Abstract
Pro-opiomelanocortin (POMC) is a prohormone that encodes multiple smaller peptide hormones within its structure. These peptide hormones can be generated by cleavage of POMC at basic-residue cleavage sites by prohormone converting enzymes in the regulated secretory pathway of POMC synthesizing endocrine cells and neurons. The peptides are stored inside the cells in dense core secretory granules until released in a stimulus dependent manner. The complexity of the regulation of the biosynthesis, trafficking and secretion of POMC and its peptides reflect an impressive level of control over many factors involved in the ultimate role of POMC expressing cells, i.e. to produce a range of different biologically active peptide hormones ready for action when signaled by the body. From the discovery of POMC as the precursor to ACTH and β-Lipotropin in the late 1970s to our current knowledge, the understanding of POMC physiology remains a monumental body of work that has provided insight into many aspects of molecular endocrinology. In this chapter, we describe the intracellular trafficking of POMC in endocrine cells, its sorting into dense core secretory granules and transport of these granules to the regulated secretory pathway. Additionally, we review the enzymes involved in the maturation of POMC to its various peptides and the mechanisms involved in the differential processing of POMC in different cell types. Finally, we highlight studies pertaining to the regulation of ACTH secretion in the anterior and intermediate pituitary and POMC neurons of the hypothalamus.
II. Introduction
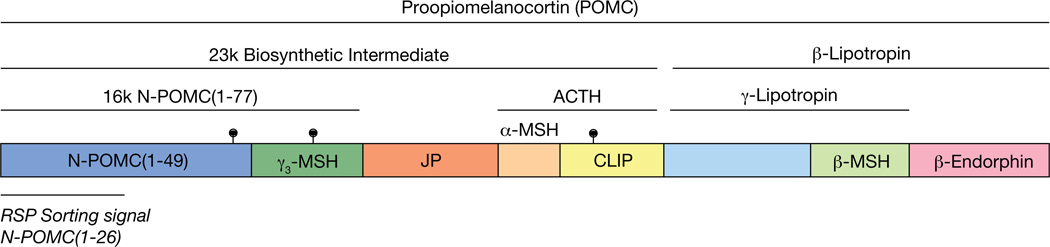
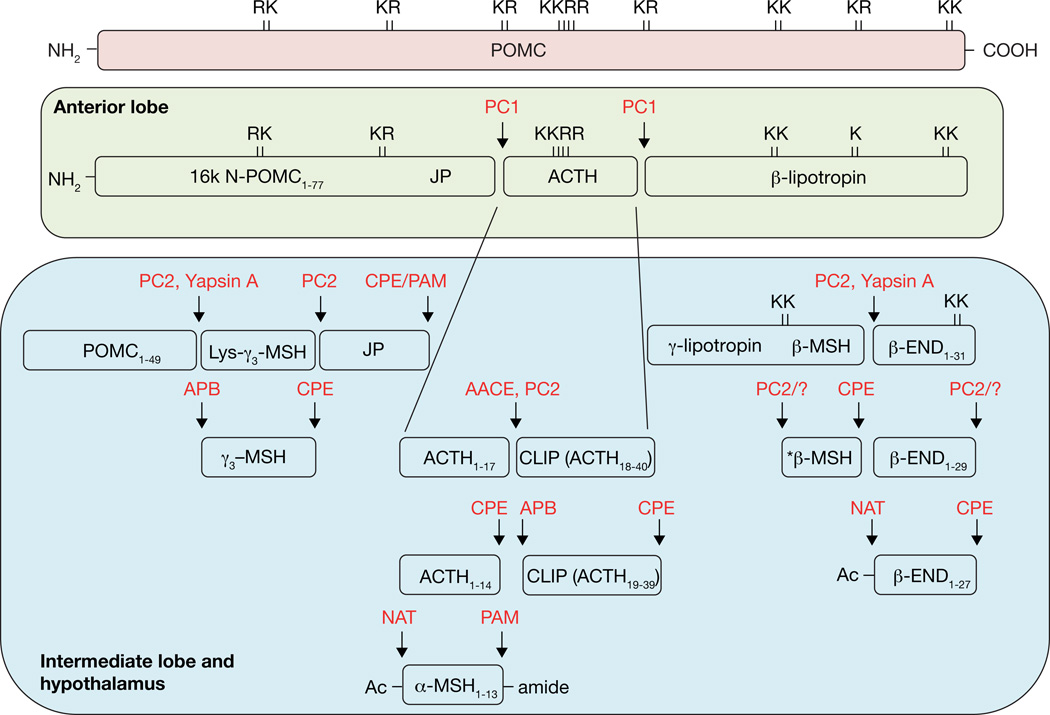
The birth of pro-opiomelanocortin stemmed from the landmark work of Dr. Choh Hao Li at the Hormone Research Laboratory at University of California at Berkeley where he first elucidated the chemistry of ACTH and subsequently β-lipotropin. Thereafter, an accumulation of peptide sequencing studies from many laboratories led to the recognition that a number of biologically active peptides such as α-MSH are derived from ACTH, and β-MSH and β-endorphin from β-lipotropin. Based on common amino acid sequences among these peptides such as α-MSH and β-MSH in ACTH and β-lipotropin, respectively, the hypothesis emerged that ACTH and β-lipotropin could be derived from a larger precursor consisting of both ACTH and β-lipotropin [for an historical perspective see (Lowry 2015)]. Subsequently, several groups, including Michel Chretien (Crine, et al. 1979), Mains and Eipper (Mains and Eipper 1979) and ours (Loh 1979), employing pulse-chase experiments provided evidence that ACTH and β-lipotropin were derived from a larger common precursor, and that the sequential processing of this precursor led to the biosynthesis of the different biologically active peptides. At about the same time, cDNA cloning studies confirmed the existence of the common precursor for ACTH and β-lipotropin (Nakanishi, et al. 1979). Hence, the name pro-opiomelanocortin was coined by Michel Chretien for the ACTH-β-lipotropin precursor (Figure 1) (Chretien and Mbikay 2016).
Figure 1. Schematic diagram of the bovine POMC protein.
The prohormone encodes multiple peptides that can be cleaved by prohormone convertases in a cell and time dependent manner. Adrenocorticotropin, ACTH; melanocyte stimulating hormone, MSH; joining peptide, JP; corticotropin-like intermediate peptide, CLIP; regulated secretory pathway, RSP; lollipop symbols represent glycosylation sites.
III. Intracellular organization of POMC maturation
1. Intracellular trafficking of POMC
POMC is synthesized in the corticotrophs and melanotrophs of the anterior and intermediate lobes of the pituitary, respectively, as well as in peptidergic neurons in the arcuate nucleus of the hypothalamus. It is post-translationally cleaved into peptide hormones that can include adrenocorticotropin (ACTH), β-endorphin, α-, β- and γ-melanocyte stimulating hormone (MSH), N-POMC1–48 and β-lipotropin, in a tissue and cell dependent manner. These peptides exhibit different physiological functions such as mitogenic activity (N-POMC1–48), steroidogenic activity (ACTH), satiety (α-MSH) and opiate-like activity (β-endorphin). After synthesis at the rough endoplasmic reticulum (ER) and folding in the ER, POMC is transported through the cell to end up ultimately in large dense core secretory granules of the regulated secretory pathway (RSP). The route involves movement of the protein through the ER and Golgi to the trans-Golgi network (TGN), where it is sorted into nascent vesicles budding from the TGN that will mature into dense core secretory granules as they are trafficked to the release sites close to the plasma membrane. During this movement within the cell, the prohormone is cleaved in a time and compartment specific way by prohormone convertases to generate the peptide hormone complement, specific for that cell type. The peptides generated in the mature granules form an electron-dense core and are stored in these granules until secreted from the cell upon stimulation by a secretagogue. How POMC is transported through and processed in the endocrine cell from the site of synthesis to the dense core secretory granules has been a long standing question and one that has been studied by many investigators.
With the discovery of POMC as the precursor to ACTH and β-LPH (Mains and Eipper 1976; Mains, et al. 1977; Crine, et al. 1978), an explosion of work followed in the 1980s and 1990s addressing the question of cellular transport and processing of the prohormone. Ideal for studying these questions were the AtT20 cells, a mouse corticotroph cell line that normally expresses POMC and processes it into ACTH, β-LPH and the 16 KDa N-POMC intermediate. Initial biochemical evidence demonstrated that the mature peptides, ACTH and β-LPH, were present in mature secretory granules of AtT20 cells purified by density gradient centrifugation on Ficoll (Gumbiner and Kelly 1981), leading to the idea that POMC must be processed in this compartment. It was subsequently found that POMC was also secreted through the constitutive secretory pathway (CSP) in these cells, i.e. in an unstimulated manner, along with an endogenous murine leukemia virus present in these cells (Gumbiner and Kelly 1982), demonstrating that the two secretory pathways were distinct in these cells, one being driven by bulk flow and tied to protein translation and the other requiring active sorting into storage granules and secretion triggered by external stimuli (Burgess and Kelly 1987). Indeed, at that time, transfection of VSVG or human growth hormone (hGH) into AtT20 cells clearly demonstrated the two secretory pathways in these cells (Moore and Kelly 1985) since the VSVG was secreted constitutively while the hGH was secreted through the RSP. In support of this, electron microscopic (EM) and immunocytochemical analyses using an anti-ACTH antibody that could label ACTH and its precursor, POMC, showed ACTH-immunoreactivity (IR) in condensing vacuoles protruding from the trans most side of the Golgi apparatus (Tooze and Tooze 1986), demonstrating that granule cargo was sorted into these “immature” granules. These condensing vacuoles and 25–30% of the mature dense core granules were shown to contain unprocessed POMC, using an ACTH-β-LPH cleavage site specific antibody, demonstrating that POMC was sorted into these immature granules where most of it was processed (Tooze, et al. 1987a). Follow on EM studies identified that the murine hepatitis virus shared this initial compartment with ACTH but diverged afterwards (Tooze, et al. 1987b), consistent with the virus being in the constitutive secretory pathway. Analysis of newly synthesized proteins labelled with 35S in methionine and sulfated proteoglycans identified several proteins in AtT20 cells that could be observed in the two distinct pathways, those that followed unprocessed POMC and those that followed ACTH (Moore, et al. 1983b), leading to the conclusion that a common signal existed for proteins destined to be directed into the RSP. The idea that maybe these proteoglycans entering the RSP could participate in the sorting process was discounted when inhibitors of chrondroitin sulfate synthesis was used, to reduce the levels of the proteoglycans, and found no difference in the processing and secretion of ACTH (Burgess and Kelly 1984). However, similar to the hGH (Moore and Kelly 1985), exogenously expressed proteins including proinsulin (Moore, et al. 1983c) and trypsinogen (Burgess, et al. 1985) were also targeted to the RSP in AtT20 cells suggesting that other prohormones and exocrine proteins contain a common signal recognized by the AtT20 cell machinery. More significantly, an important gain of function study using a fusion protein of VSVG coupled to the C-terminus of hGH demonstrated that the active sorting process of hGH could direct the constitutively secreted VSVG into the granules of the RSP in these cells (Moore and Kelly 1986), demonstrating that the sorting process for sorting into the RSP was dominant over the process of bulk flow transport through the CSP (Kelly 1985). This sorting event was believed at that time to be similar to that of the lysosomal enzymes that use the mannose-6-phosphate receptor (Sly and Fischer 1982). Support for this idea came from observations that, similar to a pH dependent sorting and recycling of lysosomal enzymes, sorting of POMC to the RSP was prevented in the presence of chloroquine (Moore, et al. 1983a), a compound that neutralizes acidic compartments. In the presence of chloroquine, reduced production of newly synthesized ACTH in the mature granules was observed with an increase in constitutive secretion indicative of mis-sorting. A similar observation was made later, when ammonium ions, that have the same pH neutralizing effect on acidic compartments, were used (Dyken and Sambanis 1994), supporting the role of pH as a very important component of the sorting process.
2. Sorting of POMC to dense-core secretory granules
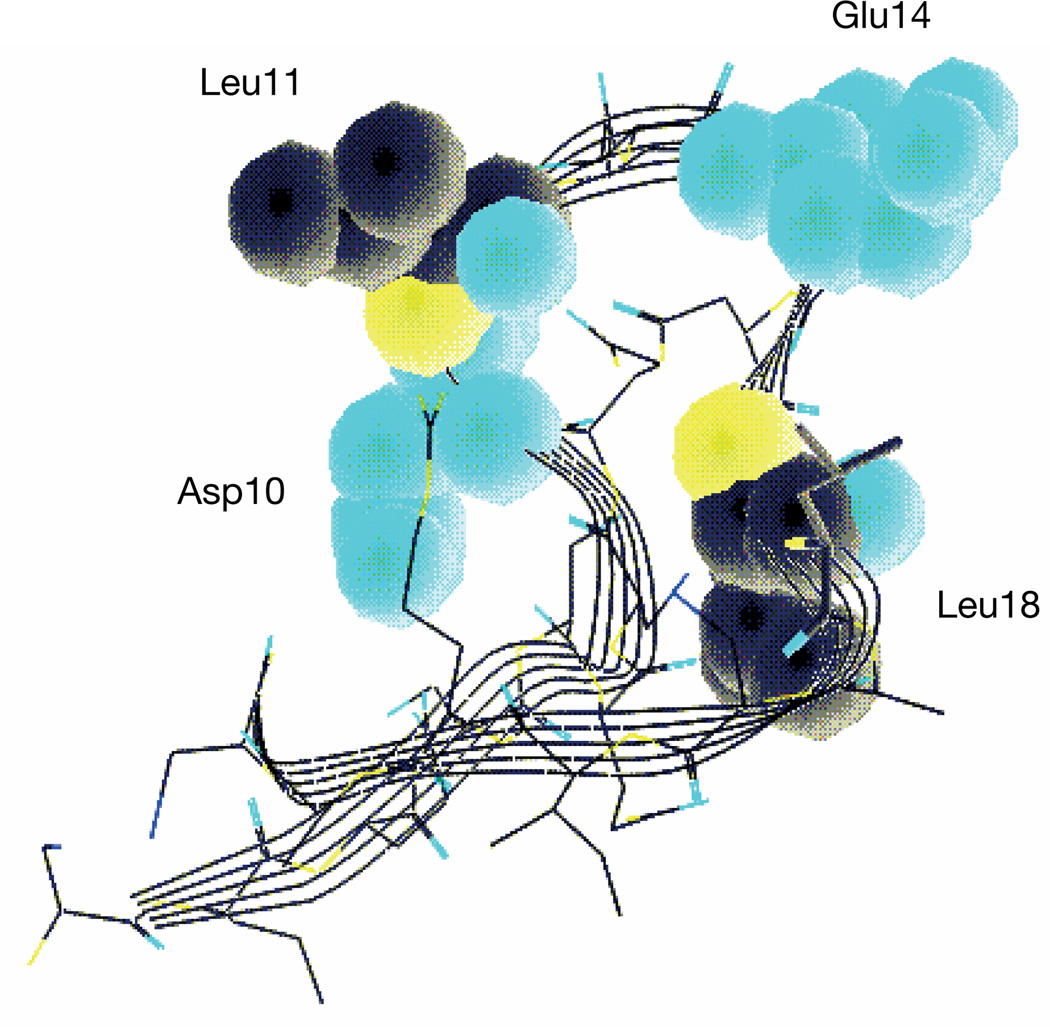
POMC is a 31 kDa protein that contains 38 positively charged (arginine and lysine) residues throughout the sequence (without the signal peptide); many of which are prohormone convertase cleavage sites. Interestingly, these positively charged residues appear to be physiologically balanced by 39 negatively charged (glutamate and aspartate) residues in the POMC protein. Thus, almost one third of the POMC protein is composed of arginine/lysine and glutamate/aspartate amino acids. Hence, POMC is a highly charged protein and very soluble in aqueous solution. While POMC is a highly charged protein, it was found to bind tightly to membranes from enriched preparations of secretory granules derived from mouse or frog neuro-intermediate lobe (NIL) pituitary suggesting it was interacting with a receptor (Loh and Tam 1985). In further studies on POMC, limiting domain transfer (gain of function) (Tam, et al. 1993; Cool and Loh 1994) and deletion or mutation (loss of function) experiments (Cool, et al. 1995) demonstrated that the N-terminus of POMC, specifically N-POMC(1–26), contained information that was sufficient and necessary for sorting POMC to the RSP in AtT20 cells and Neuro2a cells, respectively. Molecular modeling of N-POMC(1–26), made possible by earlier structural analyses of N-POMC(1–26) that solved the di-sulfide bond pairs in this domain (Bennett 1984; Bennett, et al. 1986), identified a 3D motif (Figure 2) containing two acidic (Asp10 and Glu14) and two aliphatic hydrophobic (Leu11 and Leu18) residues that were highly conserved (Cool et al. 1995) and predicted to be a consensus sorting signal motif. This motif was subsequently found in monomeric and hexameric proinsulin (Dhanvantari, et al. 2003), brain-derived neurotrophic factor but not nerve growth factor (Lou, et al. 2005) and proenkephalin (Normant and Loh 1998; Loh, et al. 2002) and predicted to bind to a prohormone sorting receptor.
