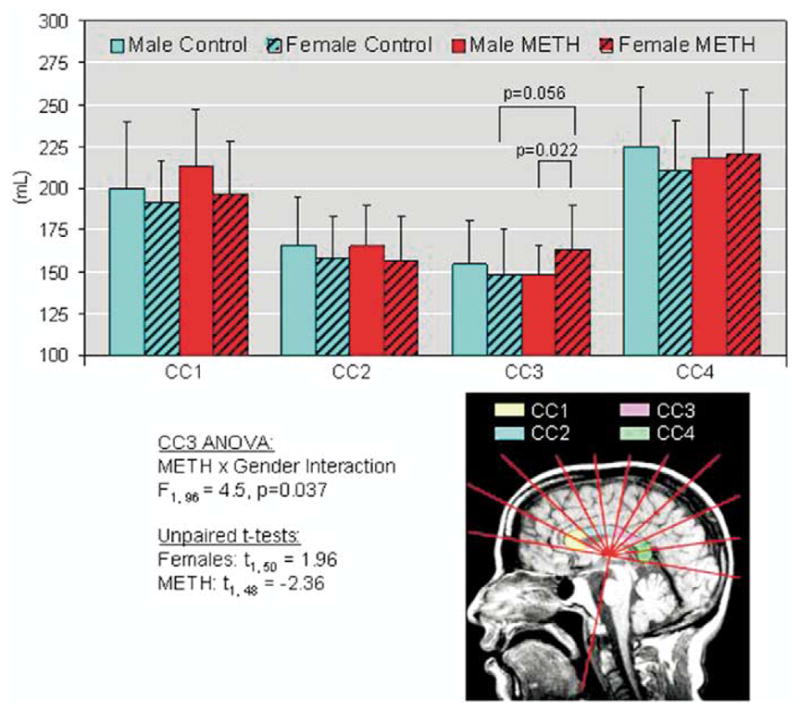
Left: Sagittal MRI showing the 4 sectors of the corpus callosum measured. Right: Bar graphs showing sex-by-region interaction in the CC3 portion of the corpus callosum. MRI, magnetic resonance imaging.
Neuropsychological Tests
A battery of neuropsychological tests designed to assess cognitive function most likely affected by injury to the striatum and frontal regions was performed in 44 of the abstinent METH-dependent subjects (31.9 ± 7.2 years old; 20 men, 23 women) and 28 comparison subjects (30.5 ± 8.2 years old, 13 males and 15 females). Although the METH subjects had lower education (12.5 ± 1.4 years) than the comparison subjects (14.3 ± 1.1 years), they were well matched by estimated verbal intelligence quotients (Table 1). The test battery included measures of gross motor functioning (Timed Gait), verbal memory (Rey Auditory Verbal Learning Test or AVLT) (Rey 1941), fine motor speed (Grooved Pegboard, testing dominant and nondominant hands) (KlØve 1963), executive function (Stroop Color Interference Tests) (Stroop 1935), estimate of verbal intelligence (New Adult Reading Test-Revised; NART) (Blair and Spreen 1989), mood (Center for Epidemiologic Studies—Depression Scale or CES-D) (Radloff 1977), and psychomotor speed [Trail Making Tests, part A and B (U.S. War Department 1944), Symbol Digit Modalities (Smith 1982), and the California Computerized Assessment Package (CalCAP) (Miller 1990)]. The CalCAP included tests for simple reaction time and psychomotor speed (choice reaction time, single digit recognition), as well as tests for working memory [1-back cued response (‘X’ only after ‘A’), sequential numbers with 1-increment, sequential numbers with 2-back], and visual discrimination and response inhibition (degraded words with distracters, response reversal/visual scanning, and form discrimination tasks). Lastly, 28 METH subjects and 22 comparison subjects additionally performed a verbal fluency test (generating words with letters FAS) and a visual construction and procedural memory test (Rey-Osterrieth complex figure test). All neuropsychological tests were performed within one month of the MRI studies.
Table 1.
