Abstract
AgRP and POMC neurons are two key cell types that regulate feeding in response to hormones and nutrients. Recently, it was discovered that these neurons are also rapidly modulated by the mere sight and smell of food. This rapid sensory regulation “resets” the activity of AgRP and POMC neurons before a single bite of food has been consumed. This surprising and counterintuitive discovery challenges longstanding assumptions about the function and regulation of these cells. Here we review these recent findings and discuss their implications for our understanding of feeding behavior. We propose several alternative hypotheses for how these new observations might be integrated into a revised model of the feeding circuit, and also highlight some of the key questions that remain to be answered.
Introduction
The body weight of most animals is remarkably stable over time, suggesting that a homeostatic system balances food intake and energy expenditure over the long-term [1]. Two neural cell types in the hypothalamus, termed Agouti-related protein (AgRP) and Proopiomelanocortin (POMC) neurons, are thought to be critical components of this homeostatic system (Figure 1a). AgRP neurons are activated by energy deficit [2] and their activity promotes food seeking and consumption [3–6]. POMC neurons, in contrast, are activated by energy surfeit, and their activity promotes fasting and weight loss [3,7]. These two cell types are often described as the “gas pedal” and the “brake” for the neural control of feeding. Together, they have been studied in far greater depth than any other neurons in the brain that have a specialized role in energy balance.
Figure 1.
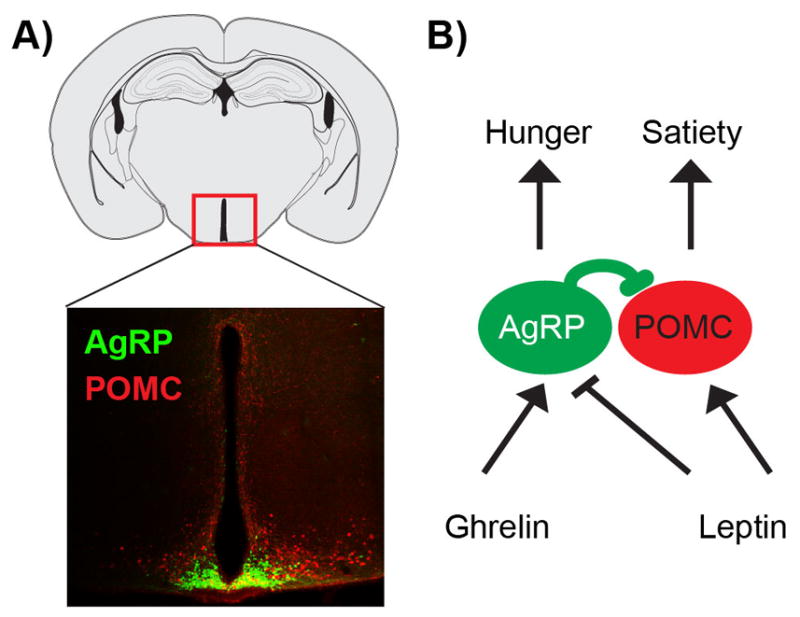
AgRP and POMC neurons regulate feeding in response to nutritional signals. A. AgRP and POMC neurons are intermingled in the arcuate nucleus of the hypothalamus. B. The anorexigenic hormone leptin and the orexigenic hormone ghrelin regulate AgRP/POMC neurons in opposite directions.
AgRP and POMC neurons are regulated by circulating signals of nutritional state, which modulate these cells in opposite directions consistent with their function. For example, the hormone leptin, which signals energy availability, activates POMC neurons and inhibits AgRP neurons [8,9]. The hormone ghrelin, which signals energy scarcity, has the opposite effect [10,11] (Figure 1b). Based on these observations, an intuitive and widely accepted model has emerged for how these two cell types control feeding. According to this model, food deprivation decreases the level of hormones that inhibit feeding, such as leptin, and increases the level of hormones that promote feeding, such as ghrelin. This hormonal switch activates AgRP neurons and inhibits POMC neurons, creating a “hunger drive” that motivates animals to find and consume food, and that persists until food intake replenishes the body of nutrients. In this way, a simple negative feedback loop is thought to explain the remarkable coordination of feeding behavior with physiologic need.
While this homeostatic model is widely accepted, one key piece of data has been missing: information about the activity dynamics of these neurons in vivo. In the past year, this gap has been closed, as three groups have reported measurements of AgRP and POMC neuron dynamics in awake, behaving mice [12–14]. Contrary to expectations, these experiments revealed that AgRP and POMC neurons are rapidly modulated by the mere sight and smell of food, in a way that “resets” their activity before any food is consumed. This paradoxical discovery has prompted reassessment of the regulation and function of these long-studied cells [12–15]. In this essay, we summarize these recent findings and discuss their implications for our understanding of the neural regulation of feeding.
The sensory detection of food rapidly resets AgRP and POMC neurons
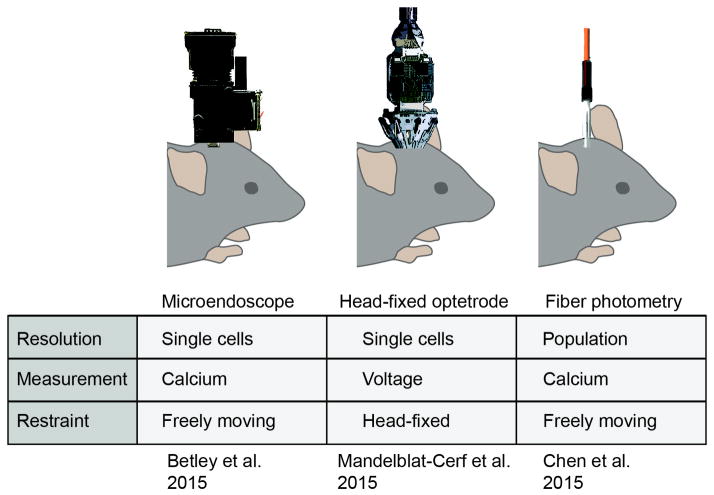
AgRP and POMC neurons were first implicated in the control of feeding almost 20 years ago [2,16–20], yet their in vivo dynamics were only described in the past year [12–14]. This long delay was due to technical challenges associated with recording neural activity from the arcuate nucleus (ARC) of the hypothalamus, which is located at the base of the forebrain and contains a diversity of intermingled neural cell types. To overcome these obstacles, it was necessary to develop new methods that enable optical [21,22] or electrophysiological [23] recordings from genetically defined cell types at deep brain sites and in freely behaving animals. Three such methods have now been developed and applied to investigate the dynamics of AgRP and POMC neurons (Figure 2).