Figure 2. An NMR confomer of the N-POMC(1–26) peptide encoding the RSP sorting signal.
Note the two acidic residues, Asp10 and Glu14, and the two hydrophobic residues, Leu11 and Leu18 comprising the sorting signal motif.
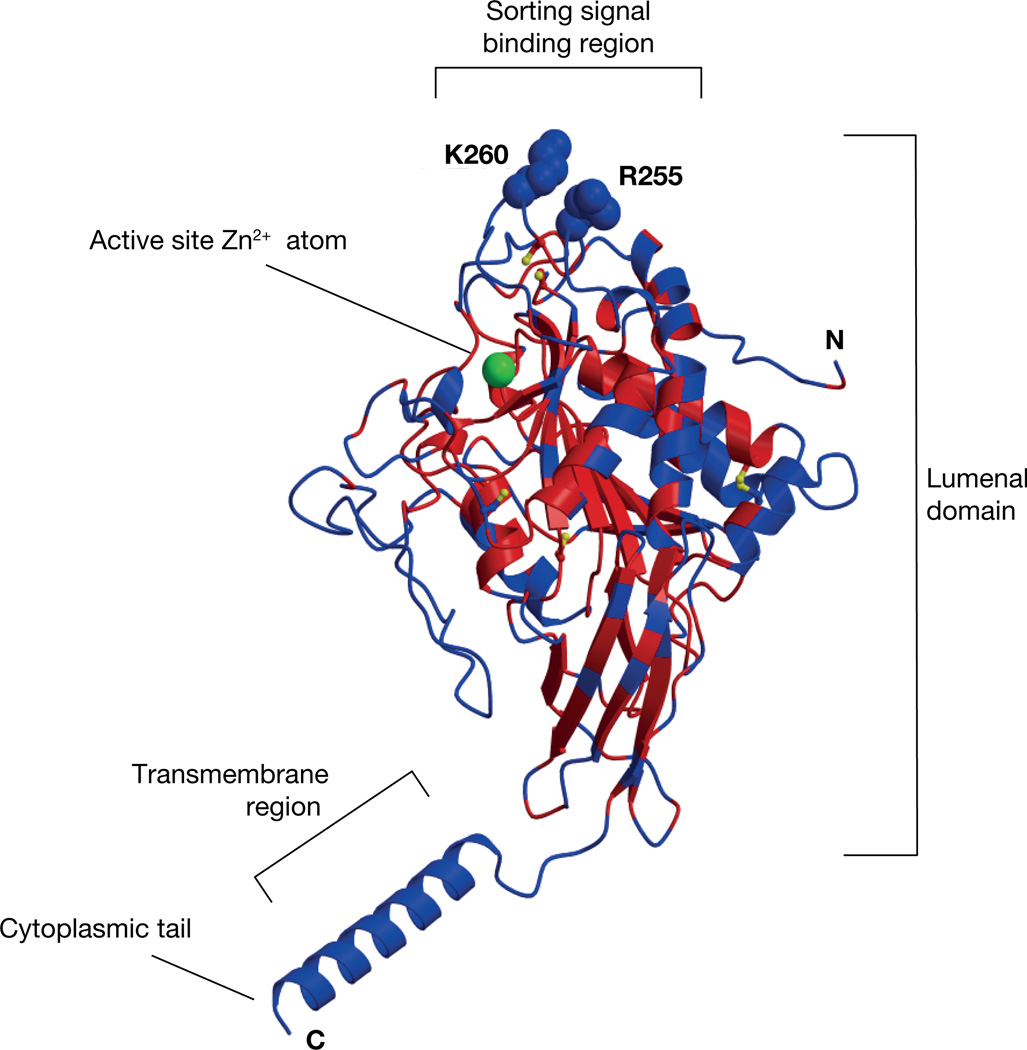
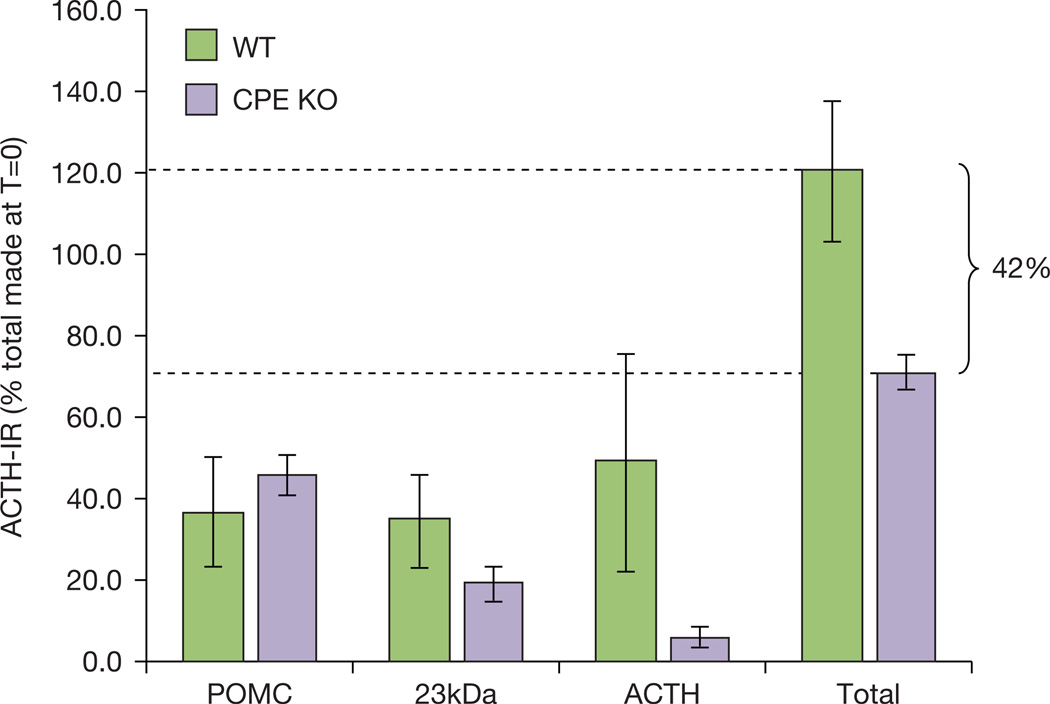
In follow up studies, Loh and colleagues coupled the N-POMC(1–26) peptide, containing the sorting signal motif of POMC, to beads and used it in affinity chromatography using NIL Golgi-derived membranes, a putative source of a prohormone sorting receptor. Solubilized membranes were applied to the column under acidic conditions (pH 5.5) and bound proteins were eluted at pH 7.4. Using this approach, a candidate sorting receptor for POMC was identified as carboxypeptidase E (CPE) (Cool, et al. 1997) since it was the major protein in the eluate identified by amino acid sequencing. CPE was classically known since the early 1980s as an enzyme involved in the maturation of peptide hormones by removing lysine and arginine amino acids from the C-termini of peptide hormone intermediates (see Section III.3.a (Fricker and Snyder 1982; Hook, et al. 1982a)). Subsequent cross-linking and binding studies confirmed its identity and characterized it with low affinity first-order binding kinetics (KD = 6 µM) (Cool and Loh 1998). In addition, binding of N-POMC(1–26) to CPE did not require the active site of CPE, as CPE with its active site mutated bound the ligand to the same extent as WT CPE (Zhang, et al. 1999). Also, addition of guanidino-ethylmercaptosuccinic acid (GEMSA), a potent inhibitor of CPE, did not prevent binding (Loh, et al. 1997), demonstrating that binding of POMC to CPE did not depend on the carboxypeptidase enzymatic activity of CPE. Indeed, molecular modelling of CPE identified a putative sorting signal binding site in Arg255 and Lys260 of CPE (Zhang et al. 1999) (Figure 3), further demonstrating the binding of N-POMC(1–26) was independent of the active site. Support for CPE as a sorting receptor for POMC came from studies on the CPEfat/fat mouse (Naggert, et al. 1995) where the mutant CPE contains a Ser202Pro mutation rendering the protein unstable and subject to degradation (Varlamov, et al. 1997; Cawley, et al. 2003). Hence the CPEfat/fat mouse was viewed as a CPE deficient mouse. Secretion studies from NIL and anterior lobe (AL) pituitary primary cells (Cool et al. 1997; Shen, et al. 1999) of these mice suggested defective sorting of POMC, indicative of the lack of a sorting receptor. Support of this comes from analysis of the CPE knockout (KO) mouse (Cawley, et al. 2010). In the complete absence of CPE, POMC processing to α-MSH in the NIL and hypothalamus of the pituitary is reduced by 81–94%, respectively, and there is a ~10-fold increase in the tissue levels of POMC and its 23kDa biosynthetic intermediate in the NIL resulting in serum levels of POMC/23kDa intermediate almost 8-fold higher in the CPE KO mice compared to WT mice (Cawley et al. 2010). This suggests a trafficking defect of POMC and accumulation in the TGN with increased constitutive secretion, although a processing defect and accumulation of the POMC likely contributes to the phenotype. In the AL of the pituitary, processing of POMC to ACTH is significantly reduced, however, in contrast to the NIL, only a small increase in tissue content of POMC was observed. Pulse-chase experiments on primary cultures of AL cells found significantly reduced ACTH production and secretion as well as a small but significant increase in the stimulated secretion of POMC, demonstrating that some POMC was sorted into the RSP but its processing to ACTH was reduced (unpublished data of the authors). Later studies in the CPEfat/fat mice suggested that compensation by another potential sorting receptor, Secretogranin III (SgIII), was possible, since ACTH was released from the AL pituitary from these mice in a CRH dependent manner, and SgIII, a known sorting receptor for chromogranin A (Hosaka, et al. 2002), was upregulated in the pituitary of these CPEfat/fat mice (Hosaka, et al. 2005). Interestingly, our pulse-chase experiments also showed that ~42% of the newly synthesized POMC was unaccounted for in the cells from the CPE KO mouse when compared to WT cells (Figure 4, unpublished data of the authors), indicative of degradation. This suggests that the corticotrophs may compensate for the poor trafficking and accumulation of POMC in the absence of CPE, by directing it to the lysosomes for degradation in vivo.
Figure 3. Molecular model of CPE based on the crystal structure of CPD.
The red areas indicate similarity of CPE and CPD, whereas the blue areas indicate unique areas to CPE. Note the N-POMC(1–26) sorting signal binding site composed of Arg255 and Lys260.
Figure 4. Pulse-chase studies of POMC in mouse anterior lobe cells.
Pituitary anterior lobe cells from wild type (WT) and CPE knockout (KO) mice were cultured and metabolically labeled with 35S-Met for 30 min and chased for 2h. Immunoreactive ACTH molecules were analyzed by immunoprecipitation and quantified. Note the reduced levels of ACTH made in the CPE KO cells. The overall recovery of total ACTH-IR was less in the CPE KO cells compared to the WT cells indicative of degradation. (unpublished data of the authors).
A major constraint in the idea of one protein being a receptor for all the prohormone in the cell was that the stoichiometry did not favor it. It was therefore proposed that homotypic and even heterotypic oligomerization of prohormones may allow concentration of the cargo followed by binding to a receptor. This phenomenon early in the sorting process would be distinguished from peptide hormone aggregation and condensation in the maturing secretory granules. In support of this, evidence suggested that POMC can loosely aggregate homotypically and heterotypically with proenkephalin to form dimers and multimers (Cawley, et al. 2000) and this aggregation is enhanced in the presence of increased calcium and reduced pH, conditions expected in the TGN (Chandra, et al. 1991; Seksek, et al. 1995). More importantly, the N-POMC(1–26) domain was not required for aggregation thus allowing it to act as a bridge to connect the aggregated cargo with the membrane form of CPE to initiate sorting into the granules of the RSP. Aggregation induced sorting into the granules of the RSP is one hypothesis proposed to answer how prohormones are sorted at the TGN away from constitutively secreted proteins, since it has been shown that many RSP cargo proteins aggregate under these mild acidic and high calcium conditions (Chanat and Huttner 1991; Colomer, et al. 1996; Jain, et al. 2002), including CPE (Rindler 1998).
RNA interference technology is a powerful tool to specifically reduce the expression of a target gene. Using siRNA to reduce CPE in Neuro2a cells, transfected POMC was secreted constitutively and no punctate staining for ACTH-IR by immunocytochemistry was seen in the knocked-down cells, supporting the findings from the CPEfat/fat mouse (Normant and Loh 1998). Later studies show conflicting results in AtT20 cells with respect to ACTH secretion. In both cases, POMC secretion through the CSP was significantly elevated when CPE was knocked down indicative of inefficient sorting to the RSP, however, in one case ACTH secretion was normal (Kemppainen and Behrend 2010) and in the other it was not (Cawley, et al. 2015). Notably, knockdown of Secretogranin III, previously shown to bind POMC (Hosaka et al. 2005) and also known as a sorting receptor for chromogranin A (Hosaka et al. 2002; Hosaka, et al. 2004), also caused significant increase in constitutive secretion of POMC and a reduction in ACTH secretion via the RSP (Cawley et al. 2015). These results suggest a mechanism involving several membrane bound proteins that can possibly interact with each other, such as CPE and SgIII (Hosaka et al. 2005), or interchange, so that the ultimate important cellular process of the endocrine cell can be carried out, i.e. to provide the peptide hormone ready for secretion upon stimulation of the mature secretory granule. Hence, sorting of POMC to the granules of the RSP likely requires interaction with multiple membrane associated molecules, of which CPE and SgIII are primary candidates, in addition to SgII (Sun, et al. 2013), at the lumenal side of the TGN during the initial budding and this interaction results in the active sorting and retention of the prohormone as the immature granule forms and matures.
3. Transport and exocytosis of POMC secretory vesicles
At the TGN, EM studies showed that POMC was sorted into condensing vacuoles that were seen to contain a clathrin coat (Tooze and Tooze 1986) which was removed during the granule maturation process as no visible structures indicative of the clathrin triskelion were found on the mature granule. Indeed, the vesicle coat contains many proteins involved in the maturation of the vesicle and storage and then fusion with the plasma membrane upon stimulation (reviewed elsewhere, (Kogel and Gerdes 2010; Bonnemaison, et al. 2013)). How POMC vesicles are transported from the TGN to their release site was an interesting question and has recently been studied using live cell imaging in AtT20 cells.