Performance on Neuropsychological Tests
| Male Comparison Subjects | Female Comparison Subjects | Male METH Subjects | Female METH Subjects | METH Effect | Sex Effect | METH × Sex Interaction | |
|---|---|---|---|---|---|---|---|
| Age (years) | 30.9 ± 7.8 | 30.0 ± 9.2 | 33.1 ± 7.8 | 30.9 ± 6.4 | ns | ns | ns |
| Education (years) | 14.4 ± 1.0 | 14.2 ± 1.1 | 12.8 ± 1.5 | 12.3 ± 1.4 | F = 27.8; p < .0001 | ns | ns |
| Estimated Verbal IQ | 100.14 ± 11.29 | 102.67 ± 11.50 | 101.11 ± 12.08 | 100.18 ± 11.22 | ns | ns | ns |
| Motor and Psychomotor Tasks | |||||||
| Time gait (s) | 9.94 ± 1.45 | 10.15 ± 1.67 | 10.11 ± 1.51 | 10.41 ± 1.21 | ns | ns | ns |
| Pegboard (dominant) (s) | 62.0 ± 5.47 | 59.08 ± 8.10 | 68.10 ± 12.06 | 62.90 ± 15.22 | ns | ns | ns |
| Pegboard (non-dominant) (s) | 77.4 ± 19.07 | 66.92 ± 11.29 | 75.95 ± 13.20 | 76.8 ± 16.1 | ns | F = 6.1; p = .02 | ns |
| Trail making A (s) | 23.91 ± 3.94 | 22.83 ± 6.48 | 26.43 ± 6.13 | 24.96 ± 10.79 | ns | ns | ns |
| Trail making B (s) | 59.82 ± 19.51 | 54.67 ± 20.69 | 71.33 ± 22.69 | 55.04 ± 19.05 | ns | ns | ns |
| Symbol digit modalities | 55.18 ± 8.02 | 61.75 ± 10.45 | 48.33 ± 7.74 | 52.00 ± 8.02 | ns | ns | ns |
| Memory and Learning - Auditory Verbal Learning Test | |||||||
| Immediate recall (s) | 6.27 ± 1.90 | 7.17 ± 2.04 | 5.57 ± 1.08 | 6.91 ± 1.91 | ns | ns | ns |
| After 5 trials (s) | 12.73 ± 1.62 | 13.25 ± 1.96 | 11.38 ± 2.33 | 13.13 ± 2.03 | ns | ns | F =5.2; p =.03 |
| With interference (s) | 11.09 ± 3.05 | 11.75 ± 2.96 | 9.57 ± 2.77 | 11.65 ± 2.01 | ns | ns | F = 8.2; p = .006 |
| Delayed recall (s) | 11.27 ± 2.10 | 11.58 ± 2.07 | 9.29 ± 2.92 | 11.61 ± 2.25 | ns | ns | F = 4.9; p = .03 |
| Executive Function-Stroop Color Interference Tests and Verbal Fluency | |||||||
| Stroop color (s) | 60.82 ± 9.25 | 57.08 ± 10.55 | 63.60 ± 10.32 | 61.65 ± 12.07 | ns | ns | ns |
| Stroop words (s) | 46.82 ± 9.64 | 41.83 ± 6.71 | 48.19 ± 8.27 | 47.39 ± 9.81 | ns | ns | ns |
| Stroop interference (s) | 116.36 ± 23.01 | 101.83 ± 22.65 | 123.80 ± 25.92 | 118.44 ± 25.06 | ns | ns | ns |
| Verbal fluency (# correct) | 40.3 ± 11.98 | 37.4 ± 12.5 | 37.5 ± 7.7 | 39.5 ± 13.97 | F = 4.1; p = .05 | ns | ns |
| Visual Construction and Visual Memory - Rey-Osterrieth Complex Figure Test | |||||||
| Copy | 29.7 ± 5.06 | 30.8 ± 3.97 | 30.3 ± 2.73 | 30.1 ± 3.48 | ns | ns | ns |
| Immediate recall | 14.9 ± 8.58 | 16.4 ± 4.18 | 16.3 ± 4.9 | 17.8 ± 7.3 | ns | ns | ns |
| Delayed recall | 18.3 ± 4.54 | 17.2 ± 6.76 | 15.8 ± 5.66 | 17.1 ± 4.51 | ns | ns | ns |
None of the group differences for neuropsychological tests are significant after Bonferroni correction. Results are presented as mean ± standard deviation; analysis of covariance (ANCOVA): F (1, 63).
METH, methamphetamine.
Statistical Analyses
Statistical analyses were performed using StatView (SAS Institute Inc., Cary, North Carolina). Two-way analysis of variance (ANOVA) was used to analyze regional brain volumes (in mL), with METH-status and sex as the two factors. Regional brain volumes relative to the whole brain were calculated and shown in Table 2. A similar analysis of covariance (ANCOVA) model was used for neuropsychological measures, which additionally included the number of years of education as a covariate. ANOVA or ANCOVA main effects of METH-status, or interactions between METH status and sex, were considered statistically significant if a Bonferroni correction for multiple comparisons showed a type I error of p < .05 (corresponding to uncorrected p < .0021 for morphometry variables; p < .0014 for neuropsychiatric variables; and p < .0028 for CalCAP variables). Variables that met p < .05 without multiple-comparison correction were defined to indicate a trend for significance. The relationship between brain volumes that showed significant group differences (after Bonferroni correction) and cognitive performance (for variables with an uncorrected METH effect of p < .05) or METH usage was evaluated using linear regression analyses. METH usage variables that were not normally distributed were log transformed for the regression analyses.
Table 2.