Figure 2.
Techniques for cell-type-specific recording of deep brain neural activity. (Left) Microendoscopic calcium imaging utilizes a head-mounted, miniaturized microscope to record fluorescence signals from a genetically encoded calcium indicator targeted to a specific cell type. This method can be used to probe deep brain structures by coupling the microscope to a gradient refractive index (GRIN) lens of appropriate length. (Middle) Optrode recordings utilize an electrode array paired with an optical fiber to perform extracellular recordings. The optical fiber enables cells expressing channelrhodopsin to be identified by their short latency responses to light stimulation. (Right) Fiber photometry uses a single optical fiber to both excite and record fluorescence from a population of neurons expressing a calcium reporter. The publications that used each of these approaches to investigate AgRP/POMC neurons are listed below.
The first study used an approach called fiber photometry, which utilizes an optical fiber to record fluorescence signals from a genetically encoded calcium reporter (e.g. GCaMP6s) targeted to a specific cell type [22]. This approach measures population-level calcium dynamics but does not resolve single cells (Figure 2). In the key experiment in this study, mice were fasted overnight and then presented with a piece of food, and during this time the calcium dynamics of AgRP and POMC neurons were optically recorded [12]. Contrary to the predictions of homeostatic models, it was found that food presentation alone rapidly inhibited AgRP neurons and activated POMC neurons. This response began the moment that food was presented and was complete within seconds, often before a single bite of food could be consumed. Thus sensory cues associated with food, rather than the post-ingestive effects of feeding, are primarily responsible for resetting the activity of these cells [12]. This finding was counterintuitive in part because it revealed that AgRP neurons, which promote food intake, are actually less active when a mouse is eating, and conversely that POMC neurons, which inhibit food intake, are more active during feeding. This paradoxical activity pattern creates a puzzle for explaining how these neurons are able to perform their presumed functions.
Further characterization of the rapid sensory modulation of these neurons revealed five important properties [12]. (1) The response depends on nutritional state, since neurons from fasted mice respond more strongly to food cues than neurons from fed mice. (2) It depends on food palatability, since energy dense foods such as peanut butter induce a stronger response than energy poor foods such as chow. (3) It depends on food accessibility, since food that is hidden or inaccessible induces a weaker and less durable response. (4) It is contingent on eventual food consumption, since removal of food before it can be consumed causes reversion of neural activity back to its prior state. (5) It is integrative, because these factors interact with each other to determine the magnitude of the response. As an example of this last property, presentation of a palatable food such as peanut butter was shown to modulate these neurons even in fed mice, which were otherwise insensitive to presentation of chow [12]. Taken together, these findings reveal that AgRP and POMC neurons are regulated in a way that anticipates the nutritional or incentive value of a forthcoming meal [12,15]. This anticipatory regulation resembles an expected value calculation, in which the brain weighs factors such as the need for food, the energy density or palatability of the food, and the likelihood that it is obtainable [12].
These findings were extended by a second study that used fluorescence microendoscopy to analyze calcium dynamics of AgRP neurons in freely behaving mice [13]. An important advantage of this approach is that it provides single cell resolution and therefore can reveal heterogeneity in responses within a genetically defined population of neurons (Figure 2). This study added two additional important pieces of information. First, it showed that essentially every AgRP neuron is rapidly inhibited by food presentation, at least at the level of calcium dynamics (106/110 cells) [13]. Thus AgRP neurons, despite having diverse projection patterns, appear to be subject to remarkably homogeneous regulation by sensory cues. Second, it showed that AgRP neurons can be modulated by an arbitrary cue, such as a light or sound, that animals have been trained to associate with food [13]. This indicates that the response to food cues is, at least in part, learned. This finding is consistent with the fact that response of these neurons to novel foods accelerates upon repeated exposure, which also indicates learning [12].
A third study reported optrode recordings of AgRP and POMC neuron activity in head fixed mice [14]. Optrodes enable the use of channelrhodopsin (ChR2) to identify cells from electrode recordings based on their light sensitivity [23] and, unlike calcium imaging, provide reliable measurement of single action potentials (Figure 2). In this study ChR2 was targeted to AgRP neurons, so that AgRP neurons could be identified as light-activated units and putative POMC neurons could be identified as light-inhibited units. Using this approach AgRP neuron firing rates were found to gradually increase throughout the course of the light phase, consistent with the slow development of energy deficit that occurs during the day [14]. Subsequent food presentation rapidly inhibited AgRP neurons and activated POMC neurons, similar to the findings from calcium imaging. One important difference between these studies, however, was that AgRP neuron activity was shown to remain somewhat elevated following food presentation in the optrode experiment [14]. This persistent elevated activity was interpreted to represent a residual “hunger drive” that is responsible for promoting subsequent food consumption. We discuss this observation and its possible implications later in the text.
Feeding is regulated by both homeostatic and anticipatory mechanisms
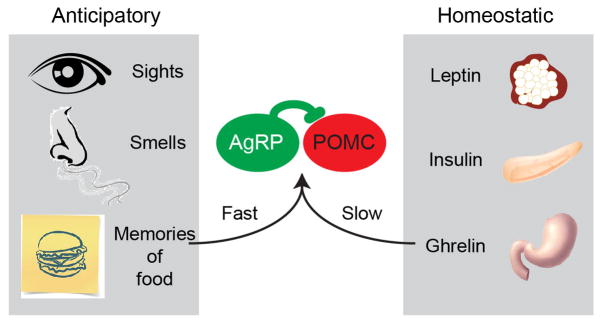
The rapid sensory modulation of AgRP and POMC neurons was unexpected in part because thinking about these cells has been dominated by the concept of homeostasis. A homeostatic mechanism is one in which a deviation from a physiologic set-point triggers a counterregulatory response [24,25]. For example, a decline in plasma leptin activates AgRP neurons to induce feeding. The sensory modulation of AgRP and POMC neurons by contrast is not homeostatic, because it occurs before any physiologic change has taken place. This response is instead “anticipatory,” because it predicts the nutritional changes that will occur in the future after the food has been consumed (Figure 3).
