Abstract
Background
Approaches to improve the immune response of immunocompromised patients to influenza vaccination are needed.
Methods
Children and young adults (3–21 years) with cancer or HIV infection were randomized to receive 2 doses of high-dose (HD) trivalent influenza vaccine (TIV) or of standard-dose (SD) TIV. Hemagglutination inhibition (HAI) antibody titers were measured against H1, H3, and B antigens after each dose and 9 months later. Seroconversion was defined as ≥ 4-fold rise in HAI titer comparing pre- and post-vaccine sera. Seroprotection was defined as a post-vaccine HAI titer ≥1:40. Reactogenicity events (RE) were solicited using a structured questionnaire 7 and 14 days after each dose of vaccine, and adverse events by medical record review for 21 days after each dose of vaccine.
Results
Eighty-five participants were enrolled in the study; 27 with leukemia, 17 with solid tumor (ST), and 41 with HIV. Recipients of HD TIV had significantly greater fold increase in HAI titers to B antigen in leukemia group and to H1 antigen in ST group compared to SD TIV recipients. This increase was not documented in HIV group. There were no differences in seroconversion or seroprotection between HD TIV and SD TIV in all groups. There was no difference in the percentage of solicited RE in recipients of HD TIV (54% after dose 1 and 38% after dose 2) compared to SD TIV (40% after dose 1 and 20% after dose 2, p=0.27 and 0.09 after dose 1 and 2, respectively).
Conclusion
HD TIV was more immunogenic than SD TIV in children and young adults with leukemia or ST, but not with HIV. HD TIV was safe and well-tolerated in children and young adults with leukemia, ST, or HIV.
Keywords: Influenza vaccine, High dose, Cancer, HIV, Immune response, Safety
INTRODUCTION
Influenza remains a major cause of morbidity and mortality among immunocompromised children, resulting in prolonged illness and viral shedding, delays in chemotherapy, and increase in the incidence of hospitalization1–7. Because of this significant burden, the Advisory Committee on Immunization Practices and the American Academy of Pediatrics recommend annual influenza vaccination of children at increased risk for hospitalization including those receiving anti-neoplastic therapy, or are HIV-infected8. However, studies evaluating the ability of immunocompromised patients to generate an immune response to standard-dose (SD) influenza vaccine indicated that the immunogenicity and efficacy of influenza vaccine varies depending on the underlying immunosuppressive disease and is generally poor compared to healthy persons9–22. For this reason, studies are needed to better understand the relationship between underlying immunocompromising diseases and immunogenicity of the vaccines; and to identify effective approaches that improve the immune response of immunocompromised patients to influenza vaccination. One proposed approach is to use higher dose influenza vaccine.
High-dose (HD) trivalent inactivated influenza vaccine (TIV) is indicated for vaccination of persons 65 years of age and older because HD TIV has been shown to be safe and more immunogenic than SDTIV in this high-risk group23–33. HD TIV contains 60 mcg of each of 3 antigens (H3N2, H1N1, and influenza B) instead of the usual 15 mcg per antigen in the SD TIV. Based on the published studies using HD TIV in the elderly, we hypothesized that HD TIV is more immunogenic than SD TIV in children and young adults with cancer or HIV infection.
MATERIALS AND METHODS
Study Design and Objectives
This was a randomized, open-label study of an HD TIV compared to an SD TIV in children and young adults with cancer (leukemia and solid tumor (ST)) or HIV (clinicaltrials.gov; NCT01205581). Patients with either cancer or HIV were separately randomized 1:1 to receive 2 doses of either HD or SD TIV, administered at least 21 days apart. Randomization was restricted with permuted blocks of size four using a computer-generated randomization schedule. Enrollment occurred during two consecutive influenza seasons, 2010–2011 and 2011–2012.
The primary objective was to compare seroconversion to influenza H1, H3, and B antigens following receipt of HD TIV to those following SD TIV, in children and young adults with cancer and HIV.
The secondary objectives were to describe the safety and reactogenicity of HD and SD TIV; to compare seroconversion to influenza H1, H3, and B antigens induced by 1 dose and 2 doses of HD or SD TIV; and to describe the relationship between baseline absolute lymphocyte count and robustness and durability of the immune response.
Study Participants
Children and young adults (3–21 years of age) who were receiving medical care at St. Jude Children’s Research Hospital (SJCRH) for cancer or HIV were enrolled. Inclusion criteria included patients with cancer receiving chemotherapy and /or radiotherapy at the time of enrollment or having received chemotherapy in the 12 weeks prior to participation in the study. Patients with personal or direct family history of Guillain-Barré syndrome, those with history of severe hypersensitivity to egg proteins or any component of TIV, or life-threatening reactions after any previous administration of influenza vaccine were excluded.
Vaccines
The SD TIV (Fluzone, Sanofi Pasteur) contained 15 mcg hemagglutinin (HA) per 0.5mL from each of the following three prototype strains: A/California/7/09 (H1N1), A/Perth/16/09 (H3N2) and B/Brisbane/60/08. The HD TIV (FluzoneHD, Sanofi Pasteur) contained 60 mcg HA per 0.5mL from each of the same three prototype strains in SD TIV. The antigens contained in the TIV administered in 2011–2012 influenza season were the same as those used in 2010–2011. All vaccine doses were administered intramuscularly.
Immunogenicity
Immunogenicity was assessed by determining the rate of seroconversion after the second dose of the vaccine using the serum hemagglutination inhibition (HAI) assay as previously described34. Influenza-specific antibodies against the 3 antigens (H1, H3, and B) were measured before vaccination, 21–42 days after each dose of the vaccine, and 9 months after the first vaccine dose, but before receipt of vaccine for the following influenza season, to assess level and durability of the immune response. Additional blood tests for baseline immune status included white blood cell counts, absolute lymphocyte counts (ALC), and immunoglobulin levels. The separated sera were stored at −80°C until batched runs of HAI assay were completed. Seroconversion was defined as post-vaccine HAI titer ≥1:40 if the pre-vaccine HAI titer was <1:10, or a four-fold rise in post-vaccine HAI titer if the pre-vaccine titer was ≥1:10. Seroprotection was defined as a post-vaccine HAI titer ≥1:40.
Safety and Reactogenicity Evaluation
Reactogenicity events (RE) were solicited using a pre-specified questionnaire administered 7 and 14 days after each dose of vaccine including local (tenderness, erythema, and swelling) and systemic reactions (myalgia, malaise, or fever defined as temperature ≥38.1°C rectal/tympanic, ≥37.8°C oral, or ≥ 37.6°C axillary). Adverse events were assessed by medical record review for 21 days after each dose of vaccine using the adapted National Cancer Institute Common Terminology Criteria for Adverse Events version 4.02. Serious adverse events (SAEs) were collected from first vaccination through the following 6 months.