In the AtT20 cells, after the initial site of budding at the TGN, the ACTH vesicles must be transported to the ends of the processes close to the plasma membrane, where they are stored until released. It was seen early on that during cell division, the mature dense core granules containing ACTH redistribute in the cell, from being localized at the Golgi and tips of the processes in interphase, randomly distributed during metaphase and anaphase but then align at the midbody as it develops during cytokinesis during telophase, a process dependent on the microtubules (Tooze and Burke 1987). Other studies using acridine orange and enhanced fluorescence microscopy, demonstrated saltatory movement of the ACTH containing vesicles mostly in the anterograde direction and some in the retrograde direction, and the movement, reported at a rate of 3–5 µm/sec, was dependent on microtubules (Kreis, et al. 1989). Transport of POMC vesicles along microtubules had not been studied in detail until recently. Previous work by Loh and colleagues identified that the C-terminus of some CPE could traverse the granule membrane and interact with Arf6, a small cytosolic GTPase involved in clathrin independent endocytosis (Arnaoutova, et al. 2003). Subsequent yeast-two-hybrid studies showed that the C-terminus of CPE interacted with dynactin (unpublished data). Confirmation of this interaction came from biochemical pull-down and co-precipitation experiments, which showed that the C-terminus of CPE specifically bound to dynactin from AtT20 cell lysates (Park, et al. 2008). The complex contained kinesin 2 and 3 as well as dynein; microtubule dependent motor proteins for anterograde and retrograde transport, respectively. Interestingly, kinesin 2 transports vesicles at a rate of ~0.5 µm/sec (Scholey 2013), a speed observed by live cell imaging of POMC-RFP containing vesicles in AtT20 cells that was eliminated when the C-terminus of CPE was constitutively over-expressed in the cytosol to act as a dominant negative molecule to inhibit endogenous CPE C-tail interaction with dynactin (Park et al. 2008). These results demonstrated that POMC vesicles were anterogradedly transported along microtubules by the motor proteins, kinesin 2 and 3 and the vesicle anchor was through the C-terminus of CPE. An additional interacting protein elucidated from the yeast-two-hybrid studies was identified as γ-adducin (Lou, et al. 2010), a protein involved in cortical actin assembly just under the plasma membrane. It was proposed that as the granule arrives at the end of the microtubules the CPE C-terminus can interact with γ-adducin to establish a storage zone for the mature granules. Over-expression of a C-terminal tail region of γ-adducin also caused an accumulation of POMC vesicles at the TGN in AtT20 cells suggestive of a role in POMC vesicle budding from the TGN through interaction with peri-Golgi F-actins (Lou, et al. 2013).
IV. Processing of POMC
Processing of POMC involves many enzymes including endoproteases, exopetidases, acetylation and amidation enzymes to generate the POMC peptides such as ACTH, α-MSH (Ac-ACTH(1–13)-NH2) and β-lipotropin and β-endorphin (Figure 5). In this section, we describe the sequential processing steps and the enzymes involved in the maturation of POMC and its derived peptides.
Figure 5. General schematic depicting the processing of bovine POMC.
In the anterior lobe, PC1/3 is the primary convertase involved in the generation of 16k N-POMC, ACTH and β-LPH. A more comprehensive processing pattern is seen in the intermediate lobe and hypothalamus yielding α-MSH and β-endorphin. APB, aminopeptidase B-like; AACE, acidic ACTH converting enzyme; CPE, carboxypeptidase E; NAT, N-acetyl transferase; PAM, peptidylglycine α-amidating monooxygenase; -END, -endorphin; JP, joining peptide; -MSH, -melanocyte stimulating hormone; PC, prohormone convertase; Ac, acetyl; K, Lysine; R, Arginine.*β-MSH in humans may not occur naturally (Scott and Lowry 1974).
1. POMC processing at paired basic residue specific sites
a. Prohormone Convertases
Processing of POMC can begin at the TGN, although the primary site of proteolytic cleavages occurs within the immature secretory granule. In the TGN, the pH is ~6.8 (Seksek et al. 1995), whereas the secretory granule pH is between 4.5 and 5.5 (Loh, et al. 1984). Hence the processing enzymes which include various endoproteases and exopeptidases involved in the maturation of POMC have to function within this pH range. The first step in POMC processing is endoproteolytic cleavage at signature pairs of basic residues (Figure 5). The major endoproteolytic enzymes for these cleavages are the prohormone convertases 1 and 2 (PC 1/3 and PC 2). These PCs are subtilisin-like enzymes related to yeast kexin, and were cloned in 1991 and shown to cleave POMC (Thomas, et al. 1991) and other prohormones at specific paired basic residues (For a review of the historical discovery of the proprotein convertases, see (Seidah 2011; Chretien and Mbikay 2016).
Both PC1/3 and PC2 enzymes are synthesized as a pro-form and are specifically trafficked to the secretory granules of the regulated secretory pathway where the majority of POMC processing occurs. The mature forms of these enzymes function at an acidic pH (Zhou and Lindberg 1993; Friedman, et al. 1994; Lindberg, et al. 1995), hence they are ideal to work in the mature granule where prohormones are fully processed. The activation of PC1/3 begins early in a pre-Golgi compartment suggestive of an autocatalytic activation on the carboxyl side of the RSKR motif in its pro-segment cleavage site (Benjannet, et al. 1993; Zhou and Lindberg 1993; Goodman and Gorman 1994). Since the ~87kDa form of PC1/3 is active and present in the Golgi, it can begin to act on the first cleavages of POMC to yield 16 kD N-POMC, ACTH and β-lipotropin (Figure 5) in this compartment although the major cleavage occurs within the immature secretory granules. PC2 is activated later in the immature secretory granule and found to be responsible for the cleavage of 16 kD N-POMC, ACTH and β-lipotropin to yield N-POMC(1–77), α-MSH and β-endorphin, respectively (Zhou, et al. 1993). Interestingly, the activity of both these PCs appears to be under the control of endogenous inhibitors/chaperones. PC1/3 pro-peptide expressed in trans is able to act as an endogenous inhibitor of PC1/3 (Lee, et al. 2004). Additionally, proSAAS (Fricker, et al. 2000) has been found to be processed by PC1/3 to yield a polypeptide that acts as an inhibitor for PC1/3 (Qian, et al. 2000) and therefore regulates its activity on other substrates. In AtT20 cells, proSAAS expression has been found to inhibit the processing of POMC under pulse/chase conditions (Lee et al. 2004). In the case of pro-PC2, a protein named 7B2 is required as a chaperone to help with its transport to the Golgi and its activation (Muller, et al. 1997). Pro-PC2 forms a complex with 7B2 in the ER and is then trafficked to the Golgi where 7B2 is cleaved by furin to generate a C-terminal 31 amino acid C-terminal (CT)-peptide. The CT-peptide then binds to pro-PC2 and acts as a potent inhibitor. Pro-PC2 is then sorted into the immature secretory granule where it is auto-catalytically processed into PC2 within the acidic environment of immature secretory granules. PC2 in turn cleaves the CT-peptide at VVAKK189↓SVP, followed by removal of the basic residues KK by the exopeptidase, CPE, to generate an inactive form of the CT-peptide that liberates the active PC2 enzyme (Fortenberry, et al. 1999; Mbikay, et al. 2001). The importance of 7B2 in POMC processing is seen in the 7B2 KO mouse. These mice, unlike PC2 KO mice, die early due to Cushing’s disease because of excessive secretion of ACTH from the pituitary intermediate lobe due to the lack of processing by an active PC2 enzyme that would normally produce α-MSH in this tissue. This indicates a role of 7B2 not only in POMC processing indirectly but also in secretion of its derived peptides (Mbikay et al. 2001). Indeed, stimulated secretion of ACTH from AtT20 cells is negatively correlated to cellular levels of 7B2, also reflecting a possible role in POMC processing and secretion in these cells (Bergeron, et al. 2002).
The physiological importance of PC1/3 in POMC processing came from several lines of evidence. This included in situ hybridization studies revealing that PC1/3 mRNA was expressed primarily in anterior pituitary corticotrophs which synthesize ACTH, whereas it was co-localized with PC2 in the intermediate pituitary which synthesizes α-MSH (Seidah, et al. 1991). This led to further understanding of the previous in vitro pulse-chase studies using AtT20 and primary cultures of anterior and intermediate pituitary cells and hypothalamic neurons (Loh 1979; Liotta, et al. 1980; Mains and Eipper 1981b) attributing cleavage of POMC at paired basic residues to generate N-POMC (16 kD), ACTH and β-lipotropin in anterior pituitary corticotrophs to PC1/3, while PC2 cleaved 16kD N-POMC to yield N-POMC(1–77), ACTH to yield α-MSH and β-lipotropin to yield β-endorphin in melanotrophs in the intermediate pituitary and hypothalamic neurons (Figure 5); observations subsequently affirmed by additional experiments (Zhou et al. 1993; Friedman et al. 1994; Friedman, et al. 1996; Paquet, et al. 1996). In vivo studies using gene knock-out in mice for PC1/3 and PC2 (Furuta, et al. 1997; Zhu, et al. 2002)) and the finding of two human patients with defects in PC1/3 (Jackson, et al. 1997; Farooqi, et al. 2007) showed that both these enzymes are not essential for life. Moreover, PC1/3 null mice process POMC poorly to ACTH (Zhu et al. 2002) yet have normal levels of circulating corticosterone. They also exhibit retarded growth and developmental defects since the enzyme is responsible for processing other prohormones. PC2 null mice look normal at birth but show retarded growth and they do not fully process POMC-derived peptides (Furuta et al. 1997). Peptidomic analyses of PC1/3 (Wardman, et al. 2010) and PC2 (Zhang, et al. 2010) KO mice revealed that the loss of PC1/3 is often compensated for by PC2, but the reverse is not always true. This corroborates with in vitro studies in GH3 cells which expresses PC2 and not PC1/3, showing that exogenously expressed POMC was completely processed to ACTH-related peptides (ACTH1–14, ACTH 1–15 and ACTH 1–17) as well as β-endorphin and Lys-γ-MSH (Friedman et al. 1996). A female patient deficient in PC1/3 protein due to both splicing and non-synonymous mutation in the PC1/3 gene, showed low expression levels of the enzyme and high circulating levels of several forms of partially processed POMC intermediate ACTH products (Jackson et al. 1997). The patient was obese and had poor glucose homeostasis. Although this patient differs from PC1/3 null mice which are not obese (Zhu et al. 2002), the current findings points to an important role of PC1/3 in vivo in POMC processing.
b. Yapsin A
In addition to the PC1/3 and PC2, another enzyme known as Yapsin A (or POMC converting enzyme), an aspartic protease, has been purified to apparent homogeneity from bovine pituitary intermediate lobe and neural lobe secretory granules as well as from adrenal chromaffin granules, have been described that can process POMC at paired basic residues to yield N-POMC(1–77), ACTH, β-lipotropin and β-endorphin (see Figure 5, (Loh, et al. 1985; Azaryan, et al. 1995)). This enzyme is related to Yapsin 1 or yeast aspartic protease 3, a gene product of the yps1 gene in yeast that has also been shown to process pro-α-mating factor at paired basic residues, similar to the yeast kex-2 enzyme, (Egel-Mitani, et al. 1990). Yapsin 1 is able to cleave POMC at paired basic residues as well (Azaryan, et al. 1993). Yapsin A has been characterized as a ~70 kD enzyme that has a pH optimum of 4.0–5.0. An antibody generated against Yapsin 1 has been used to immunologically identify mammalian yapsin 1-like proteins in bovine and mouse endocrine and neuroendocrine tissues (Cawley, et al. 1996). Yapsin 1-like immunoreactivity has also been found exclusively in human pancreatic islet α-cells and purified yapsin 1 can generate glucagon by processing proglucagon in vitro (Cawley, et al. 2011). Although Yapsin A has not been cloned, current studies suggest that a mammalian aspartic protease present in endocrine tissue, similar to Yapsin 1 in yeast, may play a role in processing of POMC and other prohormones in vivo. Additional “back-up” enzymes could be important in ensuring that prohormone processing is maintained, such that genetic defects in the PCs may not necessarily produce a phenotype.
c. Tetrabasic residue specific enzymes
A calcium activated serine protease, named acidic ACTH converting enzyme (AACE), with a pH optimum of 5.0–6.0 and is highly specific for tetrabasic residues has been reported to be present in bovine intermediate lobe secretory granules (Estivariz, et al. 1992). AACE cleaved ACTH(1–39) at the tetrabasic residues between the Arg17–Arg18 bond to yield ACTH(1–17) and CLIP, but did not cleave the paired basic residues of pro-opiomelanocortin. The enzyme has not been cloned but AACE could play a role in the processing of ACTH to α-MSH besides PC2.
2. Exopeptidases in POMC peptide processing
a. Carboxypeptidase E/H
Subsequent to endoproteolytic cleavage of POMC at paired basic residues, an exopeptidase is required to remove the C-terminal basic residues to yield the biologically active peptides. Carboxypeptidase E (CPE) or carboxypeptidase H (initially known as enkephalin convertase or carboxypeptidase B-like enzyme), was first discovered in 1982 as an enzyme capable of removing C-terminally extended lysine and arginine residues from enkephalin peptides (Fricker and Snyder 1982; Hook et al. 1982a). CPE is a metalloprotease with Zn bound at the active site. It has a pH optimum of 5.5 that is stimulated by Co++ and specifically inhibited by GEMSA. It was also shown to remove basic residues from ACTH(1–17), a peptide liberated by PC1/3 from POMC, to generate ACTH(1–16), (1–15) and (1–14) (Hook and Loh 1984). At that time, other peptides with C-terminal lysine and arginine extended residues such as vasopressin and oxytocin were also shown to be removed by CPE (Hook and Loh 1984; Kanmera and Chaiken 1985). Because of its localization and optimum activity in the acidic environment of secretory granules, where peptide hormone intermediates are processed, and because of its specificity for C-terminally extended lysine and arginine residues, CPE was considered to be the primary carboxypeptidase for most if not all peptide hormone intermediates including those derived from POMC. Indeed, proteomic analysis of pituitaries from the CPE deficient mouse, Cpefat/fat, showed a significant accumulation of the C-terminal basic residue extended POMC-derived peptides, compared to WT pituitary, indicating the role of CPE in the normal processing of these peptides in vivo (Che, et al. 2005).
There are 2 forms of CPE, a soluble form which is enzymatically much more active than the membrane form (Hook 1985). Some of the membrane form can assume a transmembrane orientation in the secretory granule membrane giving rise to a cytoplasmic tail (Dhanvantari, et al. 2002; Zhang, et al. 2003). The membrane form can act as a sorting receptor for prohormones at the TGN and the cytoplasmic tail is involved in secretory granule transport by associating with microtubule motors (see Section III.3 above). CPE, synthesized as a precursor (pro-CPE, ~55 kD in size), is trafficked to the TGN where it associates with the membrane through interaction with lipid rafts, and is subsequently sorted into immature secretory granules after budding. Some of the CPE is then processed to the mature soluble form (mol. wt ~50kD) within the secretory granule; where it can act enzymatically to cleave basic residues from peptide products liberated from POMC. CPE is secreted and several studies indicate that it plays other important non-enzymatic roles as a signaling molecule acting extracellularly, in neuroprotection and prevention of stress induced-depression (for a review, see Cawley et al 2012, (Cawley, et al. 2012)).
