Abstract
The significance of the cerebrospinal fluid (CSF) Apolipoprotein E (APOE) level and whether it might have differential effects on brain function due to the presence of APOE ε4 allele(s) in HIV-infected patients are unknown. However, APOE ε4 allele has been associated with greater incidence of HIV-associated dementia and accelerated progression of HIV infection. Here, we show further evidence for the role of APOE ε4 in promoting cognitive impairment. We measured the APOE levels in the CSF of HIV-infected individuals. HIV+ subjects showed lower CSF APOE proteins than SN controls (−19%, p=0.03). While SN subjects with or without ε4 allele showed no difference in CSF APOE levels, ε4+ HIV+ subjects had similar levels to the SN subjects but higher levels than ε4− HIV+ subjects (+34%, p=0.01). Furthermore, while HIV+ subjects with ε2 or ε3 allele(s) showed a positive relationship between their CSF APOE levels and cognitive performance on the speed of processing domain (r=+0.35, p=0.05), ε4+ HIV+ subjects, in contrast, exhibited a negative relationship such that those with higher levels of CSF APOE(4) performed worse on the HIV Dementia Scale (r=−0.61, p=0.02), had lower Global Cognitive Scores (r=−0.57, p=0.03), and had poorer performance on tests involving learning (ε4 allele × [APOE] interaction, p=0.01). Our findings also suggest that the relatively higher levels of CSF APOE in ε4+ HIV+ (having primarily APOE4 isoforms) may negatively impact the brain and lead to poorer cognitive outcomes, while those individuals without the ε4 allele (with primarily APOE2 or APOE3 isoforms) may show compensatory responses that lead to better cognitive performance.
Keywords: HIV dementia, HIV, Memory, Neuropsychological assessment, Cerebrospinal fluid: APOE
Introduction
Apolipoprotein E (APOE) ε4 is a well-established risk factor for Alzheimer's disease (AD; Corder et al. 1993; Saunders et al. 1993) and has been associated with poor cognitive outcomes in several other conditions that cause brain injury, such as postcardiac bypass surgery (Newman et al. 1995; Tardiff et al. 1997), traumatic brain injuries (Nicoll et al. 1995; Teasdale et al. 1997), and in younger individuals with multiple sclerosis (Shi et al. 2008). APOE gene polymorphism also plays a significant role in the progression of HIV disease (Burt et al. 2008). Specifically, HIV-infected individuals with the APOE ε4 allele may be more susceptible to neurological impairments, contributing to greater incidence of peripheral neuropathy and HIV-associated dementia (HAD; Corder et al. 1998; Cutler et al. 2004), and the latter may be present only in older but not younger individuals (Valcour et al. 2004). In a postmortem study, individuals with HAD and with the ε4 allele, but not those with the ε3 allele, exhibited dysregulated sterol and sphingolipid metabolism (Cutler et al. 2004) and had greater levels of lipid peroxidation, indicative of greater oxidative stress in their brains (Turchan-Cholewo et al. 2006).
The APOE gene has three alleles (ε2, ε3, and ε4), which encode for isoforms APOE2, APOE3, and APOE4, respectively. Each differs by one or two amino acid residues; hence, each has unique structural and functional properties. While APOE proteins are important in the clearance of amyloid, the APOE4 isoform, in particular, promotes amyloid aggregations that are commonly found in AD brains (Ma et al. 1994; Nicoll et al. 1995; Beffert and Poirier, 1996). However, APOE proteins also are involved in transporting and recycling cholesterols and lipids that are necessary for neuronal regenerations and repairs (Poirier et al. 1993). Although these roles are especially critical during ongoing cell damage following brain injury, APOE4 isoforms had been shown to have a dose-dependent neurotoxic effect, which is mediated partly by calcium toxicity via increased calcium influx through calcium channels on the plasma membrane (Veinbergs et al. 2002). Carriers of the ε4 allele, who express the APOE4 proteins, also may be at greater risk for the damaging effects (amyloidogenic potential) of this allele because their brains are exposed to much higher levels of the APOE4 proteins, as measured in the cerebrospinal fluid (CSF; Fukumoto et al. 2003; Wahrle and Holtzman, 2003). In the brain, APOE proteins that are secreted primarily by astrocytes are measurable in the CSF (Pitas et al. 1987). At present, how CSF APOE levels are affected and whether the different types of APOE isoforms might influence the cognitive function in HIV-infected individuals are unknown. To determine the types of APOE proteins that are expressed in the CSF of each individual, we needed to determine the APOE genotypes. Therefore, we evaluated both our seronegative controls and HIV+ subjects for the presence of APOE ε4 allele, levels of CSF APOE, and cognitive function on neuropsychological tests. Based on existing data reviewed above, we hypothesized that the presence of at least one copy of the APOE ε4 allele and hence the higher expression of APOE4 proteins in the CSF, will be associated with poorer cognitive performance in HIV+ subjects.
