Abstract
A critical role for peptide C-terminal amidation was apparent when the first bioactive peptides were identified. The conversion of POMC into ACTH and then into αMSH, an amidated peptide, provided a model system for identifying the amidating enzyme. Peptidylglycine α-amidating monooxygenase (PAM), the only enzyme that catalyzes this modification, is essential; mice lacking PAM survive only until mid-gestation. Purification and cloning led to the discovery that the amidation of peptidylglycine substrates proceeds in two steps: peptidylglycine α-hydroxylating monooxygenase (PHM) catalyzes the copper and ascorbate-dependent α-hydroxylation of the peptidylglycine substrate; peptidyl-α-hydroxyglycine α-amidating lyase (PAL) cleaves the N-C bond, producing amidated product plus glyoxylate. Both enzymes are contained in the luminal domain of PAM, a type 1 integral membrane protein. The structures of both catalytic cores have been determined, revealing how they interact with metals, molecular oxygen and substrate to catalyze both reactions. Although not essential for activity, the intrinsically disordered cytosolic domain is essential for PAM trafficking. A phylogenetic survey led to identification of bifunctional membrane PAM in Chlamydomonas, a unicellular eukaryote. Accumulating evidence points to a role for PAM in copper homeostasis and in retrograde signaling from the lumen of the secretory pathway to the nucleus. The discovery of PAM in cilia, cellular antennae that sense and respond to environmental stimuli, suggests that much remains to be learned about this ancient protein.
Keywords: amidation, peptides, cilia, copper, monooxygenase, sensory, signaling, obesity, energy homeostasis
POMC and PAM: Where it all began
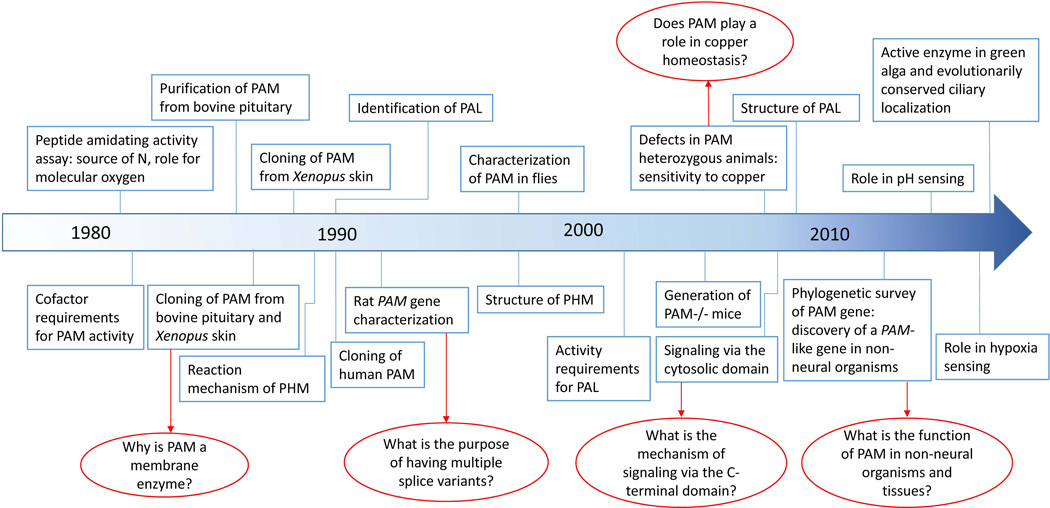
Over the last three decades, we have amassed a great deal of information on the function, trafficking and biochemical properties of the only known peptide amidating enzyme, peptidylglycine α-amidating monooxygenase (PAM). Until its discovery in 1982, even the existence of such an enzyme was questioned (Fig.1). Based on the first biologically active peptides identified (vasopressin, oxytocin, α-MSH), it was clear that a C-terminal amide group was essential, but there was no reason to suspect that a mechanism other than transamination (such as in glutamine synthesis) might be in place. The discovery of glycine-extended precursors for amidated peptides such as α-MSH, adipokinetic hormone and melittin raised the possibility that an enzyme recognizing the terminal glycine was involved in generating the mature amidated peptide (Harris and Lerner 1957; Stone, et al. 1976; Suchanek and Kreil 1977).
Figure 1. Timeline highlighting key developments leading from POMC processing studies to PAM.
The landmark study of Bradbury et al. (1982) (Bradbury et al. 1982), provided a means of assaying peptide amidating activity in tissue lysates. Purification, cloning and structural/mechanistic studies focused on PAM and then expanded to include cell biological studies on secretory granule biogenesis, retrograde signaling from the granule lumen to the nucleus and the delivery of essential cofactors (ascorbate and copper) to the secretory pathway. Key unanswered questions are marked by red arrows.
Using a synthetic radiolabeled peptidylglycine substrate (based on the last three amino acid residues of the α-MSH precursor), Bradbury et al. demonstrated the presence of an activity catalyzing the amidation reaction in secretory granules of bovine pituitaries (Bradbury, et al. 1982). In this landmark study, the amide group nitrogen was shown to be derived from the glycine residue, ruling out the possibility of a transaminase reaction; the formation of glyoxylate during the reaction pointed to a hydroxylation step in the reaction mechanism (Bradbury et al. 1982).
Around this time, our laboratory was focused on understanding the tissue-specific differential processing of POMC. The proteases that produced the longer peptides (such as pro-γ-MSH, ACTH, JP and βLPH in corticotropes) or the shorter peptides (such as αMSH, γMSH and β-endorphin in melanotropes and hypothalamic POMC neurons) were of special interest. Establishing primary rat intermediate pituitary cultures seemed like a convenient way to characterize the production of αMSH from what was then known as pro-ACTH. In order to study secretion, it was important to culture cells in serum-free medium. However, it soon became obvious that serum contained a factor that was essential for the conversion of αMSH-Gly into amidated αMSH. Antibody specific for amidated αMSH was key in realizing that otherwise healthy pituitary cells maintained in serum-free medium performed all of the processing steps required for generating active αMSH including proteolytic cleavage and acetylation, except for amidation (Eipper, et al. 1983a; Glembotski, et al. 1983). In order to identify the serum factor(s) required for amidation, we turned to the newly developed enzyme assay to take a closer look at the amidating enzyme.
As a first step, secretory granules were purified from rat and bovine anterior, intermediate and neural pituitary; β-endorphin immunoreactivity served as a convenient marker for granule fractions (Eipper, et al. 1983b). Using the assay developed by Bradbury et al. in 1982, amidation activity could be detected in the secretory granules from all three regions. A simple experiment - determining the pH optimum - proved to be key in determining the co-factor requirements of the enzyme. Activity was greatly reduced in citrate and phosphate buffers, which can chelate metals, compared to sulfonic acid buffers. Subsequent experiments confirmed an essential role for copper (Eipper et al. 1983b). Similar to other copper-dependent enzymes involved in redox reactions requiring molecular oxygen, the amidation reaction was dependent on oxygen availability. The remarkable similarity of the amidation reaction to that of dopamine β-monooxygenase (DBM), which converts dopamine to norepinephrine in a copper, molecular oxygen and ascorbate (vitamin C) dependent manner, suggested that ascorbate might provide the reducing equivalents essential for peptide amidation. As predicted, ascorbate was a potent stimulator of peptide amidation catalyzed by peptidylglycine-α-amidating monooxygenase (PAM) (Eipper et al. 1983b). Knowing the cofactors required for its catalytic activity, we were not surprised that simply adding ascorbate to our serum-free medium allowed melanotropes to produce amidated αMSH. Peptidylglycine α-amidating monooxygenase (PAM; EC 1.14.17.3) and dopamine β-monooxygenase (DBM; EC 1.14.17.1) were thought to form a family of related copper-dependent monooxygenases; this meant that the detailed structural and mechanistic studies carried out on DBM (Stewart and Klinman 1988) could guide studies of PAM.
WHAT HAVE WE LEARNED ABOUT PAM SINCE ITS IDENTIFICATION?
Structure of the PAM gene
The flurry of activity that ensued centered on cloning the gene encoding PAM (Fig.1). The protein was purified from bovine pituitaries and used to generate antibodies (Eipper, et al. 1987; Murthy, et al. 1986). Having antibodies allowed identification of a cDNA encoding PAM using a phage expression library generated from bovine intermediate pituitary RNA (Eipper et al. 1987). At the same time, a cDNA encoding PAM was cloned from Xenopus laevis skin (Mizuno, et al. 1987; Ohsuye, et al. 1988). Analysis of the protein encoded by the PAM cDNA delivered a few surprises. As expected, a cleaved signal peptide was found, allowing entry of PAM into the secretory pathway lumen. The cDNA encoded a protein more than twice the size expected. Although the enzyme purified from pituitary was soluble, the cDNA encoded what was predicted to be a type 1 integral membrane protein – its single membrane spanning domain was followed by a short stretch of hydrophilic residues predicted to reside in the cytoplasm. Several pairs of basic amino acids - recognition sites for prohormone convertase-like endoproteases - were also present in the intraluminal part of the PAM protein. Several questions arose: Why would an enzyme catalyzing amidation of bioactive peptides include a transmembrane domain? Why did it include endoproteolytic cleavage sites and how did they affect its processing and activity? Efforts spanning over two decades have unraveled the answers to some of these puzzling questions.