The physiological importance of CPE as a processing enzyme and a sorting receptor for prohormones was evident from several studies. A mutation in the Cpe gene was found in the Cpefat/fat mouse that presented with severe obesity, diabetes, and infertility (Naggert et al. 1995). In these Cpefat/fat mice, it was reported that POMC was accumulated 24-fold above normal animals in the anterior pituitary and it was poorly processed to ACTH, although larger 24 kD form of ACTH was present (Shen and Loh 1997). Furthermore, POMC was secreted constitutively at high levels, showing no response to stimulation by corticotropin-releasing hormone (Shen and Loh 1997), a finding not reproduced later by others (Hosaka et al. 2005), possibly reflecting a change in the mice within the intervening years. POMC levels were elevated in the circulation of Cpefat/fat mice versus normal mice. This poor maturation of POMC could be a result of inefficient sorting of POMC into the granules of the regulated secretory pathway for full processing since CPE acts as a sorting receptor, resulting in constitutive secretion of partially processed POMC products that accrued in the Golgi (Shen and Loh 1997). These mice also had hyperproinsulinemia (Naggert et al. 1995) and GnRH peptides with extended basic residues that were inactive, resulting in the infertility phenotype in these animals (Srinivasan, et al. 2004).
As indicated above, obesity was also an observed phenotype in the Cpefat/fat and CPE KO mice, contributed in part by autophagy due to a disruption in the hypothalamic circuitry that controls satiety (Cawley, et al. 2004). In both cases, defective processing of hypothalamic POMC to α-MSH, a major anorexigenic peptide that controls satiety in this tissue, resulted in increased food intake and obesity, demonstrating that CPE played a key role in appetite regulation and energy balance. In support of this were the observations that, ablation of Forkhead box protein O1 (FOXO1) in POMC neurons (POMC-FOXO1−/−) reduced food intake without affecting energy expenditure. The study showed that FOXO1 is a co-repressor of CPE expression at the promoter level. Consequently, increased levels of the hypothalamic neuropeptides, α-MSH and β-endorphin, were observed in the POMC-FOXO1−/− mice. FOXO1 deletion therefore protected the mice against weight gain, in a diet-induced obesity paradigm, by increasing the satiation POMC peptide, α-MSH (Plum, et al. 2009). Hence in this POMC-FOXO1−/− model, deletion of FOXO1 allowed increased expression of CPE in the POMC hypothalamic neurons that subsequently effected the levels of active PC2 by inactivation of the CT inhibitor peptide of 7B2. Concomitantly, the CPE can process the acetylated ACTH(1–16) intermediate to α-MSH (see section on PC2 above (Zhu, et al. 1996)). This study further demonstrates the important physiological function of CPE in obesity. Corroborating these mouse studies is a recent description of the first human with a truncating homozygous null mutation for CPE which showed the patient presented with obesity, type 2 diabetes, as well as intellectual disability (Alsters, et al. 2015), further emphasizing the critical role CPE plays in prohormone processing and sorting.
b. Aminopeptidases
While PC1/3 and PC2 generally cleave POMC and derived-peptides on the carboxyl side of paired basic residues, the cleavage of ACTH at the tetrabasic residues by PC2 to release CLIP (Figure 5) with a N-terminal arginine indicates that certain cleavages occurs between two basic residues. Cleaving of POMC in between basic residue doublets by Yapsin A has also been reported. Thus there is a need for an aminopeptidase B-like enzyme to remove the N-terminal basic residue. An aminopeptidase B-like enzyme has been found in bovine pituitary intermediate and neural lobe secretory granules (Gainer, et al. 1984). The enzyme activity is found both as a soluble and membrane form, has a pH optimum of 6.0, is stimulated by Co++ and Zn++ and cleaves Arg preferentially over Lys. However, it will not cleave an N-terminal Arg if it is followed by a proline such as in CLIP. Characterization of the enzyme indicates that it is a ~70 kD glycoprotein and is coordinately secreted with α-MSH indicating its co-localization in the same secretory granules (Castro, et al. 1989).
3. Acetylation and amidation of POMC-derived peptides
a. Acetylation of POMC peptides
N-Acetylation of peptide hormones may serve to increase the stability of the peptide by protecting them against the action of aminopeptidases and enhance their half-life in the circulation. Acetylation also has profound biological effects on the POMC-peptides. While the melanotropic activity of α-MSH (Guttmann and Boissonnas 1961) is potentiated and its half-life increased by N-acetylation, acetylation of β-endorphin completely abolishes its opiate activity (Deakin, et al. 1980). Since these two peptides are derived from POMC, acetylation could be used to regulate the relative amounts of melanotropic and opiate activities. Additionally, it has been reported that in mammals, while α-MSH is the predominant form, diacetylated and desacetylated forms are also present in the pituitary intermediate lobe, although the existence of these latter forms do vary among species. N-Ac-β-endorphin has been found in both anterior lobe and intermediate lobe from postnatal day 1 (P1) through adulthood. In the intermediate lobe, the level increases to 90% of the endorphins present by P14, but in the anterior lobe, N-Ac-β-endorphin drops dramatically to <5% in adult rats (Alessi, et al. 1983).
An N-acetyltransferase enzyme activity has been found in bovine and rat intermediate pituitary secretory granules that could acetylate both ACTH1–14 and β-endorphin (Chappell, et al. 1982; Glembotski 1982; Gibson and Glembotski 1985). Competition studies (Glembotski 1982) using fragments of ACTH and β-endorphin peptides and acetylation enzyme activity from bovine secretory granules, as well as comparative studies of ACTH and β-endorphin acetylation enzyme activities (Chappell et al. 1982) from rat intermediate lobe secretory granules indicate that the same acetylation enzyme is responsible for acetylating the N-terminus of both ACTH1–14 and β-endorphin to yield N-Ac-ACTH1–14 and Ac-β-endorphin, respectively. This enzyme activity specifically localized to the secretory granules of rat intermediate pituitary has been referred to as opiomelanotropin acetyltransferase (OMAT) (Chappell et al. 1982). Unlike other acetylation enzyme activities in the pituitary, OMAT has a pH optimum of 6.0–6.6 and is inhibited by detergents. Since the secretory granule acetylation enzymes in the bovine and rat intermediate pituitary have not been cloned, it remains to be determined if they are similar or identical molecules.
N-acetyltransferase activity and POMC expression have been shown to be co-regulated in the intermediate pituitary (Millington, et al. 1986). Secretion of POMC peptides from the intermediate pituitary is under inhibitory control by dopamine (Fischer and Moriarty 1977). It was found that haloperidol, a dopamine antagonist, coordinately increased acetyltransferase activity, POMC mRNA levels and POMC peptides; whereas bromocryptine, a dopamine agonist had opposite effects (Millington et al. 1986). Only acetyltransferase activity from secretory granules was affected by these pro- and anti-secretogogue activities, but not acetylation activity in the RER or Golgi. Additionally, changes in acetylation of β-endorphin have been demonstrated in rat intermediate lobe cells in culture when exposed to dopamine agonists and antagonists (Ham and Smyth 1984). These studies suggest that acetylation of these peptides may be modulated by their secretory activity. It has been reported that repeated stress selectively increased the biosynthesis and release of N-Ac-β-endorphin(1–31) from the intermediate lobe of rats and is the major form in plasma (Akil, et al. 1985). In contrast, in the anterior pituitary, after repeated stress, the major form released is β-endorphin(1–31), rather than β-lipotropin, normally released under non-stressed conditions. Thus the acetylation of POMC-derived peptides contributes another mechanism for the regulation of their hormonal activity.
b. Amidation of POMC peptides
Synthesis of α-MSH involves the amidation of N-Ac-ACTH1–14. In addition, another POMC-derived peptide, joining peptide (Figure 5) is also amidated. The enzyme involved in the amidation reaction is peptidylglycine α-amidating monooxygenase (PAM). Studies on the amidation of POMC-peptides and PAM are reviewed by Mains and Eipper (Kumar, et al. 2015) in this book issue.
4. Regulation of POMC processing
POMC is differentially processed endoproteolytically in the anterior, intermediate and arcuate nucleus of the hypothalamus to generate different end products (Figure 5). A number of factors can dictate the differential processing.
Tissue specific glycosylation of a residue close to the cleavage active site can influence the ability of the enzyme to cleave the site. Indeed, the fate of N-POMC(1–77) exemplifies such a mechanism since only ~50% of this POMC intermediate was cleaved in pituitary tissue into N-POMC(1–49) and Lys-γ3-MSH (Seger and Bennett 1986). Metabolic labelling studies and POMC fragment analysis identified that the O-linked glycosylation of Thr-45 regulated the processing of N-POMC(1–77) into N-POMC(1–49) (Bennett 1986), suggesting that stearic hindrance by the sugar moiety could prevent the processing. This has important physiological significance since the regulation of processing that this site produces, controls the level of N-POMC(1–49) and the mitogenic activity it exhibits (Pepper and Bicknell 2009).
Another mechanism for tissue specific processing of POMC in the anterior and intermediate lobe is dictated by the presence of different processing enzymes as reviewed above. Whereas the intermediate pituitary expresses PC1/3 and PC2 resulting in the processing of ACTH to α-MSH and β-lipotropin to β-endorphin, the anterior pituitary expresses primarily PC1/3 that does not catalyze theses cleavages in vivo. Additionally, the tetabasic residue specific enzyme AACE is present in much higher amounts in the intermediate lobe than the anterior lobe of the pituitary (Estivariz et al. 1992) and this contributes to the differences in processing of ACTH and β-lipotropin in these two lobes. The presence of PC2 in the hypothalamic POMC neurons could also account for the processing of ACTH to α-MSH in theses neurons (Zheng, et al. 1994; Joshi, et al. 1995).
5. Secretion of POMC-derived peptides
After post-translational processing via cleavage by prohormone convertases, POMC-derived peptides are packaged and stored as electron dense cores in secretory granules and secreted in response to simulation by secretagogues. POMC-derived peptides are mainly secreted from corticotrophs and melanotrophs of the anterior and intermediate lobes of the pituitary gland, respectively, as well as from peptidergic neurons of the arcuate nucleus of the hypothalamus. For example, ACTH, β-lipotropin and some β-endorphin are secreted by corticotrophs, whereas α-melanocyte-stimulating hormone (MSH) and β-endorphin are primarily secreted by melanotrophs and from the hypothalamus. The secretion of POMC-derived peptides is regulated by various secretagogues as discussed below. This section is not intended as a comprehensive list of effectors of POMC-derived peptide secretion, as that is exceedingly complex, but more as a summary of several pathways that play roles in this process.
a. Regulation of secretion of POMC-derived peptides from the anterior lobe of the pituitary
The pituitary gland is composed of the anterior lobe (adenohypophysis, AL) and the posterior lobe (neurohypophysis, NL) with an intermediate lobe (IL) present between the AL and NL, and is considered by many to be the master endocrine gland, although the neural lobe is technically considered an extension of the hypothalamus (Figure 6). It is the middle component of the hypothalamic-pituitary-adrenal (HPA) axis and is involved with multiple endocrine functions such as growth, stress and reproduction. Nerve fibers from the hypothalamus extend through the median eminence and infundibular stem to the pituitary through the pituitary stalk. Axons containing AVP and oxytocin from the magnocellular neurons of the hypothalamus (supraoptic nucleus (SON) and paraventricular nucleus (PVN)) innervate the capillary bed of the posterior lobe and are released to the circulation through the hypophyseal vein. Other axons from the PVN terminate earlier at the capillary network in the lower infundibular stem close to the AL and releases neurotransmitters and peptide hormones into the portal network that in turn regulates the secretion of peptide hormones from cells of the AL. Since the IL is in close proximity to the AL, molecules secreted into and through the AL effects secretion from the IL also.
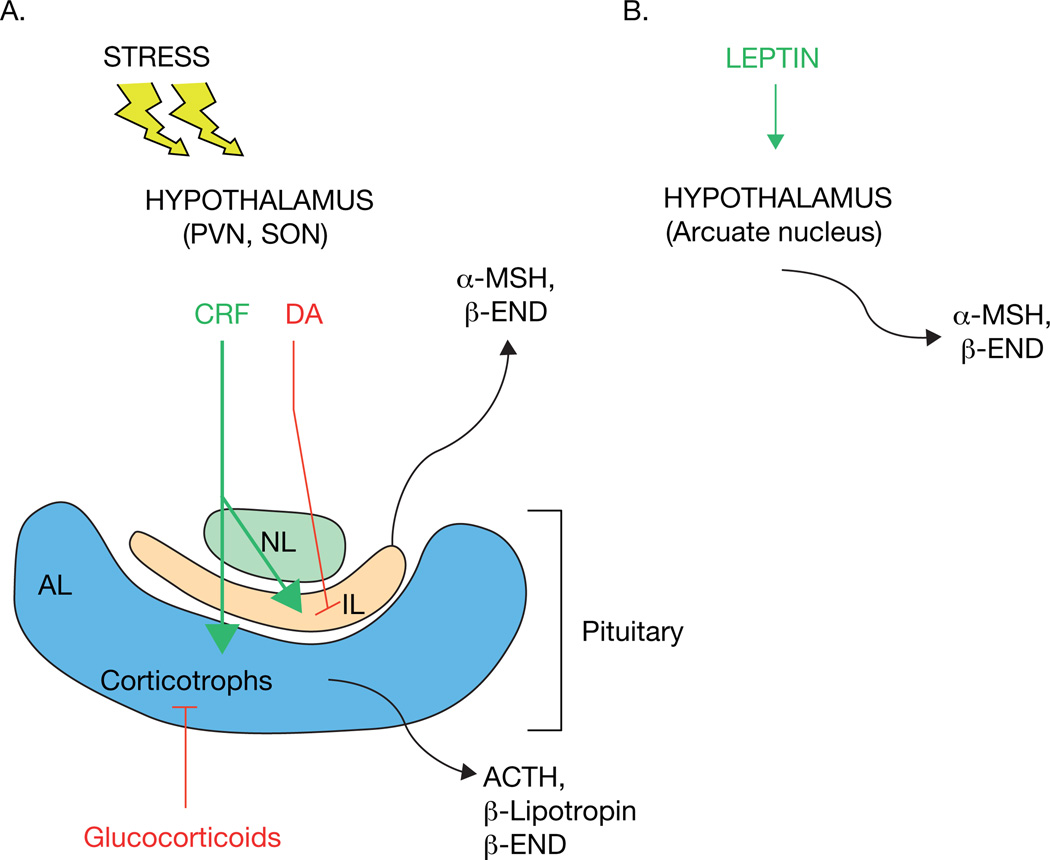
Figure 6. Simplified schematic of the hypothalamic-pituitary axis.
A. Corticotrophs in the anterior lobe (AL) are under positive regulation by CRF released from the hypothalamus under times of stress. These cells release ACTH which causes the secretion of glucocorticoids from the adrenal cortex. Glucocorticoids then inhibit ACTH, β-lipotropin and b-endorphin release in a negative feedback manner. Dopamine (DA) inhibits and CRF increases secretion of α-MSH and β-endorphin from the melanotrophs of the intermediate lobe (IL). B. Leptin secreted from adipocytes activates POMC neurons in the arcuate nucleus of the hypothalamus to release α-MSH and β-endorphin. See main text for other neurotransmitters and peptide hormones that help regulate the secretion of POMC-derived peptides from these tissues.
