Abstract
How do people get attention to operate at peak efficiency in high-pressure situations? We tested the hypothesis that the general mechanism that allows this is the maintenance of multiple target representations in working and long-term memory. We recorded subjects’ event-related potentials (ERPs) indexing the working memory and long-term memory representations used to control attention while performing visual search. We found that subjects used both types of memories to control attention when they performed the visual search task with a large reward at stake, or when they were cued to respond as fast as possible. However, under normal circumstances, one type of target memory was sufficient for slower task performance. The use of multiple types of memory representations appears to provide converging top-down control of attention, allowing people to step on the attentional accelerator in a variety of high-pressure situations.
Keywords: attention, cognitive neuroscience, evoked potentials, visual search, visual memory, open materials
How do people get attention to operate at peak efficiency in high-pressure situations? In typical laboratory tasks, the stakes for subjects are pretty low. However, when people make important sales calls or scan the field for teammates in the league championship game, they need to be able to configure attention to operate as efficiently as possible. Indeed, recent work has shown that attention can operate more efficiently in these kinds of high-pressure situations (e.g., Chelazzi, Perlato, Santandrea, & Della Libera, 2013; Lee & Shomstein, 2014). The goal of this study was to show how the human mind makes this possible.
Theories of visual attention propose that memory representations control attention. Several theories are built on the idea that visual working memory representations determine which object features will be attended (e.g., Desimone & Duncan, 1995; Olivers, Peters, Houtkamp, & Roelfsema, 2011; Soto, Hodsoll, Rotshtein, & Humphreys, 2008). However, long-term memory representations can also work to control how attention is deployed (Hutchinson & Turk-Browne, 2012; Moores, Laiti, & Chelazzi, 2003; Summerfield, Lepsien, Gitelman, Mesulam, & Nobre, 2006; Woodman, Carlisle, & Reinhart, 2013). If both working memory and long-term memory representations can control attention, what happens when these types of memory representations are simultaneously used to bias attention to the same visual inputs?
Our goal was to test the hypothesis that having working memory and long-term memory representations providing converging top-down control over visual attention is the general mechanism for getting attentional selection to operate at peak efficiency in high-pressure situations. The hypothesis that the brain uses converging memory representations to improve the rate of attentional selection was motivated by work suggesting that subjects respond to reward by recruiting both working and long-term memory systems to process task-relevant information (Reinhart & Woodman, 2014a). To determine whether memory representations were being used, we measured event-related potential (ERP) components. As we describe later, by measuring distinct ERP components indexing working memory and long-term memory, we were able to examine what was being stored in visual working memory and long-term memory while subjects performed a cued visual search task in which the identity of the target changed every seven trials. We interspersed high-pressure trials by informing subjects that extra reward was on the line on particular trials (in Experiment 1) or that certain trials were to be performed as quickly as possible (in Experiment 2). If converging memory representations underlie improved attentional selection in a variety of situations, then the same pattern of ERP effects should be evident in these different high-pressure situations.
We focused our analyses on the waveforms elicited by the cue that identified the search target on each trial. Specifically, we used the contralateral delay activity (CDA) to index the maintenance of the target in visual working memory and the anterior P1 (also known as the P170) to index the storage of the target in long-term memory. The CDA is a lateralized, posterior negativity that increases in amplitude as the amount of information stored in visual working memory increases, and its amplitude plateaus once the capacity of visual working memory is reached (Vogel & Machizawa, 2004; Vogel, McCollough, & Machizawa, 2005). The anterior P1 is a frontal positivity that becomes more negative as traces are stored in long-term memory (Voss, Schendan, & Paller, 2010; Woodman et al., 2013), or as people attempt to retrieve information from long-term memory (Diana, Vilberg, & Reder, 2005; Duarte, Ranganath, Winward, Hayward, & Knight, 2004). Because the timing and scalp distributions of these two ERP components do not overlap, we could simultaneously measure these indices of different types of memory storage to test the hypothesis that they work together to control attention in high-pressure situations.
Consistent with theories of learning and automaticity proposing that long-term memory takes over the control of attention from working memory (Logan, 2002; Rickard, 1997), studies have shown that the CDA decreases in amplitude and the anterior P1 becomes more negative across trials of searching for the same object (Carlisle, Arita, Pardo, & Woodman, 2011; Reinhart & Woodman, 2015; Woodman et al., 2013). In the present experiments, subjects searched for a given target object across a run of trials. We expected that they would therefore come to rely on long-term memory after a handful of trials in a run. Our question was what would happen after that handful of trials when subjects had come to rely on long-term memory and needed to step on the attentional “gas pedal” in searching for a new target. Would the CDA return as working memory was brought back on-line to supplement the attentional control provided by long-term memory?
