Abstract
Background
Clearance of transfused red blood cells (RBCs) is thought to be related to both storage time of the transfusion product and to inflammatory status of the recipient. We investigated these effects in a randomized “two hit” healthy volunteer transfusion model, comparing autologous RBCs stored for 35 days with RBCs stored for 2 days.
Study Design and Methods
Healthy male volunteers donated one unit of autologous RBCs either 2 (2D) or 35 days (35D) before the study day. The experiment was started by infusion of 2 ng/kg LPS (“first hit”). Two hours later the stored RBCs (“second hit”) were reinfused followed by RBCs labeled with biotin. Clearance of biotin labeled RBCs (BioRBCs) was measured during the 5 h post-transfusion endotoxemia period, along with measurement of PS-exposure, lactadherin binding and CD47 expression.
Results
In the 2D stored RBCs group, 1.5±3.4% of infused BioRBCs were cleared from the circulation 5 h post-transfusion versus 4.8±4.0% in the 35D stored group (p=0.1). There were no differences in PS-exposure, lactadherin binding, and CD47 expression between fresh and stored RBCs or between pre- and post-transfusion.
Conclusion
Our study shows a low clearance of RBCs even during endotoxemia. Furthermore, short-term clearance of BioRBCs during endotoxemia was not related to storage duration. Consistent with these observations, PS-exposure, lactadherin binding and CD47 expression did not differ between 2D and 35D stored cells before or after transfusion. We conclude that in the presence of endotoxemia, clearance of 35D stored autologous RBCs is not increased compared to 2D fresh RBCs.
Keywords: storage, transfusion, two-hit model, human model, clearance, biotin, labeling
Introduction
To improve tissue oxygenation, red blood cell (RBCs) transfusions are frequently administered for increasing oxygen delivery capacity. Although blood transfusion can be lifesaving, it also can result in serious adverse events leading to increased morbidity and mortality, especially in critically ill patients.1,2 Moreover, several randomized trials suggest that a restrictive transfusion policy results in a better outcome than a liberal transfusion policy.3-5 Storage of RBCs, which results in the RBC “storage lesion”, including reduced cellular levels of 2,3-diphosphoglycerate, adenosine triphosphate and nitric oxide, and increased oxidation of cellular lipids and proteins,6 has been implicated in transfusion induced adverse events. However, the relative contribution – if any – remains unclear.7,8 Increased post-transfusion RBC clearance caused by prolonged storage has been speculated to be detrimental; indeed, several studies have reported shortened RBC survival with increased RBC storage.9-12 This observation, and the observation that mainly critically ill patients are prone to develop adverse events of transfusion, led us to hypothesize that RBC transfusion storage time and the recipient's inflammatory status influence RBC post-transfusion clearance. This hypothesis is supported by several preclinical studies: RBC deformability diminishes in stored blood;13,14 deformability of stored RBCs has been found to impede microcirculation15,16 and in a murine model stored RBCs were found to adhere to the vasculature.17 Other studies report that RBCs express increased phosphatidylserine (PS) on the outer membrane leaflet after exposure to plasma of sepsis patients.18 PS-exposure, CD47 expression, and lactadherin binding, amongst others, have been associated with RBCs storage and in vivo RBC aging. Changes in any of these markers might influence post-transfusion RBC clearance.19-21
To test the above hypothesis in a clinically relevant setting, we examined the effect of endotoxemia and RBC storage time on the clearance of autologous biotin labeled RBCs (BioRBC) in healthy volunteers using a randomized study design. To address potential mechanisms, we also determined effect of RBC storage on RBC membrane PS-exposure, CD47 expression, and lactadherin binding.
Material and Methods
All studies have been approved by the Academic Medical Center Medical Ethical Committee (Dutch Trial Register - NTR4455) and are consistent with the Declaration of Helsinki including Good Clinical Practice and Good Manufacturing Practice. All subjects provided written informed consent as part of the informed consent process before enrolment.