Figure 3.
Fast and slow regulation of AgRP and POMC neurons by anticipatory and homeostatic signals. (Right) Circulating hormonal signals such as leptin, ghrelin, and insulin have traditionally been thought to play a primary role in the regulation of AgRP and POMC neurons. The levels of these signals fluctuate slowly over minutes to hours in accordance with changes in nutritional state. (Left) In vivo recordings however revealed a dominant role for anticipatory signals in the regulation of AgRP and POMC neurons. These anticipatory signals are triggered by sensory cues from the outside world and communiated by neural input, and therefore develop much faster than hormonal changes. In addition, these anticipatory signals precede rather than respond to changes in the nutritional state of the body.
Anticipatory mechanisms are widespread in neurobiology [24,26,27], but are often overlooked in discussions of feeding behavior. The classic example of anticipatory regulation is Pavlov’s dogs, which salivate follow ringing of a bell that predicts food availability [28]. The anticipatory response in this case is the secretion of saliva containing digestive enzymes, which functions to prepare the oral cavity for food ingestion just moments before the food arrives. By contrast, the function of the anticipatory regulation of AgRP and POMC neurons is less clear. We consider below five alternatives.
Hypothesis #1: Sensory feedback gates cephalic phase responses
Food consumption is necessary for survival but also represents an acute threat, since it floods the body with nutrients that can disrupt physiologic balance [29]. To deal with this challenge, animals have developed a large class of peripheral adaptations that are triggered by the sight, smell and taste of food and function to prepare the body to metabolize and absorb nutrients [30,31]. These anticipatory responses, which Pavlov called “psychic secretions,” are now known as cephalic phase responses because they are controlled by the brain. In addition to salivation, cephalic phase responses include the secretion of gastric acid, bile, and digestive enzymes into the stomach and intestines; the release of hormones such as insulin, cholecystokinin, and pancreatic polypeptide into the bloodstream; and an increase in body temperature [30,31]. Most of these responses can be triggered by both food cues and food absorption. For example, the cephalic phase of insulin release is triggered by sensory cues that occur at the onset of feeding and precedes changes in blood glucose [32,33]. Later, a second phase of insulin release occurs following food absorption in response to hyperglycemia.
Similarities between the sensory regulation of AgRP and POMC neurons and the activation of cephalic phase responses suggest these neurons could be part of the upstream pathway. For example, cephalic phases responses are triggered more strongly by palatable foods [34–37], by multisensory compared to unisensory food cues [38], and following food deprivation [37]. The sensory regulation of AgRP/POMC neurons shares all of these properties [12]. Cephalic phase responses can also be learned by Pavlovian conditioning [28,34,39,40], similar to the sensory modulation of AgRP/POMC neurons [13]. While the forebrain circuitry that controls cephalic phase responses is largely unknown, manipulations of the paraventricular, ventromedial and lateral hypothalamus can trigger gastric acid release and other gastrointestinal responses, indicating a role for the hypothalamus [34,41,42]. There is also some evidence that chemogenetic modulation of AgRP neurons can rapidly alter peripheral metabolism [4], although this has not been explored in detail. In future studies it will be important to measure the effects of cell-type-specific manipulations of AgRP and POMC neurons on cephalic phase responses in peripheral tissues, in order to understand the role of these neurons in gating this response.
Hypothesis #2: Sensory feedback induces anticipatory satiety
AgRP neurons are thought to promote hunger, and thus their rapid inhibition by food cues would be predicted to result in “anticipatory satiety” satiety that occurs before the food is consumed. While this seems paradoxical, there is evidence that learned associations with sensory cues contribute to the termination of feeding [43]. The most compelling data come from sham feeding experiments, performed primarily in rats, which used a gastric fistula to drain the ingested food from the stomach [44,45]. This preparation enables disconnection of the effects of gastrointestinal signals from external sensory cues on feeding behavior. These studies showed, first, that rats consume much more food during sham feeding compared to real feeding [44,45], confirming the importance of post-ingestive negative feedback signals such as gastric distension in meal termination. However, it was found that during repeated sham feeding trials the amount of food these animals consumed increased even further [44,45]. This progressive increase in sham food consumption was shown to reflect the extinction of a learned association between the sensory properties of specific foods (the conditioned stimulus) and their post-ingestive consequences (the unconditioned stimulus). This learned association functions to reduce the rate of food intake at the beginning of a meal [46,47], perhaps so that animals can anticipate at the outset of a meal some of its physiologic effects and thereby calibrate their food intake more precisely.
An implication of this finding is that food delivered directly to the stomach, thereby bypassing sensory cues, should be experienced as less satiating than food consumed orally. This prediction has been confirmed by experiments showing that enteral feeding in humans fails to fully suppress appetite [48–51]. This failure does not appear to reflect decreased production of gastrointestinal satiation signals [49,52], suggesting it involves the absence of a cognitive signal triggered by sensory cues. Consistent with this, some enteral fed human subjects report that simply chewing food decreases their residual hunger, even though the food cannot be swallowed [50,53].
The rapid inhibition of AgRP neurons by food cues could contribute to this phenomenon of anticipatory satiety. The fact that these neurons are more strongly inhibited by energy dense foods [12] and respond to Pavlovian conditioning [13] are both consistent with this possibility. One way to test this model would be to determine whether AgRP neurons can be conditioned by gastrointestinal negative feedback signals. For example, would an arbitrary cue that predicts the infusion of nutrients into the stomach attain the ability to inhibit AgRP neurons following training? Conversely, would the response of AgRP neurons to a food cue undergo extinction if the ingested food was drained from the stomach each time the cue was presented? These types of experiments would clarify the nature of the unconditioned stimulus that trains AgRP neurons to respond to food cues, and in doing so provide insight into the function of this anticipatory modulation.
