Abstract
Cross-bridge cycling kinetics play an essential role in the heart’s ability to contract and relax. The rate of tension redevelopment (ktr) slows down as a muscle length is increased in intact human myocardium. We set out to determine the effect of rapid length step changes and protein kinase A (PKA) and protein kinase C-βII (PKC-βII) inhibitors on the ktr in ultra-thin non-failing and failing human right ventricular trabeculae. After stabilizing the muscle either at L90 (90% of optimal length) or at Lopt (optimal length), we rapidly changed the length to either Lopt or L90 and measured ktr. We report that length-dependent changes in ktr occur very rapidly (in the order of seconds or faster) in both non-failing and failing muscles and that the length at which a muscle had been stabilized prior to the length change does not significantly affect ktr. In addition, at L90 and at Lopt, PKA and PKC-βII inhibitors did not significantly change ktr. Our results reveal that length-dependent regulation of cross-bridge cycling kinetics predominantly occurs rapidly and involves the intrinsic properties of the myofilament rather than post-translational modifications that are known to occur in the cardiac muscle as a result of a change in muscle/sarcomere length.
Keywords: Cross-bridge cycling kinetics, muscle length, post-translational modification, rate of tension redevelopment, trabeculae
Introduction
The beating heart utilizes the Frank-Starling mechanism to increase its cardiac output as its volume increases due to venous return of blood [1]. As the diastolic ventricular volume increases, not only does the contractile force increase, but the kinetics of contraction and relaxation are also modulated [2, 3]. Cross-bridge cycling kinetics is an important contributor in determining cardiac output [4–6] and consequently discovering how this kinetic parameter is regulated is essential to having a comprehensive understanding of how the heart regulates its pumping activity, is altered in heart failure, and discovering novel potential therapeutic targets. On a cellular level, it has been shown that increasing muscle length, an in vitro index of pre-load, decreases cross-bridge cycling kinetics in animal models [7–11]. We have recently shown that this length-dependent regulation of cross-bridge cycling kinetics is also present in both non-failing and failing human myocardium under conditions close to those in vivo [12]. The exact mechanism(s) by which muscle length affects cross-bridge cycling kinetics remains however unknown.
It has been proposed that stretching cardiomyocytes results in stretching of titin which interacts with cardiac myosin binding protein-C (cMyBP-C). This in turn exerts movement restriction on myosin heavy chain (MHC) that ultimately results in decreased cross-bridge cycling rate [5, 10, 13]. Another possible mechanism is that increasing muscle length induces post-translational modifications of contractile proteins and these modifications are responsible for the length-dependent regulation of contractile kinetics. It is well known that modifications including phosphorylation of contractile proteins, such as myosin light chain 2 (MLC-2), cMyBP-C and Troponin-I (TnI) Ser23/24, are important determinants of cross-bridge cycling kinetics [14–22]. Recent studies have shown that increasing pre-load is associated with alterations in MLC-2 phosphorylation of rat myocardium [23]. Furthermore, increasing muscle length has been shown to alter phosphorylation of MLC-2 in human [24] and both MLC-2 and TnI Ser23/24 in rabbit cardiac muscles[3], while stretching permeabilized guinea pig ventricular cardiomyocytes is associated with increased phosphorylation of MLC-2 and cMyBP-C [25]. Furthermore, we have previously shown that Protein Kinase A (PKA) and Protein Kinase C-βII (PKC-βII) pathways are essential to this length-dependent phosphorylation and their inhibition results in faster twitch kinetics at increased muscle lengths [26]. Thus, length-dependent phosphorylation of contractile proteins is a plausible explanation for the effects of muscle length on cross-bridge cycling kinetics which warrants further evaluation.
We previously developed a novel method for assessing cross-bridge cycling kinetics by measuring rate of tension redevelopment (ktr) in intact cardiac trabeculae [7]. This technique has the advantage of conducting experiments on intact cardiac preparations under near-physiological conditions, where the post-translational modification machinery remains intact. It allows the possibility of assessing whether the length-dependent regulation of contractile kinetics is solely dependent on the inherit properties of the myofilaments or that post-translational modifications also have a main role. In this study, we show that length-dependent regulation of cross-bridge cycling kinetics is a near instantaneous process suggesting that the underlying mechanism is within the myofilament proteins.
Methods
Procurement of Human Hearts
All experiments on human tissue presented in this study were performed in accordance with the Institutional Review Board (IRB) at The Ohio State University and Declaration of Helsinki. Non-failing human hearts not suitable for cardiac transplantation (n = 9) were acquired from LifeLine of Ohio Organ Procurement and failing hearts (n = 8) from patients undergoing cardiac transplantation at The Ohio State University Wexner Medical Center. Informed consent was acquired from all patients undergoing cardiac transplantation. Human hearts reported here are a subset of samples that were used in our previous study [12]. The details of the human hearts and type of experiment performed are outlined in Table 1. The acquired hearts were immediately flushed with cold cardioplegic solution containing (in mM): 110 NaCl, 16 KCl, 10 NaHCO3, 16 MgCl2, and 0.5 CaCl2 and transported promptly to the laboratory.
Table 1.