Inclusion and Randomization of Volunteers
This study is part of a larger study that has been previously described (AABB annual meeting oral presentation;22 Critical Care Medicine, article in press, DOI 10.1097/CCM.0000000000001614). In this study we recruited 15 healthy male volunteers, 18 – 35 years of age, without medical conditions. Potential subjects were excluded if they had donated or lost >500 ml blood during the preceding three months. Participants were not allowed to be concurrently enrolled in another intervention trial
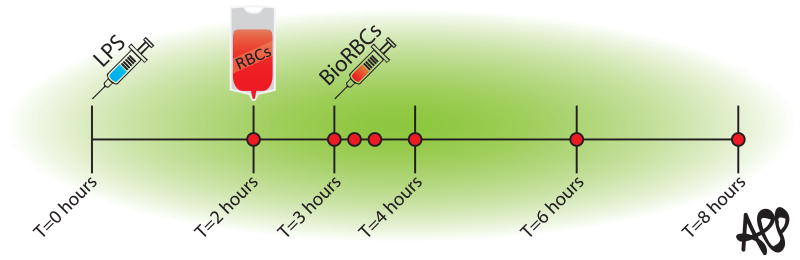
Five subjects each were randomly allocated to one of three groups: 1) the 2D RBCs group that received one unit of autologous RBC concentrate stored for 2 days, 2) the 35D RBCs group that received one unit of autologous RBC concentrate stored for 35 days, 3) the saline control group that received a similar volume of NaCl 0.9%. Each group adhered to the protocol depicted in Figure 1.
Figure 1.
Study protocol. The experiment was started with an infusion of LPS at T=0. At T=2 volunteers received an autologous blood transfusion followed by biotin labelled RBCs an hour later. The red dots represent the time-points on which blood was sampled.
All volunteers donated one unit of whole blood at the Dutch Blood Bank Sanquin, either 2 days or 35 days prior to the experiment. The whole blood unit was processed into plasma, buffy coat and leukoreduced RBC concentrate, with RBCs stored in saline, adenine, glucose and mannitol (SAGM) according to Dutch Blood Bank standards (Bottom-And-Top bag systems, CQC2988, Fresenius Kabi, Emmer Compascuum, the Netherlands; PolyVinylChloride-Di-ethyl-hexyl-phtalate bag). To monitor product quality and bacterial contamination, 6 ml of the 35D RBC concentrate was aliquoted using a sterile docking system. This sample was cultured with BacTAlert® one day before transfusion.
Biotinylation
On the day of the experiment a 25-30 ml sample was drawn with a sterile TakeSpike (Codan, Lensahn, Germany). Labeling of RBCs was done according to methods described originally and later updated by Mock and coworkers.23,24 We labelled the RBCs from 20-30 ml of RBC concentrate in order to achieve an in vivo BioRBC enrichment of 0.5%.
Briefly, RBCs were washed twice in a wash buffer consisting of 8 mL of 50-percent glucose (Braun, Melsungen, Germany), 18 ml sodium bicarbonate (Frensius Kabi, 's Hertogenbosch, The Netherlands)and 2 ml water (Braun) in 1.0 l 0.9% sodium chloride (Baxter Health Care, Deerfield, Illinois, USA). Centrifugation (1000 rcf) performed with the wash steps was done for 8 min at room temperature without breaking. Sulfo-N-hydroxysuccinimide-biotin (Pierce, Rockford, Illinois, USA) was prepared in wash buffer at a concentration of 6 μg/ml. This biotinylation labeling solution was filter-sterilized (syringe filter, 0.2 μm, acetate membrane) immediately before use. The RBCs were resuspended in wash buffer at a hematocrit of 25% and incubated with biotin labeling solution for 30 min at room temperature on a roller bench. The BioRBCs were then washed twice again to remove excess biotin and resuspended to a hematocrit of 50%. These cells were stored in a 60 ml syringe until infusion. One ml of BioRBCs was used for investigation of membrane markers and for culture with BacTAlert®.
Open-label randomized design
It was impossible to blind volunteers for group allocation as they had to donate one unit of blood either 2 or 35 days before the experiment. According to the Good Clinical Practice (GCP) research code it was also not possible to blind for saline or RBC transfusion. We thus performed an open-label randomized trial.
