Abstract
Objective
Comparing inflammatory and immunological trajectories in burned adults vs. burned elderly patients to gain novel insights and better understanding why elderly have poor outcomes.
Summary Background Data
Despite receiving the same treatment and clinical consideration as all other burn patients, elderly patients continue to have substantially poorer outcomes compared to adults. In light of an ageing population, gaining a better understanding of their susceptibility to complications and creating new treatment strategies is imperative.
Methods
We included 130 burn patients (94 adults: <65 years-old & 36 elderly: ≥65 years-old) and 10 healthy controls in this study. Immune activity and expression was assessed using bioplex at various time points. Clinical outcomes such as infection, sepsis and mortality were prospectively collected.
Results
Elderly burn patients had significantly lower burn size but significantly higher Baux scores. Morbidity and mortality was significantly increased in the elderly cohort. Immune biomarkers indicated that elderly are immune compromised and unable to respond with the expected inflammatory response during the early phase after injury. This trajectory changes to a hyper-inflammatory pattern during the later phase after burn. These findings are even more pronounced when comparing sepsis vs. non-sepsis patients as well as survivors vs. non-survivors in the elderly.
Conclusions
Elderly burned patients mount a delayed immune and dampened inflammatory response early after burn injury that changes to an augmented response at later time points. Late-onset sepsis and non-survivors had an immune exhaustion phenotype, which may represent one of the main mediators responsible for the striking mortality in elderly.
Keywords: burn, elderly, inflammation, cytokines, sepsis, morbidity, mortality
Introduction
Older adults represent the fastest growing population and presently account for over 14% of Canadians with estimations placing more elderly than children by 2016.1 Due to age-related cognitive and physical limitations, the elderly are particularly susceptible to burn injuries.2 These include thinning of the skin, decreased sensations, mental alertness and other contributing factors. Thus, the increased risk and heightened probability of the expanding elderly population suffering from burn injuries require burn treatment paradigms to incorporate the treatment tailored to older subjects. The major causes of thermal trauma in elderly include flame burn (66%), cooking accidents (33%), scald burns (15%) and contact injuries (15%).3 While the institution of early excision and grafting, implementation of ICU protocols, adequate nutrition, and improvements in wound healing markedly increased the survival of pediatric and adult burn patients, the LD50 burn size in elderly has remained the same, indicating the lack of advancements in elderly burn care.
The trauma of a severe burn injury induces distinct systemic inflammatory and immunological responses primarily mediated by cytokines and has been shown in pediatric and adult burn patients.4–6 Cytokines are a group of proteins with autocrine and endocrine activities that facilitates communication between different cell types, including those that mediate immune function, angiogenesis, cell proliferation and apoptosis. They also regulate homeostasis and cellular repair through effects on cell growth and differentiation via receptor activation. Various cytokines, such as interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF), have been utilized as markers related to the severity of burn injury.7–9 Our previous work on a large prospective study of 242 burn patients showed that the entire inflammatory response was profoundly altered up to 2–3 years post-burn.10 Pro-inflammatory cytokine synthesis or “cytokine storm” is a systemic response to manage deleterious effects of thermal injury in an attempt to restore homeostasis. However, when this response is prolonged post-trauma it can result in stress-induced hyperglycemia, insulin resistance, hypermetabolism and catabolism, which are precursors to the aforementioned negative outcomes.11–13
In general, elderly have a unique immune profile compared to adult and pediatric patients. Despite having diminished immunity, older individuals have higher baseline levels of pro-inflammatory cytokines in the absence of insult and consequently have an increased risk of complications.14,15 Some of the factors contributing to poor outcomes include pre-existing medical conditions, frailty score, immune-senescence, impaired ability to overcome post-burn infections, skin thinning leading to deeper burns, and generally decreased metabolic resources and capacity.16–21 When comparing gender differences, previous reports have shown female adult burn patients have poorer outcomes and paediatric females have increased hormone secretion and less inflammation.22–25 This has been attributed in part to the immune-enhancing effects in pre-menopausal females.26,27 However, no gender differences were reported for the increased risk of mortality for female patients over 60 years of age.28 The exact relationship between gender and outcomes in elderly burn patients remains inconclusive. Inflammation is a key factor in the progressive loss of lean tissue and impaired immune function observed in aging, suggesting a predisposition to hypermetabolism. Therefore, we hypothesize that elderly have a hyper-inflammatory response after injury associated with an over-activated immune response that would explain increased morbidity and mortality after burn. Hereon, we aim to determine the inflammatory and immune profiles in elderly with burn injuries and compare these to burned adults; and ultimately, we will extend this effort to pinpoint differences contributing towards sepsis susceptibility and non-survivorship in elderly populations.
Methods
This study was approved by the Research Ethics Board, Sunnybrook Health Sciences Centre (REB#: 194-2010). Informed consent was obtained from patients or from their Substitute Decision Makers.
Demographics
All patients were treated according to standardized protocols in our regional burn center as previously published.29 Demographic and clinical data, complications, including infection, sepsis, morbidity and mortality were all prospectively collected from 2010–2014. Patients were divided into adult, younger than 65 (<65) years and elderly (≧65) years of age, as defined by the National Institute of Health and World Health Organization. To examine the effects of elderly status, we used logistical regression analysis for dichotomous outcomes, adjusting for age, TBSA and inhalation injury. The clinical complications presently reported include respiratory, renal, liver and cardiac failures, multiple organ failure (MOF), pneumonia, bacteremia, infection and sepsis are consistent with previously established criteria.30 All subsequent cytokine analysis of immune activity was conducted using subsets of this clinical population and is described below.
Bio-specimen collection and processing
EDTA-anticoagulated samples were drawn upon admission and at various time points throughout hospital stay. Blood samples were processed using a standard Percoll-based PBMC isolation from the periphery. Timeframe of collection was measured as their days post injury. Most admissions occur within the first 48 hours after injury with samples collected upon admission, during early excision and grafting, and subsequent procedures (collection dates ranging from 0 to 140 days).
Cytokine/inflammatory profile
A subset of patient samples from the prospective tissue collection (2010–2014) was used to compare inflammatory profiles between adult and elderly patients. Using the Multiplex platform (Millipore, MA), a panel of inflammatory cytokines, chemokines and growth factors were all analyzed. Experimental kits were all conducted in accordance with manufacturers’ protocol. Plasma samples from a total of 10 healthy controls, 94 adult and 36 elderly patients were analyzed in this study. Raw data was processed using Millipore Analyst software. Samples were taken at various time points during the course of treatment. All values are presented as mean ± SEM for the respective cytokine concentration, expressed in pg/ml.