Characteristics of human hearts.
| Heart | Age | Gender | Race | Inhibitors | Rapid Length Change |
|---|---|---|---|---|---|
| Non-Failing | |||||
| 685884 | 36 | Male | Caucasian | - | X |
| 474083 | 41 | Female | African-American | - | X |
| 618200 | 58 | Female | Caucasian | X | X |
| 481043 | 65 | Female | Caucasian | - | X |
| 947200 | 63 | Female | Caucasian | X | X |
| 476074 | 29 | Female | Caucasian | X | - |
| 240603 | 51 | Female | Caucasian | X | X |
| 452192 | 55 | Female | African-American | X | - |
| 118258 | 38 | Male | Caucasian | X | - |
| Failing | |||||
| 450564 | 30 | Male | Caucasian | - | X |
| 328163 | 63 | Male | Caucasian | - | X |
| 479062 | 50 | Male | Caucasian | - | X |
| 323104 | 63 | Male | Caucasian | X | X |
| 537263 | 62 | Male | African-American | X | X |
| 522421 | 56 | Female | Caucasian | X | X |
| 214010 | 64 | Male | Caucasian | X | X |
| 597750 | 67 | Male | Caucasian | X | X |
Most heart are subset of hearts used in a previous study [12]. X indicates that the heart was used for the experiment.
Isolation of Cardiac Trabeculae
The right ventricles were transferred from the cardioplegic solution to a Krebs-Henseleit solution (K-H) previously bubbled with 95% O2-5% CO2 containing (in mM): 137 NaCl, 5 KCl, 20 NaHCO3, 10 dextrose, 1.2 NaH2PO4, 1.2 MgSO4, 0.25 CaCl2,and 20 BDM (2,3-butanedione monoxime) and pH of 7.4. Small linear trabeculae were isolated from the right ventricles. All trabeculae were submerged in this K-H solution and kept at 0–4 °C until the start of the experiments. Muscles were mounted on a custom-made setup as previously described [7, 12]. The perfusion solution was a modified K-H solution (37 °C) without BDM and containing an initial CaCl2 concentration of 0.25 mM. Muscles were stimulated at 1 Hz and the CaCl2 concentration was gradually raised to 2 mM. The muscles were then gradually stretched to optimal length (Lopt) as previously described [12] which is close to the sarcomere length of 2.2 μm at end-diastole in vivo [27].
ktr measurements with inhibitors
The data on control experiments without inhibitors presented in this study are a subset of data previously reported in another study [12]. In brief, muscles were stabilized at L90 or Lopt for 10–15 minutes without any inhibitors. The ktr was measured at sub-maximal and maximal tension levels of the K+ contracture as previously described [12]. After these control measurements, muscles were re-stabilized at Lopt, 1 Hz, 37 °C without any inhibitors for 15 minutes. Muscles were then stabilized in the presence of 20 μM H-89 and 7.5 nM PKCβII peptide inhibitor I for an additional15 minutes. These inhibitors were also added to the high K+/high Ca2+ solution (i.e. the K+ contracture solution). The K+ contracture was induced and ktr maneuvers at sub-maximal and maximal tension levels were performed. Muscles were next stabilized at L90, 1 Hz, and 37 °C with the inhibitors for 15 minutes. Afterward s, K+ contracture and ktr experiments were conducted during sub-maximal and maximal tension levels.
ktr measurements after rapid muscle length changes
The control data at Lopt and L90 reported are from a subset of experiments previously reported in our recent study [12]. Muscles were stabilized at L90 and 1 Hz for ~10–15 minutes. K+ contracture was induced and ktr was initially measured during maximal K+ contracture tension as described above. Immediately afterwards, while the muscles were still under contracture, they were quickly stretched to Lopt and ktr was measured at this length quickly (within several seconds). Similarly, muscles were allowed to stabilize at Lopt and 1 Hz for ~10–15 minutes. After the ktr at Lopt was measured during the maximal K+ contracture tension, the muscle was quickly slacked to L90 while under contracture and a ktr experiment was performed without stabilization (i.e. within seconds after slacking to L90).
Protein Analysis
For a subset of single trabeculae, total protein phosphorylation was determined by ProQ Diamond phosphoprotein staining similar to that previously described[3]. Briefly, following contractile experiments trabeculae were immediately frozen with liquid nitrogen, quickly removed from the setup frozen and stored at −80°C. Single trabeculae were solubilized by heating at 80 °C for 6 minutes with vortexing for 10 seconds every 2 minutes in 20 μl lysis buffer (8 M Urea, 2 M thiourea, 75 mM DTT, 3% SDS, 0.05% bromophenol blue and 50 mM Tris-HCl, pH 6.8). Immediately following heating, samples were clarified by centrifugation and 16 μl of the sample supernatant was fractionated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% Laemmli gel with an acrylamide-to-bisacrylamide ratio of 200:1. Total protein phosphorylation was determined in the resultant gel by staining with ProQ Diamond (Molecular Probes) according to the manufactures instructions and imaging on a Typhoon 9410 (GE Healthcare) with an excitation of 532 nm and a 580 nm BP 30 emission filter. Total protein quantification was then determined by subsequent Sypro Ruby staining (Molecular Probes) of the same gel according to the manufacturers instructions and imaging on the Typhoon with an excitation of 457-nm and a 610-nm BP 30 emission filter. Quantification of the whole-lane total protein phosphorylation and whole lane total protein was quantified by ImageQuant TL (GE Healthcare) analysis of the respective images and sample protein phosphorylation determined as the phospo-protein/total protein signal of each lane.