Endotoxemia Model
To assure that all volunteers met systemic inflammation reaction syndrome (SIRS) criteria,25 an i.v. infusion of 2 ng Escherichia coli LPS / kg body weight i.v. (National Institutes of Health Clinical Center, Bethesda, United States of America) was administered at T=0. Two hours after LPS infusion, subjects received either 2D RBCs, 35D RBCs or Saline (T=2). At completion of this infusion BioRBCs were infused over 1 min in the 2D and 35D RBC groups (Figure 1). As an additional historical control group, we compared RBC survival data of the three groups to that of healthy volunteers transfused with fresh autologous RBCs in the absence of LPS from a prior study. We used the clearance data from the first 5, 10, 20, 60 and 1440 minutes after BioRBC infusion to fit a clearance curve. From this curve we extrapolated the clearance and recovery of the 300 min time point (= 5 hours after infusion).26
Sample Collection and Analysis
All blood samples were collected from an indwelling arterial line, which was used for hemodynamic monitoring. Blood was collected prior to infusion of the BioRBCs ; 10 min after infusion of BioRBCs (T=3.1), 30 min after infusion of BioRBCs (T=3.5) and every two hours thereafter up to T=8 (Figure 1). Potassium ethylenediaminetetraacetic acid (EDTA) anticoagulated blood was used for cross matching as additional safety procedure and for analysis of BioRBCs clearance. Directly after sampling potassium-EDTA anticoagulated full blood was stored at 4°C. The day after the experiment full blood samples and untransfused BioRBC samples were shipped on wet ice to the Red Blood Cell Research Laboratory of Sanquin Blood Bank where they were analyzed with Advia 2120 Hematology System (Siemens, The Hague, Netherlands).
To determine PS-exposure on RBC membranes, RBCs were counterstained with streptavidine Qdots (Life Technologies, Carlsbad, California, USA, 2 nM final concentration) and incubated with either annexin V-FITC (VPS diagnostics, Hoeven, The Netherlands 25 μg/ml final concentration) or lactadherin-FITC (Haematologic Technologies Inc., Essex Junction, Vermont, United USA, 16 nM final concentration). Lactadherin binding was analyzed by anti-lactadherin staining (R&D Systems, Minneapolis, Minnesota USA, according to the manufacturer's protocol). Antibodies against CD47 were obtained from eBioscience (San Diego, California, USA and used according to manufacturer's protocol). RBCs were incubated with antibodies for 30 min at 4°C, washed and analyzed using a flow cytometer (LSR II, San Jose, California, USA). BioRBC enrichment expressed as a percentage of all circulating RBC was measured at all appropriate sample time-points.
Total and direct bilirubin and haptoglobin were measured as hemolysis parameters in our patient laboratory of general clinical chemistry with the Roche cobas c702 chemical analyzer (Roche Diagnostics, Indianapolis, IN, USA).
Statistical Analysis
We tracked the post-transfusion clearance of BioRBCs for 5 h after their infusion; this endpoint was selected because all symptoms of endotoxemia had abated at that time (5 h post-transfusion = 8 h after infusion of LPS, Figure 1). BioRBC clearance was determined based on the percentage of BioRBCs cleared in the circulation relative to the 10-min post-BioRBC infusion sample. Significance of differences in the post-transfusion BioRBC clearance in the 2D and 35D transfusion group was tested using Student's unpaired T-test.
We based our power calculation on previous published reports in which 22-26.5% RBC clearance was detected 24 hours after infusion.9,10 We measured clearance during the five hours of LPS induced endotoxemia and therefore expected less clearance. Assuming that removal occurred linearly over the 24 hours, we estimated that about 6% would be removed by 5 hour post-transfusion. Accordingly we powered on an expected clearance of 6% in the stored RBCs group and 1% in the fresh RBCs group (in accordance with the observations of Mock et al. using fresh autologous BioRBCs). Assuming a delta of 5%, with a pooled sigma of 2.5%, alpha of 5% and beta of 80% we required 4 volunteers. We included one extra volunteer to account for possible technical difficulties.
