Abstract
Introduction
Liver transplantation is the most effective treatment for hepatocellular carcinoma (HCC) in eligible patients, but is not accessed equally by all. We explored the effects of race and socioeconomic factors on transplantation for HCC while controlling for stage, resection status, and transplant candidacy.
Patients and Methods
All HCC patients, 2003-2013, were retrospectively analyzed using multivariate analysis was used to explore differences in transplantation rates among cohorts.
Results
Of 3,078 HCC patients, 754 (24%) were considered transplant eligible. Odds of transplantation were significantly higher for those with commercial insurance (OR=1.99, 95% CI [1.42, 2.79]) and lower for black patients (OR=0.55, 95% CI [0.33, 0.91]). Asians were more likely to be resected than white patients with similarly stage tumors and transplant criteria (p< 0.001). Patients not listed for transplantation for nonmedical reasons were more likely to be government-insured (p=0.02) and non-white (p=0.05). No step along the transplantation pathway was identified as the dominant hurdle.
Discussion
Patients who are black or government-insured are significantly less likely to undergo transplantation for HCC despite controlling for tumor stage, resection status, and transplant eligibility. Asian patients have higher rates of hepatic resection, but also appear to have lower transplantation rates beyond this effect.
Keywords: Hepatocellular carcinoma, Liver transplantation, Disparity, Socioeconomic, Minority health, Access to care
Introduction
Hepatocellular carcinoma (HCC) is now the second leading cause of death from cancer worldwide1, and its incidence has also been rising in the United States2,3. It is well known that racial/ethnic minorities and underinsured individuals tend to have worse survival from cancer4,5, and multiple studies confirm that this holds true for HCC as well6-10. The reasons behind this phenomenon are complex and multifocal. Disparities exist from the very beginning in that disadvantaged groups are more likely to develop cancer and to have their illness detected at more advanced stages6,7,11-12. However, survival differences persist even when individuals are matched by tumor stage, due in part to differences in treatment allocation6,7.
Treatments for liver cancer include liver transplantation, resection of the tumor, ablative therapies, and targeted oral agents. Of these options, liver transplantation offers the highest survival rate for eligible candidates13 – however this treatment is not equally accessed by all groups. Several studies have demonstrated that racial/ethnic minorities undergo liver transplantation for HCC about half as often as their white counterparts, even with similarly staged tumors10,12,14-19. Independent of race, lower socioeconomic status has also been shown result in lower rates of liver transplantation16,17. Patients may also be denied transplantation based on insurance status, as recently seen in the state of Arizona20. Not surprisingly, many publications demonstrate that the effects of race/ethnicity and socioeconomic status on access to care are often interrelated18,21.
The reasons for these disparities in transplantation rates have not been well elucidated, but may be related to the extensive requirements for transplant candidacy. Due to the limited supply of donor livers, strict criteria must be used to select optimal candidates for transplantation. Objective measurements of tumor number and size – known as the Milan Criteria – are a validated set of guidelines that predict which patients with HCC will benefit most from transplantation22. The United Network for Organ Sharing (UNOS) uses these criteria to allocate organs in the United States. Patients who meet these tumor criteria must then fulfill an array of additional requirements in order to proceed with transplantation, including: financial clearance, psychological assessment, abstinence from substance abuse, demonstration of support networks, and legal residency status. These non-tumor requirements for organ allocation may disproportionately affect certain demographic or socioeconomic groups, causing bias in healthcare delivery.
The eligibility for transplantation is further complicated by the fact that biologic factors can be intertwined with race/ethnicity. For example, Asians with HCC are more likely to have hepatitis B as their underlying liver disease, which causes less cirrhosis than hepatitis C or alcoholic liver disease – the etiologies most often seen in other demographic groups23. Asians with hepatitis B may have both resection and transplantation as possible therapeutic options, whereas other racial groups may be more limited to transplantation. If as a result Asians with HCC require liver transplantation less often, this could create the impression of a race-based disparity rather than a biologic reason for observed differences in transplantation rates. Prior reports on disparities in liver transplantation among minorities have not consistently accounted for this association of race with resectability10,14,15,18,19.