Data analysis
All data were collected and analyzed using custom-made programs in LabView (National Instruments). Tensions were normalized to the cross-sectional areas (mm2) of the muscles. All ktr tracings were fit to the equation F = Fmax · (1 − e−ktr(t)) + Finitial using Origin 7 (OriginLab Corp). Statistical analysis was performed using paired Student’s t-tests with statistical significance set as P < 0.05. All data is shown as mean ± S.E.M.
Results
Effects of Inhibitors on Stimulated Twitch Contraction
The combined effects of H-89 and PKCβII peptide inhibitor I was evaluated on parameters of twitch contraction in both non-failing and failing myocardium (Table 2). Of note, presence of inhibitors had no significant effect on either dF/dtmax/F (maximum rate of force increase normalized to developed force) or dF/dtmin/F (maximum rate of force decrease normalized to developed force). These twitch parameters have the advantage of providing information about purely kinetic processes in s−1 [28].
Table 2.
Effects of H-89 and PKCβII Inhibitor Peptide I on twitch kinetics.
| TTP (ms) | dF/dtmax/F (s−1) | RT50 (ms) | RT90 (ms) | dF/dtmin/F (s−1) | |
|---|---|---|---|---|---|
| Non-failing Lopt | |||||
| (−) inhibitors | 183.2 ± 11.3 | 8.4 ± 0.6 | 119.6 ± 3.0 | 261.4 ± 10.7 | 5.6 ± 0.2 |
| (+) inhibitors | 176.6 ± 11.6 | 8.3 ± 0.5 | 121.4 ± 5.5 | 314.2 ± 25.0* | 5.6 ± 0.5 |
| Failing Lopt | |||||
| (−) inhibitors | 180.4 ± 18.0 | 8.8 ± 0.9 | 123.4 ± 7.0 | 294.8 ± 52.1 | 5.7 ± 0.2 |
| (+) inhibitors | 173.4 ± 13.6 | 9.3 ± 1.0 | 136.8 ± 9.6 | 355.2 ± 34.1 | 5.0 ± 0.6 |
| Non-failing L90 | |||||
| (−) inhibitors | 171.0 ± 13.6 | 8.9 ± 0.8 | 117.5 ± 3.7 | 251.8 ± 17.4 | 6.0 ± 0.1 |
| (+) inhibitors | 154.0 ± 9.6* | 11.6 ± 1.3 | 119.0 ± 6.0 | 359.8 ± 26.7* | 5.6 ± 0.6 |
| Failing L90 | |||||
| (−) inhibitors | 154.2 ± 12.3 | 10.4 ± 0.8 | 107.0 ± 3.0 | 301.2 ± 63.4 | 6.2 ± 0.2 |
| (+) inhibitors | 146.8 ± 12.0 | 10.6 ± 1.0 | 125.2 ± 9.0* | 418.9 ± 48.9* | 5.5 ± 0.6 |
All groups were stimulated at 1 Hz and 37 °C either in the absence (−) or presence (+) of inhibitors (20 μM H-89 and 7.5 nM PKCβII peptide inhibitor I).
indicates P< 0.05 vs. no inhibitors of the same length. n = 5 non-failing for Lopt, n = 4 non-failing for L90, n = 5 failing for both Lopt and L90.
Effects of Inhibitors on Cross-Bridge Cycling Kinetics
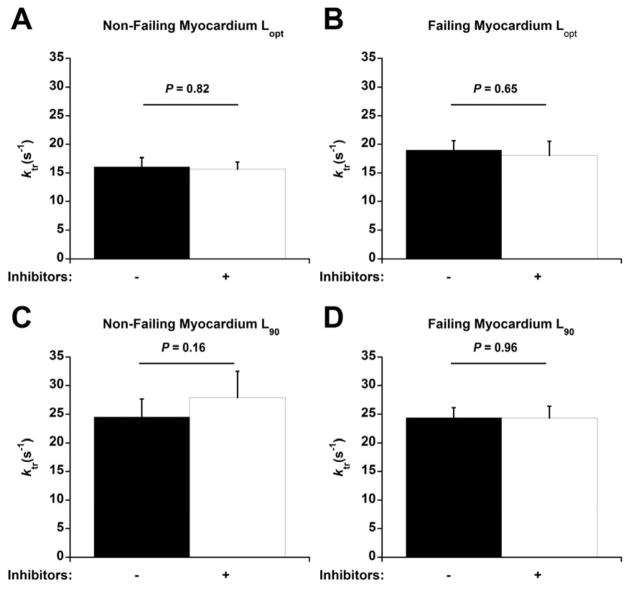
The effects of these inhibitors on cardiac contractile kinetics was further evaluated by measuring ktr, an index of cross-bridge cycling [7], during the maximal K+ contracture tensions (Figure 1). Treatment with inhibitors did not affect ktr,max at Lopt in either non-failing (Figure 1A) or failing muscles (Figure 1B) (P = 0.82 for non-failing, P = 0.65 for failing). Similarly, inhibitor treatment had no effect on ktr,max at L90 in either non-failing (Figure 1C) or failing muscles (Figure 1D) (P = 0.16 for non-failing, P = 0.96 for failing). There was no statistically significant difference between Fres:FK+ (ratio of residual tension following ktr maneuver to maximal tension obtained during contracture) within each group. The ratios of non-failing muscles at Lopt were 0.37 ± 0.07 without inhibitors and 0.23 ± 0.04 with inhibitors (P = 0.1), and at L90 were 0.03 ± 0.24 without inhibitors and 0.14 ± 0.16 with inhibitors (P = 0.72). The ratios for failing muscles at Lopt were 0.29 ± 0.07 without inhibitors and 0.25 ± 0.04 with inhibitors (P = 0.52), and at L90 were 0.19 ± 0.07 without inhibitors and 0.24 ± 0.08 with inhibitors (P = 0.68).