To investigate the role of the three membrane markers related to RBC clearance transfused BioRBCs and unlabeled RBCs collected from arterial blood samples and untransfused BioRBCs were analyzed for change in membrane biomarkers. Expression of the membrane markers was determined as either percentage of fluorescence positive cells or as mean fluorescence (MFI). Because there was no time-dependent change in membrane markers, the measurements of T=3.1 and T=8 were pooled and used for all calculations. Data are summarized in means and medians with IQR. Statistical analyses have been performed in R version 3.1.2 (R-core team, Vienna, Austria, 2014).
Results
Clinical symptoms
Approximately 1.5 h after infusion of LPS, all volunteers developed headache, muscle pain, photophobia and nausea. These symptoms were accompanied by the expected change in hemodynamic and respiratory parameters. All volunteers met the systemic inflammation reaction syndrome (SIRS) criteria.
Clearance
During endotoxemia, the percentage of RBCs removed from the circulation by 5 h was 1.5±3.4% for the 2D RBCs group and 4.8±4.0% for the 35D RBCs group (Figure 2). The difference between the storage groups was not significant (p = 0.1). In the historical control post-transfusion clearance 5 h post-transfusion of BioRBCs was 1.2±0.05% which was not different from either the 2 or 35D groups.26 We did not detect any differences in levels of total bilirubin, direct bilirubin or haptoglobin (data not shown).
Figure 2.
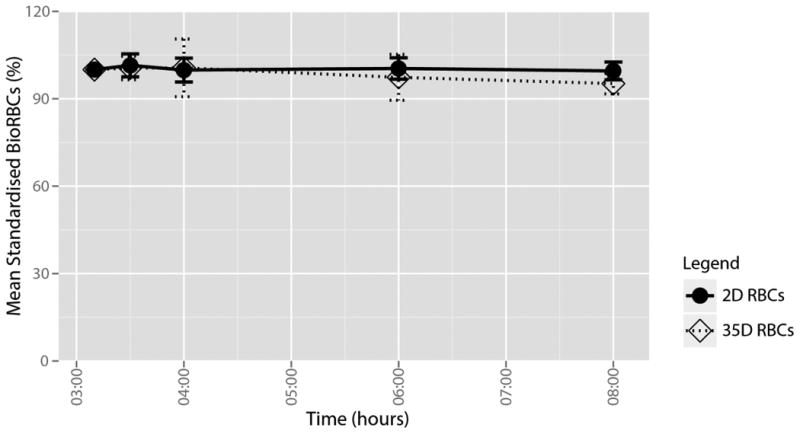
Mean clearance per transfusion group. Circles on solid lines represent 2D stored BioRBCs. Squares on dotted lines represent 35D stored BioRBCs. Bars represent 95% confidence interval.
Transfusion products
All of the 35D study subjects' autologous RBC transfusion products met Dutch Blood Bank quality standards prior to their administration. On average we measured 0.22±0.08% hemolysis, 3.69±0.26 mmol/g Hb intracellular ATP and 43±21.1% echinocytes. In two products we found >70% echinocytes which is still within the range of normal variations. None of the products had a positive bacterial culture.