Statistical Analysis
Pre- and post-vaccination HAI titers were reported as geometric mean titers (GMTs) with 95% confidence intervals. The Wilcoxon-Mann-Whitney test was applied to assess the fold increase in HAI titers after vaccination between intervention groups (HD TIV vs. SD TIV), and the Wilcoxon signed-rank test was used to compare the fold increase from pre-vaccination between 1 dose and 2 doses of vaccines. Fisher’s exact test and McNemar’s test were applied to compare the seroconversion and seroprotection rates between HD and SD TIV groups and between 1 dose and 2 doses, respectively. Statistical analyses were performed for each of the three influenza antigens separately. Wilcoxon-Mann-Whitney test and Fisher’s exact test were used for the comparisons of reactogenicity and adverse events between HD and SD TIV groups after dose 1 and 2, and for the comparison of immune responses between ALC groups. SAS (SAS Institute, Cary, NC), Windows version 9.3 was used. All p-values are 2-sided. The criterion for significance in all analyses is p-value of 0.05 or less.
RESULTS
Study Participants
Eighty-five participants were enrolled in the study. Of the 44 participants with a cancer diagnosis, 27 had leukemia and 17 had STs. Forty-one participants were HIV infected. Figure 1 shows the flow diagram of the study participants. Baseline characteristics of the study participants are reported in Table 1.
Figure 1.
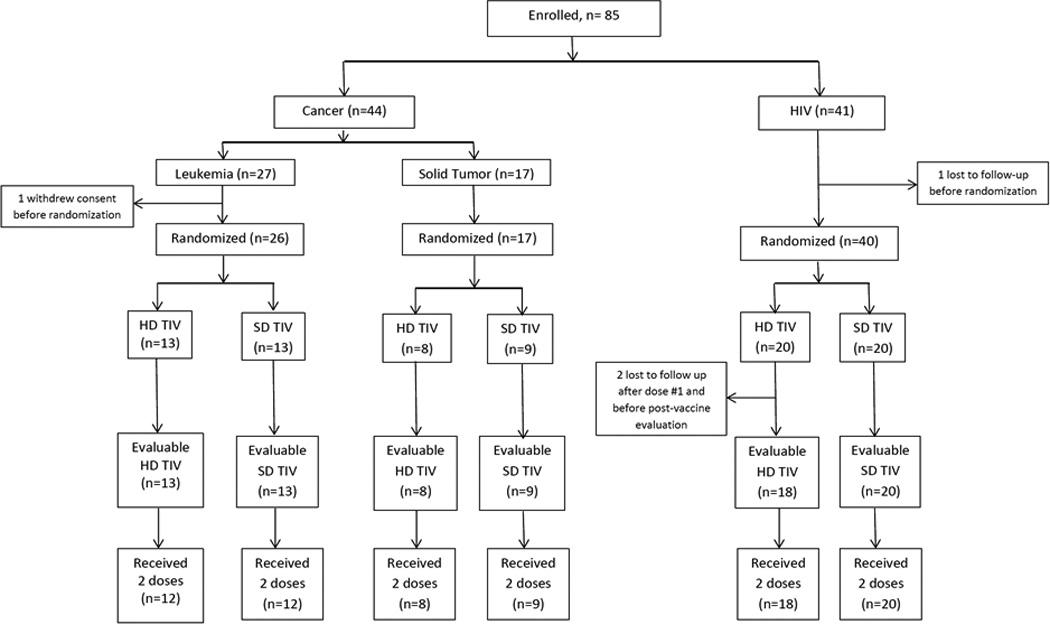
Flow Diagram of Study Participants
Table 1.
Baseline characteristics of 83* children and young adults who were randomized to receive either HD TIV or SD TIV.
| Leukemia | Solid Tumor | HIV | ||||
|---|---|---|---|---|---|---|
| Characteristics | HD (N=13) | SD (N=13) | HD (N=8) | SD (N=9) | HD (N=20) | SD (N=20) |
| Age in years, mean (Std) | 10.8 (5.7) | 11.8 (5.1) | 12.4 (4.2) | 11.7 (4.5) | 16.7 (5.6) | 19.9 (1.8) |
| Male gender, n (%) | 9 (69) | 8 (62) | 4 (50) | 5 (56) | 13 (65) | 16 (80) |
| Race, n (%) | ||||||
| White | 12 (92) | 7 (54) | 4 (50) | 4 (44) | 2 (10) | 0 (0) |
| Black | 1 (8) | 5 (38) | 4 (50) | 4 (44) | 18 (90) | 20 (100) |
| Other | 0 (0) | 1 (8) | 0 (0) | 1 (11) | 0 (0) | 0 (0) |
| Subjects with undetectable HIV viral load, n (%) | NA | NA | NA | NA | 15 (75) | 19 (95) |
| Subjects with HIV on antiretroviral therapy, n (%) | NA | NA | NA | NA | 17 (85) | 18 (90) |
| Received influenza vaccine the prior season, n (%) | 9 (75) | 7 (70) | 2 (40) | 4 (80) | 18 (100) | 17 (100) |
| Dexamethasone received | ||||||
| Prior to first dose, n (%) | 8 (100) | 8 (89) | 4 (100) | 4 (100) | NA | NA |
| Prior to second dose, n (%) | 5 (63) | 7 (78) | 2 (50) | 2 (50) | NA | NA |
| White blood cell counts * 103/mm3, median (range) | 2.5 (1.6–13.9) | 3.7 (2–5.4) | 4.4 (2.5–20.3) | 6.2 (1.3–17.5) | 5.25 (3.3–8.6) | 4.65 (3.2–14.5) |
| Absolute lymphocyte cell counts/mm3, median (range) | 518.5 (70–2363) | 725 (190–1665) | 924 (270–5590) | 1027 (78–5250) | 2128 (1755–4731) | 1610 (1120–2296) |
| Immunoglobulin levels in mg/dl, median (range) | ||||||
| IgG | 445 (153–858) | 589 (267–1094) | 744.5 (156–1115) | 948 (317–1202) | 1362.5 (899–2304) | 1246 (1041–2499) |
| IgG1 | 329 (100–502) | 376 (184–712) | 507.5 (107–752) | 641 (261–856) | 949 (623–1870) | 898.5 (651–1890) |
| IgG2 | 95 (23–306) | 118 (68–341) | 153.5 (30–363) | 160 (34–323) | 262 (104–485) | 261.5 (101–500) |
| IgM | 11 (3–70) | 17 (5–152) | 38 (5–101) | 63 (24–134) | 103.5 (41–419) | 75 (41–240) |
| IgA | 29 (9–143) | 49 (17–311) | 92 (15–320) | 120 (17–210) | 187.5 (66–419) | 190.5(93–591) |
Two patients (one with leukemia and one with HIV) were lost to follow up before randomization.