Figure 1.
H-89 and PKCβII Inhibitor Peptide I had no effect on ktr,max. The length at which muscles were stabilized and ktr experiments were performed is shown on the bottom. The presence of inhibitors is shown with – (no inhibitor) and + (20 μM H-89 and 7.5 nM PKCβII peptide inhibitor I). n = 5 non-failing and n = 5 failing.
The maximal K+ contracture tension at Lopt for the non-failing myocardium was 36.3 ± 4.8 mN/mm2 without inhibitors and 28.0 ± 3.5 mN/mm2 in the presence of inhibitors (P = 0.06). The Lopt contracture tension of the failing myocardium was 41.5 ± 5.3 mN/mm2 without inhibitors and 36.8 ± 5.8 mN/mm2 with inhibitors (P< 0.05). The L90 tensions was 16.7 ± 1.5 mN/mm2 without any inhibitors and 7.8 ± 0.9 mN/mm2 with inhibitors in non-failing myocardium (P< 0.05) and 27.0 ± 2.8 mN/mm2 without inhibitors and 19.2 ± 2.5 mN/mm2 with inhibitors in failing myocardium (P< 0.05).
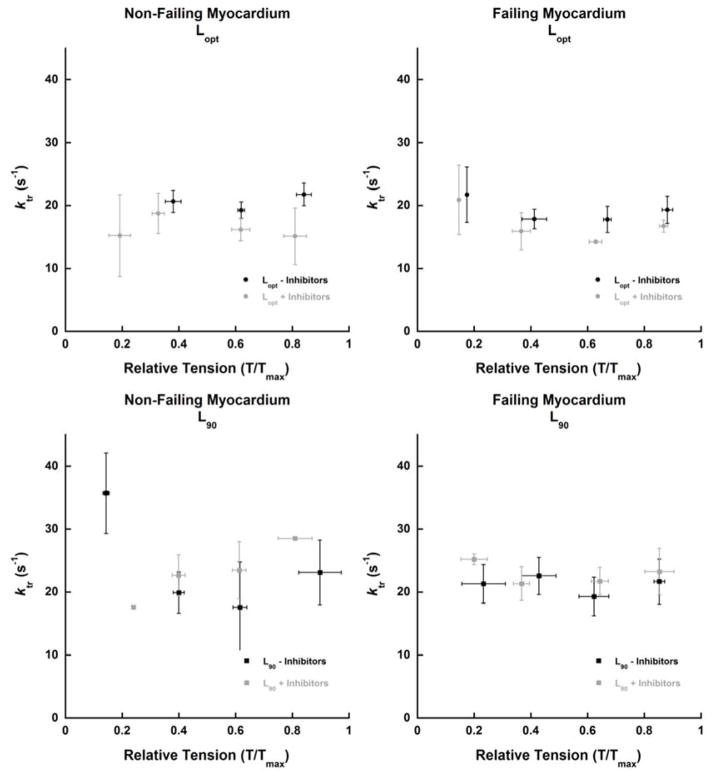
The effects of these inhibitors on ktr were also assessed at sub-maximal K+ contracture tensions (Figure 2). No significant differences could be appreciated at sub-maximal activation levels as well. It should be noted that there was a lot of variability in the relative tensions (where the ktr maneuver was performed) from experiment to experiment.
Figure 2.
Effect of H-89 and PKCβII Inhibitor Peptide I on ktr during sub-maximal K+ contracture tensions. n = 4 non-failing and n = 5 failing hearts (each point represents average of 1–4 hearts. Average of 1–3 ktrs was used for each heart).
