Abstract
Skeletal development is regulated by the coordinated activity of signaling molecules that are both produced locally by cartilage and bone cells and also circulate systemically. During embryonic development and postnatal bone remodeling, receptor tyrosine kinase (RTK) superfamily members play critical roles in the proliferation, survival, and differentiation of chondrocytes, osteoblasts, osteoclasts, and other bone cells. Recently, several molecules that regulate RTK signaling have been identified, including the four members of the Sprouty (Spry) family (Spry1–4). We report that Spry2 plays an important role in regulation of endochondral bone formation. Mice in which the Spry2 gene has been deleted have defective chondrogenesis and endochondral bone formation, with a postnatal decrease in skeletal size and trabecular bone mass. In these constitutive Spry2 mutants, both chondrocytes and osteoblasts undergo increased cell proliferation and impaired terminal differentiation. Tissue-specific Spry2 deletion by either osteoblast- (Col1-Cre) or chondrocyte- (Col2-Cre) specific drivers led to decreased relative bone mass, demonstrating the critical role of Spry2 in both cell types. Molecular analyses of signaling pathways in Spry2−/− mice revealed an unexpected upregulation of BMP signaling and decrease in RTK signaling. These results identify Spry2 as a critical regulator of endochondral bone formation that modulates signaling in both osteoblast and chondrocyte lineages.
Keywords: endochondral bone formation, chondrocytes, FGFs, BMPs, Sprouty
1. INTRODUCTION
Chondrogenesis is an essential intermediate step in endochondral ossification through which long bones and vertebrae are formed. Endochondral bone formation begins when mesenchymal cells migrate, condense at the sites of future skeletal structures, and commit to the chondrocytic lineage. Subsequent proliferation of chondrocytes leads to expansive linear growth and enlargement. Eventually, chondrocytes stop proliferating and begin to undergo hypertrophy at the center of the cartilaginous anlage, which lays the foundation for future ossification. The hypertrophic chondrocytes regulate reorganization and mineralization of the extracellular matrix (ECM) and subsequent invasion of vasculature that brings in precursors of various bone cells. The latter cells in turn mediate the osteogenic phase of endochondral bone formation (1).
Endochondral bone formation is tightly regulated by a complex system of signaling networks and feedback mechanisms controlled by local paracrine factors and systemic hormones. These factors include BMPs, FGFs, Wnts, hedgehog proteins, and insulin-like growth factor-1 (IGF-1). The spatiotemporal expression and relative concentration of these signaling factors within and around the growth plate (GP) are coordinated to regulate an orderly initiation and progression of proliferation, hypertrophy, and terminal differentiation of growth plate chondrocytes (GPCs) and osteoblasts (OBs) (1, 2). Disruptions in any of these steps result in cartilage and/or bone defects.
Sprouty (Spry) was identified in Drosophila as an inhibitor of breathless, the fly equivalent of the FGF receptor (3). Four orthologs (Spry1–4) of Drosophila Spry (dSpry) have been identified in mammals, and Spry2 exhibits the highest homology to dSpry (4–6). Sprouty gene products have been reported to function as both negative and positive regulators of MAPK signaling downstream of various receptor tyrosine kinase (RTK) signaling cascades in a cell type- and context-dependent manner. The SPRY2 protein inhibits MAPK activation induced by FGF, PDGF, and VEGF, but its effect is thought to be agonistic in signaling downstream of the epidermal growth factor (EGF) receptor (7–9). Spry2 has been shown to regulate a number of developmental processes, including limb formation (4), lung branching (5), tooth morphogenesis (10), and kidney development (11). Because FGF and other RTK family members play a critical role during endochondral bone formation (12, 13), we set out to determine if Spry2 functions in this process.
2. MATERIALS AND METHODS
2.1. Mouse lines
Mouse lines carrying the Spry1tm1.1Jdli (Spry1−/−) (14), Spry2tm1.1Mrt (Spry2−/−) (15), Spry4tm1.2Mrt (Spry4−/−) (10), and Spry2tm1Mrt (Spry2flox) alleles (15), as well as the Tg(Col1a1-cre)2Bek (Col1-Cre) (16), and Tg(Col2a1-cre)1Bhr (Col2-Cre) transgenes (17) were maintained in the CD-1 mixed background and genotyped as previously described. Conditional inactivation of Spry2 was achieved by crossing Spry2flox/flox females (15) with either Col1-CreTg/+;Spry2+/− males (16) for OB-specific KOs (Col1-Cre;Spry2flox/−) or Col2-CreTg/+;Spry2+/− males (17) for chondrocyte-specific KOs (Col2-Cre;Spry2flox/−) and genotyped as previously described. Embryos and pups from timed mating and adult male mice were studied as described in the protocols reviewed and approved by the IACUC of the University of California, San Francisco (UCSF). All animal experiments have been carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals. All mice were housed in temperature and humidity controlled rooms in animal care facilities overseen by the UCSF Laboratory Animal Resource Center (LARC), which is accredited by the Association and Accreditation of Laboratory Animal Care (AAALAC).
2.2. mRNA in situ hybridization
For mRNA in situ hybridization, hindlimbs were collected, fixed overnight in 4% paraformaldehyde at 4°C, and decalcified for 3 days in either 10% EDTA for E16.5 embryos or in Morse’s solutions for 6-week-old males at 4°C. Decalcified bones were embedded in paraffin, and sectioned at 7 µm thickness. For traditional mRNA in situ detection, anti-sense DIG-labeled RNA probes were generated from plasmids containing fragments of Spry1, Spry2, and Spry4.. Section in situ hybridization was performed according to standard protocols.
For RNAscope mRNA in situ detection, the RNAscope 2.5 High Definition (HD) RED assay kit (Advanced Cell Diagnostics, Inc., Hayward, CA) was used according to the manufacturer’s recommendation. Images were captured with a Leica DFC500 microscope using Leica Application Suite (version 4.0.0) program.
2.3. Primary GPC culture
Epiphyseal GPs from P2 – P4 Spry2 WT or KO pups were dissected free of soft tissues, and GPCs were released by enzymatic digestion and cultured as described (18). Proliferation was assessed using BrdU ELISA kit (Cell Signaling Tech., Danvers, MA) following the manufacturer’s instructions. Gene expression in GPCs was studied using qPCR.
2.4. Primary OB cultures
For primary calvarial OB cultures, calvaria were dissected from CO2-euthanized P7–P9 pups, washed in PBS, and sequentially digested in a mixture of 1.5U/ml collagenase P and 0.05% trypsin. Isolated cells were dispersed into a single-cell suspension and plated in primary medium (DMEM containing 10% FBS, 100 U/ml penicillin/streptomycin, and 0.25 µg/ml fungizone).
To measure cellular proliferation, 5000 calvarial OBs were seeded into each chamber of an 8-chamber slide. Proliferating cells were incubated with BrdU solution (1mg/ml; Sigma-Aldrich, St. Louis, MO) for 30 min in a 37°C incubator to facilitate incorporation of BrdU into the newly synthesized DNA of replicating cells. Incorporated BrdU was detected with anti-BrdU Ab (Abcam, Cambridge, MA) and visualized chromogenically with the Liquid DAB+ kit (Dako, Glostrup, Denmark). Images were captured with a Leica DFC500 microscope using Leica Application Suite (version 4.0.0) program. Both BrdU-labeled cells and unlabeled cells within each chamber were manually counted, and the percentage of BrdU-labeled cells against the total cell population was calculated.
Bone marrow osteoprogenitor cells were harvested and induced for differentiation as described previously (19) except cells were plated at 2 × 105 cells/well in 6-well plates. At day 14 of induction, cells were stained for alkaline phosphatase activity using the alkaline phosphatase, leukocyte kit (procedure no. 86; Sigma-Aldrich, St. Louis, MO) following the manufacturer’s instructions. At day 28 of culture, calcified nodules were stained with alizarin red as described previously (19). The number of alkaline phosphatase- or alizarin red-positive colonies was calculated as the percentage of stained cells/total plated area. Measurement was done using ImageJ (n=4).