A PAL for PHM
It was soon discovered that the PAM cDNA encoded two enzymatic domains, both of which were necessary to yield an amidated peptide (Fig.2). Formation of an α-hydroxyglycine intermediate by the stereo-specific hydroxylation of the glycine-extended peptide precursor was proposed as the first step in the reaction mediated by PAM (Young and Tamburini 1989). Although the second step of this reaction, cleavage of the N-C bond to yield amidated product is spontaneous in alkaline pH, it is impeded in the acidic environment of secretory granules. The stability of synthetic peptides terminating with a COOH-terminal α-hydroxyglyine residue was shown to decline at pH values above 6, with half-lives of 8 h at pH7.4 (Bundgaard and Kahns 1991). An enzyme catalyzing N-C bond cleavage was identified in bovine neurointermediate pituitaries; it was found that the bovine PAM precursor also contained this enzymatic activity. Thus, the PAM gene encodes two enzymatic domains that function sequentially to generate amidated peptides: peptidyglycine α-hydroxylating monooxygenase (PHM; EC 1.14.17.3) and peptidyl-α-hydroxyglycine α-amidating lyase (PAL; 4.3.2.5) (Katopodis, et al. 1990; Perkins, et al. 1990). Studies with purified PAL protein revealed its pH optimum to be in the acidic range and its dependence on zinc (Eipper, et al. 1991).
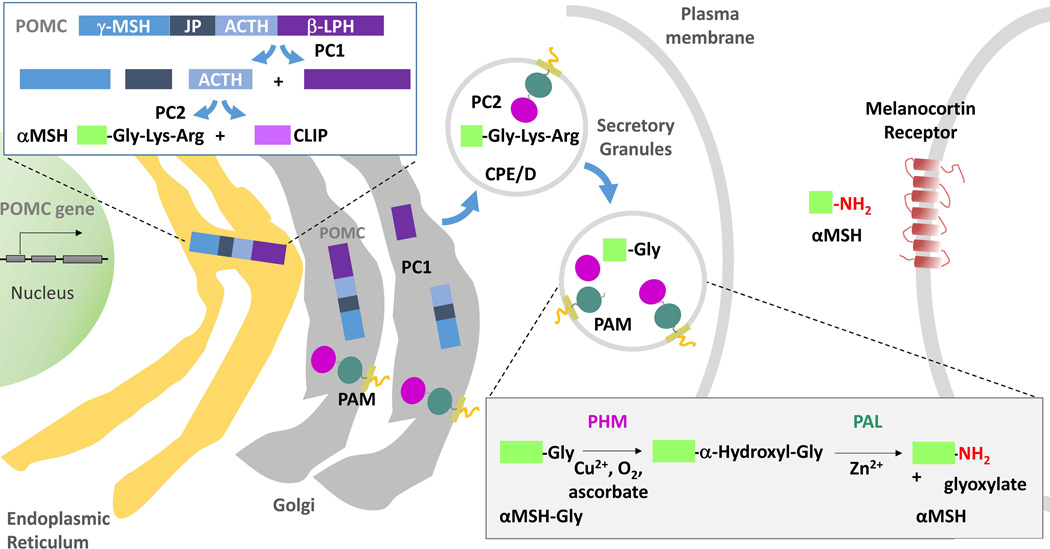
Figure 2. POMC processing: amidation of αMSH.
Following the co-translational removal of its N-terminal signal sequence, POMC moves through the Golgi complex. As luminal pH begins to fall and prohormone convertase 1 (PC1) is activated, the first POMC cleavage produces ACTH biosynthetic intermediate and β–Lipotropin. Subsequent cleavages (upper left box), which occur largely in maturing secretory granules, separate Joining Peptide (JP) from ACTH; the C-terminus of JP can be amidated. Melanotropes, which express both PC1 and PC2, cleave ACTH(1–39) to produce an N-terminal fragment (precursor to αMSH) and CLIP (corticotropin-like intermediate lobe peptide). The production of αMSH requires a carboxypeptidase, PAM and an N-acetyltransferase (not shown). The sequential actions of PHM and PAL on αMSH-Gly are illustrated (lower right box).
Apart from endoproteolytic processing, functionally different forms of PAM can also be generated by alternative splicing. The longest isoform (PAM-1) (Fig.3A) contains the two enzymatic domains, a transmembrane domain, a cytosolic domain and an endoprotease-sensitive linker region between PHM and PAL. This endoprotease-sensitive region is not included in the PAM-2 isoform, and PHM and PAL are rarely separated by cleavage. A third major isoform (PAM-3) lacks both the endoproteolytic cleavage site and the transmembrane domain, allowing soluble, bifunctional PAM to be secreted. PAM expression is not limited to neuroendocrine tissues; PAM is expressed at widely varying levels in almost all mammalian cell types, with significant expression in airway epithelium, ependymal cells in the brain, endothelial cells and adult atrium as well as brain and pituitary (Eipper, et al. 1988; Oldham, et al. 1992; Schafer, et al. 1992). PAM expression is developmentally regulated; isoform-specific regulation is especially apparent in heart and neural tissues, suggesting that different isoforms have distinct functions (Braas, et al. 1989; Eipper, et al. 1992; Stoffers, et al. 1989; Stoffers, et al. 1991). Both in neurons and endocrine cells, soluble PHM and PAL are secreted along with neuropeptides and peptide hormones upon stimulation; PHM and PAL can be detected in serum and cerebrospinal fluid (Mains, et al. 1985; Wand, et al. 1985).
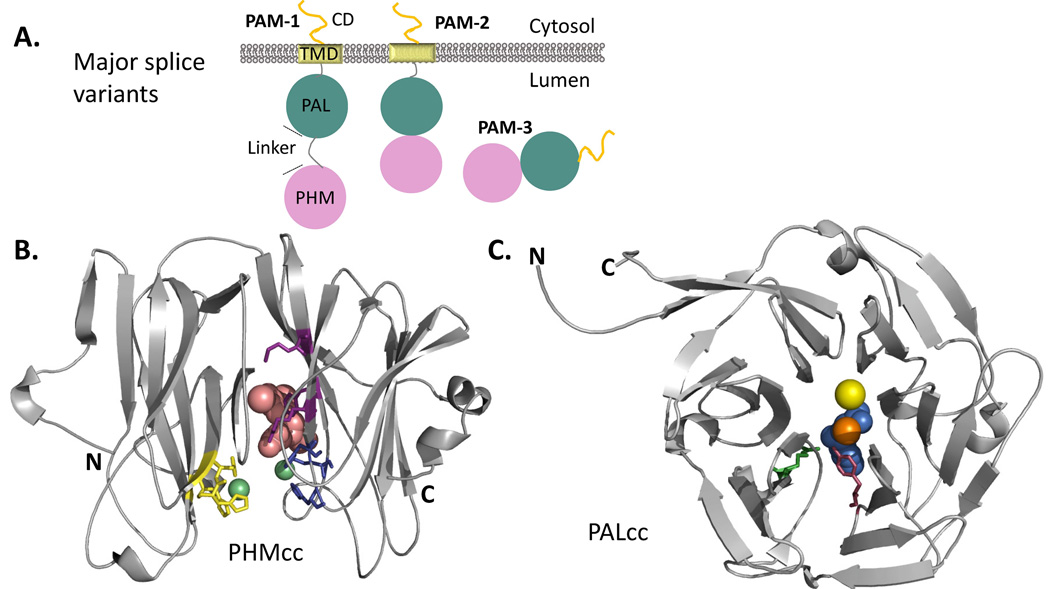
Figure 3. Major PAM Splice Variants and PHM and PAL Catalytic Core Structures.
(A) The major splice variants (isoforms) of PAM are shown. While PAM-1 and PAM-2 are type 1 integral membrane proteins, PAM-3 is a soluble, secreted protein. (B) Crystal structure of rat PHM (PDB identifier: 1OPM) in the oxidized state bound to a substrate, N-acetyl-3,5-diiodotyrosylglycine (shown in pink), rendered here using PyMOL; bound copper, green spheres. The copper binding site in the N-terminal domain of PHM (CuH, in yellow) is separated from the copper binding site in the C-terminal domain (CuM, in blue) by an 11Å solvent filled cleft; the peptidylglycine substrate and molecular oxygen bind near CuM. Other essential catalytic residues involved in substrate binding (R240, Y318, M320) are shown in purple. After considering many mechanisms, quantum mechanical tunneling is thought to facilitate electron transfer from the CuH site, through solvent, to the CuM site (Francisco et al. 2004; Klinman 2006; McIntyre et al. 2010). (B). The structure of PAL (PDB identifier: 3FW0) crystallized in the presence of mercury ion (orange) instead of zinc to capture binding of a non-peptide substrate (alpha-hydroxyhippuric acid, in blue) is shown. The six-bladed β-propeller structure of PAL positions Zn near a key Tyr residue (shown in purple) and a key Arg residue (shown in green). The structurally important calcium ion is depicted as a yellow sphere (Chufan et al. 2009).
Cellular requirements for PAM activity
PAM activity requires the delivery of copper and ascorbate to the lumen of the secretory pathway. Cells devote considerable energy to compartmentalizing and tightly regulating the flow of copper, a transition metal that can participate in free radical formation. Copper transporters deliver copper to intracellular copper chaperones such as COX17 and Atox1, which deliver copper to specific acceptor proteins. For the secretory pathway, two P-type ATPases, ATP7A and ATP7B, receive copper from Atox1 and transport it into the lumen (Prohaska 2008). No copper chaperones have been identified in the secretory pathway lumen and it has been suggested that PHM is metallated directly by the P-type ATPases (El Meskini, et al. 2001; Otoikhian, et al. 2012). While most organisms, including rodents, synthesize ascorbate from glucose, humans lack this ability and must acquire it from their diet. Two Na+-dependent ascorbate transporters (SVCT1 and SVCT2) expressed in intestinal epithelial cells and neuroendocrine cells, respectively, import ascorbate into cells (Burzle and Hediger 2012); neuroendocrine cells lack gulonolactone oxidase and must use SVCT2 to obtain ascorbate from the circulation. It is not known how ascorbate enters the secretory pathway lumen, but it is maintained in the reduced state by the action of cytochrome b561, which shuttles electrons across the secretory pathway membrane (Asada, et al. 2005; Iliadi, et al. 2008).