Statistical analysis
All data was analyzed by student’s t-test, one- and two-way ANOVA with a Tukey post-hoc test used when two or more groups were present. Mann-Whitney was also utilized for non-parametric tests and unequal distribution of data was assumed where appropriate. Categorical data was compared using Fisher exact test and survival curves via Kaplan-Meier. In order to measure the alterations over time, patients were divided into the following intervals based on sample availability and clinical representativeness: 0–6, 7–14, 15–30, 30+ days after burn injury. Further, both patient groups were divided into moderate burn (<30% TBSA) and severe burn (≧30% TBSA) in order to compare immune alterations relative to injury severity and where applicable early (<14 days after burn) and late (14+ days after burn) onset sepsis. When considering gender differences we did not observe any significant differences in the immune profiles between adults and elderly patients and as such was not included in our analysis. Pearson correlations were conducted in order to explore the association between inflammatory mediators and clinical demographics. Heatmaps are represented as the mean cytokine concentration of normal, adult and elderly burn groups respectively with data expressed as a function of advancing time after injury and were created in collaboration with Robert Tisma, M.Eng using GNU Octave. All other graphs were created using Graphpad Prism 5.0 (San Diego, CA) and analyzed statistically using SPSS 20 (IBM Corp., NY), with significance accepted at p<0.05.
Results
Demographics and clinical outcomes
When considering the entire prospective patient populace of our burn center from 2010–2014, elderly patients had slightly smaller TBSA, but significantly higher Baux scores and greater mortality (Mean ± SD: Adult: 17±16% vs. Elderly: 15±14% TBSA, t(406) = 0.76, n.s.; Adult: 61±24 & Elderly: 92±17, t(397) = 13.2, p<0.0001; Kaplan-Meier = 14.94, p<0.0001, Table 1 & Figure 1), respectively. Elderly patients also had a significantly higher percentage of combined complications (Table 2). Due to elderly having a significantly higher Baux score, despite having lower mean burn size, logistical regression analysis was used to look at the effect of elderly status on complications. After adjusting for age, TBSA, and inhalation injury, elderly status did not have a significant effect on respiratory, renal, liver, cardiac and multi-organ failures (Table 3). However, when combining the aforementioned complications into one group, the elderly were more likely to have comorbidities (p=0.009). Similar to the above findings, sepsis and pneumonia suggested a greater likelihood of older patients having a greater susceptibility, however only the later reached statistical significance (p<0.05), which collectively can be attributed to the relatively small sample size.
Table 1. Patient demographics.
Data presented as n (%) and P-values were obtained using ANOVA unless otherwise stated.
| Adult | Elderly | P | |
|---|---|---|---|
| N | 332 | 80 | |
| Age, yr | 41 ± 13 | 75 ± 8 | |
| Sex, Male | 241 (73%) | 46 (58%) | <0.01a |
| TBSA, % | 17 ± 16 | 15 ± 14 | 0.449 |
| Length of stay, days | 22 ± 22 | 23 ± 14 | 0.772 |
| Length of stay / % burn | 2.47 | 2.65 | 0.775 |
| Inhalation Injury | 68 (27%) | 12 (18%) | 0.267a |
| Baux | 61 ± 24 | 92 ± 17 | <0.0001 |
P-value from Fisher exact test.
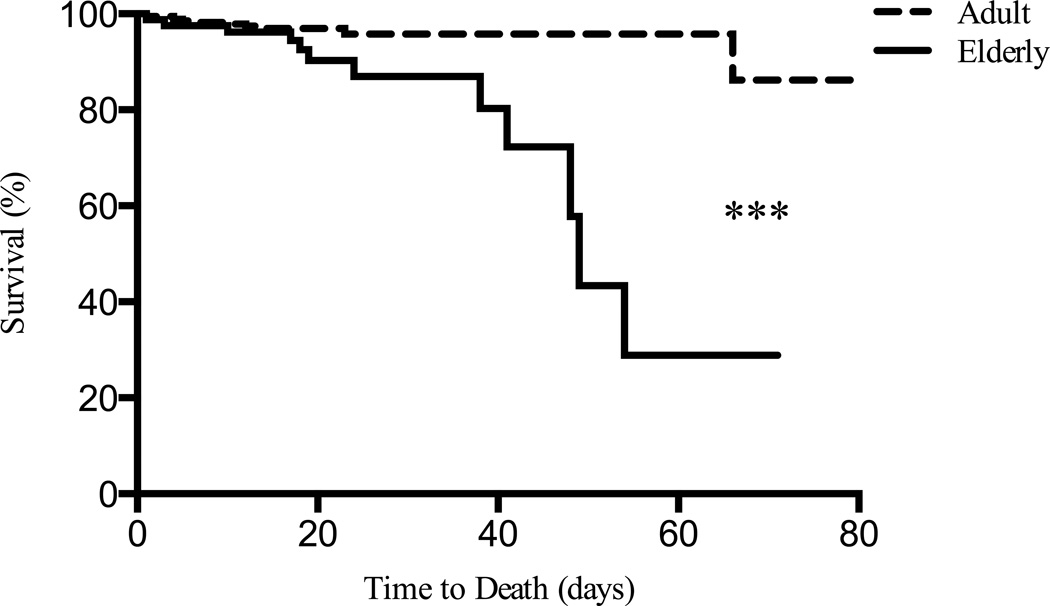
Figure 1.
Elderly patients showed decrease survival relative to adult thermally injured with divergence occurring beyond 30 days (n = 410). ***Significant difference was found between adults and elderly burns (Kaplan-Meier = 14.94, p<0.0001).
Table 2. Patient Complications.
Patient complications are presented for adult and elderly burn patients. Data presented as n (%), P-value was obtained using Fisher exact test.
| Adult | Elderly | P | |
|---|---|---|---|
| N | 332 | 80 | |
| Respiratory Failure | 10 (3.1%) | 4 (5.3%) | 0.28 |
| Renal Failure | 15 (4.8%) | 5 (6.8%) | 0.343 |
| Liver Failure | 5 (1.5%) | 0 | 0.338 |
| Cardiac Failure | 0 | 1 (1.3%) | 0.194 |
| Multi-Organ Failure | 8 (2.5%) | 5 (6.8%) | 0.086 |
| *Combined | 21 (6.8%) | 10 (14.3%) | <0.05 |
| Sepsis | 35 (11.8%) | 9 (12.9%) | 0.494 |
| Pneumonia | 60 (22.2%) | 19 (31.7%) | 0.159 |
Combined includes Respiratory, Renal, Liver, Cardiac and MOF
Table 3. Likelihood of complications by elderly status.