HIV, human immunodeficiency virus; HD, high dose; SD, standard dose; TIV, trivalent inactivated influenza vaccine; Ig, immunoglobulin; NA, non-applicable; Std, standard deviation
Immunogenicity of HD TIV Compared to SD TIV
Results of immune response to HD TIV compared to SD TIV are shown in Table 2. Participants with leukemia who received 2 doses of HD TIV had greater fold increase in HAI titers to B antigen compared to those who received SD TIV (p=0.04). The difference was not significant for H1 and H3 antigens. There were no differences in the percentages of participants with seroconversion or seroprotection after the second dose of HD TIV compared to SD TIV, except that slightly more participants who received HD TIV achieved seroprotection against H3 and B antigens than those who received SD TIV.
Table 2.
Immunogenicity of HD TIV compared to SD TIV measured using hemagglutination inhibition assay 21–42 days after each dose of vaccine and 9 months after the first dose of vaccine but before influenza vaccination for the following season§
| Antigen Type |
Immune Response | All Cancer | Leukemia | Solid Tumor | HIV | ||||
|---|---|---|---|---|---|---|---|---|---|
| HD TIV (N=21) |
SD TIV (N=22) |
HD TIV (N=13) |
SD TIV (N=13) |
HD TIV (N=8) |
SD TIV (N=9) |
HD TIV (N=18) |
SD TIV (N=20) |
||
| H1 | Pre-vaccine GMT (95% CI) | 37 (22–64) | 45 (27–76) | 29 (17–49) | 50 (22–111) | 57 (16–203) | 40 (20–81) | 71 (37–137) | 67 (40–113) |
| GMT 21–42 days after first dose of vaccine (95% CI) |
123 (59–255) | 60 (37–97) | 80 (41–155) | 55 (32–94) | 247 (43–1421) | 69 (24–198) | 435 (293–647) | 269 (172–420) | |
| GMT 21–42 days after second dose of vaccine (95% CI) |
218 (110–430) | 155 (102–236) | 117 (62–221) | 161 (82–317) | 580 (157–2137) | 148 (84–260) | 373 (264–528) | 267 (188–378) | |
| GMT 9 months after first dose of vaccine (95% CI) |
80 (25–259) | 83 (24–284) | 30 (11–87) | 85 (14–533) | 403 (33–4940) | 80 (12–533) | 279 (145–535) | 174 (93–325) | |
| GMR (post second dose vs. pre- vaccine) |
5.8 | 3.4 | 4.0 | 3.2 | 10.2$ | 3.7$ | 5.2 | 4.0 | |
| Inverse GMR (9 months vs. post second dose of vaccine) |
2.7 | 1.9 | 3.9 | 1.9 | 1.4 | 1.9 | 1.3 | 1.5 | |
| Seroconversion 21–42 days after first dose of vaccine (%) |
38 | 9 | 31 | 0 | 50 | 22 | 61 | 65 | |
| Seroconversion 21–42 days after second dose of vaccine (%) |
67 | 59 | 54 | 54 | 88 | 67 | 72 | 70 | |
| Titer ≥ 1:40* 21–42 days after first dose of vaccine (%) |
76 | 82 | 77 | 85 | 75 | 78 | 100 | 100 | |
| Titer ≥ 1:40* 21 −42 days after second dose of vaccine (%) |
95 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Titer ≥ 1:40* at 9 months after first dose of vaccine (%) |
69 | 65 | 60 | 55 | 83 | 83 | 93 | 94 | |
| H3 | Pre-vaccine GMT (95% CI) | 24 (13–44) | 23 (13–42) | 26 (12–58) | 25 (10–61) | 20 (6–69) | 22 (9–53) | 42 (24–72) | 49 (31–78) |
| GMT 21–42 days after first dose of vaccine (95% CI) |
150 (80–281) | 181 (105–315) | 144 (73–284) | 178 (85–374) | 160 (35–730) | 187 (67–520) | 226 (138–370) | 178 (111–285) | |
| GMT 21–42 days after second dose of vaccine (95% CI) |
167 (89–311) | 80 (38–170) | 139 (49–394) | 63 (21–188) | 215 (104–445) | 109 (31–381) | 154 (105–226) | 143 (97–212) | |
| GMT 9 months after first dose of vaccine (95% CI) |
64 (30–138) | 63 (28–140) | 49 (16–148) | 40 (13–123) | 101 (28–360) | 143 (49–416) | 70 (44–111) | 102 (52–199) | |
| GMR (post second dose vs. pre- vaccine) |
7.1 | 3.4 | 5.3 | 2.6 | 10.8 | 5.0 | 3.7 | 2.9 | |
| Inverse GMR (9 months vs. post second dose of vaccine) |
2.6 | 1.3 | 2.8 | 1.6 | 2.1 | 0.8 | 2.2 | 1.4 | |
| Seroconversion 21–42 days after first dose of vaccine (%) |
67 | 86 | 77 | 85 | 50 | 89 | 72 | 65 | |
| Seroconversion 21–42 days after second dose of vaccine (%) |
76 | 91 | 85 | 92 | 63 | 89 | 72 | 70 | |
| Titer ≥ 1:40* 21–42 days after first dose of vaccine (%) |
86 | 91 | 92 | 92 | 75 | 89 | 100 | 100 | |
| Titer ≥ 1:40* 21 −42 days after second dose of vaccine (%) |
86 | 73 | 80 | 67 | 100 | 78 | 100 | 100 | |
| Titer ≥ 1:40* at 9 months after first dose of vaccine (%) |
69 | 65 | 60 | 55 | 83 | 83 | 87 | 82 | |
| B | Pre-vaccine GMT (95% CI) | 16 (10–26) | 27 (17–42) | 16 (8–31) | 29 (15–57) | 17 (8–35) | 23 (12–47) | 33 (23–48) | 34 (23–48) |
| GMT 21–42 days after first dose of vaccine (95% CI) |
54 (28–104) | 88 (50–154) | 50 (24–103) | 80 (36–178) | 62 (13–290) | 101 (39–263) | 143 (99–204) | 77 (57–105) | |
| GMT 21–42 days after second dose of vaccine (95% CI) |
187 (93–374) | 145 (71–294) | 181 (89–371) | 113 (36–357) | 195 (33–1138) | 202 (83–488) | 160 (108–237) | 172 (100297) | |
| GMT 9 months after first dose of vaccine (95% CI) |
11 (7–16) | 12 (7–21) | 9 (6–13) | 13 (6–28) | 16 (6–43) | 11 (5–23) | 13 (9–19) | 13 (9–19) | |
| GMR (post second dose vs. pre- vaccine) |
11.4 | 5.5 | 11.2# | 3.9# | 11.6 | 8.6 | 4.8 | 5.1 | |
| Inverse GMR (9 months vs. post second dose of vaccine) |
17.2 | 11.8 | 20.6 | 8.8 | 12.3 | 18.0 | 12.1 | 12.9 | |
| Seroconversion 21–42 days after first dose of vaccine (%) |
48 | 45 | 38 | 46 | 63 | 44 | 67 | 35 | |
| Seroconversion 21–42 days after second dose of vaccine (%) |
81 | 86 | 85 | 85 | 75 | 89 | 78 | 75 | |
| Titer ≥ 1:40* 21–42 days after first dose of vaccine (%) |
62 | 86 | 62 | 85 | 63 | 89 | 100 | 90 | |
| Titer ≥ 1:40* 21 −42 days after second dose of vaccine (%) |
81 | 86 | 91 | 83 | 86 | 89 | 100 | 100 | |
| Titer ≥ 1:40* at 9 months after first dose of vaccine (%) |
6 | 18 | 0 | 18 | 17 | 17 | 13 | 12 | |
Three patients with HIV were lost to follow up; one before randomization and two after receiving dose #1 and before post-vaccine evaluation visit. One patient with leukemia withdrew consent before randomization.
