Abstract
The effects of chronic marijuana (MJ) use on brain function remain controversial. Because MJ is often used by human immunodeficiency virus (HIV) patients, the aim of this study was to evaluate whether chronic MJ use and HIV infection are associated with interactive or additive effects on brain chemistry and cognitive function. We evaluated 96 subjects (30 seronegative nondrug users, 24 MJ users, 21 HIV without MJ use, 21 HIV + MJ) using proton magnetic resonance spectroscopy and a battery of neuropsychological tests. The two primarily abstinent MJ user groups showed no significant differences on calculated estimates of lifetime grams of delta9-tetrahydrocannabinol exposure, despite some differences in usage pattern. The two HIV groups also had similar HIV disease severity (CD4 cell count, plasma viral load, HIV dementia staging, Karnofsky score). On two-way analyses of covariance, HIV infection (independent of MJ) was associated with trends for reduced N-acetyl aspartate (NA) in the parietal white matter and increased choline compounds (CHO) in the basal ganglia. In contrast, MJ (independent of HIV) was associated with decreased basal ganglia NA (−5.5%, p = 0.05), CHO (−10.6%, p = 0.04), and glutamate (−9.5%, p = 0.05), with increased thalamic creatine (+6.1%, p = 0.05). HIV + MJ was associated with normalization of the reduced glutamate in frontal white matter (interaction p = 0.01). After correction for age, education, or mood differences, MJ users had no significant abnormalities on neuropsychological test performance, and HIV subjects only had slower reaction times. These findings suggest chronic MJ use may lead to decreased neuronal and glial metabolites, but may normalize the decreased glutamate in HIV patients.
Keywords: proton magnetic resonance spectroscopy, neuropsychological tests, HIV, marijuana, cognitive function
Introduction
Marijuana (MJ) is the most frequently abused illegal drug in the United States. Although marijuana’s potential for medicinal use is highly debated, over the past decade, several states have legalized medical marijuana use, based on recommendations that the adverse effects of marijuana use are not more problematic than those of other common medications (Watson et al. 2000). Several recent reviews on marijuana’s numerous effects on the neuroimmune system, particularly macrophages, highlighted variable effects depending on the methodologies (Cabral and Dove Pettit 1998; Klein et al. 1998; Klein et al. 2000). As a significant number of immunocompromised or human immunodeficiency virus (HIV) positive individuals are using marijuana for pain management, nausea associated with antiretroviral treatments or to increase appetite, it is imperative to evaluate whether marijuana use might influence HIV-associated brain injury. Neuroimaging techniques and neuropsychological testing can be applied to evaluate possible brain changes in these HIV patients.
Recent neuroimaging studies using positron emission tomography (PET) found that chronic marijuana users have decreased regional cerebral blood flow (rCBF) or glucose metabolism in the cerebellum, but increased rCBF and metabolism in the frontal and cerebellar regions after delta9-tetrahydrocannabinol (THC) administration or marijuana smoking (Volkow et al. 1996; O’Leary et al. 2002). Brain activation studies using PET or functional magnetic resonance imaging (MRI) found altered brain activation patterns during cognitive tasks (Eldreth et al. 2004; Bolla et al. 2005; Gruber and Yurgelun-Todd 2005). Similar techniques have been employed to evaluate HIV-associated brain injury; these studies found abnormalities in brain perfusion or metabolism, as well as altered brain activation (Rottenberg et al. 1996; Chang et al. 2000, 2004). Another MR technique, proton magnetic resonance spectroscopy (1H MRS), measures several cerebral metabolite concentrations in vivo, including the glial marker, myoinositol (MI), the neuronal marker, N-acetyl aspartate (NA), choline compounds (CHO), total creatine (CR), glutamate (GLU), and glutamine. 1H MRS is highly sensitive for detecting subclinical brain injury (i.e., alteration of these brain metabolites) associated with other drugs of abuse, including methamphetamine (Ernst et al. 2000), ecstasy (Chang et al. 1999b), and cocaine (Chang et al. 1999a; Ke et al. 2004). However, there are no data regarding how marijuana use affects these brain metabolites. In contrast, over 30 studies have used MRS to evaluate brain metabolite abnormalities in HIV patients. Elevated glial markers (choline and myoinositol), and decreased neuronal marker NA, have been shown to correlate with the severity of HIV cognitive motor complex (Chang et al. 1999c), and with poorer performance on neuropsychological measures (Chang et al. 2002; Ernst et al. 2003). These findings suggest that HIV-associated neuroinflammatory processes may be related to cognitive deficits in these patients. However, despite the known immunosuppressive effects of marijuana, no MRS study has been performed to evaluate whether marijuana use is associated with altered brain metabolism in HIV patients.
The neuropsychological deficits of psychomotor slowing and attention deficits in HIV patients are well documented (Grant et al. 1987; Miller et al. 1990; Heaton et al. 1995; Martin et al. 1995). In contrast, the effects of marijuana on cognitive function remain somewhat controversial. Most studies concur that deficits in attention and memory are present within the first 7 days of marijuana use (Pope and Yurgelun-Todd 1996; Solowij et al. 2002); however, some found normalization of these cognitive deficits after 28 days of abstinence (Pope et al. 2001), whereas others found persistent deficits (Bolla et al. 2002). A recent meta-analysis of 11 studies that included over 1,000 marijuana users found only mild cognitive deficits on learning and memory (Grant et al. 2003). Again, there are little data regarding possible interactive or additive effects of HIV and marijuana on cognition; only one study reported more cognitive deficits in symptomatic HIV patients with regular MJ use than those without MJ use (Cristiani et al. 2004). Therefore, using a well-validated 1H MRS technique and a detailed battery of neuropsychological tests, we performed a study to evaluate whether chronic marijuana use is associated with persistent brain chemistry and cognitive abnormalities in individuals with or without HIV infection.
Based on prior studies, we expected that HIV subjects would demonstrate metabolite abnormalities reflecting glial activation (elevated myoinositol, choline compounds, and creatine), whereas chronic marijuana users would show attenuated levels of these glial markers, and HIV subjects who used marijuana would show interactive effects on these glial metabolites. In addition, as both cannabinoids (Shen and Thayer 1999; Pistis et al. 2002; Tomasini et al. 2002) and HIV (Ferrarese et al. 2001; Chang et al. 2002) have been shown to affect the glutamatergic system, we also expected to observe interactive effects between HIV and chronic marijuana use on brain glutamate concentrations.