Another important factor necessary for amidation is the low pH of the secretory pathway lumen. Both PHM and PAL exhibit optimal activity under acidic conditions. Intraluminal pH is maintained by the proton pumping activity of the multi-subunit vacuolar or V-ATPase. In addition to its role in acidification, the V-ATPase plays a role in secretory granule biogenesis through its role in sorting of vesicular cargo (Sobota, et al. 2009). This proton pump also relays information about amino acid levels in the interior of lysosomes to mTOR (mammalian Target of Rapamycin), a cytosolic Ser/Thr kinase that serves as a master energy regulator (Jewell, et al. 2013). It is intriguing to consider a role for PAM in signaling nutritional status information from distal parts of the secretory pathway (glycine levels, for example) to mTOR through the V-ATPase.
Crystal structures and insights into mechanism of peptide amidation
As mentioned previously, the cofactor requirements for the PHM domain of PAM are strikingly similar to those of dopamine β-monooxygenase (DβM). The similarity extends to their amino acid sequences, with about 30% identity in the catalytic cores of the two molecules (Prigge, et al. 1997). Another member of this small family of copper-dependent monooxygenases is the endoplasmic reticulum localized Monooxygenase X (MOX), predicted to hydroxylate an unidentified hydrophobic substrate (Xin, et al. 2004). Of these three monooxygenases, PHM is the only one with a solved crystal structure (Fig.3B and C), providing key insights into reaction mechanism (Prigge, et al. 2004; Prigge et al. 1997; Prigge, et al. 1999). Only small structural differences were observed when the protein was crystallized in the reduced, oxidized or substrate bound form. The catalytic core of PHM has two domains, each composed primarily of eight antiparallel β-strands (Fig.3B). Resembling a jelly roll motif, each domain has a hydrophobic interior held together by disulfide linkages; the two domains are linked by a single polypeptide chain. Each domain has one copper binding site: the three His residues of the CuH site are in the N-terminal domain; the two His and one Met of the CuM site are in the C-terminal domain. An 11 Å solvent exposed hydrophilic cleft separates CuH from CuM; in two single electron steps, both sites are reduced. Molecular oxygen binds to the CuM site, with the peptidylglycine substrate bound close by; glycine and D-alanine are the only amino acids that can be accommodated and there is no indication that the distance between CuH and CuM varies during the reaction. The active site of PHM can accommodate large substrates (e.g. ubiquitin and selected immunoglobulin heavy chains) as well as fatty acyl glycines and other non-peptide substrates (Chew, et al. 2005; Skulj, et al. 2014; Wilcox, et al. 1999).
The crystal structure of the catalytic core of PAL was solved about ten years later (Fig.3C): it is a six-bladed β-propeller, with long loops extending from the propeller surface (Chufan, et al. 2009). The central cavity houses a calcium ion required for structural integrity and a zinc ion required for catalytic activity. The peptidyl-α-hydroxyglycine substrate binds close to the zinc, which is coordinated by three His residues. Well conserved tyrosine (Tyr654) and arginine (Arg706) residues play a critical role in catalysis (Chufan et al. 2009; De, et al. 2006). As in the catalytic core of PHM, disulfide linkages play an essential role in the structural integrity of PAL. The N- and C-termini of PAL are positioned close to each other and its C-terminus is tethered to the membrane; the unique geometry of the β-propeller structure positions PHM close to the membrane to receive copper and ascorbate from transmembrane P-type ATPases and cytochrome b561.
With the ability to produce active PHMcc and site directed mutants in mammalian cells, the unique properties of the two essential copper binding sites and the effects of pH on PHM were determined (Evans, et al. 2006; Jaron, et al. 2002; Kline, et al. 2013; Siebert, et al. 2005). The detailed mechanistic studies carried out previously on purified dopamine β-monooxygenase guided similar studies on PHMcc (Stewart and Klinman 1988). The initial focus was on the 11 Å solvent filled gap separating the two essential copper binding sites. CuH, which has the properties expected of an electron transfer site, and CuM, which binds O2 and is adjacent to the peptidylglycine substrate, are both essential. Current evidence indicates that the two copper domains are directly coupled through a solvent bridge which facilitates redox and catalysis, a remarkable level of precision for a solvent accessible active site (Francisco et al., 2002; Bauman et al., 2006; Jaron et al., 2002). The effects of temperature on intrinsic isotope effects and modeling studies have led to the consensus that PHM-dependent C(α)-H bond activation is dominated by quantum mechanical tunneling and tightly coupled with oxygen activation (Francisco, et al. 2002; Francisco, et al. 2004; Klinman 2006; McIntyre, et al. 2010). Efforts to develop inhibitors of PHM and PAL continue (Langella, et al. 2010; Merkler, et al. 2008). Disulfiram, a copper chelator reduces levels of amidated peptides in rat pituitary and cerebral cortex, presumably by inhibiting PHM activity (Mueller, et al. 1993). A mechanism based suicide inhibitor, 4-phenyl-3-butenoic acid has been shown to inhibit PHM activity in vivo (Mueller, et al. 1999). Other potent inhibitors include hippurate analogs and N-substituted homocysteine analogs (Erion, et al. 1994; Merkler et al. 2008).
MORE THAN JUST AN ENZYME - A MULTI-TASKING PROTEIN
Evolutionary distribution of PAM
Amidated peptides are widespread, with roles in organisms with simple nervous systems, such as Hydra and Aplysia (Fujisawa, et al. 1999; Grunder and Assmann 2015). Furthermore, PAM has been characterized in sea anemone, fly and flatworm (Han, et al. 2004; Kolhekar, et al. 1997; Mair, et al. 2004; Williamson, et al. 2000). Although it was assumed that PAM co-evolved with the nervous system, recent studies suggest otherwise. A phylogenetic study identified PAM-like sequences in non-neural organisms such as Amphimedon queenslandica, a sponge, and Trichoplax adhaerens, a placozoan. Neuropeptide like sequences have been found in the Trichoplax genome and immunoreactivity to amidated peptides has been demonstrated in cells of Trichoplax (Jekely 2013; Smith, et al. 2014). Perhaps more surprising was finding PAM-like sequences in several green algal genomes; the presence of PAM in Chlamydomonas reinhardtii, Volvox carteri and Ostreococcus tauri raised the possibility that PAM evolved before the divergence of plants and animals (Attenborough, et al. 2012). Active PAM enzyme has been demonstrated in the unicellular green alga, Chlamydomonas reinhardtii raising the question of ancestral function (Kumar, et al. 2015). Although amidated peptides have not been identified in green algae, the evolutionary conservation of components essential for PAM activity (copper homeostatic machinery, ascorbate synthesis, the V-ATPase) in eukaryotes certainly supports the possibility of amidation reactions occurring in these non-neural organisms. It is also possible that PAM amidates non-peptide substrates, or has a completely novel role distinct from those discovered in mammals.
The cytosolic domain of PAM
Although a cytosolic domain is not essential for PHM or PAL activity, the PAM gene in Chlamydomonas reinhardtii encodes a protein of identical topology (Kumar et al. 2015). Unlike soluble granule content proteins, granule membrane proteins are not secreted; after exocytosis and insertion into the plasma membrane, membrane PAM can be retrieved, traveling through the endocytic pathway in a regulated manner. In Drosophila, gene duplication is thought to have produced separate genes encoding soluble PHM, soluble PAL and membrane PAL.
Unlike the catalytic domains of PAM, its cytosolic domain is protease sensitive; the specific activity of PHM is increased following removal of the cytosolic domain. The cytosolic domain of PAM plays an essential role in its trafficking in mammalian cells; although soluble PHM and soluble PAL accumulate in secretory granules, PAM lacking its cytosolic domain accumulates on the plasma membrane (Milgram, et al. 1993; Tausk, et al. 1992). Furthermore, an epitope-tagged protein consisting of the transmembrane and cytosolic domains of PAM localizes to the trans Golgi network and secretory granules, indicating that it contains key trafficking information (El Meskini et al. 2001).
The cytosolic domain (86 residues in mammalian PAM) is unstructured and contains no characteristic motifs. It is sensitive to proteolytic degradation, a feature characteristic of intrinsically disordered proteins involved in signaling, where the signal must be turned off just as rapidly as it is turned on (Rajagopal, et al. 2009). Unstructured domains are often hubs of protein/protein interactions and multiple PAM cytosolic domain interactors have been identified. Kalirin and Trio, members of the Rho GDP/GTP exchange factor (Rho-GEF) family, play roles in cytoskeletal control and the maintenance of synapses. Uhmk1, a Ser/Thr kinase, phosphorylates the C-terminus of PAM and Rassf9, a member of the Ras-association domain family, plays a role in epidermal homeostasis (Alam, et al. 1996; Lee, et al. 2011). More recently, PAM has been shown to interact with the Adaptor Protein-1 (AP-1) Complex (Bonnemaison, et al. 2015). The AP-1 complex belongs to a family of endosomal and secretory granule coat proteins that link cargo proteins to clathrin; both PAM and Atp7a, the P-type ATPase that transports copper into the TGN, interact with AP-1. Diminished AP-1 function affects PAM and Atp7a trafficking through the endocytic pathway. Antibodies to amidated (18 kDa fragment) and non-amidated POMC products allowed development of an assay to measure PAM activity in cells following manipulation of copper levels; cells with reduced AP-1 levels were more sensitive to copper restriction than control cells (Bonnemaison, et al. 2014; Bonnemaison et al. 2015).