Logistical regression was used to compare adults (n = 332) and elderly (n = 80) that adjusted for patient demographics and injury characteristics with odds ratio.
| Odds Ratio | P | |
|---|---|---|
| Respiratory Failure | 2.59 | 0.143 |
| Renal Failure | 2.60 | 0.165 |
| Liver Failure | - | - |
| Cardiac Failure | - | - |
| Multi-Organ Failure | - | - |
| *Combined | 3.85 | 0.009 |
| Sepsis | 1.65 | 0.305 |
| Pneumonia | 1.98 | 0.046 |
Delayed inflammatory response in elderly burned patients
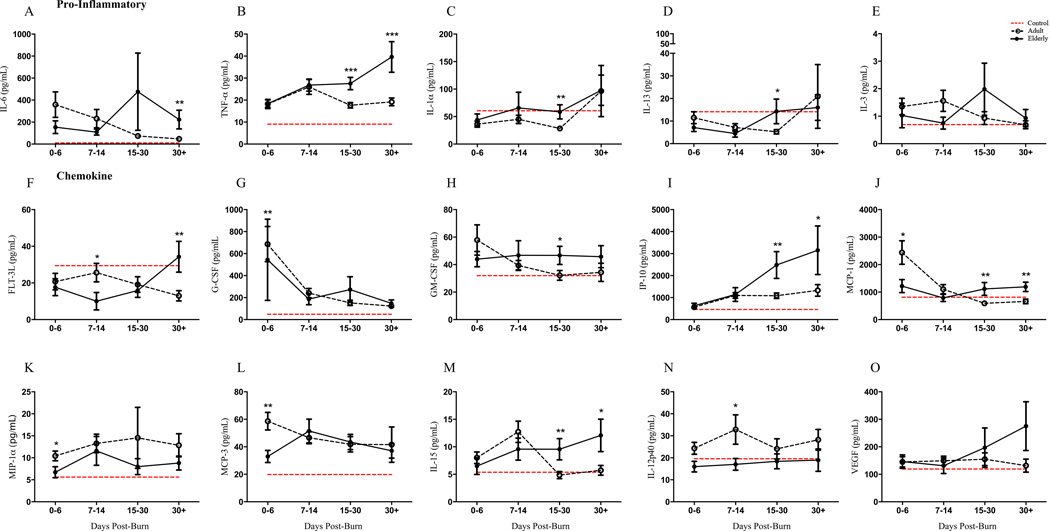
Relative to healthy controls, burn patients of both groups had up to a ten-fold up-/down-regulation of inflammatory mediators. Cytokine profile showed the emergence of delayed immune response in elderly patients and was consistent for chemokine, pro- and anti- inflammation biomarkers indicating a systemic pathophysiology (Figure 2A–O). The acute phase of burn injury was characterized by lower activation of critical immune factors and beyond the third week, inflammatory markers such as TNF-α, IL-1α, IL-6, FLT-3L, GM-CSF, MCP-1, IP-10 and IL-15 were significantly altered (refer to Supplemental Table 1 for statistics).
Figure 2.
Plasma cytokine profiling of healthy controls (n = 10), adult (n = 94) and elderly (n = 36) groups over the course of hospital stay (0–6, 7–14, 15–30, 30+ days post burn) showed dramatic alterations relative to healthy controls. The emergence of a delayed immune response in the older group becomes apparent beyond the second week after injury. Comparing both burn groups, pro-inflammatory cytokines were increased in elderly beyond 14-days after injury for IL-6 (A), TNF-α (B), IL-1α (C), IL-13 (D) and IL-3 (E). FMS-like tyrosine kinase 3 ligand (FLT-3L) (F), Granulocyte-colony stimulating factor (G-CSF) (G), Granulocyte-macrophage colony-stimulating factor (GM-CSF) (H), interferon gamma-induced protein 10 (IP-10) (I) and monocyte chemotactic protein 1 (MCP-1) (J) showed similar delayed upregulation. Other significant alterations were observed in MIP-1α (K), MCP-3 (L), IL-15 (M), IL-12p40 (N). Dashed red lines represent healthy control mean values and data is represented as mean ± SEM, *p<0.05, **p<0.01 and ***p<0.001 relative to adult burn group.
Next, we validated our biomarkers by determining whether these candidates correlated with severity of injury. A positive Pearson correlation was found between injury severity and the following cytokines: G-CSF (p=0.004), Frantalkine (p<0.001), IFN-α2 (p<0.05), GRO (p=0.002), IL-10 (p<0.001), IL-12p70 (p<0.05), IL-15 (p<0.05), IL-1RA (p<0.001), IL-1β (p=0.008), IL-2 (p<0.05), IL-6 (p<0.05), IL-8 (p<0.001), IP-10 (p=0.004), MCP-1 (p<0.05), MIP-1α (p<0.05), MIP-1β (p<0.05) and TNF-α (p<0.001). All other cytokines and immune measures that did not reach statistical significance are found in Supplemental Figure 1. Moreover, when exclusively comparing pneumonia-, bacteremia- and infectious-positive adult and elderly burn patients; the delayed phenotype was also upheld further supporting the prevalence of this effect in older patients (Supplemental Figure 2).
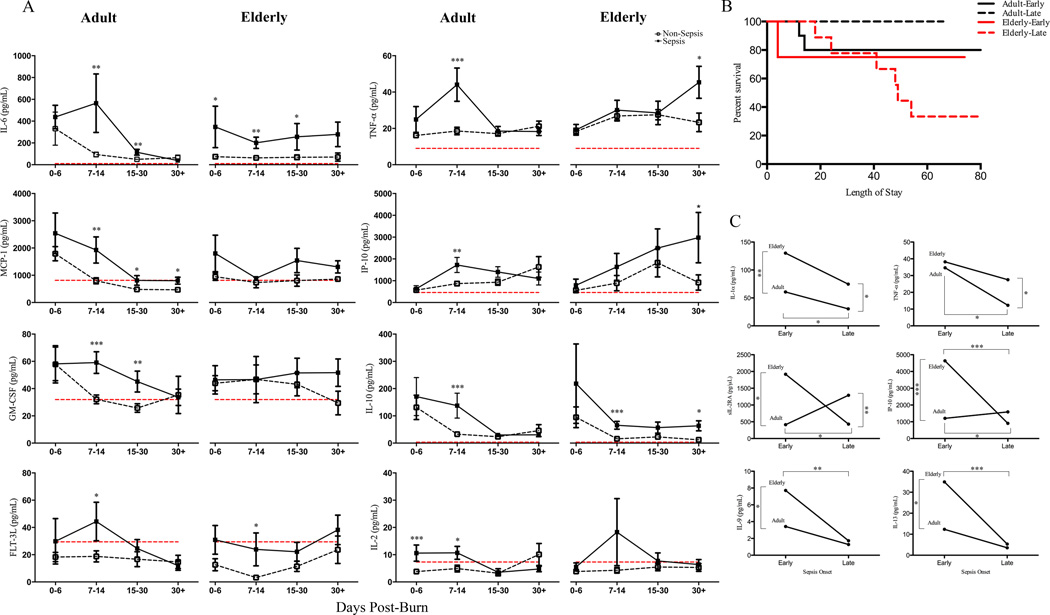
Sepsis
When adult and elderly burned patients were stratified into non-sepsis and sepsis cohorts, we found similar increases in cytokine expression in the septicemia groups for both ages (Figure 3A). Exclusively comparing sepsis patients between each age cohort, similar to earlier findings, sepsis did not distinguish the elderly group until after the second week post-injury (Supplemental Figure 3). An ANOVA test comparing non-sepsis and sepsis adults revealed adult sepsis had greater levels of chemokine expression during early time points (<14 days) including MCP-1 (p<0.05), IP-10 (p=0.003), GM-CSF (p=0.006), EGF (p=0.002), FGF-2 (p=0.007) and MIP-1β (p=0.008). Similarly, pro-inflammatory and other immune cytokines (IL-6, TNF-α, FLT-3L and IL-2) had similar patterns. Extending this analysis and comparing non-septicemia and septicemia-positive elderly, despite the sepsis group having slight increases at each time point there were no significant alterations in early chemokine activity suggesting a delayed immune responsiveness and inability to respond to initial stress. Pro-inflammatory IL-6 was elevated in elderly with sepsis throughout all time points after injury, however TNF-α did not distinguish the groups until 30+ days. Lastly, IL-10 was consistently higher for all time points beyond the first week in sepsis elderly relative to age-matched non-sepsis with student’s t-test revealing significance at 7–14 days (p=0.001) and 30+ days (p=0.021). This suggests a persistent anti-inflammatory state in sepsis elderly and may be contributing to the dampened/delayed