Instantaneous Acceleration and Deceleration of Cross-Bridge Cycling Kinetics in Response to Muscle Length Change
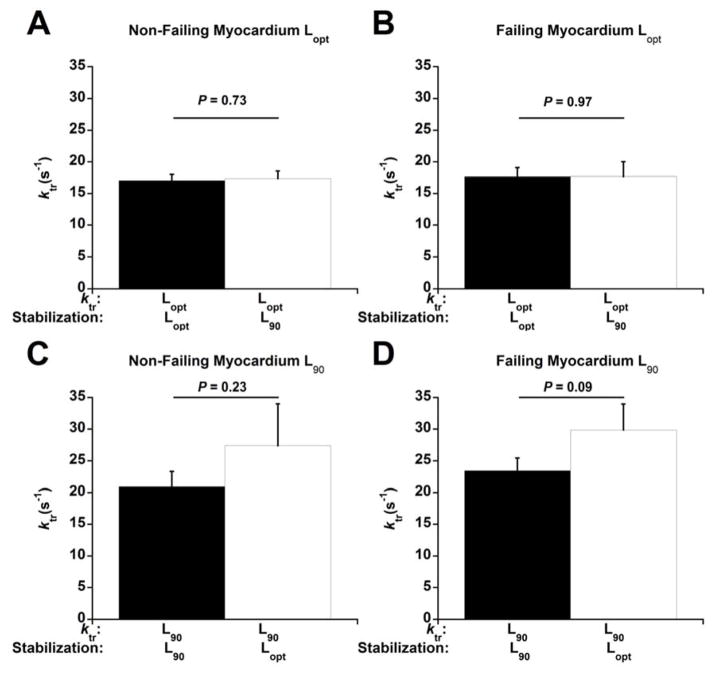
We next determined whether stabilization at a particular muscle length is essential for the effects of muscle length on cardiac contractile kinetics (Figure 3). In both non-failing (Figure 3A) and failing myocardium (Figure 3B), ktr,max at Lopt was very similar regardless of whether the muscles were stabilized at Lopt or L90 and then rapidly stretched to Lopt (P = 0.73 for non-failing, P = 0.97 for failing). Similarly, the ktr,max at L90 was similar whether the muscle was initially stabilized at L90 or Lopt and then rapidly slacked to L90 in both non-failing (Figure 3C) and failing myocardium (Figure 3D) (P = 0.23 for non-failing, P = 0.09 for failing). The Fres:FK+ were similar within each respective muscle length regardless of the length at which the muscles were stabilized. The Fres:FK+ of ktrs measured at Lopt were 0.29 ± 0.04 (stabilized at Lopt) and 0.32 ± 0.04 (stabilized at L90) for the non-failing muscles (P = 0.72), and 0.21 ± 0.08 (stabilized at Lopt) and 0.30 ± 0.07 (stabilized at L90) for the failing muscles (P = 0.19). The ratios of ktrs measured at L90 were 0.34 ± 0.03 (stabilized at L90) and 0.22 ± 0.06 (stabilized at Lopt) for the non-failing muscles (P = 0.1), and 0.19 ± 0.06 (stabilized at L90) and 0.07 ± 0.15 (stabilized at Lopt) for the failing muscles (P = 0.43).
Figure 3.
ktr after rapid length changes. The length at which muscles were stabilized and ktrs performed is shown on the bottom of each figure. All experiments were performed at 1 Hz and 37 °C. n = 6 non-failing for ktrs at both Lopt and L90. n = 7 failing for ktrs at Lopt and n = 8 failing for ktrs at L90.
The maximal developed tensions during the K+ contracture at Lopt of both non-failing and failing myocardium were not affected by the rapid length changes. The Lopt tensions for the non-failing myocardium were 37.3 ± 5.7 mN/mm2 (stabilized at Lopt) and 40.3 ± 6.5 mN/mm2 (stabilized at L90) (P = 0.22). The Lopt tensions for the failing myocardium were 41.0 ± 4.5 mN/mm2 (stabilized at Lopt) and 40.3 ± 3.9 mN/mm2 (stabilized at L90) (P = 0.87). Stabilizing muscles at Lopt and quickly slacking them to L90 during the contracture did, however, cause a decrease in the tensions as compared to contracture tensions when stabilized at L90. The L90 tensions for the non-failing myocardium was 20.4 ± 3.9 mN/mm2 when stabilized at L90 and 9.9 ± 2.3 mN/mm2 when stabilized at Lopt and rapidly slacked to L90 (P< 0.05). The L90 tensions for the failing myocardium was 24.8 ± 3.1 mN/mm2 when stabilized at L90 and 16.1 ± 14.2 mN/mm2 when stabilized at Lopt and rapidly slacked to L90 (P< 0.05).
Discussion
In this study we show that the regulation of cardiac cross-bridge cycling kinetics by muscle length is 1) not affected by inhibitors that target the pathway involved in the length-dependent phosphorylation of myofilament proteins, 2) stabilization at a particular muscle length is not essential for this length-dependent regulation. These results collectively suggest that the mechanism underlying length-dependent regulation of cross-bridge cycling kinetics is a rapid process and inherent to the myofilaments properties themselves.