Subjects/materials and methods
Participants
Forty-eight HIV+ and 39 healthy seronegative (SN) control subjects with similar age and education were evaluated (Table 1). A majority of the study participants were males, 92% in the controls and 94% in the HIV-infected participants. Based on self-identification, the three largest ethnic groups were Whites (40%), Native or part-Native Hawaiians (28%), and Asians (13%). All subjects were recruited from the local communities in Honolulu, Hawaii between December 2004 and August 2006, and were enrolled in a longitudinal observational study of HIV-associated brain injury (Chang et al. 2008b; Ernst et al. 2009). Each subject provided a written informed consent approved by the Institutional Review Board at our institution prior to the study and was evaluated with a standardized neuropsychiatric assessment to ensure they fulfill study criteria for the longitudinal study. SN controls were included in the study if they were ≥18 years of age, negative for HIV by a blood test (ELISA), negative for urine toxicology, and had no history of drug dependence. HIV+ subjects were included if they were also ≥18 years of age, tested positive for HIV, had nadir CD4 count of <500 cells/mm3, and fulfilled other study criteria (Chang et al. 2008a,b). Since only 60% of the subjects enrolled in the longitudinal HIV study consented to the lumbar puncture and blood draw, APOE analyses in CSF and APOE genotyping in blood were conducted on all of these available banked specimens.
Table 1.
Subject characteristics (mean ± SE)
| Seronegative controls |
HIV+ subjects |
||||
|---|---|---|---|---|---|
|
n=39 |
n=48 |
||||
| ε4+ (n=11) | ε4− (n=28) | ε4+ (n=15) | ε4− (n=33) | p Value | |
| Age (years) | 47.2±3.4 | 47.0±2.5 | 48.7±3.7 | 45.8±1.6 | 0.88 |
| Education (years) | 15.5±0.7 | 15.0±0.4 | 14.1±0.6 | 14.5±0.5 | 0.43 |
| Males (%)/females (%) | 9(82)/2(18) | 27(96)/1(4) | 13 (87)/2(13) | 32(97)/1(3) | 0.16 |
| Ethnicity | |||||
| White | 9 | 9 | 7 | 10 | |
| Asian | 1 | 5 | 3 | 2 | |
| Black/African-American | 0 | 2 | 1 | 1 | |
| Native Hawaiian/Native American | 1/0 | 10/0 | 2/0 | 12/2 | |
| Pacific Islander | 0 | 2 | 2 | 2 | |
| Others (mix, Hispanic, not reported) | 0 | 0 | 0 | (1,1,2) | |
| APOE genotypes | |||||
| ε2/ε3 | 0 | 3 | 0 | 4 | |
| ε3/ε3 | 0 | 25 | 0 | 29 | |
| ε2/ε4 | 1 | 0 | 1 | 0 | |
| ε3/ε4 | 10 | 0 | 11 | 0 | |
| ε4/ε4 | 0 | 0 | 3 | 0 | |
| CD4 count (cells/mm3) | 411.2±66.3 | 487.4±43.8 | 0.34 | ||
| nadir CD4 (cells/mm3) | 165.8±49.2 | 197.9±27.4 | 0.54 | ||
| HIV duration (months) | 129.4±19.9 | 148.8±15.0 | 0.46 | ||
| HIV viral load (copies/mL) | 25,220±14,2189 | 15,524±8,876 | 0.55 | ||
| Log viral load | 2.5±0.4 | 2.6±0.2 | 0.85 | ||
| Karnofsky score (max. 100) | 89.3±2.5 | 92.8±1.6 | 0.24 | ||
p≤0.05 is significant
Cognitive assessments
Briefly, each participant underwent a battery of comprehensive neuropsychological tests, as described previously (Chang et al. 2008a, b). The tests assessed seven cognitive domains: fluency, executive function, learning, memory, speed of processing, attention, and motor function (Ernst et al. 2009). Cognitive domain scores were calculated based on age- and education-adjusted normative data of 273 SN healthy subjects and transformed into Z-scores (Paul et al. 2007; Chang et al. 2008b). The Global Cognitive Score (GCS) is presented as the average of the seven cognitive domain Z-scores. The positive Z-scores indicate better performance while negative Z-scores indicate worse performance than the mean performance (at 0 Z-score) of age- and education-adjusted norms. The cognitive status of these subjects was also evaluated by a neurologist or a psychiatrist with a structured neurological examination, including the Mini-Mental Status Examination (MMSE; Folstein et al. 1975), the HIV dementia scale (Power and Johnson, 1995), and the activity of daily living scale (Pfeffer et al. 1982). HIV dementia scale is based on a 16-point scale with scores ≤10 suggestive of HIV dementia (Power and Johnson, 1995). Using both the neuropsychological test findings and the clinical assessments, the HIV+ subjects also were determined whether they fulfilled the diagnostic criteria for HIV-associated neurocognitive disorder (HAND; Antinori et al. 2007).
APOE genotyping
Genomic DNA was first extracted from previously frozen whole blood samples that were stored in EDTA using Quick-gDNA™ MiniPrep (Zymo Research Corporation, Orange, CA, USA). Following DNA extraction, the polymorphic regions of the APOE gene were amplified using a PCR-based method (Zivelin et al. 1997). The PCR-amplified DNA products were then visualized on 2% agarose gels, from which the DNA bands were extracted, cleaned, and subsequently sequenced. APOE genotype was then determined based on the DNA sequencing results.
Assays
The concentration of APOE in previously frozen CSF samples were measured using AssayMax Human Apolipoprotein E ELISA Kit (Assaypro, St. Charles, MO, USA), based on a quantitative sandwich enzyme immunoassay technique. The antibody used for this assay recognizes all three APOE isoforms: APOE2, APOE3, and APOE4. APOE assays were conducted per manufacturer's protocol. We used diluted CSF samples that were within the linear range of the standard curve. A standard curve was generated for every assay run and triplicate measurements were conducted for each sample and standard solution.