Changes in phosphatidylserine exposure, lactadherin binding and CD47 expression
Phosphatidylserine exposure
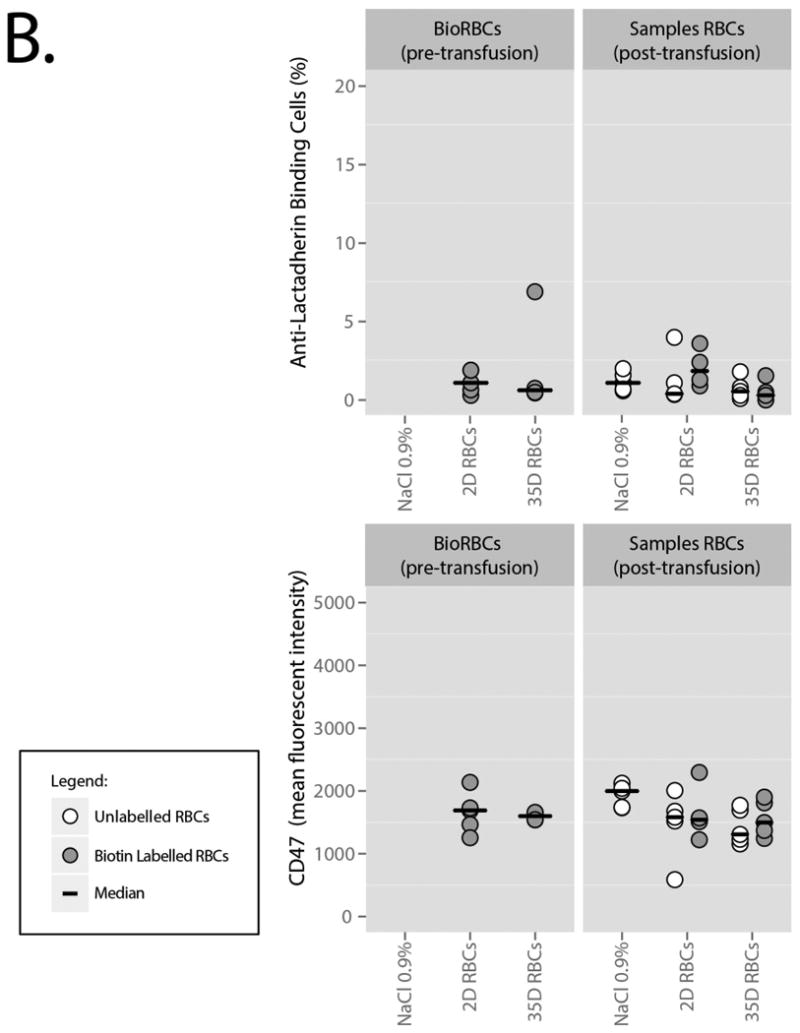
Before infusion, BioRBCs expressed low levels of PS as measured by staining with lactadherin and annexin V (Figure 3A; Table 1). PS-exposure between 2D and 35D stored BioRBCs was similar and confidence intervals overlapped with unlabeled, untransfused RBCs. After transfusion, PS-exposure on both the 2D BioRBCs and 35D BioRBCs remained low throughout the experiment. In addition, lactadherin binding to the RBC was not detectable on BioRBCs before infusion, as determined by anti-lactadherin antibody staining (Figure 3A, Table 2), and did not increase after transfusion. Binding was comparable to unlabeled RBCs. Lastly, the level of CD47 on BioRBCs did not change after transfusion and was not different from circulating, unlabeled cells.
Figure 3.
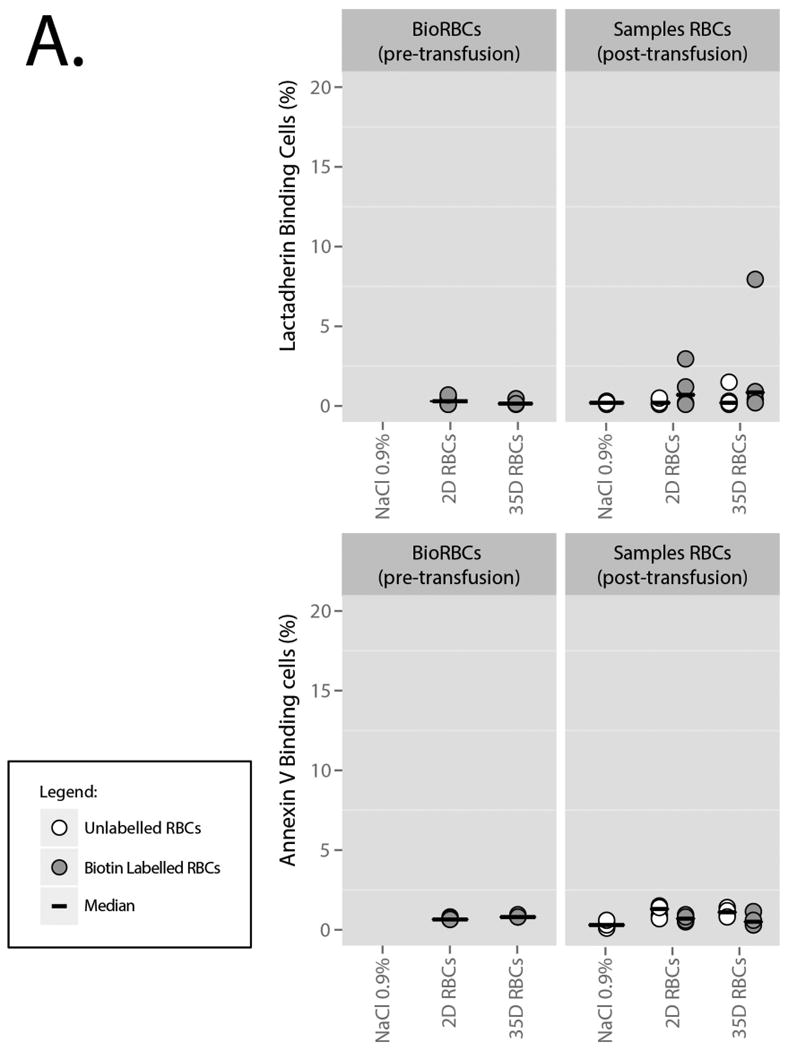
A. PS-exposure, measured by annexin V and lactadherin staining. The grey dots represent biotin labelled cells. The white dots represent untransfused and unlabeled cells in full blood samples from which the BioRBCs were extracted. Bars represent medians.
B. Lactadherin binding and CD47 expression. The grey dots represent Biotin Labelled cells. The white dots represent untransfused and unlabeled cells in full blood samples from which the BioRBCs were extracted. Bars represent medians.