Given the complex interactions between variables, specific mechanisms leading to poor outcomes can be difficult to isolate. Disentangling these mechanisms is critical, since only targeted interventions are likely to be successful at improving healthcare across sociodemographic groups24. Accordingly, the primary aim of this study was to explore transplantation rates at a large HCC treatment center with specific focus on the effects of race and socioeconomic factors, while controlling for tumor stage and resectability. We also sought to identify at what point along the transplantation pipeline these effects were exerted.
Materials and Methods
Study Population and Data Collection
After IRB approval, a query of the hospital electronic medical records at The Mount Hospital was performed to identify all patients with HCC diagnosed 1/1/03-12/31/13. This institution is a leading HCC treatment center with an active liver transplantation program that serves a heterogeneous urban population. We selected 2003 as the starting year because UNOS policy on organ allocation for HCC underwent significant change in 200225. All patients aged ≥ 18 years at the time of diagnosis were included. Patients initially treated or ultimately transplanted elsewhere were excluded.
An initial list of patients was generated using ICD-9 code 155.0 and manual review of the entire cohort was performed to confirm HCC as defined by accepted radiographic and/or pathologic criteria26.
Sociodemographic data were extracted on age, gender, years of education, marital status, and preferred language for medical visits. The patient's home zip code was used as a proxy for median annual income27. Self-declared race was categorized into white, black, Asian, and other. Patients declaring Hispanic ethnicity within any race group were classified as Hispanic. Insurance type was classified as Commercial (any commercial insurance), Government (Medicare without supplement, or Medicaid), Government + Supplement (Medicare with a commercial supplement), other, and none.
Underlying liver disease was divided into hepatitis C, hepatitis B, alcoholic liver disease in the absence of viral hepatitis, non-alcoholic steatohepatitis (NASH), none, and other. Patients who were co-infected with both hepatitis B and C were classified under hepatitis C. Date from initial diagnostic imaging to the date of the first transplant specialist visit was noted, and a wait time greater than 90 days was defined as a delay in care.
In order to compare patients’ medical fitness for transplantation, all ICD-9 codes for each patient were collected. In consultation with a liver transplant surgeon (M.E.S.), patients were considered not eligible for transplantation if they carried ICD-9 diagnoses for any non-HCC malignancy, pulmonary hypertension, dementia, congestive heart failure, or chronic obstructive pulmonary disease. In addition, age > 70 years was used as a cut-off for transplant eligibility for the purposes of this analysis. These designations were made to control for medical fitness for transplantation, recognizing that individual patients may or may not be actual candidates based on other reasons.
Radiology reports were used to determine tumor number and size, as well as assess for the presence of gross vascular invasion and metastatic disease, defined as distant metastases or perihepatic lymphadenopathy greater than 2cm. Patients were considered eligible for transplantation if they met the Milan Criteria: a single lesion ≤5 cm or up to three separate lesions, with none >3cm, and with no evidence of gross vascular invasion or metastases22.
Steps along the transplant pathway were: diagnosis; specialist visit (defined as any transplant hepatologist, transplant surgeon, or surgical oncologist); formal transplant evaluation; UNOS listing; and transplantation. The records of individuals who were not listed for transplantation were examined. Reasons for non-listing were divided into “clinical” and “social” explanations. Clinical reasons included early stage disease (tumors less than 2cm in size), prohibitive comorbidities, and progression of disease beyond Milan criteria. Examples of social reasons included active substance abuse, inconsistent follow-up, failure of psychological screening, lack of financial clearance, and patient refusal.
Statistical Analyses
The Student t test or Wilcoxon rank sum test was used for the univariate analyses of the continuous variables, and the Pearson Chi Square or Fisher exact test for the categorical variables. Statistical significance was expressed by p-values and/or 95% confidence intervals. For all the analyses p-values ≤0.05 were considered statistically significant; all hypothesis tests were two-sided unless noted otherwise.
The primary outcome was the relationship between sociodemographic variables and liver transplantation. Multivariable logistic regression was used to model the relationship between the predictors of interest and liver transplantation28. Any predictors that were significant at the 0.2 level during the univariate analysis were included in the logistic model. Race/ethnicity and underlying liver diagnosis were included a priori regardless of the results of the univariate analysis. The models were built using backward selection of covariates when at each run the least significant covariate was excluded from the consequent model. C score, Hosmer and Lemeshow Goodness-of-Fit (GOF) test, and the stability of the estimates were used as model assessment methods.