inflammation (Figure 3A). By comparing the immune profile of adult sepsis cohort to elderly sepsis over time, adults peaked at earlier time points where as in elderly it occurred later (TNF-α: adult (7–14 days) - 48 pg/mL vs. elderly (30+ days) - 45 pg/mL), which is consistent with previously mentioned findings. Similarly, other early indicators of sepsis such as proliferative T-cell factor IL-2 and dendritic cell stimulator FLT-3L appeared to be upregulated in both adult and elderly relative to non-sepsis counterparts with notable increases within the first 14-days post injury (Adult IL-2: 0–6 days – p=0.001; 7–14 days – p=0.026; FLT-3L: 7–14 days – p=0.022; Elderly FLT-3L: 7–14 days - p=0.031). The consequence of uncoordinated inflammatory response in elderly with sepsis was further explored by comparing early (0–14 days) versus late sepsis onset (>14 days post-injury). While late onset of sepsis in adults had no deaths, elderly had a 75% mortality rate (Figure 3B). This observation was further explored by extending these parameters to cytokine proportions. Student’s t-test and Mann-Whitney analysis revealed early onset sepsis was a better measure to differentiate the age cohorts with significance obtained for IL-1α (p=0.009) MCP-3 (p<0.05), sIL-2RA (p<0.05), IP-10 (p<0.0001), IL-9 (p<0.05), IL-13 (p<0.05) (Figure 3C). Late onset sepsis showed differences in sIL-2RA (p<0.05) and TNF-α (p<0.001) between adults and elderly. When comparing onset of sepsis in elderly patients alone, late onset sepsis in elderly had significantly lower concentrations of FLT-3L, IL-12p40, IL-12p70, IL-4, IL-5, IL-9, IL-13 and IP-10 (Figure 3C & Supplemental Figure 4). Taken together, this data suggests that sepsis is not as transparent as the observed physiological response under non-complication conditions and the persistent pro- and anti-inflammatory response presently described may drive this vulnerable population to poorer outcomes. Further, the onset of sepsis should be taken into account when making distinctions between populations.
Figure 3.
Immunological profile of adult and elderly septicemia patients. Comparing adult non-sepsis and sepsis burn patient’s showed significant increases in inflammatory (IL-6, TNF-α, IL-10), chemokine (MCP-1, IP-10, GM-CSF) and other immune mediators (FLT-3L, IL-2) early after injury (0–6 & 7–14 days). Elderly non-sepsis and sepsis patients showed very similar cytokine expression over time with early significance found for IL-6, IL-10, FLT-3L and later (>14 days) for TNF-α, and IP-10. (A). Kaplan-Meier survival curves for early and late onset sepsis and for both adult and elderly groups. Early-onset sepsis for both age group resulted in approximately 80% survival, however a striking difference was observed when comparing late-onset sepsis with elderly supporting a 30% survival (B). Extending the comparison of sepsis onset to cytokine analysis, early (0–14 days) versus late (>14 days) sepsis onset was compared for various immune mediators. All cytokine presented were found to be significant and the lower concentration of late-onset sepsis elderly suggest a dampened response contributing to mortality (C). Dashed red lines represent healthy control mean values and sepsis data is represented as mean ± SEM, *p<0.05, **p<0.01 and ***p<0.001 relative to non-sepsis for each respective burn age group.
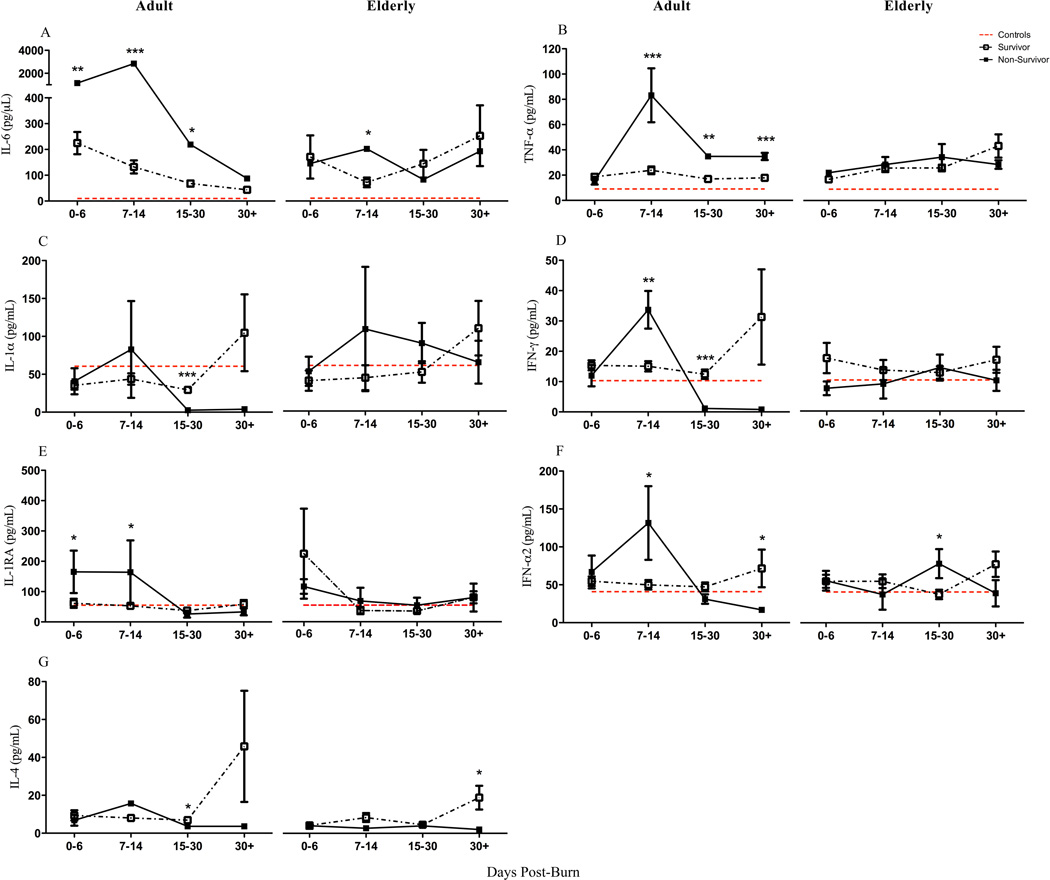
Mortality
The comparison between survivors and non-survivors for both age groups reaffirmed not only the aforementioned delayed inflammatory response, but also other substantial differences. A repeated measures ANOVA of pro-inflammatory cytokine expression between adult survivors and non-survivors showed group differences over the length of hospital stay [mortality × days post-burn groups: IL-6: F(1,212) = 36.02, p<0.001) and TNF-α: (F(1,212) = 6.16, p=0.014). Specifically, the most profound upregulation in IL-6 (7–14 days: p<0.001) and TNF-α (7–14 days: p=0.001) was early during the course of hospital stay (Figure 4). These observations were not present in elderly non-survivors relative to age-matched survivors. Beyond the second week, adult non-survivors had substantially decreased inflammatory markers relative to earlier time points. Elderly non-survivors did not display the same response as adults with most markers remaining invariable, with students t-test revealing exceptions for IL-6 (7–14 days: p<0.05), IFN-α2 (15–30 days: p<0.05), and IL-4 (30+: p<0.05). Overall, the elderly immune profile reflected an immune incapacitation. In summary, our data indicate that elderly non-survivors do not have an enhanced or augmented inflammatory response, but rather a dampened immune activation.