General Method
Subjects
A different group of 20 subjects (18–35 years of age, 45% women) with normal color vision, normal or corrected-to-normal visual acuity, and no reported history of neurological problems volunteered for each experiment. All subjects gave informed consent to procedures approved by the Vanderbilt University Institutional Review Board prior to their participation.
Our power calculations were based on a pilot experiment in which we simultaneously measured the CDA and anterior P1 during visual search in which extra reward was possible on a subset of the trials (n = 10). Conservatively pooling mean differences and standard deviations across the behavioral responses and across the electrophysiological responses, we estimated Cohen’s ds using paired-samples two-tailed t tests (reaction time, or RT: d = 0.89; CDA: d = 0.75; anterior P1: d = 0.34). We found that a sample size of 20 subjects would be sufficient to detect effects of the same magnitude with 80% power at the .05 significance level.
Stimuli
Stimuli were viewed on a gray background (54.3 cd/m2) from a distance of 114 cm. A black fixation cross (< 0.01 cd/m2, 0.4° × 0.4° of visual angle) was visible throughout each trial. In Experiment 1, the first display in a trial was a blue (x = 0.140, y = 0.720, 6.41 cd/m2), yellow (x = 0.408, y = 0.505, 54.1 cd/m2), or magenta (x = 0.289, y = 0.151, 42 cd/m2) circle around the fixation point (all chromaticity coordinates are in the CIE 1931 color space). In Experiment 2, trials started with either a blue or a yellow circle. The colored circles indicated the reward value (Experiment 1) or deadline (Experiment 2) for the trial (see Procedure).
The target-cue stimuli and search stimuli were Landolt Cs (diameter: 0.88°; thickness: 0.13°; gap width: 0.22°) with eight possible gap orientations (0°, 22.5°, 45°, 67.5°, 90°, 112.5°, 135°, 157.5°). Target-cue stimuli were presented 2.2° to the left and right of the center of the monitor. Each target-cue array contained one red item (x = 0.612, y = 0.333, 15.1 cd/m2) and one green item (x = 0.281, y = 0.593, 45.3 cd/m2). These colors indicated which stimulus identified the target and which was irrelevant to the task. Search stimuli were arranged similarly to the number locations on a clock face, 4.4° from the center of the monitor. Each search array contained 1 red, 1 green, and 10 black (distractor; < 0.01 cd/m2) items. The task-relevant color (i.e., red or green) was counterbalanced across subjects, and the gap orientation of the target Landolt C varied across trials within subjects, to rule out physical-stimulus confounds that would make the lateralized CDA in response to the task-relevant cue difficult to interpret (Woodman, 2010).
Procedure
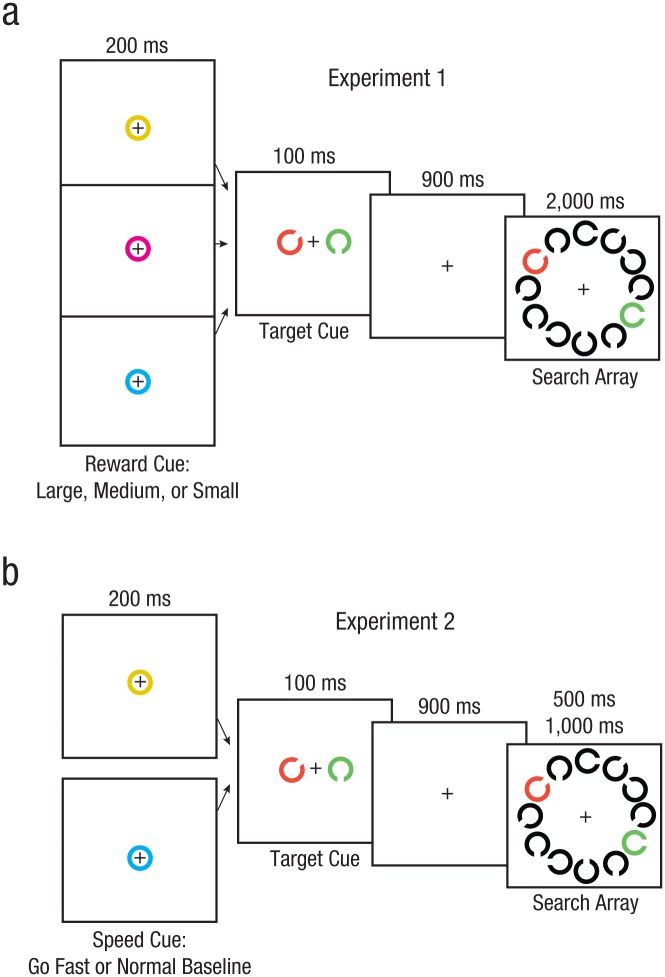
Figure 1 illustrates the trial sequence for both experiments. Each trial began with the presentation of the fixation point for 1,200 to 1,600 ms (duration randomly drawn from a rectangular distribution of times). Next, a central cue stimulus, a colored circle, was presented for 200 ms. The color of the circle indicated the type of trial: large-, medium-, or small-reward trial in Experiment 1 (Fig. 1a), and fast or normal trial in Experiment 2 (Fig. 1b). Then, the target-cue stimuli were presented for 100 ms, followed by a 900-ms interval. The search array appeared next for 2,000 ms (all trials in Experiment 1), 1,000 ms (normal baseline trials in Experiment 2), or 500 ms (fast trials in Experiment 2). The intertrial interval was 1,200 to 1,600 ms (duration randomly drawn from a rectangular distribution). A target (Landolt C matching the orientation and color of the task-relevant cue) was present on half of the trials and was absent on the other half (i.e., the item matching the cue color was of a different orientation than the cue). Subjects responded as quickly and accurately as possible to each search array using a handheld game pad (Logitech Precision Gamepad, Logitech, Newark, CA). They pressed one button when the target was present and a different button when the target was absent, using different fingers on their right hand.
