Abstract
Background
Cerebral pressure passivity (CPP) in sick newborns can be detected by evaluating coupling between mean arterial pressure (MAP) and cerebral blood flow measured by NIRS hemoglobin difference (HbD). However, continuous MAP monitoring requires invasive catheterization with its inherent risks. We tested whether heart rate (HR) could serve as a reliable surrogate for MAP in the detection of CPP in sick newborns.
Methods
Continuous measurements of MAP, HR, and HbD were made and partitioned into 10-minute epochs. Spectral coherence (COH) was computed between MAP and HbD (COHMAP-HbD) to detect CPP, between HR and HbD (COHHR-HbD) for comparison, and between MAP and HR (COHMAP-HR) to quantify baroreflex function (BRF). The agreement between COHMAP-HbD and COHHR-HbD was assessed using ROC analysis.
Results
We found poor agreement between COHMAP-HbD and COHHR-HbD in left hemisphere (area under the ROC curve (AUC) 0.68) and right hemisphere (AUC 0.71). Baroreflex failure (COHMAP-HR not significant) was present in 79% of epochs. Confining comparison to epochs with intact BRF showed an AUC of 0.85 for both hemispheres.
Conclusions
In these sick newborns, HR was an unreliable surrogate for MAP required for the detection of CPP. This is likely due to the prevalence of BRF failure in these infants.
Introduction
Brain injury is a major long-term consequence of critical illness in the newborn and young infant. In infants with unstable systemic hemodynamics and loss of cerebral pressure autoregulation, cerebral hypoperfusion and reperfusion are important mechanisms of injury. Cerebral pressure passivity (CPP) has been shown to be prevalent in high risk newborns,(1) has been associated with brain injury, (2) and is currently impossible to predict accurately with routine bedside monitoring.
To date, the ability to monitor infants continuously for the emergence of CPP has been complicated by several factors. The major obstacle continues to be the lack of a reliable, non-invasive technique for continuous blood pressure (BP) measurement. Indwelling arterial catheters are used for invasive BP monitoring in some, but not all, critically ill infants. This relates to the technical challenges of catheter placement, particularly in the smallest premature infants, and the risk of infection, hemorrhage and regional ischemia. (3, 4) Non-invasive techniques for continuous BP monitoring have been applied successfully in adults, but have not found widespread application in newborns. (5, 6) Thus, the lack of a reliable surrogate for continuous invasive BP monitoring remains a significant impediment for CPP monitoring in infants.
In healthy, mature subjects, changes in MAP are associated with opposite changes in heart rate (HR) mediated through the baroreflex. (7) HR changes are easily and non-invasively measured by continuous cutaneous ECG recordings. The NIRS hemoglobin difference (HbD) signal has been shown to be highly correlated with cerebral blood flow in animal models. (1, 2, 8, 9) In previous high-risk newborn populations we (2) and others (10) have used the coherence between changes in mean arterial pressure (MAP) and HbD to identify CPP. In the current study we use previously acquired datasets from studies in which critically ill preterm and term infants underwent invasive arterial BP monitoring, to test the hypothesis that the coherence between HR and HbD will reliably predict the coherence between MAP and HbD, allowing HR changes to serve as a reliable surrogate BP changes for detecting CPP.
In the current study our objectives were to quantify coherence (COH) between MAP and HbD (COHMAP-HbD) to study CPP, between HR and HbD (COHHR-HbD) for comparison and between MAP and HR (COHMAP-HR) to quantify baroreflex function (BRF) (i) to test the hypothesis that measurements of HR are a reliable surrogate (interchangeable) for changes in MAP when monitoring for CPP in critically-ill infants, and (ii) to evaluate the effect of the BRF on the ability of HR to serve as a surrogate for MAP when monitoring CPP.
Results
Clinical
In this study we included data from 82 infants ranging from 23 to 41 weeks of GA. These infants were studied during a broad spectrum of critical illness, and were representative of cases in which CPP might be prevalent. Specifically, 43 subjects were term infants undergoing therapeutic hypothermia for neonatal encephalopathy; (11) 19 newborns with congenital heart disease prior to corrective surgery; 12 premature infants undergoing surgical PDA ligation, and 8 premature infants in the early postnatal period. The duration of each study varied between 2 to 90 hours. The clinical characteristics of these subjects are given in Table 1. The median postnatal age at the onset of study was 0.79 days. Pressor-inotrope support was required in 34 infants, and 38 had respiratory failure requiring positive pressure ventilation, for either all or part of the study period. Brain injury was diagnosed in 25 infants, and 9 infants died prior to intensive care unit discharge. There was no significant difference in the variance of the MAP measured from peripheral artery from 13 infants and the variance of the MAP measured from umbilical artery in similar age group infants (p>0.05).
