Abstract
Feeding is a highly complex behavior that is influenced by learned associations between external and internal cues. The type of excessive feeding behavior contributing to obesity onset and metabolic deficit may be based, in part, on conditioned appetitive and ingestive behaviors that occur in response to environmental and/or interoceptive cues associated with palatable food. Therefore, there is a critical need to understand the neurobiology underlying learned aspects of feeding behavior. The stomach-derived “hunger” hormone, ghrelin, stimulates appetite and food intake and may function as an important biological substrate linking mnemonic processes with feeding control. The current review highlights data supporting a role for ghrelin in mediating the cognitive and neurobiological mechanisms that underlie conditioned feeding behavior. We discuss the role of learning and memory on food intake control (with a particular focus on hippocampal-dependent memory processes) and provide an overview of conditioned cephalic endocrine responses. A neurobiological framework is provided through which conditioned cephalic ghrelin secretion signals in neurons in the hippocampus, which then engage orexigenic neural circuitry in the lateral hypothalamus to express learned feeding behavior.
Keywords: appetite, learning, obesity, hippocampus, GHSR, cephalic
I. Introduction
In light of the current obesity pandemic, there is a recent surge of interest in investigating the biological and psychological antecedents of feeding behavior, in particular the type of excessive food intake that contributes to weight gain and metabolic disorders. Food seeking and ingestion rarely occur as passive behaviors in response to energy deficit. Rather, for humans, food intake is more often initiated as a result of either habitual feeding patterns (e.g., meals consumed at approximately the same time each day) or in response to environmental stimuli associated with food consumption, including cues associated with palatable foods such as fast food billboards and TV commercials. Indeed, learned associations between food and interoceptive or external cues have the capability to drive or alter feeding behavior [1–5]. However, despite the robust influence of mnemonic processes on food intake, very little is understood about the neurobiological substrates of learned feeding behavior. In this review we discuss psychological and biological mechanisms underlying learned feeding behavior, focusing primarily on the gut-derived hormone, ghrelin as being a critical interface linking memory and ingestion.
II. Mnemonic control of food intake
Learning and memory processes have powerful control over appetite and food intake. Through experience, food-associated cues (e.g. environmental cues or interoceptive cues) become associated with rewarding or aversive postingestive consequences, and these learned associations robustly affect future feeding behaviors. An example is conditioned flavor avoidance (or aversion) learning, a process through which animals learn to avoid (or reject) neutral flavors that were previously associated with nausea or malaise [6]. Studies have also demonstrated appetite promoting effects through learned associations between neutral flavor cues and nutritive postingestive consequences. More specifically, animals can learn to prefer a neutral flavor that was previously paired with gastric or intestinal infusion of nutrients [7]. Thus, even simple associative learning mechanisms have the capacity to powerfully modulate feeding either by stimulating appetite or by inducing avoidance/aversive behaviors. This review will focus on the role of ghrelin, an appetite-promoting hormone released primarily from the gastric P/D1 cells, on learned aspects of eating behavior.
In addition to associations between food-relevant stimuli and postingestive consequences, learned relationships between food access and other types of interoceptive cues, including circadian rhythms, also have the capacity to guide and alter feeding behavior. Many humans and nonhuman animals have habitual feeding patterns in which meals are consumed at approximately the same time each day (e.g., breakfast, lunch, and dinner), and accordingly, conditioned interoceptive circadian cues can acquire the capacity to elicit preparatory cephalic release of ghrelin and other endocrine signals that influence appetitive behavior [8–10] (a phenomenon discussed in more depth below). In some cases feeding behavior is entrained, not based on convenient habitual feeding patterns, but rather, based on limited food availability. This type of entrainment can be demonstrated experimentally in rodents placed on restricted meal schedules in which they are only allowed access to food at a fixed time and duration each day. Animals under these feeding conditions learn across days to rapidly consume a large quantity of food to meet their metabolic requirements [10–12].
Food-associated environmental cues are also capable of driving hyperphagia under conditions where food access is not limited or restricted. Previously neutral cues (e.g. visual or auditory stimuli) that become associated with access to palatable food can potently stimulate appetite and excessive feeding behavior (i.e., “cue potentiated feeding”) in both humans and nonhuman animal models. For example, rodents can learn to associate discrete auditory stimuli with rewarding foods, and subsequent exposure to the food-associated cue(s) when the same food reinforcement is freely available stimulates intake in nonrestricted rats in excess of that occurring during or after exposure to control cues [1, 2, 5, 13–17]. In fully sated humans, the sight of palatable foods enhances reported desire and intake for the given food [1], and exposure to recognizable food advertisements and other food cues increases subsequent food intake in children [5, 18]. Importantly, the processes through which food-associated external cues promote excess palatable food consumption involve ghrelin signaling [19–21].
In addition to influencing feeding through simple associations and learned circadian entrainment patterns, food-related conditioned cues can influence food intake through their integration into more complex mnemonic constructs. Declarative memory, comprised of the conscious recollection of factual information (semantic memory) and autobiographical memories (episodic memory), requires neural processing in the hippocampus [22, 23]. Declarative memories of previous meals or eating occasions can be consolidated and retrieved at a later time to influence subsequent feeding behaviors. This is perhaps most powerfully illustrated by research in amnesic patients with bilateral damage to the hippocampus and surrounding medial temporal lobe. These patients are impaired in episodic memory formation, and under some experimental conditions they will consume a second or third meal offered only minutes after consuming a meal [24, 25]. Parent and colleagues (2013) have elegantly modeled this phenomenon in rodent models, demonstrating that reversible inactivation of hippocampal neurons immediately following a scheduled, anticipated sucrose meal reduces meal initiation latency and increases the size of the subsequent meal [26]. This group more recently demonstrated that orosensory stimulation through either intake of sucrose or non-caloric saccharin robustly increases hippocampal synpatic plasticity in an experience-dependent manner [27]. Together, these results suggest that 1) hippocampal neural processing can regulate the consolidation of food-related declarative memories, and 2) disrupted hippocampal processing can potentially lead to excessive eating. Indeed, rodents with selective bilateral hippocampal lesions are hyperphagic and gain excess body weight relative to control rats [28].
An alternative and complementary framework for the influence of hippocampal-dependent memory processes on feeding behavior – one that does not evoke the use of complex mnemonic constructs like declarative memory – is provided by Davidson and colleagues (see [3, 28–31] for reviews on the role of hippocampal neural processing in the mnemonic regulation of feeding behavior). In this model, hippocampal-dependent memory processes influence feeding by utilizing interoceptive energy status cues (e.g., different levels of food restriction) to resolve ambiguous learned relationships between food-related stimuli and their postingestive consequences [32]. Within this framework, energy status cues (e.g., hunger, satiety) modulate the strength of learned associations between food-related stimuli and postingestive nutritive reinforcement. These energy status cues are likely communicated to the hippocampus, in part, by ghrelin and other endocrine signals (e.g., leptin, glucagon-like peptide-1) whose receptors are expressed on hippocampal neurons [33].