Understanding the mechanisms that are involved in the length-dependent regulation of cross-bridge cycling kinetics is essential to further advance our understanding of cardiac physiology and pathology. One proposed mechanism is that at longer muscle lengths, titin exerts a straining effect on cMyBP-C which in turn through its interaction with MHC results in slowing of cross-bridge cycling kinetics [5, 10, 13]. We recently re-analyzed data from a previously published study on mice harboring truncated MyBP-C [29]. The data shows that dF/dtmax/F (maximal rate of force rise normalized to force) of mice with this truncated protein does not decelerate as muscles were stretched from 85% to 100% of optimal length (data not shown) [29]. A recent study also showed that ktr does not decrease as sarcomere length is increased from 1.9 um to 2.1 um in permeabilized cardiac preparations isolated from cMyBP-C deficient mice as opposed to their wild-type counterparts [11]. These studies have provided a plausible mechanism for the length-dependent regulation of cross-bridge cycling kinetics; however, the use of permeabilized muscle preparations is a limiting factor in these studies. The permeabilization process results in preparations that are physiologically “not living”, and typically necessitate the use of non-physiological low temperature, and loss of post-translational modification pathways in these preparations.
Another potential regulatory mechanism is that muscle length induces post-translational modifications of contractile proteins that result in alterations in cross-bridge cycling kinetics. Indeed, it has been shown in several studies that modifications such as phosphorylation are essential to regulation of cross-bridge cycling kinetics [14–21]. Furthermore, increasing muscle length is associated with regulation of phosphorylation of TnI at Ser23/24, MLC-2, and cMyBP-C [3, 23–25]. The use of the intact cardiac trabeculae in this study permitted assessing whether length-dependent post-translational modifications are to any extent involved in length-dependent regulation of cross-bridge cycling.
Our previous study delineated that PKA and PKCβII pathways are essential in length-induced phosphorylation of TnI and MLC-2 in rabbit myocardium [26]. Treatment of human cardiac trabeculae with these inhibitors in this study did not significantly affect ktr at either L90 or Lopt. In accordance with these results, the inhibitors did not significantly alter the twitch kinetics parameters, dF/dtmax/F and dF/dtmin/F which are pure kinetic measurements (force-independent, unlike dF/dt) of cardiac contraction and relaxation, respectively [28]. Several factors can contribute to the apparent discrepancy between the results here and the expectation that inhibition of PKA and PKCβII pathways, based on our previous study [26], should alter length-dependent regulation of contractile kinetics. The previous study was performed in rabbit myocardium [26] while the current study is in human myocardium. There are significant differences between human and rabbit myocardium which can affect expected outcomes as reviewed elsewhere [30]. We measured kinetics at Lopt and L90 muscle lengths in the current study in order to simulate the end-diastolic and end-systolic conditions in vivo [27]. However, in the previous study, the phosphorylation of myofilament proteins was compared at Lopt versus slacked length [26] which is far below the physiological equivalent of L90 used in the present study. Additionally, the capacity to perform multiple types of experiments in human hearts is limited by the number of available human hearts, time constraint to perform experiments within several hours after explantation, and inherent variability between human myocardial tissues. In the previous study on rabbit myocardium [26], we had the ability to perform experiments with H-89 and PKCβII inhibitor peptide separately. However, due to the mentioned constraints, the combined effects of these inhibitors were assessed in human myocardium in this current study. It is possible that the two inhibitors can, to some extent, counteract the effects of each other which could be investigated in future studies.
The lack of effect of inhibition of PKA and PKCβII on either twitch kinetics or ktr in human myocardium argues against a primary role of their downstream targets in the length-dependent regulation of cross-bridge cycling kinetics. Length-dependent changes in myofibril-relaxation properties occur within only a few milliseconds[31]. Conversely, it has been shown that the slow-phase response of Frank-Starling relationship takes minutes and is dependent on multiple mechanisms such as increase in calcium transient [32, 33]. Additionally, during this slow phase response, contractile proteins are also phosphorylated [24, 34]. Therefore, in our second approach, we measured ktr at L90 after it was rapidly slacked from Lopt and vice versa. The ktr was dependent on the length at which the ktr experiment was actually executed, and not on the length the muscle was stabilized. This provides insight that the regulation of cross-bridge cycling kinetics by muscle length is not a gradual process rather it is a near-instantaneous property that is inherent to the myofilaments. It would be expected that the cMyBP-C’s role in length-dependent regulation of cross-bridge kinetics as put forth by others [5, 10, 11, 13] is also a near instantaneous process. While previous studies have utilized myocardium of animal models, to our knowledge this is the first study that has shown this process in human myocardium.