Table 1.
Clinical data (n=82)
| Clinical Characteristic | Median [range] or Number (Percentage) |
|---|---|
| GA at birth, in weeks | 38 [23 -- 41] |
| Post-natal age at study, in days | 0.79 [0.14 51] |
| Male (n, %) | 34 (41) |
| Inotropic support (n, %) | 34 (41) |
| Invasive ventilator support (n, %) | 38 (46) |
| Brain injurya (n, %) | 25 (30) |
| Died (n, %) | 7 (9) |
18 diagnosed using magnetic resonance image and 7 diagnosed using ultrasound
Cerebral Pressure Passivity
There were a total of 19165 10-minute epochs from all of the studies. Of those, 3295 epochs (17%) on the left cerebral hemisphere and 3438 (18%) on the right cerebral hemisphere showed significant COHMAP-HbD. These epochs served as gold standard to test agreement between COHMAP-HbD and COHHR-HbD. There was no correlation between significant COHMAP-HbD (pressure passivity) and COHHR-HbD with GA at birth (p>0.05).
Comparison of MAP and HR in detecting CPP
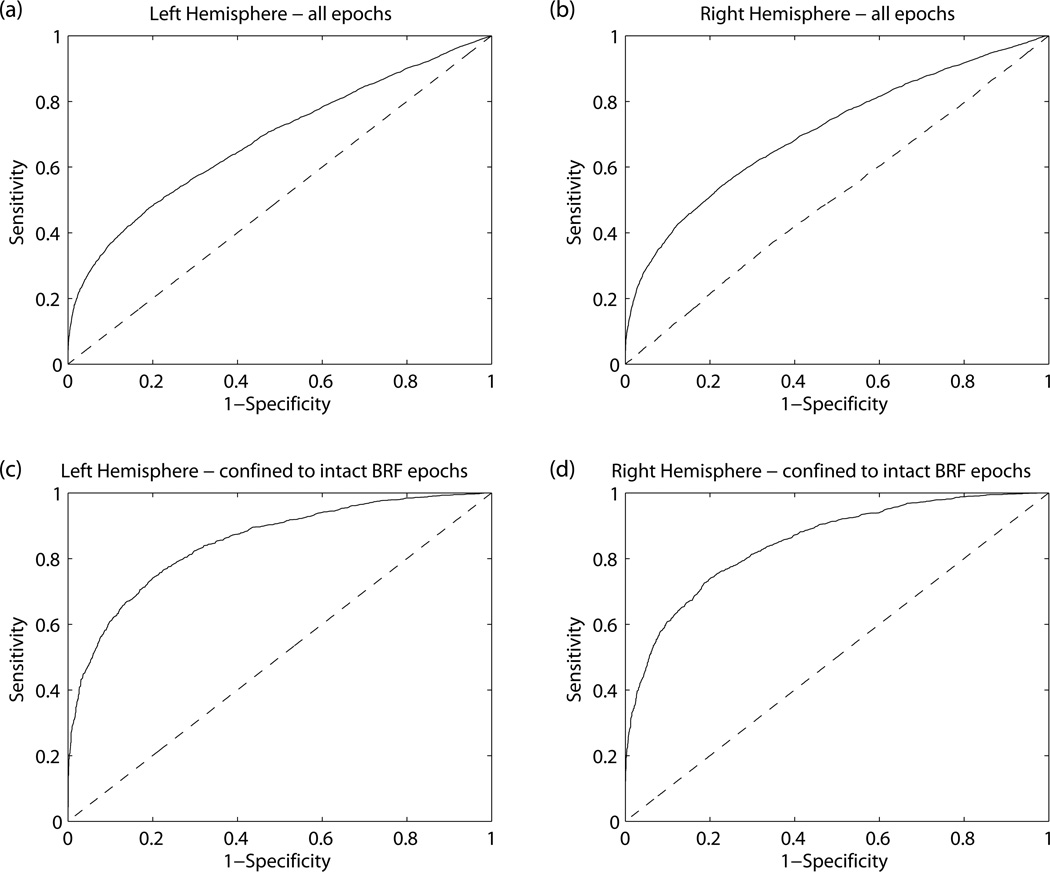
In Figure 1, we show the agreement between COHMAP-HbD and COHHR-HbD. The comparison was poor in both the left (Figure 1a) and right cerebral hemispheres (Figure 1b). The results of the ROC analysis, sensitivity, specificity, and area under the curve (AUC) are 0.47, 0.81, 0.68 and 0.54, 0.78, 0.71 for the left and right hemispheres, respectively.