Regional Brain Volumes
| Hemisphere or Region |
Male Comparison Subjects |
Female Comparison Subjects |
Male METH Subjects |
Female METH Subjects |
METH Effect | Sex Effect (M>F) |
METH × Sex Interaction |
|
|---|---|---|---|---|---|---|---|---|
| Whole Brain | Full Brain | 1357.32 ± 102.35 | 1191.77 ± 108.01 | 1365.30 ± 124.14 | 1207.87 ± 124.63 | ns |
F = 49.0 p < .0001 |
ns |
| Cerebellum | Right | 65.26 ± 8.69 (4.81 ± .54) | 58.55 ± 4.76 (4.93 ± .34) | 59.79 ± 8.01 (4.41 ± .65) | 58.73 ± 8.82 (4.87 ± .62) | ns |
F = 6.5 p = .01 |
ns |
| Left | 63.16 ± 8.32 (4.66 ± .55) | 56.95 ± 4.51 (4.79 ± .28) | 59.53 ± 8.63 (4.37 ± .59) | 57.98 ± 9.32 (4.81 ± .63) | ||||
| Vermis | 9.38 ± 1.39 (.69 ± .08) | 8.60 ± .93 (.72 ± .07) | 9.34 ± 1.04 (.69 ± .07) | 8.93 ± 1.47 (.74 ± .11) | ns |
F = 6.0 p = .02 |
ns | |
| Corpus Callosum | Genu (CC1) | 199.99 ± 39.90 (14.74 ± 2.81) | 191.23 ± 24.95 (16.21 ± 2.78) | 212.62 ± 34.68 (15.60 ± 2.29) | 196.37 ± 30.90 (16.30 ± 2.20) | ns | ns | ns |
| Anterior-Mid. (CC2) | 165.45 ± 29.21 (12.26 ± 2.37) | 157.80 ± 24.81 (13.35 ± 2.45) | 164.80 ± 25.16 (12.11 ± 1.74) | 156.61 ± 25.67 (13.08 ± 2.45) | ns | ns | ns | |
| Posterior-Mid. (CC3) | 154.21 ± 26.11 (11.37 ± 1.76) | 148.54 ± 26.79 (12.62 ± 2.77) | 147.80 ± 17.71 (10.92 ± 1.64) | 162.94 ± 26.33 (13.61 ± 2.44) | ns | ns |
F = 4.5 p = .037 |
|
| Splenium (CC4) | 225.11 ± 34.98 (16.67 ± 2.78) | 211.01 ± 29.49 (17.82 ± 2.83) | 218.01 ± 39.27 (16.01 ± 2.69) | 220.00 ± 38.39 (18.29 ± 3.05) | ns | ns | ns | |
| Thalamus | Right | 6.42 ± .61 (.47 ± .05) | 6.16 ± .41 (.52 ± .05) | 6.67 ± .61 (.49 ± .05) | 6.08 ± .65 (.51 ± .05) | ns |
F = 13.9 p = .0003 |
ns |
| Left | 6.67 ± .55 (.49 ± .05) | 6.30 ± .47 (.53 ± .05) | 6.93 ± .58 (.51 ± .05) | 6.45 ± .76 (.54 ± .06) | ||||
| Midbrain | Right | 3.79 ± .39 (.28 ± .03) | 3.47 ± .29 (.29 ± .03) | 3.65 ± .47 (.27 ± .03) | 3.68 ± .55 (.31 ± .05) | ns | ns | ns |
| Left | 3.77 ± .39 (.28 ± .03) | 3.49 ± .32 (.29 ± .03) | 3.64 ± .52 (.27 ± .05) | 3.57 ± .52 (.30 ± .05) | ||||
| Globus Pallidus | Right | 1.74 ± .21 (.13 ± .02) | 1.73 ± .23 (.15 ± .03) | 1.88 ± .23 (.14 ± .02) | 1.83 ± .26 (.15 ± .03) |
F = 10.5 1p = .0017a |
ns | ns |
| Left | 1.75 ± .22 (.13 ± .02) | 1.67 ± .15 (.14 ± .02) | 1.90 ± .30 (.14 ± .03) | 1.85 ± .28 (.015 ± .03) | ||||
| Putamen | Right | 5.23 ± .65 (.39 ± .06) | 5.22 ± .65 (.44 ± .06) | 5.87 ± .75 (.43 ± .06) | 5.60 ± .87 (.47 ± .08) |
F = 13.1 1p = .0005a |
ns | ns |
| Left | 5.52 ± .74 (.41 ± .04) | 5.24 ± .61 (.44 ± .06) | 6.08 ± .80 (.45 ± .06) | 5.78 ± .93 (.48 ± .09) | ||||
| Caudate | Right | 4.25 ± .51 (.31 ± .04) | 3.91 ± .45 (.33 ± .03) | 4.25 ± .48 (.31 ± .04) | 4.09 ± .50 (.34 ± .05) | ns |
F = 7.5 1p = .007 |
ns |
| Left | 4.50 ± .57 (.33 ± .04) | 4.14 ± .58 (.35 ± .04) | 4.53 ± .52 (.33 ± .05) | 4.30 ± .53 (.36 ± .05) |
Regional brain volumes in mL (and % of whole brain) and ANOVA F (dF = 1,96) and p values.
ANOVA, analysis of variance; METH, methamphetamine.
Significant after Bonferroni correction.