2.5. Quantitative PCR
For qPCR analysis, RNA was extracted from either primary GP chondrocytes or primary calvarial OBs using the RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Expression levels of Sprouty genes were measured using the GoTaq qPCR Master Mix (Promega, Madison, WI) with a Mastercycler Realplex (Eppendorf, Hamburg, Germany). RNA levels were normalized to L19, which encodes a ribosomal protein, and displayed as percent of expression. Sequences are available upon request.
2.6. Analyses of growth and skeletal phenotype in mice
At 4 or 6 weeks of age, Spry2−/−, Col1-Cre;Spry2flox/−, or Col2-Cre;Spry2flox/− males and their control littermates were weighed individually prior to CO2 euthanasia. Both femurs and tibias were collected from 4- and 6-week-old male mice and their control littermates free of soft tissues, fixed in 10% neutral buffered formalin (NBF, 10% formalin in PBS) overnight, and stored in 70% ethanol. Bone length of both femurs and tibias was recorded by measuring the longest distance between two epiphyses with a caliper.
2.7. MicroCT (µCT) scans of long bones
Femurs were isolated from 6-week-old Spry1−/−−, Spry2−/−, Spry4−/−, Col1-Cre;Spry2flox/− or Col2-Cre;Spry2flox/− males and their control littermates, fixed in 10% NBF overnight, and stored in 70% ethanol. Distal femurs were scanned to analyze microarchitectural structures of Tb bones on a SCANCO vivaCT 40 scanner (SCANCO Medical, Basserdorf, Switzerland) with 10.5 µm voxel size and 55 kV X-ray energy, as per Bouxsein et al. (20). For Tb bone, 100 serial cross-sectional scans (1.05 mm) were obtained from the end of the GP extending proximally. A threshold of 300 mg hydroxyapatite (HA)/mm3 was applied to segment total mineralized bone matrix from soft tissue. Image analysis and 3D reconstructions were performed with the manufacturer's software (SCANCO Medical AG, Bassersdorf, Switzerland) by the SF-VAMC Bone Imaging Core facilities.
2.8. Histomorphometric analyses of Tb bones
To study adult bones, femurs were isolated from 6-week-old Spry2−/− and WT littermate males, fixed overnight in 10% NBF, dehydrated with ethanol, defatted with xylene, and embedded in MMA (Sigma-Aldrich, St. Louis, MO). Adjacent sections (5 or 10 µm in thickness) were cut on an automated microtome (LEICA RM2255, Germany) and mounted on gelatin-coated slides for various staining procedures. Digital images of stained bone sections were acquired. For histomorphometry, the region of interest began approximately 150 µm below the femoral GP, extended 1 mm distally and flanked the two sides that are 100 µm apart from cortical bone. Two sections (approximately 50–100 µm apart) per bone sample were analyzed for each staining method, and the average was used for statistical analyses. The histomorphometric analysis was done by the SF-VAMC Bone Imaging Core. The terminology and units used are those recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (21).
The VK staining method was performed to detect the phosphate-containing minerals and calculate static bone parameters: Tb.BV/TV, Tb.N, and Tb.Sp. To quantify structural parameters of OC-positive resorbing surface, sections were stained with TRAP reagents. The deduced indices include ES/BS, N.Oc/BS, and N.Oc/ES.
For dynamic bone formation indices, calcein (15 mg/kg body weight) and xylenol orange (15 mg/kg body weight) were administered sequentially to both control and experimental groups at 14 and 7 days before sample collection, respectively. Unstained MMA-embedded bone sections were obtained as described above and used to quantify MS/BS, MAR, and BFR/BS. Bone images were acquired by Zeiss AXIO Imager M1 Microscope with an automated stage and analyzed using BioQuant OSTEO 2009 software (Version 9.00, BIOQUANT Image Analysis Co., Nashville, TN).
2.9. Histological, histomorphometric, and immunohistological analyses of GP cartilage
To study GP cartilage in embryonic limbs, E18.5 hindlimbs were collected from both Spry2−/− and WT littermate embryos, cleaned of soft tissues, and fixed prior to embedding in glycol methacrylate (GMA). GMA-embedded limbs were sectioned at 2.5µm thickness and stained using the VK with Safranin-O (SafO) reagents. Average numbers of chondrocytes within the proliferative zone were calculated by manually counting cells within randomly selected four 1”×1” grids per sample and averaging total number of cells within each grid. The average height of the hypertrophic zone was calculated by measuring the distance between top row of hypertrophic chondrocytes and chondro-osseous junctions at 10 different locations along the width of the GP.
For immunohistochemistry, hindlimbs were processed in the same fashion as samples for in situ hybridization except that decalcification was done in 10% EDTA for all specimens. Sections were probed with commercially available antibodies against phospho-MEK1/2 and phospho-SMAD1/5/8 (Cell Signaling Tech., Danvers, MA).
For the BrdU cell proliferation and TUNEL analyses, pregnant dams with E18.5 embryos were injected with BrdU solution (50mg/g body weight; Sigma-Aldrich, St. Louis, MO) 2 hours prior to euthanasia. BrdU-incorporated chondrocytes in limb sections were detected with anti-BrdU antibody (Abcam, Cambridge, MA). Bound antibody was visualized chromogenically with the Liquid DAB+ kit (Dako, Glostrup, Denmark). Apoptotic cells in embryonic limb sections were detected with the in situ cell death detection kit, TMR red (Roche, Indianapolis, IN) according to the manufacturer’s instructions.
To quantitate cellular proliferation, four 1 inch × 1 inch grids were randomly selected from each image and numbers of BrdU-labeled cells and unlabeled cells within each grid were obtained to calculate the percentage of BrdU-labeled cells against the total cell numbers.
2.10. Statistical analysis
All experiments were performed independently at least three times, and data were presented as mean ± SD. Student's t-test was used to determine p values and p<0.05 was deemed to be significant. P-values less than 0.001 were presented as p 0.001.
3. RESULTS
3.1. Spry1, Spry2, and Spry4 are expressed in both cartilage and bone cells
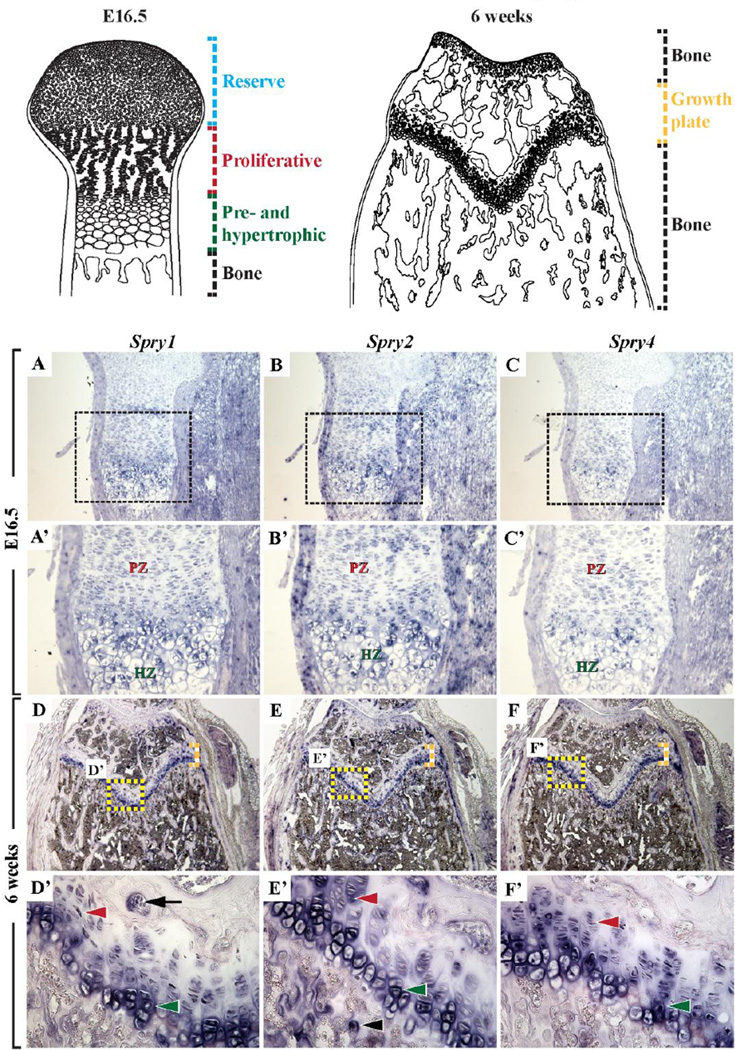
To determine the mRNA expression pattern of the Sprouty gene family, in situ hybridization analysis was performed on long bones of E16.5 and 6-week-old CD1 mice. Spry1, Spry2, and Spry4 RNA transcripts were detected in the proliferating, prehypertrophic, and hypertrophic chondrocytes in the embryonic (Figure 1A–1C’) and postnatal GPs (Figure 1D–1F), and in the OBs and osteoclasts (OCs) in the primary and secondary spongiosa (Figure 1D’–1F’). Spry3 mRNA was not detected in these tissues (data not shown).
