Abstract
Hospital systems increasingly utilize pharmacogenomic testing to inform clinical prescribing. Successful implementation efforts have been modeled at many academic centers. In contrast, this report provides insights into the formation of a pharmacogenomics consultation service at a safety-net hospital, which predominantly serves low-income, uninsured, and vulnerable populations. The report describes the INdiana GENomics Implementation: an Opportunity for the UnderServed (INGENIOUS) trial and addresses concerns of adjudication, credentialing, and funding.
THE INGENIOUS TRIAL
Pharmacogenomic-guided therapy has been successfully implemented at several academic medical centers.1–3 However, a different model may be required to deliver clinical support to providers (physicians, advanced practice nurses, and pharmacists) outside of academic centers.4 The Indiana Institute for Personalized Medicine (IIPM), at Indiana University School of Medicine (IUSM), functions as the home for implementation of genotype-guided prescribing in Indiana. IIPM established a Clinical Laboratory Improvement Amendments (CLIA)-approved laboratory that utilizes a custom polymerase chain reaction (PCR)-based OpenArray genotyping platform to assess 43 germline variants in 14 genes known to affect 28 medications. The laboratory provides genotyping services for all IUSM-related initiatives.
In March 2015, the INGENIOUS trial (NCT02297126) began recruitment within the Eskenazi Health System, Indianapolis’ safety-net hospital system servicing predominantly low-income and uninsured patients.5 The trial tests whether pharmaco-genotyping impacts annual healthcare costs and adverse event incidence. The hospital’s 10 clinics maintain common electronic medical record systems (EMR). When any Eskenazi physician enters a new prescription for one of 28 drugs, an EMR interruptive alert prompts the prescriber to request enrollment in the INGENIOUS trial. With prescriber approval, a patient is randomized to receive pharmaco-genotyping or standard care (no pharmaco-genotyping). For patients in the genotyping arm, the EMR notifies a research coordinator who approaches patients to obtain consent and DNA from agreeing patients.
Within 7 days of sample submission, genotype reports are delivered to the enrolling prescriber and uploaded as searchable documents in the EMR. Reports contain recommendations for selecting alternative agents or dosing of the medication that triggered enrollment, following guidelines from the Clinical Pharma-cogenetics Implementation Consortium (CPIC). Supplemental Document 1 contains an example report. Future e-prescribing of any of the 28 drugs leads to an interruptive automated alert that notifies clinicians a genotype report is available and provides dosing recommendations (trial details are available in Supplemental Document 2).
ADJUDICATION IN THE INGENIOUS TRIAL
The INGENIOUS trial maintains an adjudication committee that reviews genotypes and medication lists for enrollees to ensure clinicians receive actionable results and to protect against liability associated with incidental findings. This committee includes a physician, a co-investigator of the INGENIOUS trial, and a clinical pharmacology fellow. If the enrolling prescriber is no longer involved with a patient’s care when the genotyping results are reported, the adjudication committee identifies the most appropriate provider and relays actionable results. For example, an enrolling hospitalist may no longer treat a patient after discharge and results would be relayed to the primary care physician (PCP) instead. If a PCP cannot be identified, a specialist providing longitudinal care or the prescribing physician is contacted.
The adjudication committee also alerts the appropriate physician of incidental findings. For example, a patient genotyped for a new prescription may have actionable results relevant to a second existing prescription. The committee alerts the appropriate provider of these incidental findings. Results that do not impact currently prescribed medications are uploaded to the EMR’s problem list and will prompt future alerts, but providers are not contacted directly in these scenarios. The committee requires ~15 minutes to evaluate a single patient’s genotype in the context of the medication list. To summarize, the following support is provided by the INGENIOUS trial: (1) enrolling prescribers are directly sent genotyping reports; (2) all Eskenazi prescribers may access the genotype report in the EMR, view abnormal genotypes in the problem list, and receive automated interruptive alerts for future prescriptions; (3) for actionable results, the adjudication committee contacts appropriate physicians. In addition to this process, some clinicians request additional support in applying pharmacogenomic data to their patients.