Figure 1.
Results of testing the ability of HR to serve as a surrogate for MAP to detect CPP from all epochs are shown in a) for left hemisphere, b) for right hemisphere. For the comparison confined to intact BRF epochs, the results are shown in c) and d) for left and right hemispheres, respectively. The specificity, sensitivity, AUC and optimal COHHR-HbD (Threshold) that delineated the coherent COHMAP-HbD epochs from non-coherent ones, are as follows (Specificity, Sensitivity, AUC, Threshold): a) (0.81, 0.47, 0.68, 0.23) b) (0.78, 0.54, 0.71, 0.21) c) (0.8, 0.74, 0.85, 0.26) and d) (0.8, 74, 0.85, 0.27).
Baroreflex analysis
Only 3935 epochs (20.53%) had intact BRF as defined by COHMAP-HR criterion. When we confined our comparison to these epochs, the agreement between COHMAP-HbD and COHHR-HbD improved significantly (AUC=0.85). The results of this comparison during intact BRF epochs are displayed in Figure 1c and Figure 1d for left and right hemispheres, respectively. A COHHR-HbD cut-point value of 0.26 and 0.27 for left and right cerebral hemispheres respectively had a sensitivity of 0.74 and specificity of 0.8 for defining coherent COHMAP-HbD.
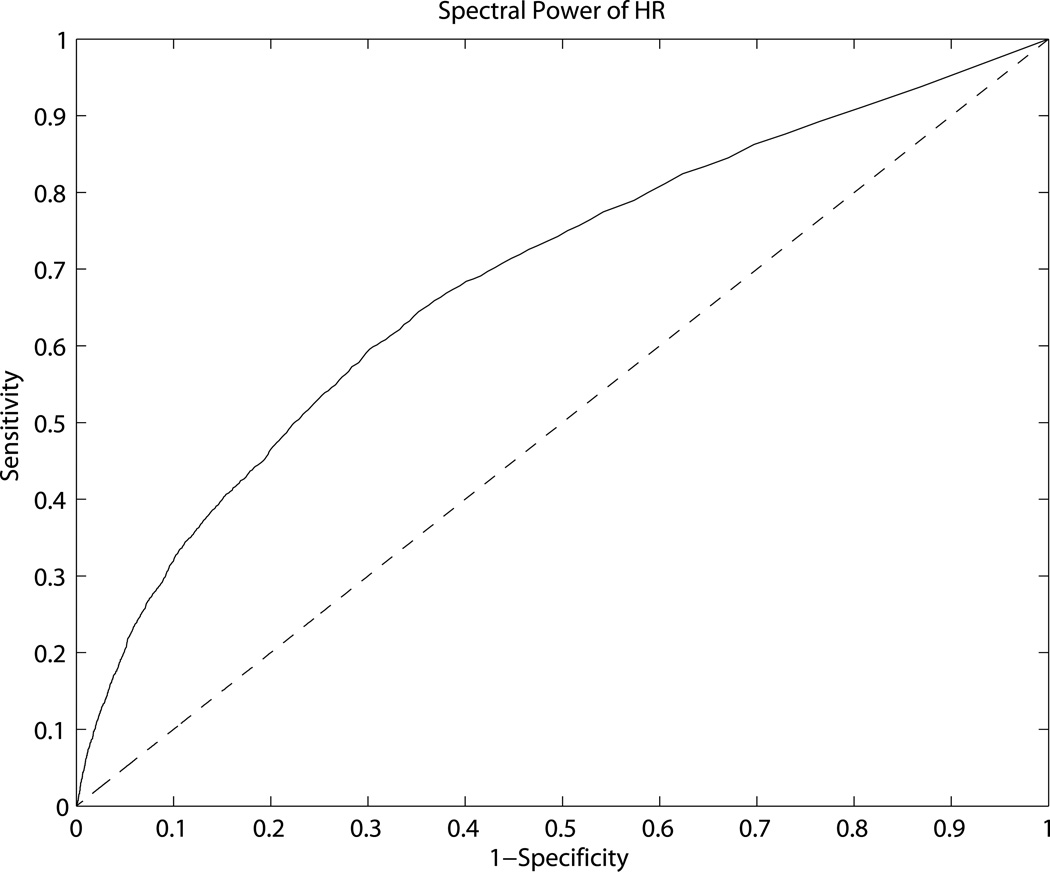
Finally, we sought to evaluate, whether degree of HR variability could identify epochs of intact BRF. To answer this question, we studied the relationship between HR spectral power and COHMAP-HR. The cut-point for HR variability that distinguished coherent COHMAP-HR epochs from non-coherent epochs was 6.85 beats/minute which had a sensitivity, specificity, and AUC of 0.6, 0.7 and 0.69, respectively (see Figure 2).