Figure 4.
Cytokine distribution of survivors and non-survivors over time showed an early (<14 days) elevated expression in adult non-survivors for IL-6, TNF-α, IL-1α, IFN-γ, IL-1RA & IFN-α2. They further supported immune exhaustion in this group with IL-1α, IFN-γ, IFN-α2 & IL-4 showing lower expression than survivors and healthy controls beyond 14-days after injury. Elderly survivors and non-survivors showed near-indistinguishable difference for all cytokines over the coarse of time and further proposed a non-responsive immune profile compared to adults for inflammation (TNF-α, IL-1RA, IFN-γ, IL-4). Dashed red lines represent healthy control mean values and non-survivor data is represented as mean ± SEM, *p<0.05, **p<0.01 and ***p<0.001 relative to survivors for each respective burn age group.
Predictors of mortality
Lastly, we aimed to predict whether cytokines are able to differentiate survivors and non-survivors in adult and elderly patients. The practice of early wound excision resulting in improved outcomes has been widely accepted for quite some time.31,32 With this in mind, we aimed to discover blood markers during early collection time points that could differentiate mortality groups. Within the first 72-hours after injury, using Mann-Whitney nonparametric analysis adult patients showed significant differences between survivors and non-survivors for IL-6 (177.6 ± 46.8 vs. 3427.5 ± 2258, p<0.01), FGF-2 (p<0.01), FLT-3L (p<0.05), Fractalkine (p<0.01), IL-10 (p<0.01), IL-12p70 (p<0.05), IL-15 (p<0.05), IL-1RA (p<0.01), IL-2 (p<0.05), IL-8 (p<0.01), MCP-1 (p<0.05), MIP-1α (p<0.05). Non-survivors had a notable elevation in all immune mediators and may suggest a predisposition to immune exhaustion and subsequent susceptibility to death. Lastly, pro-inflammatory cytokine expression in adult non-survivors negatively correlated with length of stay (IL-6, IL-1α & IL-1β). When conducting the same comparison in elderly patients, there were no significant differences reported between survivors and non-survivors in the entire cytokine profile. Thus, the lack of early predictors further demonstrates the complexity and difficulty in understanding this pathogenic process in older burn patients.
Discussion
The present study aimed to characterize and differentiate the immune inflammatory response of adult and elderly burned patients throughout their hospital course. In contrast to our initial prediction, relative to adults, the elderly cytokine profile reflected a delayed and divergent immune response beyond the second week after injury consistent for pro-inflammatory, anti-inflammatory, chemokines and other immune effectors alike. In addition, sepsis in elderly demonstrated a slightly greater presence of particular cytokines and a differential profile based on the onset of sepsis. Lastly, mortality generated a unique characteristic in adults that shows early over-activation followed by depletion predisposing this population to adverse outcomes, however no definitive immune signature could be concluded in the elderly.
Aging is a distinctive event that permanently alters the systemic capacity by interfering with homeostatic function. Also known as “inflamm-aging”, this chronic inflammatory state of deregulated IL-6, TNF-α and C-reactive protein expression consequently impairing the ability to fight infections and causing greater complications.14,33 This inflammation also manifests neurologically occurring alongside the production of astrocytes and microglia.34 Other models of the immune response taking place after injury have shown that the initial systemic inflammation is greater, followed by a parallel anti-inflammatory response.15 Presently, we report that the delayed “cytokine storm” and characteristic over-expression of immune mediators in elderly patients several weeks after initial insult may explain the increased incidence of infection, sepsis, complications and mortality, which has been extensively supported.18,20,35
Consistent with a previous report showing differences between the inflammatory profile of pediatric and adult burn patients,36 our present data support and extend the notion of an uncoordinated and over-activation response to insult when comparing patients from age-disparate groups. This was consistent for numerous cytokines in both burn and sepsis conditions as shown in Figures 2–4. Although the precise mechanism is not well understood, it has been proposed that “priming” of the immune system due to age-induced chronic inflammation as a driving force increasing vulnerability to negative outcomes.15 These findings were not limited to serological measures. We have previously published that inflammasome activity increases as a function of injury severity both at the gene and protein levels.37 Other unpublished data from our group suggests a mild effect showing a dampened immune response in elderly using excised white adipose tissue taken during the first week after injury characterized by lower proportions of IL-1β+ and caspase-1+ macrophage compared to adults. Concurrently, these observations directly relate the alterations in blood to adipose tissue-specific inflammatory mediators from the site of injury. With these alterations in tissue directly correlating with length of hospital stay it suggests a prospective approach to predictive indictors of clinical course.