The association between race/ethnicity and liver resection was analyzed using the contingency table and Pearson's Chi Square test. Only those patients who were eligible for transplantation but instead underwent liver resection were considered. The sociodemographic differences between subjects who were not listed for transplantation for social vs. clinical reasons were examined by using the Student t test and Pearson's Chi Square test. All statistical analyses were performed using SAS version 9.3 for Windows (SAS Institute Inc., Cary, North Carolina).
Results
Features of the entire HCC cohort
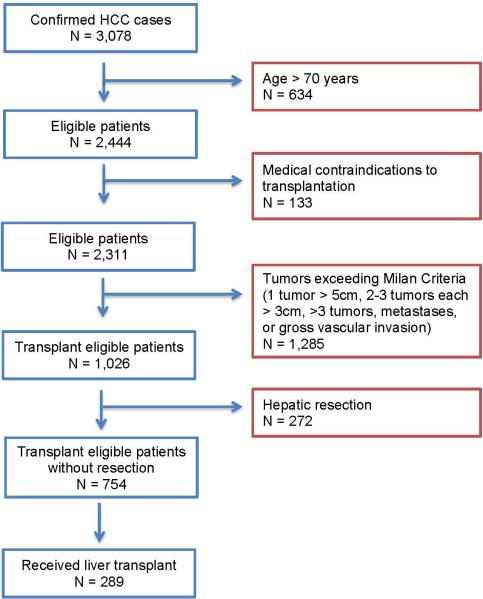
A total of 3,078 individuals were identified as having undergone index treatment for HCC at our institution (Figure 1). Mean age was 61±11 years; 23% (n=703) were women, 42% (n=1,243) were white, 24% (n=730) were Asian, 20% (n=589) were Hispanic, and 14% (n=416) were black. Median income was $55,588 (first (Q1) and third (Q3) quartiles [$38,750, $80,724]), median distance of residence from the hospital was 34 miles (Q1,Q3 [5.6, 17.5]); and 73% (n=2,251) were married or living with a partner. Most of the cohort had only government insurance (45%, n=1,300), 40% (n=1,150) had commercial insurance, and the remaining 15% (n=628) had no insurance or no payer information on record. Hepatitis C was present in 54% (n=1,519) of patients; 26% (n=743) had hepatitis B; 7% (n=193) had alcoholic cirrhosis in the absence of viral hepatitis; and 4% (n=106) had NASH. A single tumor was present in 58% of patients (n=1,804); 15% (n=482) had two tumors, 7% (n=198) had three tumors, and 20% (589) had ≥ 4 tumors. On presentation the majority of subjects had no metastases (84%, n=2,582) and/or no gross vascular invasion (80%, n=2,435).
Figure 1.
Selection of transplant eligible individuals from the cohort of patients with HCC
Transplant eligible patients, not undergoing hepatic resection
Of the entire cohort, 754 (25%) individuals were considered eligible for liver transplantation, and did not under liver resection as their treatment. As followed over the treatment pathway, 740 patients had a transplant specialist visit, 593 had formal transplant evaluation, and 475 individuals were listed by UNOS. Ultimately, 289 (38%) patients were transplanted, and 465 (62%) patients did not receive a liver transplant. Univariate analyses of the demographic and socioeconomic characteristics of these patients are presented in Table 1. Patients who remained active on the transplant list, but not yet transplanted at last follow-up were excluded from the analysis.
Table 1.