Figure 2.
Result of the comparison of spectral power of HR between intact BRF (coherent MAP-HR) epochs and non-intact BRF (non-coherent) epochs. The specificity, sensitivity, AUC, and the HR spectral power that demarcated intact BRF epochs from non-intact epochs are 0.7, 0.6, 0.69 and 6.85 beats per minute, respectively.
Discussion
Reliable monitoring for CPP would be a major advance toward the prevention of brain injury in critically ill newborns. (1, 2, 12, 13) Currently, the continuous integration of BP and cerebral measurements needed to detect CPP is impeded by the lack of non-invasive continuous BP monitoring techniques in the newborn. (6, 14, 15) The significant infectious, hemorrhagic, and thrombotic risks of indwelling arterial catheters are a serious concern in these fragile infants. (16–21) In this study, we explore the reliability of non-invasive HR as a surrogate for invasive MAP in monitoring for CPP. Not surprisingly we show that when the BRF is intact, HR changes are a reliable surrogate for MAP changes. Conversely, when BRF is impaired, HR cannot reliably be used to evaluate CPP. Unfortunately, in this broad spectrum of critically ill newborn infants, we show an almost 80% prevalence of impaired BRF. The high prevalence of impaired BRF in these infants suggests an inability to maintain a stable cardiac output and further emphasizes the urgent need for continuous MAP monitoring in sick infants. (22–24) Our results suggest that HR spectral power <6.85 beats per minute may be a marker for impaired BRF, indicating a need for invasive MAP measurements to allow for reliable CPP monitoring.
A recent study quantified the association between HR and cerebral tissue oxygenation index, using a time domain metric. (25) Based on the correlation between HR and both tissue oxygenation and Doppler flow velocity, their findings suggest that HR may be a reliable indicator of systemic hemodynamics. The reasons for discrepancy between the two studies are not immediately clear. However, there were several important methodological differences. In our study, we tested how reliably COHHR-HbD would predict COHMAP-HbD in every 10-minute window of data, instead of metrics averaged over the entire study period, as in Mitra et al. (25). Furthermore the time domain metric used was not assessed for its statistical significance. In contrast, we used a mathematically derived threshold to assess the statistical significance of coherence.