Table 1. Percentage of RBCs with PS-exposure as measured with annexin V and lactadherin on transfused, untransfused and in vitro biotin labelled RBCs.
| Before Transfusion | After Transfusion | ||||
|---|---|---|---|---|---|
|
| |||||
| PS-exposure | Unlabeled and untransfused RBCs sampled from circulation | BioRBCs only | Unlabeled RBCs from circulation | BioRBCs isolated from circulation | |
| Lactadherin (%) | Control | 0.20 (0.19-0.29) | N/A | 0.20 (0.14-0.26) | N/A |
| 2D RBCs | 0.20 (0.15-0.39) | 0.30 (0.11-0.57) | 0.17 (0.12-0.42) | 0.70 (0.00-2.43) | |
| 35D RBCs | 0.20 (0.01-0.76) | 0.20 (0.05-0.37) | 0.59 (0.00-0.98) | 0.85 (0.00-5.02) | |
|
| |||||
| Annexin V (%) | Control | 0.30 (0.18-0.58) | N/A | 0.30 (0.15-0.49) | N/A |
| 2D RBCs | 1.30 (0.90-1.44) | 0.65 (0.63-0.76) | 1.30 (0.85-1.47) | 0.70 (0.51-0.91) | |
| 35D RBCs | 1.20 (0.37-0.97) | 0.80 (0.76-0.91) | 1.10 (0.87-1.29) | 0.50 (0.32-0.90) | |
The column “Before Transfusion” describes the background lactadherin and CD47 expression on naïve RBCs drawn from the circulation and BioRBCs directly after labeling. The column “After Transfusion” describes the sample which has been drawn after transfusion from which both BioRBCs and unlabeled RBCs have been isolated. Values are expressed as medians and confidence intervals between brackets.
Table 2. Percentage of RBCs expressing lactadherin and CD47 expression in mean fluorescent intensity (MFI) on transfused, untransfused and in vitro biotin labelled RBCs.