Based on the work described above, it is apparent that both simple learned associations and more complex hippocampal-dependent mnemonic processes are critical for regulating decisions about when and where to feed, as well as preparing an organism to consume food when feeding is imminent or anticipated. Regarding the latter, sensory stimuli associated with meal anticipation elicit preparatory physiological “cephalic” reflexes that occur prior to feeding [34–38]. These responses are particularly advantageous when environmental factors, such as limited food availability or access, restrict food consumption such that an organism is required to consume a large amount of food in a short period of time. Research has demonstrated that circadian, olfactory, gustatory, and cognitive food-related cues are capable of generating these cephalic phase responses, characterized by transient upregulation of key endocrine feeding signals such as ghrelin and the pancreatic hormone insulin [10, 34, 38]. The integration of ghrelin and other cephalic-released hormones with higher-order neural substrates that control learning and memory processes may represent a mechanistic biological link between food-related mnemonics, interoceptive energy status cues, and the neural control of ingestive behavior.
III. Learned cephalic-phase biological responses and feeding behavior
Cephalic-phase responses are anticipatory physiological and metabolic adjustments in preparation for the digestion and absorption of ingested nutrients and reflect a biological mechanism through which external cues interact with the central nervous system to express learned feeding behavior. Nutrient ingestion, while obviously beneficial, can also be thought of as perturbing physiological homeostasis. By eliciting cephalic-phase responses, disruption of homeostasis during feeding is attenuated and food intake during a meal is enhanced (reviewed in [39]). For example, rats that are placed on a restricted feeding schedule involving random meal times have been shown to eat smaller meals compared to ad libitum-fed rats [39]. In addition to circadian entrainment cues, exposure to sensory related food cues (e.g., orosensory, visual) and cognitive factors (e.g., thinking about food) can generate cephalic-phase responses (discussed below). These cephalic-phase responses involve Pavlovian conditioning principles in the sense that animals learn through experience that a certain food cue occurs prior to or during feeding (unconditioned stimulus), and that this food cue subsequently acts as a conditioned stimulus that acquires the capacity to evoke conditioned responses; the cephalic-phase responses. Indeed, a classic example of a conditioned cephalic response is salivation; an effect famously demonstrated in dogs in Pavlov’s pioneering work in classical conditioning theory.
The most extensively studied cephalic-phase response is cephalic insulin release. In humans, preprandial insulin infusion significantly reduces postprandial glucose levels [40], thereby supporting an important role of preabsorptive insulin release contributing to meal related glucose homeostasis. The vagus nerve (10th cranial nerve), which relays gastrointestinal food-related sensory signaling to the brain, plays a critical role in cephalic insulin release. Vagal lesions in sheep decrease the insulin response after presentation of food and increase postprandial glucose levels [41]. Similarly, truncal vagotomy has been shown to disrupt cephalic insulin release in rats [42].
In addition to insulin secretion, gastric emptying is another vagally-mediated cephalic-phase response. Myoelectric activity of the stomach, which correlates to gastric motility, increases in sham fed humans (sight, smell, and taste of food without ingestion) but not in vagotomized individuals [43]. Similarly, sham feeding improves gastric motility in humans with impaired gastric digestion [44]. These findings suggest that orosensory food-related cues, commonly modeled experimentally with sham feeding, affect vagus nerve signaling to elicit cephalic-phase responses such as cephalic insulin release and gastric motility.
Olfactory and orosensory food-related cues are perhaps the most robust stimulators of cephalic-phase responses. In humans, increased insulin secretion is elicited within two minutes after orosensory stimulation (without ingestion) and returns to baseline levels ten minutes after the stimulus was initiated [36]. Similarly, in the rat, continuous sham feeding via gastric fistulas produces robust cephalic insulin release and this response is blocked when vagal signaling is disrupted with atropine administration [45]. In addition to the robust cephalic-phase responses produced from orosensory and olfactory cues, exposure to visual food-related cues also influences cephalic-phase responses. For example, in dogs, gastric acid secretion occurs following the sight of food, however, this response is less robust than that occurring following sham feeding via esophageal fistulas stimulation [46]. Presentation of pictures of highly palatable foods also elicits an increase in salivation compared to normal, resting salivary levels in both rats [47] and humans [48]. In this latter study, subjects that were able to consume the food that was viewed compared to those that were told they couldn’t consume food showed higher salivary responses [48], suggesting that under some conditions cephalic-phase responses are also under cognitive control.
Circadian cues have also been demonstrated to initiate cephalic insulin secretion, thereby allowing an animal to exhibit conditioned physiological changes at consistent time points with relation to habitual or restricted feeding schedules. Rats placed on a restricted feeding schedule of food access for two hours everyday secrete large levels of insulin at the time of day associated with feeding (even without exposure to the food) compared to rats with ad libitum access to food [8, 49]. At a cellular level, transcription factors CLOCK and BMAL1 are circadian “clock genes” expressed in the beta cells of the pancreas, and their functional elimination in mutant mice results in decreased insulin production and high levels of blood glucose [50, 51]. Thus, cephalic insulin release based on circadian cues may be due to entrainment of these clock genes.
In addition to insulin, there are several other feeding relevant hormonal cues that have the capacity for conditioned cephalic phase release. For example, circulating glucagon-like peptide-1 (GLP-1) levels have been shown to increase prior to anticipated meals in rodents [9, 52]. Studies have also suggested possible cephalic leptin responses, as sham-feeding procedures augment circulating leptin levels [53] and leptin levels are regulated by circadian rhythms, with robust prandial peaks in scheduled-fed humans and rodents [54–56]. However, experiments examining the cephalic leptin response have been inconsistent and require further research. Glucagon also has a potential cephalic role as experiments have demonstrated that levels of glucagon decrease in the hours preceding an anticipated meal in rats [57], an effect in the opposite direction of most other cephalic-phase endocrine responses. Evidence for cephalic pancreatic polypeptide (PP) release has also been demonstrated through robust increases in circulating PP levels during sham feeding [58–60]. Collectively, these studies and others [36, 61, 62] have begun to elucidate the physiological and endocrine mechanisms that mediate learned food intake. Below we focus on the gut-derived “hunger” hormone ghrelin as a particularly important link between conditioned cephalic-phase endocrine responses and neural regulation of feeding behavior.
IV. Ghrelin: a cephalic signal for the anticipation of feeding
Ghrelin is the only known circulating hormone that increases food intake [63, 64] and has prominent stimulatory roles in modulating meal size, meal frequency, body weight, and food-motivated reward behaviors ([21, 65–67]; see reviews [68, 69]). While ghrelin is commonly referred to as a “hunger” hormone, a growing body of literature in both human and animal models indicates that ghrelin is more appropriately described as a signal for the anticipation of food intake. For example, rodents who learn to anticipate meals when placed on a meal entrainment schedule (e.g., fixed 4 hour food access every day at the same time) exhibit increasing levels of circulating ghrelin prior to the ingestion of the meal, followed by rapid postprandial suppression. Importantly, levels of ghrelin measured immediately prior to the learned food access period were elevated in comparison to animals that were equally food-restricted but had not received previous meal entrainment conditioning [10]. Comparable results are also found in sheep that were fed once daily at the same time each day, where experimenters showed that immediately prior to the animals’ meal, there is a transient surge of plasma ghrelin levels that rapidly declines during feeding. Interestingly, this study also showed a similar cephalic ghrelin response to “pseudo-feeding”, where the animals were given the diet in a nylon bag that could not be ingested [70]. Similarly, in humans who are instructed to anticipate a large meal following a 14-hour fast and then exposed to the presentation of a breakfast buffet, a rapid postprandial suppression of plasma ghrelin levels occurs that is in excess of that observed in equally fasted-controls [71]. While the study showed no differences in preprandial ghrelin levels compared to equally fasted-controls (potentially caused by knowledge of future food intake in control subjects), the results suggest that meal anticipation can strongly modulate peripheral ghrelin secretion in humans.