Mass spectrometry and two dimensional gel electrophoresis revealed multiple phosphorylation sites (Ser and Thr) in the cytosolic domain of PAM (Rajagopal et al. 2009). Phosphomimetic mutants revealed a role for phosphorylation in PAM trafficking through various biosynthetic and endocytic compartments. Development of an antibody specific to the C-terminal domain of PAM allowed identification of the various proteolytic fragments generated. Prohormone convertase-mediated cleavage of PAM in the secretory granules generates a slightly larger transmembrane/cytosolic domain fragment than α-secretase-mediated cleavage of PAM. Both transmembrane/cytosolic domain fragments can be cleaved by γ-secretase; this intramembrane cleavage produces a short-lived, soluble 16 kDa fragment (sf-CD, soluble fragment of PAM cytosolic domain) that accumulates in the nucleus (Rajagopal et al. 2009; Rajagopal, et al. 2010). Upregulation of PAM-1 levels in a corticotrope cell line caused alterations in cell morphology, a reduction in secretagogue-responsiveness and altered expression of a subset of genes, including copper chaperones, aquaporin 1 and Slpi, a protease inhibitor (Francone, et al. 2010). In addition to its enzymatic role, these studies indicate that PAM relays signals from the lumen of the secretory/endocytic pathway to the nucleus, in a manner similar to other type I integral membrane proteins in the endoplasmic reticulum such as SREBP and ATF6 (Rajagopal, et al. 2012).
Lessons learned from knockout and heterozygous mice
Mice lacking both copies of Pam show no detectable peptide amidation activity and do not survive past mid-gestation, dying at e14.5 to e15.5 (Czyzyk, et al. 2005). The Pam null embryos display severe edema, cardiac malformations and poor vasculature compared to wild type littermates. The heterozygous animals on the other hand, have half the wild type level of PAM activity and are viable. The heterozygous animals showed only minor changes in the levels of the amidated peptides tested, suggesting that the reduced levels of PAM in PAM+/− mice were sufficient to catalyze the amidation reaction (Czyzyk et al. 2005).
As they age, PAM+/− mice display increased adiposity compared to age matched wild type littermates. Glucose metabolism, which is regulated in part by amidated peptides, is impaired in older PAM heterozygous mice compared to wild type controls (Czyzyk et al. 2005). The PAM+/− animals demonstrate other behavioral deficits: they are unable to regulate body temperature, show increased anxiety-like behavior and are more susceptible to seizures compared to wild type mice. Moreover, wild type mice maintained on a copper restricted diet mimic many of the behavioral deficits observed in PAM+/− mice, and supplemental dietary copper reverses some of the behavioral defects observed in PAM+/− animals (Bousquet-Moore, et al. 2009; Bousquet-Moore, et al. 2010). These data suggest that altered copper homeostasis contributes to the behavioral defects observed in PAM heterozygous mice. A role for PAM in copper homeostasis had not previously been suspected.
Sensory roles of PAM
Comparative genomic analyses suggest that cuproproteins evolved following oxygenation of the Earth; thus PAM belongs to a small set of cuproenzymes with primitive roles predating multicellularity. As one of the few proteins dependent on a steady supply of copper and molecular oxygen for activity, PAM is well positioned to function as a sensor for these critical factors. As mentioned above, studies with mice lacking one copy of Pam and signaling mediated by PAM sf-CD suggest an involvement in copper sensing. Additional support for this hypothesis comes from studies using corticotrope cell lines. Depleting or overloading these cells with copper alters the trafficking of PAM through the secretory and endocytic pathways, but does not lead to its degradation, as in the case of other cuproproteins (De, et al. 2007).
The absolute dependence of the amidation reaction on molecular oxygen has been known for a long time, but whether physiologically relevant changes in oxygen levels affected PHM activity was not known. A recent study shows that the ability of PAM to produce amidated products is as sensitive to changes in oxygen level as the prolyl hydroxylases that control the stability of hypoxia inducible factor (HIF), a key oxygen signaling protein. The speed and sensitivity with which PAM activity responds to hypoxia suggest a novel, paracrine signaling mechanism that could operate during oxygen homeostasis in vivo (Simpson, et al. 2015).
The pH gradient in the lumen of the secretory pathway plays an essential role in the biosynthesis and processing of proteins. Enzymes like PAM, which operate late in the secretory pathway, display acidic pH optima, and the pH gradient in the luminal compartment often dictates which biosynthetic enzymes are active and which processing steps can occur. The protease-sensitive linker region that connects the catalytic core of PHM to the catalytic core of PAL contains a cluster of histidine residues (His-Gly-His-His) that confer pH sensitivity (Vishwanatha, et al. 2014). PAM lacking these histidine residues (Ala-Gly-Ala-Ala) is handled differently in corticotrope cell lines; instead of being recycled, endocytosed PAM lacking this His-cluster is rapidly degraded. The γ-secretase-mediated production of PAM sf-CD is largely eliminated and the morphological changes associated with PAM expression no longer occur.
WHAT THE FUTURE HOLDS FOR PAM BIOLOGY
The involvement of PAM in sensing environmental signals and generating factors involved in transcriptional regulation led us to expand our studies beyond the role of PAM as an amidating enzyme. Thus, there has been a paradigm shift in our understanding of this multi-tasking protein. We see understanding the role of PAM in non-neural tissues and organisms as key to uncovering all of the functions of this ancient, evolutionarily conserved protein. For instance, it will be interesting to determine the significance of abundant levels of PAM in the atrium, where it comprises about 1% of the total proteome (O'Donnell, et al. 2003).
Mutations in the luminal copper transporting P-type ATPase, ATP7A, cause Menkes disease, a copper metabolism disorder characterized by neurodegeneration, mental retardation, connective tissue abnormalities and early childhood lethality. Several cuproenzymes including PAM are affected by the loss of functional ATP7A. Using the mottled-brindled mouse, an animal model for Menkes disease, normal levels of PAM expression, but reduced levels of amidated peptides such as joining peptide, α-MSH and cholecystokinin were shown, suggesting that alterations in PAM activity might contribute to some of the phenotypes observed in these patients (Steveson, et al. 2003). Altered PAM levels/activity have also been reported in the cerebrospinal fluid of multiple sclerosis and post-polio syndrome patients (Gonzalez, et al. 2009; Tsukamoto, et al. 1995). The ability to assay PAM activity in serum makes it an attractive biomarker candidate (Gaier, et al. 2014).
During its endocytic trafficking, PAM enters the intraluminal vesicles that characterize multivesicular bodies (Fig.4); the fusion of multivesicular bodies with the plasma membrane results in release of these intraluminal vesicles, which are then known as exosomes (Rajagopal et al. 2010). Since they can be isolated from a variety of sources such as saliva, serum and urine, exosomes are being explored as a diagnostic tool (Simpson, et al. 2009). PAM was identified in exosomes derived from human saliva and prostate cancer cells (Gonzalez-Begne, et al. 2009; Minciacchi, et al. 2015). It will be interesting to determine if PAM-containing exosome release is differentially regulated, especially during genetic or metabolic alterations.
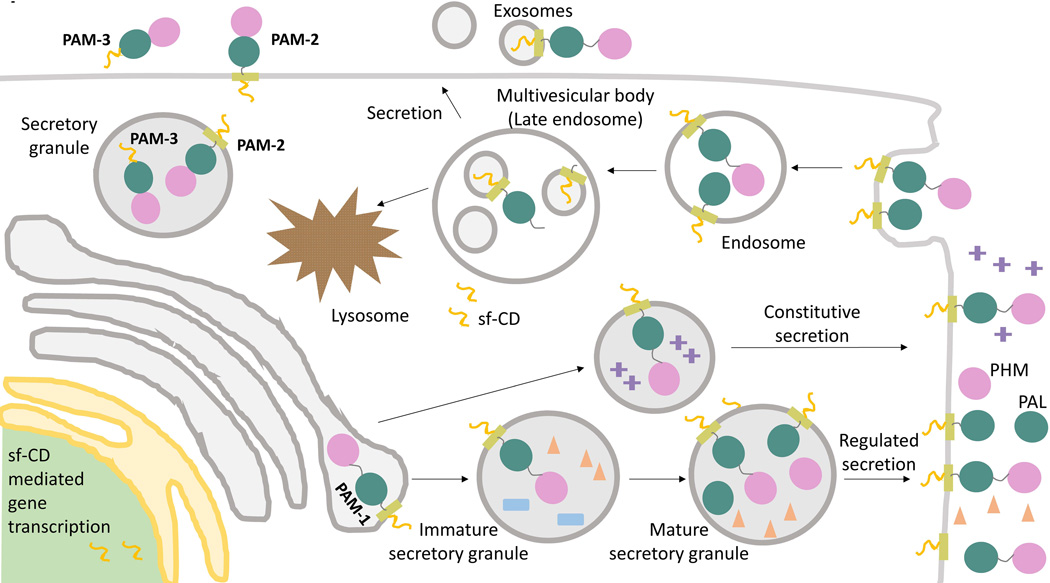
Figure 4. PAM trafficking.