Figure 1. Sprouty gene expression in embryonic and adult long bones.
In situ hybridization of proximal tibial sections with corresponding RNA probes showing robust expression of Spry1 (A, A’, D, D’), Spry2 (B, B’, E, E’), and Spry4 (C, C’, F, F’), but not Spry3 (data not shown), in proliferating (red arrowhead), prehypertrophic, and hypertrophic (green arrowhead) chondrocytes in E16.5 embryonic (A–C’) and 6-week-old GPs (D–F and D’–F’), and in OCs (black arrow) and OBs (black arrowhead) in the primary spongiosa (D–F and D’–F’) of WT mice. Top schematic panels: Blue dashed lines – reserve chondrocytes, red dashed lines – proliferative chondrocytes, green dashed lines – pre- and hypertrophic chondrocytes, black dashed lines – bone, yellow dashed lines – GP.
Quantitative PCR analyses of cultured GPCs (Figure S1A–S1C) and calvarial OBs (Figure S1D–S1F) confirmed the expression of Spry1, Spry2, and Spry4 RNA and the lack of expression of Spry3 RNA (data not shown) in these cells. As expected, Spry2 mRNA expression was not detected in GPCs and OBs isolated from the Spry2−/− mice (Figure S1B). Interestingly, the expression of Spry1 and Spry4 was upregulated significantly in the Spry2−/− mice (Figure S1A–S1F), suggesting a compensatory increase in expression of these genes in the absence of Spry2.
3.2. Spry2−/− mice are smaller and have stunted postnatal skeletal growth
Although the Sprouty genes share a similar expression pattern in long bones, only the Spry2 KO mice showed growth retardation and a skeletal phenotype (Figure S2A and data not shown); the Spry1 and Spry4 mutants were grossly normal in terms of their skeletons. While Spry2−/− pups were indistinguishable from their WT littermates in body weight at birth, their postnatal growth was stunted. By P7, Spry2−/− pups were significantly smaller than control littermates (Figure S2A,B), and at 6 weeks of age, their body weight was only 39% of their control littermates (Figure S2C). These growth defects were accompanied by reduction of femur and tibia length, by 14% and 11%, respectively (Figure S2D, S2E).
3.3. Spry2−/− mice have less trabecular (Tb) bone due to slow bone turnover
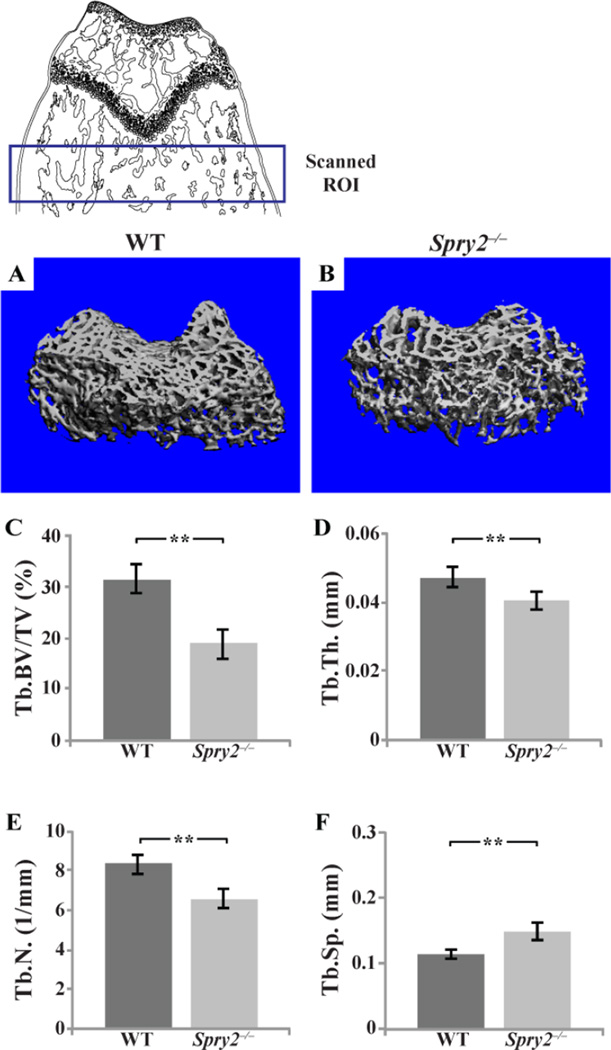
In addition to the abnormalities in longitudinal bone growth, we found abnormal microarchitecture of the long bones in Spry2−/− mice. MicroCT (µCT) analyses of the distal femurs showed decreased ratio of Tb bone volume to tissue volume (Tb.BV/TV), Tb number (Tb.N), and Tb thickness (Tb.Th), by 42, 25, and 13%, respectively, and increased Tb spacing (Tb.Sp) by 32% of the Spry2−/− mice compared to control littermates (Figure 2C–F). These bone parameters were unchanged in the Spry1−/− and Spry4−/− mice (Figure S3), indicating a minimal role for these two genes in skeletal development.
Figure 2. Spry2−/− mice have less Tb bone.
µCT analysis of distal femurs from 6-week-old Spry2−/− and WT males. 3D reconstructed images of trabecular bones from µCT analysis of WT (A) and Spry2−/− (B) distal femoral metaphyses (scanned ROI in top schematic panel). (C–F) Spry2−/− mice have decreased Tb. BV/TV (C, p = 0.001), average Tb.Th (D, p = 0.002), and Tb.N (E, p = 0.001) and increased spacing between trabeculae (F, p = 0.001). n=8 per genotype.