The immune-senescent state of the elderly cohort (as defined by the decrease in number of whole body leukocyte count relative to adults) entails their hampered ability to mount a robust immune response against external insults.38 Thus, in attempt to produce a potent immune response, theoretically, each individual white blood cell of the elderly’s immune system would have to function at a greater-than-normal capacity in order to achieve an immune output comparable to adults (due to the differential leukocyte count). This overload on the elderly leukocytes, despite the delayed production of effector molecules (as shown by the surge of cytokines in the later stages of the pathophysiology from our data) bankrupts the elderly’s immune potential consequently making them susceptible to future insults, rendering them to an “immune exhausted” state. As immune activation post injury is required to ensure protection and survival of patients post thermal injury, we postulate the overall immune phenotype and the clinical outcomes observed in the elderly may be attributed to the phenomenon of the aforementioned “immune exhaustion”. This notion would explain why elderly burn patients with late onset sepsis had such a striking mortality rate (Figure 3B), a consequence of depleting the necessary immune reserve required to mount an adequate response and ultimately succumb to their injuries. Other reports have also shown that early rapid decline is one of the best predictors of fatal outcome.39
The immune response assessed within 72-hours post injury between adult survivors and non-survivors suggest an exaggerated response for pro-inflammatory, anti-inflammatory and chemokines, although with no distinguishable differences between the two groups. Albeit interesting, the difficulty with this finding is that no parallels can be drawn from the results of the adult cohort to the immune output of elderly patients. In fact, older individuals display an inverse relationship with decreased responsiveness at the characteristic early phase (within the first 14-days) and an overall “immune exhausted phenotype” that is likely the culprit predisposing this vulnerable population to complications and inevitable mortality.40 Previous studies have shown that the first three days after injury are associated with alterations in IL-6, IL-12p70 and TNF-α, which collectively predicted mortality in pediatric burn patients.41 Presently, we expanded our initial survivorship analysis (Figure 4) to compare cytokine alterations within 72-hours after hospitalization to determine if initial tissue collection would distinguish the elderly groups. This approach accounted for the possibility that this timeline may not reflect the same dampened response as observed within the 0–6 day group. Much to our surprise, there were no significant differences between elderly survivors and non-survivors and further supports older patients warranting independent characterization. At this time we are unable to speculate the underlying processes driving this response in the elderly. Rather, we presently report the observed pattern/trajectory of immune response to burn in elderly and support the necessity for new approaches to be implemented in order to predict outcomes due to cytokine appraisal of blood may be too late. In summary, the ability for the cytokine storm occurring within the first few days to differentiate outcomes in adults only supports that the dampened immune response in elderly not only assimilates complication groups but it also supports the digression of the survival curve beyond the first month.
An elegant study conducted by Faist and colleagues showed that immune stimulation in elderly patients undergoing surgery results in increased lymphocyte proliferation and cellular immune response.42 Perioperative administration of immune activating thymopentin also reduced severity of infection in elderly.43 Similarly, septic shock has been shown to result in depression in the capacity of the pro- and anti-inflammatory response.44 Anticipating a delayed immune hyperactivity does not reflect mortality predisposition and rather can be described as a “failure to launch” phenotype. However, the complete absence of cytokine production may predispose this cohort to fatal outcomes. Both sepsis and mortality sub-groups supported the exhaustion phenotype presently observed (Figures 3–4). Ongoing investigations are being conducted to determine the culprits responsible for the prevention of early immune responsiveness in the elderly. Our present findings suggest that exploration of immune activating agents is lacking. Additionally, we needs to conclusively determine whether "jump-starting" the immune system would promote homeostatic balance during the acute phase. Other tissues may also prove to be better outcome predictors that overcome the temporal delays of cytokine detection in blood. Importantly, the unique profile of elderly burn patients warrants its own clinical management and supports the previous notion of “age-adapted” therapies to target morbidity and outcomes.45
This study demonstrates a delayed and divergent immune response in elderly burn patients. It also supports the notion of an increased susceptibility to secondary infections and complications due to this late over-activation in chemokines and pro-inflammation. However, some of the limitations of the present study include that elderly have various comorbidities and it is unclear how these comorbidities affect the inflammatory a priori. Premorbid conditions have been shown to be present in 85% of patients over 65 years old.46 In addition, our present cytokine absolute proportions differ from previous publications in burn patients.10,36 This discrepancy may be attributed to the individual multiplex platform/instrument used per study and the variations in handling/processing of blood collection. These factors will collectively contribute to alterations in the absolute concentration reported in the independent studies; thus cross-study comparisons of absolute cytokine concentrations are not recommended. While noting this technical limitation, we report concentrations and compare adults relative to elderly burn patients in our present study. Furthermore, healthy control samples were used as an added measure to compare the inherent relative values and should be treated for analysis as such. Although equal proportions of age-matched controls were not presently used, alterations between healthy adult and elderly have been extensively reported with older individuals having greater inflammation and baseline cytokine concentrations.47–49 Retrospectively, the time points of our blood sampling may have missed crucial “windows” where an exacerbated inflammatory response would have been observed. Also, serum and plasma represent an accumulation of many sources from the body and it is not clear how these cytokines correlate with actual cell functions. Efforts overcoming these translational hurdles should be investigated in subsequent studies to further our understanding and implementation for the pathology and care for elderly patients’, respectively.
In summary, this study shows an impaired immune response and exhaustion phenotype in elderly burn patients, which unfortunately, lacks biomarkers to predict sepsis and mortality. It further demonstrates the urgent need for new and age-specific treatment options that will stimulate both the immune responsiveness during the acute phase of injury and subsequent vigilance against infection and sepsis for this vulnerable population. Early excision and autografting of all wounds to avoid immune depression in the elderly patient cohort is paramount. Subsequent analyses should be designed to unravel the mechanisms suppressing normal immune activation and the culprit driving the exhaustion profile in non-survivors.
Supplementary Material
Supplemental Table 1: Statistical analysis of adult versus elderly burns for various cytokines for 0–6 (R1), 7–14 (R2), 15–13 (R3) & 30+ (R4) days post-injury. Dashed red lines represent healthy control mean values and non-survivor data is represented as mean ± SEM, *p<0.05, **p<0.01 and ***p<0.001 relative to adult burn group.
Supplemental Figure 1: Complete cytokine profile of burn patients used in the study that either did not reach statistical significance or were not previously reported. Comparison is between healthy controls (dashed red line), adult and elderly burned patients during the length of hospital stay. Data is represented as mean ± SEM and where applicable, *p<0.05, **p<0.01 and ***p<0.001 relative to adult burn group.
Supplemental Figure 2: Pro-inflammatory, chemokine and immune mediator cytokine profile of adult and elderly burn patients that all encountered pneumonia, bacteremia or infection complications. Elderly burn patients showed a delayed increase in cytokine activation compared to adults with significance occurring beyond 14 days after injury (A–F). Bacteremia (G–L) and infection (M–R) showed consistent immune activation profiles as described previously. All cytokines presented were divided into post-injury groups (0–6, 7–14, 15–30 & 30+ days post-burn). Dashed red lines represent healthy control mean values and data is represented as mean ± SEM, *p<0.05, **p<0.01 and ***p<0.001 relative to adult burns patients for each respective complication.