Results
METH Usage and Incomes
The 50 abstinent METH-dependent subjects had used METH 6.3 ± 1.3 days/week at 1.6 ± 1.6 g/day (median 1.0 g/day, range .25–10 g/day) for 110 ± 68 months, and had an average lifetime METH use of 4,519 ± 5,730 g (Log 3.41 ± .5, median 3,240 g, range 103–36,000 g). They were abstinent for 4.0 ± 6.2 months (median 2.0 months, range .25–36 months). The 43 METH subjects who also completed the neuropsychological tests had similar METH usage as a group: METH 6.2 ± 1.4 days/week at 1.5 ± 1.7 g/day (median 1.0 g/day) for 118 ± 68 months, and a lifetime METH use of mean 4,870 ± 6,101 g (Log 3.42 ± .5; median 3,394 g, range 103 g–36 kg); average duration of METH abstinence was 4.0 ± 6.5 months (median 2.0 months, range .25–36 months). While these subjects were using METH regularly, their income was $1,459 ± 1,759/month; however, during their abstinence and rehabilitation period, their income decreased to $703 ± 809/month.
Volumetric Analyses
Whole brain volumes on MRI were not different between METH and comparison subjects. However, as expected, whole brain and regional volumes had significant sex effects. Men had substantially greater whole brain volumes (Controls: + 13.9%, F1,48 = 30.83, p < .0001; METH: + 13.1 %, F1,48 = 19.99, p < .0001), and larger cerebellum (hemisphere and vermis), thalami, and caudate nuclei than women (Table 2). However, since these specific regions of interest were only slightly larger in men, they typically had lower % regional volumes relative to the whole brain than women (Table 2).
A significant METH effect was observed in the striatal brain regions after Bonferroni correction for multiple comparisons (Table 2, Figure 1). Specifically, the METH subjects showed larger averaged globus pallidus volumes (+ 9.6%; F1,96 = 10.5, p = .0017) and averaged putamen volumes (+ 9.9%; F1,96 =13.1, p = .0005). No significant sex effects or METH-by-sex interactions were observed in these two regions.
However, a trend for significant METH-by-sex interactions was observed in the posterior middle portion of the corpus callosum (CC3, F1,96 = 4.46, p = .037, Table 2). In particular, female METH users had larger CC3 (+ 9.7%, p = .05) than the nondrug using women.
Neuropsychological Test Performance (Tables 1 and 3)
Table 3.
Reaction Times (RT) on CalCAP Tasks (in Milliseconds)
| CalCAP Tasks | Male Comparison Subjects | Female Comparison Subjects | Male METH Subjects | Female METH Subjects | METH Effect | Sex Effect | METH × Sex Interaction |
|---|---|---|---|---|---|---|---|
| Simple RT | 300.54 ± 40.33 | 303.40 ± 57.45 | 327.65 ± 78.07 | 366.13 ± 109.48 | F = 5.1; p = .03 | ns | ns |
| Choice (‘7’) RT | 394.46 ± 39.44 | 392.00 ± 38.20 | 395.40 ± 52.41 | 410.17 ± 46.27 | ns | ns | ns |
| Cued (‘X’ only after ‘A’) RT | 378.00 ± 56.47 | 403.53 ± 144.81 | 420.50 ± 138.00 | 433.00 ± 84.12 | ns | ns | ns |
| Sequential RT (1-back) | 509.46 ± 94.26 | 471.40 ± 67.08 | 512.60 ± 98.08 | 583.74 ± 98.81 | F = 6.6; p = .01 | ns | F = 5.9; p = .02 |
| Sequential RT (1-increment) | 568.69 ± 109.05 | 581.13 ± 125.48 | 613.40 ± 124.08 | 669.52 ± 68.58 | F = 5.2; p = .03 | ns | ns |
| Sequential RT (2-back) | 757.39 ± 187.05 | 677.00 ± 144.18 | 815.20 ± 171.83 | 781.22 ± 131.64 | F = 4.5; p = .04 | ns | ns |
| RT for Degraded Words | 495.77 ± 50.45 | 499.53 ± 71.94 | 509.70 ± 78.38 | 556.96 ± 92.07 | ns | ns | ns |
| RT for Word Reversal | 615.62 ± 84.06 | 615.40 ± 97.32 | 644.60 ± 110.10 | 662.17 ± 98.57 | ns | ns | ns |
| RT for Form Discrimination | 729.92 ± 101.71 | 691.20 ± 92.02 | 739.45 ± 137.30 | 713.78 ± 98.51 | ns | ns | ns |
None of the group differences for neuropsychological tests are significant after Bonferroni correction. ANOVA F (df = 1,61) and p values.
ANOVA, analysis of variance; CalCAP, California Computerized Assessment Package; METH, methamphetamine.
Despite the difference in education, the four subject groups were well matched by age and on the NART estimated-verbal intelligence quotient (IQ). Using education as a co-variate in the ANCOVA, none of the neuropsychological tests showed significant group differences after Bonferroni correction. We observed only trends for METH effect for verbal fluency and sex effect on the Pegboard nondominant test (F1,63 = 6.1, p = .02), with the nondrug using women performing faster than the nondrug using men, but no sex-difference in the METH subject group. On the Auditory verbal learning test (AVLT), trends for METH-by-sex interaction were observed for recall after 5 trials (F1,63 = 5.2, p = .03), with interference (F1,63 = 8.2, p = .006) and after a delayed period (F1,63 = 4.9, p = .03, Table 1). The trends for these METH-by-sex interactions in the AVLT trials were due to the poorer performance by the male METH subjects than the female METH subjects, while no sex difference was observed in the comparison group. No significant sex-differences were observed on the CalCAP tests (Table 3).