In further support of ghrelin as an anticipatory signal for food intake, Cummings and colleagues (2001) measured plasma ghrelin levels in humans with meals provided on a fixed schedule throughout a 24-hour period. Their results revealed a robust increase in ghrelin levels immediately before each scheduled meal (breakfast, lunch, and dinner), followed by a suppression occurring 1 hour afterwards [72]. Other studies using human subjects have also demonstrated that varying habitual feeding times (e.g., short or long inter-meal intervals) strongly affects temporal patterns of circulating ghrelin concentrations, where peak ghrelin levels occur immediately prior to an individual’s habitual meal time [73]. These results suggest that ghrelin levels rise in anticipation of food intake based on learned, habitual feeding patterns as opposed to being exclusively a “hunger signal” whose release is linked to the magnitude of food restriction. Scheduled feeding regiments (3 meals a day at fixed times) in rodents also induce spontaneous activity and increased acylated ghrelin levels at the time of scheduled meal presentation [74]. Collectively, these data suggest that ghrelin acts as an anticipatory signal for meal initiation, and in these examples its release is potentially stimulated, in part, by learned circadian cues. This notion is further corroborated by studies demonstrating that stomach cells that secrete ghrelin also express circadian clock proteins (PER1 and PER2) that exhibit rhythmic activity in response to food entrainment [75]. Moreover, in one study ghrelin levels increased before requested meals in subjects that were deprived of time- and food-related cues [76], suggesting a circadian entrainment. However, an alternative (and not mutually exclusive) explanation of these findings is that ghrelin levels rise with hunger rather than (or in addition to) in response to circadian rhythms.
Other food related stimuli are also capable of generating cephalic ghrelin responses. In humans undergoing a modified sham-feeding paradigm, serum ghrelin levels exhibit a typical cephalic response in which there is a rise immediately following sham feeding, followed by rapid suppression that in some cases can occur despite the lack of actual ingestion [34, 77]. These findings suggest that ghrelin release triggered by orosensory food cues can return to baseline without postingestive nutritive consequences, potentially through a yet unidentified cephalic phase response contributing to the clearance of ghrelin from circulation. Another study revealed an increase in circulating ghrelin levels occurred following sham feeding and this response was markedly higher in individuals with anorexia than age-matched healthy controls [78]. Similarly, research from the same group reported that in women with bulimia there is a higher cephalic increase in ghrelin levels relative to healthy controls in response to sham feeding, a result that is positively correlated with binge eating frequency [79]. These results taken together suggest that orosensory stimulation can have a powerful affect on circulating ghrelin levels, however, more research is needed to resolve the temporal dynamics and other factors contributing to ghrelin secretion vs. clearance immediately following orosensory stimulation.
The cephalic-phase ghrelin response is also differentially regulated depending on macronutrient content. Zhu and colleagues [80] demonstrated that modified sham feeding of different types of diet (e.g. high-fat, carbohydrate, or protein) all induce a cephalic ghrelin response, however, there is a substantially greater increase in circulating ghrelin levels with high-protein foods. These data indicate that the cephalic ghrelin response is not only sensitive to previous experience, but are also flexible to changes in macronutrient composition and/or the type of diet of that is being consumed. Whether palatability and/or flavor of the diet modulates ghrelin release independently of macronutrient content will require further investigation.
While these studies strongly implicate ghrelin as an anticipatory feeding signal released in response to conditioned circadian and/or orosensory cues, it is not yet known whether cephalic ghrelin responses occur in response to visual and other food cues (e.g., cognitive, interoceptive). However, as indicated above ghrelin signaling has an important role in external, cue-driven feeding. For example, peripheral ghrelin receptor blockade [19] or genetic deletion [20] in rodents blocks the ability of Pavlovian conditioned stimuli to produce hyperphagia. Whether these types of external cues generate cephalic ghrelin responses requires deeper investigation. Moreover, the role of cephalic ghrelin secretion in hyperphagia and obesity generally speaking is not established. Several studies have shown that obese humans [81, 82] and rodents maintained on an obesogenic (e.g., high fat) diet exhibit impaired ghrelin secretion [83–85] and central ghrelin signaling [21]. Future systematic studies will be required to determine whether impairments in cephalic ghrelin signaling contribute to obesity development.
It is also unclear whether cephalic ghrelin responses require vagal signaling. Vagal afferents are not required for the hyperphagic effects following peripheral (intraperitoneal) ghrelin injections [86], nor is the vagus required for postprandial suppression of ghrelin [87]. However, total subdiaphragmatic vagotomy eliminated the hyperhpagic effects of intravenous ghrelin administration in rats [88], and the increases in circulating ghrelin in response to 48hr food restriction [85]. These mixed findings give little clear insight as to whether cephalic phase ghrelin release operates independent of the vagus, and to our knowledge this has not yet been directly tested. Regardless, it is clear that learned processes involving food access (or eating) can entrain the secretion of ghrelin, which may act as an important signal for meal initiation and anticipatory feeding behavior through activity within the central nervous system.
The data reviewed above provides strong evidence for the role of cephalic ghrelin signaling in meal anticipation, and together with activation of ghrelin receptors (growth hormone secretagogue receptor, GHSR), represents a biological signaling system that allows for learned feeding behavior. Previous data has shown that genetic deletion of GHSRs results in deficits in meal anticipatory behavior under meal entrainment schedules ([89–91], but see [92]) and as indicated above, both pharmacological blockade and genetic knockout of GHSRs disrupts the expression of cue potentiated feeding [19, 20]. These findings suggest that not only is ghrelin receptor signaling important for the expression of learned feeding behavior based on internal cues (e.g., circadian), but also for feeding driven by external, food-associated stimuli. Interestingly, Davis and colleagues [89] showed that GHSR null mice can meet metabolic requirements under meal-entrained schedules, but are impaired in meal anticipatory locomotor activity. While these data suggest that ghrelin signaling is not required for food intake control in response to predictable metabolic deficits, we note that compensatory mechanisms are likely to arise during development as a result of genetic deletion of GHSRs. Thus, conclusions with regards to the ghrelin’s functional relevance to meal anticipation (and to learned consummatory responses) that are made exclusively based on transgenic GHSR null mice should be interpreted with caution. It should also be noted that the preceding studies did not systematically determine whether transgenic or pharmacological-induced deficits in ghrelin signaling impair distinct associative processes related to food cues (e.g. acquisition, consolidation, and retrieval), nor do they identify a specific neuronal substrate that potentially regulates ghrelin’s effects on learned feeding behavior.
V. CNS Regulation: Hippocampal control of ghrelin-mediated conditioned food intake
Ghrelin readily crosses the blood brain barrier [84, 93] and acts through the G protein-coupled receptor, GHSR. GHSRs are densely expressed in many feeding and reward-related neural substrates [94, 95] and studies have shown that ghrelin acts within the limbic and mesolimbic reward systems [e.g., ventral tegmental area (VTA), nucleus accumbens (NAcc) and amygdala] to express a myriad of ingestive and food-motivated behaviors [96–100]. For example, direct ghrelin administration to either the VTA or NAcc increases food intake, whereas motivated responding for food reward is increased following VTA (but not NAcc) ghrelin delivery [98, 99].