With respect to POMC, in vivo, in response to short-term or long-term stress, the neurons in the hypothalamic PVN secrete corticotroph releasing factor/hormone (CRF, also known as CRH) into the hypophyseal portal system (Swanson, et al. 1983), which then stimulates the secretion of POMC-related peptides from the corticotrophs of the AL, including primarily ACTH (Chan, et al. 1982). The ACTH exits the pituitary via the hypophyseal vein and in turn activates the adrenal cortex to produce glucocorticoids in times of stress. In addition, β-lipotropin/β-endorphin is released to activate opioid pathways in the body in response to pain. Among their many physiological roles, glucocorticoids in turn exhibit a negative feedback inhibition on the corticotrophs in the AL and the hypothalamic neurons to keep the levels of circulating ACTH under control. Hence, the AL is primarily under the positive regulation of CRF and negative regulation of glucocorticoids although there are many other hormones and compounds that contribute to the net secretion of POMC-derived peptides from the corticotrophs. Indeed, dexamethasone, a glucocorticoid homologue, was shown to reduce the serotonergic induced secretion of β-LPH in vivo suggesting that serotonin neurons may regulate and contribute to the release of β-LPH (and by association ACTH) from anterior pituitary corticotrophs in vivo (Sapun-Malcolm, et al. 1983).
In addition to glucocorticoids, other factors, such as atrial natriuretic factor (ANF) (Shibasaki, et al. 1986) and somatostatin (Invitti, et al. 1991), have been shown to inhibit POMC-derived peptides secretion by affecting CRF function. For ANF, it had been proposed that its inhibition was through cGMP signaling, however later studies did not support this (Bowman, et al. 1997). For somatostatin, the selective inhibition of CRF-induced secretion of β-endorphin in vivo, and not ACTH and β-lipotropin, was confounding, however, it was proposed that treatment with somatostatin possibly reduced the processing of POMC to β-endorphin in corticotrophs to account for the reduced levels. Alternatively, the effect could be through regulation on pituicytes or other non-pituitary tissue specifically expressing β-endorphin and not corticotrophs. This was considered an example of dissociated secretion of POMC-derived peptides. Gamma-aminobutyric acid (GABA) can also inhibit β-endorphin secretion, possibly through an interaction between GABAergic neurons and CRF neurons and indicated that GABA exerts a tonic inhibitory role on the CRF-regulated corticotroph secretion (Petraglia, et al. 1986).
In contrast to those molecules involved in the negative regulation of POMC-derived peptides secretion, many other factors have been shown to stimulate their secretion via effects on CRF, e.g., clusterin, a peptide secreted from the pituitary and hypothalamus after stress, increased basal and CRF-stimulated POMC promoter activities and intracellular cAMP levels, thus augmenting CRF-stimulated ACTH production and secretion (Shin, et al. 2013). Indeed the regulation of expression and synthesis of POMC by secretagogues (Aoki, et al. 1997) affects the cellular content and hence secretion profiles, e.g. melatonin and bone morphogenetic protein-4 (Tsukamoto, et al. 2010; Tsukamoto, et al. 2013). Some hormones or compounds also appear to stimulate secretion of POMC-related peptides from the anterior pituitary via diverse mechanisms. For example, Melittin, the major peptide component of bee venom and a powerful stimulator of phospholipase A2, generated a signal in corticotrophs of rat AL resulting in the stimulated secretion of ACTH and β-endorphin, although the mechanism appeared to be independent of the phospholipase A2 activation (Knepel and Gerhards 1987). Interleukin-1, a cytokine released from cells of the immune system in response to infection, enhanced secretion of β-endorphin by inducing protein kinase C (Fagarasan, et al. 1989). In addition, the renin-angiotensin system increased the secretion of ACTH, β-lipotropin and β-endorphin by stimulating the secretion of arginine vasopressin (AVP) from neurons of the supraoptic nuclei (SON) and paraventricular nuclei (PVN) of the hypothalamus to exert its stimulatory effect on the AL, thus increasing secretion of β-endorphin (Beuers, et al. 1982). AVP had been shown to induce the secretion of POMC-derived peptides from primary cultures of human anterior pituitary cells; however, it was not as potent as CRF (Chan et al. 1982).
Small molecules have also identified further levels of control on the secretion of POMC-derived peptides from the AL. It was observed that the calcium antagonist, nimodipine, increased β-endorphin secretion through an action on the adrenal glands. It was proposed that, since glucocorticoids exhibit feedback inhibition on the regulation of biosynthesis and secretion of POMC, nimodipine, which reduced adrenal gland responsiveness to ACTH, might increase β-endorphin release from the anterior pituitary gland by reducing glucocorticoid secretion from the adrenal cortex (Costa, et al. 1984). Hence, regulation of adrenal responsiveness to ACTH affects corticotroph behavior. Other small molecules such as Cyclosporin A and Tacrolimus (FK506), immunosuppressant drugs, stimulated POMC-derived peptide secretion and potentiated phorbol ester and CRF stimulated secretion (Sheppard 1995) demonstrating that these immunosuppressant drugs act at a common point in these pathways.
As indicated in Section II, AtT20 cells have been used extensively in the study of POMC biosynthesis and trafficking as well as in the regulation of secretion of its POMC-derived peptides. These cells, as noted previously, store and secrete ACTH and β-endorphin-related peptides in a calcium dependent manner in response to secretagogues or by membrane depolarization with high levels of K+. Membrane depolarization by action potentials and calcium influx was shown to be closely linked to the regulated secretion of the mature granules in AtT20 cells (Surprenant 1982). Using isoproterenol, a non-selective β-adrenergic agonist, or raising the external calcium concentrations, increased both action potential frequency and ACTH/β-endorphin-like peptide secretion in AtT20 cells. However, a complete blockade of action potential activity had no effect on basal hormone secretion in these cells, indicating that the mechanisms underlying stimulated hormone secretion were different from those responsible for basal secretory activity. Indeed, norepinephrine, a member of the catecholamine family, stimulated the release of ACTH, β-endorphin, β-lipotropin, and 16K N-POMC from AtT20 cells, an effect that was fully blocked by cobalt, demonstrating the stimulated secretion was calcium dependent, the hallmark of regulated secretion (Mains and Eipper 1981a). Also in AtT20 cells, phorbol ester, vasoactive intestinal peptide, forskolin, beta-adrenergic agonist, as well as the calcium ionophore stimulated the secretion of a dynorphin-converting enzyme found in these cells, in parallel with CPE and ACTH, demonstrating the wide responsiveness of the granules containing ACTH in these cells to many secretagogues (Devi 1992).
As with the feedback inhibition by glucocorticoids on the corticotrophs in vivo, glucocorticoids rapidly inhibit secretion of these peptides from these cells in vitro by increasing transcription and translation of proteins that inhibit synthesis or increase catabolism of the peptides (Sabol 1980). The fast inhibitory effect may partly be due to a glucocorticoid-dependent reduction in CRF stimulation by blocking the CRF dependent calcium signaling (Antoni 1996). Other studies in AtT20 cells showed that CRF at concentrations which stimulated ACTH secretion also increased phospholipid methylation. These effects were blocked not only by dexamethasone, a synthetic glucocorticoid which selectively inhibits corticotroph secretion in vitro, but also by the phospholipid methyltransferase inhibitors, 3-deazaadenosine and L-homocysteine thiolactone, suggesting that phospholipid methylation might be a CRF receptor-mediated event associated with ACTH secretion (Hook, et al. 1982b).
In contrast to the negative regulation by glucocorticoids on the AtT20 cells, no evidence for auto-inhibition of secretion by accumulated secreted peptides (i.e., ultra-short feedback) was found. Furthermore, synthetic human ACTH and synthetic camel β-endorphin did not alter secretion of peptides when added to the culture medium at up to 10,000 times above physiological levels (Mains and Eipper 1981a).
b. Regulation of secretion of POMC-derived peptides from the intermediate lobe of the pituitary
Mammals and lower vertebrates have a well-developed intermediate lobe (IL) whereby α-MSH is released into the blood stream through the hypophyseal vein to effect the regulation of pigmentation by melanocytes. However, in humans, the intermediate lobe is only present in the fetus as a distinct area but in adults is reduced to a thin layer of cells between the anterior and posterior lobes of the pituitary or it is entirely absent (McNicol 1986). As such, the relevance to human physiology is limited since most work on the IL has been done in tissue obtained from a variety of animals including frog, mouse, rat and dogs. As mentioned above, the IL is in close proximity to neurotransmitters and peptide hormones released from the hypothalamic neurons in the capillary network of the hypophyseal artery in addition to being at the interface of the neurohypophysis. Since the IL is composed primarily of a homogeneous population of melanotrophs, its main function is to provide MSH-like peptides, in addition to β-endorphin, to the circulation in response to hypothalamic signals.
Early on, dopamine was shown to effectively inhibit release of ACTH-like material from isolated rat NIL, while other compounds were reported as potent secretagogues such as acetylcholine, serotonin and AVP (Fischer and Moriarty 1977), demonstrating that net secretion was likely a balance between stimulatory and inhibitory signaling. Indeed, release of ACTH and MSH from dog IL was inhibited by dopamine, somatostatin, norepinephrine and epinephrine; the last two however were blocked by haloperidol (a dopamine D2 receptor antagonist), suggesting signaling through the dopamine receptor (Kemppainen, et al. 1989). In addition, haloperidol increased POMC mRNA expression in rat IL (Hollt and Bergmann 1982), but decreased the CRF receptor expression (Shiver, et al. 1992), whereas bromocriptine, a dopamine receptor agonist did the opposite. Indeed, CRF receptors are present on both lobes of the pituitary (Aguilera, et al. 1987) but show different levels of expression in response to dopamine agonists and antagonists (Shiver et al. 1992). Notably, these two compounds did not affect CRF receptor levels in the AL and demonstrated a tight regulation of POMC expression and secretion of POMC-derived peptides from the IL by specific tonic inhibition of dopamine D2 receptors and subsequent regulation of CRF receptor expression (Beaulieu, et al. 1984) although other neurotransmitters eg. GABA, also participate (Tomiko, et al. 1983). Similar to the regulation of corticotrophs in the AL, release of POMC-derived peptides from the IL is stimulated by CRF and CRF-like peptides e.g. urotensin I and sauvagine (Tran, et al. 1990). Hence, CRF stimulates corticotrophs to secrete ACTH and β-LPH and melanotrophs to secrete α-MSH and β-endorphin.
Further studies in perifused rat IL demonstrated that the amounts of spontaneously secreted ACTH- and lipotropin- related peptides were proportional to the amounts in which these peptides were found in extracts of intermediate lobe (Tilders, et al. 1981). This high basal rate of secretion was presumably due to the lack of tonic inhibition by dopamine in this in vitro system. However, isoproterenol could stimulate the release of various peptides, including α-MSH, ACTH and β-endorphin-like peptides above this baseline (Tilders et al. 1981). Studies in bovine IL indicated that 8-bromo-cAMP significantly increased and bromocriptine significantly reduced secretion of α-MSH (Castro et al. 1989).
There are many studies investigating the regulation of secretion of peptide hormones, specifically POMC-derived peptides, from the IL; too many to adequately describe here, however, it can be seen from this section that the interplay between signaling molecules from the hypothalamus and peripheral tissues results in controlling the levels of POMC peptides delivered to the circulation. Taken together, the regulation of secretion of POMC-derived peptides from the AL and IL of the pituitary gland involves many factors with the final outcome depending on the competing action of these stimulatory and inhibitory factors.
c. Regulation of secretion of POMC-derived peptides from the arcuate nucleus of the hypothalamus
The arcuate nucleus is the third major site for POMC expression; other tissues have also been identified where POMC is expressed e.g. skin, placenta and others (for review and references within see (Stevens and White 2010)). As already stated, the final peptides produced in the hypothalamus are similar to those from the IL; α-MSH and β-endorphin. These neurons are responsive to leptin and insulin, and other neural and humeral signals, as indicators of peripheral energy stores in vivo, and are primarily known to centrally regulate food intake through α-MSH action on the melanocortin 4 receptors (MC4R) in other hypothalamic areas and in areas of the brainstem important in regulating energy balance (Cowley, et al. 2001; Lin and Salton 2013). Mutations in MC4R were first reported to be associated with inherited human obesity in 2008 (Loos, et al. 2008) demonstrating the importance of this signaling pathway in human health. Indeed, defective POMC processing in humans and mouse models leads to severe obesity, as well as ACTH deficiency and hypopigmentation (Jackson et al. 1997; Krude, et al. 1998; Jackson, et al. 1999; Yaswen, et al. 1999). In this regard, POMC is a particularly interesting molecule in the homeostatic regulation of appetite and obesity. For a more comprehensive review on POMC neurons in the arcuate nucleus and their regulation, readers are directed to a recent review by Sharon Wardlaw (Wardlaw 2011) and the chapter in this book by Prof. Roger Cone (Cone 2016).
d. Secretion of ACTH in tumors
When secretion of POMC-derived peptides is abnormal, a disease state can occur. For instance, positive regulation of ACTH secretion by CRF, vasopressin as well as other factors leads to excessive levels of ACTH when the feedback inhibition of glucocorticoids is faulty (Imura 1985). This leads to excessive glucocorticoids (cortisol) in circulation, leading to Cushing’s syndrome. A similar dysregulation may occur with an ACTH producing pituitary adenoma giving rise to Cushing’s disease. Another example is Nelson’s syndrome, which results from a rapid enlargement of a pre-existing ACTH-secreting pituitary adenoma that occurs after the removal of the adrenal glands thus lacking the negative feedback of cortisol on the production of ACTH (Biller, et al. 2008). A study on the cultured pituitary adenoma from a patient with Nelson’s syndrome showed that somatostatin-14 and somatostatin-28 suppressed the secretion of POMC-derived peptides; but other neuropeptides such as arginine vasopressin, vasoactive intestinal polypeptide, and oxytocin stimulated the secretion of POMC-derived peptides. Substance P, thyrotropin-releasing factor, Met-enkephalin and Leu-enkephalin were also found to modulate the secretion of POMC-derived peptides, suggesting that adenomas of this nature may have multiple receptors to various neuropeptides (Shibasaki and Masui 1982) that could regulate POMC peptide secretion in vivo.
V. Summary
Pro-opiomelanocortin is a multi-valent prohormone capable of producing at least 7 peptide hormones depending on its processing by prohormone converting enzymes. The prohormone is sorted into the nascent immature granules of the regulated secretory pathway at the TGN, through interaction with at least two membrane associated proteins, carboxypeptidase E and secretograinin III. As the granules mature, POMC is cleaved into its complement of peptide hormone intermediates which are further processed by exopeptidases and amidating and acetylating enzymes to produce the bioactive peptide hormones. The granules containing POMC are transported in a microtubule dependent anterograde manner through interaction of the carboxypeptidase E cytoplasmic tail with dynactin and the motor proteins, kinesin 2 and 3. The mature granules are stored close to the plasma membrane and are released in a secretagogue dependent manner that depends on calcium. Stimulated release of the mature granules is regulated by multiple contributing factors; primarily among them is corticotrophin releasing factor acting on the corticotrophs that in turn are inhibited by glucocorticoids. Melanotrophs are under the tonic inhibition of dopamine, while POMC neurons in the hypothalamus respond to leptin. The subject of the biosynthesis, sorting, trafficking and secretion of POMC and its bioactive peptides has been studied extensively and represents an area of physiology that now has a broad and substantial foundation in knowledge thanks to the pioneers in this field. This strong base allows future investigators to ask more stringent questions about the generation, roles and regulation of these peptides in normal and diseased states in vivo.
Acknowledgments
Funding: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, USA (Grant #HD000056).