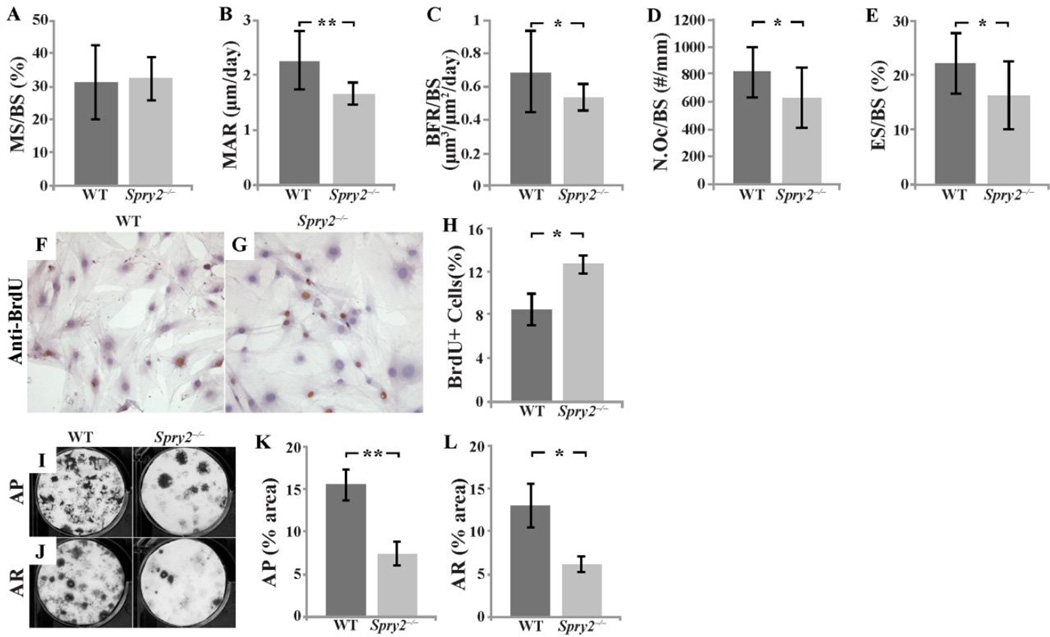
Histomorphometric analyses of undecalcified femurs from 6-week-old Spry2−/− and control mice supported the µCT findings. Static histomorphometry with von Kossa (VK)/tetrachrome staining showed a reduced Tb.BV/TV, Tb.N, and Tb.Th, and increased Tb.Sp in the Spry2−/− bones (Figure S4). The decreased Tb bone mass in the Spry2−/− mice was likely due to a reduction in bone formation rather than an increase in bone resorption, as the mineral apposition rate (MAR) and bone formation rate (BFR/BS) in the calcein/xylenol orange-labeled femurs were decreased by 26% and 23%, respectively (Figure 3B,3C), and OC numbers (N.Oc/Bs) and bone resorption surface (ES/BS) in tartrate resistant acid phosphatase (TRAP)-stained bone sections were decreased by 23% and 27%, respectively (Figure 3D,3E). The unchanged ratio of mineralizing surface over bone surface (MS/BS; Figure 3A) further suggested that the decreased mineral apposition rate was likely due to reduced mineralizing function in Spry2−/− OBs per unit of mineralizing surface. In support of this notion of OB dysfunction, calvarial OBs and bone marrow stromal cells (BMSCs) isolated from Spry2−/− mice had a higher rate of proliferation as assessed by BrdU incorporation (Figure 3F–H) but a reduced capacity to form alkaline phosphatase expressing and mineralized colonies (Figure 3I–L).
Figure 3. Deletion of Spry2 leads to slow bone turnover.
Trabecular bone formation was assessed by dual fluorochrome labeling (n= 14 for WT and n= 14 for Spry2−/−). Result showed that MAR (B, p = 0.001) and BFR/BS (C, p = 0.02) are reduced in 6-week-old Spry2−/− males without evidence of decreased OB numbers. TRAP staining of distal femurs of 6-week-old Spry2−/− males showing decreased N.Oc/BS (D, p = 0.022) and ES/BS (E, p = 0.01). Calvarial OBs from Spry2−/− mice have increased proliferation as evidenced by increased number of BrdU positive cells (brown-stained cells) (F–H, p = 0.005). Alkaline phosphatase (AP; I,K, p = 0.001) and mineralization (AR; J,L, p = 0.012) staining of induced BMSCs showing that OB differentiation is delayed or disrupted in Spry2−/− mice. p-values are calculated using the Student’s t-test (*p<0.05, **p<0.005).
3.4. Conditional inactivation of Spry2 in chondrocytes or OBs partly recapitulates skeletal defects seen in Spry2−/− mice
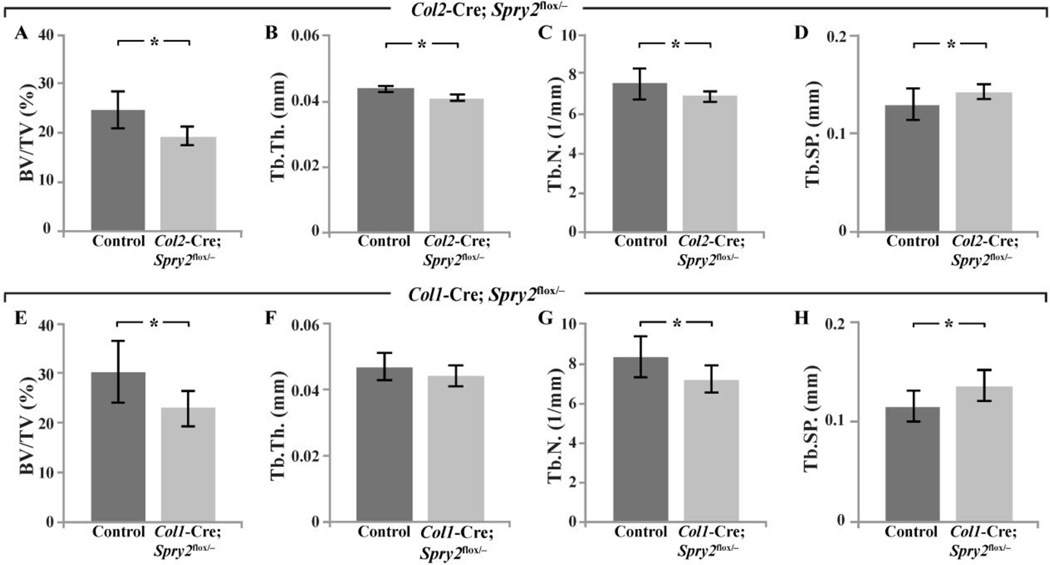
To determine whether the skeletal defects in Spry2−/− mice were due to the loss of Spry2 in chondrocytes and/or OBs, we generated either chondrocyte- (Col2-Cre;Spry2flox/−) or OB-specific (Col1-Cre;Spry2flox/−) KOs of Spry2. Unlike Spry2−/− animals, both Col2-Cre;Spry2flox/− and Col1-Cre;Spry2flox/− mice were indistinguishable from their littermate controls in body weight and femur and tibia length at 6 weeks of age (Figure S2I–S2K). We also analyzed Tb bone in the distal femurs of 6-week-old mice with either chondrocyte- (Col2-Cre;Spry2flox/−) or OB-specific (Col1-Cre;Spry2flox/−) inactivation of Spry2. Tissue-specific inactivation of Spry2 in chondrocytes or OBs at early stages of cartilage and bone development recapitulated, albeit to a lesser extent, the Tb bone defects of global Spry2 KOs. Col2-Cre;Spry2flox/− and Col1-Cre;Spry2flox/− mice had decreased Tb.BV/TV by 23% and 26%, respectively (Figure 4A,E), in contrast to a 42% reduction in Spry2−/− mice. The trabeculae of Col2-Cre;Spry2flox/− mice were significantly thinner in size (Tb.Th) by 7%, fewer in number (Tb.N) by 10%, and with greater spacing (Tb.Sp) by 10% (Figure 4B–D). There was a similar phenotype in the Col1-Cre;Spry2flox/− mice, with a significant decrease in Tb.N by 15%, an increase in Tb.Sp by 14%, and a trend towards thinner trabeculae (Figure 4F–H). The skeletal phenotype in both of the conditional Spry2 inactivation models shows that Spry2 expression in both chondrocytes and OBs is vital for optimal trabecular bone formation. The more pronounced phenotype of the global Spry2 KO suggests that the roles of Spry2 in chondrocytes and OBs are independent and additive with respect to endochondral bone formation.
Figure 4. Osteoblast- and chondrocyte-specific Spry2 KO mice partly recapitulate Tb bone phenotype of the global Spry2 null mice.
µCT analyses of Tb bone in distal femurs of 6-week-old Col2-Cre;Spry2flox/− (A–D) and Col1-Cre;Spry2flox/− (E–H) show decreased BV/TV (A and E), Tb.Th. (B and F), and Tb.N (C and G) and increased Tb spacing (D and H) which are relatively milder (by ≈50%) than those in global Spry2 KO mice. Spry2flox/+ mice from the same litter were used as control. (*p<0.05, **p<0.005). n=8 mice per genotype.