Hypothesis #3: Sensory feedback suppresses appetitive behaviors
AgRP neurons regulate not only food intake but also appetitive behaviors that promote food obtainment. For example, optogenetic or chemogenetic activation of AgRP neurons motivates animals to engage in vigorous lever pressing or nose poking for a food reward [4,54]. AgRP neuron activation also stimulates locomotor activity specifically in the absence of food, which has been interpreted as representing foraging [4,55]. Pharmacologic experiments have shown that delivery of AgRP or NPY into the brain can preferentially promote appetitive rather than consummatory behaviors under some conditions [56–59]. Together, these observations highlight the importance of AgRP neurons for promoting behaviors that lead to food discovery, and raise the question of how the transition from appetitive to consummatory behaviors is controlled.
The discovery that AgRP neurons are rapidly inhibited by food cues suggested that this inhibition may gate the transition from foraging to feeding [12,14]. Indeed, the response properties of AgRP neurons to sensory cues appear almost perfectly designed to serve this function. As described above, food cues modulate AgRP neurons in a way that resembles an expected value calculation, in which the animal weighs factors such as the accessibility of the food, the energy density of the food, and its own need for nutrition. This integration would enable animals to make foraging decisions that are adaptive in the face of changing internal and external circumstances. For example, discovery of a suboptimal source of nutrition by a starving animal would nonetheless inhibit AgRP neurons, thereby ensuring that foraging is blocked when food is of greatest value [12–14]. By contrast AgRP neurons from a well-fed animal would be insensitive to food cues unless the food was particularly palatable [12].
While this model is appealing, it is inconsistent in its simplest form with all of the available evidence. One problem is that if AgRP neuron inhibition is required for the transition from foraging to feeding, then continuous optogenetic stimulation of AgRP neurons should result in animals that perpetually forage and as a result do not eat. However this is not the case [3]. One way to explain this discrepancy might be that AgRP neuron inhibition promotes, but is not required, for the transition from foraging to feeding. Alternatively, it is possible that food presentation sends a sufficiently strong inhibitory signal to AgRP neurons that they become silenced even in the presence of continuous optogenetic stimulation. Consistent with this second possibility, it has been shown that AgRP activation induced by high-dose ghrelin treatment can be largely reversed by presentation of a single piece of chow [12], highlighting the potency of this inhibitory sensory input.
An important unresolved question is whether AgRP neurons are required for appetitive behaviors in food deprived mice. One study found that neonatal ablation of AgRP neurons reduced food anticipatory activity (FAA), which is an increase in locomotor activity that occurs before food availability and resembles foraging in mice subjected to scheduled feeding protocols [60]. By contrast, adult ablation of AgRP neurons reduced food intake primarily due to “visceral malaise,” a defect that is consummatory in nature since AgRP neuron ablated mice will reject food delivered directly into their mouths [61]. However, this nausea may be specific to the extreme case of acute AgRP neuron ablation in adults, since otherwise the mere sight and smell of food would trigger sickness and eating would be impossible.
Experiments that test the requirement for AgRP neurons in specific appetitive behaviors will be important in order to clarify how the sensory modulation of these cells influences the transition from foraging to feeding. While AgRP neuron dynamics appear well-suited to serve this function, it is possible that these anticipatory dynamics are merely a consequence of this behavioral transition rather than its cause, as has been proposed for other cell types [62].
Hypothesis #4: Sensory feedback acts as a teaching signal
Hunger is an unpleasant state, and one reason animals eat is to eliminate this negative feeling. Recently, it was shown that AgRP neuron activity has negative valence, meaning that mice find it aversive and therefore learn to avoid places and flavors that are associated with elevated AgRP neuron activity [13]. It has been proposed that, by alleviating this negative state, the rapid sensory inhibition of AgRP neurons may function as a teaching signal that trains animals to search for and consume food [13]. This mechanism for encouraging a behavior by linking it to the removal of an aversive stimulus is known as negative reinforcement [63].
A prediction of this negative reinforcement model is that mice should learn to perform instrumental responses that lead to a reduction in AgRP neuron activity. However this is not the case [13], as it has been shown that mice fail to execute simple operant tasks that have been experimentally paired with AgRP neuron inactivation. For example, fed mice will neither lever press nor nose poke in order to pause optogenetic stimulation of AgRP neurons [13]. Similarly, fasted mice fail to nose poke in order to induce optogenetic silencing of naturally elevated AgRP neuron activity [13]. This indifference to AgRP neuron silencing is in stark contrast to the dramatic lever pressing and nose poking that AgRP neuron stimulated animals will perform for an actual food reward [4,54]. Therefore negative reinforcement is not the primary motivational mechanism that AgRP neurons utilize to achieve their remarkable behavioral effects.
An alternative possibility is that AgRP neurons function by increasing the positively rewarding properties of food, such as its sight, smell, and taste [15]. It is well known that food deprivation makes food more appealing, a concept known as alliesthesia [64], and that this enhanced palatability and incentive salience can promote food seeking and consumption [15]. Consistent with this, palatable foods have enhanced ability to modulate AgRP neurons [12,15]. At present, however, there is little data that directly addresses this hypothesis.