Statistical analysis
Statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). Student's t test or analysis of variance was used for group comparisons. Pearson correlation was used to examine the relationship between CSF APOE concentration and cognitive scores. Multiple linear regression was used to examine interactions between APOE genotype, CSF APOE concentration and HIV serostatus. Data are presented as mean ± SE. P values ≤0.05 were considered statistically significant.
Results
Subject characteristics
Only 18 (nine ANI, nine MND) of the 48 HIV+ subjects had cognitive deficits that fulfilled the diagnostic criteria for HAND. There was no difference in the proportion of individuals with ε4+ genotypes between the SN controls (n=11 or 28%) and the HIV+ subjects (n=15 or 31%). Among the ε4− subjects, the majority were homozygous ε3/ε3 (89% of the ε4− SN subjects and 88% of the ε4− HIV+ subjects). In those with ε4 alleles, the majority had ε3/ε4 genotype (91% of the ε4+ SN subjects and 73% of the ε4+ HIV+ subjects). Only three subjects were homozygous ε4/ε4, and all were HIV+. Between ε4+ and ε4− HIV+ participants, there were no differences in their CD4 cell counts (p=0.34), nadir CD4 cell counts (p=0.54), duration of HIV diagnosis (p=0.46), scores on Karnofsky scale (p=0.24), HIV viral load (p=0.55), and log viral load (p=0.85; Table 1).
CSF APOE levels
HIV-1-infected individuals had significantly lower levels of CSF APOE compared to the SN controls (4.2±0.2 vs. 5.2±0.4 μg/mL, p=0.03; Fig. 1a). No difference in the CSF APOE levels was observed between ε4+ SN controls and ε4− SN controls (p=0.53; Fig. 1b). In contrast, among the HIV+ subjects, those with the ε4 alleles had higher CSF APOE levels than the non-ε4 carriers (5.1±0.5 vs. 3.8±0.3 μg/mL, p=0.01). Figure 1b further shows that ε4+ HIV+ subjects had CSF APOE levels comparable to the two control groups.
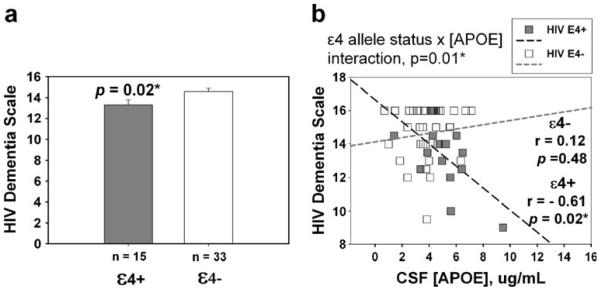
Fig. 1.
a CSF APOE levels in HIV+ individuals (n=48) and SN controls (n=39). b CSF APOE levels by HIV serostatus and ε4 allele
Cognitive function in relation to CSF APOE levels and APOE ε4 genotype
Figure 2a (left panel) demonstrates that ε4+ HIV-infected individuals had lower mean HIV Dementia Score than ε4− HIV-infected individuals (13.3±0.5 vs. 14.6±0.3, p=0.02). Also, an inverse correlation between HIV Dementia Scale Score and CSF APOE levels was found in ε4+ HIV+ subjects (r=−0.61, p=0.02), but not in ε4− HIV+ subjects (r=0.12, p=0.48; interaction, p=0.01, Fig. 2b, right panel).
Fig. 2.
Relationship between CSF APOE levels and cognitive performance. a HIV Dementia Scale in HIV+ subjects by ε4 allele. b Relationship between CSF APOE and HIV Dementia Scale by ε4 allele
In general, ε4+ HIV+ subjects performed poorer in all seven cognitive domains, reaching statistical significance in four of the domains, and hence had poorer GCS as well (Table 2). Three of these four cognitive domains (learning, memory, fluency), and the speed of information processing were affected by the ε4 genotype alone or in combination with the APOE protein levels and HIV status. Significant interaction between the ε4 allele and CSF APOE concentration was observed on the learning domain (p=0.01; Fig. 3a). Higher CSF APOE levels tended to be associated with greater learning deficits in all individuals harboring the ε4 allele, regardless of the subject's HIV status (ε4+ HIV+: r=−0.47, p=0.08; ε4+ SN controls: r=−0.51; p=0.11). Similar trends for higher levels of CSF APOE and greater memory deficits were also observed in both ε4+ HIV+ participants and ε4+ SN controls (ε4+ HIV+: r=−0.35, p=0.20; ε4+ SN control: r=−0.50; p=0.12; ε4 allele × CSF APOE interaction, p=0.09 trend). Additionally, trends for three-way interactions [ε4 allele×CSF APOE×HIV status] were observed in the fluency (p=0.08) and speed domains (p=0.09). While ε4+ HIV+ subjects with higher CSF APOE levels tended to perform worse on the fluency and speed domains (fluency: r=−0.44, p=0.10; speed: r=−0.41, p=0.13), ε4+ SN controls with higher CSF APOE levels tended to perform better on the fluency domain (r=0.55, p=0.08) but not on the speed domain (r=0.25, p=0.46). CSF APOE and the fluency domain did not correlate in individuals without the ε4 allele. However, higher CSF APOE levels was associated with better performance on the speed domain among HIV+ individuals without the ε4 allele (ε4− HIV+: r=+0.35, p=0.05). ε4− HIV+ subjects with higher CSF APOE levels also performed slightly better on the executive functioning test (r=+0.34, p=0.06). Lastly, an inverse correlation between CSF APOE level and GCS was observed in ε4+ HIV subjects (r=−0.57, p=0.03), but not in ε4− HIV subjects or control subjects (interactions, p=0.04; Fig. 3b).