Demographic and socioeconomic characteristics of patients with HCC meeting eligibility criteria for liver transplantation (tumor staging within Milan criteria, age ≤70, and an absence of contraindicated comorbidities), who did not undergo liver resection
| Transplant Eligible Patients | |||||
|---|---|---|---|---|---|
| Transplanted (N=289) | Not transplanted (N=465) | P-value | |||
| N | % | N | % | ||
| Age (mean, sd) | 57 | 7 | 58 | 8 | 0.001 |
| Median income, USD (median, IQR) | 56537 | [42051,84048] | 61710 | [37897,80000] | 0.06 |
| Miles from hospital (median, IQR) | 12 | [7.1,26.7] | 9 | [5.0,16.7] | <0.001 |
| Gender (female) | 69 | 23.9 | 106 | 22.8 | 0.79 |
| Race/ethnicity | 0.01 | ||||
| White, non-Hispanic | 145 | 50.2 | 174 | 37.4 | |
| Black, non-Hispanic | 29 | 10 | 66 | 14.2 | |
| Hispanic | 73 | 25.3 | 128 | 27.5 | |
| Asian, non-Hispanic | 42 | 14.5 | 95 | 20.4 | |
| English proficiency | 0.89 | ||||
| Non-proficient | 61 | 21.1 | 68 | 14.6 | |
| Proficient | 190 | 65.7 | 218 | 46.9 | |
| U.S. Citizenship status | 0.65 | ||||
| Citizen | 240 | 83 | 260 | 55.9 | |
| Resident | 28 | 9.7 | 29 | 6.2 | |
| Non-documented resident | 5 | 1.7 | 9 | 1.9 | |
| Education level | 0.61 | ||||
| College degree | 99 | 34.3 | 103 | 22.2 | |
| High school | 114 | 39.4 | 145 | 31.2 | |
| Grade school or less | 30 | 10.4 | 36 | 7.7 | |
| Marital Status | 0.52 | ||||
| Married/With partner | 228 | 78.9 | 346 | 74.4 | |
| Separated/Divorced/Widowed | 61 | 21.1 | 104 | 22.4 | |
| Insurance | <0.001 | ||||
| Commercial | 183 | 63.3 | 205 | 44.1 | |
| Government + Supplement | 22 | 7.6 | 47 | 10.1 | |
| Government Only | 80 | 27.7 | 188 | 40.4 | |
| None / Other | 3 | 1 | 4 | 0.8 | |
| Liver diagnosis | 0.43 | ||||
| Alcoholic liver disease | 22 | 7.6 | 39 | 8.4 | |
| Hepatitis B | 43 | 14.9 | 81 | 17.4 | |
| Hepatitis C | 189 | 65.4 | 304 | 65.4 | |
| NASH | 9 | 3.1 | 15 | 3.2 | |
| Other | 26 | 9 | 26 | 5.6 | |
A multivariable logistic model was then used to determine predictors for receiving transplantation in these groups. A total of 742 (98.4%) patients had no missing values for the predictors of interest and were included in the multivariate analysis. The final model included age, race/ethnicity, income, insurance type, and was adjusted for underlying liver disease (Table 2). The p-values for the Hosmer and Lemeshow GOF statistic and C-statistic were 0.11 and 0.66, respectively, indicating that the final model was a good fit to the data. Having commercial insurance was associated with significantly higher odds (OR=1.99, 95% CI [1.42, 2.79], p<0.001) of undergoing transplantation compared to having only Medicaid or Medicare. The odds of undergoing transplantation were lower for black patients (OR=0.55, 95% CI [0.33, 0.91], p-value=0.02) when compared to white patients. The odds for transplantation were lower for Asians when compared to whites, however the difference did not reach significance (OR=0.58, 95% CI [0.33, 1.02], p-value=0.06). Every year increase in age was associated with significantly lower odds of undergoing transplantation (OR=0.98, 95% CI [0.96,1.00], p-value=0.046). The remaining socioeconomic features did not significantly predict transplantation.
Table 2.
Multivariate analysis of transplantation rates for HCC (N=742)
| Predictor | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Race (reference: white) | |||
| Black | 0.55 | (0.33, 0.91) | 0.02 |
| Asian | 0.58 | (0.33, 1.03) | 0.06 |
| Hispanic | 0.83 | (0.57, 1.22) | 0.34 |
| Insurance (reference: government) | |||
| Commercial | 1.99 | (1.42, 2.79) | <0.001 |
| Government + supplement | 1.30 | (0.72, 2.35) | 0.38 |
| Age | 0.98 | (0.96, 1.0) | 0.05 |
| Liver diagnosis (reference: hepatitis B) | |||
| Alcoholic liver disease | 0.84 | (0.39, 1.81) | 0.66 |
| Hepatitis C | 0.99 | (0.56, 1.75) | 0.98 |
| NASH | 1.31 | (0.47, 3.65) | 0.61 |
| Other | 1.86 | (0.84, 4.12) | 0.12 |
When steps leading up transplantation were evaluated, patients with only Medicare or Medicaid coverage, and non-white patients experienced greater drop-off rates when compared to all transplant eligible patients (Figure 2). These patients fall off the treatment pipeline at increasingly higher rates, beginning after the specialist visit. No individual step in the transplantation pathway was identified as being predominantly responsible for this drop-off.