On most CalCAP tasks, the METH subjects tended to perform slower than the comparison subjects, but none of these tests remained significant after Bonferroni correction (Table 3).
Correlations Between Brain Volumes, Cognitive Performance, and Drug Usage
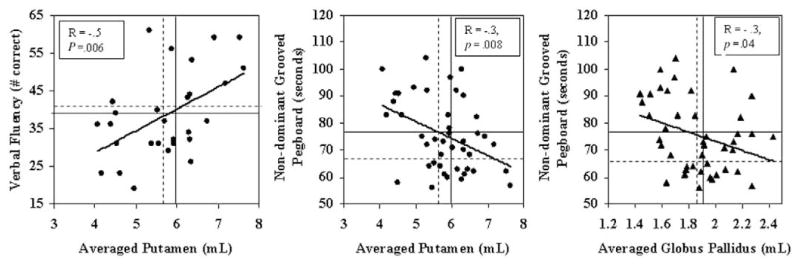
To minimize multiple correlations, averaged volumes of the left and right putamen or globus pallidus (the two regions that showed significant METH effect) were correlated only with cognitive tasks that showed trend group differences. In the METH users, smaller putamen volume was associated with poorer performance on verbal fluency (F1,27 = 8.82, r = .5, p = .006) and Grooved Pegboard (nondominant hand: F1,42 = 7.92, r = −.30, p = .008, Figure 3).
Figure 3.
Correlations between cognitive function and striatal structures in the METH users. Smaller putamen and globus pallidus, averaged between left and right, are associated with poorer performance. Solid lines in graph indicate mean values from male control subjects while dotted lines indicated mean values from female subjects. METH, methamphetamine.
Similarly, smaller globus pallidus volumes in METH users were related to slower performance on nondominant Grooved Pegboard (F1,42 = 4.49, r = −.31, p = .04, Figure 3).
Cumulative METH usage (log-transformed) correlated inversely with putamen (F1,48 = 3.89; r = −.27, p = .05) and globus pallidus volumes (F1,48 = 4.09, r = −.28, p = .05) (Figure 4). We also evaluated whether older subjects might have used the drug longer; however, no correlation between age and Log cumulative METH used was observed (F1,48 =1.57, r = .06, p = .7). No correlations were observed between other METH use parameters (recency of use, duration of use, or frequency of use per week) and striatal volumes.
Figure 4.
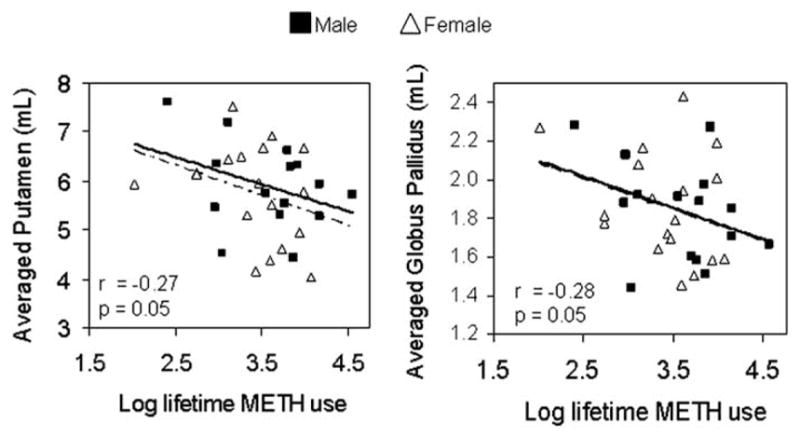
Correlations between cumulative METH usage and volumes of putamen and globus pallidus in METH users. Solid line indicates male METH users and dotted line indicates female METH users. Correlation values are for both male and female METH users. METH, methamphetamine.
Discussion
This study found enlarged striatal structures (globus pallidus and putamen), but normal cognitive performance, in both male and female METH users relative to comparison subjects. Since METH affects the dopaminergic terminals, which have the highest density in the putamen and globus pallidus, abnormalities in these brain regions are expected. Prior studies in abstinent METH-dependent subjects also found abnormalities in the striatal regions, including decreased dopamine transporters (Volkow et al 2001c), and decreased neuronal marker N-acetylaspartate (NAA) or NAA-to-creatine ratio on 1H MRS (Ernst et al 2000). Only one study evaluated brain morphometry in METH users, but surprisingly, the striatal regions were not examined; the authors found decreased hippocampal volume and increased frontal white matter instead (Thompson et al 2004). Contrary to our initial hypothesis, we found larger striatal volumes in the METH users as a group. However, those with the smallest striatal volumes had the greatest cumulative lifetime METH use and poorest cognitive performance; this finding is consistent with our second hypothesis that smaller striatal volume would be associated with poorer cognitive performance. Taken together, these findings suggest that striatal enlargement occur in METH users possibly as a compensatory response to repeated METH-induced striatal injury during early phases of drug dependence, but greater cumulative METH usage might lead to decreased striatal volumes and poorer cognitive performance.