Table 2.
Cognitive function (mean ± SE)
| Seronegative controls |
HIV+ subjects |
||||
|---|---|---|---|---|---|
|
n=39 |
n=48 |
||||
| ε4+ (n=11) | ε4− (n=28) | ε4+ (n=15) | ε4− (n=33) | p Value | |
| Learning | 0.45±0.17 | 0.27±0.10 | −0.37±0.25*, ** | 0.11±0.14 | 0.02 |
| Fluency | 0.54±0.17 | 0.26±0.09 | −0.21±0.18*, ** | 0.30±0.15 | 0.05 |
| Memory | 0.60±0.16 | 0.27±0.11 | −0.34±0.23*, ** | 0.10±0.15 | 0.01 |
| Attention | −0.15±0.23 | 0.05±0.14 | −0.70±0.17** | −0.37±0.14*** | 0.02 |
| Executive Function | 0.09±0.26 | 0.07±0.14 | −0.35±0.17 | 0.14±0.20 | 0.39 |
| Motor | 0.01±0.22 | −0.18±0.16 | −0.25±0.25 | −0.15±0.17 | 0.91 |
| Speed | 0.05±0.22 | −0.08±0.12 | −0.26±0.16 | −0.14±0.12 | 0.67 |
| Global cognitive score | 0.23±0.14 | 0.10±0.08 | −0.35±0.12*, ** | −0.002±0.11 | 0.02 |
p<0.05, for ε4+ HIV+ vs. ε4+ SN control
p<0.05, for ε4+ HIV+ vs. ε4− SN control
p<0.05 for ε4− HIV+ vs. ε4− SN control
Fig. 3.
Relationship between CSF APOE concentration and learning (a) and Global Cognitive Scores (b) by ε4 allele and HIV serostatus
Discussion
Here, we report for the first time APOE concentrations in the CSF of HIV-infected individuals. In this study, HIV-infected participants had lower CSF APOE levels than controls. However, our HIV-infected subjects carrying the ε2 and the ε3 alleles had even lower CSF APOE levels than HIV-infected subjects with the ε4 allele, suggesting that HIV and the APOE alleles may interact to yield differential expression of the various APOE proteins.
We also found that the CSF APOE levels of HIV-infected individuals with the ε4 allele remains high, comparable to normal levels. However, these ε4+ HIV+ subjects with relatively “normal” levels of CSF APOE protein showed poorer cognitive performance. Additionally, they also exhibited negative “dose-dependent” effects on cognition, such that HIV subjects with the ε4 allele(s) and higher CSF APOE levels had poorer performance on the HIV Dementia Scale and on the Global Cognitive Scores. In contrast, HIV+ individuals with the ε2 and/or ε3 alleles (ε4− HIV+ subjects) showed a significant positive “dose-dependent” effect only on the domain involving speed of processing and a weak effect on executive functioning. These findings suggest that higher levels of CSF APOE in HIV+ subjects with APOE ε4 allele could negatively impact the brain and lead to poorer cognitive outcomes, while those individuals without the ε4 allele may show compensatory responses that lead to better cognitive performance.
Similarities and differences with prior studies
Our measurements of CSF APOE levels, with a mean concentration of 5.2±0.4 μg/mL for SN controls are consistent with those previously reported by others (Landen et al. 1996; Lefranc et al. 1996; Song et al. 1997). Numerous studies had examined the role of CSF APOE levels in the neuropathogenesis of AD. To date, the findings on CSF APOE levels in AD remain inconclusive. Some studies found higher concentrations of CSF APOE in patients with AD (Lindh et al. 1997; Merched et al. 1997), while others found reduced levels of CSF APOE (Blennow et al. 1994; Landen et al. 1996; Pirttila et al. 1998) when compared to the levels in control subjects. Other groups even observed no significant difference in the CSF APOE levels between controls and AD patients (Lefranc et al. 1996; Hahne et al. 1997). The differences in these findings could be attributed to experimental variations (e.g., handling of CSF APOE samples) (Hesse et al. 2000) as well as heterogeneity of the AD patient populations (Song et al. 1997; Fukuyama et al. 2000). One study that evaluated CSF APOE in relation to cognitive function in AD patients (Fukuyama et al. 2000) found higher levels of CSF APOE in AD patients than age-matched controls, and the subjects with higher CSF APOE levels had lower scores on the MMSE. The patients studied included early-onset AD and late-onset AD, and this inverse relationship between the CSF APOE levels and MMSE was even more significant in the early onset AD group. Similar to the findings reported in this AD study (Fukuyama et al. 2000), we also observed worse cognitive performance with higher levels of CSF APOE, especially in our HIV subjects with the ε4 allele. In addition, we observed an inverse relationship on some but not all cognitive tasks among SN controls who carry the ε4 allele. Prior reports of healthy subjects similarly found that those with the ε4 allele may perform better on some cognitive tasks than those without the ε4 allele, especially at younger age (Alexander et al. 2007; Mondadori et al. 2007). Since our SN subjects are relatively young (average age <50 years), it is not surprising that some of our SN subjects have not started to show this inverse relationship. Our findings are also consistent with prior reports that found accelerated progression of HIV disease (Burt et al. 2008) and an increased risk for developing HAND in HIV patients with the ε4 allele (Corder et al. 1998; Spector et al. 2010). However, several studies did not find that APOE ε4 allele had increased risk on cognitive deficits in HIV subjects (Dunlop et al. 1997; Pomara et al. 2008; Joska et al. 2010). One of these studies that evaluated the response to an acute administration of lorazepam in small sample of non-demented HIV subjects even found that those with ε4 allele(s) had better memory performance than those without the ε4 allele (Pomara et al. 2008). Several factors including differences in age, population (i.e., race or ethnicity of subjects), HIV viral strain (e.g., clade C in the South African study (Joska et al. 2010)), and anti-HIV treatments are potential variables that may contribute to the discrepancies in these reports.