We are unaware of previous studies evaluating HR as a surrogate for BP in young infants. In adults with unilateral carotid obstruction, HR has proved to be a reliable surrogate for BP in the study of CPP. (26) This study differed in several important ways from ours. First, this was a mature and relatively stable population, unlike our subjects with systemic immaturity and critical illness. Second, the adult study used a paced-breathing paradigm over three minutes, and analyses were confined to the respiratory frequency. (26) In fact, most previous studies of BRF in mature populations have made point measurements in time to characterize HR responses to pharmacologic, (27), Valsalva, (28) or neck-chamber, (29) pressure perturbations. Typically, these techniques would produce a sigmoid (S-shaped) curve from which the BRF is defined using the linear region of the curve. (30) These techniques are neither feasible nor suitable for the fundamental goal of our work, which is to develop continuous monitoring of hemodynamics in critically ill newborns. Hence, in this work we used transfer function analysis of spontaneous MAP and HR changes to assess the BRF in sick infants, an approach used previously in other studies. (26, 31, 32) Furthermore, transfer function analysis has correlated well with BRF assessments using pharmacological perturbations. (7, 27) Point measurements of BRF are not useful for continuous monitoring of sick infants, given the fluctuating nature of systems control (such as cerebral pressure autoregulation) (1) and the changing clinical nature of critical illness. Perhaps not surprisingly, our study of critically ill infants shows a high prevalence of BRF impairment, contradicting our premise that MAP changes would be reliably reflected in the HR signal. This impaired BRF may be attributable to failure of the underdeveloped parasympathetic arm of the autonomic nervous system. (7, 33)
The threshold of 0.3842 used for COHMAP-HbD may be too stringent for COHHR-HbD. The weak COHHR-HbD may be due to one or more of the following reasons: there are other possible technical reasons for the failure of HR changes to serve as a reliable surrogate for changing MAP when monitoring for CPP. One possibility is that the power of HR variability may have lacked that of MAP variability, thereby precluding the COHHR-HbD reaching significant level during CPP. Further, latency between the signals has shown to affect the coherence estimation. (34) The impulse-response latency between MAP and HR is longer in premature infants and shortens with maturation. (7) Because of these factors, we derived a new threshold for the COHHR-HbD using ROC analysis. As anticipated, COHHR-HbD required a lower significance level compared to the COHMAP-HbD to study CPP.
In addition, there are numerous intrinsic and extrinsic factors that can further affect BRF in sick newborn infants. These include vasoactive medications, (35), hypothermia, (36), and pre-existing brain injury. (37) The influence of such factors on BRF is beyond the scope of this study given our heterogeneous population.
Our work has several limitations. The BP measurements made using umbilical artery have been shown to be more reliable than that measured using the peripheral artery. Since, our work relies on the temporal nature of changes in the BP rather than the absolute values, we have included infants with peripheral arterial BP in our study. We have used HbD as a marker of cerebral blood flow in our work. Although controlled human and animal studies have validated the relationship between cerebral blood flow and NIRS measurements, it is valid only if oxygen extraction is constant. If the extraction is variable, then this assumption may not hold true. But the extraction is unlikely to change significantly in the relatively brief 10-minute epochs. Since the primary goal was to test how reliably COHHR-HbD could identify 10-minute epochs with known significant COHMAP-HbD, we considered each 10-minute epoch as independent and used ROC analysis for the comparison. The effect of high intra-subject correlation and the possible inflation of BRF dysfunction from records of longer durations from sick infants might have influenced our results, which we have not adjusted for in our report. Our goal was to test the ability of HR to serve as a surrogate for BP in sick infants over a broad range of GA. Even though we have shown that our results are independent of GA, we have not adjusted for clinical variables such as medications and clinical conditions (such as congenital heart disease), which may have affected our results.
In summary, we tested the hypothesis that continuous HR measurements could serve as a surrogate for invasive BP in the development of continuous CPP monitoring devices. Data from a broad spectrum of critically ill newborns failed to support this hypothesis. The primary reason for this is the unexpectedly high prevalence of autonomic impairment, with baroreflex failure. Our study highlights the risk of potentially injurious impairment of systemic hemodynamics regulation in this high-risk population. The high prevalence of impaired BRF in this retrospective study emphasizes the need for prospective studies of the autonomic nervous system in critically ill newborns.
Methods
In this study, we included data collected as part of a number of studies on brain development in critically ill newborns. Informed written consent for neuromonitoring during periods of critical illness was obtained from the parent of each participant, and the studies were approved by the Children’s National Health System Institutional Review Board in all original studies. We acquired continuous BP (69 umbilical artery line and 13 peripheral artery (12 radial and 1 tibial)) and ECG measures from the analog port of the bedside monitors (Philips Intelli Vue MP-70, Andover, MA). Cerebral oximetry was obtained from the left and right fronto-temporal regions using NIRS (NIRO 200, Hamamatsu Photonics, Hamamatsu City, Japan). All of the signals were acquired in analog format and digitized simultaneously at 1 KHz using Labview 2011 Professional version (National Instruments, Austin, TX) software. The physiological signals were stored in a computer and analyzed off-line.