Fig. 1.
Illustration of the task and stimuli used in Experiments 1 and 2. Each trial of Experiment 1 began with a cue indicating the reward level for that trial and then a cue identifying the target (a). After a blank screen, the search array was presented for 2,000 ms. A given target object was cued and searched for across seven trials, and then a new target was selected. The large- and medium-reward cues could appear anywhere across the target repetitions within a run, but were mostly clustered at Target Repetition 5. The trials and procedure of Experiment 2 were similar (b), except that the first cue indicated whether the trial was to be performed with a balance of speed and accuracy (normal baseline) or as fast as possible (go fast).
The cued target orientation, whether the target was present or absent, and the target location (on target-present trials) were all randomly selected on each trial, with the exception that a given target orientation was cued across a run of 7 trials in a row. Each subject completed 1,260 trials (prior to rejection of trials with artifacts).
In both experiments, the meaning of the initial color cues was counterbalanced across subjects. In Experiment 1, these cues indicated whether subjects could earn 50 points (high reward), 25 points (medium reward), or 1 point (small reward) for the correct answer. The majority of trials were cued for small reward. Subjects were informed that the number of points they accumulated would translate to bonus money added to their hourly compensation of $10 (50 points = 5¢, 25 points = 2.5¢, 1 point = 1¢). The average reward bonus earned was approximately $15, so subjects received a total compensation of approximately $45 for the 3-hr experiment. In Experiment 2, the colored circles indicated whether the trial was to be performed fast (500-ms deadline) or normally (i.e., 1,000-ms deadline, so that speed and accuracy should be balanced). The majority of trials were cued for normal speed.
As in recent studies (Reinhart, McClenahan, & Woodman, 2015; Reinhart & Woodman, 2014a), in most runs, subjects searched for a given object with the small level of reward or normal speed stress for the first four trials. In Experiment 1, the reward level than changed to large reward on 33% of the fifth trials and to medium reward on 33% of the fifth trials; on the other 33% of the fifth trials, the reward level remained low. In Experiment 2, the fifth trial was preceded by a fast-speed cue in 50% of the runs and by a normal-speed cue in the remaining runs. So that subjects would be prepared for changes in reward and speed cues within runs, we had large-reward and fast-speed cues distributed at the other six serial positions (i.e., Target Repetitions 1–4, 6, and 7) in 5% of the runs. We focused on data from the critical fifth target repetitions, for which we had the most power to determine whether the averaged ERPs indicated that the high-pressure cues of reward or speed stress triggered subjects to use both working memory and long-term memory target representations to control attention.
Electrophysiology
The electroencephalogram (EEG) was recorded from 21 tin electrodes (250-Hz sampling rate, 0.01- to 100-Hz bandpass filter) and amplified with a gain of 20,000 (SA Instruments, San Diego, CA). The electrodes were embedded in an elastic cap (Electro-Cap International, Eaton, OH). Recordings were taken at three midline sites (Fz, Cz, Pz), seven pairs of lateral sites (F3/F4, C3/C4, P3/P4, PO3/PO4, T3/T4, T5/T6, O1/O2), and two nonstandard sites (OL, halfway between O1 and T5; OR, halfway between O2 and T6), in an array based on the International 10/20 System. The right mastoid served as the on-line reference, and signals were rereferenced off-line to the average of the left and the right mastoids (Nunez & Srinivasan, 2006).