Possible biological bases of our findings
Lower CSF APOE levels in HIV+ individuals, especially in those without the ε4 alleles, than SN controls may be due to one of the following mechanisms. It may be due to the downregulation of the APOE gene. A recent report showed that HIV-1 downregulated Apoe gene expression and Nef transgenic mice also exhibited reduced expression of Apoe (Arora et al. 2009). Hence, the lower CSF APOE levels found in HIV+ individuals with the ε2 and ε3 alleles, but not in those with the ε4 alleles, might be due to downregulaton of the APOE gene by the HIV-1 virus, and further suggest that the virus may differentially regulate the expression of the APOE alleles. Alternatively, the lower CSF APOE levels in the ε4− HIV+ individuals may reflect more active recycling of the APOE proteins and cholesterol in the brain, which occurs during repair processes (Rellin et al. 2008).
However, the apparently normal levels of CSF APOE in ε4+ HIV+ subjects may also occur due to opposing mechanisms. First, the re-uptake and reutilization of the APOE4 isoforms may occur at a slower rate compared to those with the APOE2 and APOE3 isoforms. In fact, the presence of HIV-Tat disrupts LRP (LDLR-related protein) receptor function, dysregulating the binding, uptake, and degradation of APOE4, as well as clearance of other ligands like amyloid precursor protein, and β-amyloid protein from the extracellular space (Liu et al. 2000). Such disruption of the APOE uptake may likely contribute to higher levels of APOE4 circulating in the extracellular space and the CSF. However, if HIV-1 also downregulates the production of APOE, as that seen in the mouse study (Arora et al. 2009), the combination of decreased clearance (leading to increased levels) and downregulation (leading to decreased expression and synthesis) may have counteracting effects, leading to this apparently normal level of CSF APOE. Despite this normal level of APOE, however, continued exposure to the APOE4 isoforms may lead to neurotoxic effects (Veinbergs et al. 2002) and the subsequent neuroinflammatory responses (Vitek et al. 2009), which would lead to further brain injury and hence cognitive deficits.
In support of this hypothesis, ε4+ neuronal cultures were found to be more vulnerable to the toxic effects of Tat and gp120 than non-ε4+ cultures (Turchan-Cholewo et al. 2006), and postmortem brains of ε4 individuals with HIV dementia also showed higher levels of oxidative markers, cholesterol and sphingolipids, than HIV patients with the ε3 allele (Cutler et al. 2004; Haughey et al. 2004; Sacktor et al. 2004). Therefore, the greater oxidative stress among the ε4+ subjects also could contribute to brain injury and cognitive deficits (Turchan-Cholewo et al. 2006). Taken together, the APOE ε4 genotype, CSF APOE levels particularly the APOE4 isoform, and HIV-1 may interact and hasten the neurotoxicity in these individuals.
Overlapping indicators for both AD and HAND
Some of the markers in the neuropathogenesis of AD are now implicated in promoting HAND. For instance, low CSF Aβ42 has been identified as a reliable biomarker for amyloid deposition in the brains of AD patients (Fagan et al. 2005). Similarly, Aβ42 was found to be lower in the CSF of individuals with HAND (Brew et al. 2005; Clifford et al. 2009). Furthermore, postmortem studies showed that HIV-1-infected brains exhibited greater than normal amounts of β-amyloid deposition (Green et al. 2005; Rempel and Pulliam, 2005). However, another postmortem study did not find β-amyloid accumulation in the HIV-infected brains (Gelman and Schuenke, 2004) and a recent small 11C-PiB imaging study also found no evidence of fibrillar amyloid plaques in living patients with HIV infection (Ances et al. 2010). Since APOE is known to be crucial in the clearance of amyloids (Fagan et al. 2002) and that APOE4 binds more avidly than APOE3 to β amyloids, enhancing their aggregation and deposition into amyloid fibrils (Ma et al. 1994; Nicoll et al. 1995), it is reasonable to assume that similar pathways for β-amyloid deposition and the brain's repair processes may exist in both AD and HAND. We did not examine the levels of CSF APOE in relation to other CSF markers for AD (Aβ42, p-tau, t-tau) in our HIV+ subjects. It will be crucial to conduct a longitudinal study to determine how these CSF markers (APOE, Aβ42, p-tau, t-tau) might contribute to the progression of, or might serve as biomarkers for predicting HAND.
There are several limitations in the current study. First, although we had CSF samples from 87 individuals who consented to lumbar punctures, the sample size for each subgroup was much smaller after we classified the subjects by their HIV serostatus and by their APOE genotypes. Therefore, some of the findings are yielding only trends for significance (e.g., correlations between CSF APOE levels and some of the cognitive scores). Second, the ELISA assay does not distinguish between the three different isoforms; therefore, in the heterozygotes, we cannot determine what fraction of the total CSF APOE is due to each specific APOE isoform. An ELISA assay that recognizes only the APOE4 isoform would provide a better quantification of the APOE4 protein concentration found in the CSF, as a large percentage of the ε4+ groups in our study (73% HIV+ individuals and 91% SN controls) are ε3/ε4 heterozygotes. However, in ε3/ε4 heterozygotes, 60–70% of the CSF APOE consists of APOE4 isoforms (Fukumoto et al. 2003). Third, since the HIV+ subjects in our study had relatively mild cognitive deficits, including more HIV subjects with HAND may provide stronger effects and validation for these preliminary observations. Fourth, our study participants are relatively young (mean age <49 years), and are mostly males, given the prior data implicating further interactions between APOE ε4 genotype with age (Valcour et al. 2004; Alexander et al. 2007; Mondadori et al. 2007) and sex (Payami et al. 1996), including older individuals and females are warranted in future studies.