3.5. Mechanisms underlying chondrogenic defects in Spry2−/− mice
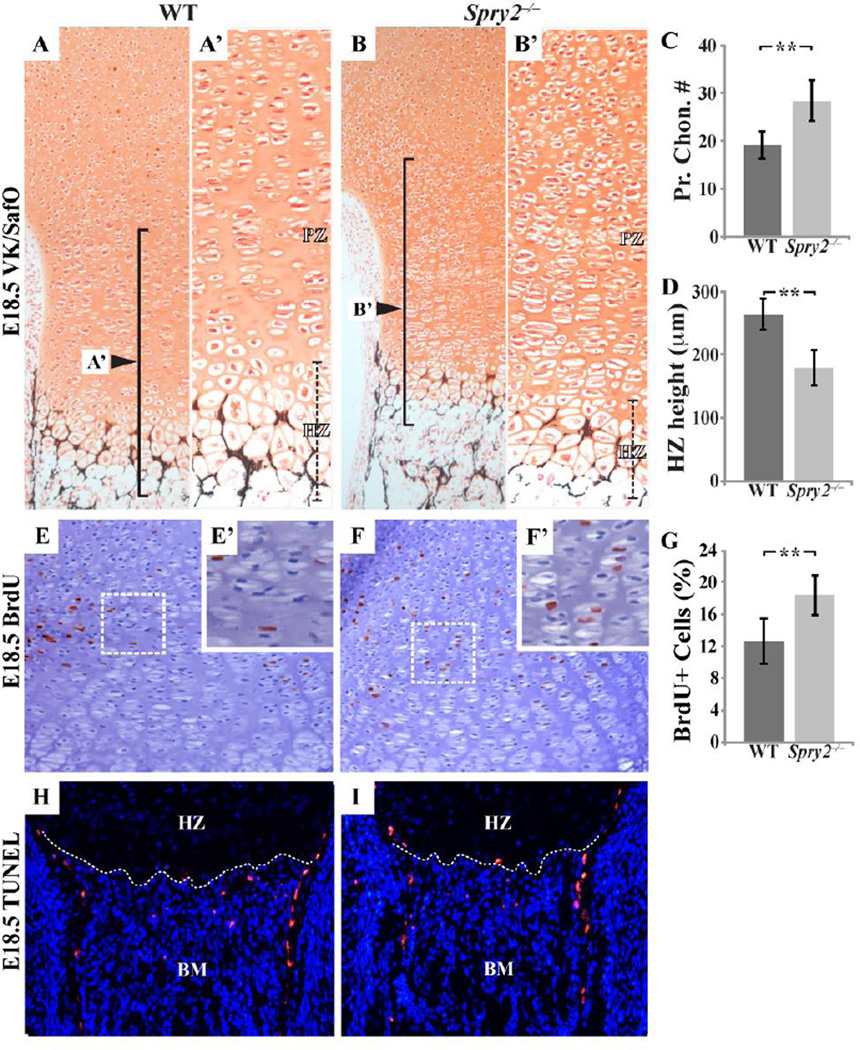
In addition to its role in post-natal endochondral bone formation, Spry2 is involved in the regulation of chondrogenesis. Histological analysis of the GPs of the distal femurs in E18.5 Spry2−/− embryos demonstrated a 32% increase in the number of proliferating chondrocytes with typical flattened cell morphology (Figure 5C). In contrast, chondrocyte differentiation was retarded, as evidenced by a 32% decreased length of the hypertrophic zone of the mutant GP (Figure 5A’,B’,D). The hypercellularity of the proliferative zone in Spry2−/− embryos was due to an increase in cell proliferation, as indicated by 34% more BrdU-positive cells in this zone (Figure 5E–G), but not cell apoptosis, as the number of TUNEL-positive cells in the hypertrophic zones or in the primary spongiosa of Spry2−/− embryos was unchanged (Figure 5H,I).
Figure 5. Spry2−/− embryos have disrupted chondrogenesis due to increased proliferation.
(A–B’) VK/SafO staining of E18.5 femur sections shows there are more proliferating chondrocytes in the GP of Spry2−/− (B and B’) than in that of WT (A and A’). Within the proliferative zone (PZ) of Spry2−/− embryos, a 32% increase in chondrocyte number is detected. (C, p <0.001) In addition, Spry2−/− embryos (B’) have thinner hypertrophic zone (HZ; black dashed lines) than that of WT littermates (A’). Average height of the hypertrophic zone is decreased by 32% in Spry2−/− embryos (D, p <0.001). Measurements (unit=micrometer) are averaged and P values are calculated using the Student’s t-test (*p<0.05, **p<0.005). n = 4/genotype. Spry2−/− embryos show elevated level of chondrocyte proliferation (E–F’), an increased number of BrdU-positive cells (brown-stained cells) by 34% (G, p < 0.001). n = 3/genotype. Similar rates of apoptosis in WT and Spry2−/− embryos are demonstrated by similar numbers of TUNEL (red)-positive cells within the chondro-osseous junction (white dashed lines): HZ = hypertrophic zone, BM = Bone Marrow (H and I).
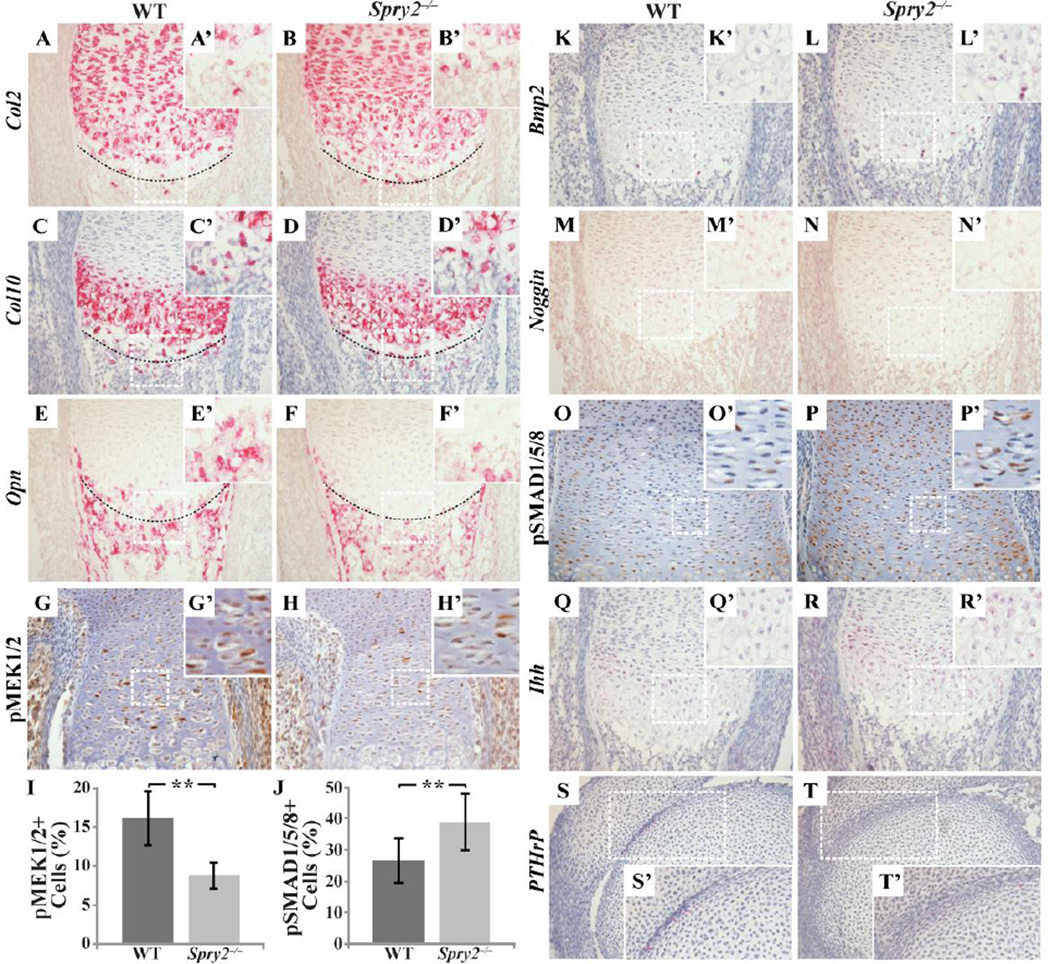
To characterize the molecular changes within the GPCs associated with the abnormalities of chondrogenesis in Spry2−/− embryos, we assessed the expression of cartilage extracellular matrix molecules that indicate stages of chondrocyte differentiation by immunohistochemical and RNAscope in situ hybridization analyses on sections from control and Spry2−/− embryonic limbs. We observed no change in expression level of either the alpha-1 subunit of the type II collagen (Col2a1; Col2), a marker of early resting and proliferative chondrocytes, or the alpha-1 subunit of type X collagen (Col10a1), a hypertrophic chondrocyte marker encoded by Col10a1 (Col10), in Spry2−/− specimens (Figure 6A–D’). However, more Col2-expressing cells can be seen in the chondro-osseous junction of Spry2−/− (Figure 6B,B’) than in that of WT (Figure 6A,A’). Furthermore, in agreement with the size reduction of the hypertrophic zone of Spry2−/− embryos (Figure 5A’,B’), the expression of osteopontin (Opn), a marker of terminally differentiated hypertrophic chondrocytes, was altered and decreased in mutants (Figure 6E–F’). These data indicate that chondrocyte proliferation and initial differentiation occur normally in the absence of Spry2, but the terminal differentiation of chondrocytes is delayed in Spry2−/− embryos, and this may contribute to retarded bone growth.