The electrooculogram (EOG) was recorded using bipolar electrodes placed 1 cm lateral to the external canthi (to measure horizontal eye movements) and bipolar electrodes above and below the left eye (to measure vertical eye movements and blinks). Trials with incorrect behavioral responses or ocular or myogenic artifacts were excluded. A two-step ocular-artifact rejection method was implemented (Woodman & Luck, 2003). Two subjects from Experiment 1 and 2 subjects from Experiment 2 were removed for excessive eye movements (a subject was removed for having either > 25% of individual trials rejected or residual systematic eye movement in the grand-average waveforms that resulted in horizontal EOG voltage deflections > 3.2 µV, which corresponded to an ocular deviation of ±0.1°). Grand-average waveforms were low-pass-filtered at 35 Hz for the graphs in this article, but all analyses were performed on unfiltered voltages.
Analysis
The CDA, our index of visual working memory, was measured across the lateral parietal, occipital, and temporal electrodes (PO3/PO4, O1/O2, OL/OR, and T5/T6) as the mean difference in amplitude between the ipsilateral and contralateral waveforms (with respect to the target cue) from 300 through 1,000 ms after the onset of the target cue, corrected to the baseline from 200 to 0 ms prior to that cue’s onset (Carlisle et al., 2011; Reinhart & Woodman, 2014a, 2014b; Vogel & Machizawa, 2004; Vogel et al., 2005; Woodman et al., 2013; Woodman & Vogel, 2008). The anterior P1 amplitude was measured at the fronto-central electrode site (Fz) from 170 through 250 ms following the onset of the target cue (Reinhart & Woodman, 2014a; Voss et al., 2010; Woodman et al., 2013). In addition, we replicated all anterior P1 results in Experiments 1 and 2 using a broader time window (150–300 ms) as well as a narrower time window (170–-200 ms).
RTs and ERP amplitudes in the search task were examined with planned comparisons (two-tailed t tests) of the trials with different reward levels (large vs. medium vs. small; Experiment 1) and the trials with different speed stress (fast vs. normal speed; Experiment 2). These analyses included only the critical Target Repetition 5 trials. We verified that the same conclusions would be drawn when the RT data and ERP amplitudes were analyzed using analyses of variance (ANOVAs) with the factor of condition (reward level in Experiment 1; speed stress in Experiment 2). Target-repetition effects were assessed by binning data according to the number of trials that had occurred since a change in target identity (i.e., 1–2, 3–4, or 5–7), as in previous work (Carlisle et al., 2011; Reinhart & Woodman, 2014a, 2014b; Woodman et al., 2013).
Experiment 1
In Experiment 1, we manipulated the pressure that subjects experienced by using cues that indicated the reward value of each trial. Our logic was that if subjects could step on the attentional gas pedal to perform visual search more quickly, then they would do this more on large-reward trials than on the medium-reward trials; the small-reward trials served as our baseline condition. We expected increased reward value to speed subjects’ behavioral response to targets in the cluttered arrays of objects, but how would this be possible? If the mechanism for increasing the efficiency of search is the simultaneous use of working memory and long-term memory to push attention to targetlike objects, then the CDA and anterior P1 data would indicate that both working memory and long-term memory representations of the target were more active on the large-reward trials relative to the small-reward trials, and these effects would be reduced on the medium-reward trials.
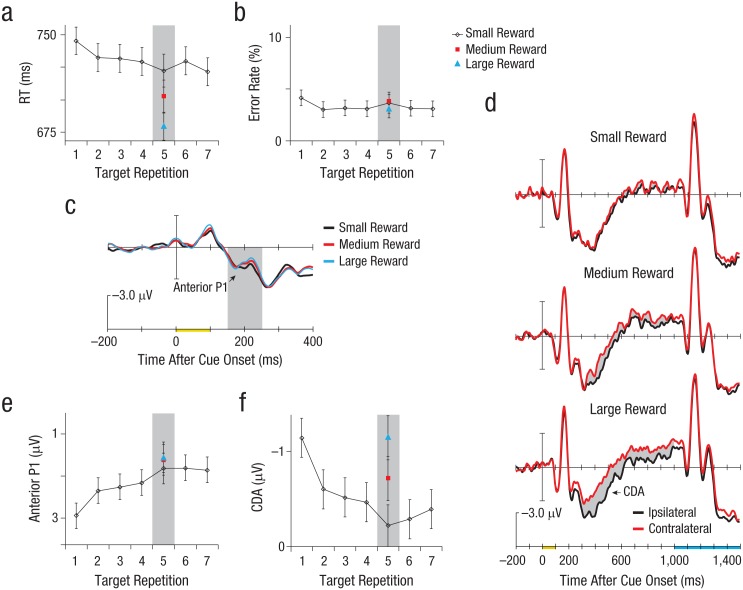
Figure 2a shows that RTs got faster across target repetitions on the small-reward trials, F(2, 38) = 3.275, d = 0.59, p = .049. However, the important observation is that a cue indicating that additional reward could be earned resulted in faster RTs. Search was significantly faster on Target Repetition 5 following a medium-reward cue (25 points) than following a small-reward cue (1 point), t(19) = 2.119, d = 0.53, p = .048, and search was even faster following a large-reward cue (50 points) than following a medium-reward cue, t(19) = 2.322, d = 0.61, p = .032 (Fig. 2a). These RT effects were not due to a speed-accuracy trade-off across conditions, as accuracy was universally high (M = 96.6% correct; see Fig. 2b for the error rates) and did not differ across different reward levels or target repetitions (ts < 0.84, ps > .41).