Defined as Seroprotection for HAI values
p-value for this comparison=0.04
p-value for this comparison=0.04
GMT, geometric mean titer; GMR, geometric mean ratio; HIV, human immunodeficiency virus; NA, non-applicable; HD, high dose; SD, standard dose; TIV, trivalent inactivated influenza vaccine; CI, Confidence interval
Patients with ST who received 2 doses of HD TIV had significantly greater fold increase in titers to H1 antigen but not H3 and B antigens when compared to those who received SD TIV (p=0.04). There were no differences in the percentages of participants with seroconversion or seroprotection after the second dose of HD TIV compared to SD TIV.
In the HIV group, the fold increase in HAI titers after 2 doses of HD TIV was not significantly different from SD TIV against all three antigens. In addition, there were no differences in the percentages of participants with seroconversion or seroprotection after the second dose of HD TIV compared to SD TIV.
In general, higher geometric mean ratios (GMR) were achieved after 2 doses of HD TIV than SD TIV in all groups against all antigens.
Immunogenicity of Two Doses Compared to One Dose of TIV
About 70 percent of participants, HD and SD TIV combined, in all three groups of underlying diagnosis had seroconversion against H3 antigen after 1 dose of TIV, and the rate of seroconcersion was not significantly improved with the second dose. On the other hand, seroconversion was significantly more likely to be achieved after 2 doses than after 1 dose for H1 and B antigens (p<0.0001 for both), and the magnitude of improvement in seroconversion was more prominent in participants with leukemia or ST than in those with HIV (Table 2). Most participants had seroprotection after 1 dose of either HD or SD TIV, and 100% seroprotection was documented among HIV patients against all three antigens as well as participants with leukemia or ST against H1 antigen after 2 doses (Table 2).
Significant rises in HAI titers against all 3 antigens were found in all groups after 1 dose, and the HAI titers against B antigen significantly further increased in all groups after 2 doses and those against H1 antigen in the leukemia and ST groups.
Durability of Immune Response
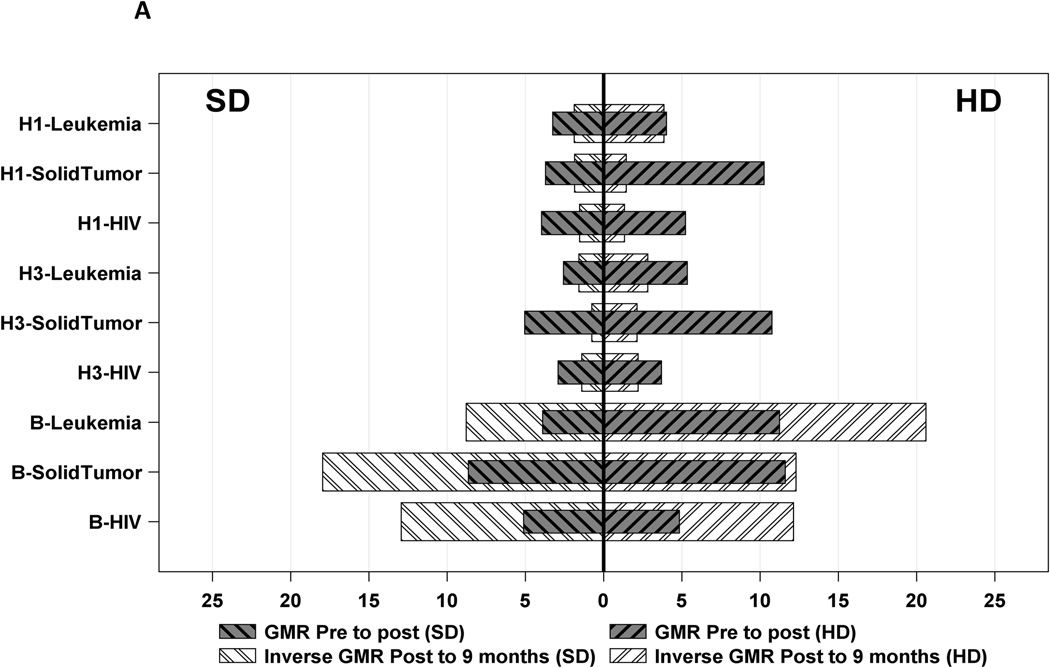
Table 2 and figure 2 show that the GMT at 9 months after the first dose of vaccine had decreased to much lower levels that those detected after the second dose of either type of vaccine in all groups, reported as inverse GMR. The most significant decrease in titers, represented by higher inverse GMR, was documented against B antigen in all 3 groups of underlying diagnosis and with both HD and SD TIV. Immunity against B antigen was more likely to be lost by 9 months after vaccination compared to H1 and H3 antigens.