Figure 6. Deletion of Spry2 leads to increased BMP signaling and decreased RTK signaling during chondrogenesis.
RNAscope assay shows changes in expression levels of molecular markers for different stages of chondrogenesis in femur sections from E16.5 Spry2−/− embryos (A–F’, K–N’, Q–T’).. Immunohistochemical staining for pMEK1/2 (G–H’) and pSMAD1/5/8 (O–P’) reveals a decrease in the number of pMEK1/2-positive cells (I, p 0.001), but an increase in pSMAD1/5/8-positive cells (J, p 0.001) in Spry2−/− embryos. In Spry2−/− samples, more Col2-expressing cells are found extending to the chondro-osseous junction (B and B’). Also, Opn expression is only detected in primary spongiosa of Spry2−/− (F and F’) while in WT, Opn expression starts from hypertrophic zone (E and E’). Noggin (M–N’) expression is decreased while expression of Bmp (K–L’) and Ihh (Q–R’) is increased in Spry2−/−. The PTHrP expression seems similar to that of WT control (S–T’). Magnification = 200×
3.6. Aberrant signaling in Spry2−/− chondrocytes
Since Sproutys have been found to be negative feedback modulators of growth factor (GF)-mediated MAPK activation, we expected that deletion of Spry2 would augment RTK signaling. However, immunohistochemical analysis of embryonic GPs revealed a significant decrease in the number of phospho-MEK (pMEK) 1/2-positive cells, pointing to a decrease of RTK signaling in the absence of Spry2 (Figure 6G–I). In light of this unexpected effect on RTK signaling, we investigated the impact of Spry2 deletion on other signaling cascades using the more sensitive RNAscope technology. We showed that bones from Spry2−/− embryos had increased expression of Bmp2 in the perichondrium and hypertrophic zone and reduced expression of Noggin, a BMP antagonist, throughout the GP (Figure 6K–N’). Consistent with these findings, there was a significant increase in the number of cells staining for phospho-Smad (pSMAD)1/5/8, indicating increased BMP signaling (Figure 6J,O–P’). Previous studies have shown that BMP signaling stimulates Indian hedgehog (Ihh) expression, which in turn increases expression of PTHrP (22, 23). As expected with an increase in BMP signaling, we observed an increase in Ihh expression in the Spry2−/− mice (Figure 6Q–R’). However, there was no increase in expression of PTHrP (Figure 6S–T’), suggesting an uncoupling between Ihh signaling and PTHrP expression in the absence of Spry2. Thus, in the absence of Spry2, the failure of chondrocytes to terminally differentiate is due, at least in part, to increased BMP signaling and decreased RTK signaling.
4. DISCUSSION
We have investigated the role of Sprouty genes during endochondral bone development. Spry1, Spry2, and Spry4 were expressed in cartilage and bone, but mice carrying mutant alleles of either Spry1 or Spry4 did not manifest any obvious long bone phenotype. In contrast, Spry2 null mice developed profound postnatal growth and skeletal defects. These observations suggest that only Spry2 plays a non-redundant role in supporting skeletal development and may explain why upregulation of Spry1 and Spry4 in the Spry2−/− chondrocytes and bone cells was unable to compensate for the loss of Spry2. Since defects in the Spry2 null mice were recapitulated, albeit to a lesser degree, in Col2-Cre;Spry2flox/− and Col1-Cre;Spry2flox/− mice, the skeletal defects in Spry2−/− mice are due, at least in part, to the absence of Spry2 from chondrocytes and OBs. In contrast to the shortened skeleton in the Spry2−/− mice, normal bone lengths in Col2-Cre;Spry2flox/− and Col1-Cre;Spry2flox/− mice implicate additional actions of Spry2, perhaps in chondro- and/or osteoprogenitors prior, to the activation of Col2 or Col1 promoter to support post-natal bone elongation. Alternatively, other Spry2-mediated growth signals (local or systemic) may also be needed for complete skeletal development.
Defective GP histology and endochondral bone formation in Spry2−/− mice indicate a role for Spry2 during chondrocyte development. This is consistent with our previous report of the requirement of Spry1 and Spry2 in proper formation of the temporomandibular joint (24). The loss of Spry2 expression does not affect the initiation of chondrogenesis, as limbs of Spry2−/− mice appear to develop normally until late gestation. Thus, Spry2 does not significantly impact mesenchymal cell condensation or the subsequent initial differentiation of mesenchymal precursors to chondrocytes. Rather, it regulates chondrocyte proliferation and differentiation, as indicated by the phenotypes in late-gestation Spry2−/− embryos.
The increased proliferation of the Spry2−/− GPCs in vivo is consistent with previous work showing that increased Spry2 expression inhibited chondrocyte proliferation in chicks (4). This hyperproliferative Spry2−/− phenotype was also observed in the GPC cultures, which were deprived of systemic factors and other cell types, indicating that this is a cell autonomous phenomenon. Reduced size of the hypertrophic zone and decreased expression of the terminal hypertrophic chondrocyte marker Opn, as well as expansion of Col2 expression to the chondro-osseous junction and absence of Opn expression within the hypertrophic zone, all suggest a delay in the terminal differentiation of chondrocytes in the absence of Spry2.
The Spry2 gene product has been reported to be a negative regulator of RTK signaling, including the FGF pathway, in several contexts. SPRY2 can inhibit FGF signaling by binding to GRB2 or RAF1 and preventing FGF-mediated MAPK activity (25, 26). Also, the Fgfr3 missense mutant mice (Fgfr-TDII) have reduced proliferating chondrocytes with decreased Ihh and ColX expression (27) and the mutant gain-of-function phenotype is thought to be modulated by Spry2 (28). It was therefore surprising to find the opposite: a significant decrease in pMEK1/2 activity in Spry2−/− chondrocytes. It is possible that this could due to a compensatory increase in the expression of Spry1 and Spry4 in chondrocytes, which we have previously reported a compensatory upregulation of Spry1 in the absence of Spry2 during tongue development (29). However, we do not yet know whether Spry1 or Spry4, or both, can compensate for the absence of Spry2, as any increased expression of Spry1 and Spry4 was insufficient to compensate for the inactivation of Spry2. This suggests that Spry2 has other critical actions during endochondral bone formation beyond regulation of FGF signaling.