Fig. 2.
Results from Experiment 1. The graphs in (a) and (b) show reaction time (RT) and error rate in the small-reward condition as a function of target repetition, along with mean RT and error rate on Target Repetition 5 in the other two reward conditions. The grand-average waveforms in (c) are from the fronto-central electrode and show the anterior P1 amplitude (within the shaded area) on the critical Target Repetition 5 trials in each reward condition. The grand-average waveforms in (d) are from the lateral parietal, occipital, and temporal electrodes on Target Repetition 5 trials. The contralateral delay activity (CDA) is the difference (indicated by the gray shading) between waveforms from sites contralateral and ipsilateral to the target cue; separate waveforms are shown for each reward condition. The x-axes in (c) and (d) show the timing of stimulus presentation relative to the waveforms, with presentation of the target cue marked in yellow, from 0 to 100 ms, and presentation of the search array marked in blue. The graphs in (e) and (f) show the mean amplitudes of the anterior P1 and CDA as a function of target repetition in the small-reward condition, along with their mean amplitudes on Target Repetition 5 in the other two reward conditions. In (a), (b), (e), and (f), error bars represent ±1 SEM, and the shaded areas highlight results for Target Repetition 5.
Figures 2c and 2e show results for the anterior P1 component. The anterior P1 became progressively more negative as subjects searched for the same target across a run of small-reward trials, F(2, 38) = 3.675, d = 0.62, p = .035. This is consistent with the idea that each trial of search laid down a long-term memory trace that was used to help guide attention to the target item, and that the cumulative effect of these traces was to speed RT with each additional target repetition. We found that the different reward cues had no significant effect on the amplitude of the anterior P1 (ts < 0.35, ps > .73), which suggests that the strength of the trace laid down in long-term memory was not modulated by the reward value of the trial.
Figure 2f shows that the CDA decreased in amplitude as subjects searched for the same target across a run of trials, and a one-way ANOVA revealed a significant effect of target repetition across small-reward trials, F(2, 38) = 7.607, d = 0.89, p = .002. This is consistent with theories of learning and automaticity proposing that the role of working memory decreases as people perform the same task trial after trial (Logan, 2002). The key result is what happened on the Target Repetition 5 trials following high- and medium-reward cues relative to those following the small-reward cue. As illustrated in Figure 2d, the amplitude of the CDA elicited by the target-cue stimulus was significantly larger after medium-reward cues than after small-reward cues, t(19) = 2.140, d = 0.48, p = .046, and the CDA was even larger following large-reward than medium-reward cues, t(19) = 2.323, d = 0.50, p = .031, such that it returned to full amplitude following large-reward cues.
In summary, our index of working memory demonstrated a parametric effect of the reward value of the trials, which resulted in faster search milliseconds later. This is notable because after a handful of trials, long-term memory appeared to be largely in control of attention; the mean CDA amplitude approached zero on small-reward trials by the fifth target repetition (see Fig. 2f). These findings are consistent with the idea that subjects responded to the reward cues by bringing working memory representations of the target back on-line to complement the attentional control provided by the long-term memory representations that had accumulated across trials of searching for the same target object.
It is appealing to conclude that the medium-reward cue resulted in subjects pushing the attentional gas pedal down halfway, relative to the pressure they exerted on large-reward trials. This could have been due to subjects using working memory representations to supplement the attentional control provided by long-term memory on about half of the trials overall, and on all of the large-reward trials. However, this could also have been due to the use of a graded working memory representation worth half an object on the medium-reward trials, if one assumes a resource model of working memory in which half a target representation is possible (Luck & Vogel, 2013). What is clear is that the high-pressure trials in which greater reward was at stake resulted in the subjects using working memory representations of the target to supplement the attentional control provided by long-term memory.