CONSULTATION SUPPORT FOR THE INGENIOUS TRIAL
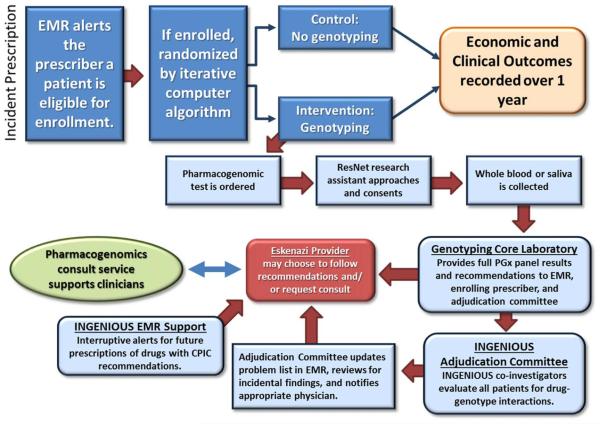
The strongest impetus for establishing a pharmacogenomics consult service was to support the INGENIOUS trial. Figure 1 summarizes the progression of patients from enrollment to adjudication to potential consultation. Six physicians, all American Board of Clinical Pharmacology (ABCP) board-certified or eligible (Supplemental Table 1), obtained privileges to interpret pharmacogenomic testing, staff clinics, and provide inpatient and outpatient consults. Eskenazi had no established guidelines for clinical pharmacology privileges and a new set of requirements was submitted and accepted by hospital leadership (Supplemental Table 2), which included completion of an ABCP-accredited fellowship.
Figure 1.
The interface between the INGENIOUS trial and the pharmacogenomics consult service. Any Eskenazi prescriber may enroll her or his patient following an incident new prescription of one of the 28 drugs. Patients are randomized to the standard care control arm (no genotyping) or the intervention arm (genotyping). Patients randomized to the genotyping arm are consented for enrollment by a research assistant, and DNA is collected. The genotyping core laboratory provides all pharmacogenomic results and recommendations to the EMR, enrolling physician, and the adjudication committee. The adjudication committee reviews the genotype for incidental findings and notifies an appropriate physician of actionable genotypes. Eskenazi prescribers may choose to follow recommendations or not. They may also choose to request a consult if they require additional information or support. Consults are derived from provider referrals.
Consults are requested by Eskenazi providers. Genotyping reports include a common phone/pager number to request pharmacogenomics consults. Outpatient clinic slots were obtained at Eskenazi hospital in existing subspecialty clinics to provide patient counseling should physicians request this service. Traditional inpatient consultations are available; however, due to the time required to report a genotype in the EMR, inpatient consults may lose relevance except in emergent cases and prolonged hospital stays.
The consultants principally provide support for Eskenazi clinicians outside of a traditional clinic or hospital, in a role analogous to that of a pathologist. In this role, the consultant enters a clinical pathology consult (CPC) into the EMR, without performing a history and physical. The pharmacogenomics consult service interprets the genotype report in the clinical context, analogous to a pathologist reporting a biopsy result. These clinical pathology consults (CPT 80500 or 80502) allow the fiexibility to scale the workload to demands. In contrast to scheduling an outpatient consultation, the requesting provider, patient, and pharmacogenomics consultant achieve their respective goals more efficiently. The requesting provider obtains documentation from a consultant validating her or his clinical management; the patient achieves the proper dose or alternate medication expeditiously; and the consultant can staff these consults around his or her other clinical and research duties. No defined documentation is required for an 80500 or 80502 consultation (Supplemental Document 3 provides an example). Consults stemming from the initial genotyping are offered without charge given the socioeconomic status of this patient population. Written notes are placed in the EMR within 24 hours of the consult request.
As education is a core mission of the division, six fellows from our clinical pharmacology program rotate on service. These consults provide excellent clinical applications for fellows and increase awareness of clinical pharmacology across different medical specialties.