Hypothesis #5: Sensory feedback connects the present to the future
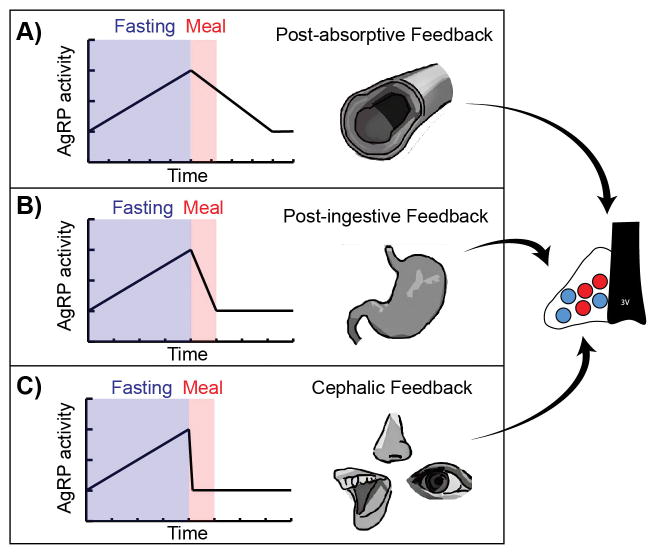
It is possible that the rapid sensory modulation of AgRP and POMC neurons may not trigger any immediate behavioral transition, motivational change, or physiologic response. Instead, this sensory modulation could function primarily to synchronize rapid feeding behavior with slower nutritional changes. To illustrate why this may be necessary, consider three types of signals that could communicate ongoing meal status to AgRP and POMC neurons (Figure 4). The first is post-absorptive signals, such as leptin, that report on the nutritional state of the body. These signals have been the focus of most studies of the regulation of AgRP and POMC neurons, yet they act too slowly to control behavior directly (Figure 4A). For example, changes in plasma leptin require metabolic and transcriptional responses in adipocytes which develop over hours, and therefore leptin could not directly terminate a “hunger drive” following feeding. A second candidate is post-ingestive signals that arise from the stomach and intestine following feeding, including gastric distension and gut derived satiation peptides such as cholecystokinin. These signals have the right kinetics for controlling feeding behavior (Figure 4B), but there is little evidence that they actually regulate AgRP and POMC neurons, with the possible exception of peptide YY ([65,66] but see also [67]).
Figure 4.
Three models for the inactivation of AgRP neurons by feeding. A. Post-absorptive feedback reports on the nutritional state of the body and includes circulating signals such as leptin. These signals evolve over hours and therefore are too slow to explain the rapid suppression of hunger by food intake. B. Post-ingestive signals arise from the stomach and intestine immediately following food intake, and including gastric distension as well as gut-derived satiation peptides. These signals directly control meal termination, and therefore would be well-suited to inactivate AgRP neurons after feeding. However current data does not support a prominent role for these gastrointestinal signals in the actual regulation of AgRP neuron activity. C. Cephalic feedback involves sight, smell, taste, and possibly other external sensory cues. In vivo recordings reveal that this is the major mechanism for the regulation of AgRP and POMC neurons during meals.
In the absence of post-absorptive and post-ingestive signals, external sensory cues are the primary remaining type of information that AgRP and POMC neurons could use to learn about the status of an ongoing meal (Figure 4C). Because these sensory cues are detected before food has been consumed, they can most easily be used to make predictions about impending food consumption, which is how they are utilized in practice [12]. Thus the anticipatory regulation of AgRP and POMC neurons may have evolved primarily as a facile mechanism for the brain to coordinate rapid food ingestion with much slower homeostatic changes, rather than as a way to trigger a specific behavioral or metabolic transition upon the discovery of food.
Whatever the reason, it is clear that AgRP and POMC neurons are rapidly modulated by food associated sensory cues. This raises another question: if these neurons are “reset” by the sight and smell of food, then how do they control feeding at all?
How do AgRP and POMC neurons control feeding?
A vast body of evidence shows that AgRP and POMC neurons control food intake. This includes the results of optogenetic, chemogenetic, and cell ablation studies in mice demonstrating that bidirectional manipulation of these cells alters feeding [3–7], as well as pharmacologic and genetic studies showing that the neurotransmitters produced by these cells (gamma-aminobutyric acid (GABA), neuropeptide Y (NPY), AgRP, and POMC) modulate feeding in ways consistent with their putative functions [16–18,68–71]. These findings have been extended to humans by the discovery that loss-of-function mutations in the melanocortin 4-receptor (MC4R) are a common cause of extreme obesity [72–75]. Thus a mechanism must exist by which the activity of these neurons is translated into changes in food consumption.
Nonetheless, the natural activity patterns of these neurons are difficult to reconcile with their presumed functions. It is generally assumed that, if a neuron drives a behavior, then the neuron should be more active during or immediately preceding the behavior’s execution. Yet this is not the case for AgRP and POMC neurons. AgRP neurons, which are thought to drive food intake, are much less active during feeding compared to minutes before. POMC neurons, which are thought to inhibit food intake, are more active during the act of feeding itself. These counterintuitive trends apply to even the small fluctuations in AgRP and POMC neuron activity that surround individual bouts of eating, which often have the opposite sign relative to what would be predicted based on the known functions of these cells [12,14].
How can we explain these paradoxical findings? We consider below two hypotheses, which are not mutually exclusive, for how these cells may regulate feeding.
Mechanism #1: Residual AgRP neuron activation persists after food presentation and drives feeding
An important question is whether food presentation completely resets the activity of AgRP and POMC neurons to baseline, or whether a residual orexigenic activity pattern persists in these cells. Microendoscopic calcium imaging found that essentially all AgRP neurons were rapidly inhibited by food presentation (106/110 cells inhibited versus 1/110 cells activated) [13]. By contrast, optrode recordings found that only 64% of AgRP neurons were inhibited by food presentation (14/22 cells), whereas 23% of AgRP neurons were activated (5/22 cells) [14]. Consistent with this mixed response, optrode recordings showed that food presentation to food restricted mice in the dark phase did not reduce the firing rate of AgRP neurons all the way to the level of fed mice at the start of the light phase, a time that mice generally do not eat. This residual activity of AgRP neurons was proposed to represent a hunger drive that persists after food discovery [14].
Why would optrode recordings reveal residual AgRP activity that was not detected by calcium imaging? One possibility is that the difference is technical. Calcium dynamics are only a surrogate for neural firing and provide relative, not absolute, measurements of neural activity. Calcium sensors can also display non-linearity in their response properties outside a certain range. By contrast, electrophysiologic recordings provide data on absolute firing rates by measuring individual spikes. Thus it is possible that a residual activation of AgRP neurons following food presentation was simply not detected in the calcium imaging experiments due to a technical limitation of the approach.