Materials and methods
Subject selection
Ninety-six subjects were studied: 42 HIV-seropositive subjects [21 with regular marijuana use (HIV + MJ) and 21 with little (less than 1 joint/month) or no marijuana exposure (HIV)] and 54 seronegative subjects [24 with a history of chronic MJ use (MJ) and 30 nondrug using controls (CON)] were assessed. HIV patients were recruited from local HIV clinics and the community. The seronegative subjects were recruited from the same local communities by advertisement and through word-of-mouth from subjects already enrolled in our studies. Prior to the study, each subject was verbally informed of the study protocol and signed a consent form approved by the Institutional Review Board at the Brookhaven National Laboratory. Each subject was evaluated by detailed medical and neuropsychiatric history and examination, screening blood tests, and urine toxicology screens. The screening blood tests included routine chemistry, routine blood count, thyroid panel, and HIV test if the serostatus was unknown. The most recent CD4 cell count and plasma viral load measurements of each HIV subjects were obtained or measured. Detailed marijuana usage and history of other drug use were also recorded. Karnofsky scale, HIV dementia scale, and AIDS dementia complex (ADC) stage were assessed in each HIV patient.
Based on the screening evaluation, male or female subjects of any ethnicity, age 18–75 years, who were able to provide consent, were willing to participate in the study, and met the following additional inclusion/exclusion criteria were enrolled. HIV patients (with or without MJ use) were enrolled based on these inclusion criteria: (1) HIV seropositive; (2) CD4 < 500/mm3; (3) either on no HIV antiretroviral medication or on a stable HIV antiretroviral drug regimen for at least 4 weeks; and (4) AIDS dementia complex stage ≤ 2. MJ subjects (with or without HIV) fulfilled the following criteria: (1) regular MJ use (>4 days/week) for at least 12 months; (2) negative urine toxicology screen for other drugs of abuse (e.g., cocaine, amphetamines, barbiturates, benzodiazepines, and opiates); and (3) for the MJ-only group: HIV seronegative and healthy, and on no medications (except vitamins and/or oral contraceptives). Criteria for CON subjects were: (1) healthy and on no medications (except vitamins and oral contraceptives); (2) no history of marijuana abuse; (3) negative urine toxicology screen for drugs of abuse (marijuana, cocaine, amphetamines, barbiturates, benzodiazepines, and opiates); (4) seronegative for HIV. Subjects who met any of the following criteria were excluded: 1) history of comorbid psychiatric illness, which may confound the analysis of the study (e.g., schizophrenia, bipolar disorder, major depression); (2) confounding neurological disorder (e.g., multiple sclerosis, degenerative brain diseases, any brain infections, neoplasms, or cerebral palsy); (3) abnormal screening laboratory tests that might affect MRS measures (e.g., significant renal or hepatic dysfunction); (4) current or history of other drug dependence (including, but not limited to, amphetamines, cocaine, alcohol, opiates, and barbiturates); (5) history of head trauma with loss of consciousness for more than 30 min; (6) contraindications for MR studies: metallic or electronic implants in the body (e.g., pacemaker, surgical clips, pumps, etc.), significant claustrophobia, pregnant or breastfeeding in female subjects; (7) less than an 8th-grade level English reading skills.
Proton magnetic resonance spectroscopy
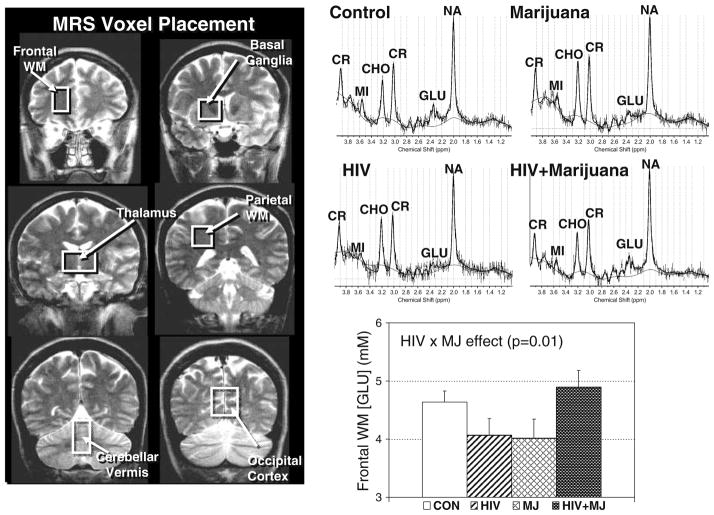
Subjects were studied with MRI and localized 1H MRS, using a Varian 4.0 Tesla (T) scanner equipped with an actively shielded Siemens Sonata whole-body gradient set. The imaging protocol consisted of three standard structural imaging sequences: a sagittal T1-weighted localizer (TE/TR = 11/500 ms, 5 mm slice thickness, 1 mm gap, 24 cm FOV, 192 phase encoding steps, 1.5 min scan time), followed by an axial 3D modified driven equilibrium Fourier transform (MDEFT) sequence (Lee et al. 1995) and a set of T2-weighted fast coronal images. Voxel locations for MRS were chosen based on prior studies that demonstrated the site of action for cannabinoids in rodents (Patel et al. 1998) and in nonhuman primates (Charalambous et al. 1991). Localized 1H MRS was performed in the thalamus and the right basal ganglia of all 96 subjects; in addition, measurements were performed in 1–3 more of the following brain regions: frontal white matter (n = 56), cerebellar vermis (n = 47), right parietal white matter (n = 49), and occipital gray matter (n = 45). Voxel locations are shown in Figure 1 and voxel sizes ranged between 3 and 5 mL depending on the subject’s anatomy and brain region. Data were acquired using a double spin echo sequence, point resolved spectroscopy (or PRESS), with TR/TE = 3,000/30 ms. Metabolite concentrations were determined using a method developed by one of the authors (Ernst et al. 1993; Kreis et al. 1993). Briefly, the T2 decay of the unsuppressed water signal from the PRESS experiment was measured at 10 different echo times, to calculate the metabolite concentrations corrected for the partial volume of cerebrospinal fluid. The data were processed using the LCModel program (Provencher 1993), which yielded molar metabolite concentrations of NA, CR, CHO, MI, and GLU. Typical interindividual variabilities in the metabolite concentrations were 10–15%. There were no differences between subject groups in spectral quality (as indicated by full-width at half maximum and signal-to-noise ratio measures).
Fig. 1.