Acknowledgments
We thank our research participants and our community physicians (Dr. Drew Kovach, Dr. Cyril Goshima, Dr. Jennifer Frank, Dr. Arthur Johnson, Dr. Alan Tice, and others) who referred many of these participants to our research. We also thank Dr. Helenna Nakama, Dr. Michael Watters, Dr. Mary Ricardo-Dukelow, and our research coordinators for their participation in data collection.
This work was supported by research grants (2R01MH61427, 5R25MH080661, 2K24DA016170 and 1U54-NS056883) from the National Institutes of Health.
Footnotes
None of the authors has conflicts of interest regarding the work presented.
References
- Alexander DM, Williams LM, Gatt JM, Dobson-Stone C, Kuan SA, Todd EG, Schofield PR, Cooper NJ, Gordon E. The contribution of apolipoprotein E alleles on cognitive performance and dynamic neural activity over six decades. Biol Psychol. 2007;75:229–238. doi: 10.1016/j.biopsycho.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Ances BM, Christensen JJ, Teshome M, Taylor J, Xiong C, Aldea P, Fagan AM, Holtzman DM, Morris JC, Mintun MA, Clifford DB. Cognitively unimpaired HIV-positive subjects do not have increased 11C-PiB: a case–control study. Neurology. 2010;75:111–115. doi: 10.1212/WNL.0b013e3181e7b66e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S, Husain M, Kumar D, Patni H, Pathak S, Mehrotra D, Reddy VK, Reddy LR, Salhan D, Yadav A, Mathieson PW, Saleem MA, Chander PN, Singhal PC. Human immunodeficiency virus downregulates podocyte apoE expression. Am J Physiol Ren Physiol. 2009;297:F653–F661. doi: 10.1152/ajprenal.90668.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Poirier J. Apolipoprotein E, plaques, tangles and cholinergic dysfunction in Alzheimer's disease. Ann NY Acad Sci. 1996;777:166–174. doi: 10.1111/j.1749-6632.1996.tb34415.x. [DOI] [PubMed] [Google Scholar]
- Blennow K, Hesse C, Fredman P. Cerebrospinal fluid apolipoprotein E is reduced in Alzheimer's disease. NeuroReport. 1994;5:2534–2536. doi: 10.1097/00001756-199412000-00032. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65:1490–1492. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- Burt TD, Agan BK, Marconi VC, He W, Kulkarni H, Mold JE, Cavrois M, Huang Y, Mahley RW, Dolan MJ, McCune JM, Ahuja SK. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc Natl Acad Sci USA. 2008;105:8718–8723. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Nakama H, Stokes B, Ernst T. Antiretroviral treatment is associated with increased attentional load-dependent brain activation in HIV patients. J Neuroimmune Pharmacol. 2008a;3:95–104. doi: 10.1007/s11481-007-9092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Wong V, Nakama H, Watters M, Ramones D, Miller EN, Cloak C, Ernst T. Greater than age-related changes in brain diffusion of HIV patients after 1 year. J Neuroimmune Pharmacol. 2008b;3:265–274. doi: 10.1007/s11481-008-9120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB, Fagan AM, Holtzman DM, Morris JC, Teshome M, Shah AR, Kauwe JS. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73:1982–1987. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Corder EH, Robertson K, Lannfelt L, Bogdanovic N, Eggertsen G, Wilkins J, Hall C. HIV-infected subjects with the E4 allele for APOE have excess dementia and peripheral neuropathy. Nat Med. 1998;4:1182–1184. doi: 10.1038/2677. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Haughey NJ, Tammara A, McArthur JC, Nath A, Reid R, Vargas DL, Pardo CA, Mattson MP. Dysregulation of sphingolipid and sterol metabolism by ApoE4 in HIV dementia. Neurology. 2004;63:626–630. doi: 10.1212/01.wnl.0000134662.19883.06. [DOI] [PubMed] [Google Scholar]
- Dunlop O, Goplen AK, Liestol K, Myrvang B, Rootwelt H, Christophersen B, Kvittingen EA, Maehlen J. HIV dementia and apolipoprotein E. Acta Neurol Scand. 1997;95:315–318. doi: 10.1111/j.1600-0404.1997.tb00217.x. [DOI] [PubMed] [Google Scholar]
- Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, Ricardo-Dukelow ML, Chang L. Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann Neurol. 2009;65:316–325. doi: 10.1002/ana.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM. Human and murine ApoE markedly alters A beta metabolism before and after plaque formation in a mouse model of Alzheimer's disease. Neurobiol Dis. 2002;9:305–318. doi: 10.1006/nbdi.2002.0483. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Csernansky CA, Morris JC, Holtzman DM. The search for antecedent biomarkers of Alzheimer's disease. J Alzheimers Dis. 2005;8:347–358. doi: 10.3233/jad-2005-8404. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fukumoto H, Ingelsson M, Garevik N, Wahlund LO, Nukina N, Yaguchi Y, Shibata M, Hyman BT, Rebeck GW, Irizarry MC. APOE epsilon 3/epsilon 4 heterozygotes have an elevated proportion of apolipoprotein E4 in cerebrospinal fluid relative to plasma, independent of Alzheimer's disease diagnosis. Exp Neurol. 2003;183:249–253. doi: 10.1016/s0014-4886(03)00088-8. [DOI] [PubMed] [Google Scholar]