Supplemental Figure 3: Complete cytokine profile of sepsis burn patients. Adult and elderly patients that encountered septicemia were included in the analysis. All cytokines presented reached statistical significance and were divided into post-injury groups (0–6, 7–14, 15–30 & 30+ days post-burn). As evident by the intensity of G-CSF, IFN-α2, IL-1α, IL-6 & IL-8 the cytokine in elderly burns increased at later time points (15–30 & 30+) reflecting an early-dampened response to insult. Increasing intensity is represented as green to red, with black reflecting no change (note: color bar is asymmetrical and color intensity should be compared directly to controls and burn injury groups).
Supplemental Figure 4: Comparison of sepsis onset (early: 0–14 vs. late: >14 days post-injury) in adult and elderly burns showed that late onset sepsis in older patients had lower proportions for all cytokines relative to early onset. This comparison was not significant in adults and in some cases was reversed (IL-12p70, IL-4, IL-5). Sepsis data is represented as mean ± SEM, *p<0.05, **p<0.01 and ***p<0.001.
Acknowledgements
We would like to thank Marjorie Burnett and Sarah Rehou for their assistance with the collection of clinical demographics.
This work is supported by grants from the National Institutes of Health (R01 GM087285-01); CIHR Funds (123336), CFI Leader’s Opportunity Fund (Project #25407); Physician’s Services Incorporated Foundation: Health Research Grant Program.
References
- 1.Milan A. Age and sex structure: Canada, provinces and territories, 2010. 2011 Retrieved from http://www.statcan.gc.ca. [Google Scholar]
- 2.Barillo DJ, Goode R. Fire fatality study: demographics of fire victims. Burns. 1996;22:85–88. doi: 10.1016/0305-4179(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 3.Pham TN, Kramer CB, Wang J, et al. Epidemiology and outcomes of older adults with burn injury: an analysis of the National Burn Repository. J Burn Care Res. 2009;30:30–36. doi: 10.1097/BCR.0b013e3181921efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauglitz GG, Song J, Herndon DN, et al. Characterization of the inflammatory response during acute and post-acute phases after severe burn. Shock. 2008;30:503–507. doi: 10.1097/SHK.0b013e31816e3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyhlen K, Gautam C, Andersson R, et al. Modulation of cytokine-induced production of IL-8 in vitro by interferons and glucocorticosteroids. Inflammation. 2004;28:77–88. doi: 10.1023/b:ifla.0000033023.76110.51. [DOI] [PubMed] [Google Scholar]
- 7.de Bandt JP, Chollet-Martin S, Hernvann A, et al. Cytokine response to burn injury: relationship with protein metabolism. J Trauma. 1994;36:624–628. doi: 10.1097/00005373-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Endo S, Inada K, Kikuchi M, et al. Are plasma endotoxin levels related to burn size and prognosis? Burns. 1992;18:486–489. doi: 10.1016/0305-4179(92)90181-s. [DOI] [PubMed] [Google Scholar]
- 9.Ueyama M, Maruyama I, Osame M, et al. Marked increase in plasma interleukin-6 in burn patients. J Lab Clin Med. 1992;120:693–698. [PubMed] [Google Scholar]
- 10.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeschke MG, Gauglitz GG, Finnerty CC, Kraft R, Mlcak RP, Herndon DN. Survivors versus nonsurvivors postburn: differences in inflammatory and hypermetabolic trajectories. Ann Surg. 2014;259:814–823. doi: 10.1097/SLA.0b013e31828dfbf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeschke MG, Herndon DN. Burns in children: standard and new treatments. Lancet. 2014;383:1168–1178. doi: 10.1016/S0140-6736(13)61093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van den Berghe G, de Zegher F, Veldhuis JD, et al. The somatotropic axis in critical illness: effect of continuous growth hormone (GH)-releasing hormone and GH-releasing peptide-2 infusion. J Clin. Endocrinol. Metab. 1997;82:590–599. doi: 10.1210/jcem.82.2.3736. [DOI] [PubMed] [Google Scholar]
- 14.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 15.Nomellini V, Gomez CR, Gamelli RL, et al. Aging and animal models of systemic insult: trauma, burn, and sepsis. Shock. 2009;31:11–20. doi: 10.1097/SHK.0b013e318180f508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masud D, Norton S, Smailes S, et al. The use of a frailty scoring system for burns in the elderly. Burns. 2013;39:30–36. doi: 10.1016/j.burns.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Albornoz CR, Villegas J, Sylvester M, et al. Burns are more aggressive in the elderly: proportion of deep burn area/total burn area might have a role in mortality. Burns. 2011;37:1058–1061. doi: 10.1016/j.burns.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Grimble RF. Inflammatory response in the elderly. Curr Opin Clin Nutr Metab Care. 2003;6:21–29. doi: 10.1097/00075197-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Lundgren RS, Kramer CB, Rivara FP, et al. Influence of comorbidities and age on outcome following burn injury in older adults. J Burn Care Res. 2009;30:307–314. doi: 10.1097/BCR.0b013e318198a416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rani M, Schwacha MG. Aging and the pathogenic response to burn. Aging Dis. 2012;3:171–180. [PMC free article] [PubMed] [Google Scholar]
- 21.Solana R, Tarazona R, Gayoso I, et al. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24:331–341. doi: 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Wasiak J, Lee SJ, Paul E, et al. Predictors of health status and age-related quality of life 12 months after severe burn. Burns. 2014;40:568–574. doi: 10.1016/j.burns.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Kerby JD, McGwin G, Jr, George RL, et al. Sex differences in mortality after burn injury: results of analysis of the National Burn Repository of the American Burn Association. J Burn Care Res. 2006;27:452–456. doi: 10.1097/01.BCR.0000225957.01854.EE. [DOI] [PubMed] [Google Scholar]
- 24.Jeschke MG, Barrow RE, Mlcak RP, et al. Endogenous anabolic hormones and hypermetabolism: effect of trauma and gender differences. Ann Surg. 2005;241:759–767. doi: 10.1097/01.sla.0000161028.43338.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeschke MG, Mlcak RP, Finnerty CC, et al. Gender differences in pediatric burn patients: does it make a difference. Ann Surg. 2008;248:126–136. doi: 10.1097/SLA.0b013e318176c4b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wohltmann CD, Franklin GA, Boaz PW, et al. A multicenter evaluation of whether gender dimorphism affects survival after trauma. Am J Surg. 2001;181:297–300. doi: 10.1016/s0002-9610(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 27.Mostafa G, Huynh T, Sing RF, et al. Gender-related outcomes in trauma. J Trauma. 2002;53:340–434. doi: 10.1097/00005373-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 28.McGwin G, George RL, Cross JM, et al. Gender differences in mortality following burn injury. Shock. 2002;18:311–315. doi: 10.1097/00024382-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Silver GM, Klein MB, Herndon DN, et al. Standard operating procedures for the clinical management of patients enrolled in a prospective study of inflammation and the host response to thermal injury. J Burn Care Res. 2007;28:222–230. doi: 10.1097/BCR.0B013E318031AA44. [DOI] [PubMed] [Google Scholar]