Localized MRS was performed in six brain regions, indicated by white boxes on coronal T2-weighted MRI scans (left). The graphs at the top right show representative spectra from the frontal white matter of one subject from each group. Also, the glutamate concentration ([GLU]) in the frontal white matter region showed a significant interaction between HIV serostatus and marijuana use, in that HIV infection or marijuana use alone were associated with reduced [GLU], whereas seropositive MJ users had essentially normal [GLU]. WM: white matter; NA: N-acetyl peak; GLU: glutamate; CR: total creatine; CHO: choline-containing compounds; MI: myo-inositol; MJ: marijuana; CON: control group
Neuropsychological tests
A battery of neuropsychological tests designed to assess cognitive function most likely affected by injury to the striatum and frontal regions was performed. The test battery included measures of gross motor functioning (Timed Gait), verbal memory [Rey Auditory Verbal Learning Test (AVLT)] (Rey 1941), fine motor speed (Grooved Pegboard, testing dominant and non-dominant hands) (Kløve 1963), executive function (Stroop Color Interference Tests) (Stroop 1935), estimate of verbal intelligence [New Adult Reading Test-Revised (NART); Blair and Spreen 1989], mood (Center for Epidemiologic Studies—Depression Scale or CES-D; Radloff 1977), and psychomotor speed (Trail Making Tests, parts A and B; Anonymous 1944; Symbol Digit Modalities; Smith 1982; and the California Computerized Assessment Package, CalCAP; Miller 1990). The CalCAP included tests for simple reaction time and psychomotor speed (choice reaction time, single digit recognition), as well as tests for working memory [1-back cued response (“X” only after “A”), sequential numbers with 1-increment, sequential numbers with 2-back], and visual discrimination and response inhibition (degraded words with distracters, response reversal/visual scanning, and form discrimination tasks). All neuropsychological tests were performed in all subjects, except for one HIV subject who could not perform the Timed Gait, and two subjects (1 HIV and 1 CON) who were color-blind and could not perform the Stroop tasks. Neuropsychological tests were performed within 1 month of the MRI studies.
Statistical analyses
Statistical analyses were performed in StatView (SAS Institute Inc., Cary, NC, USA). A two-way analysis of variance (ANOVA) was performed for each metabolite, with marijuana and HIV serostatus as the two factors. For variables that showed significance (p < 0.05), or trends for significance (p < 0.10), on the ANOVA main effects (HIV or MJ status) or interactions between the two categories, post-hoc t-tests were also performed. A type I error probability of <0.05 was used to determine statistical significance. To minimize multiple correlations, only metabolites that had trends or significant effects were tested for correlations with MJ usage (frequency, duration, and last use) or with HIV disease severity (CD4 and viral loads), using simple regression analysis and Spearman correlations as needed.
Results
Clinical and demographic variables
Table 1 shows the demographic data and clinical variables related to HIV disease severity and MJ drug usage. Age was not significantly different among groups. The HIV subjects had lower education than the seronegative subjects (p = 0.0007), although the difference was only by 1–2 years of education. The two HIV-positive groups had similar HIV disease severity, as measured by CD4 count, plasma viral loads, Karnofsky scores, and AIDS dementia stage, but the HIV-positive groups had more depressive symptoms (CES-D) compared to the seronegative groups (p < 0.0001). Most of the HIV positive subjects were on combination therapies (17 of 21 HIV and 20 of 21 HIV + MJ). The types of antiretroviral medications used were similar between the two groups (nonnucleoside reverse transcriptase inhibitors were used by 94% HIV and 100% HIV + MJ; nucleoside reverse transcriptase inhibitors were used by 53% HIV and 45% HIV + MJ; and protease inhibitors were used only by 41% HIV and 45% HIV + MJ). Subjects not on medications were not outliers on any MRS or neuropsychological measures; therefore, no distinction between those off and on medications were made in further analysis.
Table 1.
Subject characteristics
| No history of chronic marijuana use
|
History of chronic marijuana use
|
Two-way ANOVA
|
|||||
|---|---|---|---|---|---|---|---|
| HIV-negative subjects (n = 30), mean ± SEM | HIV-positive subjects (n = 21) mean ± SEM | HIV-negative subjects (n = 24) mean ± SEM | HIV-positive subjects (n = 21) mean ± SEM | HIV effect | Marijuana effect | HIV × Marijuana effect | |
| Age (years) | 42.20 ± 2.20 | 44.19 ± 2.95 | 36.33 ± 2.30 | 42.67 ± 1.66 | n.s. | n.s. | n.s. |
| Education (years) | 14.33 ± 0.32 | 13.62 ± 0.46 | 15.00 ± 0.43 | 12.91 ± 0.41 | F1, 92 = 12.2, p = 0.0007 | n.s. | n.s. |
| Females | 6 (20%) | 4 (19%) | 4 (17%) | 3 (14%) | |||
| Antiretroviral medications | – | 17 (81%) | – | 20 (95%) | |||
| CD4 count/mm3 | – | 274.89 ± 38.20 | – | 343.93 ± 37.33 | NA | n.s. | NA |
| Plasma viral load (copies/mL) | – | 65,999 ± 47,073 | – | 28,087 ± 24,058 | NA | n.s. | NA |
| Logarithm of the viral load | – | 3.12 ± 0.29 | – | 3.04 ± 0.27 | NA | n.s. | NA |
| HIV Dementia Scale Score (range = 0–16) | – | 13.80 ± 0.68 | – | 14.39 ± 0.56 | NA | n.s. | NA |
| Karnofsky Performance Scale score (range = 0–100) | – | 86.11 ± 2.16 | – | 89.33 ± 2.06 | NA | n.s. | NA |
| AIDS Dementia Stage (range = 0–4) | – | 0.88 ± 0.14 | – | 0.69 ± 0.11 | NA | n.s. | NA |
| Center for Epidemiologic Studies—Depression Scale (range = 0–30) | 7.92 ± 1.19 | 18.29 ± 2.96 | 7.79 ± 1.51 | 15.24 ± 1.87 | F1, 87 = 21.6, p < 0.0001 | n.s. | n.s. |
| Age of first marijuana use | 20.73 ± 2.32 (13–35) | 18.22 ± 0.52 (16–21) | 17.25 ± 1.06 (13–35) | 17.71 ± 1.20 (8–33) | n.s. | n.s. | n.s. |
| Time since last use of marijuana (months) | 199.73 ± 29.34 (1–312) | 215.44 ± 57.87 (0–420) | 51.75 ± 16.73 (0–240) | 21.95 ± 9.43 (0–132) | n.s. | F1, 61 = 46.3, p < 0.0001 | n.s. |
| Total estimated lifetime of THC exposed (g)a | 0.10 ± 0.04 (0–0.75) | 0.29 ± 0.16 (0–2.6) | 164.00 ± 71.13 (3.6–1,680) | 230.06 ± 62.34 (3.6–1,666) | n.s. | F1, 92 = 18.9, p < 0.0001 | n.s. |
| Logarithm of estimated lifetime THC exposed (g) | −1.11 ± 0.26 | −0.74 ± 0.28 | 1.68 ± 0.15 | 1.95 ± 0.16 | n.s. | F1, 61 = 178, p < 0.0001 | n.s. |
| Frequency of use (days/month) | 0.41 ± 0.11 (0–2) | 0.52 ± 0.16 (0–3) | 19.46 ± 2.24 (1–30) | 12.52 ± 2.31 (1–30) | F1, 92 = 5.0, p = 0.03 | F1, 92 = 103.3, p < 0.0001 | F1, 92 = 5.3, p = 0.02 |
| Duration of use (months) | 16.0 ± 6.35 (0–144) | 25.43 ± 15.24 (0–300) | 175.29 ± 22.26 (36–448) | 302.00 ± 25.22 (36–624) | F1, 92 = 14.9, p = 0.0002 | F1, 92 = 152.4, p < 0.0001 | F1, 92 = 11.0, p = 0.001 |
One HIV + MJ subject was also using Marinol; this was not included in the THC exposure calculations.