The ventral subregion of the hippocampus (vHP) has also been recently identified as playing a role in food-motivated and ingestive behaviors [3, 28–31, 33]. The hippocampus is activated by gastric distension and nutrient infusion [101, 102] and has been implicated in the modulation of learned meal anticipation [103–106]. Neurons in the vHP express receptors for various endocrine feeding-relevant signals, including leptin, GLP-1, and ghrelin [94, 107, 108], and activation of these receptors in the vHP is one mechanism through which hippocampal neural processing bi-directionally influences energy balance [21, 109, 110]. Interactions between these vHP neuroendocrine signals is a strong possibility and a deeper discussion on this topic can be found in [33]. Recent work from Kanoski, Grill, and colleagues [21] showed that pharmacological activation of GHSRs in the vHP potently increases food intake and food motivated behaviors. They further demonstrated that vHP GHSR activation increases meal initiation frequency in response to external food-associated cues, while having no hyperphagic feeding effects in response to neutral stimuli [21]. This is strong evidence for vHP ghrelin signaling as a neural substrate that underlies learned feeding behavior in response to external food-associated cues.
To expand these findings, a recent study from our group investigated the endogenous relevance of this system in circadian entrained feeding behavior [111]. Rats were placed on a meal entrainment schedule in which food was presented at the same time every day for 4 hours. Similar to previous work [10], these animals learned to utilize internal circadian cues to anticipate meals, and steadily increased their caloric intake over successive days to meet their metabolic needs. Pharmacological blockade of vHP GHSRs (via JMV 2959, a GHSR antagonist) prior to meal access reduced 4-hour food consumption during the scheduled meal time, while having no effects in animals that were similarly food deprived, but not meal entrained. These data strongly suggest that vHP ghrelin signaling is a neurobiological substrate that is endogenously relevant for learned food intake. It is also important to note that GHSRs have high levels of constitutive activity, and there is emerging research suggesting that constitutively active GHSRs might play a role in regulating energy balance [112, 113]. Thus, while ghrelin release and vHP ghrelin signaling is important for learned feeding behavior, there is the yet unexplored possibility that vHP GHSR constitutive activity is also involved in regulating meal anticipation and subsequent food intake control. This hypothesis could be tested using a GHSR inverse agonist (e.g., [D-Arg1, D-Phe5, D-Trp7,9, Leu11]-substance P [114]) to silence constitutive activity in vHP GHSR-expressing neurons.
Field CA1 pyramidal neurons in the vHP ipsilaterally project to the dorsal perifornical subregion of the lateral hypothalamic area (dpLHA) [111, 115–118], a region that mediates orexigenic aspects of feeding behavior [119–126]. Hsu et al. [111] demonstrated that ~85% of vCA1 neurons that project to the dpLHA also express GHSR [111], thus providing anatomical evidence identifying the LHA as a downstream target for vHP ghrelin signaling. Taking advantage of the exclusively ipsilateral connection between these brain regions, a neuropharmacological neural disconnection approach was utilized that eliminated communication between the vHP and dpLHA via unilateral excitotoxic lesions of the dpLHA. In unilateral dpLHA-lesioned and sham control rats, food intake was measured following unilateral injections of ghrelin either ipsilateral (vHP-dpLHA connection compromised) or contralateral (vHP-dpLHA connection intact) to the dpLHA lesion. A hyperphagic response was observed when ghrelin was administered to the vHP contralateral to the dpLHA lesions, and in animals with sham lesions; however, when ghrelin was administered ipsilaterally to the dpLHA lesion the hyperphagic response was robustly attenuated [111]. These data strongly suggest that the LHA is a critical downstream target for vHP ghrelin-mediated feeding and therefore highlight a novel neural circuitry mediating some aspects of learned food intake.
Consistent with this model, delivery of ghrelin to the vHP activates dpLHA neurons that produce orexin, a neuropeptide that stimulates appetite and feeding [127–133], and lateral ventricle delivery of an orexin-1 receptor antagonist (using a dose with no effect on feeding alone) blocked the hyperphagic effects of vHP ghrelin delivery [111]. Thus, we hypothesize that orexin is a critical downstream target for ghrelin-mediated conditioned feeding effects. As is the case with ghrelin receptor knockout mice, orexin receptor mutation also reduces food anticipatory activity [127]. Moreover, it appears as though orexin, like ghrelin, can be entrained in response to restricted meal schedules and meal anticipation [134, 135]. The VTA is one putative site through which orexin signaling influences learned feeding behavior, as blockade of orexin receptors in the VTA attenuates the hyperphagic response following ventricular delivery of ghrelin [136]. In addition to being responsive to vHP ghrelin signaling, orexin-producing neurons in the LHA express GHSR and are activated by direct LHA ghrelin delivery [137], thus raising that possibility that ghrelin may be stimulating learned feeding behavior (and downstream VTA orexin signaling) though direct action on LHA orexin neurons. However, blockade of GHSRs in the LHA has no effects on food intake in meal-entrained animals [111], indicating that the vHP ghrelin signaling is a potentially a unique neural substrate for learned feeding behavior.
Taken together, these data suggest that through monosynaptic neural circuitry between the vHP and LHA, vHP ghrelin-activated glutamatergic pyramidal CA1 neurons stimulate LHA orexin secretion, which in turn engages the expression of learned feeding through activation of orexin receptors in downstream regions, potentially including the VTA. However, in addition to the VTA, orexin receptor signaling at several brain regions has diverse effects on feeding and food-motivated behaviors [119, 130, 132, 133, 138–141]. Thus, there is a need for future studies to explore which of the various potential downstream orexin targets are relevant for learned feeding effects. Furthermore, vHP field CA1 neurons also have projections to other feeding relevant regions, such as the amygdala and medial prefrontal cortex (mPFC) [142, 143], which then provides topographical input to the LHA [115, 116]. Additionally, amygdala-LHA and mPFC-LHA neural pathways are required for the expression of cue potentiated feeding [13, 15]. Whether these polysynaptic pathways are also involved in vHP ghrelin-mediated feeding effects will require deeper examination.
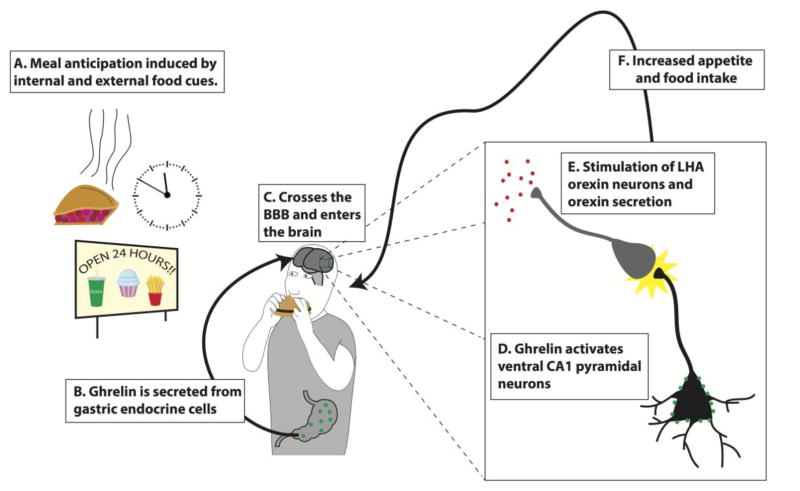
Based on our recent work and other previous findings, we propose the following model through which ghrelin acts to regulate conditioned aspects of feeding (Fig. 1; modified from [111]): A) Meal anticipation is induced by learned external or internal food associated cues, which B) stimulates the secretion of ghrelin from the P/D1 cells in the stomach. C) Ghrelin then enters the vascular system, where it crosses the blood-brain barrier and accesses the brain. D) Ghrelin activates vHP field CA1 pyramidal neurons, which project monosynaptically to LHA orexin neurons. E) The activated vCA1 pyramidal neurons then stimulate orexin-producing LHA neurons and initiates downstream orexin signaling to feeding and reward-relevant brain regions (e.g. VTA). F) Activity within this system then stimulates learned appetite and food intake.