Membrane PAM (PAM-1, PAM-2) travels through both the biosynthetic and endocytic pathways; soluble PAM (PAM-3) is efficiently packaged into secretory granules and secreted. Cleavage of PAM in the biosynthetic pathway involves the prohormone convertases and cleavages at pairs of basic residues in the linker region between PHM and PAL and immediately following PAL. Cleavages on the cell surface and in the endocytic pathway involve α-secretase and γ-secretase. Phosphorylation of its cytosolic domain affects the ability of PAM to move from the limiting membrane of MVBs into the intraluminal vesicles. Endocytosed PAM can be returned to the trans-Golgi network, for re-entry into secretory granules, or degraded in lysosomes.
PAM, cilia and POMC in obesity
A close look at the phylogenetic distribution of PAM suggested a strong correlation with the presence of cilia, leading us to explore the presence of PAM in this signaling organelle (Kumar et al. 2015). PAM localizes to primary and motile cilia in mammalian cells and to the motile cilium of Chlamydomonas reinhardtii, a unicellular eukaryote, suggesting an important, evolutionarily conserved role for PAM in this organelle (Fig.5). Cilia are tiny hair-like, microtubule-based organelles that extend from the cell surface of almost all mammalian cells. Primary cilia are critical sensory and signaling structures, important for sensing and responding to environmental stimuli. Multiple, motile cilia in unicellular eukaryotes, tracheal and ependymal cells play additional roles in cell motility and fluid propulsion. Acting as tiny reaction chambers, cilia compartmentalize signaling pathways and proteins. Ciliary protein entry and exit is tightly regulated by specialized trafficking pathways. Only proteins destined for the cilium are recognized by the intraflagellar trafficking machinery. Ciliary dysfunction affects a number of different tissues including kidney, heart and eyes, resulting in disorders collectively known as ciliopathies (Fliegauf, et al. 2007).
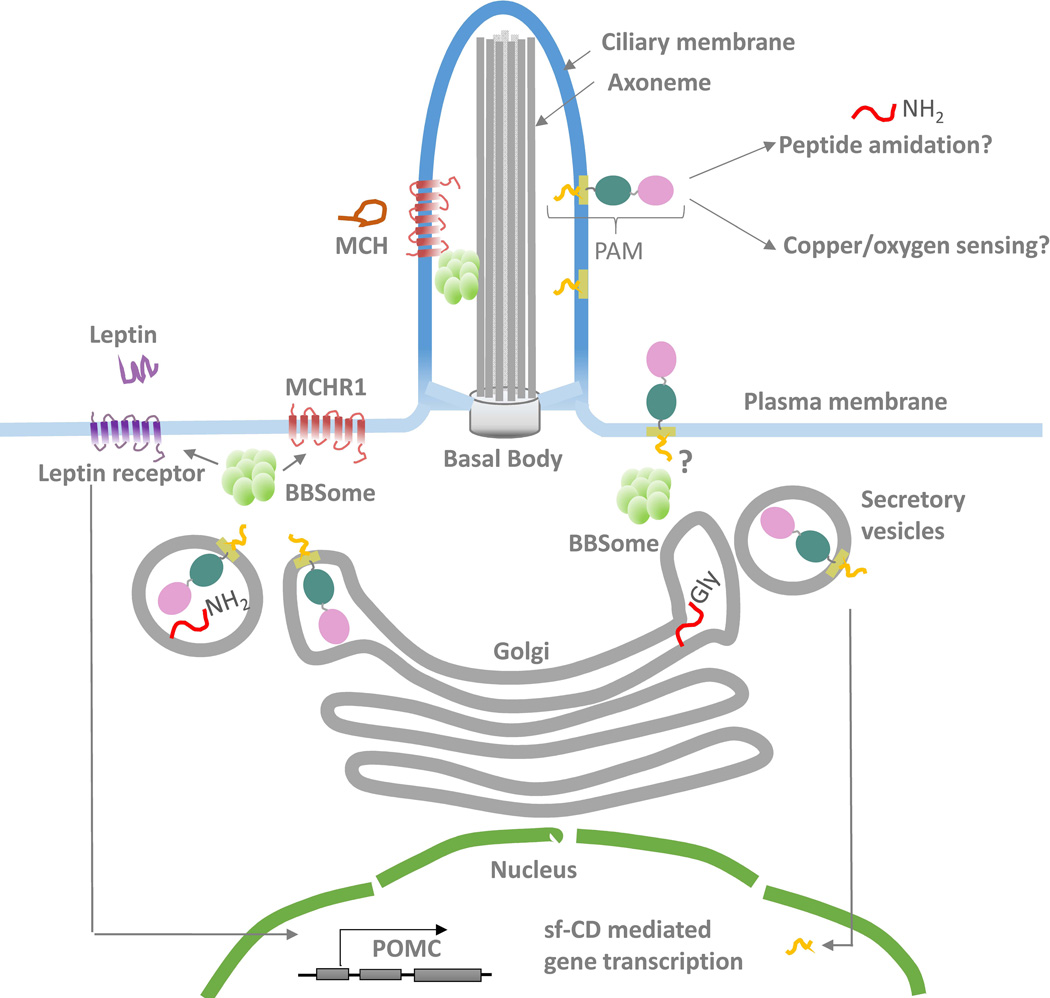
Figure 5. Model of PAM function in cilia.
PAM localizes to cilia, where it may play roles in peptide amidation and sensing of copper or oxygen. Ciliary PAM may also be subjected to cleavages leading to the release of sf-CD and changes in gene expression. It is not known if the BBSome is essential for PAM trafficking into the ciliary compartment. However, trafficking of the melanocortin concentrating hormone receptor (MCHR1) into the cilium requires the BBSome and leptin receptor localization is affected by loss of BBS components. The leptin/melanocortin system is critical for establishing energy homeostasis. Leptin binding to its receptor leads to up-regulation of POMC and satiety responses. It is not known if PAM also plays a role in energy balance in POMC neurons.
The significance of POMC in energy homeostasis is clear: loss of POMC or melanocortin receptors, MC3R and MC4R, causes an obesity phenotype in mice, and mutations in POMC result in obesity in humans (Challis, et al. 2004; Hinney, et al. 1998; Yaswen, et al. 1999). The leptin/melanocortin system plays a central role in regulating feeding behavior and obesity. Leptin, a satiety hormone, is secreted by adipocytes in proportion to body fat and acts on POMC and NPY neurons in the hypothalamus to reduce food intake and promote thermogenesis. Increased POMC production as a result of leptin signaling leads to a rise in αMSH and βMSH levels; their binding to melanocortin receptors regulates energy balance (Sen Gupta, et al. 2009).
A number of recent studies have shown that cilia play an important role in energy homeostasis. Several GPCRs, including melanin-concentrating hormone receptor-1 (MCHR1), localize to cilia (Fig.5), suggesting that G-protein signaling might occur in cilia (Berbari, et al. 2008a). Furthermore, this localization is perturbed in mice lacking components of the BBSome (Berbari, et al. 2008b). The BBSome, a multi-protein complex comprised of seven Bardet-Biedl Syndrome (BBS) proteins, is implicated in membrane protein trafficking and ciliary biogenesis (Jin and Nachury 2009). Mutations in these BBS genes result in a ciliopathy (Bardet-Biedl Syndrome) characterized by mental retardation, retinopathy and renal defects. Obesity is also observed in these patients and mouse models of the disease, further strengthening the link between energy homeostasis and cilia (Sen Gupta et al. 2009).
Leptin signaling has recently been linked to the cilium - hyperphagic BBS mutant mice have increased levels of circulating leptin and are resistant to exogenous leptin treatment, even when weight matched with control animals (Rahmouni, et al. 2008; Seo, et al. 2009). Although the leptin receptor has not been localized to cilia, it interacts with components of the BBSome and loss of BBS components causes mislocalization of the leptin receptor in large vesicles (Seo et al. 2009). Further support for the importance of cilia in energy regulation comes from the conditional knockout of intraflagellar transport proteins, which leads to the loss of primary cilia in adult mice. Ablation of cilia only on POMC neurons causes mice to become hyperphagic and obese; a model proposing the disruption of a feedback mechanism between leptin and somatostatin signaling that regulates satiety responses has been proposed (Davenport, et al. 2007; Satir 2007). These data are interesting, especially in light of the localization of PAM to cilia. It is currently not known if PAM localizes to cilia in POMC neurons, or if this localization is BBSome-mediated (Fig.5). It is also unclear if PAM performs a catalytic or signaling role in the cilium, but there is certainly a link between PAM and energy metabolism, as seen in the increased adiposity phenotype in older PAM+/− mice. Two genome wide association studies linked PAM to altered insulinogenic index and susceptibility to type 2 diabetes in Finnish and Icelandic populations, necessitating a closer look at altered energy homeostasis in the context of PAM (Huyghe, et al. 2013; Steinthorsdottir, et al. 2014).
Acknowledgments
Funding
This work has been funded continuously by the National Institutes of Health, DK-32948 and DK-32949.
We thank the graduate students, post-doctoral fellows and technicians with whom we have worked over the years for sharing their curiosity and passion for science. The expertise of our various collaborators has allowed us to expand our studies in ways we could not have imagined; special thanks go to Mario Amzel, Ninian Blackburn, Paul Taghert and Nils Back, without whom much of this work could not have been imagined, and to Neil McIntyre for sharing his knowledge of reaction mechanisms.