n.s. = not significant; NA = not applicable.
MJ and other drug usage
The two chronic MJ groups were primarily abstinent from MJ use, except for 12 subjects in the MJ only group and 6 subjects in the HIV + MJ group, who remained regular users (>4 times/week). The two MJ groups had similar ages of first use; although the HIV + MJ subjects had a shorter average time since last use (21 months) than the MJ only group (51 months), the difference was not significant (p = 0.14; Table 1). Some of the subjects in the two groups without a history of chronic MJ use also used MJ recreationally (11 CON and 9 HIV); however, they tended to first use the drug at later ages and had significantly longer time since last use (p < 0.0001) compared to the two chronic MJ user groups. Although the HIV + MJ group used MJ fewer days per month (p = 0.04) than the seronegative MJ group, the estimated lifetime THC exposure did not differ significantly between groups (p = 0.32), because the HIV + MJ group had more years of use (p = 0.0005).
There is a trend for fewer CON subjects who smoked nicotine daily (n = 4, 13%) compared to the MJ subjects (n = 9, 42%) and the HIV-positive subjects (8 HIV, 38% and 11 HIV + MJ, 57%). Although subjects with history of other drug dependence, including alcohol, were excluded, four of the HIV + MJ subjects and two of the MJ subjects also admitted to past recreational usage of cocaine. Alcohol use was less than 7 drinks/week in all subjects, except for one CON, one MJ, one HIV, and two HIV + MJ subjects. All marijuana-using subjects indicated that marijuana was their primary drug of abuse and their drug of choice, and the other drugs used were minimal in comparison.
Group differences in brain metabolites
Based on ANOVA, HIV infection (independent of MJ) was associated with trends for reduced [NA] in the parietal white matter (−3%, p = 0.09) and increased [CHO] in the basal ganglia (7.4%, p = 0.1). In contrast, MJ (independent of HIV serostatus) was associated with decreased basal ganglia [NA] (−5.5%, p = 0.05), [CHO] (−10.6%, p = 0.04), and [GLU] (−9.5%, p = 0.05), with increased thalamic [CR] (+6.1%, p = 0.05), and trends for increased [MI] in occipital gray matter (+5.6%, p = 0.1) and in thalamus (+10.2%, p = 0.1). Two-way ANOVA also showed a significant interaction in the frontal white matter [GLU] (p = 0.01) (Fig. 1) and trends for interactions for cerebellar (p = 0.07) and basal ganglia [MI] (p = 0.08).
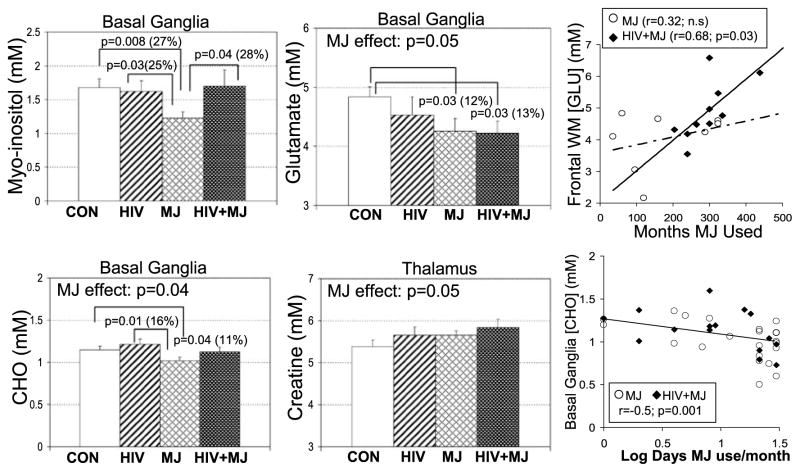
Post-hoc t-tests were conducted on those metabolite concentrations that showed significant differences, or trends toward significance, by two-way ANOVA. In the basal ganglia, HIV-seronegative MJ users had lower [MI] (−27%, p = 0.008), lower [CHO] (−11%, p = 0.04), and lower [GLU] (−12%, p = 0.03) compared to CON (Fig. 2). Because some MRS metabolites have been reported to show age effects (e.g., [CR], [CHO], and [MI]) (Chang et al. 1996; Soher et al. 1996) and MJ users were younger than the other groups, analysis of covariance (ANCOVA) was performed with age as a covariate for these metabolites. After covarying for age, the decreased basal ganglia [CHO] and decreased [MI] initially observed in MJ subjects were no longer significant. Also, in the basal ganglia, HIV subjects showed a nonsignificantly decreased [GLU] (−6%), whereas HIV + MJ showed significantly decreased [GLU] (−13%, p = 0.03) compared to CON (Fig. 2). In the thalamus, a trend for additive effects of HIV and MJ were observed on [CR], with the MJ subjects or HIV subjects showing slightly elevated [CR] (+5%, p = 0.1) and the HIV + MJ had the highest level of [CR] relative to CON (+9%, p = 0.06) (Fig. 2). All other post-hoc tests were nonsignificant.
Fig. 2.