Footnotes
Conflict of interest: The authors declare there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Bibliography
- Aguilera G, Millan MA, Hauger RL, Catt KJ. Corticotropin-releasing factor receptors: distribution and regulation in brain, pituitary, and peripheral tissues. Ann N Y Acad Sci. 1987;512:48–66. doi: 10.1111/j.1749-6632.1987.tb24950.x. [DOI] [PubMed] [Google Scholar]
- Akil H, Shiomi H, Matthews J. Induction of the intermediate pituitary by stress: synthesis and release of a nonopioid form of beta-endorphin. Science. 1985;227:424–426. doi: 10.1126/science.3155575. [DOI] [PubMed] [Google Scholar]
- Alessi NE, Khachaturian H, Watson S, Akil H. Postnatal ontogeny of acetylated and non-acetylated B-endorphin in rat pituitary. Life Sci. 1983;33(Suppl 1):57–60. doi: 10.1016/0024-3205(83)90443-5. [DOI] [PubMed] [Google Scholar]
- Alsters SI, Goldstone AP, Buxton JL, Zekavati A, Sosinsky A, Yiorkas AM, Holder S, Klaber RE, Bridges N, van Haelst MM, et al. Truncating Homozygous Mutation of Carboxypeptidase E (CPE) in a Morbidly Obese Female with Type 2 Diabetes Mellitus, Intellectual Disability and Hypogonadotrophic Hypogonadism. PLoS One. 2015;10:e0131417. doi: 10.1371/journal.pone.0131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni FA. Mortyn Jones Memorial Lecture--1995. Calcium checks cyclic AMP--corticosteroid feedback in adenohypophysial corticotrophs. J Neuroendocrinol. 1996;8:659–672. [PubMed] [Google Scholar]
- Aoki Y, Iwasaki Y, Katahira M, Oiso Y, Saito H. Regulation of the rat proopiomelanocortin gene expression in AtT-20 cells. I: Effects of the common secretagogues. Endocrinology. 1997;138:1923–1929. doi: 10.1210/endo.138.5.5121. [DOI] [PubMed] [Google Scholar]
- Arnaoutova I, Jackson CL, Al-Awar OS, Donaldson JG, Loh YP. Recycling of Raft-associated prohormone sorting receptor carboxypeptidase E requires interaction with ARF6. Mol Biol Cell. 2003;14:4448–4457. doi: 10.1091/mbc.E02-11-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azaryan AV, Schiller MR, Hook VY. Chromaffin granule aspartic proteinase processes recombinant proopiomelanocortin (POMC) Biochem Biophys Res Commun. 1995;215:937–944. doi: 10.1006/bbrc.1995.2554. [DOI] [PubMed] [Google Scholar]
- Azaryan AV, Wong M, Friedman TC, Cawley NX, Estivariz FE, Chen HC, Loh YP. Purification and characterization of a paired basic residue-specific yeast aspartic protease encoded by the YAP3 gene. Similarity to the mammalian pro-opiomelanocortin-converting enzyme. J Biol Chem. 1993;268:11968–11975. [PubMed] [Google Scholar]
- Beaulieu M, Goldman ME, Miyazaki K, Frey EA, Eskay RL, Kebabian JW, Cote TE. Bromocriptine-induced changes in the biochemistry, physiology, and histology of the intermediate lobe of the rat pituitary gland. Endocrinology. 1984;114:1871–1884. doi: 10.1210/endo-114-5-1871. [DOI] [PubMed] [Google Scholar]
- Benjannet S, Rondeau N, Paquet L, Boudreault A, Lazure C, Chretien M, Seidah NG. Comparative biosynthesis, covalent post-translational modifications and efficiency of prosegment cleavage of the prohormone convertases PC1 and PC2: glycosylation, sulphation and identification of the intracellular site of prosegment cleavage of PC1 and PC2. Biochem J. 1993;294(Pt 3):735–743. doi: 10.1042/bj2940735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett HP. Isolation and characterization of the 1 to 49 amino-terminal sequence of pro-opiomelanocortin from bovine posterior pituitaries. Biochem Biophys Res Commun. 1984;125:229–236. doi: 10.1016/s0006-291x(84)80358-7. [DOI] [PubMed] [Google Scholar]
- Bennett HP. Biosynthetic fate of the amino-terminal fragment of pro-opiomelanocortin within the intermediate lobe of the mouse pituitary. Peptides. 1986;7:615–622. doi: 10.1016/0196-9781(86)90036-7. [DOI] [PubMed] [Google Scholar]
- Bennett HP, Seidah NG, Benjannet S, Solomon S, Chretien M. Reinvestigation of the disulfide bridge arrangement in human pro-opiomelanocortin N-terminal segment (hNT 1–76) Int J Pept Protein Res. 1986;27:306–313. doi: 10.1111/j.1399-3011.1986.tb01825.x. [DOI] [PubMed] [Google Scholar]
- Bergeron F, Sirois F, Mbikay M. ACTH secretion by mouse corticotroph AtT20 cells is negatively modulated by the intracellular level of 7B2. FEBS Lett. 2002;512:259–262. doi: 10.1016/s0014-5793(02)02277-9. [DOI] [PubMed] [Google Scholar]
- Beuers U, Hertting G, Knepel W. Release of beta-lipotropin- and beta-endorphin-like material induced by angiotensin in the conscious rat. Br J Pharmacol. 1982;76:579–585. doi: 10.1111/j.1476-5381.1982.tb09257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, et al. Treatment of adrenocorticotropin-dependent Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab. 2008;93:2454–2462. doi: 10.1210/jc.2007-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnemaison ML, Eipper BA, Mains RE. Role of adaptor proteins in secretory granule biogenesis and maturation. Front Endocrinol (Lausanne) 2013;4:101. doi: 10.3389/fendo.2013.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman ME, Robinson PJ, Smith R. Atrial natriuretic peptide, cyclic GMP analogues and modulation of guanylyl cyclase do not alter stimulated POMC peptide release from perifused rat or sheep corticotrophs. J Neuroendocrinol. 1997;9:929–936. doi: 10.1046/j.1365-2826.1997.00665.x. [DOI] [PubMed] [Google Scholar]
- Burgess TL, Craik CS, Kelly RB. The exocrine protein trypsinogen is targeted into the secretory granules of an endocrine cell line: studies by gene transfer. J Cell Biol. 1985;101:639–645. doi: 10.1083/jcb.101.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess TL, Kelly RB. Sorting and secretion of adrenocorticotropin in a pituitary tumor cell line after perturbation of the level of a secretory granule-specific proteoglycan. J Cell Biol. 1984;99:2223–2230. doi: 10.1083/jcb.99.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess TL, Kelly RB. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Castro MG, Birch NP, Loh YP. Regulated secretion of pro-opiomelanocortin converting enzyme and an aminopeptidase B-like enzyme from dispersed bovine intermediate lobe pituitary cells. J Neurochem. 1989;52:1619–1628. doi: 10.1111/j.1471-4159.1989.tb09217.x. [DOI] [PubMed] [Google Scholar]
- Cawley NX, Normant E, Chen A, Loh YP. Oligomerization of pro-opiomelanocortin is independent of pH, calcium and the sorting signal for the regulated secretory pathway. FEBS Lett. 2000;481:37–41. doi: 10.1016/s0014-5793(00)01961-x. [DOI] [PubMed] [Google Scholar]
- Cawley NX, Portela-Gomes G, Lou H, Loh YP. Yapsin 1 immunoreactivity in {alpha}-cells of human pancreatic islets: implications for the processing of human proglucagon by mammalian aspartic proteases. J Endocrinol. 2011;210:181–187. doi: 10.1530/JOE-11-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley NX, Pu LP, Loh YP. Immunological identification and localization of yeast aspartic protease 3-like prohormone-processing enzymes in mammalian brain and pituitary. Endocrinology. 1996;137:5135–5143. doi: 10.1210/endo.137.11.8895388. [DOI] [PubMed] [Google Scholar]
- Cawley NX, Rathod T, Young S, Lou H, Birch N, Loh YP. Carboxypeptidase E and Secretogranin III coordinately facilitate efficient sorting of pro-opiomelanocortin to the regulated secretory pathway in AtT20 cells. Mol Endocrinol. 2015 doi: 10.1210/me.2015-1166. me20151166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley NX, Rodriguez YM, Maldonado A, Loh YP. Trafficking of mutant carboxypeptidase E to secretory granules in a beta-cell line derived from Cpe(fat)/Cpe(fat) mice. Endocrinology. 2003;144:292–298. doi: 10.1210/en.2002-220588. [DOI] [PubMed] [Google Scholar]
- Cawley NX, Wetsel WC, Murthy SR, Park JJ, Pacak K, Loh YP. New roles of carboxypeptidase E in endocrine and neural function and cancer. Endocr Rev. 2012;33:216–253. doi: 10.1210/er.2011-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley NX, Yanik T, Woronowicz A, Chang W, Marini JC, Loh YP. Obese carboxypeptidase E knockout mice exhibit multiple defects in peptide hormone processing contributing to low bone mineral density. Am J Physiol Endocrinol Metab. 2010;299:E189–E197. doi: 10.1152/ajpendo.00516.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley NX, Zhou J, Hill JM, Abebe D, Romboz S, Yanik T, Rodriguiz RM, Wetsel WC, Loh YP. The carboxypeptidase E knockout mouse exhibits endocrinological and behavioral deficits. Endocrinology. 2004;145:5807–5819. doi: 10.1210/en.2004-0847. [DOI] [PubMed] [Google Scholar]
- Chan JS, Lu CL, Seidah NG, Chretien M. Corticotropin releasing factor (CRF): effects on the release of pro-opiomelanocortin (POMC)-related peptides by human anterior pituitary cells in vitro. Endocrinology. 1982;111:1388–1390. doi: 10.1210/endo-111-4-1388. [DOI] [PubMed] [Google Scholar]
- Chanat E, Huttner WB. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991;115:1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Kable EP, Morrison GH, Webb WW. Calcium sequestration in the Golgi apparatus of cultured mammalian cells revealed by laser scanning confocal microscopy and ion microscopy. J Cell Sci. 1991;100(Pt 4):747–752. doi: 10.1242/jcs.100.4.747. [DOI] [PubMed] [Google Scholar]
- Chappell MC, Loh YP, O'Donohue TL. Evidence for an opiomelanotropin acetyltransferase in the rat pituitary neurointermediate lobe. Peptides. 1982;3:405–410. doi: 10.1016/0196-9781(82)90100-0. [DOI] [PubMed] [Google Scholar]
- Che FY, Biswas R, Fricker LD. Relative quantitation of peptides in wild-type and Cpe(fat/fat) mouse pituitary using stable isotopic tags and mass spectrometry. J Mass Spectrom. 2005;40:227–237. doi: 10.1002/jms.742. [DOI] [PubMed] [Google Scholar]
- Chretien M, Mbikay M. 60 YEARS OF POMC: From the prohormone theory to proopiomelanocortin and to proprotein convertases (PCSK1 to PCSK9) J Mol Endocrinol. 2016 doi: 10.1530/JME-15-0261. [DOI] [PubMed] [Google Scholar]
- Colomer V, Kicska GA, Rindler MJ. Secretory granule content proteins and the luminal domains of granule membrane proteins aggregate in vitro at mildly acidic pH. J Biol Chem. 1996;271:48–55. doi: 10.1074/jbc.271.1.48. [DOI] [PubMed] [Google Scholar]
- Cone R. 60 YEARS OF POMC: Alpha-MSH and its role in apetite regulation. J Mol Endocrinol. 2016 doi: 10.1530/JME-16-0014. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool DR, Fenger M, Snell CR, Loh YP. Identification of the sorting signal motif within pro-opiomelanocortin for the regulated secretory pathway. J Biol Chem. 1995;270:8723–8729. doi: 10.1074/jbc.270.15.8723. [DOI] [PubMed] [Google Scholar]
- Cool DR, Loh YP. Identification of a sorting signal for the regulated secretory pathway at the N-terminus of pro-opiomelanocortin. Biochimie. 1994;76:265–270. doi: 10.1016/0300-9084(94)90156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool DR, Loh YP. Carboxypeptidase E is a sorting receptor for prohormones: binding and kinetic studies. Mol Cell Endocrinol. 1998;139:7–13. doi: 10.1016/s0303-7207(98)00081-1. [DOI] [PubMed] [Google Scholar]
- Cool DR, Normant E, Shen F, Chen HC, Pannell L, Zhang Y, Loh YP. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- Costa G, Saija A, Padovano I, Trimarchi GR, De Pasquale R, Caputi AP. The calcium antagonist nimodipine increases beta-endorphin release from rat hypophysis through an action on adrenal glands. An"in vivo" and "in vitro" study. Pharmacol Res Commun. 1984;16:959–968. doi: 10.1016/s0031-6989(84)80060-0. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Crine P, Gianoulakis C, Seidah NG, Gossard F, Pezalla PD, Lis M, Chretien M. Biosynthesis of beta-endorphin from beta-lipotropin and a larger molecular weight precursor in rat pars intermedia. Proc Natl Acad Sci U S A. 1978;75:4719–4723. doi: 10.1073/pnas.75.10.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crine P, Gossard F, Seidah NG, Blanchette L, Lis M, Chretien M. Concomitant synthesis of beta-endorphin and alpha-melanotropin from two forms of pro-opiomelanocortin in the rat pars intermedia. Proc Natl Acad Sci U S A. 1979;76:5085–5089. doi: 10.1073/pnas.76.10.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JF, Dostrovsky JO, Smyth DG. Influence of N-terminal acetylation and C-terminal proteolysis on the analgesic activity of beta-endorphin. Biochem J. 1980;189:501–506. doi: 10.1042/bj1890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L. Secretion and regulation of a neuropeptide-processing enzyme by AtT-20 cells. Endocrinology. 1992;131:1930–1935. doi: 10.1210/endo.131.4.1327723. [DOI] [PubMed] [Google Scholar]
- Dhanvantari S, Arnaoutova I, Snell CR, Steinbach PJ, Hammond K, Caputo GA, London E, Loh YP. Carboxypeptidase E, a prohormone sorting receptor, is anchored to secretory granules via a C-terminal transmembrane insertion. Biochemistry. 2002;41:52–60. doi: 10.1021/bi015698n. [DOI] [PubMed] [Google Scholar]
- Dhanvantari S, Shen FS, Adams T, Snell CR, Zhang C, Mackin RB, Morris SJ, Loh YP. Disruption of a receptor-mediated mechanism for intracellular sorting of proinsulin in familial hyperproinsulinemia. Mol Endocrinol. 2003;17:1856–1867. doi: 10.1210/me.2002-0380. [DOI] [PubMed] [Google Scholar]
- Dyken JJ, Sambanis A. Ammonium selectively inhibits the regulated pathway of protein secretion in two endocrine cell lines. Enzyme Microb Technol. 1994;16:90–98. doi: 10.1016/0141-0229(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Egel-Mitani M, Flygenring HP, Hansen MT. A novel aspartyl protease allowing KEX2-independent MF alpha propheromone processing in yeast. Yeast. 1990;6:127–137. doi: 10.1002/yea.320060206. [DOI] [PubMed] [Google Scholar]
- Estivariz FE, Friedman TC, Chikuma T, Loh YP. Processing of adrenocorticotropin by two proteases in bovine intermediate lobe secretory vesicle membranes. A distinct acidic, tetrabasic residue-specific calcium-activated serine protease and a PC2-like enzyme. J Biol Chem. 1992;267:7456–7463. [PubMed] [Google Scholar]
- Fagarasan MO, Eskay R, Axelrod J. Interleukin 1 potentiates the secretion of beta-endorphin induced by secretagogues in a mouse pituitary cell line (AtT-20) Proc Natl Acad Sci U S A. 1989;86:2070–2073. doi: 10.1073/pnas.86.6.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Volders K, Stanhope R, Heuschkel R, White A, Lank E, Keogh J, O'Rahilly S, Creemers JW. Hyperphagia and early-onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. J Clin Endocrinol Metab. 2007;92:3369–3373. doi: 10.1210/jc.2007-0687. [DOI] [PubMed] [Google Scholar]