Indeed, we observed an upregulation of BMP signaling in Spry2 KOs. These observations included increased Bmp2 expression, decreased Noggin expression, and increased pSMAD1/5/8 activities in Spry2−/− GPCs. The increased Ihh expression and enhanced chondrocyte proliferation in Spry2−/− mice are consistent with increased BMP signaling, as previous studies showed that addition of BMP2 to limb cultures increased Ihh expression (30). The delayed terminal differentiation of hypertrophic chondrocytes and decreased Opn expression are also compatible with increased BMP signaling in the Spry2−/− mice, as previous studies showed that blocking BMP signaling by chondrocyte-specific deletion of Bmpr1a led to increased expression of Opn and Mmp13, markers of terminal differentiation (23). Additionally, treatment of embryonic limb cultures with Noggin increased Opn expression (30). We were surprised to see that PTHrP expression was not markedly suppressed in the presence of Ihh upregulation in Spry2−/− GPs. One potential explanation for this observation is that more dense cartilage ECM, resulting from the increase in proliferating chondrocyte numbers, effectively blocked IHH diffusion to the perichondrium and caused disruption of PTHrP expression. Alternatively, deletion of Spry2 could disrupt signaling responses that are required to couple the PTHrP and Ihh pathways. Future studies will be needed to tease apart the detailed dynamics of these signaling interactions.
Analyses of postnatal Spry2−/− long bones revealed a reduced bone turnover, with concurrent decreases in bone formation and resorption. Overall, Tb bone mass was significantly decreased, likely due to impaired chondrocyte maturation. Hypertrophic chondrocytes are the key cells that induce osteogenesis; they synthesize ECM components and GFs to initiate vascularization of the GP, recruitment of osteogenic precursors, and calcification. Terminal hypertrophic chondrocytes at the chondro-osseous junction are marked by the expression of Opn and Mmp-13 and the loss of Col10 expression (23, 31, 32). In late gestation Spry2−/− embryos, we observed high Col10 expression within the hypertrophic zone that is similar to that of WT littermates, but a downregulation of Opn expression at the chondro-osseous junction of the GP. Also, our preliminary data showed approximately 60% decrease in Mmp13 and 40% decrease in alkaline phosphatase (Ap) mRNA levels when relative transcript levels were measured in both WT and Spry2−/− BMSC by qPCR (data not shown). Hence, these data indicate that without Spry2 expression, terminal differentiation of hypertrophic chondrocytes is disrupted, which negatively affects bone formation. Furthermore, a decrease in OC activity seen in long bones of 6-week-old Spry2−/− mice could be due in part to reduced Opn expression within the ECM, as Opn is involved in the regulation of osteoclastic activities (33). This is different from published in vitro data showing that BMP2 induces RANKL expression in hypertrophic chondrocytes to regulate osteoclastogenesis in vitro (34). Complex interconnected molecular signaling pathways required for chondrogenesis might account for the discrepancy between our in vivo data and previously reported in vitro results, as loss of Spry2 induced an increase in Bmp2 expression sufficient to downregulate Opn expression, but not to affect RANKL expression.
The decrease in Tb bone in Spry2−/− mice was not exclusively due to impaired chondrocyte maturation. Osteoprogenitor cells from Spry2−/− mice had increased proliferation and impaired differentiation, similar to Spry2−/− chondrocytes. These findings indicate that Spry2 has a role during OB differentiation in addition to its regulation of chondrogenesis. Furthermore, µCT analysis of Col2-Cre;Spry2flox/− and Col1-Cre;Spry2flox/− mice showed that both conditional KOs had a decrease in Tb.BV/TV that was approximately half that of Spry2−/− mice. Thus, the Spry2−/− skeletal phenotype was due to defects in both chondrocyte and OB function.
The mechanism that causes upregulation of Bmp2 in Spry2−/− mice is not clear, since the role of Spry2 in regulating the expression of Bmps or in BMP signaling is not known. Also, we cannot rule out the possibility of signaling pathways other than BMP or RTK being affected by the inactivation of Spry2. However, our data suggest that SPRY2 interacts with both FGF and BMP signaling pathways to control chondrocyte development. Loss of Spry2 causes upregulation of Spry1 and Spry4 expression, which leads to suppression of FGF signaling. Either through this suppression of RTK signaling or through loss of a negative effect of Spry2 directly on the BMP signaling pathway, BMP signaling is subsequently increased, which manifests as upregulated Ihh expression, increased chondrocyte proliferation, and impaired terminal chondrocyte differentiation. Together, our data show that Spry2 is important for normal chondrocyte proliferation and differentiation, and loss of Spry2 leads to defects in endochondral bone formation and bone mass accrual, likely due to an upregulation of BMP signaling. The Tb bone mass defect is further influenced by the role of Spry2 in OB differentiation and function.
Supplementary Material
Highlights.
Spry1, Spry2, and Spry4 are expressed in both cartilage and bone cells.
Deletion of Spry2 leads to smaller and weaker trabecular bones in limbs.
Inactivation of Spry2 leads to aberrant BMP and RTK signaling during chondrogenesis, which affects terminal differentiation of hypertrophic chondrocytes.
Acknowledgments
We thank Dong-Kha Tran, Sarah Alto, Rebecca D’Urso, and Asoka Rathnayake for technical assistance, Wei-Dar Lu and Tsui-Hua Chen for the calvarial OB primary culture system, and the SF-VAMC Bone Imaging Core facilities for help with µCT scans and histomorphometric analyses. We also thank Dr. Amnon Sharir for his review of the manuscript and helpful comments. This work was supported by NIH T32 DE007306 to AJ, R03AR057121 and R01-DE024988 to ODK and R01AR067291 and P30AR066262 to WC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
All authors state that they have no conflicts of interest.
Authors’ roles: Study design: AJ, RL, WC, and ODK. Study conduct: AJ, RL, ZC, and WC. Data collection: AJ, RL, ZC, CA, and WC. Data analysis: AJ, RL, ZC, WC, and ODK. Data interpretation: AJ, RL, WC, and ODK. Draft manuscript: AJ and RL. Revising manuscript content: AJ, RL, WC, and ODK. Approving final version of manuscript: AJ, RL, CA, WC, and ODK. ODK takes responsibility for the integrity of the data analysis.
REFERENCES
- 1.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003 May 15;423(6937):332–336. doi: 10.1038/nature01657. PubMed PMID: 12748651. eng. [DOI] [PubMed] [Google Scholar]
- 2.Mackie EJ, Tatarczuch L, Mirams M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J Endocrinol. 2011 Nov;211(2):109–121. doi: 10.1530/JOE-11-0048. PubMed PMID: 21642379. Epub 2011/06/07. eng. [DOI] [PubMed] [Google Scholar]
- 3.Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998 Jan 23;92(2):253–263. doi: 10.1016/s0092-8674(00)80919-8. PubMed PMID: 9458049. [DOI] [PubMed] [Google Scholar]
- 4.Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, et al. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999 Oct;126(20):4465–4475. doi: 10.1242/dev.126.20.4465. PubMed PMID: 10498682. Epub 1999/09/28. eng. [DOI] [PubMed] [Google Scholar]
- 5.Tefft JD, Lee M, Smith S, Leinwand M, Zhao J, Bringas P, Jr, et al. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr Biol. 1999 Feb 25;9(4):219–222. doi: 10.1016/s0960-9822(99)80094-3. PubMed PMID: 10074434. Epub 1999/03/13. eng. [DOI] [PubMed] [Google Scholar]
- 6.de Maximy AA, Nakatake Y, Moncada S, Itoh N, Thiery JP, Bellusci S. Cloning and expression pattern of a mouse homologue of drosophila sprouty in the mouse embryo. Mech Dev. 1999 Mar;81(1–2):213–216. doi: 10.1016/s0925-4773(98)00241-x. PubMed PMID: 10330503. [DOI] [PubMed] [Google Scholar]