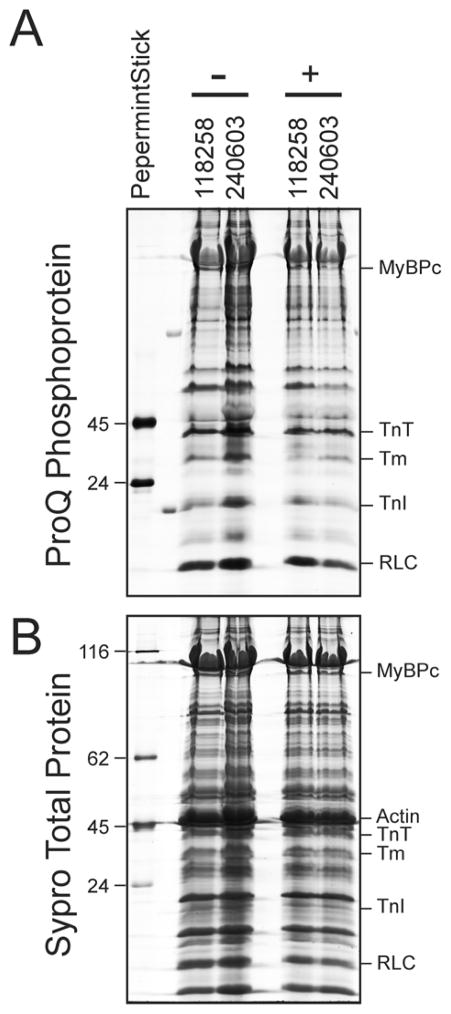
One of the limitations of this current study is that we were not able to comprehensively evaluate the phosphorylation of myofilament proteins in response to length changes and inhibitors in the current study. Unlike in inbred animals, there is very substantial variability between human hearts due to multiple factors such as co-morbid disorders, life-style behaviors, medications, and genetics [35]. Therefore, the phosphorylation status in response to inhibitors needs to be compared within the same hearts. Both the quantity of available human hearts and the number of free running thin cardiac trabeculae that can be excised from each heart is limited. Since from each heart at least 4 muscles would have to undergo the protocol (2 different lengths, each with and without inhibitor), this was logistically not possible for the majority of hearts studied. In a subset of 2 non-failing hearts, we had (for each heart) a muscle frozen, stretched to optimal length in presence of inhibitors, and a second muscle stretched to optimal length without inhibitors (Figure 4). Although n=2 is insufficient for a per-protein statistical analysis, it clearly illustrates that for future studies it is possible to study specific myofilament protein phosphorylation in these small samples, and that the kinase inhibitors depress or block the increase of protein phosphorylation upon lengthening of the muscle, since the overall muscle protein phosphorylation is ~20% lower when stretch takes place in presence of the inhibitors. Although we can not provide a detailed biochemical analysis at different muscle lengths with sufficient power, the outcome of the rapid length change experiments however showed that the impact of the length change on the ktr was quantitatively nearly identical to the long-time length impact, suggesting that even if post-translational impact of length would be present, it would represent merely a negligible quantitative impact.
Figure 4.
Protein phosphorylation of representative single trabeculae collected from individual experiments without (−) or with (+) of kinase inhibitor treatment was determined by ProQ Diamond staining of Laemmli gels (A) followed by Sypro Ruby staining for total protein determination (B). Quantification of the trabeculae total phosphate signal in the lane/the total protein signal in the lane demonstrates that trabeculae stretched in presence of kinase inhibitors had overall decreased protein phosphorylation.
It should be noted that in some experiments, rapid muscle length changes and/or inhibitors resulted in changes in tensions in respect to their control counterparts. It has been previously shown that this phenomenon is mainly, or even exclusively, due to muscle run-down of the preparation due to alterations in sarcoplasmic-reticulum calcium content [35] while the measured ktr remains stable throughout the course of the several experiments [7, 12, 35]. These previous studies suggest that the run-down that was observed in some of our experiments is not primarily due to the myofilament proteins and has minimal or no effect on our ktr measurements.
Conclusions
Overall, our results show that the length-dependent mechanism of cardiac cross-bridge cycling kinetics is likely due to the properties within the myofilaments rather than cellular signaling cascades that are induced by a change in muscle length.
Acknowledgments
We would like to thank the Cardiothoracic Surgery Department of The Ohio State University Wexner Medical Center and LifeLine of Ohio Organ Procurement for providing the human hearts. This study was financially supported by NIH R01HL113084 to PMLJ, American Heart Association Great Rivers Affiliate Pre-doctoral Fellowship 1148008 to NM, and The Ohio State University Graduate School Fellowship to JHC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen DG, Kentish JC. J Mol Cell Cardiol. 1985;17:821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- 2.Monasky MM, Varian KD, Davis JP, Janssen PM. Pflugers Archiv : European journal of physiology. 2008;456:267–276. doi: 10.1007/s00424-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 3.Monasky MM, Biesiadecki BJ, Janssen PM. J Mol Cell Cardiol. 2010;48:1023–1028. doi: 10.1016/j.yjmcc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maughan DW. Heart Fail Rev. 2005;10:175–185. doi: 10.1007/s10741-005-5248-2. [DOI] [PubMed] [Google Scholar]
- 5.Hanft LM, Korte FS, McDonald KS. Cardiovasc Res. 2008;77:627–636. doi: 10.1093/cvr/cvm099. [DOI] [PubMed] [Google Scholar]