Footnotes
Declaration of interest
All three authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- Alam MR, Caldwell BD, Johnson RC, Darlington DN, Mains RE, Eipper BA. Novel proteins that interact with the COOH-terminal cytosolic routing determinants of an integral membrane peptide-processing enzyme. J Biol Chem. 1996;271:28636–28640. doi: 10.1074/jbc.271.45.28636. [DOI] [PubMed] [Google Scholar]
- Asada A, Orii H, Watanabe K, Tsubaki M. Planarian peptidylglycine-hydroxylating monooxygenase, a neuropeptide processing enzyme, colocalizes with cytochrome b561 along the central nervous system. FEBS J. 2005;272:942–955. doi: 10.1111/j.1742-4658.2004.04528.x. [DOI] [PubMed] [Google Scholar]
- Attenborough RM, Hayward DC, Kitahara MV, Miller DJ, Ball EE. A "neural" enzyme in nonbilaterian animals and algae: preneural origins for peptidylglycine alpha-amidating monooxygenase. Mol Biol Evol. 2012;29:3095–3109. doi: 10.1093/molbev/mss114. [DOI] [PubMed] [Google Scholar]
- Bauman AT, Jaron S, Yukl ET, Burchfiel JR, Blackburn NJ. pH Dependence of peptidylglycine monooxygenase. Mechanistic implications of Cu-methionine binding dynamics. Biochemistry. 2006;45:11140–11150. doi: 10.1021/bi060905a. [DOI] [PubMed] [Google Scholar]
- Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell. 2008a;19:1540–1547. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci U S A. 2008b;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnemaison M, Back N, Lin Y, Bonifacino JS, Mains R, Eipper B. AP-1A controls secretory granule biogenesis and trafficking of membrane secretory granule proteins. Traffic. 2014;15:1099–1121. doi: 10.1111/tra.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnemaison ML, Back N, Duffy ME, Ralle M, Mains RE, Eipper BA. Adaptor Protein-1 Complex Affects the Endocytic Trafficking and Function of Peptidylglycine alpha-Amidating Monooxygenase, a Luminal Cuproenzyme. J Biol Chem. 2015;290:21264–21279. doi: 10.1074/jbc.M115.641027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet-Moore D, Ma XM, Nillni EA, Czyzyk TA, Pintar JE, Eipper BA, Mains RE. Reversal of physiological deficits caused by diminished levels of peptidylglycine alpha-amidating monooxygenase by dietary copper. Endocrinology. 2009;150:1739–1747. doi: 10.1210/en.2008-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet-Moore D, Prohaska JR, Nillni EA, Czyzyk T, Wetsel WC, Mains RE, Eipper BA. Interactions of peptide amidation and copper: novel biomarkers and mechanisms of neural dysfunction. Neurobiol Dis. 2010;37:130–140. doi: 10.1016/j.nbd.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braas KM, Stoffers DA, Eipper BA, May V. Tissue specific expression of rat peptidylglycine alpha-amidating monooxygenase activity and mRNA. Mol Endocrinol. 1989;3:1387–1398. doi: 10.1210/mend-3-9-1387. [DOI] [PubMed] [Google Scholar]
- Bradbury AF, Finnie MD, Smyth DG. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982;298:686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Bundgaard H, Kahns AH. Chemical stability and plasma-catalyzed dealkylation of peptidyl-alpha-hydroxyglycine derivatives--intermediates in peptide alpha-amidation. Peptides. 1991;12:745–748. doi: 10.1016/0196-9781(91)90127-b. [DOI] [PubMed] [Google Scholar]
- Burzle M, Hediger MA. Functional and physiological role of vitamin C transporters. Curr Top Membr. 2012;70:357–375. doi: 10.1016/B978-0-12-394316-3.00011-9. [DOI] [PubMed] [Google Scholar]
- Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, et al. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3–36) Proc Natl Acad Sci U S A. 2004;101:4695–4700. doi: 10.1073/pnas.0306931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew GH, Galloway LC, McIntyre NR, Schroder LA, Richards KM, Miller SA, Wright DW, Merkler DJ. Ubiquitin and ubiquitin-derived peptides as substrates for peptidylglycine alpha-amidating monooxygenase. FEBS Lett. 2005;579:4678–4684. doi: 10.1016/j.febslet.2005.06.089. [DOI] [PubMed] [Google Scholar]
- Chufan EE, De M, Eipper BA, Mains RE, Amzel LM. Amidation of bioactive peptides: the structure of the lyase domain of the amidating enzyme. Structure. 2009;17:965–973. doi: 10.1016/j.str.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyzyk TA, Ning Y, Hsu MS, Peng B, Mains RE, Eipper BA, Pintar JE. Deletion of peptide amidation enzymatic activity leads to edema and embryonic lethality in the mouse. Dev Biol. 2005;287:301–313. doi: 10.1016/j.ydbio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De M, Bell J, Blackburn NJ, Mains RE, Eipper BA. Role for an essential tyrosine in peptide amidation. J Biol Chem. 2006;281:20873–20882. doi: 10.1074/jbc.M513886200. [DOI] [PubMed] [Google Scholar]
- De M, Ciccotosto GD, Mains RE, Eipper BA. Trafficking of a secretory granule membrane protein is sensitive to copper. J Biol Chem. 2007;282:23362–23371. doi: 10.1074/jbc.M702891200. [DOI] [PubMed] [Google Scholar]
- Eipper BA, Glembotski CC, Mains RE. Selective loss of alpha-melanotropin-amidating activity in primary cultures of rat intermediate pituitary cells. J Biol Chem. 1983a;258:7292–7298. [PubMed] [Google Scholar]
- Eipper BA, Green CB, Campbell TA, Stoffers DA, Keutmann HT, Mains RE, Ouafik L. Alternative splicing and endoproteolytic processing generate tissue-specific forms of pituitary peptidylglycine alpha-amidating monooxygenase (PAM) J Biol Chem. 1992;267:4008–4015. [PubMed] [Google Scholar]
- Eipper BA, Mains RE, Glembotski CC. Identification in pituitary tissue of a peptide alpha-amidation activity that acts on glycine-extended peptides and requires molecular oxygen, copper, and ascorbic acid. Proc Natl Acad Sci U S A. 1983b;80:5144–5148. doi: 10.1073/pnas.80.16.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper BA, May V, Braas KM. Membrane-associated peptidylglycine alpha-amidating monooxygenase in the heart. J Biol Chem. 1988;263:8371–8379. [PubMed] [Google Scholar]
- Eipper BA, Park LP, Dickerson IM, Keutmann HT, Thiele EA, Rodriguez H, Schofield PR, Mains RE. Structure of the precursor to an enzyme mediating COOH-terminal amidation in peptide biosynthesis. Mol Endocrinol. 1987;1:777–790. doi: 10.1210/mend-1-11-777. [DOI] [PubMed] [Google Scholar]
- Eipper BA, Perkins SN, Husten EJ, Johnson RC, Keutmann HT, Mains RE. Peptidyl-alpha-hydroxyglycine alpha-amidating lyase. Purification, characterization, and expression. J Biol Chem. 1991;266:7827–7833. [PubMed] [Google Scholar]
- El Meskini R, Galano GJ, Marx R, Mains RE, Eipper BA. Targeting of membrane proteins to the regulated secretory pathway in anterior pituitary endocrine cells. J Biol Chem. 2001;276:3384–3393. doi: 10.1074/jbc.M008062200. [DOI] [PubMed] [Google Scholar]
- Erion MD, Tan J, Wong M, Jeng AY. Inhibition of peptidylglycine alpha-amidating monooxygenase by N-substituted homocysteine analogs. J Med Chem. 1994;37:4430–4437. doi: 10.1021/jm00052a002. [DOI] [PubMed] [Google Scholar]
- Evans JP, Blackburn NJ, Klinman JP. The catalytic role of the copper ligand H172 of peptidylglycine alpha-hydroxylating monooxygenase: a kinetic study of the H172A mutant. Biochemistry. 2006;45:15419–15429. doi: 10.1021/bi061734c. [DOI] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- Francisco WA, Knapp MJ, Blackburn NJ, Klinman JP. Hydrogen tunneling in peptidylglycine alpha-hydroxylating monooxygenase. J Am Chem Soc. 2002;124:8194–8195. doi: 10.1021/ja025758s. [DOI] [PubMed] [Google Scholar]
- Francisco WA, Wille G, Smith AJ, Merkler DJ, Klinman JP. Investigation of the pathway for inter-copper electron transfer in peptidylglycine alpha-amidating monooxygenase. J Am Chem Soc. 2004;126:13168–13169. doi: 10.1021/ja046888z. [DOI] [PubMed] [Google Scholar]
- Francone VP, Ifrim MF, Rajagopal C, Leddy CJ, Wang Y, Carson JH, Mains RE, Eipper BA. Signaling from the secretory granule to the nucleus: Uhmk1 and PAM. Mol Endocrinol. 2010;24:1543–1558. doi: 10.1210/me.2009-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa Y, Furukawa Y, Ohta S, Ellis TA, Dembrow NC, Li L, Floyd PD, Sweedler JV, Minakata H, Nakamaru K, et al. The Aplysia mytilus inhibitory peptide-related peptides: identification, cloning, processing, distribution, and action. J Neurosci. 1999;19:9618–9634. doi: 10.1523/JNEUROSCI.19-21-09618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaier ED, Kleppinger A, Ralle M, Covault J, Mains RE, Kenny AM, Eipper BA. Genetic determinants of amidating enzyme activity and its relationship with metal cofactors in human serum. BMC Endocr Disord. 2014;14:58. doi: 10.1186/1472-6823-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glembotski CC, Eipper BA, Mains RE. Adrenocorticotropin(1–14)OH-related molecules in primary cultures of rat intermediate pituitary cells. Identification and role in the biosynthesis of alpha-melanotropin. J Biol Chem. 1983;258:7299–7304. [PubMed] [Google Scholar]