- Fischer JL, Moriarty CM. Control of bioactive corticotropin release from the neuro-intermediate lobe of the rat pituitary in vitro. Endocrinology. 1977;100:1047–1054. doi: 10.1210/endo-100-4-1047. [DOI] [PubMed] [Google Scholar]
- Fortenberry Y, Liu J, Lindberg I. The role of the 7B2 CT peptide in the inhibition of prohormone convertase 2 in endocrine cell lines. J Neurochem. 1999;73:994–1003. doi: 10.1046/j.1471-4159.1999.0730994.x. [DOI] [PubMed] [Google Scholar]
- Fricker LD, McKinzie AA, Sun J, Curran E, Qian Y, Yan L, Patterson SD, Courchesne PL, Richards B, Levin N, et al. Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing. J Neurosci. 2000;20:639–648. doi: 10.1523/JNEUROSCI.20-02-00639.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker LD, Snyder SH. Enkephalin convertase: purification and characterization of a specific enkephalin-synthesizing carboxypeptidase localized to adrenal chromaffin granules. Proc Natl Acad Sci U S A. 1982;79:3886–3890. doi: 10.1073/pnas.79.12.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman TC, Cool DR, Jayasvasti V, Louie D, Loh YP. Processing of pro-opiomelanocortin in GH3 cells: inhibition by prohormone convertase 2 (PC2) antisense mRNA. Mol Cell Endocrinol. 1996;116:89–96. doi: 10.1016/0303-7207(95)03702-0. [DOI] [PubMed] [Google Scholar]
- Friedman TC, Loh YP, Birch NP. In vitro processing of proopiomelanocortin by recombinant PC1 (SPC3) Endocrinology. 1994;135:854–862. doi: 10.1210/endo.135.3.8070378. [DOI] [PubMed] [Google Scholar]
- Furuta M, Yano H, Zhou A, Rouille Y, Holst JJ, Carroll R, Ravazzola M, Orci L, Furuta H, Steiner DF. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci U S A. 1997;94:6646–6651. doi: 10.1073/pnas.94.13.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainer H, Russell JT, Loh YP. An aminopeptidase activity in bovine pituitary secretory vesicles that cleaves the N-terminal arginine from beta-lipotropin60–65. FEBS Lett. 1984;175:135–139. doi: 10.1016/0014-5793(84)80586-4. [DOI] [PubMed] [Google Scholar]
- Gibson TR, Glembotski CC. Acetylation of alpha MSH and beta-endorphin by rat neurointermediate pituitary secretory granule-associated acetyltransferase. Peptides. 1985;6:615–620. doi: 10.1016/0196-9781(85)90162-7. [DOI] [PubMed] [Google Scholar]
- Glembotski CC. Characterization of the peptide acetyltransferase activity in bovine and rat intermediate pituitaries responsible for the acetylation of beta-endorphin and alpha-melanotropin. J Biol Chem. 1982;257:10501–10509. [PubMed] [Google Scholar]
- Goodman LJ, Gorman CM. Autoproteolytic activation of the mouse prohormone convertase mPC1. Biochem Biophys Res Commun. 1994;201:795–804. doi: 10.1006/bbrc.1994.1771. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Kelly RB. Secretory granules of an anterior pituitary cell line, AtT-20, contain only mature forms of corticotropin and beta-lipotropin. Proc Natl Acad Sci U S A. 1981;78:318–322. doi: 10.1073/pnas.78.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B, Kelly RB. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982;28:51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- Guttmann S, Boissonnas RA. Influence of the structure of the n-terminal extremity of alpha-MSH on the melanophore stimulating activity of this hormone. Experientia. 1961;17:265–267. doi: 10.1007/BF02161433. [DOI] [PubMed] [Google Scholar]
- Ham J, Smyth DG. Regulation of bioactive beta-endorphin processing in rat pars intermedia. FEBS Lett. 1984;175:407–411. doi: 10.1016/0014-5793(84)80778-4. [DOI] [PubMed] [Google Scholar]
- Hollt V, Bergmann M. Effects of acute and chronic haloperidol treatment on the concentrations of immunoreactive beta-endorphin in plasma, pituitary and brain of rats. Neuropharmacology. 1982;21:147–154. doi: 10.1016/0028-3908(82)90154-x. [DOI] [PubMed] [Google Scholar]
- Hook VY. Differential distribution of carboxypeptidase-processing enzyme activity and immunoreactivity in membrane and soluble components of chromaffin granules. J Neurochem. 1985;45:987–989. doi: 10.1111/j.1471-4159.1985.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Hook VY, Eiden LE, Brownstein MJ. A carboxypeptidase processing enzyme for enkephalin precursors. Nature. 1982a;295:341–342. doi: 10.1038/295341a0. [DOI] [PubMed] [Google Scholar]
- Hook VY, Heisler S, Axelrod J. Corticotropin-releasing factor stimulates phospholipid methylation and corticotropin secretion in mouse pituitary tumor cells. Proc Natl Acad Sci U S A. 1982b;79:6220–6224. doi: 10.1073/pnas.79.20.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook VY, Loh YP. Carboxypeptidase B-like converting enzyme activity in secretory granules of rat pituitary. Proc Natl Acad Sci U S A. 1984;81:2776–2780. doi: 10.1073/pnas.81.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka M, Suda M, Sakai Y, Izumi T, Watanabe T, Takeuchi T. Secretogranin III binds to cholesterol in the secretory granule membrane as an adapter for chromogranin A. J Biol Chem. 2004;279:3627–3634. doi: 10.1074/jbc.M310104200. [DOI] [PubMed] [Google Scholar]
- Hosaka M, Watanabe T, Sakai Y, Kato T, Takeuchi T. Interaction between secretogranin III and carboxypeptidase E facilitates prohormone sorting within secretory granules. J Cell Sci. 2005;118:4785–4795. doi: 10.1242/jcs.02608. [DOI] [PubMed] [Google Scholar]
- Hosaka M, Watanabe T, Sakai Y, Uchiyama Y, Takeuchi T. Identification of a chromogranin A domain that mediates binding to secretogranin III and targeting to secretory granules in pituitary cells and pancreatic beta-cells. Mol Biol Cell. 2002;13:3388–3399. doi: 10.1091/mbc.02-03-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura H. ACTH and related peptides: molecular biology, biochemistry and regulation of secretion. Clin Endocrinol Metab. 1985;14:845–866. doi: 10.1016/s0300-595x(85)80080-3. [DOI] [PubMed] [Google Scholar]
- Invitti C, Pecori Giraldi F, Dubini A, Piolini M, Cavagnini F. Effect of sandostatin on CRF-stimulated secretion of ACTH, beta-lipotropin and beta-endorphin. Horm Metab Res. 1991;23:233–235. doi: 10.1055/s-2007-1003660. [DOI] [PubMed] [Google Scholar]
- Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT, Hutton JC, O'Rahilly S. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16:303–306. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- Jackson RS, O'Rahilly S, Brain C, Nussey SS. Proopiomelanocortin products and human early-onset obesity. J Clin Endocrinol Metab. 1999;84:819–820. doi: 10.1210/jcem.84.2.5472-1. [DOI] [PubMed] [Google Scholar]
- Jain RK, Chang WT, Geetha C, Joyce PB, Gorr SU. In vitro aggregation of the regulated secretory protein chromogranin A. Biochem J. 2002;368:605–610. doi: 10.1042/BJ20021195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D, Miller MM, Seidah NG, Day R. Age-related alterations in the expression of prohormone convertase messenger ribonucleic acid (mRNA) levels in hypothalamic proopiomelanocortin mRNA neurons in the female C57BL/6J mouse. Endocrinology. 1995;136:2721–2729. doi: 10.1210/endo.136.6.7750497. [DOI] [PubMed] [Google Scholar]
- Kanmera T, Chaiken IM. Pituitary enzyme conversion of putative synthetic oxytocin precursor intermediates. J Biol Chem. 1985;260:10118–10124. [PubMed] [Google Scholar]
- Kelly RB. Pathways of protein secretion in eukaryotes. Science. 1985;230:25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Kemppainen RJ, Behrend EN. Acute inhibition of carboxypeptidase E expression in AtT-20 cells does not affect regulated secretion of ACTH. Regul Pept. 2010;165:174–179. doi: 10.1016/j.regpep.2010.07.162. [DOI] [PubMed] [Google Scholar]
- Kemppainen RJ, Zerbe CA, Sartin JL. Regulation and secretion of proopiomelanocortin peptides from isolated perifused dog pituitary pars intermedia cells. Endocrinology. 1989;124:2208–2217. doi: 10.1210/endo-124-5-2208. [DOI] [PubMed] [Google Scholar]
- Knepel W, Gerhards C. Stimulation by melittin of adrenocorticotropin and beta-endorphin release from rat adenohypophysis in vitro. Prostaglandins. 1987;33:479–490. doi: 10.1016/0090-6980(87)90027-x. [DOI] [PubMed] [Google Scholar]
- Kogel T, Gerdes HH. Maturation of secretory granules. Results Probl Cell Differ. 2010;50:1–20. doi: 10.1007/400_2009_31. [DOI] [PubMed] [Google Scholar]
- Kreis TE, Matteoni R, Hollinshead M, Tooze J. Secretory granules and endosomes show saltatory movement biased to the anterograde and retrograde directions, respectively, along microtubules in AtT20 cells. Eur J Cell Biol. 1989;49:128–139. [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- Kumar D, Mains RE, Eipper E. 60 YEARS OF POMC: From POMC and alphaMSH to PAM, molecular oxygen, copper and vitamin C. J Mol Endocrinol. 2015 doi: 10.1530/JME-15-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SN, Prodhomme E, Lindberg I. Prohormone convertase 1 (PC1) processing and sorting: effect of PC1 propeptide and proSAAS. J Endocrinol. 2004;182:353–364. doi: 10.1677/joe.0.1820353. [DOI] [PubMed] [Google Scholar]
- Lin WJ, Salton SR. The regulated secretory pathway and human disease: insights from gene variants and single nucleotide polymorphisms. Front Endocrinol (Lausanne) 2013;4:96. doi: 10.3389/fendo.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg I, van den Hurk WH, Bui C, Batie CJ. Enzymatic characterization of immunopurified prohormone convertase 2: potent inhibition by a 7B2 peptide fragment. Biochemistry. 1995;34:5486–5493. doi: 10.1021/bi00016a020. [DOI] [PubMed] [Google Scholar]
- Liotta AS, Loudes C, McKelvy JF, Krieger DT. Biosynthesis of precursor corticotropin/endorphin-, corticotropin-, alpha-melanotropin-, beta-lipotropin-, and beta-endorphin-like material by cultured neonatal rat hypothalamic neurons. Proc Natl Acad Sci U S A. 1980;77:1880–1884. doi: 10.1073/pnas.77.4.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YP. Immunological evidence for two common precursors to corticotropins, endorphins, and melanotropin in the neurointermediate lobe of the toad pituitary. Proc Natl Acad Sci U S A. 1979;76:796–800. doi: 10.1073/pnas.76.2.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YP, Maldonado A, Zhang C, Tam WH, Cawley N. Mechanism of sorting proopiomelanocortin and proenkephalin to the regulated secretory pathway of neuroendocrine cells. Ann N Y Acad Sci. 2002;971:416–425. doi: 10.1111/j.1749-6632.2002.tb04504.x. [DOI] [PubMed] [Google Scholar]
- Loh YP, Parish DC, Tuteja R. Purification and characterization of a paired basic residue-specific pro-opiomelanocortin converting enzyme from bovine pituitary intermediate lobe secretory vesicles. J Biol Chem. 1985;260:7194–7205. [PubMed] [Google Scholar]
- Loh YP, Snell CR, Cool DR. Receptor-mediated targeting of hormones to secretory granules: role of carboxypeptidase E. Trends Endocrinol Metab. 1997;8:130–137. doi: 10.1016/s1043-2760(97)00010-6. [DOI] [PubMed] [Google Scholar]
- Loh YP, Tam WW. Association of newly synthesized pro-opiomelanocortin with secretory granule membranes in pituitary pars intermedia cells. FEBS Lett. 1985;184:40–43. doi: 10.1016/0014-5793(85)80648-7. [DOI] [PubMed] [Google Scholar]
- Loh YP, Tam WW, Russell JT. Measurement of delta pH and membrane potential in secretory vesicles isolated from bovine pituitary intermediate lobe. J Biol Chem. 1984;259:8238–8245. [PubMed] [Google Scholar]
- Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron. 2005;45:245–255. doi: 10.1016/j.neuron.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Lou H, Park JJ, Cawley NX, Sarcon A, Sun L, Adams T, Loh YP. Carboxypeptidase E cytoplasmic tail mediates localization of synaptic vesicles to the pre-active zone in hypothalamic pre-synaptic terminals. J Neurochem. 2010;114:886–896. doi: 10.1111/j.1471-4159.2010.06820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H, Park JJ, Phillips A, Loh YP. gamma-Adducin promotes process outgrowth and secretory protein exit from the Golgi apparatus. J Mol Neurosci. 2013;49:1–10. doi: 10.1007/s12031-012-9827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry P. 60 YEARS OF POMC: Purification and biological characterisation of melanotrophins and corticotrophins. J Mol Endocrinol. 2015 doi: 10.1530/JME-15-0260. [DOI] [PubMed] [Google Scholar]
- Mains RE, Eipper BA. Biosynthesis of adrenocorticotropic hormone in mouse pituitary tumor cells. J Biol Chem. 1976;251:4115–4120. [PubMed] [Google Scholar]
- Mains RE, Eipper BA. Synthesis and secretion of corticotropins, melanotropins, and endorphins by rat intermediate pituitary cells. J Biol Chem. 1979;254:7885–7894. [PubMed] [Google Scholar]
- Mains RE, Eipper BA. Coordinate, equimolar secretion of smaller peptide products derived from pro-ACTH/endorphin by mouse pituitary tumor cells. J Cell Biol. 1981a;89:21–28. doi: 10.1083/jcb.89.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains RE, Eipper BA. Differences in the post-translational processing of beta-endorphin in rat anterior and intermediate pituitary. J Biol Chem. 1981b;256:5683–5688. [PubMed] [Google Scholar]
- Mains RE, Eipper BA, Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977;74:3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbikay M, Seidah NG, Chretien M. Neuroendocrine secretory protein 7B2: structure, expression and functions. Biochem J. 2001;357:329–342. doi: 10.1042/0264-6021:3570329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol AM. A study of intermediate lobe differentiation in the human pituitary gland. J Pathol. 1986;150:169–173. doi: 10.1002/path.1711500304. [DOI] [PubMed] [Google Scholar]
- Millington WR, O'Donohue TL, Chappell MC, Roberts JL, Mueller GP. Coordinate regulation of peptide acetyltransferase activity and proopiomelanocortin gene expression in the intermediate lobe of the rat pituitary. Endocrinology. 1986;118:2024–2033. doi: 10.1210/endo-118-5-2024. [DOI] [PubMed] [Google Scholar]
- Moore HH, Kelly RB. Re-routing of a secretory protein by fusion with human growth hormone sequences. Nature. 1986;321:443–446. doi: 10.1038/321443a0. [DOI] [PubMed] [Google Scholar]