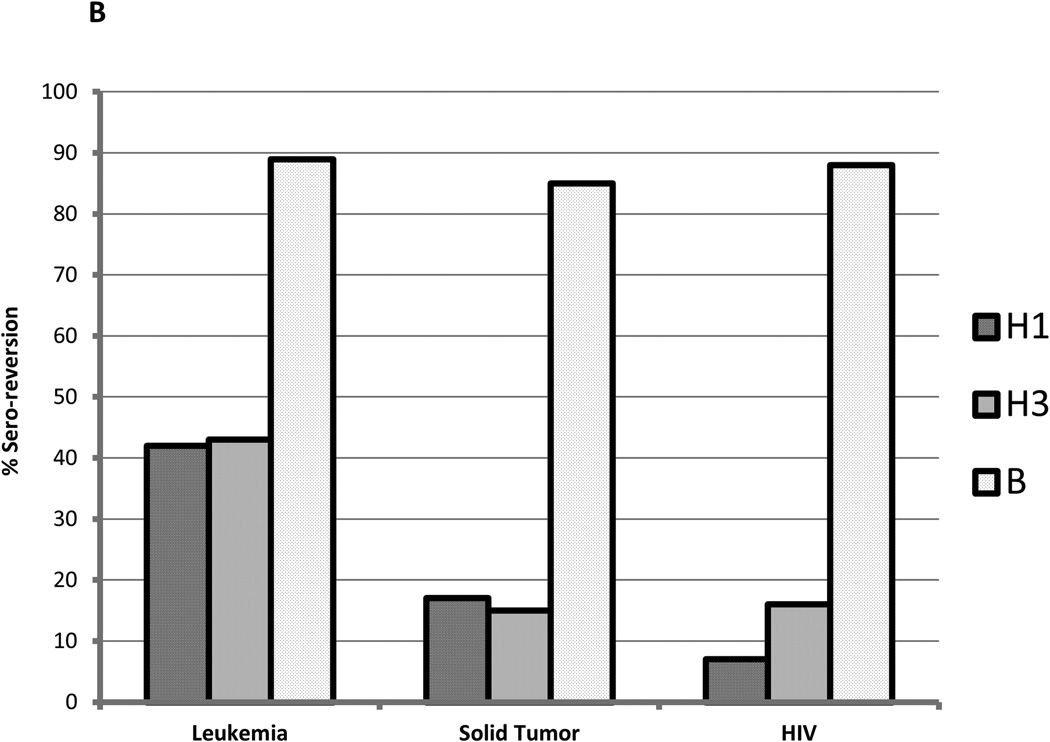
Figure 2.
Durability of immune response to 3 influenza vaccine antigens H1, H3, and B in children and young adults with leukemia, solid tumor, or HIV who received HD TIV or SD TIV, represented by (A) Geometric Mean Ratios and Inverse GMR; and (B) Sero-reversion
Similarly, seroprotective titers were lost by 9 months after the vaccine. High rates of sero-reversion (drop from titer ≥1:40 after 2 doses to <1:40 at 9 months) were documented in patients with leukemia compared to ST or HIV against H1 and H3 antigens; almost all participants sero-reverted to B antigen (Table 2, Figure 2 (B)).
Immunogenicity by Baseline Absolute Lymphocyte Count
Table 3 shows that patients with ALC ≥ 1000 cells/mm3 had significantly higher pre-vaccine GMT, a trend for higher post-vaccine GMT, significantly higher GMT at 9 months, and were also more likely to achieve seroconversion after 1 dose, compared to those with ALC < 1000 cells/mm3.
Table 3.
Immune Responses by Baseline Absolute Lymphocyte Count
| Absolute lymphocyte count <1000 cells/mm3 (N=26) |
Absolute lymphocyte count ≥1000 cells/mm3 (N=28) |
P-value* | |
|---|---|---|---|
| Pre-vaccine GMT (95% CI) | 36.0 (23.7–54.5) | 70.7 (41.5–120.4) | 0.045 |
| Post-vaccine GMT (95% CI) † | 155.3 (100.2–240.6) | 274.9 (183.4–412.2) | 0.066 |
| 9 month GMT (95% CI) † | 58.6 (22.3–154.1) | 249.3 (118.6–524.1) | 0.008 |
| GMR, pre to post | 4.3 | 3.9 | - |
| Inverse GMR, post to 9 months | 2.7 | 1.1 | - |
| Seroconversion (%) | 16 (62) | 19 (68) | 0.777 |
| Seroprotection (%) | 25 (96) | 28 (100) | 0.482 |
| Seroconversion after 1 dose (%) | 4 (15) | 16 (57) | 0.002 |
| Seroprotection after 1 dose (%) | 21 (81) | 26 (93) | 0.243 |
| Seroconversion 9 months vs. post † (%) | 2 (11) | 2 (8) | 1.000 |
| 9 months Seroprotection † (%) | 13 (65) | 22 (88) | 0.083 |
Wilcoxon-Mann-Whitney tests for continuous variables, Fisher’s exact tests for categorical variables
Missing values were excluded.
GMT, geometric mean titer; GMR, geometric mean ratio; CI, confidence interval
Safety and Reactogenicity of HD TIV compared to SD TIV
A summary of RE following dose 1 and dose 2 of each of HD and SD TIV is reported in Table 4. Although participants who received HD TIV reported more frequent RE (54% after dose 1 and 38% after dose 2) compared to those who received SD TIV (40% after dose 1 and 20% after dose 2), these differences did not reach statistical significance (p=0.27 and 0.09 after dose 1 and dose 2, respectively). Solicited REs were mild to moderate.
Table 4.
Reactogenicity and Adverse Events after Dose 1 and Dose 2 of HD TIV Compared to SD TIV
| Event | Dose 1 of TIV | Dose 2 of TIV | ||||
|---|---|---|---|---|---|---|
| HD TIV (N=41 doses) |
SD TIV (N=42 doses) |
P-value* | HD TIV (N=39 doses) |
SD TIV (N=40 doses) |
P-value* | |
| Reactogenicity event occurred in, n (%) | 22 (54) | 17 (40) | 0.27 | 15 (38) | 8 (20) | 0.09 |
| Muscle ache | 3 (7) | 1 (2) | 0.36 | 1 (3) | 1 (3) | 1.00 |
| Fatigue | 5 (12) | 3 (7) | 0.48 | 4 (10) | 1 (3) | 0.20 |
| Pain | 19 (46) | 15 (36) | 0.38 | 11 (28) | 7 (18) | 0.29 |
| Redness | 3 (7) | 4 (10) | 1.00 | 1 (3) | 0 (0) | 0.49 |
| Induration | 4 (10) | 1 (2) | 0.20 | 1 (3) | 0 (0) | 0.49 |
| Fever | 4 (10) | 4 (10) | 1.00 | 3 (8) | 1 (3) | 0.36 |
| Median Temperature of Fever in °F (PO) (range) | 101.5 (100.2–102.9) | 101.0 (100.2–102) | 0.49 | 102 (101.2–102.9) | 100.7 | 0.50 |
| Vaccine-related adverse event, n (%) | 1# (2) | 0 | 0.49 | 0 | 0 | - |
| Serious adverse events, n (%) | 1# (2) | 0 | 0.49 | 0 | 0 | - |
Exact Wilcoxon-Mann-Whitney tests for continuous variables, Fisher’s exact tests for categorical variables
This adverse event was classified as grade 2 somnolence using the adapted National Cancer Institute Common Terminology Criteria for Adverse Events version 4.02.