ECG was filtered between 0.5–60 Hz, the R-wave was identified reliably using the Hilbert transform approach (38) and the HR was calculated. Using continuous BP monitors, systolic and diastolic pressures were computed, and the MAP was calculated as the linear combination of one third of systolic and two thirds of diastolic pressures. HR and MAP were interpolated to derive continuous evenly spaced samples using spline technique. All calculations were done using MATLAB (Mathworks Inc, Natick, MA). Data were then partitioned into 10-minute epochs. The decision to parcellate the datasets into 10-minute epochs is based on the fact that the impulse-response time for cerebral pressure autoregulation ranges from 5–20 seconds. (2) Therefore, to reliably quantify this function we aimed to include at least 30 cycles in our calculation, which required approximately 10 minutes of data. We have developed and validated an automated artifact detection/rejection program. Periods of missing MAP (e.g., arterial blood sampling or transport of the NICU) were removed from the analysis. In earlier work, we proposed a modification to the coherence analysis technique (39) that reliably deals with non-stationarities in the signal caused by artifacts. We used the modified coherence approach in this work and calculated coherence between MAP and HbD (COHMAP-HbD), between HR and HbD (COHHR-HbD) and MAP and HR (COHMAP-HR). In these ways we have reduced the vulnerability of our data to artifact.
COHMAP-HbD was quantified in the frequency band 0.05–0.25Hz. (2, 39) COHMAP-HbD above a pre-defined threshold of 0.3842 was considered statistically significant and defined as CPP. (39, 40) To test our primary hypothesis, i.e., that HR changes would be a reliable surrogate for changes in MAP, we then quantified COHHR-HbD for each epoch using the same frequency. We used ROC analysis to compare the agreement between COHMAP-HbD and COHHR-HbD and identified the optimal COHHR-HbD that would demarcate pressure passive epochs with COHMAP-HbD >0.3842 and epochs with COHMAP-HbD≤0.3842.
To evaluate the influence of BRF integrity on our findings, we quantified BRF as COHMAP-HR. BRF was considered intact if COHMAP-HR in the 0.05–0.25Hz band was greater than 0.3842. (39) We then compared the agreement between COHMAP-HbD and COHHR-HbD only for the epochs with intact BRF. Finally, we sought to further characterize the BRF by determining the critical HR spectral power that signifies intact BRF.
For each subject, we calculated the percent time the COHMAP-HbD was greater than the threshold of 0.3842, which was defined as pressure passivity index (PPI) in our earlier work. (1). We studied the Pearson’s correlation between PPI and GA at birth.
Spectral power is related to the variance of the signal. Using t-test, we compared the variance of the BP measurements made using peripheral artery in 13 infants to the variance of BP measurements from umbilical artery in infants in the same age group. A value of p<0.05 was considered statistically significant.
Acknowledgments
Financial support:
This study was funded by an internal special purpose fund. This publication was supported by the Clinical and Translational Science Institute at Children’s National Health System (NIH UL1TR000075, KL2TR000076), and the Intellectual and Developmental Disabilities Research Consortium (NIH P30HD040677).
Footnotes
Conflict of Interest: The authors have no potential conflicts of interests.
Disclaimer: This study was presented in part at the 2013 Pediatric Academic Society.
References Cited
- 1.Soul JS, Hammer PE, Tsuji M, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61:467–473. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- 2.O'Leary H, Gregas MC, Limperopoulos C, et al. Elevated cerebral pressure passivity is associated with prematurity-related intracranial hemorrhage. Pediatrics. 2009;124:302–309. doi: 10.1542/peds.2008-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmann PA, Dykes FD, Lazzara A, Holt PJ, Giddens DP, Carrigan TA. Relationship between pressure passivity and subependymal/intraventricular hemorrhage as assessed by pulsed Doppler ultrasound. Pediatrics. 1983;72:665–669. [PubMed] [Google Scholar]