Bar graphs of several metabolites that show significant effects of HIV infection and/or marijuana use (left and middle columns). The graphs on the right depict some of the significant correlations between brain metabolite levels and measures of marijuana use. GLU: glutamate; CHO: choline-containing compounds; MJ: marijuana; CON: control group
Neuropsychological test performance
The HIV subjects (both with and without MJ use) performed significantly poorer in all cognitive domains tested, whereas the MJ subjects (both with and without HIV) performed significantly better, or showed trends for better performance, in all cognitive domains except for the memory tests (Table 2). As the HIV subjects also had modest but significantly lower education and higher CES-D scores (which could influence performance and motivation) compared to the seronegative groups, the neuropsychological data were additionally analyzed using education and CES-D as covariates in an ANCOVA. After correcting for education and mood, HIV subjects were slower only on five of the seven reaction times on the CalCAP tasks (Table 2). As there was also a trend for the MJ users to be younger (with the MJ only group significantly younger than the other groups, p = 0.04), reanalyses using age as a covariate were performed; all tasks in which chronic MJ users performed faster or better were no longer significant. No interactive effects between HIV and MJ were observed for any of the neuropsychological tests.
Table 2.
Neuropsychological test performance
| Neuropsychological test | No chronic marijuana use
|
Chronic marijuana use
|
Two-way ANOVA
|
||||
|---|---|---|---|---|---|---|---|
| HIV-negative subjects (n = 30), mean ± SEM | HIV-positive subjects (n = 21), mean ± SEM | HIV-negative subjects (n = 24), mean ± SEM | HIV-positive subjects (n = 21), mean ± SEM | HIV effect | Marijuana effect | HIV × Marijuana interaction | |
| NART | 43.23 ± 1.77 | 35.67 ± 2.92 | 47.33 ± 1.71 | 40.24 ± 2.85 | p = 0.002 | (p = 0.06) | n.s. |
| Est VIQ | 112.88± 1.57 | 106.16 ± 2.60 | 116.50 ± 1.49 | 110.23 ± 2.54 | p = 0.002 | (p = 0.06) | n.s. |
| Gross motor | |||||||
| Timed Gait | 8.59 ± 0.20 | 10.32 ± 0.42 | 8.45 ± 0.26 | 9.45 ± 0.23 | p < 0.0001 | (p = 0.07) | n.s. |
| Fine motor | |||||||
| Pegboard Dominant hand | 65.67 ± 2.10 | 74.19 ± 3.19 | 62.75 ± 1.69 | 64.86 ± 2.66 | p = 0.03 | p = 0.01 | n.s. |
| Pegboard Nondominant | 72.47 ± 3.17 | 87.95 ± 5.92 | 71.67 ± 2.52 | 73.48 ± 2.60 | p = 0.02 | p = 0.04 | p = 0.07 |
| Psychomotor | |||||||
| Symbol Digit | 52.23 ± 1.07 | 45.67 ± 2.35 | 56.58 ± 2.28 | 47.75 ± 3.07 | p = 0.0006 | (p = 0.1) | n.s. |
| Trail Making A | 31.10 ± 2.03 | 34.57 ± 2.31 | 28.04 ± 1.98 | 30.43 ± 2.54 | n.s. | (p = 0.1) | n.s. |
| Trail Making B | 58.13 ± 3.20 | 81.10 ± 8.28 | 52.46 ± 2.92 | 68.19 ± 4.68 | p = 0.0001 | (p = 0.06) | n.s. |
| Memory | |||||||
| AVLT 1 | 6.60 ± 0.32 | 5.81 ± 0.41 | 6.92 ± 0.45 | 6.48 ± 0.40 | (p = 0.1) | n.s. | n.s. |
| AVLT 5 | 11.77 ± 0.53 | 10.71 ± 0.57 | 12.08 ± 0.50 | 11.76 ± 0.44 | n.s. | n.s. | n.s. |
| AVLT6 | 10.70 ± 0.54 | 8.91 ± 0.76 | 10.67 ± 0.54 | 9.33 ± 0.91 | p = 0.02 | n.s. | n.s. |
| AVLT7 | 10.30 ± 0.49 | 9.10 ± 0.77 | 10.46 ± 0.61 | 8.40 ± 0.94 | p = 0.02 | n.s. | n.s. |
| Executive | |||||||
| Stroop Color | 61.54 ± 2.19 | 71.25 ± 4.27 | 57.09 ± 2.15 | 62.48 ± 2.74 | p = 0.009 | p = 0.02 | n.s. |
| Stroop Word | 46.21 ± 1.53 | 49.29 ± 3.11 | 42.09 ± 1.16 | 47.38 ± 1.75 | p = 0.04 | (p = 0.1) | n.s. |
| Stroop Interference | 114.21± 4.55 | 133.65 ± 9.48 | 99.09 ± 3.82 | 124.71 ± 8.96 | p = 0.001 | (p = 0.08) | n.s. |
| CalCAP tasks—reaction time (RT) | |||||||
| Simple RT | 303.20± 13.59 | 349.62 ± 18.51 | 303.96 ± 18.27 | 354.29 ± 44.42 | p = 0.05** | n.s. | n.s. |
| Choice RT | 380.43± 6.02 | 413.05 ± 10.92 | 373.21 ± 5.72 | 416.33 ± 17.76 | p = 0.0004*** | n.s. | n.s. |
| Cued RT | 416.33± 17.29 | 423.10 ± 20.51 | 365.63 ± 9.59 | 408.43 ± 17.01 | p = 0.1** | p = 0.05 | n.s. |
| 1Back RT | 528.43± 16.09 | 600.10 ± 31.17 | 499.58 ± 15.59 | 543.81 ± 26.68 | p = 0.01** | (p = 0.06) | n.s. |
| 2Back RT | 733.00± 23.65 | 877.71 ± 32.71 | 643.08 ± 29.19 | 758.91 ± 58.03 | p = 0.0005 | p = 0.005 | n.s. |
| 1Incr RT | 590.87± 17.97 | 656.81 ± 25.03 | 553.63 ± 26.59 | 617.33 ± 25.12 | p = 0.007 | (p = 0.1) | n.s. |
| DegWord RT | 515.23± 12.63 | 578.38 ± 32.83 | 492.63 ± 11.54 | 584.95 ± 29.71 | p = 0.0006** | n.s. | n.s. |
| Form RT | 704.70± 25.55 | 824.91 ± 34.00 | 662.63 ± 19.82 | 813.67 ± 44.51 | p < 0.0001* | n.s. | n.s. |
| RevWord RT | 617.20± 15.35 | 695.86 ± 25.83 | 598.83 ± 17.83 | 697.76 ± 32.29 | p = 0.0002* | n.s. | n.s. |
p ≤ 0.05 after covarying for education and CES-D;
p ≤ 0.005 after covarying for education and CES-D.
p Values in parentheses indicate trends for significance.