Experiment 2
In Experiment 1, we showed that subjects respond to reward cues by bringing working memory representations of the targets back on-line to supplement the attentional control provided by long-term memory. But how general is this effect? If the use of multiple memory representations is a general mechanism that people use to speed attentional selection of task-relevant information, then the same effect should emerge in other high-pressure situations. In Experiment 2, we kept the search task the same, but simply cued people to respond quickly on a subset of trials by enforcing a 500-ms response deadline (see Fig. 1b). We call these go-fast trials. The rest of the trials were preceded by a cue indicating that the response deadline was 1,000 ms and subjects should balance speed and accuracy. We call these normal baseline trials.
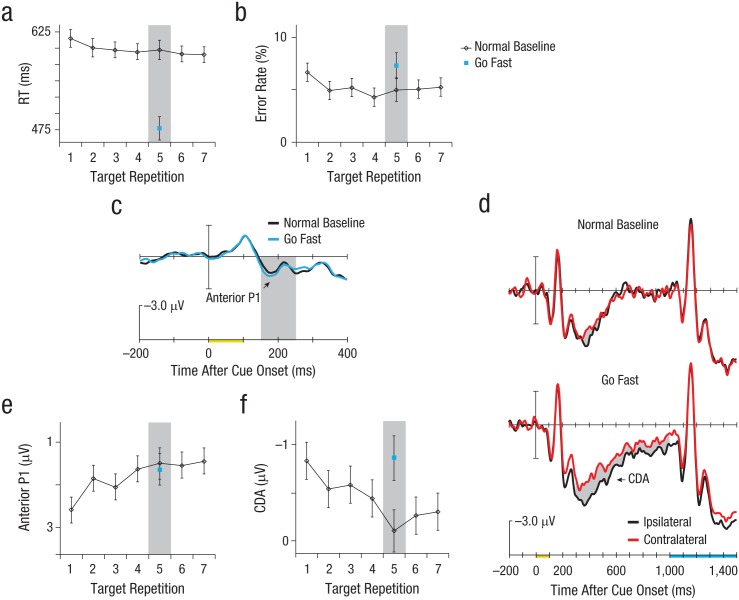
Figure 3a summarizes the RT results. The RTs got faster across target repetitions of the normal baseline trials, F(2, 38) = 4.219, d = 0.67, p = .040. Critically, subjects were faster on Target Repetition 5 following the go-fast cue, t(19) = 7.402, d = 1.49, p < .01, at the cost of a slight reduction in accuracy (see Fig. 3b for error rates), t(19) = 2.114, d = 0.62, p = .048.
Fig. 3.
Results from Experiment 2. The graphs in (a) and (b) show reaction time (RT) and error rate in the normal baseline condition as a function of target repetition, along with mean RT and error rate on Target Repetition 5 in the go-fast condition. The grand-average waveforms in (c) are from the fronto-central electrode and show the anterior P1 amplitude (within the shaded area) on the critical Target Repetition 5 trials in each speed condition. The grand-average waveforms in (d) are from the lateral parietal, occipital, and temporal electrodes on Target Repetition 5 trials. The contralateral delay activity (CDA) is the difference (indicated by the gray shading) between waveforms from sites contralateral and ipsilateral to the target cue; separate waveforms are shown for each speed condition. The x-axes in (c) and (d) show the timing of stimulus presentation relative to the waveforms, with presentation of the target cue marked in yellow, from 0 to 100 ms, and presentation of the search array marked in blue. The graphs in (e) and (f) show the mean amplitudes of the anterior P1 and CDA as a function of target repetition in the normal baseline condition, along with their mean amplitudes on Target Repetition 5 in the go-fast condition. In (a), (b), (e), and (f), error bars represent ±1 SEM, and the shaded areas highlight results for Target Repetition 5.
Figure 3e shows that the anterior P1 became progressively more negative across target repetitions in the normal baseline trials. The effect of target repetition on mean amplitude was significant, F(2, 38) = 4.865, d = 0.71, p = .014. As illustrated in Figure 3c, there was no significant effect of the type of cue (i.e., go-fast vs. normal baseline cue) on this index of long-term memory, t(19) = 0.279, d = 0.12, p = .783. These findings demonstrate the same pattern of long-term memory ERPs as we found in Experiment 1, with a different kind of pressure (speed, rather than large reward). We verified the similarity of these observations with a 2 (experiment: Experiment 1 vs. Experiment 2) × 2 (cue type: go-fast or large-reward cue vs. baseline cue) × 3 (target repetition: 1–2, 3–4, 5–7) ANOVA. This analysis yielded an effect of experiment, F(1, 19) = 7.269, d = 0.87, p = .014; mean anterior P1 amplitude was larger in Experiment 1 than in Experiment 2. However, experiment did not interact with target repetition, F(2, 38) = 0.022, d = 0.15, p = .970, or cue type, F(1, 19) = 0.449, d = 0.22, p = .511.