- Fukuyama R, Mizuno T, Mori S, Yanagisawa K, Nakajima K, Fushiki S. Age-dependent decline in the apolipoprotein E level in cerebrospinal fluid from control subjects and its increase in cerebrospinal fluid from patients with Alzheimer's disease. Eur Neurol. 2000;43:161–169. doi: 10.1159/000008157. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Schuenke K. Brain aging in acquired immunodeficiency syndrome: increased ubiquitin–protein conjugate is correlated with decreased synaptic protein but not amyloid plaque accumulation. J Neurovirol. 2004;10:98–108. doi: 10.1080/13550280490279816. [DOI] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- Hahne S, Nordstedt C, Ahlin A, Nyback H. Levels of cerebrospinal fluid apolipoprotein E in patients with Alzheimer's disease and healthy controls. Neurosci Lett. 1997;224:99–102. doi: 10.1016/s0304-3940(97)13477-2. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- Hesse C, Larsson H, Fredman P, Minthon L, Andreasen N, Davidsson P, Blennow K. Measurement of apolipoprotein E (apoE) in cerebrospinal fluid. Neurochem Res. 2000;25:511–517. doi: 10.1023/a:1007516210548. [DOI] [PubMed] [Google Scholar]
- Joska JA, Combrinck M, Valcour VG, Hoare J, Leisegang F, Mahne AC, Myer L, Stein DJ. Association between apolipoprotein E4 genotype and human immunodeficiency virus-associated dementia in younger adults starting antiretroviral therapy in South Africa. J Neurovirol. 2010;16:377–383. doi: 10.3109/13550284.2010.513365. [DOI] [PubMed] [Google Scholar]
- Landen M, Hesse C, Fredman P, Regland B, Wallin A, Blennow K. Apolipoprotein E in cerebrospinal fluid from patients with Alzheimer's disease and other forms of dementia is reduced but without any correlation to the apoE4 isoform. Dementia. 1996;7:273–278. doi: 10.1159/000106892. [DOI] [PubMed] [Google Scholar]
- Lefranc D, Vermersch P, Dallongeville J, Daems-Monpeurt C, Petit H, Delacourte A. Relevance of the quantification of apolipoprotein E in the cerebrospinal fluid in Alzheimer's disease. Neurosci Lett. 1996;212:91–94. doi: 10.1016/0304-3940(96)12774-9. [DOI] [PubMed] [Google Scholar]
- Lindh M, Blomberg M, Jensen M, Basun H, Lannfelt L, Engvall B, Scharnagel H, Marz W, Wahlund LO, Cowburn RF. Cerebrospinal fluid apolipoprotein E (apoE) levels in Alzheimer's disease patients are increased at follow up and show a correlation with levels of tau protein. Neurosci Lett. 1997;229:85–88. doi: 10.1016/s0304-3940(97)00429-1. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Ma J, Yee A, Brewer HB, Jr, Das S, Potter H. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994;372:92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- Merched A, Blain H, Visvikis S, Herbeth B, Jeandel C, Siest G. Cerebrospinal fluid apolipoprotein E level is increased in late-onset Alzheimer's disease. J Neurol Sci. 1997;145:33–39. doi: 10.1016/s0022-510x(96)00234-1. [DOI] [PubMed] [Google Scholar]
- Mondadori CR, de Quervain DJ, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, Boesiger P, Hock C, Nitsch RM, Papassotiropoulos A, Henke K. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cereb Cortex. 2007;17:1934–1947. doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- Newman MF, Croughwell ND, Blumenthal JA, Lowry E, White WD, Spillane W, Davis RD, Jr, Glower DD, Smith LR, Mahanna EP, et al. Predictors of cognitive decline after cardiac operation. Ann Thorac Surg. 1995;59:1326–1330. doi: 10.1016/0003-4975(95)00076-w. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Roberts GW, Graham DI. Apolipoprotein E epsilon 4 allele is associated with deposition of amyloid beta-protein following head injury. Nat Med. 1995;1:135–137. doi: 10.1038/nm0295-135. [DOI] [PubMed] [Google Scholar]
- Paul RH, Yiannoutsos CT, Miller EN, Chang L, Marra CM, Schifitto G, Ernst T, Singer E, Richards T, Jarvik GJ, Price R, Meyerhoff DJ, Kolson D, Ellis RJ, Gonzalez G, Lenkinski RE, Cohen RA, Navia BA. Proton MRS and neuropsychological correlates in AIDS dementia complex: evidence of subcortical specificity. J Neuropsychiatry Clin Neurosci. 2007;19:283–292. doi: 10.1176/jnp.2007.19.3.283. [DOI] [PubMed] [Google Scholar]
- Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, Yu CE, Wijsman EM, Heston LL, Litt M, Schellenberg GD. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet. 1996;58:803–811. [PMC free article] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Pirttila T, Koivisto K, Mehta PD, Reinikainen K, Kim KS, Kilkku O, Heinonen E, Soininen H, Riekkinen P, Sr, Wisniewski HM. Longitudinal study of cerebrospinal fluid amyloid proteins and apolipoprotein E in patients with probable Alzheimer's disease. Neurosci Lett. 1998;249:21–24. doi: 10.1016/s0304-3940(98)00381-4. [DOI] [PubMed] [Google Scholar]