- 4.Adams MA, Pasternak JF, Kupfer BM, Gardner TH. A computerized system for continuous physiologic data collection and analysis: initial report on mean arterial blood pressure in very low- birth-weight infants. Pediatrics. 1983;71:23–30. [PubMed] [Google Scholar]
- 5.Blaber AP, Bondar RL, Stein F, et al. Transfer function analysis of cerebral autoregulation dynamics in autonomic failure patients. Stroke. 1997;28:1686–1692. doi: 10.1161/01.str.28.9.1686. [DOI] [PubMed] [Google Scholar]
- 6.Andriessen P, Schoffelen RL, Berendsen RC, et al. Noninvasive assessment of blood pressure variability in preterm infants. Pediatr Res. 2004;55:220–223. doi: 10.1203/01.PDR.0000104152.85296.4F. [DOI] [PubMed] [Google Scholar]
- 7.Andriessen P, Oetomo SB, Peters C, Vermeulen B, Wijn PF, Blanco CE. Baroreceptor reflex sensitivity in human neonates: the effect of postmenstrual age. J Physiol. 2005;568:333–341. doi: 10.1113/jphysiol.2005.093641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuji M, duPlessis A, Taylor G, Crocker R, Volpe JJ. Near infrared spectroscopy detects cerebral ischemia during hypotension in piglets. Pediatr Res. 1998;44:591–595. doi: 10.1203/00006450-199810000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Roberts IG, Fallon P, Kirkham FJ, et al. Measurement of cerebral blood flow during cardiopulmonary bypass with near-infrared spectroscopy. J Thorac Cardiovasc Surg. 1998;115:94–102. doi: 10.1016/s0022-5223(98)70447-7. [DOI] [PubMed] [Google Scholar]
- 10.Panerai RB, Hudson V, Fan L, et al. Assessment of dynamic cerebral autoregulation based on spontaneous fluctuations in arterial blood pressure and intracranial pressure. Physiol Meas. 2002;23:59–72. doi: 10.1088/0967-3334/23/1/306. [DOI] [PubMed] [Google Scholar]
- 11.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 12.Howlett JA, Northington FJ, Gilmore MM, et al. Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2013;74:525–535. doi: 10.1038/pr.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Costa CS, Czosnyka M, Smielewski P, Mitra S, Stevenson GN, Austin T. Monitoring of cerebrovascular reactivity for determination of optimal blood pressure in preterm infants. J Pediatr. 2015;167:86–91. doi: 10.1016/j.jpeds.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 14.Andriessen P, Schraa O, van den Bosch-Ruis W, et al. Feasibility of non-invasive continuous finger arterial blood pressure measurements in very young children, aged 0–4 years. Pediatr Res. 2008 doi: 10.1203/PDR.0b013e31816c8fe3. [DOI] [PubMed] [Google Scholar]
- 15.Drouin E, Gournay V, Calamel J, Mouzard A, Roze JC. Feasibility of using finger arterial pressure in neonates. Arch Dis Child Fetal Neonatal Ed. 1997;77:F139–F140. doi: 10.1136/fn.77.2.f139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman J, Fabre J, Netscher D, Jaksic T. Treatment of acute neonatal vascular injuries--the utility of multiple interventions. J Pediatr Surg. 1999;34:940–945. doi: 10.1016/s0022-3468(99)90764-9. [DOI] [PubMed] [Google Scholar]
- 17.Denkler KA, Cohen BE. Reversal of dopamine extravasation injury with topical nitroglycerin ointment. Plast Reconstr Surg. 1989;84:811–813. doi: 10.1097/00006534-198911000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Vasquez P, Burd A, Mehta R, Hiatt M, Hegyi T. Resolution of peripheral artery catheter-induced ischemic injury following prolonged treatment with topical nitroglycerin ointment in a newborn: a case report. J Perinatol. 2003;23:348–350. doi: 10.1038/sj.jp.7210870. [DOI] [PubMed] [Google Scholar]
- 19.Braly BD. Neonatal arterial thrombosis and embolism. Surgery. 1965;58:869–873. [PubMed] [Google Scholar]
- 20.Subhani M, Sridhar S, DeCristofaro JD. Phentolamine use in a neonate for the prevention of dermal necrosis caused by dopamine: a case report. J Perinatol. 2001;21:324–326. doi: 10.1038/sj.jp.7200493. [DOI] [PubMed] [Google Scholar]
- 21.Wong AF, McCulloch LM, Sola A. Treatment of peripheral tissue ischemia with topical nitroglycerin ointment in neonates. J Pediatr. 1992;121:980–983. doi: 10.1016/s0022-3476(05)80356-7. [DOI] [PubMed] [Google Scholar]
- 22.Amin RS, Carroll JL, Jeffries JL, et al. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2004;169:950–956. doi: 10.1164/rccm.200309-1305OC. [DOI] [PubMed] [Google Scholar]