Figure 3f shows that the CDA in Experiment 2 returned to full amplitude following go-fast cues on Target Repetition 5 trials, just as we observed with the large-reward cues in Experiment 1. As in Experiment 1, we found significant effects of target repetition, F(2, 38) = 4.536, d = 0.69, p = .019 (see Fig. 3f), and cue type, t(19) = 2.649, d = 0.88, p = .016. A 2 (experiment: Experiment 1 vs. Experiment 2) × 2 (cue type: go-fast or large-reward cue vs. baseline cue) × 3 (target repetition: 1–2, 3–4, 5–7) ANOVA verified that go-fast cues and large-reward cues had similar effects on CDA amplitude relative to their respective baseline cues (cf. Figs. 2d and 3d). This mixed-model ANOVA yielded no effect of experiment, F(1, 19) = 0.267, d = 0.17, p = .611; mean CDA amplitudes were roughly equivalent between the groups of subjects in Experiments 1 and 2. Also, experiment did not interact with target repetition, F(2, 38) = 0.583, d = 0.25, p = .541, or cue type, F(1, 19) = 0.500, d = 0.23, p = .488.
Conclusion
Our findings support the hypothesis that people overrepresent targets in memory to make attention operate at peak efficiency. That is, they simultaneously use memory representations of targets in working memory and in long-term memory to bias attention to select targetlike objects. This conclusion is supported by our observation of essentially identical patterns in our electrophysiological indices of working memory and long-term memory across two different situations that induced higher pressure during the visual search task.
We note that the present study reveals but a slice of how the human brain reconfigures cognitive processing on the basis of changes in motivational state. For example, we used speed stress and monetary incentives to change the motivational state of the subjects. However, some people might operationally define high-pressure situations as those that result in costs, ascribing greater subjective value to costs than to gains (Tversky & Kahneman, 1992). In addition, human electrophysiology can reveal only what occurs in cortical structures (Luck, 2005). But it is clear that neural circuitry in subcortical structures, such as the basal ganglia (Frank, Loughry, & O’Reilly, 2001), is critical for changes in brain states and memory representations, particularly as motivational factors wax and wane. Integrating the present findings into the broader literature will require combining tasks that can reveal effects of factors such as reward value, motivation, arousal, learning, and attention with techniques that measure activity across the brain.
Supplementary Material
Acknowledgments
We thank Gordon Logan for useful discussions and for pointing out that it is odd that we refer to the attentional “gas pedal,” given that we are electric-vehicle drivers.
Footnotes
Action Editor: Edward S. Awh served as action editor for this article.
Declaration of Conflicting Interests: The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Funding: This work was made possible by grants from the National Institutes of Health (R01-EY025275, R01-EY019882, P30-EY08126, F31-MH102042, and T32-EY007135).
Open Practices:

All materials have been made publicly available via Open Science Framework and can be accessed at https://osf.io/4y9xf/. The complete Open Practices Disclosure for this article can be found at http://pss.sagepub.com/content/by/supplemental-data. This article has received the badge for Open Materials. More information about the Open Practices badges can be found at https://osf.io/tvyxz/wiki/1.%20View%20the%20Badges/ and http://pss.sagepub.com/content/25/1/3.full.