Figure 1.
A model for ventral hippocampus (vHP) ghrelin-mediated conditioned feeding behavior (adapted from [111]): A) Humans and animals learn to anticipate feeding based on external or internal food cues (e.g. habitual circadian cues, food advertisements, and sight and/or smell of food). B) Ghrelin secreted from the stomach in anticipation of feeding crosses the blood-brain barrier and enters the central nervous system. C) Ghrelin activates ghrelin receptors expressed on vHP pyramidal neurons, which directly engages D) downstream activation of dpLHA orexin neurons and orexin secretion. E) Activation of this neural pathway and downstream orexin receptor signaling enhances learned feeding behavior.
VI. Conclusions
Feeding is a highly complex behavior and several studies have now shown an intimate relationship between learning and memory processes and their underlying neural and physiological substrates in the control of energy balance. The gut-derived hormone ghrelin is a biological signal that appears to be a critical link between previous experience and food intake control. Like insulin and other endocrine signals, ghrelin is released in a cephalic manner based on learned meal entrainment and orosensory stimulation, and may also be released in response to other interoceptive and external food-related cues. Circulating ghrelin crosses the blood-brain barrier and acts in several brain regions to stimulate appetite and feeding behavior. The ventral subregion of the hippocampus, vHP, is one critical site mediating ghrelin’s stimulatory effects on conditioned feeding behavior, and ghrelin signaling in this brain region engages orexin signaling originating in the lateral hypothalamus to stimulate appetite and ingestion. Future studies will aim to comprehensively understand other neural targets and interactions between endocrine neuropeptide systems through which ghrelin acts as a learned signal to feed.
Highlights.
Learning and memory processes strongly influence feeding behavior
Learned, cephalic---phase endocrine responses are a physiological substrate for conditioned feeding.
The cephalic ghrelin response is a learned, anticipatory signal for feeding.
Ghrelin acts in hippocampus---lateral hypothalamic neural circuitry to express learned feeding behavior.
Acknowledgments
We would like to acknowledge our support from the National Institute of Health grants DK104897 (SEK) and SSIB for the New Investigator Travel Award (TMH). We report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cornell CE, Rodin J, Weingarten H. Stimulus-induced eating when satiated. Physiol Behav. 1989;45:695–704. doi: 10.1016/0031-9384(89)90281-3. [DOI] [PubMed] [Google Scholar]
- 2.Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiology & behavior. 2002;76:117–29. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- 3.Davidson TL, Tracy AL, Schier LA, Swithers SE. A view of obesity as a learning and memory disorder. Journal of experimental psychology Animal learning and cognition. 2014;40:261–79. doi: 10.1037/xan0000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benoit SC, Davis JF, Davidson TL. Learned and cognitive controls of food intake. Brain research. 2010;1350:71–6. doi: 10.1016/j.brainres.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halford JC, Gillespie J, Brown V, Pontin EE, Dovey TM. Effect of television advertisements for foods on food consumption in children. Appetite. 2004;42:221–5. doi: 10.1016/j.appet.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–8. [PubMed] [Google Scholar]
- 7.Sclafani A. Post-ingestive positive controls of ingestive behavior. Appetite. 2001;36:79–83. doi: 10.1006/appe.2000.0370. [DOI] [PubMed] [Google Scholar]
- 8.Woods SC, Vasselli JR, Kaestner E, Szakmary GA, Milburn P, Vitiello MV. Conditioned insulin secretion and meal feeding in rats. Journal of comparative and physiological psychology. 1977;91:128–33. doi: 10.1037/h0077307. [DOI] [PubMed] [Google Scholar]
- 9.Vahl TP, Drazen DL, Seeley RJ, D’Alessio DA, Woods SC. Meal-anticipatory glucagon-like peptide-1 secretion in rats. Endocrinology. 2010;151:569–75. doi: 10.1210/en.2009-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147:23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- 11.Mistlberger R, Rusak B. Palatable daily meals entrain anticipatory activity rhythms in free-feeding rats: dependence on meal size and nutrient content. Physiol Behav. 1987;41:219–26. doi: 10.1016/0031-9384(87)90356-8. [DOI] [PubMed] [Google Scholar]
- 12.Abe H, Rusak B. Anticipatory activity and entrainment of circadian rhythms in Syrian hamsters exposed to restricted palatable diets. Am J Physiol. 1992;263:R116–24. doi: 10.1152/ajpregu.1992.263.1.R116. [DOI] [PubMed] [Google Scholar]
- 13.Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci. 2002;22:8748–53. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:6436–41. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25:8295–302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrovich GD, Hobin MP, Reppucci CJ. Selective Fos induction in hypothalamic orexin/hypocretin, but not melanin-concentrating hormone neurons, by a learned food-cue that stimulates feeding in sated rats. Neuroscience. 2012;224:70–80. doi: 10.1016/j.neuroscience.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson AW. Eating beyond metabolic need: how environmental cues influence feeding behavior. Trends in neurosciences. 2013;36:101–9. doi: 10.1016/j.tins.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Birch LL, McPhee L, Sullivan S, Johnson S. Conditioned meal initiation in young children. Appetite. 1989;13:105–13. doi: 10.1016/0195-6663(89)90108-6. [DOI] [PubMed] [Google Scholar]
- 19.Dailey MJ, Moran TH, Holland PC, Johnson AW. The antagonism of ghrelin alters the appetitive response to learned cues associated with food. Behavioural brain research. 2016;303:191–200. doi: 10.1016/j.bbr.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 20.Walker AK, Ibia IE, Zigman JM. Disruption of cue-potentiated feeding in mice with blocked ghrelin signaling. Physiology & behavior. 2012;108:34–43. doi: 10.1016/j.physbeh.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin Signaling in the Ventral Hippocampus Stimulates Learned and Motivational Aspects of Feeding via PI3K-Akt Signaling. Biol Psychiatry. 2013;73:915–23. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83:764–70. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen NJ, Poldrack RA, Eichenbaum H. Memory for items and memory for relations in the procedural/declarative memory framework. Memory. 1997;5:131–78. doi: 10.1080/741941149. [DOI] [PubMed] [Google Scholar]
- 24.Higgs S, Williamson AC, Rotshtein P, Humphreys GW. Sensory-Specific Satiety Is Intact in Amnesics Who Eat Multiple Meals. Psychological Science. 2008;19:623–8. doi: 10.1111/j.1467-9280.2008.02132.x. [DOI] [PubMed] [Google Scholar]
- 25.Rozin P, Dow S, Moscovitch M, Rajaram S. What Causes Humans to Begin and End a Meal? A Role for Memory for What Has Been Eaten, as Evidenced by a Study of Multiple Meal Eating in Amnesic Patients. Psychological Science. 1998;9:392–6. [Google Scholar]
- 26.Henderson YO, Smith GP, Parent MB. Hippocampal neurons inhibit meal onset. Hippocampus. 2013;23:100–7. doi: 10.1002/hipo.22062. [DOI] [PubMed] [Google Scholar]
- 27.Henderson YO, Nalloor R, Vazdarjanova A, Parent MB. Sweet orosensation induces Arc expression in dorsal hippocampal CA1 neurons in an Experience-dependent manner. Hippocampus. 2015 doi: 10.1002/hipo.22532. [DOI] [PubMed] [Google Scholar]