- 23.Axelrod FB, Chelimsky GG, Weese-Mayer DE. Pediatric autonomic disorders. Pediatrics. 2006;118:309–321. doi: 10.1542/peds.2005-3032. [DOI] [PubMed] [Google Scholar]
- 24.Harrington C, Kirjavainen T, Teng A, Sullivan CE. Altered autonomic function and reduced arousability in apparent life-threatening event infants with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1048–1054. doi: 10.1164/ajrccm.165.8.2102059. [DOI] [PubMed] [Google Scholar]
- 25.Mitra S, Czosnyka M, Smielewski P, O'Reilly H, Brady K, Austin T. Heart rate passivity of cerebral tissue oxygenation is associated with predictors of poor outcome in preterm infants. Acta Paediatr. 2014;103:e374–e382. doi: 10.1111/apa.12696. [DOI] [PubMed] [Google Scholar]
- 26.Sommerlade L, Schelter B, Timmer J, Reinhard M. Grading of dynamic cerebral autoregulation without blood pressure recordings: a simple Doppler-based method. Ultrasound Med Biol. 2012;38:1546–1551. doi: 10.1016/j.ultrasmedbio.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Bonyhay I, Risk M, Freeman R. High-pass filter characteristics of the baroreflex--a comparison of frequency domain and pharmacological methods. PLoS One. 2013;8:e79513. doi: 10.1371/journal.pone.0079513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Airaksinen KE, Hartikainen JE, Niemela MJ, Huikuri HV, Mussalo HM, Tahvanainen KU. Valsalva manoeuvre in the assessment of baroreflex sensitivity in patients with coronary artery disease. Eur Heart J. 1993;14:1519–1523. doi: 10.1093/eurheartj/14.11.1519. [DOI] [PubMed] [Google Scholar]
- 29.Eckberg DL, Cavanaugh MS, Mark AL, Abboud FM. A simplified neck suction device for activation of carotid baroreceptors. J Lab Clin Med. 1975;85:167–173. [PubMed] [Google Scholar]
- 30.La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol. 2008;13:191–207. doi: 10.1111/j.1542-474X.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andriessen P, Koolen AM, Berendsen RC, et al. Cardiovascular fluctuations and transfer function analysis in stable preterm infants. Pediatr Res. 2003;53:89–97. doi: 10.1203/00006450-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Yiallourou SR, Sands SA, Walker AM, Horne RS. Postnatal development of baroreflex sensitivity in infancy. J Physiol. 2010;588:2193–2203. doi: 10.1113/jphysiol.2010.187070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yiallourou SR, Witcombe NB, Sands SA, Walker AM, Horne RS. The development of autonomic cardiovascular control is altered by preterm birth. Early Hum Dev. 2012;89:145–152. doi: 10.1016/j.earlhumdev.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Govindan RB, Raethjen J, Kopper F, Claussen JC, Deuschl G. Estimation of time delay by coherence analysis. Physica A: Statistical Mechanics and its Applications. 2005;350:277–295. [Google Scholar]
- 35.Nachar RA, Booth EA, Friedlich P, et al. Dose-dependent hemodynamic and metabolic effects of vasoactive medications in normotensive, anesthetized neonatal piglets. Pediatr Res. 2011;70:473–479. doi: 10.1203/PDR.0b013e31822e178e. [DOI] [PubMed] [Google Scholar]
- 36.Lasky RE, Parikh NA, Williams AL, Padhye NS, Shankaran S. Changes in the PQRST intervals and heart rate variability associated with rewarming in two newborns undergoing hypothermia therapy. Neonatology. 2009;96:93–95. doi: 10.1159/000205385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vergales BD, Zanelli SA, Matsumoto JA, et al. Depressed heart rate variability is associated with abnormal EEG, MRI, and death in neonates with hypoxic ischemic encephalopathy. Am J Perinatol. 2014;31:855–862. doi: 10.1055/s-0033-1361937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulusar UD, Govindan RB, Wilson JD, Lowery CL, Preissl H, Eswaran H. Adaptive rule based fetal QRS complex detection using hilbert transform. Conf Proc IEEE Eng Med Biol Soc. 2009;1:4666–4669. doi: 10.1109/IEMBS.2009.5334180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Govindan RB, Massaro AN, Andescavage NN, Chang T, du Plessis A. Cerebral pressure passivity in newborns with encephalopathy undergoing therapeutic hypothermia. Front Hum Neurosci. 2013;8:266. doi: 10.3389/fnhum.2014.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data--theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]