- Gonzalez-Begne M, Lu B, Han X, Hagen FK, Hand AR, Melvin JE, Yates JR. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT) J Proteome Res. 2009;8:1304–1314. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H, Ottervald J, Nilsson KC, Sjogren N, Miliotis T, Von Bahr H, Khademi M, Eriksson B, Kjellstrom S, Vegvari A, et al. Identification of novel candidate protein biomarkers for the post-polio syndrome - implications for diagnosis, neurodegeneration and neuroinflammation. J Proteomics. 2009;71:670–681. doi: 10.1016/j.jprot.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Grunder S, Assmann M. Peptide-gated ion channels and the simple nervous system of Hydra. J Exp Biol. 2015;218:551–561. doi: 10.1242/jeb.111666. [DOI] [PubMed] [Google Scholar]
- Han M, Park D, Vanderzalm PJ, Mains RE, Eipper BA, Taghert PH. Drosophila uses two distinct neuropeptide amidating enzymes, dPAL1 and dPAL2. J Neurochem. 2004;90:129–141. doi: 10.1111/j.1471-4159.2004.02464.x. [DOI] [PubMed] [Google Scholar]
- Harris JI, Lerner AB. Amino-acid sequence of the alpha-melanocyte-stimulating hormone. Nature. 1957;179:1346–1347. doi: 10.1038/1791346a0. [DOI] [PubMed] [Google Scholar]
- Hinney A, Becker I, Heibult O, Nottebom K, Schmidt A, Ziegler A, Mayer H, Siegfried W, Blum WF, Remschmidt H, et al. Systematic mutation screening of the pro-opiomelanocortin gene: identification of several genetic variants including three different insertions, one nonsense and two missense point mutations in probands of different weight extremes. J Clin Endocrinol Metab. 1998;83:3737–3741. doi: 10.1210/jcem.83.10.5298. [DOI] [PubMed] [Google Scholar]
- Huyghe JR, Jackson AU, Fogarty MP, Buchkovich ML, Stancakova A, Stringham HM, Sim X, Yang L, Fuchsberger C, Cederberg H, et al. Exome array analysis identifies new loci and low-frequency variants influencing insulin processing and secretion. Nat Genet. 2013;45:197–201. doi: 10.1038/ng.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliadi KG, Avivi A, Iliadi NN, Knight D, Korol AB, Nevo E, Taylor P, Moran MF, Kamyshev NG, Boulianne GL. nemy encodes a cytochrome b561 that is required for Drosophila learning and memory. Proc Natl Acad Sci U S A. 2008;105:19986–19991. doi: 10.1073/pnas.0810698105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaron S, Mains RE, Eipper BA, Blackburn NJ. The catalytic role of the copper ligand H172 of peptidylglycine alpha-hydroxylating monooxygenase (PHM): a spectroscopic study of the H172A mutant. Biochemistry. 2002;41:13274–13282. doi: 10.1021/bi020404z. [DOI] [PubMed] [Google Scholar]
- Jekely G. Global view of the evolution and diversity of metazoan neuropeptide signaling. Proc Natl Acad Sci U S A. 2013;110:8702–8707. doi: 10.1073/pnas.1221833110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Nachury MV. The BBSome. Curr Biol. 2009;19:R472–R473. doi: 10.1016/j.cub.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Katopodis AG, Ping D, May SW. A novel enzyme from bovine neurointermediate pituitary catalyzes dealkylation of alpha-hydroxyglycine derivatives, thereby functioning sequentially with peptidylglycine alpha-amidating monooxygenase in peptide amidation. Biochemistry. 1990;29:6115–6120. doi: 10.1021/bi00478a001. [DOI] [PubMed] [Google Scholar]
- Kline CD, Mayfield M, Blackburn NJ. HHM motif at the CuH-site of peptidylglycine monooxygenase is a pH-dependent conformational switch. Biochemistry. 2013;52:2586–2596. doi: 10.1021/bi4002248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman JP. The copper-enzyme family of dopamine beta-monooxygenase and peptidylglycine alpha-hydroxylating monooxygenase: resolving the chemical pathway for substrate hydroxylation. J Biol Chem. 2006;281:3013–3016. doi: 10.1074/jbc.R500011200. [DOI] [PubMed] [Google Scholar]
- Kolhekar AS, Roberts MS, Jiang N, Johnson RC, Mains RE, Eipper BA, Taghert PH. Neuropeptide amidation in Drosophila: separate genes encode the two enzymes catalyzing amidation. J Neurosci. 1997;17:1363–1376. doi: 10.1523/JNEUROSCI.17-04-01363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Blaby-Haas CE, Merchant SS, Mains RE, King SM, Eipper BA. Cilia-associated bioactive peptide amidating activity preceded the emergence of multicellularity. American Society for Cell Biology meeting, San Diego, California. 2015 Dec 12–16; [Google Scholar]
- Langella E, Pierre S, Ghattas W, Giorgi M, Reglier M, Saviano M, Esposito L, Hardre R. Probing the peptidylglycine alpha-hydroxylating monooxygenase active site with novel 4-phenyl-3-butenoic acid based inhibitors. Chem Med Chem. 2010;5:1568–1576. doi: 10.1002/cmdc.201000214. [DOI] [PubMed] [Google Scholar]
- Lee CM, Yang P, Chen LC, Chen CC, Wu SC, Cheng HY, Chang YS. A novel role of RASSF9 in maintaining epidermal homeostasis. PLoS One. 2011;6:e17867. doi: 10.1371/journal.pone.0017867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains RE, Myers AC, Eipper BA. Hormonal, drug, and dietary factors affecting peptidyl glycine alpha-amidating monooxygenase activity in various tissues of the adult male rat. Endocrinology. 1985;116:2505–2515. doi: 10.1210/endo-116-6-2505. [DOI] [PubMed] [Google Scholar]
- Mair GR, Niciu MJ, Stewart MT, Brennan G, Omar H, Halton DW, Mains R, Eipper BA, Maule AG, Day TA. A functionally atypical amidating enzyme from the human parasite Schistosoma mansoni. FASEB J. 2004;18:114–121. doi: 10.1096/fj.03-0429com. [DOI] [PubMed] [Google Scholar]
- McIntyre NR, Lowe EW, Jr, Belof JL, Ivkovic M, Shafer J, Space B, Merkler DJ. Evidence for substrate preorganization in the peptidylglycine alpha-amidating monooxygenase reaction describing the contribution of ground state structure to hydrogen tunneling. J Am Chem Soc. 2010;132:16393–16402. doi: 10.1021/ja1019194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkler DJ, Asser AS, Baumgart LE, Carballo N, Carpenter SE, Chew GH, Cosner CC, Dusi J, Galloway LC, Lowe AB, et al. Substituted hippurates and hippurate analogs as substrates and inhibitors of peptidylglycine alpha-hydroxylating monooxygenase (PHM) Bioorg Med Chem. 2008;16:10061–10074. doi: 10.1016/j.bmc.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram SL, Mains RE, Eipper BA. COOH-terminal signals mediate the trafficking of a peptide processing enzyme in endocrine cells. J Cell Biol. 1993;121:23–36. doi: 10.1083/jcb.121.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minciacchi VR, You S, Spinelli C, Morley S, Zandian M, Aspuria PJ, Cavallini L, Ciardiello C, Reis Sobreiro M, Morello M, et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget. 2015;6:11327–11341. doi: 10.18632/oncotarget.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Ohsuye K, Wada Y, Fuchimura K, Tanaka S, Matsuo H. Cloning and sequence of cDNA encoding a peptide C-terminal alpha-amidating enzyme from Xenopus laevis. Biochem Biophys Res Commun. 1987;148:546–552. doi: 10.1016/0006-291x(87)90911-9. [DOI] [PubMed] [Google Scholar]
- Mueller GP, Driscoll WJ, Eipper BA. In vivo inhibition of peptidylglycine-alpha-hydroxylating monooxygenase by 4-phenyl-3-butenoic acid. J Pharmacol Exp Ther. 1999;290:1331–1336. [PubMed] [Google Scholar]
- Mueller GP, Husten EJ, Mains RE, Eipper BA. Peptide alpha-amidation and peptidylglycine alpha-hydroxylating monooxygenase: control by disulfiram. Mol Pharmacol. 1993;44:972–980. [PubMed] [Google Scholar]
- Murthy AS, Mains RE, Eipper BA. Purification and characterization of peptidylglycine alpha-amidating monooxygenase from bovine neurointermediate pituitary. J Biol Chem. 1986;261:1815–1822. [PubMed] [Google Scholar]
- O'Donnell PJ, Driscoll WJ, Back N, Muth E, Mueller GP. Peptidylglycine-alpha-amidating monooxygenase and pro-atrial natriuretic peptide constitute the major membrane-associated proteins of rat atrial secretory granules. J Mol Cell Cardiol. 2003;35:915–922. doi: 10.1016/s0022-2828(03)00171-8. [DOI] [PubMed] [Google Scholar]
- Ohsuye K, Kitano K, Wada Y, Fuchimura K, Tanaka S, Mizuno K, Matsuo H. Cloning of cDNA encoding a new peptide C-terminal alpha-amidating enzyme having a putative membrane-spanning domain from Xenopus laevis skin. Biochem Biophys Res Commun. 1988;150:1275–1281. doi: 10.1016/0006-291x(88)90767-x. [DOI] [PubMed] [Google Scholar]