- 7.Impagnatiello MA, Weitzer S, Gannon G, Compagni A, Cotten M, Christofori G. Mammalian sprouty-1 and -2 are membrane-anchored phosphoprotein inhibitors of growth factor signaling in endothelial cells. J Cell Biol. 2001 Mar 5;152(5):1087–1098. doi: 10.1083/jcb.152.5.1087. PubMed PMID: 11238463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kajita M, Ikeda W, Tamaru Y, Takai Y. Regulation of platelet-derived growth factor-induced Ras signaling by poliovirus receptor Necl-5 and negative growth regulator Sprouty2. Genes Cells. 2007 Mar;12(3):345–357. doi: 10.1111/j.1365-2443.2007.01062.x. PubMed PMID: 17352739. Epub 2007/03/14. eng. [DOI] [PubMed] [Google Scholar]
- 9.Egan JE, Hall AB, Yatsula BA, Bar-Sagi D. The bimodal regulation of epidermal growth factor signaling by human Sprouty proteins. Proceedings of the National Academy of Sciences of the United States of America. 2002 Apr 30;99(9):6041–6046. doi: 10.1073/pnas.052090899. PubMed PMID: 11983899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein OD, Minowada G, Peterkova R, Kangas A, Yu BD, Lesot H, et al. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Developmental cell. 2006 Aug;11(2):181–190. doi: 10.1016/j.devcel.2006.05.014. PubMed PMID: 16890158. Pubmed Central PMCID: 2847684. Epub 2006/08/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi L, Zhang S, Lin Y, Prunskaite-Hyyrylainen R, Vuolteenaho R, Itaranta P, et al. Sprouty proteins regulate ureteric branching by coordinating reciprocal epithelial Wnt11, mesenchymal Gdnf and stromal Fgf7 signalling during kidney development. Development. 2004 Jul;131(14):3345–3356. doi: 10.1242/dev.01200. PubMed PMID: 15201220. Epub 2004/06/18. eng. [DOI] [PubMed] [Google Scholar]
- 12.Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Developmental cell. 2002 Sep;3(3):439–449. doi: 10.1016/s1534-5807(02)00261-7. PubMed PMID: 12361605. eng. [DOI] [PubMed] [Google Scholar]
- 13.Ornitz DM. FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev. 2005 Apr;16(2):205–213. doi: 10.1016/j.cytogfr.2005.02.003. PubMed PMID: 15863035. Pubmed Central PMCID: 3083241. Epub 2005/05/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Developmental cell. 2005 Feb;8(2):229–239. doi: 10.1016/j.devcel.2004.12.004. PubMed PMID: 15691764. eng. [DOI] [PubMed] [Google Scholar]
- 15.Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a Mouse Deafness Gene, Regulates Cell Fate Decisions in the Auditory Sensory Epithelium by Antagonizing FGF Signaling. Developmental cell. 2005 Apr;8(4):553–564. doi: 10.1016/j.devcel.2005.02.009. PubMed PMID: 15809037. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Woitge HW, Braut A, Kronenberg MS, Lichtler AC, Mina M, et al. Expression and activity of osteoblast-targeted Cre recombinase transgenes in murine skeletal tissues. Int J Dev Biol. 2004 Sep;48(7):645–653. doi: 10.1387/ijdb.041816fl. PubMed PMID: 15470637. [DOI] [PubMed] [Google Scholar]
- 17.Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000 Feb;26(2):145–146. PubMed PMID: 10686612. [PubMed] [Google Scholar]
- 18.Rodriguez L, Tu C, Cheng Z, Chen TH, Bikle D, Shoback D, et al. Expression and functional assessment of an alternatively spliced extracellular Ca2+-sensing receptor in growth plate chondrocytes. Endocrinology. 2005 Dec;146(12):5294–5303. doi: 10.1210/en.2005-0256. PubMed PMID: 16166224. Epub 2005/09/17. eng. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Nishida S, Boudignon BM, Burghardt A, Elalieh HZ, Hamilton MM, et al. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007 Sep;22(9):1329–1337. doi: 10.1359/jbmr.070517. PubMed PMID: 17539737. Epub 2007/06/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010 Jul;25(7):1468–1486. doi: 10.1002/jbmr.141. PubMed PMID: 20533309. Epub 2010/06/10. [DOI] [PubMed] [Google Scholar]
- 21.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013 Jan;28(1):2–17. doi: 10.1002/jbmr.1805. PubMed PMID: 23197339. Epub 2012/12/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Retting KN, Song B, Yoon BS, Lyons KM. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009 Apr;136(7):1093–1104. doi: 10.1242/dev.029926. PubMed PMID: 19224984. Pubmed Central PMCID: 2668702. Epub 2009/02/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon BS, Pogue R, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, et al. BMPs regulate multiple aspects of growth-plate chondrogenesis through opposing actions on FGF pathways. Development. 2006 Dec;133(23):4667–4678. doi: 10.1242/dev.02680. PubMed PMID: 17065231. eng. [DOI] [PubMed] [Google Scholar]
- 24.Purcell P, Jheon A, Vivero MP, Rahimi H, Joo A, Klein OD. Spry1 and spry2 are essential for development of the temporomandibular joint. Journal of dental research. 2012 Apr;91(4):387–393. doi: 10.1177/0022034512438401. PubMed PMID: 22328578. Pubmed Central PMCID: 3310757. Epub 2012/02/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanafusa H, Torii S, Yasunaga T, Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nature cell biology. 2002 Nov;4(11):850–858. doi: 10.1038/ncb867. PubMed PMID: 12402043. Epub 2002/10/29. eng. [DOI] [PubMed] [Google Scholar]
- 26.Yusoff P, Lao DH, Ong SH, Wong ES, Lim J, Lo TL, et al. Sprouty2 inhibits the Ras/MAP kinase pathway by inhibiting the activation of Raf. J Biol Chem. 2002 Feb 1;277(5):3195–3201. doi: 10.1074/jbc.M108368200. PubMed PMID: 11698404. Epub 2001/11/08. eng. [DOI] [PubMed] [Google Scholar]
- 27.Li C, Chen L, Iwata T, Kitagawa M, Fu XY, Deng CX. A Lys644Glu substitution in fibroblast growth factor receptor 3 (FGFR3) causes dwarfism in mice by activation of STATs and ink4 cell cycle inhibitors. Hum Mol Genet. 1999 Jan;8(1):35–44. doi: 10.1093/hmg/8.1.35. PubMed PMID: 9887329. [DOI] [PubMed] [Google Scholar]
- 28.Guo C, Degnin CR, Laederich MB, Lunstrum GP, Holden P, Bihlmaier J, et al. Sprouty 2 disturbs FGFR3 degradation in thanatophoric dysplasia type II: a severe form of human achondroplasia. Cell Signal. 2008 Aug;20(8):1471–1477. doi: 10.1016/j.cellsig.2008.04.001. PubMed PMID: 18485666. Pubmed Central PMCID: PMC2675614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen CI, Jheon AH, Mostowfi P, Charles C, Ching S, Thirumangalathu S, et al. FGF signaling regulates the number of posterior taste papillae by controlling progenitor field size. PLoS genetics. 2011 Jun;7(6):e1002098. doi: 10.1371/journal.pgen.1002098. PubMed PMID: 21655085. Pubmed Central PMCID: 3107195. Epub 2011/06/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minina E, Wenzel HM, Kreschel C, Karp S, Gaffield W, McMahon AP, et al. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development. 2001 Nov;128(22):4523–4534. doi: 10.1242/dev.128.22.4523. PubMed PMID: 11714677. Epub 2001/11/21. eng. [DOI] [PubMed] [Google Scholar]
- 31.Wu CW, Tchetina EV, Mwale F, Hasty K, Pidoux I, Reiner A, et al. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002 Apr;17(4):639–651. doi: 10.1359/jbmr.2002.17.4.639. PubMed PMID: 11918221. Epub 2002/03/29. [DOI] [PubMed] [Google Scholar]
- 32.Kozhemyakina E, Lassar AB, Zelzer E. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 2015 Mar 1;142(5):817–831. doi: 10.1242/dev.105536. PubMed PMID: 25715393. Pubmed Central PMCID: 4352987. Epub 2015/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franzen A, Hultenby K, Reinholt FP, Onnerfjord P, Heinegard D. Altered osteoclast development and function in osteopontin deficient mice. J Orthop Res. 2008 May;26(5):721–728. doi: 10.1002/jor.20544. PubMed PMID: 18050311. Epub 2007/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 34.Usui M, Xing L, Drissi H, Zuscik M, O'Keefe R, Chen D, et al. Murine and chicken chondrocytes regulate osteoclastogenesis by producing RANKL in response to BMP2. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008 Mar;23(3):314–325. doi: 10.1359/JBMR.071025. PubMed PMID: 17967138. Pubmed Central PMCID: PMC2636701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.