References
- Carlisle N. B., Arita J. T., Pardo D., Woodman G. F. (2011). Attentional templates in visual working memory. Journal of Neuroscience, 31, 9315–9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelazzi L., Perlato A., Santandrea E., Della Libera C. (2013). Rewards teach visual selective attention. Vision Research, 85, 58–72. [DOI] [PubMed] [Google Scholar]
- Desimone R., Duncan J. (1995). Neural mechanisms of selective visual attention. Annual Review of Neuroscience, 18, 193–222. [DOI] [PubMed] [Google Scholar]
- Diana R. A., Vilberg K. L., Reder L. M. (2005). Identifying the ERP correlate of a recognition memory search attempt. Cognitive Brain Research, 24, 674–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A., Ranganath C., Winward L., Hayward D., Knight R. T. (2004). Dissociable neural correlates for familiarity and recollection during the encoding and retrieval of pictures. Cognitive Brain Research, 18, 255–272. [DOI] [PubMed] [Google Scholar]
- Frank M. J., Loughry B., O’Reilly R. C. (2001). Interactions between frontal cortex and basal ganglia in working memory: A computational model. Cognitive, Affective, & Behavioral Neuroscience, 1, 137–160. [DOI] [PubMed] [Google Scholar]
- Hutchinson J. B., Turk-Browne N. B. (2012). Memory-guided attention: Control from multiple memory systems. Trends in Cognitive Sciences, 16, 576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Shomstein S. (2014). Reward-based transfer from bottom-up to top-down search tasks. Psychological Science, 25, 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan G. D. (2002). An instance theory of attention and memory. Psychological Review, 109, 376–400. [DOI] [PubMed] [Google Scholar]
- Luck S. J. (2005). An introduction to the event-related potential technique. Cambridge, MA: MIT Press. [Google Scholar]
- Luck S. J., Vogel E. K. (2013). Visual working memory: From psychophysics and neurobiology to individual differences. Trends in Cognitive Sciences, 17, 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores E., Laiti L., Chelazzi L. (2003). Associative knowledge controls deployment of visual selective attention. Nature Neuroscience, 6, 182–185. [DOI] [PubMed] [Google Scholar]
- Nunez P. L., Srinivasan R. (2006). Electric fields of the brain: The neurophysics of EEG (2nd ed.). Oxford, England: Oxford University Press. [Google Scholar]
- Olivers C. N. L., Peters J. C., Houtkamp R., Roelfsema P. R. (2011). Different states in visual working memory: When it guides attention and when it does not. Trends in Cognitive Sciences, 15, 327–334. [DOI] [PubMed] [Google Scholar]
- Reinhart R. M. G., McClenahan L. J., Woodman G. F. (2015). Visualizing trumps vision when training attention. Psychological Science, 26, 1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart R. M. G., Woodman G. F. (2014a). High stakes trigger the use of multiple memories to enhance the control of attention. Cerebral Cortex, 24, 2022–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart R. M. G., Woodman G. F. (2014b). Oscillatory coupling reveals the dynamic reorganization of networks processing reward, maintaining working memory and controlling attention. Journal of Cognitive Neuroscience, 26, 175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart R. M. G., Woodman G. F. (2015). Enhancing long-term memory with stimulation tunes visual attention in one trial. Proceedings of the National Academy of Sciences, USA, 112, 625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard T. C. (1997). Bending the power law: A CMPL theory of strategy shifts and the automatization of cognitive skills. Journal of Experimental Psychology: General, 126, 288–311. [Google Scholar]
- Soto D., Hodsoll J., Rotshtein P., Humphreys G. W. (2008). Automatic guidance of attention from working memory. Trends in Cognitive Sciences, 12, 342–348. [DOI] [PubMed] [Google Scholar]
- Summerfield J. J., Lepsien J., Gitelman D. R., Mesulam M. M., Nobre A. C. (2006). Orienting attention based on long-term memory experience. Neuron, 49, 905–916. [DOI] [PubMed] [Google Scholar]
- Tversky A., Kahneman D. (1992). Advances in prospect theory: Cumulative representation of uncertainty. Journal of Risk and Uncertainty, 5, 297–323. [Google Scholar]
- Vogel E. K., Machizawa M. G. (2004). Neural activity predicts individual differences in visual working memory capacity. Nature, 428, 748–751. [DOI] [PubMed] [Google Scholar]
- Vogel E. K., McCollough A. W., Machizawa M. G. (2005). Neural measures reveal individual differences in controlling access to working memory. Nature, 438, 500–503. [DOI] [PubMed] [Google Scholar]
- Voss J. L., Schendan H. E., Paller K. A. (2010). Finding meaning in novel geometric shapes influences electrophysiological correlates of repetition and dissociates perceptual and conceptual priming. NeuroImage, 49, 2879–2889. [DOI] [PubMed] [Google Scholar]
- Woodman G. F. (2010). A brief introduction to the use of event-related potentials (ERPs) in studies of perception and attention. Attention, Perception, & Psychophysics, 72, 2031–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman G. F., Carlisle N. B., Reinhart R. M. G. (2013). Where do we store the memory representations that guide attention? Journal of Vision, 13(3), Article 1. doi: 10.1167/13.3.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman G. F., Luck S. J. (2003). Serial deployment of attention during visual search. Journal of Experimental Psychology: Human Perception and Performance, 29, 121–138. [DOI] [PubMed] [Google Scholar]
- Woodman G. F., Vogel E. K. (2008). Selective storage and maintenance of an object’s features in visual working memory. Psychonomic Bulletin & Review, 15, 223–229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.