However there are reasons to suspect this is not the whole story. One reason is that technical differences would not obviously explain why the two studies found a different direction of modulation for a significant subset of AgRP neurons; i.e. why would calcium imaging show that 96% of AgRP neurons were inhibited by food cues if 23% were actually activated? In response to other stimuli, AgRP activation was robustly detected by calcium imaging [13]. Second, photometry recordings showed that presentation of palatable food to fed mice can reduce calcium signals considerably below the ad libitum fed baseline [12]. Therefore calcium imaging is not inherently limited by linear range or sensitivity in its ability to detect activity reductions below the baseline level of fed animals.
An alternative possibility is that these discrepancies reflect differences in experimental paradigm. One important difference is that the optrode study used head-fixed mice presented with a liquid diet [14], whereas the calcium imaging studies used freely behaving mice presented with solid food [12,13]. It has been shown that the magnitude and durability of the response of AgRP and POMC neurons to food cues is very sensitive to factors such as the accessibility and palatability of the food [12]. Thus it may be that the use of a head-fixed preparation or liquid diet reduces the anticipatory response of these neurons, either due to stress or some change in the animal’s expectation of food availability or value. This would explain why the calcium imaging and electrophysiologic measurements showed different percentages of AgRP neurons that were inhibited by food cues.
To clarify these issues, it will be important to perform optrode recordings from AgRP neurons in freely behaving mice and measure whether any residual activation persists after food presentation. In addition, it will be important to test the functional importance of any residual AgRP activity by using methods with high temporal control, such as optogenetic silencers, to selectively inhibit AgRP neurons after food presentation and measure the kinetics of the cessation of feeding. These experiments will enable dissection of the contribution of residual AgRP neuron activity to subsequent food consumption.
Mechanism #2: AgRP neurons transmit a sustained hunger signal
An alternative hypothesis is that AgRP neurons act through a sustained or persistent mechanism that enables the activity of these neurons before food presentation to “spill over” and influence the food intake that occurs later. There have been hints that such a sustained mechanism might operate. One clue is that feeding following optogenetic stimulation of AgRP neurons has an unusually long latency (six minutes) [3]. By contrast, optogenetic stimulation of inputs to the lateral hypothalamus can induce feeding within ten seconds [76]. This suggests that AgRP neurons do not control the food intake machinery directly, but rather produce some factor that must “build up” in the downstream circuit before feeding is triggered.
How could such a mechanism operate? It has been shown that a single injection of the AgRP neuropeptide into the brain can increase feeding for up to one week, indicating that the effects of AgRP peptide release can extend far beyond the duration of AgRP neuron activation [77]. Similarly, activation of POMC neurons inhibits feeding on a time-scale of hours to days [3,7]. Thus modulation of the melanocortin system can have delayed and chronic effects on feeding. However this mechanism cannot explain feeding that results from release of NPY and GABA, which in many contexts are more important than release of AgRP itself [3,54,61,68,70,78]. In this regard, activation of AgRP neurons lacking both GABA and NPY does not promote feeding in the first two hours after stimulation [70]. This implies that any mechanism that regulates acute feeding would necessarily be mediated by a melanocortin independent signal (either GABA or NPY).
A prediction of this model is that there should exist a population of feeding-regulatory neurons that serve as the substrate for this sustained response. These neurons would be located downstream of AgRP neurons in the feeding circuit and integrate AgRP neuron activity over time, so that their response to sudden changes in AgRP neuron firing rate was delayed. As a result, these downstream cells would appear to “remember” the history of AgRP neuron activity, enabling them to promote feeding even after AgRP neurons have been silenced by sensory cues. If such neurons exist, their dynamics would correlate more closely with the subjective notion of hunger than AgRP neurons themselves.
Conclusions and outlook
Over the past year we have witnessed some of the first glimpses into the dynamics of the key neural cell types that control feeding, including AgRP and POMC neurons. This has revealed that these cell types, long regarded as merely sensors of the internal state of the body, in fact respond rapidly to sensory cues from the outside world. They use this sensory information to predict nutritional changes that will occur in the future, revealing anticipatory dynamics that are both remarkable and puzzling, since they confound traditional explanations for how these neurons control behavior. An important challenge for the field will be to integrate these new observations into a revised model of the feeding circuit, and we have highlighted here some of the key questions that remain to be answered. As AgRP and POMC neurons represent only a small piece of the neural network that controls feeding, it is likely that additional surprises lie ahead as we uncover the dynamics of this circuitry.
Acknowledgments
Y.C. is an HHMI International Student Research Fellow. Z.A.K. is a New York Stem Cell Foundation Robertson Investigator and acknowledges support from the New York Stem Cell Foundation, the Rita Allen Foundation, the McKnight Foundation, the Alfred P. Sloan Foundation, the Brain and Behavior Research Foundation, the Esther A. & Joseph Klingenstein Foundation, the Program for Breakthrough Biological Research, and the UCSF Diabetes Center (P30 DK063720). This work was supported by an NIH New Innovator Award (DP2-DK109533) and R01-DK106399.