HD, high dose; SD, standard dose; TIV, trivalent inactivated influenza vaccine
One patient with non-Hodgkin lymphoma who received dose 1 of HD TIV was classified to have Grade 2 somnolence SAE. This was a 9 year old boy with known history of neurological disorder who underwent sedation for a routine imaging study. He received HD TIV in the afternoon after recovery from sedation, was observed and discharged home. Six hours after discharge, this patient returned to the hospital for concerns of increased sleepiness that started approximately 4 hours after vaccination. Although he was at baseline state upon presentation for evaluation, he was hospitalized for testing and observation. Tests including electroencephalography, CT scan, and neurological examination have been unremarkable. He spontaneously recovered within 2–3 hours and was back at his baseline upon presentation to the hospital.
DISCUSSION
This randomized, open-label study demonstrated that HD TIV was significantly more immunogenic than SD TIV against B antigen in leukemia patients and H1 antigen in ST patients. However, there was no significant difference between HD and SD TIV against all antigens in HIV-infected patients. Our findings suggest that using a HD influenza vaccine is a potential approach to improve the immune response of children and young adults with malignancy to influenza vaccine, but not those with HIV infection. FluzoneHD, which contains four times the antigen dose as that in SD TIV, was FDA approved in December 2009 for use in the elderly, and has been shown to be safe and more immunogenic in this risk group in whom SD TIV vaccines are poorly immunogenic25–33. Two recent studies have evaluated the safety of HD TIV in children with acute lymphoblastic leukemia35 and in pediatric solid organ transplant (SOT) recipients36. Although these 2 studies were not designed to compare immunogenicity results, their findings suggested that HD TIV was not significantly different from SD TIV in children with ALL35, but more immunogenic to H3N2 in pediatric SOT recipients36. Inconsistent with the findings of McManus and colleagues35, we have shown that HD TIV was significantly more immunogenic than SD TIV against B antigen in leukemia patients. Our study was the first to evaluate immunogenicity and safety of HD compared to SD TIV in children and young adults with ST or HIV infection. We have found no significant difference in the immune response to HD TIV compared to SD TIV in HIV-infected patients; who were of older age than participants with malignancy. A recent randomized double blind trial that enrolled HIV-infected adults showed that seroprotection rates after vaccination were higher in HD TIV than SD TIV recipients37. Our findings were not consistent with this study probably because of the relatively high pre-vaccine titers detected in both groups of HIV-infected children and young adults randomized to either HD or SD TIV, especially against H1 antigen which could be due to prior influenza vaccination or infection. Taking into consideration the higher, but nonsignificant, proportion of patients reporting reactogenicity events, and the absence of improved immune response to HD TIV compared to SD TIV, SD TIV remains the appropriate approach for HIV-infected children and young adults.
Another proposed strategy to improve immune response is administration of 2 doses of influenza vaccine at least 4 weeks apart. We have shown that two doses of TIV were more immunogenic than one dose in children and young adults with leukemia or ST, but no added benefit was demonstrated in HIV-infected children and young adults. These findings were consistent with previous studies9,13,38 using older formulations of influenza vaccine. However, a large study designed to compare immunogenicity of 2 doses to 1 dose of contemporary formulation of influenza vaccine is highly needed.
The durability of influenza antibodies after vaccination remains undefined. In a previous study, we have shown that the GMTs measured 6 months after the last dose of monovalent 2009 pandemic H1N1 vaccine were higher than those measured after the last dose of vaccine in children with cancer or HIV infection14. However, the current study has demonstrated sero-reversion by 9 months after vaccination. The most significant decrease in titers was documented against B antigen in all 3 groups of underlying diagnosis and with both HD and SD TIV. Several interventions in the 9-month period after influenza vaccination could have contributed to the loss of immune response including subsequent cycles of chemotherapy and/or corticosteroids. A recent study that prospectively measured HAI titers in adult persons at serial time points during the 2009 H1N1 pandemic showed that antibodies induced by natural infection maintained high titer for at least 15 months39. However, the titers significantly decreased at approximately 10 months compared to 3 months after vaccination39. Our study showed that patients with ALC ≥ 1000 cells/mm3 had significantly higher pre-vaccine GMT, higher GMT at 9 months, and higher seroconversion rate compared to those with ALC < 1000 cells/mm3. Previous studies have attempted to investigate factors associated with improved immune responses to influenza vaccines in children and young adults with cancer, including age, white blood cell count, lymphocyte count, serum immunoglobulin levels, type of malignancy, intensity and type of chemotherapy, vaccine dose, prior influenza vaccination, and timing of vaccination in relation to chemotherapy9,10,12,14,17,19,22,40. We have found that higher ALC is associated with improved immune response to influenza vaccine. However, ALC was measured at the pre-vaccination visit and subsequent changes of ALC in relation to chemotherapy were not evaluated at the time of vaccination.
This study demonstrated that HD TIV is safe and well tolerated in children and young adults with cancer or HIV infection. Although patients who received HD TIV reported more solicited REs than those who received SD TIV, this difference was not significant. There was no difference between the two groups regarding local injection and systemic reactions. This finding was consistent with the safety results of HD TIV in children with ALL reported by McManus et al35. However, GiaQuinta and colleagues reported that pediatric SOT recipients had more local reactions to HD TIV than SD TIV36. Most of these reactions were reported as being mild or moderate in severity, and many resolved within three days. In addition, our findings differed from those of previous larger studies in the elderly population, in which higher rates of local reactions were reported in HD TIV compared to SD TIV recipients25–27,30,31. However, our findings were similar to a previous study that compared HD to SD TIV in adults infected with HIV37. Our study as well as the other 2 studies35,36 has not reported any SAEs attributed to the vaccine, either HD or SD TIV. Although one patient in our study was classified to have SAE, this event was most likely not attributed to HD TIV because of other possible causes of increased sleepiness including recent sedation and known history of neurologic disorder.