Correlations between metabolite levels, cognitive function, drug usage, and HIV disease severity
Of the metabolite concentrations that showed significant group effects, frontal white matter [GLU] was positively correlated with duration of MJ use in the HIV + MJ group, but not in the MJ-only group (Fig. 2). The basal ganglia [CHO] was inversely correlated with frequency of marijuana usage (Fig. 2), and showed a similar trend for negative correlation with the log of the estimated lifetime THC exposure (r = −0.23, p = 0.09). Some HIV disease severity measures also showed a positive correlation with basal ganglia [CHO], including ADC stage (r = 0.39, p = 0.02; Spearman corrected) and Karnofsky (r = −0.40, p = 0.02; Spearman corrected), whereas CD4 (r = 0.32, p = 0.06) had a similar trend. These clinical variables did not correlate with any of the self-reported drug usage measures.
Subjects with lower basal ganglia [MI] tended to have poorer performance on two of the CalCAP tests (simple and choice RT: r = 0.2, p = 0.07 for both). The trend for correlation between simple RT test and basal ganglia [MI] was primarily attributable to a significant positive correlation in the nonmarijuana-using subjects (r = 0.36, p = 0.02).
The logarithm of the estimated lifetime marijuana usage positively correlated with performance on the same two CalCAP tests (simple RT: r = 0.27, p = 0.05; choice RT: r = 0.30; p = 0.04). Longer MJ use was associated with longer reaction times on choice RT (r = 0.53, p = 0.0002); this positive correlation was stronger in the MJ group (r = 0.53, p = 0.008) than in the HIV + MJ group (r = 0.37, p = 0.1). Because older subjects might have used MJ longer, we also evaluated whether age might be related to drug usage. Although there was no correlation between age and estimated lifetime THC exposure (r = 0.13, p = 0.4), age did correlate positively with months of use (r = 0.62, p < 0.0001), which could account for the longer reaction times in MJ users.
Discussion
This study demonstrates that marijuana and HIV have individual and combined effects on brain metabolites, but only HIV affects neuropsychological test measures. Because our HIV subjects were relatively neuroasymptomatic, their cognitive deficits were mild and were detected only on the reaction times of the more sensitive computerized tests. However, we observed several metabolite abnormalities (decreased GLU, CHO, and MI) in the basal ganglia and (elevated CR) in the thalamus of chronic marijuana users. Although HIV subjects with chronic MJ use also had decreased GLU in the basal ganglia and elevated CR in the thalamus, the glial markers MI and CHO were normal in the basal ganglia of HIV + MJ subjects.
Chronic marijuana use (independent of HIV serostatus) was associated with decreased basal ganglia [NA], [CHO], and [GLU], but increased thalamic [CR]. The MJ-only group also showed decreased [GLU]. Because the neuronal marker NA is typically decreased in various brain disorders, including neurotoxicity associated with cocaine (Chang et al. 1999a) and methamphetamine (Ernst et al. 2000), the decreased NA in our MJ users suggests neuronal loss or dysfunction in the basal ganglia. However, as elevated CHO and especially elevated MI are typically associated with conditions of glial activation, the decreased levels of these metabolites in both groups of MJ users suggest less glial activation. GLU is present primarily in the vesicles within neurons, and once released is immediately taken up again by astroglia, which converts GLU into glutamine (GLN) that is in turn transferred back to the neurons for conversion to GLU; this process is called the glutamate–glutamine shuttle. Therefore, the decreased GLU may result either from neuronal dysfunction (hence decreased conversion of GLN to GLU) or from glial dysfunction (decreased GLU reuptake), or both processes. The decreased [NA] and [GLU] in the basal ganglia suggest neuronal dysfunction, whereas decreased [MI] suggests decreased glial function, possibly related to the immunomodulatory effects of THC.
Possible neuronal injury and suppression of the neuroimmune response as a result of chronic marijuana abuse are supported by preclinical studies. The endogenous cannabinoid anandamide, or arachidonylethanolamide (AEA), is a lipid mediator that blocks proliferation and induces apoptosis in many cell types, including neurons (Maccarrone and Finazzi-Agro 2003). Because cannabinoid (CB)1 receptors have been shown to be neuroprotective by blocking the apoptotic effects of AEA, chronic marijuana use downregulates CB1 receptors, which might lead to decreased neuroprotection in response to oxidative stress associated with normal cellular metabolism. A recent study also showed that activation of CB1 receptors by THC may increase cell death induced by oxidative stress (Goncharov et al. 2005). However, an earlier study argued against the role of CB1 receptors in neuroprotection against oxidative stress (Marsicano et al. 2002). In our study, the brain regions that showed metabolite alterations, specifically basal ganglia and thalamus, correspond to those that were shown to have high THC uptake in a PET study on nonhuman primates (Charalambous et al. 1991), as well as regions that showed FOS immunoreactivity to AEA (Patel et al. 1998). Although the cerebellum is also rich in CB1 receptors and was affected by THC in prior studies, we did not observe metabolite abnormalities in the cerebellum, most likely a result of the smaller sample size studied.
Consistent with prior reports, our HIV subjects, regardless of MJ use status, had trends for reduced [NA] in the parietal white matter and increased [CHO] in the basal ganglia, but the effects were not significant (most likely because of the neuroasymptomatic status of the HIV subjects). However, these trends again support possible neuronal dysfunction (with decreased NA) and glial activation (with elevated CHO), as is often observed in HIV-associated brain injury, both from prior MRS studies (Chang et al. 1999c) and from neuropathological findings (Budka et al. 1987; Price et al. 1988; Adle-Biassette et al. 1999). Unlike prior MRS studies in HIV patients (Chang et al. 1999c, 2002), however, we did not observe elevated glial marker myoinositol in the frontal white matter of our HIV patients. This may be attributable to the smaller sample sizes in the HIV subgroups, the neuroasymptomatic status of these subjects, and the larger variability in myoinositol measurements at 4-T field strength, as each of the myoinositol multiplet peaks at 4 T has a lower signal than the larger MI pseudo-singlet at 1.5 T. Further evidence for neuronal loss or dysfunction is supported by the decreased [GLU] in the basal ganglia of the HIV subjects, because [GLU] is primarily stored in neuronal vesicles (Copper et al. 1996).
The combined effects of HIV infection and MJ were consistent with an additive model for some metabolites. Specifically, HIV + MJ demonstrated trends for additive effects of decreased basal ganglia [GLU] and increased thalamic [CR]. The decreased [GLU] suggests exacerbation of neuronal dysfunction in HIV subjects with chronic MJ use, whereas the additive effect on the increased thalamic [CR] suggests increased cellular metabolism. Because glial cells have higher concentrations of [CR] and higher metabolism (Brand et al. 1993), the elevated [CR] suggest glial activation in this brain region.