Figure 2.
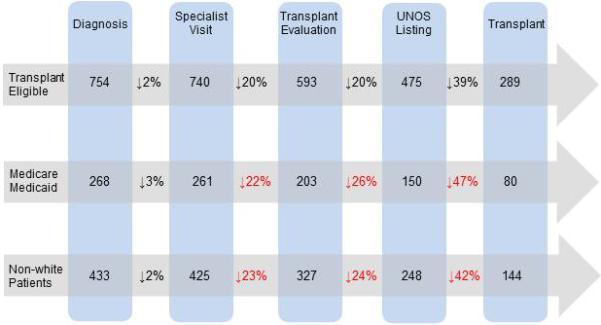
Progress of eligible patients through the pre-transplantation process: individuals with only Medicare or Medicaid, and non-white patients experience greater drop-off rates when compared to all eligible patients
Transplant eligible patients who underwent hepatic resection instead
A total of 268 patients were considered transplant eligible, but underwent hepatic resection instead. The demographics of these subjects were compared to those patients who underwent liver transplantation (n=289). More than half (52%) of eligible but resected patients were Asian, while the majority of those who received a liver transplantation was white (50%). The association between race and intervention (resection vs. transplantation) was statistically significant (p-value < 0.001), demonstrating that Asians were more likely to undergo resection versus transplantation than their non-Asian counterparts with similarly staged tumors and transplant eligibility characteristics. Logistic regression using only race as a predictor demonstrates that the odds of undergoing resection versus transplantation is seven times higher for Asians compared to whites (OR=7.20, 95% CI [4.594, 11.27], p-value <0.001).
Non-listing reasons
Several patients who were evaluated for liver transplantation, but ultimately not transplanted had documented non-listing reasons available for review (n= 310). Univariate analysis revealed that the “social reasons” category contained significantly more non-white (p=0.05) and government insured individuals (p=0.02) than those not listed for “clinical reasons”. Patients in the social reasons group were also noted to have a higher rate of delay (greater than 90 days) from diagnosis to specialist visit (p=0.009).
Discussion
Since transplantation is the most effective treatment for HCC, differences in access to care are likely to play a significant role in the survival disparity experienced by minorities with HCC. Our study confirms prior reports that liver transplantation is not accessed equally by all sociodemographic groups.
First, our study confirms that racial minorities are less likely to undergo liver transplantation for HCC when compared to white patients, even with similarly staged tumors and transplant eligibility criteria8,10,14,16-19. We found that this disparity reached statistical significance in blacks. This disparity was seen across all non-white groups, although individual breakdown by race showed a non-significant trend (p=0.06) for Asian patients. Lower rates of transplantation in Asian patients have been reported in previous studies10,15,18. Indeed, Asian patients with HCC are much more likely to have hepatitis B as their underlying liver disease; which is associated with better liver function and higher resectability, making transplantation less strongly indicated. We demonstrate that Asian patients have significantly higher hepatic resection rates than white patients for similarly staged tumors and transplant eligibility criteria. While prior reports have suggested that the lower rates of transplantation in Asians may be attributable to higher rates of resection10,15,18, we consider it likely that Asian patients have lower transplantation rates above and beyond that accountable by increased resectability.
Another main finding is that coverage with Medicare or Medicaid insurance alone is associated with decreased rates of transplantation, independent of other socioeconomic variables such as income. This finding corroborates similar reports by other investigators, suggesting that it is a true phenomenon16,17,29. For example, El-Serag and colleagues reported that HCC patients who were Medicare recipients underutilized transplantation and liver resection despite having early stage tumors; and Yu et al reported that patients with commercial insurance had higher transplantation rates than those with Medicaid29. Additional investigation is required to identify specifically how the presence of government insurance creates a disincentive for transplantation. We hypothesize that the presence of commercial insurance may facilitate transplantation by providing more comprehensive coverage for the many tests, procedures, and clinician visits that are required to complete a formal transplant evaluation. In the course of a typical transplantation evaluation, each patient is required to undergo numerous blood tests, upper endoscopy, repeated imaging, as well as clinician visits with a psychiatrist, hepatologist, and transplant surgeon. Efforts to equalize access to and coverage for these events may lead to a reduction in the observed insurance-based disparity in care.