The etiology for the enlargement of these striatal structures, especially in those with lesser METH usage, remains unclear. One potential mechanism for increased striatal volume is inflammation and reactive gliosis due to METH-induced striatal injury. This hypothesis is consistent with in vitro findings that METH might contribute to CNS inflammation by stimulating increased release and/or activation of matrix-degrading proteinases (Conant et al 2004), which allow influx of inflammatory cytokines and macrophages into the brain. Rodent and nonhuman primate studies also found METH-induced astroglial or microglial responses in addition to dopaminergic neuronal injury (Hebert and O’Callaghan 2000; Seiden and Sabol 1996); one study even found dose-dependent attenuation of METH-induced microglial activation by ketoprofen, a nonsteroidal anti-inflammatory drug (Asanuma et al 2003).
Another possibility for the enlarged lentiform nuclei might be related to glial-mediated neurotrophic effects associated with METH-induced injury to the striatum. In a rodent model of striatal injury, activated macrophage and microglia quickly accumulated and induced dopaminergic neuron sprouting at the wound site by expressing brain-derived neurotrophic factor and glial cell-line derived neurotrophic factor (Batchelor et al 1999). Prior MRS studies in a different group of abstinent METH users, however, found decreased neuronal marker NAA in the striatum, which suggested neuronal loss rather than increased neuronal content (Ernst et al 2000). Therefore, it is more likely that recurrent METH-induced injury leads to inflammatory changes described above and the associated increased striatal water content and volume.
With regards to our third aim, the only region that showed a trend for sex-by-METH interaction was the mid-posterior portion of the corpus callosum (CC3). Female METH users showed enlarged, while male METH users had slightly smaller, CC3 volumes relative to corresponding comparison subjects. Similar to the enlarged striatal structures, the enlarged CC3 in the female METH users suggests inflammation in this portion of the corpus callosum. Since the parietal fibers traverse the CC3 regions, future studies should evaluate whether hypertrophy also occurs in the parietal regions. It is interesting to note that prior imaging studies consistently found increased regional perfusion and glucose metabolism in the parietal brain regions of recently abstinent METH subjects (Chang et al 2002; Volkow et al 2001b), and the increased perfusion was more prominent in female METH users (Chang et al 2002).
These findings might represent a sex-specific neuroprotective response since induction of aromatase, the enzyme that produces estrogen de novo in astrocytes, is involved in the glial repair response to brain injury (Garcia-Segura et al 1999). Increased reactive astrocytes in female compared to male rodents after neurotoxic doses of METH were thought to be partly responsible for the lesser dopamine depletion and injury (Dluzen et al 2003). Therefore, female METH users may have a stronger glial response, hence more inflammatory changes, due to the neuroprotective effect of estrogen in specific brain regions.
METH induced dopaminergic neurotoxicity has been well documented in preclinical studies; however, persistent damage to the dopaminergic terminals in humans is less clear. Recent studies in nonhuman primates and humans demonstrated reversible changes in the dopaminergic terminal markers after prolonged abstinence from METH (Volkow et al 2001d). Cognitive tests in abstinent METH-dependent individuals have suggested a hypodopaminergic state, including slower psychomotor function and reaction times on computerized tasks (Chang et al 2002; Simon et al 2002). However, unlike other hypodopaminergic states, such as Parkinson’s disease, which are characterized by decreased putamen and globus pallidus volumes (O’Neill et al 2002), chronic METH users as a group showed enlarged basal ganglia. The inverse correlations between cumulative METH usage and striatal volume or cognitive performance further suggest that the enlarged striatal volumes might represent a compensatory response to the METH exposure.
Enlarged striatal structures have been observed in several other conditions. For example, cocaine-dependent subjects also showed enlarged putamen (Jacobsen et al 2001). Since METH and cocaine are both psychostimulant drugs that cause recurrent increases in extracellular dopamine levels, the mechanism for the enlarged lentiform nuclei in both METH and cocaine may be similar. Patients with schizophrenia also have enlarged striatum. However, numerous studies clarified that subcortical hypertrophy in schizophrenia occurred only after treatment with neuroleptics (Gur et al 1998), while those who are medication-naive had normal or reduced caudate volumes (Corson et al 1999; Gur et al 1998). One study even demonstrated reversal of the caudate enlargement after the patients were switched from typical neuroleptics to clozapine (Chakos et al 1995). Based on these studies, the mechanism for subcortical hypertrophy in schizophrenic patients is likely due to the tropic effect of typical neuroleptics on striatal D2 receptors. Only one study of neuroleptic-naive schizophrenic patients from an Indian village reported enlarged putamen (+11%), but these patients also had dyskinesia (McCreadie et al 2002), which suggests aberrant dopaminergic function. Larger thalamus and putamen were associated with more severe positive symptoms in one group of schizophrenic patients (Gur et al 1998), but a recent study found larger relative putamen in those with better outcome, nonKraepelinian subtype, than poor outcome patients or controls (Buchsbaum et al 2003). Therefore, consistent with our current observation in the METH users, larger putamen may represent a compensatory response to maintain function.