References
- 1.Weigle DS. Appetite and the regulation of body composition. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1994;8:302–10. doi: 10.1096/fasebj.8.3.8143936. [DOI] [PubMed] [Google Scholar]
- 2.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature neuroscience. 1998;1:271–2. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 3.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature neuroscience. 2011;14:351–5. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krashes MJ, Koda S, Ye C, Rogan SC, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. The Journal of clinical investigation. 2011;121:1424–8. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–5. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 6.Gropp E, Shanabrough M, Borok E, Xu AW, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nature neuroscience. 2005;8:1289–91. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 7.Zhan C, Zhou J, Feng Q, Zhang JE, et al. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:3624–32. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–4. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 9.Pinto S, Roseberry AG, Liu H, Diano S, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–5. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 10.Cowley MA, Smith RG, Diano S, Tschop M, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–61. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 11.Nakazato M, Murakami N, Date Y, Kojima M, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160:829–41. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betley JN, Xu S, Cao ZF, Gong R, et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–5. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandelblat-Cerf Y, Ramesh RN, Burgess CR, Patella P, et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife. 2015;4 doi: 10.7554/eLife.07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seeley RJ, Berridge KC. The hunger games. Cell. 2015;160:805–6. doi: 10.1016/j.cell.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Fan W, Boston BA, Kesterson RA, Hruby VJ, et al. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–8. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 17.Ollmann MM, Wilson BD, Yang YK, Kerns JA, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–8. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 18.Seeley RJ, Yagaloff KA, Fisher SL, Burn P, et al. Melanocortin receptors in leptin effects. Nature. 1997;390:349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 19.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–41. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 20.Lu DS, Willard D, Patel IR, Kadwell S, et al. Agouti Protein Is an Antagonist of the Melanocyte-Stimulating-Hormone Receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 21.Ziv Y, Burns LD, Cocker ED, Hamel EO, et al. Long-term dynamics of CA1 hippocampal place codes. Nature neuroscience. 2013;16:264–6. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–51. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lima SQ, Hromadka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PloS one. 2009;4:e6099. doi: 10.1371/journal.pone.0006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berridge KC. Motivation concepts in behavioral neuroscience. Physiology & behavior. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Cannon WB. Organization for Physiological Homeostasis. Physiological reviews. 1929;9:399–431. [Google Scholar]
- 26.Woods SC, Ramsay DS. Homeostasis: beyond Curt Richter. Appetite. 2007;49:388–98. doi: 10.1016/j.appet.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stricker EM, Hoffmann ML. Presystemic signals in the control of thirst, salt appetite, and vasopressin secretion. Physiology & behavior. 2007;91:404–12. doi: 10.1016/j.physbeh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Pavlov IP. The work of the digestive glands. London: Charles Griffin Co. Ltd; 1902. [Google Scholar]
- 29.Woods SC. The eating paradox: how we tolerate food. Psychological review. 1991;98:488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- 30.Power ML, Schulkin J. Anticipatory physiological regulation in feeding biology: cephalic phase responses. Appetite. 2008;50:194–206. doi: 10.1016/j.appet.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zafra MA, Molina F, Puerto A. The neural/cephalic phase reflexes in the physiology of nutrition. Neuroscience and biobehavioral reviews. 2006;30:1032–44. doi: 10.1016/j.neubiorev.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Steffens AB. Influence of the oral cavity on insulin release in the rat. The American journal of physiology. 1976;230:1411–5. doi: 10.1152/ajplegacy.1976.230.5.1411. [DOI] [PubMed] [Google Scholar]
- 33.Strubbe JH, Steffens AB. Rapid insulin release after ingestion of a meal in the unanesthetized rat. The American journal of physiology. 1975;229:1019–22. doi: 10.1152/ajplegacy.1975.229.4.1019. [DOI] [PubMed] [Google Scholar]
- 34.Powley TL. The ventromedial hypothalamic syndrome, satiety, and a cephalic phase hypothesis. Psychological review. 1977;84:89–126. [PubMed] [Google Scholar]
- 35.Brand JG, Cagan RH, Naim M. Chemical senses in the release of gastric and pancreatic secretions. Annual review of nutrition. 1982;2:249–76. doi: 10.1146/annurev.nu.02.070182.001341. [DOI] [PubMed] [Google Scholar]
- 36.Janowitz HD, Hollander F, Orringer D, Levy MH, et al. A quantitative study of the gastric secretory response to sham feeding in a human subject. Gastroenterology. 1950;16:104–16. [PubMed] [Google Scholar]
- 37.Klajner F, Herman CP, Polivy J, Chhabra R. Human obesity, dieting, and anticipatory salivation to food. Physiology & behavior. 1981;27:195–8. doi: 10.1016/0031-9384(81)90256-0. [DOI] [PubMed] [Google Scholar]
- 38.Feldman M, Richardson CT. Role of thought, sight, smell, and taste of food in the cephalic phase of gastric acid secretion in humans. Gastroenterology. 1986;90:428–33. doi: 10.1016/0016-5085(86)90943-1. [DOI] [PubMed] [Google Scholar]
- 39.Woods SC, Kuskosky PJ. Classically conditioned changes of blood glucose level. Psychosomatic medicine. 1976;38:201–19. doi: 10.1097/00006842-197605000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Woods SC, Vasselli JR, Kaestner E, Szakmary GA, et al. Conditioned insulin secretion and meal feeding in rats. Journal of comparative and physiological psychology. 1977;91:128–33. doi: 10.1037/h0077307. [DOI] [PubMed] [Google Scholar]
- 41.Kermani M, Eliassi A. Gastric acid secretion induced by paraventricular nucleus microinjection of orexin A is mediated through activation of neuropeptide Yergic system. Neuroscience. 2012;226:81–8. doi: 10.1016/j.neuroscience.2012.08.052. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi N, Okumura T, Yamada H, Kohgo Y. Stimulation of gastric acid secretion by centrally administered orexin-A in conscious rats. Biochemical and biophysical research communications. 1999;254:623–7. doi: 10.1006/bbrc.1998.9994. [DOI] [PubMed] [Google Scholar]
- 43.Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;16:814–20. doi: 10.1016/s0899-9007(00)00457-3. [DOI] [PubMed] [Google Scholar]