Our study has some limitations. Firstly, this was a single center study with a small sample size in each sub-group of underlying diagnosis. A larger multicenter study is needed to validate our findings. Secondly, this was an open label study. To minimize bias, the HAI assays were completed in batched runs using de-identified samples with no information provided about the type of vaccine. And, if any related bias was introduced to the interpretation of safety and reactogenicity data, the slightly higher percentage of events solicited in HD TIV recipients was not significantly different from that in SD TIV recipients. Thirdly, enrollment occurred during two influenza seasons. However, the antigenic vaccine composition was not changed. Also, participants enrolled in the first influenza season were not enrolled in the second season to prevent any boosting effect. Fourthly, data about timing and intensity of chemotherapy or corticosteroids administration in relation to influenza vaccination was not collected; which might affect the ability to generate an adequate immune response. Similarly, the regimens of antineoplastic therapy for patients within the same underlying malignancy group (leukemia or solid tumor) were not compared between HD TIV and SD TIV groups. Fifthly, the patient population enrolled in the study is heterogeneous including patients with leukemia, ST, and HIV. However, the analysis was stratified by the underlying diagnosis to further minimize this limitation. Finally, the effectiveness of the vaccine in preventing proven influenza or influenza-like illnesses was not evaluated. In conclusion, our study revealed that the HD TIV is more immunogenic than SD TIV in children and young adults with leukemia or ST, and safe and well-tolerated in children and young adults with leukemia, ST, or HIV. However, randomized controlled trials with larger sample size are needed to evaluate the effectiveness of the vaccine in preventing proven influenza or influenza-like illnesses, and to determine the optimal influenza vaccine regimen in immunocompromised children and young adults, evaluating timing, dosing, and number of doses.
Key Points.
High dose influenza vaccine was more immunogenic than standard dose influenza vaccine in children and young adults with leukemia or solid tumor, but not with HIV.
High dose influenza vaccine was safe and well-tolerated in children and young adults with leukemia, solid tumor, or HIV.
Acknowledgments
The authors would like to acknowledge Judy Glenn for data abstraction and Kris Branum for regulatory support.
Funding:
This work was supported by a National Institutes of Health Grant CA21765 and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers: All authors report no conflict of interest.
REFERENCES
- 1.Feldman S, Webster RG, Sugg M. Influenza in children and young adults with cancer: 20 cases. Cancer. 1977 Jan;39(1):350–353. doi: 10.1002/1097-0142(197701)39:1<350::aid-cncr2820390153>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Gooskens J, Jonges M, Claas EC, Meijer A, Kroes AC. Prolonged influenza virus infection during lymphocytopenia and frequent detection of drug-resistant viruses. The Journal of infectious diseases. 2009 May 15;199(10):1435–1441. doi: 10.1086/598684. [DOI] [PubMed] [Google Scholar]
- 3.Kempe A, Hall CB, MacDonald NE, et al. Influenza in children with cancer. The Journal of pediatrics. 1989 Jul;115(1):33–39. doi: 10.1016/s0022-3476(89)80325-7. [DOI] [PubMed] [Google Scholar]
- 4.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. The Lancet Infectious diseases. 2009 Aug;9(8):493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljungman P, Andersson J, Aschan J, et al. Influenza A in immunocompromised patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1993 Aug;17(2):244–247. doi: 10.1093/clinids/17.2.244. [DOI] [PubMed] [Google Scholar]
- 6.Madhi SA, Ramasamy N, Bessellar TG, Saloojee H, Klugman KP. Lower respiratory tract infections associated with influenza A and B viruses in an area with a high prevalence of pediatric human immunodeficiency type 1 infection. The Pediatric infectious disease journal. 2002 Apr;21(4):291–297. doi: 10.1097/00006454-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Tasian SK, Park JR, Martin ET, Englund JA. Influenza-associated morbidity in children with cancer. Pediatric blood & cancer. 2008 May;50(5):983–987. doi: 10.1002/pbc.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grohskopf LA, Olsen SJ, Sokolow LZ, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) -- United States, 2014–15 influenza season. MMWR. Morbidity and mortality weekly report. 2014 Aug 15;63(32):691–697. [PMC free article] [PubMed] [Google Scholar]
- 9.Bektas O, Karadeniz C, Oguz A, Berberoglu S, Yilmaz N, Citak C. Assessment of the immune response to trivalent split influenza vaccine in children with solid tumors. Pediatric blood & cancer. 2007 Dec;49(7):914–917. doi: 10.1002/pbc.21106. [DOI] [PubMed] [Google Scholar]
- 10.Chisholm J, Howe K, Taj M, Zambon M. Influenza immunisation in children with solid tumours. European journal of cancer. 2005 Oct;41(15):2280–2287. doi: 10.1016/j.ejca.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Goossen GM, Kremer LC, van de Wetering MD. Influenza vaccination in children being treated with chemotherapy for cancer. The Cochrane database of systematic reviews. 2013;8 doi: 10.1002/14651858.CD006484.pub3. CD006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross PA, Gould AL, Brown AE. Effect of cancer chemotherapy on the immune response to influenza virus vaccine: review of published studies. Reviews of infectious diseases. 1985 Sep-Oct;7(5):613–618. doi: 10.1093/clinids/7.5.613. [DOI] [PubMed] [Google Scholar]
- 13.Gross PA, Lee H, Wolff JA, Hall CB, Minnefore AB, Lazicki ME. Influenza immunization in immunosuppressed children. The Journal of pediatrics. 1978 Jan;92(1):30–35. doi: 10.1016/s0022-3476(78)80065-1. [DOI] [PubMed] [Google Scholar]
- 14.Hakim H, Allison KJ, Van De Velde LA, Li Y, Flynn PM, McCullers JA. Immunogenicity and safety of inactivated monovalent 2009 H1N1 influenza A vaccine in immunocompromised children and young adults. Vaccine. 2012 Jan 20;30(5):879–885. doi: 10.1016/j.vaccine.2011.11.105. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh YC, Lu MY, Kao CL, et al. Response to influenza vaccine in children with leukemia undergoing chemotherapy. Journal of the Formosan Medical Association = Taiwan yi zhi. 2002 Oct;101(10):700–704. [PubMed] [Google Scholar]