- 6.McDonald KS. Pflugers Arch. 2011;462:61–67. doi: 10.1007/s00424-011-0949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milani-Nejad N, Xu Y, Davis JP, Campbell KS, Janssen PM. J Gen Physiol. 2013;141:133–139. doi: 10.1085/jgp.201210894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adhikari BB, Regnier M, Rivera AJ, Kreutziger KL, Martyn DA. Biophys J. 2004;87:1784–1794. doi: 10.1529/biophysj.103.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stelzer JE, Moss RL. J Gen Physiol. 2006;128:461–471. doi: 10.1085/jgp.200609634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korte FS, McDonald KS. J Physiol. 2007;581:725–739. doi: 10.1113/jphysiol.2007.128199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mamidi R, Gresham KS, Stelzer JE. Front Physiol. 2014;5:461. doi: 10.3389/fphys.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milani-Nejad N, Canan BD, Elnakish MT, Davis JP, Chung JH, Fedorov V, Binkley PF, Higgins RS, Kilic A, Mohler PJ, Janssen PM. Am J Physiol Heart Circ Physiol. 2015 doi: 10.1152/ajpheart.00685.2015. ajpheart 00685 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanft LM, Greaser ML, McDonald KS. Arch Biochem Biophys. 2013 doi: 10.1016/j.abb.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biesiadecki BJ, Kobayashi T, Walker JS, Solaro RJ, de Tombe PP. Circ Res. 2007;100:1486–1493. doi: 10.1161/01.RES.0000267744.92677.7f. [DOI] [PubMed] [Google Scholar]
- 15.Turnbull L, Hoh JF, Ludowyke RI, Rossmanith GH. J Physiol. 2002;542:911–920. doi: 10.1113/jphysiol.2002.022707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ. Circ Res. 2001;88:1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- 17.Scruggs SB, Solaro RJ. Arch Biochem Biophys. 2011;510:129–134. doi: 10.1016/j.abb.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stelzer JE, Patel JR, Moss RL. J Gen Physiol. 2006;128:261–272. doi: 10.1085/jgp.200609547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morano I. Basic Res Cardiol. 1992;87(Suppl 1):129–141. doi: 10.1007/978-3-642-72474-9_11. [DOI] [PubMed] [Google Scholar]
- 20.Toepfer C, Caorsi V, Kampourakis T, Sikkel MB, West TG, Leung MC, Al-Saud SA, MacLeod KT, Lyon AR, Marston SB, Sellers JR, Ferenczi MA. J Biol Chem. 2013;288:13446–13454. doi: 10.1074/jbc.M113.455444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colson BA, Locher MR, Bekyarova T, Patel JR, Fitzsimons DP, Irving TC, Moss RL. J Physiol. 2010;588:981–993. doi: 10.1113/jphysiol.2009.183897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stelzer JE, Patel JR, Moss RL. Circ Res. 2006;99:884–890. doi: 10.1161/01.RES.0000245191.34690.66. [DOI] [PubMed] [Google Scholar]
- 23.Hidalgo C, Wu Y, Peng J, Siems WF, Campbell KB, Granzier H. Arch Biochem Biophys. 2006;456:216–223. doi: 10.1016/j.abb.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Kockskamper J, von Lewinski D, Khafaga M, Elgner A, Grimm M, Eschenhagen T, Gottlieb PA, Sachs F, Pieske B. Prog Biophys Mol Biol. 2008;97:250–267. doi: 10.1016/j.pbiomolbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ait Mou Y, le Guennec JY, Mosca E, de Tombe PP, Cazorla O. Pflugers Arch. 2008;457:25–36. doi: 10.1007/s00424-008-0501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monasky MM, Taglieri DM, Jacobson AK, Haizlip KM, Solaro RJ, Janssen PM. Arch Biochem Biophys. 2013;535:22–29. doi: 10.1016/j.abb.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez EK, Hunter WC, Royce MJ, Leppo MK, Douglas AS, Weisman HF. Am J Physiol Heart Circ Physiol. 1992;263:H293–306. doi: 10.1152/ajpheart.1992.263.1.H293. [DOI] [PubMed] [Google Scholar]
- 28.Janssen PM. Am J Physiol Heart Circ Physiol. 2010;299:H1092–1099. doi: 10.1152/ajpheart.00417.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer BM, Georgakopoulos D, Janssen PM, Wang Y, Alpert NR, Belardi DF, Harris SP, Moss RL, Burgon PG, Seidman CE, Seidman JG, Maughan DW, Kass DA. Circ Res. 2004;94:1249–1255. doi: 10.1161/01.RES.0000126898.95550.31. [DOI] [PubMed] [Google Scholar]
- 30.Milani-Nejad N, Janssen PM. Pharmacol Ther. 2014;141:235–249. doi: 10.1016/j.pharmthera.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mateja RD, de Tombe PP. Biophys J. 2012;103:L13–15. doi: 10.1016/j.bpj.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cingolani HE, Perez NG, Cingolani OH, Ennis IL. Am J Physiol Heart Circ Physiol. 2013;304:H175–182. doi: 10.1152/ajpheart.00508.2012. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez BV, Perez NG, Ennis IL, Camilion de Hurtado MC, Cingolani HE. Circ Res. 1999;85:716–722. doi: 10.1161/01.res.85.8.716. [DOI] [PubMed] [Google Scholar]
- 34.Kockskamper J, Khafaga M, Grimm M, Elgner A, Walther S, Kockskamper A, von Lewinski D, Post H, Grossmann M, Dorge H, Gottlieb PA, Sachs F, Eschenhagen T, Schondube FA, Pieske B. Cardiovasc Res. 2008;79:642–651. doi: 10.1093/cvr/cvn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milani-Nejad N, Brunello L, Gyorke S, Janssen PM. J Muscle Res Cell Motil. 2014;35:225–234. doi: 10.1007/s10974-014-9386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]