- 28.Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiol Behav. 2005;86:731–46. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Kanoski SE. Cognitive and neuronal systems underlying obesity. Physiol Behav. 2012;106:337–44. doi: 10.1016/j.physbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benoit SC, Davis JF, Davidson TL. Learned and cognitive controls of food intake. Brain Res. 2010;1350:71–6. doi: 10.1016/j.brainres.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tracy AL, Jarrard LE, Davidson TL. The hippocampus and motivation revisited: appetite and activity. Behav Brain Res. 2001;127:13–23. doi: 10.1016/s0166-4328(01)00364-3. [DOI] [PubMed] [Google Scholar]
- 32.Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behavioral neuroscience. 2010;124:97–105. doi: 10.1037/a0018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanoski SE, Grill HJ. Hippocampus Contributions to Food Intake Control: Mnemonic, Neuroanatomical, and Endocrine Mechanisms. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arosio M, Ronchi CL, Beck-Peccoz P, Gebbia C, Giavoli C, Cappiello V, et al. Effects of modified sham feeding on ghrelin levels in healthy human subjects. The Journal of clinical endocrinology and metabolism. 2004;89:5101–4. doi: 10.1210/jc.2003-032222. [DOI] [PubMed] [Google Scholar]
- 35.Robertson MD, Jackson KG, Williams CM, Fielding BA, Frayn KN. Prolonged effects of modified sham feeding on energy substrate mobilization. The American journal of clinical nutrition. 2001;73:111–7. doi: 10.1093/ajcn/73.1.111. [DOI] [PubMed] [Google Scholar]
- 36.Teff K. Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite. 2000;34:206–13. doi: 10.1006/appe.1999.0282. [DOI] [PubMed] [Google Scholar]
- 37.Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behavioural brain research. 2000;110:175–82. doi: 10.1016/s0166-4328(99)00194-1. [DOI] [PubMed] [Google Scholar]
- 38.Woods SC, Hutton RA, Makous W. Conditioned insulin secretion in the albino rat. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1970;133:964–8. doi: 10.3181/00379727-133-34605. [DOI] [PubMed] [Google Scholar]
- 39.Woods SC. The eating paradox: how we tolerate food. Psychological review. 1991;98:488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- 40.Teff KL, Townsend RR. Early phase insulin infusion and muscarinic blockade in obese and lean subjects. The American journal of physiology. 1999;277:R198–208. doi: 10.1152/ajpregu.1999.277.1.R198. [DOI] [PubMed] [Google Scholar]
- 41.Herath CB, Reynolds GW, MacKenzie DD, Davis SR, Harris PM. Vagotomy suppresses cephalic phase insulin release in sheep. Experimental physiology. 1999;84:559–69. [PubMed] [Google Scholar]
- 42.Powley TL. Vagal circuitry mediating cephalic-phase responses to food. Appetite. 2000;34:184–8. doi: 10.1006/appe.1999.0279. [DOI] [PubMed] [Google Scholar]
- 43.Stern RM, Crawford HE, Stewart WR, Vasey MW, Koch KL. Sham feeding. Cephalic-vagal influences on gastric myoelectric activity. Digestive diseases and sciences. 1989;34:521–7. doi: 10.1007/BF01536327. [DOI] [PubMed] [Google Scholar]
- 44.Lunding JA, Nordstrom LM, Haukelid AO, Gilja OH, Berstad A, Hausken T. Vagal activation by sham feeding improves gastric motility in functional dyspepsia. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2008;20:618–24. doi: 10.1111/j.1365-2982.2007.01076.x. [DOI] [PubMed] [Google Scholar]
- 45.Berthoud HR, Jeanrenaud B. Sham feeding-induced cephalic phase insulin release in the rat. The American journal of physiology. 1982;242:E280–5. doi: 10.1152/ajpendo.1982.242.4.E280. [DOI] [PubMed] [Google Scholar]
- 46.IPP . The work of the digestive glands. London: Charles Griffin Co LTD; 1902. [Google Scholar]
- 47.Wooley SC, Wooley OW. Salivation to the sight and thought of food: a new measure of appetite. Psychosomatic medicine. 1973;35:136–42. doi: 10.1097/00006842-197303000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi T. Experimental evidence of the second signaling system of man. Conditional reflex. 1968;3:18–28. doi: 10.1007/BF03001133. [DOI] [PubMed] [Google Scholar]
- 49.Wiley JH, Leveille GA. Significance of insulin in the metabolic adaptation of rats to meal ingestion. The Journal of nutrition. 1970;100:1073–80. doi: 10.1093/jn/100.9.1073. [DOI] [PubMed] [Google Scholar]
- 50.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–4. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dailey MJ, Stingl KC, Moran TH. Disassociation between preprandial gut peptide release and food-anticipatory activity. Endocrinology. 2012;153:132–42. doi: 10.1210/en.2011-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konturek SJ, Konturek JW. Cephalic phase of pancreatic secretion. Appetite. 2000;34:197–205. doi: 10.1006/appe.1999.0281. [DOI] [PubMed] [Google Scholar]
- 54.Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. The Journal of clinical investigation. 1998;101:1020–7. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. The Journal of clinical endocrinology and metabolism. 2005;90:2537–44. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahren B. Diurnal variation in circulating leptin is dependent on gender, food intake and circulating insulin in mice. Acta physiologica Scandinavica. 2000;169:325–31. doi: 10.1046/j.1365-201x.2000.00746.x. [DOI] [PubMed] [Google Scholar]
- 57.Davidson AJ, Stephan FK. Plasma glucagon, glucose, insulin, and motilin in rats anticipating daily meals. Physiology & behavior. 1999;66:309–15. doi: 10.1016/s0031-9384(98)00308-4. [DOI] [PubMed] [Google Scholar]
- 58.Veedfald S, Plamboeck A, Deacon CF, Hartmann B, Knop FK, Vilsboll T, et al. Cephalic phase secretion of insulin and other enteropancreatic hormones in humans. American journal of physiology Gastrointestinal and liver physiology. 2016;310:G43–51. doi: 10.1152/ajpgi.00222.2015. [DOI] [PubMed] [Google Scholar]
- 59.Taylor IL, Feldman M. Effect of cephalic-vagal stimulation on insulin, gastric inhibitory polypeptide, and pancreatic polypeptide release in humans. The Journal of clinical endocrinology and metabolism. 1982;55:1114–7. doi: 10.1210/jcem-55-6-1114. [DOI] [PubMed] [Google Scholar]
- 60.Wisen O, Bjorvell H, Cantor P, Johansson C, Theodorsson E. Plasma concentrations of regulatory peptides in obesity following modified sham feeding (MSF) and a liquid test meal. Regulatory peptides. 1992;39:43–54. doi: 10.1016/0167-0115(92)90007-h. [DOI] [PubMed] [Google Scholar]
- 61.Zafra MA, Molina F, Puerto A. The neural/cephalic phase reflexes in the physiology of nutrition. Neuroscience and biobehavioral reviews. 2006;30:1032–44. doi: 10.1016/j.neubiorev.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Smeets PA, Erkner A, de Graaf C. Cephalic phase responses and appetite. Nutrition reviews. 2010;68:643–55. doi: 10.1111/j.1753-4887.2010.00334.x. [DOI] [PubMed] [Google Scholar]
- 63.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 64.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–61. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 65.Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–7. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 66.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. The Journal of clinical endocrinology and metabolism. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 67.Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–5. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- 68.Skibicka KP, Dickson SL. Ghrelin and food reward: the story of potential underlying substrates. Peptides. 2011;32:2265–73. doi: 10.1016/j.peptides.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 69.Perello M, Dickson SL. Ghrelin signalling on food reward: a salient link between the gut and the mesolimbic system. Journal of neuroendocrinology. 2015;27:424–34. doi: 10.1111/jne.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sugino T, Hasegawa Y, Kikkawa Y, Yamaura J, Yamagishi M, Kurose Y, et al. A transient ghrelin surge occurs just before feeding in a scheduled meal-fed sheep. Biochemical and biophysical research communications. 2002;295:255–60. doi: 10.1016/s0006-291x(02)00654-x. [DOI] [PubMed] [Google Scholar]