- 44.Young RC, Gibbs J, Antin J, Holt J, et al. Absence of satiety during sham feeding in the rat. Journal of comparative and physiological psychology. 1974;87:795–800. doi: 10.1037/h0037210. [DOI] [PubMed] [Google Scholar]
- 45.Davis JD, Campbell CS. Peripheral control of meal size in the rat. Effect of sham feeding on meal size and drinking rate. Journal of comparative and physiological psychology. 1973;83:379–87. doi: 10.1037/h0034667. [DOI] [PubMed] [Google Scholar]
- 46.Weingarten HP, Kulikovsky OT. Taste-to-postingestive consequence conditioning: is the rise in sham feeding with repeated experience a learning phenomenon? Physiology & behavior. 1989;45:471–6. doi: 10.1016/0031-9384(89)90060-7. [DOI] [PubMed] [Google Scholar]
- 47.Davis JD, Smith GP. Learning to sham feed: behavioral adjustments to loss of physiological postingestional stimuli. The American journal of physiology. 1990;259:R1228–35. doi: 10.1152/ajpregu.1990.259.6.R1228. [DOI] [PubMed] [Google Scholar]
- 48.Stratton RJ, Stubbs RJ, Elia M. Bolus tube feeding suppresses food intake and circulating ghrelin concentrations in healthy subjects in a short-term placebo-controlled trial. Am J Clin Nutr. 2008;88:77–83. doi: 10.1093/ajcn/88.1.77. [DOI] [PubMed] [Google Scholar]
- 49.Stratton RJ, Stubbs RJ, Elia M. Short-term continuous enteral tube feeding schedules did not suppress appetite and food intake in healthy men in a placebo-controlled trial. J Nutr. 2003;133:2570–6. doi: 10.1093/jn/133.8.2570. [DOI] [PubMed] [Google Scholar]
- 50.Stratton RJ, Elia M. The effects of enteral tube feeding and parenteral nutrition on appetite sensations and food intake in health and disease. Clin Nutr. 1999;18:63–70. doi: 10.1016/s0261-5614(99)80053-3. [DOI] [PubMed] [Google Scholar]
- 51.Cecil JE, Castiglione K, French S, Francis J, et al. Effects of intragastric infusions of fat and carbohydrate on appetite ratings and food intake from a test meal. Appetite. 1998;30:65–77. doi: 10.1006/appe.1997.0109. [DOI] [PubMed] [Google Scholar]
- 52.LeGall-Salmon E, Stevens WD, Levy JR. Total parenteral nutrition increases serum leptin concentration in hospitalized, undernourished patients. JPEN J Parenter Enteral Nutr. 1999;23:38–42. doi: 10.1177/014860719902300138. [DOI] [PubMed] [Google Scholar]
- 53.Wolf SG, Wolff HG. Human gastric function, an experimental of a man and his stomach. London, New York etc: Oxford university press; 1943. [Google Scholar]
- 54.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–7. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jerlhag E, Egecioglu E, Dickson SL, Andersson M, et al. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addiction biology. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 56.Sederholm F, Ammar AA, Sodersten P. Intake inhibition by NPY: role of appetitive ingestive behavior and aversion. Physiology & behavior. 2002;75:567–75. doi: 10.1016/s0031-9384(02)00648-0. [DOI] [PubMed] [Google Scholar]
- 57.Day DE, Keen-Rhinehart E, Bartness TJ. Role of NPY and its receptor subtypes in foraging, food hoarding, and food intake by Siberian hamsters. American journal of physiology Regulatory, integrative and comparative physiology. 2005;289:R29–36. doi: 10.1152/ajpregu.00853.2004. [DOI] [PubMed] [Google Scholar]
- 58.Ammar AA, Nergardh R, Fredholm BB, Brodin U, et al. Intake inhibition by NPY and CCK-8: A challenge of the notion of NPY as an “Orexigen”. Behavioural brain research. 2005;161:82–7. doi: 10.1016/j.bbr.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 59.Woods SC, Figlewicz DP, Madden L, Porte D, Jr, et al. NPY and food intake: discrepancies in the model. Regulatory peptides. 1998;75–76:403–8. doi: 10.1016/s0167-0115(98)00095-0. [DOI] [PubMed] [Google Scholar]
- 60.Tan K, Knight ZA, Friedman JM. Ablation of AgRP neurons impairs adaption to restricted feeding. Molecular metabolism. 2014;3:694–704. doi: 10.1016/j.molmet.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Q, Howell MP, Cowley MA, Palmiter RD. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2687–92. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 63.Skinner BF. The behavior of organisms. New York: Appleton-Century-Crofts; 1938. [Google Scholar]
- 64.Cabanac M. Physiological role of pleasure. Science. 1971;173:1103–7. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- 65.Parkinson JR, Dhillo WS, Small CJ, Chaudhri OB, et al. PYY3–36 injection in mice produces an acute anorexigenic effect followed by a delayed orexigenic effect not observed with other anorexigenic gut hormones. American journal of physiology Endocrinology and metabolism. 2008;294:E698–708. doi: 10.1152/ajpendo.00405.2007. [DOI] [PubMed] [Google Scholar]
- 66.Batterham RL, Cowley MA, Small CJ, Herzog H, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 67.Halatchev IG, Ellacott KL, Fan W, Cone RD. Peptide YY3–36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology. 2004;145:2585–90. doi: 10.1210/en.2003-1754. [DOI] [PubMed] [Google Scholar]
- 68.Tong Q, Ye CP, Jones JE, Elmquist JK, et al. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nature neuroscience. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark JT, Kalra PS, Kalra SP. Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Endocrinology. 1985;117:2435–42. doi: 10.1210/endo-117-6-2435. [DOI] [PubMed] [Google Scholar]
- 70.Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell metabolism. 2013;18:588–95. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poggioli R, Vergoni AV, Bertolini A. ACTH-(1–24) and alpha-MSH antagonize feeding behavior stimulated by kappa opiate agonists. Peptides. 1986;7:843–8. doi: 10.1016/0196-9781(86)90104-x. [DOI] [PubMed] [Google Scholar]
- 72.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. The New England journal of medicine. 2003;348:1085–95. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 73.Vaisse C, Clement K, Durand E, Hercberg S, et al. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. The Journal of clinical investigation. 2000;106:253–62. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farooqi IS, Yeo GS, Keogh JM, Aminian S, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. The Journal of clinical investigation. 2000;106:271–9. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hinney A, Schmidt A, Nottebom K, Heibult O, et al. Several mutations in the melanocortin-4 receptor gene including a nonsense and a frameshift mutation associated with dominantly inherited obesity in humans. The Journal of clinical endocrinology and metabolism. 1999;84:1483–6. doi: 10.1210/jcem.84.4.5728. [DOI] [PubMed] [Google Scholar]
- 76.Jennings JH, Rizzi G, Stamatakis AM, Ung RL, et al. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341:1517–21. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, et al. Long-term orexigenic effects of AgRP-(83---132) involve mechanisms other than melanocortin receptor blockade. American journal of physiology Regulatory, integrative and comparative physiology. 2000;279:R47–52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- 78.Qian S, Chen H, Weingarth D, Trumbauer ME, et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Molecular and cellular biology. 2002;22:5027–35. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]