To date, consult volume has proved modest (Table 1). The INGENIOUS trial is the only source of consults at Eskenazi. The physician accrual rate for patient enrollment exceeds 80%, and 58% of patients approached consent to enroll in the study. To date, 106 patients are enrolled in the genotyping arm. Across the Eskenazi health system, the mean combined number of “new” prescriptions for all of the 28 drugs is 375 per month. The key enrollment challenge is the ability of research assistants to make contact with patients before their discharge from the hospital or clinic. To improve enrollment, saliva was added an alternative to blood and missed patients are now compensated for travel back to the hospital for study enrollment.
Table 1.
INGENIOUS enrollment and consultation data
| Statistic | Value prior to 7/31/15 |
Value 8/1/15 to 11/30/15 |
Total |
|---|---|---|---|
| Months enrollinga | 4 | 4 | 8 |
| Total alerts to Eskenazi prescribers prompted by new prescriptions |
1402 | 1269 | 2671 |
| Clinicians clicking “Yes” to enrollb |
539 (38%) | 1040 (79%) | 1579 (59%) |
| Patients refusing enrollment |
21 | 56 | 77 |
| Patients enrolled in the control armc |
354 | 252 | 606 |
| Patients enrolled in the intervention armd |
28 | 78 | 106 |
| Actionable genotypese | 7 (25%) | 18 (23%) | 25 (24%) |
| Consults requested | 5 (18%) | 5 (6%) | 10 (9%) |
Enrollment began March 20, 2015, although the first intervention arm patient was enrolled in May 2015.
The default selection marked on the physician’s alert was “no to enrollment” prior to 7/31/15 and “yes to enrollment” after 8/1/15.
No consent is required to enroll in the control arm. The randomization process is designed to place patients 2:1 in the control to pharmaco-genotyping intervention arms. The process is iterative; when the ratio of patients accrued is greater than 2:1, the randomization algorithm shifts to a lower ratio, to drive the balance back toward 2:1 to the extent possible.
Consent is required to enroll patients in the pharmaco-genotyping intervention arm. To improve enrollment since 12/1/15, saliva was added as an alternative to blood and missed patients are now compensated for returning to the hospital to enroll in the study.
An actionable genotype is defined as any mutation that affects the dose or drug choice for any currently prescribed medications. This includes significant incidental findings. All genotype findings deemed actionable by the adjudication committee are relayed to an appropriate Eskenazi provider.
Among the 106 patients, 25 actionable genotypes have been reported and 10 consults have been requested by Eskenazi physicians. Providers have requested consults for 9% of all genotyped patients and 40% of patients with actionable genotypes. While this represents low utilization of the consult service, a flexible approach is required to prepare for potential spikes in workload when the INGENIOUS trial reaches its monthly target enrollment of 80 patients. At target enrollment, we predict the pharmacogenomics consult service will require 3–4% relative value unit-based effort of a full-time equivalent physician (calculations in Supplemental Document 4). Actual time required may exceed this effort.
A UNIQUE MODEL
Academic centers have modeled both physician and pharmacistled pharmacogenomics consult services. Five unique features of the INGENIOUS trial precipitated development of a new model: (1) the implementation occurs in a safety-net hospital system with low-income patients and community providers; (2) the genotypes associated with 28 medications are available to the entirety of the hospital system; (3) the patients’ genotypes and automated EMR support will persist after the trial ends; (4) the consult volume is low and unpredictable; and (5) consultation support is not funded by the trial, Eskenazi health system, or IUSM. Resultant from these features, we felt it necessary to ensure an additional layer of support, beyond the INGENIOUS trial’s adjudication committee. Our consult service was built as a flexible model with the ability to adapt to workload changes by staffing consults remotely. The service offers a sustainable mechanism for clinical questions after the conclusion of the trial.