- 71.Ott V, Friedrich M, Zemlin J, Lehnert H, Schultes B, Born J, et al. Meal anticipation potentiates postprandial ghrelin suppression in humans. Psychoneuroendocrinology. 2012;37:1096–100. doi: 10.1016/j.psyneuen.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 72.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 73.Frecka JM, Mattes RD. Possible entrainment of ghrelin to habitual meal patterns in humans. American journal of physiology Gastrointestinal and liver physiology. 2008;294:G699–707. doi: 10.1152/ajpgi.00448.2007. [DOI] [PubMed] [Google Scholar]
- 74.Verbaeys I, Tolle V, Swennen Q, Zizzari P, Buyse J, Epelbaum J, et al. Scheduled feeding results in adipogenesis and increased acylated ghrelin. American journal of physiology Endocrinology and metabolism. 2011;300:E1103–11. doi: 10.1152/ajpendo.00551.2010. [DOI] [PubMed] [Google Scholar]
- 75.LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13582–7. doi: 10.1073/pnas.0906426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab. 2004;287:E297–304. doi: 10.1152/ajpendo.00582.2003. [DOI] [PubMed] [Google Scholar]
- 77.Heath RB, Jones R, Frayn KN, Robertson MD. Vagal stimulation exaggerates the inhibitory ghrelin response to oral fat in humans. The Journal of endocrinology. 2004;180:273–81. doi: 10.1677/joe.0.1800273. [DOI] [PubMed] [Google Scholar]
- 78.Monteleone P, Serritella C, Martiadis V, Maj M. Deranged secretion of ghrelin and obestatin in the cephalic phase of vagal stimulation in women with anorexia nervosa. Biological psychiatry. 2008;64:1005–8. doi: 10.1016/j.biopsych.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 79.Monteleone P, Serritella C, Scognamiglio P, Maj M. Enhanced ghrelin secretion in the cephalic phase of food ingestion in women with bulimia nervosa. Psychoneuroendocrinology. 2010;35:284–8. doi: 10.1016/j.psyneuen.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 80.Zhu Y, Hsu WH, Hollis JH. Modified sham feeding of foods with different macronutrient compositions differentially influences cephalic change of insulin, ghrelin, and NMR-based metabolomic profiles. Physiology & behavior. 2014;135:135–42. doi: 10.1016/j.physbeh.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 81.Monteleone P, Fabrazzo M, Tortorella A, Martiadis V, Serritella C, Maj M. Circulating ghrelin is decreased in non-obese and obese women with binge eating disorder as well as in obese non-binge eating women, but not in patients with bulimia nervosa. Psychoneuroendocrinology. 2005;30:243–50. doi: 10.1016/j.psyneuen.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 82.Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10434–9. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perreault M, Istrate N, Wang L, Nichols AJ, Tozzo E, Stricker-Krongrad A. Resistance to the orexigenic effect of ghrelin in dietary-induced obesity in mice: reversal upon weight loss. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2004;28:879–85. doi: 10.1038/sj.ijo.0802640. [DOI] [PubMed] [Google Scholar]
- 84.Banks WA, Burney BO, Robinson SM. Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood-brain barrier. Peptides. 2008;29:2061–5. doi: 10.1016/j.peptides.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams DL, Grill HJ, Cummings DE, Kaplan JM. Overfeeding-induced weight gain suppresses plasma ghrelin levels in rats. Journal of endocrinological investigation. 2006;29:863–8. doi: 10.1007/BF03349188. [DOI] [PubMed] [Google Scholar]
- 86.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–60. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams DL, Grill HJ, Cummings DE, Kaplan JM. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology. 2003;144:5184–7. doi: 10.1210/en.2003-1059. [DOI] [PubMed] [Google Scholar]
- 88.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–8. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 89.Davis JF, Choi DL, Clegg DJ, Benoit SC. Signaling through the ghrelin receptor modulates hippocampal function and meal anticipation in mice. Physiology & behavior. 2011;103:39–43. doi: 10.1016/j.physbeh.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, et al. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience. 2009;164:351–9. doi: 10.1016/j.neuroscience.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lamont EW, Bruton J, Blum ID, Abizaid A. Ghrelin receptor-knockout mice display alterations in circadian rhythms of activity and feeding under constant lighting conditions. The European journal of neuroscience. 2014;39:207–17. doi: 10.1111/ejn.12390. [DOI] [PubMed] [Google Scholar]
- 92.Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2010;67:880–6. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. The Journal of pharmacology and experimental therapeutics. 2002;302:822–7. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 94.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. The Journal of comparative neurology. 2006;494:528–48. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mani BK, Walker AK, Lopez Soto EJ, Raingo J, Lee CE, Perello M, et al. Neuroanatomical characterization of a growth hormone secretagogue receptor-green fluorescent protein reporter mouse. J Comp Neurol. 2014;522:3644–66. doi: 10.1002/cne.23627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.St-Onge V, Watts A, Abizaid A. Ghrelin enhances cue-induced bar pressing for high fat food. Hormones and behavior. 2015;78:141–9. doi: 10.1016/j.yhbeh.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 97.King SJ, Isaacs AM, O’Farrell E, Abizaid A. Motivation to obtain preferred foods is enhanced by ghrelin in the ventral tegmental area. Hormones and behavior. 2011;60:572–80. doi: 10.1016/j.yhbeh.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 98.Skibicka KP, Shirazi RH, Rabasa-Papio C, Alvarez-Crespo M, Neuber C, Vogel H, et al. Divergent circuitry underlying food reward and intake effects of ghrelin: dopaminergic VTA-accumbens projection mediates ghrelin’s effect on food reward but not food intake. Neuropharmacology. 2013;73:274–83. doi: 10.1016/j.neuropharm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 99.Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–37. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 100.Alvarez-Crespo M, Skibicka KP, Farkas I, Molnar CS, Egecioglu E, Hrabovszky E, et al. The amygdala as a neurobiological target for ghrelin in rats: neuroanatomical, electrophysiological and behavioral evidence. PloS one. 2012;7:e46321. doi: 10.1371/journal.pone.0046321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Min DK, Tuor UI, Koopmans HS, Chelikani PK. Changes in differential functional magnetic resonance signals in the rodent brain elicited by mixed-nutrient or protein-enriched meals. Gastroenterology. 2011;141:1832–41. doi: 10.1053/j.gastro.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 102.Min DK, Tuor UI, Chelikani PK. Gastric distention induced functional magnetic resonance signal changes in the rodent brain. Neuroscience. 2011;179:151–8. doi: 10.1016/j.neuroscience.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 103.Inglis FM, Day JC, Fibiger HC. Enhanced acetylcholine release in hippocampus and cortex during the anticipation and consumption of a palatable meal. Neuroscience. 1994;62:1049–56. doi: 10.1016/0306-4522(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 104.Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S. Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. The European journal of neuroscience. 2001;13:1190–6. doi: 10.1046/j.0953-816x.2001.01483.x. [DOI] [PubMed] [Google Scholar]