Another disorder that involves enlarged striatum is velo-cardio-facial syndrome (a genetic disorder with 22q11.2 deletion); approximately 30% of these patients also present with psychosis (Eliez et al 2002). The caudate head volumes in patients with velo-cardio-facial syndrome were increased independent of medication (Eliez et al 2002); however, the other striatal structures were not measured. Likewise, some METH users may develop persistent and recurrent symptoms of paranoid psychosis even after years of abstinence (Sato 1992), but the relationship between striatal volume and psychosis has not been evaluated in METH users. A speculative view of these observations is that enlarged striatal structures in schizophrenia, velo-cardio-facial syndrome, as well as METH or cocaine-dependence might partly reflect the neuropathological substrate of psychosis.
There are several limitations to the current study. First, we cannot rule out the possibility that enlarged striatum might be a premorbid phenotype prior to METH usage; future longitudinal studies may clarify whether enlarged striatal volumes are reversible with prolonged abstinence (although we did not observe a correlation with duration of abstinence in the current cohort). Second, despite the co-variation of years of education in the ANCOVA, our neuropsychological test results might be confounded by the different education levels between groups, and the fact that only a subgroup of the subjects had the cognitive testing. Third, the large number of variables may lead to type I errors; validation of these findings with another cohort is needed.
In summary, we observed enlarged putamen and globus pallidus in a group of abstinent METH-dependent subjects, especially those who had lesser cumulative METH usage and relatively normal cognitive function. These findings suggest a compensatory response after chronic METH exposure and might reflect inflammatory changes in these striatal regions. Furthermore, only female METH-users showed a trend for larger volumes in the mid-posterior callosum, which suggests that women may have stronger inflammatory responses than male METH users. Future studies that evaluate inflammatory markers in relation to these structures might help to clarify these relationships.
Acknowledgments
This work was supported in part by funds from National Institute of Health [K24-DA16170; K02-DA16991; R01-DA12734, T32 DA07316 and General Clinical Research Center MO1-RR00425].
We are grateful to the research subjects who participated in this study. We also thank Dr. M. Leonido-Yee and N. Trivedi for subject recruitment, Dolores Lyttle from the South Bay Human Services Center for subject referral, and the technical support from the Harbor-University of California-Los Angeles Imaging Center. ENM is the owner and author of the California Computerized Assessment Package (CalCAP).
References
- U.S. War Department. Army Individual Test Battery: Manual of directions and scoring. Washington, D.C: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- Asanuma M, Tsuji T, Miyazaki I, Miyoshi K, Ogawa N. Methamphetamine-induced neurotoxicity in mouse brain is attenuated by ketoprofen, a non-steroidal anti-inflammatory drug. Neuroscience Lett. 2003;352:13–16. doi: 10.1016/j.neulet.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Batchelor P, Liberatore G, Wong J, Porritt M, Frerichs F, Donnan G, Howells D. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair J, Spreen O. The New Adult Reading Test - Revised Manual. Victoria, Canada: University of Victoria; 1989. [Google Scholar]
- Buchsbaum M, Shihabuddin L, Brickman A, Miozzo R, Prikryl R, Shaw R, Davis K. Caudate and putamen volumes in good and poor outcome patients with schizophrenia. Schizophr Res. 2003;64:53–62. doi: 10.1016/s0920-9964(02)00526-1. [DOI] [PubMed] [Google Scholar]
- Chakos M, Lieberman J, Alvir J, Bilder R, Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet. 1995;345:456–457. doi: 10.1016/s0140-6736(95)90441-7. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller E. Perfusion MRI Abnormalities and Computerized Cognitive Deficits in Abstinent Methamphetamine Users. Psychiatry Res: Neuroimaging. 2002;114:65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Conant K, St Hillaire C, Anderson C, Galey D, Wang J, Nath A. Human immunodeficiency virus type 1 Tat and methamphetamine affect the release and activation of matrix-degrading proteinases. J Neurovirol. 2004;10:21–28. doi: 10.1080/13550280490261699. [DOI] [PubMed] [Google Scholar]
- Corson P, Nopoulos P, Andreasen N, Heckel D, Arndt S. Caudate size in first-episode neuroleptic-naive schizophrenic patients measured using an artificial neural network. Biol Psychiatry. 1999;46:712–720. doi: 10.1016/s0006-3223(99)00079-7. [DOI] [PubMed] [Google Scholar]
- Dluzen D, Tweed C, Anderson L, Laping N. Gender differences in methamphetamine-induced mRNA associated with neurodegeneration in the mouse nigrostriatal dopaminergic system. Neuroendocrinology. 2003;77:232–238. doi: 10.1159/000070278. [DOI] [PubMed] [Google Scholar]