We did not observe significant abnormalities in HIV subjects or MJ users on the standard neuropsychological test measures, after taking into account group differences in age, education, or depression scores. The lack of group difference in HIV subjects is likely due to the relatively small sample size and the asymptomatic stages of the HIV subjects. Prior studies using larger samples of HIV patients have clearly demonstrated psychomotor slowing and impairment on tasks that involve attention and working memory, both prior to the availability of potent antiretroviral treatments (Grant et al. 1987; Miller et al. 1990; Heaton et al. 1995; Martin et al. 1995), and in those maintained on highly active antiretroviral therapy (Tozzi et al. 2005). Our MJ users performed faster on several motor and psychomotor tasks, but the differences were accounted for by their younger age. Therefore, our findings of relatively normal cognitive performance in our MJ users are consistent with those reported previously (Pope et al. 2001; Grant et al. 2003). We also did not observe additive or interactive effects on cognitive performance in our HIV + MJ users. This finding may be due to the relatively asymptomatic status of our HIV subjects, because a prior study reported increased cognitive deficits only in symptomatic HIV patients who use marijuana regularly compared to HIV patients who are not chronic marijuana users (Cristiani et al. 2004).
There are several limitations and considerations in the current study. (1) Subject groups in this study were not matched on all demographic measures (e.g., age, education, and depression scores); however, we reanalyzed group differences using these variables as covariates whenever necessary. (2) Because our chronic MJ users all smoked the drug, other ingredients in the smoke might have contributed to the brain metabolite abnormalities. Therefore, we could not confidently attribute the abnormal metabolites to THC, but only as an association to chronic MJ smoking. (3) The carefully estimated cumulative THC exposure may conceal transient changes associated with drug peak level effects or variable frequency of drug use during the subjects’ lifetime, which are not possible to assess in such a cross-sectional study of primarily abstinent subjects. (4) As we did not study these subjects prior to their usage of MJ, we cannot exclude the possibility that the brain metabolite abnormalities observed might be a premorbid condition or a phenotype for predisposition to drug use. (5) Lastly, despite a fairly substantial sample size for an MRS study, sample sizes in the subgroups were small to moderate in some of the brain regions measured (e.g., cerebellum and frontal white matter). Therefore, future studies using larger sample sizes and longitudinal follow-ups in the subgroups for measuring these brain regions are needed.
Despite these limitations, this study demonstrates abnormal brain chemistry in healthy chronic marijuana users, and some additive and interactive effects on brain chemistry in HIV patients who used marijuana chronically. Further research is warranted in this area to determine whether marijuana, or active ingredients of marijuana (e.g., cannabinol, cannabindiol), may have negative or beneficial influence on brain injury associated with HIV patients or in other inflammatory disorders.
Acknowledgments
This work was supported in part by funds from NIH [K24-DA16170, K02-DA16991, and T32 DA07316] and the Department of Energy (Office of Environmental Research). We are grateful to the research subjects who participated in this study. We also thank K. Warren and L. Zimmerman for subject recruitment, K. Leckova for subject evaluation, and S. Arnold, D. Tomasi, C. Lozar, and E. Caparelli for some of the MRS data acquisition.
References
- Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol. 1999;25:123–133. doi: 10.1046/j.1365-2990.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- Anonymous. Army individual test battery: manual of directions and scoring. War Department, Adjutant General’s Office; Washington, DC: 1994. [Google Scholar]
- Blair J, Spreen O. The new adult reading test—revised manual. University of Victoria; Victoria, Canada: 1989. [Google Scholar]
- Bolla K, Brown K, Eldreth D, Tate K, Cadet J. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;9:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bolla K, Eldreth D, Matochik J, Cadet J. Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Budka H, Costanzi G, Cristina S, Lechi A, Parravicini C, Trabattoni R, Vago L. Brain pathology induced by infection with the human immunodeficiency virus (HIV). A histological, immunocytochemical and electron microscopical study of 100 autopsy cases. Acta Neuropathol. 1987;75:185–198. doi: 10.1007/BF00687080. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Dove Pettit DA. Drugs and immunity: cannabinoids and their role in decreased resistance to infectious disease. J Neuroimmunol. 1998;83:116–123. doi: 10.1016/s0165-5728(97)00227-0. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Poland R, Jenden D. In vivo proton magnetic resonance spectroscopy of the normal human aging brain. Life Sci. 1996;58:2049–2056. doi: 10.1016/0024-3205(96)00197-x. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Strickland T, Mehringer CM. Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am J Psychiatry. 1999a;156:716–722. doi: 10.1176/ajp.156.5.716. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Grob CS, Poland RE. Cerebral 1H MRS abnormalities in 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) users. J Magn Reson Imaging. 1999b;10:521–526. doi: 10.1002/(sici)1522-2586(199910)10:4<521::aid-jmri4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E. Cerebral metabolite abnormalities correlate with clinical severity of HIV-cognitive motor complex. Neurology. 1999c;52:100–108. doi: 10.1212/wnl.52.1.100. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Leonido-Yee M, Speck O. Perfusion MRI detects rCBF abnormalities in early stages of HIV-cognitive motor complex. Neurology. 2000;54:389–396. doi: 10.1212/wnl.54.2.389. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt M, Ames N, Jocivich J, Speck O, Gaiefsky M, Walot I, Miller E. Relationships among cerebral metabolites, cognitive function and viral loads in antiretroviral-naïve HIV patients. NeuroImage. 2002;17:1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E, Ernst T. Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol. 2004;56:259–272. doi: 10.1002/ana.20190. [DOI] [PubMed] [Google Scholar]
- Charalambous A, Marciniak G, Shiue C, Dewey S, Schlyer D, Wolf A, Makriyannis A. PET studies in the primate brain and biodistribution in mice using (−)-5′-18F-delta 8-THC. Pharmacol Biochem Behav. 1991;40:503–507. doi: 10.1016/0091-3057(91)90354-5. [DOI] [PubMed] [Google Scholar]
- Copper J, Bloom F, Roth R. The Biochemical Basis of Neuropharmacology. 7. Oxford University Press, Inc; New York: 1996. [Google Scholar]