- 105.Verwey M, Amir S. Variable restricted feeding disrupts the daily oscillations of Period2 expression in the limbic forebrain and dorsal striatum in rats. Journal of molecular neuroscience : MN. 2012;46:258–64. doi: 10.1007/s12031-011-9529-z. [DOI] [PubMed] [Google Scholar]
- 106.Poulin AM, Timofeeva E. The dynamics of neuronal activation during food anticipation and feeding in the brain of food-entrained rats. Brain research. 2008;1227:128–41. doi: 10.1016/j.brainres.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 107.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, et al. Leptin targets in the mouse brain. The Journal of comparative neurology. 2009;514:518–32. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. The Journal of comparative neurology. 1999;403:261–80. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 109.Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, Gianessi CA, Gilbert JR, et al. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1859–70. doi: 10.1038/npp.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE. Hippocampal GLP-1 Receptors Influence Food Intake, Meal Size, and Effort-Based Responding for Food through Volume Transmission. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hsu TM, Hahn JD, Konanur VR, Noble EE, Suarez AN, Thai J, et al. Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. Elife. 2015;4 doi: 10.7554/eLife.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Petersen PS, Woldbye DP, Madsen AN, Egerod KL, Jin C, Lang M, et al. In vivo characterization of high Basal signaling from the ghrelin receptor. Endocrinology. 2009;150:4920–30. doi: 10.1210/en.2008-1638. [DOI] [PubMed] [Google Scholar]
- 113.Mear Y, Enjalbert A, Thirion S. GHS-R1a constitutive activity and its physiological relevance. Frontiers in neuroscience. 2013;7:87. doi: 10.3389/fnins.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor--identification of a potent inverse agonist. Molecular endocrinology. 2003;17:2201–10. doi: 10.1210/me.2003-0069. [DOI] [PubMed] [Google Scholar]
- 115.Hahn JD, Swanson LW. Connections of the juxtaventromedial region of the lateral hypothalamic area in the male rat. Frontiers in systems neuroscience. 2015;9:66. doi: 10.3389/fnsys.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hahn JD, Swanson LW. Connections of the lateral hypothalamic area juxtadorsomedial region in the male rat. The Journal of comparative neurology. 2012;520:1831–90. doi: 10.1002/cne.23064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cenquizca LA, Swanson LW. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. The Journal of comparative neurology. 2006;497:101–14. doi: 10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kishi T, Tsumori T, Ono K, Yokota S, Ishino H, Yasui Y. Topographical organization of projections from the subiculum to the hypothalamus in the rat. The Journal of comparative neurology. 2000;419:205–22. doi: 10.1002/(sici)1096-9861(20000403)419:2<205::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 119.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 120.Dube MG, Kalra SP, Kalra PS. Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain research. 1999;842:473–7. doi: 10.1016/s0006-8993(99)01824-7. [DOI] [PubMed] [Google Scholar]
- 121.Berthoud HR, Munzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav. 2011;104:29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behavioural brain research. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Legrand R, Lucas N, Breton J, Dechelotte P, Fetissov SO. Dopamine release in the lateral hypothalamus is stimulated by alpha-MSH in both the anticipatory and consummatory phases of feeding. Psychoneuroendocrinology. 2015;56:79–87. doi: 10.1016/j.psyneuen.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 124.Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. The Yale journal of biology and medicine. 1951;24:123–40. [PMC free article] [PubMed] [Google Scholar]
- 125.Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341:1517–21. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science. 1962;135:375–7. doi: 10.1126/science.135.3501.375. [DOI] [PubMed] [Google Scholar]
- 127.Akiyama M, Yuasa T, Hayasaka N, Horikawa K, Sakurai T, Shibata S. Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. The European journal of neuroscience. 2004;20:3054–62. doi: 10.1111/j.1460-9568.2004.03749.x. [DOI] [PubMed] [Google Scholar]
- 128.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 129.Sakurai T. The role of orexin in motivated behaviours. Nature reviews Neuroscience. 2014;15:719–31. doi: 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- 130.Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain research. 2005;1050:156–62. doi: 10.1016/j.brainres.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 131.Rodgers RJ, Ishii Y, Halford JC, Blundell JE. Orexins and appetite regulation. Neuropeptides. 2002;36:303–25. doi: 10.1016/s0143-4179(02)00085-9. [DOI] [PubMed] [Google Scholar]
- 132.Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology. 2013;226:155–65. doi: 10.1007/s00213-012-2902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parise EM, Lilly N, Kay K, Dossat AM, Seth R, Overton JM, et al. Evidence for the role of hindbrain orexin-1 receptors in the control of meal size. American journal of physiology Regulatory, integrative and comparative physiology. 2011;301:R1692–9. doi: 10.1152/ajpregu.00044.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10493–501. doi: 10.1523/JNEUROSCI.3171-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kaur S, Thankachan S, Begum S, Blanco-Centurion C, Sakurai T, Yanagisawa M, et al. Entrainment of temperature and activity rhythms to restricted feeding in orexin knock out mice. Brain research. 2008;1205:47–54. doi: 10.1016/j.brainres.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cone JJ, Roitman JD, Roitman MF. Ghrelin regulates phasic dopamine and nucleus accumbens signaling evoked by food-predictive stimuli. J Neurochem. 2015;133:844–56. doi: 10.1111/jnc.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides. 2003;24:597–602. doi: 10.1016/s0196-9781(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 138.Zhu Y, Yamanaka A, Kunii K, Tsujino N, Goto K, Sakurai T. Orexin-mediated feeding behavior involves both leptin-sensitive and -insensitive pathways. Physiology & behavior. 2002;77:251–7. doi: 10.1016/s0031-9384(02)00843-0. [DOI] [PubMed] [Google Scholar]
- 139.Goforth PB, Leinninger GM, Patterson CM, Satin LS, Myers MG., Jr Leptin acts via lateral hypothalamic area neurotensin neurons to inhibit orexin neurons by multiple GABA-independent mechanisms. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:11405–15. doi: 10.1523/JNEUROSCI.5167-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–82. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thorpe AJ, Doane DF, Sweet DC, Beverly JL, Kotz CM. Orexin A in the rostrolateral hypothalamic area induces feeding by modulating GABAergic transmission. Brain Res. 2006;1125:60–6. doi: 10.1016/j.brainres.2006.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. Journal of neurophysiology. 2006;96:2134–8. doi: 10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- 143.Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain research reviews. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]