- 30.Klein MB, Goverman J, Hayden DL, et al. Benchmarking outcomes in critically injured burn patients. Ann Surg. 2014;259:833–841. doi: 10.1097/SLA.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janzekovic Z. A new concept in the early excision and immediate grafting of burns. J Trauma. 1970;10:1103–1109. [PubMed] [Google Scholar]
- 32.Herndon DN, Park DH. Comparison of serial debridement and autografting and early massive excision with cadaver skin overlay in the treatment of large burns in children. J Trauma. 1986;26:149–152. doi: 10.1097/00005373-198602000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Caruso C, Lio D, Cavallone L, et al. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- 34.Morgan TE, Wong AM, Finch CE. Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. Interdiscip Top Gerontol. 2007;35:83–97. doi: 10.1159/000096557. [DOI] [PubMed] [Google Scholar]
- 35.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 36.Finnerty CC, Jeschke MG, Herndon DN, et al. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008;14:553–560. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanojcic M, Chen P, Harrison RA, et al. Leukocyte infiltration and activation of the NLRP3 inflammasome in white adipose tissue following thermal injury. Crit Care Med. 2014;42:1357–1364. doi: 10.1097/CCM.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pritz T, Weinberger B, Grubeck-Loebenstein B. The aging bone marrow and its impact on immune responses in old age. Immunol Lett. 2014;162:310–315. doi: 10.1016/j.imlet.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Swanson JW, Otto AM, Gibran NS, et al. Trajectories to death in patients with burn injury. J Trauma Acute Care Surg. 2013;74:282–288. doi: 10.1097/TA.0b013e3182788a1c. [DOI] [PubMed] [Google Scholar]
- 40.Rymkiewicz PD, Heng YX, Vasudev A, et al. The immune system in the aging human. J Immunol Res. 2012;53:235–250. doi: 10.1007/s12026-012-8289-3. [DOI] [PubMed] [Google Scholar]
- 41.Finnerty CC, Herndon DN, Chinkes DL, et al. Serum cytokine differences in severely burned children with and without sepsis. Shock. 2007;27:4–9. doi: 10.1097/01.shk.0000235138.20775.36. [DOI] [PubMed] [Google Scholar]
- 42.Faist E, Ertel W, Salmen B, et al. The immune-enhancing effect of perioperative thymopentin administration in elderly patients undergoing major surgery. Arch Surg. 1988;123:1449–1453. doi: 10.1001/archsurg.1988.01400360019001. [DOI] [PubMed] [Google Scholar]
- 43.Braga M, Costantini E, Di Francesco A, et al. Impact of thymopentin on the incidence and severity of postoperative infection: a randomized controlled trial. Br J Surg. 1994;81:205–208. doi: 10.1002/bjs.1800810216. [DOI] [PubMed] [Google Scholar]
- 44.Haupt W, Zirngibl H, Riese J, et al. Depression of tumor necrosis factor-alpha, interleukin-6, and interleukin-10 production: a reaction to the initial systemic hyperactivation in septic shock. J Invest Surg. 1997;10:349–355. doi: 10.3109/08941939709099598. [DOI] [PubMed] [Google Scholar]
- 45.Keck M, Lumenta DB, Andel H, et al. Burn treatment in elderly. Burns. 2009;35:1071–1079. doi: 10.1016/j.burns.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Khadim MF, Rashid A, Fogarty B, et al. Mortality estimates in the elderly burned patients: the Northern Ireland experience. Burns. 2009;35:7–13. doi: 10.1016/j.burns.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Fagiolo U, Cossarizza A, Scala E, et al. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- 48.Forsey RJ, Thompson JM, Ernerudh J, et al. Plasma cytokine levels in elderly humans. Mech Ageing Dev. 2003;124:487–493. doi: 10.1016/s0047-6374(03)00025-3. [DOI] [PubMed] [Google Scholar]
- 49.Stowe RP, Peek MK, Cutchin MP, et al. Plasma cytokine levels in a population-based study: relation to age and ethnicity. J Gerontol A Biol Sci Med Sci. 2010;65:429–433. doi: 10.1093/gerona/glp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Statistical analysis of adult versus elderly burns for various cytokines for 0–6 (R1), 7–14 (R2), 15–13 (R3) & 30+ (R4) days post-injury. Dashed red lines represent healthy control mean values and non-survivor data is represented as mean ± SEM, *p<0.05, **p<0.01 and ***p<0.001 relative to adult burn group.
Supplemental Figure 1: Complete cytokine profile of burn patients used in the study that either did not reach statistical significance or were not previously reported. Comparison is between healthy controls (dashed red line), adult and elderly burned patients during the length of hospital stay. Data is represented as mean ± SEM and where applicable, *p<0.05, **p<0.01 and ***p<0.001 relative to adult burn group.
Supplemental Figure 2: Pro-inflammatory, chemokine and immune mediator cytokine profile of adult and elderly burn patients that all encountered pneumonia, bacteremia or infection complications. Elderly burn patients showed a delayed increase in cytokine activation compared to adults with significance occurring beyond 14 days after injury (A–F). Bacteremia (G–L) and infection (M–R) showed consistent immune activation profiles as described previously. All cytokines presented were divided into post-injury groups (0–6, 7–14, 15–30 & 30+ days post-burn). Dashed red lines represent healthy control mean values and data is represented as mean ± SEM, *p<0.05, **p<0.01 and ***p<0.001 relative to adult burns patients for each respective complication.
Supplemental Figure 3: Complete cytokine profile of sepsis burn patients. Adult and elderly patients that encountered septicemia were included in the analysis. All cytokines presented reached statistical significance and were divided into post-injury groups (0–6, 7–14, 15–30 & 30+ days post-burn). As evident by the intensity of G-CSF, IFN-α2, IL-1α, IL-6 & IL-8 the cytokine in elderly burns increased at later time points (15–30 & 30+) reflecting an early-dampened response to insult. Increasing intensity is represented as green to red, with black reflecting no change (note: color bar is asymmetrical and color intensity should be compared directly to controls and burn injury groups).
Supplemental Figure 4: Comparison of sepsis onset (early: 0–14 vs. late: >14 days post-injury) in adult and elderly burns showed that late onset sepsis in older patients had lower proportions for all cytokines relative to early onset. This comparison was not significant in adults and in some cases was reversed (IL-12p70, IL-4, IL-5). Sepsis data is represented as mean ± SEM, *p<0.05, **p<0.01 and ***p<0.001.