Interventions to reduce disparities are inherently more successful when the underlying mechanism is well understood24. We sought to breakdown the complex process of undergoing liver transplantation into discrete steps, in the hopes of identifying the major barrier faced by disadvantaged patients. Patients with only Medicare or Medicaid, and non-white patients appear to fall off the treatment pipeline at increasingly higher rates, beginning after the specialist visit. While no specific stage in the transplantation pathway appeared to be the dominant hurdle to patients, our findings do suggest that targeted interventions towards individuals with government insurance and black patients could reduce this disparity in transplantation rates for HCC.
Also, patients who were excluded from the transplantation list for social reasons were more likely to be a racial/ethnic minority or covered by government insurance only. This suggests that efforts to increase transplantation rates in these groups should focus on combatting the most common nonlisting reasons, which in our study included active substance abuse, inconsistent follow-up, failure of psychological screening, and lack of financial clearance.
This study has certain limitations. As a retrospective study it is subject to the same weaknesses of all observational studies. Also, since all the patients in this study were treated at a single hospital in New York City, it is possible that the results are not generalizable to the greater population. In addition, it is possible that some of the patients who were lost to follow-up at our institution were seen and treated at other hospitals. However, given the finding that these patients had more advanced disease, we do not believe this is the case for most. Finally, due to the inconsistent long-term follow-up information on our patient cohort, we were unable to reliably assess whether our findings resulted in differences in survival. The strengths of this project include the robust socioeconomic and clinical data available for use in our analysis: as compared to prior reports that used large population data registries, we were able to rigorously control for cancer stage, the presence of all other comorbidities, resection status, and socioeconomic variables - in a large cohort of patients with confirmed HCC.
In conclusion, our study confirms that liver transplantation for HCC is not accessed equally by all demographic and socioeconomic groups. While the reasons for worse HCC survival among disadvantaged groups are complex and multifactorial, some of the observed disparity is due to decreased rates of transplantation by patients who are a racial minority, and those who are covered by government insurance. Since liver transplantation is the single most effective treatment for HCC, efforts to improve transplantation rates in these groups should help reduce the survival disparity.
Acknowledgments
Funding: We gratefully acknowledge that this study was supported by grant 1R03CA164546-01A1 from the National Cancer Institute.
Footnotes
Conflict of interest disclosures: All authors declare no disclosures.
Author contributions:
Study conception and design: Sarpel U, Bagiella E, Schwartz ME
Acquisition of data: Berger Y, Tedjasukmana A, Sekendiz Z
Analysis and interpretation of data: Sarpel U, Bagiella E, Suprun M
Drafting of manuscript: Sarpel U, Suprun M
Critical revision: Sarpel U, Schwartz ME
References
- 1. [2/10/15];GloboCan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 2.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004 Nov;127(5 Suppl 1):S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Hepatocellular carcinoma - United States, 2001-2006. MMWR Morb Mortal Wkly Rep. 2010 May 7;59(17):517–20. [PubMed] [Google Scholar]
- 4.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002 Mar 6;94(5):334–57. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 5.Ward E, Halpern M, Schrag N, et al. Association of Insurance with Cancer Care Utilization and Outcomes. CA Cancer J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 6.Davila JA, El-Serag HB. Racial differences in survival of HCC in the United States. Clin Gastroenterol Hepatol. 2006 Jan;4(1):104–10. [PubMed] [Google Scholar]