- Pitas RE, Boyles JK, Lee SH, Hui D, Weisgraber KH. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B, E(LDL) receptors in the brain. J Biol Chem. 1987;262:14352–14360. [PubMed] [Google Scholar]
- Poirier J, Baccichet A, Dea D, Gauthier S. Cholesterol synthesis and lipoprotein reuptake during synaptic remodelling in hippocampus in adult rats. Neuroscience. 1993;55:81–90. doi: 10.1016/0306-4522(93)90456-p. [DOI] [PubMed] [Google Scholar]
- Pomara N, Belzer KD, Silva R, Cooper TB, Sidtis JJ. The apolipoprotein E varepsilon4 allele and memory performance in HIV-1 seropositive subjects: differences at baseline but not after acute oral lorazepam challenge. Psychopharmacol Berl. 2008;201:125–135. doi: 10.1007/s00213-008-1253-1. [DOI] [PubMed] [Google Scholar]
- Power C, Johnson RT. HIV-1 associated dementia: clinical features and pathogenesis. Can J Neurol Sci. 1995;22:92–100. doi: 10.1017/s0317167100040154. [DOI] [PubMed] [Google Scholar]
- Rellin L, Heeren J, Beisiegel U. Recycling of apolipoprotein E is not associated with cholesterol efflux in neuronal cells. Biochim Biophys Acta. 2008;1781:232–238. doi: 10.1016/j.bbalip.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005;19:127–135. doi: 10.1097/00002030-200501280-00004. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Haughey N, Cutler R, Tamara A, Turchan J, Pardo C, Vargas D, Nath A. Novel markers of oxidative stress in actively progressive HIV dementia. J Neuroimmunol. 2004;157:176–184. doi: 10.1016/j.jneuroim.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao CB, Vollmer TL, Tyry TM, Kuniyoshi SM. APOE epsilon 4 allele is associated with cognitive impairment in patients with multiple sclerosis. Neurology. 2008;70:185–190. doi: 10.1212/01.wnl.0000264004.62612.44. [DOI] [PubMed] [Google Scholar]
- Song H, Saito K, Seishima M, Noma A, Urakami K, Nakashima K. Cerebrospinal fluid apo E and apo A-I concentrations in early- and late-onset Alzheimer's disease. Neurosci Lett. 1997;231:175–178. doi: 10.1016/s0304-3940(97)00558-2. [DOI] [PubMed] [Google Scholar]
- Spector SA, Singh KK, Gupta S, Cystique LA, Jin H, Letendre S, Schrier R, Wu Z, Hong KX, Yu X, Shi C, Heaton RK. APOE epsilon4 and MBL-2 O/O genotypes are associated with neurocognitive impairment in HIV-infected plasma donors. AIDS. 2010;24:1471–1479. doi: 10.1097/QAD.0b013e328339e25c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardiff BE, Newman MF, Saunders AM, Strittmatter WJ, Blumenthal JA, White WD, Croughwell ND, Davis RD, Jr, Roses AD, Reves JG. Preliminary report of a genetic basis for cognitive decline after cardiac operations. The Neurologic Outcome Research Group of the Duke Heart Center. Ann Thorac Surg. 1997;64:715–720. doi: 10.1016/s0003-4975(97)00757-1. [DOI] [PubMed] [Google Scholar]
- Teasdale GM, Nicoll JA, Murray G, Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet. 1997;350:1069–1071. doi: 10.1016/S0140-6736(97)04318-3. [DOI] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Liu Y, Gartner S, Reid R, Jie C, Peng X, Chen KC, Chauhan A, Haughey N, Cutler R, Mattson MP, Pardo C, Conant K, Sacktor N, McArthur JC, Hauser KF, Gairola C, Nath A. Increased vulnerability of ApoE4 neurons to HIV proteins and opiates: protection by diosgenin and l-deprenyl. Neurobiol Dis. 2006;23:109–119. doi: 10.1016/j.nbd.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes OA, Grove J, Liu Y, Abdul-Majid KB, Gartner S, Sacktor N. Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii aging with HIV Cohort. J Neuroimmunol. 2004;157:197–202. doi: 10.1016/j.jneuroim.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Veinbergs I, Everson A, Sagara Y, Masliah E. Neurotoxic effects of apolipoprotein E4 are mediated via dysregulation of calcium homeostasis. J Neurosci Res. 2002;67:379–387. doi: 10.1002/jnr.10138. [DOI] [PubMed] [Google Scholar]
- Vitek MP, Brown CM, Colton CA. APOE genotype-specific differences in the innate immune response. Neurobiol Aging. 2009;30:1350–1360. doi: 10.1016/j.neurobiolaging.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrle SE, Holtzman DM. Differential metabolism of ApoE isoforms in plasma and CSF. Exp Neurol. 2003;183:4–6. doi: 10.1016/s0014-4886(03)00185-7. [DOI] [PubMed] [Google Scholar]
- Zivelin A, Rosenberg N, Peretz H, Amit Y, Kornbrot N, Seligsohn U. Improved method for genotyping apolipoprotein E polymorphisms by a PCR-based assay simultaneously utilizing two distinct restriction enzymes. Clin Chem. 1997;43:1657–1659. [PubMed] [Google Scholar]