- Eliez S, Barnea-Goraly N, Schmitt J, Liu Y, Reiss A. Increased basal ganglia volumes in velo-cardio-facial syndrome (deletion 22q11.2) Biol Psychiatry. 2002;52:68–70. doi: 10.1016/s0006-3223(02)01361-6. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, William J. Structured Clinical Interview For DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press, Inc; 1997. [Google Scholar]
- Garcia-Segura LM, Naftolin F, Hutchison JB, Azcoitia I, Chowen JA. Role of astroglia in estrogen regulation of synaptic plasticity and brain repair. J Neurobiol. 1999;40:574–584. [PubMed] [Google Scholar]
- Gur R, Maany V, Mozley P, Swanson C, Bilker W, Gur R. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry. 1998;155:1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- Hebert M, O’Callaghan J. Protein phosphorylation cascades associated with methamphetamine-induced glial activation. Ann NY Acad Sci. 2000;914:238–262. doi: 10.1111/j.1749-6632.2000.tb05200.x. [DOI] [PubMed] [Google Scholar]
- Hoffman E, Ricci A, van der Stee L, Phelps M. ECAT–Basic Design Considerations. IEEE Trans Nucl Sci. 1983;NS-30:729–733. [Google Scholar]
- Itti L, Chang L, Mangin JF, Darcourt J, Ernst T. Robust multimodality registration for brain mapping. Hum Brain Mapp. 1997;5:3–17. doi: 10.1002/(SICI)1097-0193(1997)5:1<3::AID-HBM2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Jacobsen L, Giedd JN, Gottschalk C, Kosten TR, Krystal JH. Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am J Psychiatry. 2001;158:486–489. doi: 10.1176/appi.ajp.158.3.486. [DOI] [PubMed] [Google Scholar]
- Kaufer D, Miller B, Itti L, Fairbanks L, Li J, Fishman J, et al. Midline cerebral morphometry distinguishes frontotemporal dementia and Alzheimer’s disease. Neurology. 1997;48:978–985. doi: 10.1212/wnl.48.4.978. [DOI] [PubMed] [Google Scholar]
- KlØve H. Clinical Neuropsychology. In: Forster F, editor. The Medical Clinics of North America. Vol. 46. New York: WB Saunders; 1963. pp. 110–125. [PubMed] [Google Scholar]
- McCreadie R, Thara R, Padmavati R, Srinivasan T, Jaipurkar S. Structural brain differences between never-treated patients with schizophrenia, with and without dyskinesia, and normal control subjects: a magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59:332–336. doi: 10.1001/archpsyc.59.4.332. [DOI] [PubMed] [Google Scholar]
- Miller EN. California Computerized Assessment Package, Custom ed. Los Angeles: Norland Software; 1990. [Google Scholar]
- Moszczynska A, Fitzmaurice P, Ang L, Kalasinsky KS, Schmunk GA, Peretti FJ, et al. Why is parkinsonism not a feature of human methamphetamine users? Brain. 2004;127:363–370. doi: 10.1093/brain/awh046. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Schuff N, Marks W, Jr, Feiwell R, Aminoff M, Weiner M. Quantitative 1H magnetic resonance spectroscopy and MRI of Parkinson’s disease. Mov Disord. 2002;17:917–927. doi: 10.1002/mds.10214. [DOI] [PubMed] [Google Scholar]
- Paulus M, Hozack N, Zauscher B, Frank L, Brown G, Braff D, Schuckit M. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;20:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Radloff LL. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Rey A. L’examen psycholoqiue dans les cas d’encephalopathie traumatique. Arch Psychol. 1941;28:286–340. [Google Scholar]
- Sato M. A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann NY Acad Sci. 1992;654:160–170. doi: 10.1111/j.1749-6632.1992.tb25965.x. [DOI] [PubMed] [Google Scholar]
- Seiden L, Sabol K. Methamphetamine and methylenedioxymethamphetamine neurotoxicity: possible mechanisms of cell destruction. NIDA Res Monograph. 1996;163:251–276. [PubMed] [Google Scholar]
- Simon S, Domier C, Sim T, Richardson K, Rawson R, Ling W. Cognitive performance of current methamphetamine and cocaine abusers. J Addict Dis. 2002;21:61–74. doi: 10.1300/j069v21n01_06. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test. Western Psychological Services; 1982. [Google Scholar]
- Stroop J. Studies of interference in serial verbal reaction. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- Substance Abuse and Mental Health Services Administration S. National Survey on Drug Use and Health. Rockville, MD: Office of Applied Studies; 2002. [Google Scholar]
- Thompson P, Hayashi K, Simon S, Geaga J, Hong M, Sui Y, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Chang L, Wang G, Fowler J, Ding Y, Sedler M, et al. Low level of brain dopamine d(2) receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow N, Chang L, Wang G, Fowler J, Franceschi D, Sedler M, et al. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry. 2001b;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Volkow N, Chang L, Wang G, Fowler J, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001c;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow N, Chang L, Wang G-J, Fowler J, Franceschi D, Sedler M, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001d;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]