- 7.Sloane D, Chen H, Howell C. Racial disparity in primary HCC: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006 Dec;98(12):1934–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Artinyan A, Mailey B, Sanchez-Luege N, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with HCC in the United States. Cancer. 2010;116(5):1367–77. doi: 10.1002/cncr.24817. [DOI] [PubMed] [Google Scholar]
- 9.Kwong SL, Stewart SL, Aoki CA, et al. Disparities in HCC survival among Californians of Asian ancestry, 1988 to 2007. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2747–57. doi: 10.1158/1055-9965.EPI-10-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathur AK, Osborne NH, Lynch RJ, et al. Racial/ethnic disparities in access to care and survival for patients with early-stage HCC. Arch Surg. 2010 Dec;145(12):1158–63. doi: 10.1001/archsurg.2010.272. [DOI] [PubMed] [Google Scholar]
- 11.Zaydfudim V, Whiteside MA, Griffin MR, et al. Health insurance status affects staging and influences treatment strategies in patients with HCC. Ann Surg Oncol. 2010 Dec;17(12):3104–11. doi: 10.1245/s10434-010-1181-2. [DOI] [PubMed] [Google Scholar]
- 12.Wong LL, Hernandez B, Kwee S, et al. Healthcare disparities in Asians and Pacific Islanders with HCC. Am J Surg. 2012 Jun;203(6):726–32. doi: 10.1016/j.amjsurg.2011.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for HCC. Semin Liver Dis. 2005;25(2):181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 14.Robbins AS, Daily MF, Aoki CA, et al. Decreasing disparity in liver transplantation among white and Asian patients with HCC: California, 1998–2005. Cancer. 2008;113:2173–2179. doi: 10.1002/cncr.23766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel AB, McBride RB, El-Serag HB, et al. Racial disparities in utilization of liver transplantation for HCC in the United States, 1998–2002. Am J Gastroenterol. 2008;103:120–127. doi: 10.1111/j.1572-0241.2007.01634.x. [DOI] [PubMed] [Google Scholar]
- 16.Bryce CL, Chang CC, Angus DC, et al. The Effect of Race, Sex, and Insurance Status on Time-to-Listing Decisions for Liver Transplantation. J Transplant. 2010;2010:467976. doi: 10.1155/2010/467976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu JC, Neugut AI, Wang S, et al. Racial and insurance disparities in the receipt of transplant among patients with HCC. Cancer. 2010;116:1801–9. doi: 10.1002/cncr.24936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins AS, Cox DD, Johnson LB, et al. Persistent Disparities in Liver Transplantation for Patients with HCC in the United States, 1998 Through 2007. Cancer. 2011 Oct 1;117(19):4531–9. doi: 10.1002/cncr.26063. [DOI] [PubMed] [Google Scholar]
- 19.Wong RJ, Cheung R, Devaki, et al. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era. Liver Transpl. 2014 May;20(5):528–35. doi: 10.1002/lt.23820. [DOI] [PubMed] [Google Scholar]
- 20.Lacey M. Arizona Cuts Financing for Transplant Patients. The New York Times; Published Dec 2, 2010. “ http://www.nytimes.com/2010/12/03/us/03transplant.html?_r=0”. [Google Scholar]
- 21.Kish JK, Yu M, Percy-Laurry A, et al. Racial and Ethnic Disparities in Cancer Survival by Neighborhood Socioeconomic Status in SEER Registries. Journal of the National Cancer Institute Monographs. 2014;49:236–243. doi: 10.1093/jncimonographs/lgu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 23.Wan DW, Tzimas D, Smith JA, et al. Risk factors for early-onset and late-onset hepatocellular carcinoma in Asian immigrants with hepatitis B in the United States. Am J Gastroenterol. 2011 Nov;106(11):1994–2000. doi: 10.1038/ajg.2011.302. [DOI] [PubMed] [Google Scholar]
- 24.Halpern MT. Cancer Disparities: It Is Time to Come of Age. Cancer. 2015 Apr 15;121(8):1158–9. doi: 10.1002/cncr.29192. [DOI] [PubMed] [Google Scholar]
- 25.Sharma P, Balan V, Hernandez JL, et al. Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transpl. 2004 Jan;10(1):36–41. doi: 10.1002/lt.20012. [DOI] [PubMed] [Google Scholar]
- 26.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005 Nov;42(5):1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Census Bureau . U.S. Summary: 2000, Census US Profile. Washington, D.C.: [Google Scholar]
- 28.Sauerbrei W, Meier-Hirmer C, Benner A, et al. Multivariable regression model building by using fractional polynomials. Comput Stat Data Anal. 2006 Aug;50(12):3464–3485. [Google Scholar]
- 29.El-Serag HB, Siegel AB, Davila JA, et al. Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States. J Hepatol. 2006 Jan;44(1):158–66. doi: 10.1016/j.jhep.2005.10.002. [DOI] [PubMed] [Google Scholar]