- Oldham CD, Li C, Girard PR, Nerem RM, May SW. Peptide amidating enzymes are present in cultured endothelial cells. Biochem Biophys Res Commun. 1992;184:323–329. doi: 10.1016/0006-291x(92)91196-w. [DOI] [PubMed] [Google Scholar]
- Otoikhian A, Barry AN, Mayfield M, Nilges M, Huang Y, Lutsenko S, Blackburn NJ. Lumenal loop M672–P707 of the Menkes protein (ATP7A) transfers copper to peptidylglycine monooxygenase. J Am Chem Soc. 2012;134:10458–10468. doi: 10.1021/ja301221s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins SN, Husten EJ, Eipper BA. The 108-kDA peptidylglycine alpha-amidating monooxygenase precursor contains two separable enzymatic activities involved in peptide amidation. Biochem Biophys Res Commun. 1990;171:926–932. doi: 10.1016/0006-291x(90)90772-f. [DOI] [PubMed] [Google Scholar]
- Prigge ST, Eipper BA, Mains RE, Amzel LM. Dioxygen binds end-on to mononuclear copper in a precatalytic enzyme complex. Science. 2004;304:864–867. doi: 10.1126/science.1094583. [DOI] [PubMed] [Google Scholar]
- Prigge ST, Kolhekar AS, Eipper BA, Mains RE, Amzel LM. Amidation of bioactive peptides: the structure of peptidylglycine alpha-hydroxylating monooxygenase. Science. 1997;278:1300–1305. doi: 10.1126/science.278.5341.1300. [DOI] [PubMed] [Google Scholar]
- Prigge ST, Kolhekar AS, Eipper BA, Mains RE, Amzel LM. Substrate-mediated electron transfer in peptidylglycine alpha-hydroxylating monooxygenase. Nat Struct Biol. 1999;6:976–983. doi: 10.1038/13351. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Role of copper transporters in copper homeostasis. Am J Clin Nutr. 2008;88:826S-–829S. doi: 10.1093/ajcn/88.3.826S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest. 2008;118:1458–1467. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal C, Mains RE, Eipper BA. Signaling from the secretory granule to the nucleus. Crit Rev Biochem Mol Biol. 2012;47:391–406. doi: 10.3109/10409238.2012.694845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal C, Stone KL, Francone VP, Mains RE, Eipper BA. Secretory granule to the nucleus: role of a multiply phosphorylated intrinsically unstructured domain. J Biol Chem. 2009;284:25723–25734. doi: 10.1074/jbc.M109.035782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal C, Stone KL, Mains RE, Eipper BA. Secretion stimulates intramembrane proteolysis of a secretory granule membrane enzyme. J Biol Chem. 2010;285:34632–34642. doi: 10.1074/jbc.M110.145334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P. Cilia biology: stop overeating now! Curr Biol. 2007;17:R963–R965. doi: 10.1016/j.cub.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Stoffers DA, Eipper BA, Watson SJ. Expression of peptidylglycine alpha-amidating monooxygenase (EC 1.14.17.3) in the rat central nervous system. J Neurosci. 1992;12:222–234. doi: 10.1523/JNEUROSCI.12-01-00222.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen Gupta P, Prodromou NV, Chapple JP. Can faulty antennae increase adiposity? The link between cilia proteins and obesity. J Endocrinol. 2009;203:327–336. doi: 10.1677/JOE-09-0116. [DOI] [PubMed] [Google Scholar]
- Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18:1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert X, Eipper BA, Mains RE, Prigge ST, Blackburn NJ, Amzel LM. The catalytic copper of peptidylglycine alpha-hydroxylating monooxygenase also plays a critical structural role. Biophys J. 2005;89:3312–3319. doi: 10.1529/biophysj.105.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson PD, Eipper BA, Katz MJ, Gandara L, Wappner P, Fischer R, Hodson EJ, Ratcliffe PJ, Masson N. Striking Oxygen Sensitivity of the Peptidylglycine alpha-Amidating Monooxygenase (PAM) in Neuroendocrine Cells. J Biol Chem. 2015;290:24891–24901. doi: 10.1074/jbc.M115.667246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- Skulj M, Pezdirec D, Gaser D, Kreft M, Zorec R. Reduction in C-terminal amidated species of recombinant monoclonal antibodies by genetic modification of CHO cells. BMC Biotechnol. 2014;14:76. doi: 10.1186/1472-6750-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, Varoqueaux F, Kittelmann M, Azzam RN, Cooper B, Winters CA, Eitel M, Fasshauer D, Reese TS. Novel cell types, neurosecretory cells, and body plan of the early-diverging metazoan Trichoplax adhaerens. Curr Biol. 2014;24:1565–1572. doi: 10.1016/j.cub.2014.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobota JA, Back N, Eipper BA, Mains RE. Inhibitors of the V0 subunit of the vacuolar H+ATPase prevent segregation of lysosomal- and secretory-pathway proteins. J Cell Sci. 2009;122:3542–3553. doi: 10.1242/jcs.034298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinthorsdottir V, Thorleifsson G, Sulem P, Helgason H, Grarup N, Sigurdsson A, Helgadottir HT, Johannsdottir H, Magnusson OT, Gudjonsson SA, et al. Identification of low-frequency and rare sequence variants associated with elevated or reduced risk of type 2 diabetes. Nat Genet. 2014;46:294–298. doi: 10.1038/ng.2882. [DOI] [PubMed] [Google Scholar]
- Steveson TC, Ciccotosto GD, Ma XM, Mueller GP, Mains RE, Eipper BA. Menkes protein contributes to the function of peptidylglycine alpha-amidating monooxygenase. Endocrinology. 2003;144:188–200. doi: 10.1210/en.2002-220716. [DOI] [PubMed] [Google Scholar]
- Stewart LC, Klinman JP. Dopamine beta-hydroxylase of adrenal chromaffin granules: structure and function. Annu Rev Biochem. 1988;57:551–592. doi: 10.1146/annurev.bi.57.070188.003003. [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Green CB, Eipper BA. Alternative mRNA splicing generates multiple forms of peptidyl-glycine alpha-amidating monooxygenase in rat atrium. Proc Natl Acad Sci U S A. 1989;86:735–739. doi: 10.1073/pnas.86.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers DA, Ouafik L, Eipper BA. Characterization of novel mRNAs encoding enzymes involved in peptide alpha-amidation. J Biol Chem. 1991;266:1701–1707. [PubMed] [Google Scholar]
- Stone JV, Mordue W, Batley KE, Morris HR. Structure of locust adipokinetic hormone, a neurohormone that regulates lipid utilisation during flight. Nature. 1976;263:207–211. doi: 10.1038/263207a0. [DOI] [PubMed] [Google Scholar]
- Suchanek G, Kreil G. Translation of melittin messenger RNA in vitro yields a product terminating with glutaminylglycine rather than with glutaminamide. Proc Natl Acad Sci U S A. 1977;74:975–978. doi: 10.1073/pnas.74.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tausk FA, Milgram SL, Mains RE, Eipper BA. Expression of a peptide processing enzyme in cultured cells: truncation mutants reveal a routing domain. Mol Endocrinol. 1992;6:2185–2196. doi: 10.1210/mend.6.12.1491698. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Noguchi M, Kayama H, Watanabe T, Asoh T, Yamamoto T. Increased peptidylglycine alpha-amidating monooxygenase activity in cerebrospinal fluid of patients with multiple sclerosis. Intern Med. 1995;34:229–232. doi: 10.2169/internalmedicine.34.229. [DOI] [PubMed] [Google Scholar]
- Vishwanatha K, Back N, Mains RE, Eipper BA. A histidine-rich linker region in peptidylglycine alpha-amidating monooxygenase has the properties of a pH sensor. J Biol Chem. 2014;289:12404–12420. doi: 10.1074/jbc.M113.545947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Ney RL, Mains RE, Eipper BA. Characterization of peptide alpha-amidation activity in human cerebrospinal fluid and central nervous system tissue. Neuroendocrinology. 1985;41:482–489. doi: 10.1159/000124223. [DOI] [PubMed] [Google Scholar]
- Wilcox BJ, Ritenour-Rodgers KJ, Asser AS, Baumgart LE, Baumgart MA, Boger DL, DeBlassio JL, deLong MA, Glufke U, Henz ME, et al. N-acylglycine amidation: implications for the biosynthesis of fatty acid primary amides. Biochemistry. 1999;38:3235–3245. doi: 10.1021/bi982255j. [DOI] [PubMed] [Google Scholar]
- Williamson M, Hauser F, Grimmelikhuijzen CJ. Genomic organization and splicing variants of a peptidylglycine alpha-hydroxylating monooxygenase from sea anemones. Biochem Biophys Res Commun. 2000;277:7–12. doi: 10.1006/bbrc.2000.3629. [DOI] [PubMed] [Google Scholar]
- Xin X, Mains RE, Eipper BA. Monooxygenase X, a member of the copper-dependent monooxygenase family localized to the endoplasmic reticulum. J Biol Chem. 2004;279:48159–48167. doi: 10.1074/jbc.M407486200. [DOI] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Young SD, Tamburini PP. Enzymatic peptidyl .alpha.-amidation proceeds through formation of an .alpha.-hydroxyglycine intermediate. Journal of the American Chemical Society. 1989;111:1933–1934. [Google Scholar]