The model affords framework to build toward future implementation efforts across Indiana. The IUSM is affiliated with 19 hospitals and 67 clinics across the state of Indiana. The IIPM was tasked by the university to create a pharmacogenomics consult framework capable of servicing satellite centers should broad-based genotyping be extended throughout the state. With the exception of the EMR support, parallel infrastructure to that described above has been established in the Indiana University Health system. The CPC provides a tool to support clinicians at satellite hospitals across Indiana, when patients cannot travel to Indianapolis.
In contrast to our system, other models employ pharmacists as the primary consultants.2 The use of CPCs presently limits our service to physician consultants, as pharmacists are not recognized Medicare Part B providers of CPCs. Ultimately, we predict a transition toward greater pharmacist engagement. As providers become familiar with pharmacogenomics, most questions will be fielded by knowledgeable pharmacists. With this expectation, the role of the physician clinical pharmacologist may be relegated to complicated or unusual cases. However, given the circumstances outlined above, the pharmacogenomics consultation service is vital during this transitional time. Our model should provide an adaptable solution for health systems to meet unpredictable clinical pharmacology demands.
Supplementary Material
ACKNOWLEDGMENTS
The INGENIOUS trial (NCT02297126) is sponsored by an NIH/NHGRI U01-grant (HG007762). Z.D., K.D.L., B.S.D., T.C.S., R.V., V.M.P., M.B.R., J.S.C., A.M.H., P.R.D., R.P.K., D.M.H., and D.A.F. are supported by the NIH-U01 HG007762. MTE was supported by the PhRMA foundation (Clinical Pharmacology Young Investigator Award) and the Norman S. Coplon Satellite Health Extramural Grant Program. This project was also supported by NIH-NIGMS: Indiana University Comprehensive Training in Clinical Pharmacology (5T32GM008425) which provided stipend support to R.C.P., B.T.G., J.D.R., N.D., and M.A.H. This publication was made possible by the Indiana University Health – Indiana University School of Medicine Strategic Research Initiative (V.M.P., R.P.K.). The content is solely the responsibility of the authors. The supporting organizations had no role in data acquisition, analysis, or interpretation, article creation, or decision to submit for publication.
Footnotes
CONFLICT OF INTEREST/DISCLOSURE
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Article preparation: M.T.E., B.S.D., K.D.L., T.C.S., E.A.B., J.T.C., A.S.P., R.V., V.M.P., Z.D., D.A.F. Conceptualization: M.T.E., Z.D., C.A.M., D.A.F. Trial Design: Z.D., K.D.L., T.C.S., J.T.C., P.R.D., V.M.P., M.B.R., J.S.C., A.M.H., D.A.F. Implementation: M.T.E., B.S.D., C.A.M., E.A.B., J.S.C., J.T.C., A.S.P., R.V., R.C.P., M.A.H., B.T.G., N.D., J.D.R., S.K.G., D.M.H., R.P.K., Z.D., D.A.F.
Additional Supporting Information may be found in the online version of this article.
References
- 1.O’Donnell PH, et al. Adoption of a clinical pharmacogenomics implementation program during outpatient care—initial results of the University of Chicago “1,200 Patients Project. Am. J. Med. Genet. C Semin. Med. Genet. 2014;166C:68–75. doi: 10.1002/ajmg.c.31385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crews KR, et al. Development and implementation of a pharmacist-managed clinical pharmacogenetics service. Am. J. Health Syst. Pharm. 2011;68:143–150. doi: 10.2146/ajhp100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson JF, et al. Electronic health record design and implementation for pharmacogenomics: a local perspective. Genet. Med. 2013;15:833–841. doi: 10.1038/gim.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shuldiner AR, et al. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: overcoming challenges of real-world implementation. Clin. Pharmacol. Ther. 2013;94:207–210. doi: 10.1038/clpt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy KD, et al. Prerequisites to implementing a pharmacogenomics program in a large health-care system. Clin. Pharmacol. Ther. 2014;96:307–309. doi: 10.1038/clpt.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.