- 16.Madhi SA, Maskew M, Koen A, et al. Trivalent inactivated influenza vaccine in African adults infected with human immunodeficient virus: double blind, randomized clinical trial of efficacy, immunogenicity, and safety. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Jan 1;52(1):128–137. doi: 10.1093/cid/ciq004. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki A, Suminoe A, Koga Y, Kinukawa N, Kusuhara K, Hara T. Immune response after influenza vaccination in children with cancer. Pediatric blood & cancer. 2005 Nov;45(6):831–837. doi: 10.1002/pbc.20470. [DOI] [PubMed] [Google Scholar]
- 18.Miotti PG, Nelson KE, Dallabetta GA, Farzadegan H, Margolick J, Clements ML. The influence of HIV infection on antibody responses to a two-dose regimen of influenza vaccine. Jama. 1989 Aug 11;262(6):779–783. [PubMed] [Google Scholar]
- 19.Porter CC, Edwards KM, Zhu Y, Frangoul H. Immune responses to influenza immunization in children receiving maintenance chemotherapy for acute lymphoblastic leukemia. Pediatric blood & cancer. 2004 Jan;42(1):36–40. doi: 10.1002/pbc.10459. [DOI] [PubMed] [Google Scholar]
- 20.Reilly A, Kersun LS, McDonald K, Weinberg A, Jawad AF, Sullivan KE. The efficacy of influenza vaccination in a pediatric oncology population. Journal of pediatric hematology/oncology. 2010 Jul;32(5):e177–e181. doi: 10.1097/MPH.0b013e3181d869f3. [DOI] [PubMed] [Google Scholar]
- 21.Shahgholi E, Ehsani MA, Salamati P, Maysamie A, Sotoudeh K, Mokhtariazad T. Immunogenicity of trivalent influenza vaccine in children with acute lymphoblastic leukemia during maintenance therapy. Pediatric blood & cancer. 2010 May;54(5):716–720. doi: 10.1002/pbc.22421. [DOI] [PubMed] [Google Scholar]
- 22.Stiver HG, Weinerman BH. Impaired serum antibody response to inactivated influenza A and B vaccine in cancer patients. Canadian Medical Association journal. 1978 Oct 7;119(7):733–735. 738. [PMC free article] [PubMed] [Google Scholar]
- 23.Tsang P, Gorse GJ, Strout CB, et al. Immunogenicity and safety of Fluzone((R)) intradermal and high-dose influenza vaccines in older adults >/=65 years of age: a randomized, controlled, phase II trial. Vaccine. 2014 May 1;32(21):2507–2517. doi: 10.1016/j.vaccine.2013.09.074. [DOI] [PubMed] [Google Scholar]
- 24.Chit A, Becker DL, DiazGranados CA, Maschio M, Yau E, Drummond M. Cost-effectiveness of high-dose versus standard-dose inactivated influenza vaccine in adults aged 65 years and older: an economic evaluation of data from a randomised controlled trial. The Lancet. Infectious diseases. 2015 Sep 8; doi: 10.1016/S1473-3099(15)00249-2. [DOI] [PubMed] [Google Scholar]
- 25.Couch RB, Winokur P, Brady R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine. 2007 Nov 1;25(44):7656–7663. doi: 10.1016/j.vaccine.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiazGranados CA, Dunning AJ, Jordanov E, Landolfi V, Denis M, Talbot HK. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity and relative efficacy during the 2009–2010 season. Vaccine. 2013 Jan 30;31(6):861–866. doi: 10.1016/j.vaccine.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 27.DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. The New England journal of medicine. 2014 Aug 14;371(7):635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 28.DiazGranados CA, Dunning AJ, Robertson CA, Talbot HK, Landolfi V, Greenberg DP. Efficacy and immunogenicity of high-dose influenza vaccine in older adults by age, comorbidities, and frailty. Vaccine. 2015 Aug 26;33(36):4565–4571. doi: 10.1016/j.vaccine.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 29.DiazGranados CA, Robertson CA, Talbot HK, Landolfi V, Dunning AJ, Greenberg DP. Prevention of serious events in adults 65 years of age or older: A comparison between high-dose and standard-dose inactivated influenza vaccines. Vaccine. 2015 Sep 11;33(38):4988–4993. doi: 10.1016/j.vaccine.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. The Journal of infectious diseases. 2009 Jul 15;200(2):172–180. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 31.Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Archives of internal medicine. 2006 May 22;166(10):1121–1127. doi: 10.1001/archinte.166.10.1121. [DOI] [PubMed] [Google Scholar]
- 32.Izurieta HS, Thadani N, Shay DK, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using Medicare data: a retrospective cohort analysis. The Lancet. Infectious diseases. 2015 Mar;15(3):293–300. doi: 10.1016/S1473-3099(14)71087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson DM, Medvedeva EL, Roberts CB, Linkin DR Centers for Disease C, Prevention Epicenter P. Comparative effectiveness of high-dose versus standard-dose influenza vaccination in community-dwelling veterans. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015 Jul 15;61(2):171–176. doi: 10.1093/cid/civ261. [DOI] [PubMed] [Google Scholar]
- 34.McCullers JA, Van De Velde LA, Allison KJ, Branum KC, Webby RJ, Flynn PM. Recipients of vaccine against the 1976 “swine flu” have enhanced neutralization responses to the 2009 novel H1N1 influenza virus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010 Jun 1;50(11):1487–1492. doi: 10.1086/652441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McManus M, Frangoul H, McCullers JA, Wang L, O’Shea A, Halasa N. Safety of high dose trivalent inactivated influenza vaccine in pediatric patients with acute lymphoblastic leukemia. Pediatric blood & cancer. 2014 May;61(5):815–820. doi: 10.1002/pbc.24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.GiaQuinta S, Michaels MG, McCullers JA, et al. Randomized, double-blind comparison of standard-dose vs. high-dose trivalent inactivated influenza vaccine in pediatric solid organ transplant patients. Pediatric transplantation. 2015 Mar;19(2):219–228. doi: 10.1111/petr.12419. [DOI] [PubMed] [Google Scholar]
- 37.McKittrick N, Frank I, Jacobson JM, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Annals of internal medicine. 2013 Jan 1;158(1):19–26. doi: 10.7326/0003-4819-158-1-201301010-00005. [DOI] [PubMed] [Google Scholar]
- 38.Allison JE, Glezen WP, Taber LH, Paredes A, Webster RG. Reactogenicity and immunogenicity of bivalent influenza A and monovalent influenza B virus vaccines in high-risk children. The Journal of infectious diseases. 1977 Dec;(136 Suppl):S672–S676. doi: 10.1093/infdis/136.supplement_3.s672. [DOI] [PubMed] [Google Scholar]
- 39.Sridhar S, Begom S, Hoschler K, et al. Longevity and determinants of protective humoral immunity after pandemic influenza infection. American journal of respiratory and critical care medicine. 2015 Feb 1;191(3):325–332. doi: 10.1164/rccm.201410-1798OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chisholm JC, Devine T, Charlett A, Pinkerton CR, Zambon M. Response to influenza immunisation during treatment for cancer. Archives of disease in childhood. 2001 Jun;84(6):496–500. doi: 10.1136/adc.84.6.496. [DOI] [PMC free article] [PubMed] [Google Scholar]