- Cristiani SA, Pukay-Martin ND, Bornstein RA. Marijuana use and cognitive function in HIV-infected people. J Neuropsychiatry Clin Neurosci. 2004;16:330–335. doi: 10.1176/jnp.16.3.330. [DOI] [PubMed] [Google Scholar]
- Eldreth D, Matochik J, Cadet J, Bolla K. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain. I. Compartments and water. J Magn Reson. 1993;B102:1–8. [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Arnold S. Increased glial markers predict increased working memory network activation in HIV patients. NeuroImage. 2003;19:1686–1693. doi: 10.1016/s1053-8119(03)00232-5. [DOI] [PubMed] [Google Scholar]
- Ferrarese C, Aliprandi A, Tremolizzo L, De Micheli A, Dolara A, Frattola L. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2001;57:671–675. doi: 10.1212/wnl.57.4.671. [DOI] [PubMed] [Google Scholar]
- Goncharov I, Weiner L, Vogel Z. Delta9-tetrahydrocan-nabinol increases C6 glioma cell death produced by oxidative stress. Neuroscience. 2005;134:567–574. doi: 10.1016/j.neuroscience.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Grant I, Atkinson J, Hesselink J. Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other immunodeficiency virus (HIV) infections. Ann Intern Med. 1987;107:828–836. doi: 10.7326/0003-4819-107-6-828. [DOI] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc. 2003;9:679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Heaton R, Grant I, Butters N, White D, Kirson D, Atkinson J, McCutchan J, Taylor M, Kelly M, Ellis R. The HNRC 500—neuropsychology of HIV infection at different stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Ke Y, Streeter C, Nassar L, Sarid-Segal O, Hennen J, Yurgelun-Todd D, Awad L, Rendall M, Gruber S, Nason A, Mudrick M, Blank S, Meyer A, Knapp C, Ciraulo D, Renshaw P. Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatry Res. 2004;130:283–293. doi: 10.1016/j.pscychresns.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Klein TW, Friedman H, Specter S. Marijuana, immunity and infection. J Neuroimmunol. 1998;83:102–115. doi: 10.1016/s0165-5728(97)00226-9. [DOI] [PubMed] [Google Scholar]
- Klein TW, Lane B, Newton CA, Friedman H. The cannabinoid system and cytokine network. Proc Soc Exp Biol Med. 2000;225:1–8. doi: 10.1177/153537020022500101. [DOI] [PubMed] [Google Scholar]
- Kløve H. Clinical neuropsychology. In: Forster F, editor. The Medical Clinics of North America. WB Saunders; New York: 1963. pp. 110–125. [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD. Absolute quantitation of water and metabolites in the human brain. II. Metabolite concentrations. J Magn Reson. 1993;B102:9–19. [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, Ugurbil K. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Finazzi-Agro A. The endocannabinoid system, anandamide and the regulation of mammalian cell apoptosis. Cell Death Differ. 2003;10:946–955. doi: 10.1038/sj.cdd.4401284. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Moosmann B, Hermann H, Lutz B, Behl C. Neuroprotective properties of cannabinoids against oxidative stress: role of the cannabinoid receptor CB1. J Neurochem. 2002;80:448–456. doi: 10.1046/j.0022-3042.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- Martin E, Pitrak D, Pursell K, Mullane K, Novak R. Delayed recognition memory span in HIV-1 infection. J Int Neuropsychol Soc. 1995;1:575–580. doi: 10.1017/s1355617700000710. [DOI] [PubMed] [Google Scholar]
- Miller EN. Custom Edition. Norland Software; Los Angeles: 1990. California Computerized Assessment Package. [Google Scholar]
- Miller EN, Selnes OA, McArthur JC, Satz P, Becker JT, Cohen BA, Sheridan K, Machado AM, Visscher B, Gorp WGVan. Neuropsychological performance in HIV-1 infected homosexual men: the Multicenter AIDS Cohort Study (MACS) Neurology. 1990;40:197–203. doi: 10.1212/wnl.40.2.197. [DOI] [PubMed] [Google Scholar]
- O’Leary D, Block R, Koeppel J, Flaum M, Schultz S, Andreasen N, Ponto L, Watkins G, Hurtig R, Hichwa R. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology. 2002;26:802–816. doi: 10.1016/S0893-133X(01)00425-0. [DOI] [PubMed] [Google Scholar]
- Patel N, Moldow R, Patel J, Wu G, Chang S. Arachidonylethanolamide (AEA) activation of FOS proto-oncogene protein immunoreactivity in the rat brain. Brain Res. 1998;797:225–233. doi: 10.1016/s0006-8993(98)00364-3. [DOI] [PubMed] [Google Scholar]
- Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, Devoto P. Delta(9)-tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Res. 2002;948:155–158. doi: 10.1016/s0006-8993(02)03055-x. [DOI] [PubMed] [Google Scholar]
- Pope HJ, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. J Am Med Assoc. 1996;275:521–527. [PubMed] [Google Scholar]
- Pope HJ, Gruber A, Hudson J, Huestis M, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Provencher S. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Radloff LL. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Rey A. L’examen psycholoqiue dans les cas d’encephalopathie traumatique. Arch Psychol. 1941;28:286–340. [Google Scholar]
- Rottenberg DA, Sidtis JJ, Strother SC, Schaper KA, Anderson JR, Nelson MJ, Price RW. Abnormal cerebral glucose metabolism in HIV-1 seropositive subjects with and without dementia. J Nucl Med. 1996;37:1133–1141. [PubMed] [Google Scholar]
- Shen M, Thayer SA. Delta9-tetrahydrocannabinol acts as a partial agonist to modulate glutamatergic synaptic transmission between rat hippocampal neurons in culture. Mol Pharmacol. 1999;55:8–13. doi: 10.1124/mol.55.1.8. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test. Western Psychological Services; 1982. [Google Scholar]
- Soher B, Hurd R, Sailasuta N, Barker P. Quantitation of automated single-voxel proton MRS using cerebral water as an internal reference. Magn Reson Med. 1996;36:335–339. doi: 10.1002/mrm.1910360302. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. J Am Med Assoc. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Stroop J. Studies of interference in serial verbal reaction. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- Tomasini MC, Ferraro L, Bebe BW, Tanganelli S, Cassano T, Cuomo V, Antonelli T. Delta(9)-tetrahydrocannabinol increases endogenous extracellular glutamate levels in primary cultures of rat cerebral cortex neurons: involvement of CB(1) receptors. J Neurosci Res. 2002;68:449–453. doi: 10.1002/jnr.10242. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Serraino D, Bellagamba R, Corpolongo A, Piselli P, Lorenzini P, Visco-Comandini U, Vlassi C, Quartuccio M, Giulianelli M, Noto P, Galgani S, Ippolito G, Antinori A, Narciso P. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res Hum Retrovir. 2005;21:706–713. doi: 10.1089/aid.2005.21.706. [DOI] [PubMed] [Google Scholar]
- Volkow N, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res. 1996;67:29–38. doi: 10.1016/0925-4927(96)02817-x. [DOI] [PubMed] [Google Scholar]
- Watson SJ, Benson JA, Jr, Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry. 2000;57:547–552. doi: 10.1001/archpsyc.57.6.547. [DOI] [PubMed] [Google Scholar]