- Moore HP, Gumbiner B, Kelly RB. Chloroquine diverts ACTH from a regulated to a constitutive secretory pathway in AtT-20 cells. Nature. 1983a;302:434–436. doi: 10.1038/302434a0. [DOI] [PubMed] [Google Scholar]
- Moore HP, Gumbiner B, Kelly RB. A subclass of proteins and sulfated macromolecules secreted by AtT-20 (mouse pituitary tumor) cells is sorted with adrenocorticotropin into dense secretory granules. J Cell Biol. 1983b;97:810–817. doi: 10.1083/jcb.97.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore HP, Kelly RB. Secretory protein targeting in a pituitary cell line: differential transport of foreign secretory proteins to distinct secretory pathways. J Cell Biol. 1985;101:1773–1781. doi: 10.1083/jcb.101.5.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore HP, Walker MD, Lee F, Kelly RB. Expressing a human proinsulin cDNA in a mouse ACTH-secreting cell. Intracellular storage, proteolytic processing, and secretion on stimulation. Cell. 1983c;35:531–538. doi: 10.1016/0092-8674(83)90187-3. [DOI] [PubMed] [Google Scholar]
- Muller L, Zhu X, Lindberg I. Mechanism of the facilitation of PC2 maturation by 7B2: involvement in ProPC2 transport and activation but not folding. J Cell Biol. 1997;139:625–638. doi: 10.1083/jcb.139.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979;278:423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Normant E, Loh YP. Depletion of carboxypeptidase E, a regulated secretory pathway sorting receptor, causes misrouting and constitutive secretion of proinsulin and proenkephalin, but not chromogranin A. Endocrinology. 1998;139:2137–2145. doi: 10.1210/endo.139.4.5951. [DOI] [PubMed] [Google Scholar]
- Paquet L, Zhou A, Chang EY, Mains RE. Peptide biosynthetic processing: distinguishing prohormone convertases PC1 and PC2. Mol Cell Endocrinol. 1996;120:161–168. doi: 10.1016/0303-7207(96)03834-8. [DOI] [PubMed] [Google Scholar]
- Park JJ, Cawley NX, Loh YP. Carboxypeptidase E cytoplasmic tail-driven vesicle transport is key for activity-dependent secretion of peptide hormones. Mol Endocrinol. 2008;22:989–1005. doi: 10.1210/me.2007-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper DJ, Bicknell AB. The stimulation of mitogenic signaling pathways by N-POMC peptides. Mol Cell Endocrinol. 2009;300:77–82. doi: 10.1016/j.mce.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia F, Cella SG, Radice L, Genazzani AR, Muller EE. gamma-Aminobutyric acid inhibits beta-endorphin secretion from the anterior pituitary but not the neurointermediate lobe in the rat. Endocrinology. 1986;118:360–366. doi: 10.1210/endo-118-1-360. [DOI] [PubMed] [Google Scholar]
- Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik JH, Loh YP, et al. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med. 2009;15:1195–1201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Devi LA, Mzhavia N, Munzer S, Seidah NG, Fricker LD. The C-terminal region of proSAAS is a potent inhibitor of prohormone convertase 1. J Biol Chem. 2000;275:23596–23601. doi: 10.1074/jbc.M001583200. [DOI] [PubMed] [Google Scholar]
- Rindler MJ. Carboxypeptidase E, a peripheral membrane protein implicated in the targeting of hormones to secretory granules, co-aggregates with granule content proteins at acidic pH. J Biol Chem. 1998;273:31180–31185. doi: 10.1074/jbc.273.47.31180. [DOI] [PubMed] [Google Scholar]
- Sabol SL. Storage and secretion of beta-endorphin and related peptides by mouse pituitary tumor cells: regulation by glucocorticoids. Arch Biochem Biophys. 1980;203:37–48. doi: 10.1016/0003-9861(80)90151-4. [DOI] [PubMed] [Google Scholar]
- Sapun-Malcolm D, Farah JM, Jr, Mueller GP. Evidence for serotonergic stimulation of pituitary beta-endorphin release: preferential release from the anterior lobe in vivo. Life Sci. 1983;33:95–102. doi: 10.1016/0024-3205(83)90716-6. [DOI] [PubMed] [Google Scholar]
- Scholey JM. Kinesin-2: a family of heterotrimeric and homodimeric motors with diverse intracellular transport functions. Annu Rev Cell Dev Biol. 2013;29:443–469. doi: 10.1146/annurev-cellbio-101512-122335. [DOI] [PubMed] [Google Scholar]
- Scott AP, Lowry PJ. Adrenocorticotrophic and melanocyte-stimulating peptides in the human pituitary. Biochem J. 1974;139:593–602. doi: 10.1042/bj1390593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger MA, Bennett HP. Structure and bioactivity of the amino-terminal fragment of pro-opiomelanocortin. J Steroid Biochem. 1986;25:703–710. doi: 10.1016/0022-4731(86)90298-0. [DOI] [PubMed] [Google Scholar]
- Seidah NG. The proprotein convertases, 20 years later. Methods Mol Biol. 2011;768:23–57. doi: 10.1007/978-1-61779-204-5_3. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Marcinkiewicz M, Benjannet S, Gaspar L, Beaubien G, Mattei MG, Lazure C, Mbikay M, Chretien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol. 1991;5:111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- Seksek O, Biwersi J, Verkman AS. Direct measurement of trans-Golgi pH in living cells and regulation by second messengers. J Biol Chem. 1995;270:4967–4970. doi: 10.1074/jbc.270.10.4967. [DOI] [PubMed] [Google Scholar]
- Shen FS, Aguilera G, Loh YP. Altered biosynthesis and secretion of pro-opiomelanocortin in the intermediate and anterior pituitary of carboxypeptidase E-deficient, Cpe(fat)/ Cpe(fat)mice. Neuropeptides. 1999;33:276–280. doi: 10.1054/npep.1999.0045. [DOI] [PubMed] [Google Scholar]
- Shen FS, Loh YP. Intracellular misrouting and abnormal secretion of adrenocorticotropin and growth hormone in cpefat mice associated with a carboxypeptidase E mutation. Proc Natl Acad Sci U S A. 1997;94:5314–5319. doi: 10.1073/pnas.94.10.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard KE. Cyclosporin A and FK506 are potent activators of proopiomelanocortin-derived peptide secretion without affecting corticotrope glucocorticoid receptor function. J Neuroendocrinol. 1995;7:833–840. doi: 10.1111/j.1365-2826.1995.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Masui H. Effects of various neuropeptides on the secretion of proopiomelanocortin-derived peptides by a cultured pituitary adenoma causing Nelson's syndrome. J Clin Endocrinol Metab. 1982;55:872–876. doi: 10.1210/jcem-55-5-872. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Naruse M, Yamauchi N, Masuda A, Imaki T, Naruse K, Demura H, Ling N, Inagami T, Shizume K. Rat atrial natriuretic factor suppresses proopiomelanocortin-derived peptides secretion from both anterior and intermediate lobe cells and growth hormone release from anterior lobe cells of rat pituitary in vitro. Biochem Biophys Res Commun. 1986;135:1035–1041. doi: 10.1016/0006-291x(86)91032-6. [DOI] [PubMed] [Google Scholar]
- Shin MS, Chang H, Namkoong C, Kang GM, Kim HK, Gil SY, Yu JH, Park KH, Kim MS. Hypothalamic and pituitary clusterin modulates neurohormonal responses to stress. Neuroendocrinology. 2013;98:233–241. doi: 10.1159/000355625. [DOI] [PubMed] [Google Scholar]
- Shiver T, Familari M, Aguilera G. Regulation of intermediate pituitary corticotropin-releasing hormone receptors by dopamine. Endocrinology. 1992;130:2299–2304. doi: 10.1210/endo.130.4.1347742. [DOI] [PubMed] [Google Scholar]
- Sly WS, Fischer HD. The phosphomannosyl recognition system for intracellular and intercellular transport of lysosomal enzymes. J Cell Biochem. 1982;18:67–85. doi: 10.1002/jcb.1982.240180107. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Bunch DO, Feng Y, Rodriguiz RM, Li M, Ravenell RL, Luo GX, Arimura A, Fricker LD, Eddy EM, et al. Deficits in reproduction and pro-gonadotropin-releasing hormone processing in male Cpefat mice. Endocrinology. 2004;145:2023–2034. doi: 10.1210/en.2003-1442. [DOI] [PubMed] [Google Scholar]
- Stevens A, White A. ACTH: cellular peptide hormone synthesis and secretory pathways. Results Probl Cell Differ. 2010;50:63–84. doi: 10.1007/400_2009_30. [DOI] [PubMed] [Google Scholar]
- Sun M, Watanabe T, Bochimoto H, Sakai Y, Torii S, Takeuchi T, Hosaka M. Multiple sorting systems for secretory granules ensure the regulated secretion of peptide hormones. Traffic. 2013;14:205–218. doi: 10.1111/tra.12029. [DOI] [PubMed] [Google Scholar]
- Surprenant A. Correlation between electrical activity and ACTH/beta-endorphin secretion in mouse pituitary tumor cells. J Cell Biol. 1982;95:559–566. doi: 10.1083/jcb.95.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tam WW, Andreasson KI, Loh YP. The amino-terminal sequence of pro-opiomelanocortin directs intracellular targeting to the regulated secretory pathway. Eur J Cell Biol. 1993;62:294–306. [PubMed] [Google Scholar]
- Thomas L, Leduc R, Thorne BA, Smeekens SP, Steiner DF, Thomas G. Kex2-like endoproteases PC2 and PC3 accurately cleave a model prohormone in mammalian cells: evidence for a common core of neuroendocrine processing enzymes. Proc Natl Acad Sci U S A. 1991;88:5297–5301. doi: 10.1073/pnas.88.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilders FJ, Post M, Jackson S, Lowry PJ, Smelik PG. beta-Adrenergic stimulation of the release of ACTH- and LPH-related peptides from the pars intermedia of the rat pituitary gland. Acta Endocrinol (Copenh) 1981;97:343–351. doi: 10.1530/acta.0.0970343. [DOI] [PubMed] [Google Scholar]
- Tomiko SA, Taraskevich PS, Douglas WW. GABA acts directly on cells of pituitary pars intermedia to alter hormone output. Nature. 1983;301:706–707. doi: 10.1038/301706a0. [DOI] [PubMed] [Google Scholar]
- Tooze J, Burke B. Accumulation of adrenocorticotropin secretory granules in the midbody of telophase AtT20 cells: evidence that secretory granules move anterogradely along microtubules. J Cell Biol. 1987;104:1047–1057. doi: 10.1083/jcb.104.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Hollinshead M, Frank R, Burke B. An antibody specific for an endoproteolytic cleavage site provides evidence that pro-opiomelanocortin is packaged into secretory granules in AtT20 cells before its cleavage. J Cell Biol. 1987a;105:155–162. doi: 10.1083/jcb.105.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Tooze SA. Clathrin-coated vesicular transport of secretory proteins during the formation of ACTH-containing secretory granules in AtT20 cells. J Cell Biol. 1986;103:839–850. doi: 10.1083/jcb.103.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Tooze SA, Fuller SD. Sorting of progeny coronavirus from condensed secretory proteins at the exit from the trans-Golgi network of AtT20 cells. J Cell Biol. 1987b;105:1215–1226. doi: 10.1083/jcb.105.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TN, Fryer JN, Lederis K, Vaudry H. CRF, urotensin I, and sauvagine stimulate the release of POMC-derived peptides from goldfish neurointermediate lobe cells. Gen Comp Endocrinol. 1990;78:351–360. doi: 10.1016/0016-6480(90)90025-h. [DOI] [PubMed] [Google Scholar]
- Tsukamoto N, Otsuka F, Miyoshi T, Yamanaka R, Inagaki K, Yamashita M, Otani H, Takeda M, Suzuki J, Ogura T, et al. Effects of bone morphogenetic protein (BMP) on adrenocorticotropin production by pituitary corticotrope cells: involvement of up-regulation of BMP receptor signaling by somatostatin analogs. Endocrinology. 2010;151:1129–1141. doi: 10.1210/en.2009-1102. [DOI] [PubMed] [Google Scholar]
- Tsukamoto N, Otsuka F, Ogura-Ochi K, Inagaki K, Nakamura E, Toma K, Terasaka T, Iwasaki Y, Makino H. Melatonin receptor activation suppresses adrenocorticotropin production via BMP-4 action by pituitary AtT20 cells. Mol Cell Endocrinol. 2013;375:1–9. doi: 10.1016/j.mce.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Varlamov O, Fricker LD, Furukawa H, Steiner DF, Langley SH, Leiter EH. Beta-cell lines derived from transgenic Cpe(fat)/Cpe(fat) mice are defective in carboxypeptidase E and proinsulin processing. Endocrinology. 1997;138:4883–4892. doi: 10.1210/endo.138.11.5506. [DOI] [PubMed] [Google Scholar]
- Wardlaw SL. Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur J Pharmacol. 2011;660:213–219. doi: 10.1016/j.ejphar.2010.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardman JH, Zhang X, Gagnon S, Castro LM, Zhu X, Steiner DF, Day R, Fricker LD. Analysis of peptides in prohormone convertase 1/3 null mouse brain using quantitative peptidomics. J Neurochem. 2010;114:215–225. doi: 10.1111/j.1471-4159.2010.06760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Zhang CF, Dhanvantari S, Lou H, Loh YP. Sorting of carboxypeptidase E to the regulated secretory pathway requires interaction of its transmembrane domain with lipid rafts. Biochem J. 2003;369:453–460. doi: 10.1042/BJ20020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CF, Snell CR, Loh YP. Identification of a novel prohormone sorting signal-binding site on carboxypeptidase E, a regulated secretory pathway-sorting receptor. Mol Endocrinol. 1999;13:527–536. doi: 10.1210/mend.13.4.0267. [DOI] [PubMed] [Google Scholar]
- Zhang X, Pan H, Peng B, Steiner DF, Pintar JE, Fricker LD. Neuropeptidomic analysis establishes a major role for prohormone convertase-2 in neuropeptide biosynthesis. J Neurochem. 2010;112:1168–1179. doi: 10.1111/j.1471-4159.2009.06530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Streck RD, Scott RE, Seidah NG, Pintar JE. The developmental expression in rat of proteases furin, PC1, PC2, and carboxypeptidase E: implications for early maturation of proteolytic processing capacity. J Neurosci. 1994;14:4656–4673. doi: 10.1523/JNEUROSCI.14-08-04656.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A, Bloomquist BT, Mains RE. The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing. J Biol Chem. 1993;268:1763–1769. [PubMed] [Google Scholar]
- Zhou Y, Lindberg I. Purification and characterization of the prohormone convertase PC1(PC3) J Biol Chem. 1993;268:5615–5623. [PubMed] [Google Scholar]
- Zhu X, Rouille Y, Lamango NS, Steiner DF, Lindberg I. Internal cleavage of the inhibitory 7B2 carboxyl-terminal peptide by PC2: a potential mechanism for its inactivation. Proc Natl Acad Sci U S A. 1996;93:4919–4924. doi: 10.1073/pnas.93.10.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhou A, Dey A, Norrbom C, Carroll R, Zhang C, Laurent V, Lindberg I, Ugleholdt R, Holst JJ, et al. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci U S A. 2002;99:10293–10298. doi: 10.1073/pnas.162352599. [DOI] [PMC free article] [PubMed] [Google Scholar]