| Before Transfusion | After Transfusion | ||||
|---|---|---|---|---|---|
|
| |||||
| Unlabeled and untransfused RBCs sampled from circulation | BioRBCs only | Unlabeled RBCs from circulation | BioRBCs isolated from circulation | ||
| Membrane bound Lactadherin (%) | Control | 1.10 (0.07-1.43) | N/A | 1.10 (0.67-1.73) | N/A |
| 2D RBCs | 0.40 (0-2.02) | 1.10 (0.05-1.82) | 0.40 (0.00-2.65) | 1.85 (0.84-3.26) | |
| 35D RBCs | 0.60 (0.03-1.29) | 0.63 (0-5.32) | 0.55 (0.12-1.30) | 0.30 (0.00-1.06) | |
|
| |||||
| CD47 (MFI) | Control | 2383 (2091-2446) | N/A | 1998 (1766-2088) | N/A |
| 2D RBCs | 1582 (890-1820) | 1690 (1360-1951) | 1582 (1001-1948) | 1542 (1196-2106) | |
| 35D RBCs | 1309 (1161-1567) | 1599 (1533-1666) | 1309 (1180-1684) | 1497 (1313-1817) | |
The column “Before Transfusion” describes the background lactadherin and CD47 expression on naïve RBCs drawn from the circulation and BioRBCs directly after labeling. The column “After Transfusion” describes the sample which has been drawn after transfusion from which both BioRBCs and unlabeled RBCs have been isolated. Values are expressed as medians and confidence intervals between brackets.
Discussion
In this study we transfused fresh (2D) and stored (35D) biotin labeled red blood cells into humans in the presence of LPS-induced inflammation, sufficient to meet clinical SIRS criteria. We did so to investigate the effect of RBC storage on clearance of transfused RBCs under conditions of inflammation. We observed that: 1) clearance of transfused RBCs is low even in the presence of marked inflammation and similar to clearance in the absence of inflammation; 2) clearance of transfused autologous RBCs is not measurably dependent on storage time in presence of inflammation; 3) PS-exposure, lactadherin binding, and CD47 expression on RBCs is not altered during storage and does not change after transfusion in LPS-treated healthy volunteers.
Several studies have reported reduced survival of RBCs after transfusion.9-11 Although the factors influencing post-transfusion recovery remain unclear, storage time and inflammation in the recipient have been hypothesized to play an important role. In 2008, a Dutch research group investigated whether survival of RBCs was storage-time dependent by identification of transfused cells with minor-antigen mismatch. The study included 10 hematology patients who required transfusion after high-dose chemotherapy. For RBCs <10 days, a mean 24 hour post transfusion recovery of 86.4% was reported. For RBCs stored 25-35 days the average survival after 24 hours was 73.5%.The authors hypothesized that the high clearance in both transfusion groups might be caused by disease severity of the recipients.10
We designed a clinically relevant model to investigate the effect of RBC storage and inflammation on RBC clearance in humans. To mimic clinical sepsis, we primed volunteers with LPS and successfully induced SIRS in all the volunteers. Labeling with biotin was used both to assess RBC clearance and to isolate RBCs for determination of membrane markers implicated in cell clearance. The number of RBCs cleared during endotoxemia was low and was not dependent on storage time, which is in contrast with previous findings.10 We used extrapolation of a historical control group of volunteers without LPS-exposure to investigate the additional effect of endotoxemia.26 In this historical study post-transfusion clearance 5 hours post transfusion of BioRBCs was similar to our results.
We also investigated several “eat me” and “don't eat me” erythrophagocytosis signals which have been related to clearance of aged RBCs. We investigated PS-exposure, lactadherin binding, and CD47 expression which all have been related to RBC clearance.
In healthy cells, PS is retained on the inner side of the cell-membrane. During senescence, the asymmetric distribution of lipids in the cell-membrane changes and PS is exposed on the outer leaflet which can be measured by staining with annexin or lactadherin. PS has been proposed to be a marker for erythrophagocytosis.27 Several studies have investigated PS-exposure as function of storage. In some studies PS-exposure did not change during storage,19 while in others expression increased.28,29
Lactadherin is a PS-binding glycoprotein that promotes erythrophagocytosis by macrophages in a concentration-dependent manner.21 We hypothesized that lactadherin might also play a role in clearance of senescent and stored RBCs in individuals suffering from inflammation. Contrary to our hypothesis, we did not find any evidence for lactadherin binding to the transfused RBC, as assessed by anti-lactadherin staining, nor did we find any evidence for increased PS-exposure, a prerequisite for lactadherin binding, assayed by both annexin V and lactadherin staining.
CD47 is a crucial “don't eat me” signal on the erythrocyte membrane. CD47 binds to signal regulatory protein-alpha (SIRP ) on macrophages, which leads to the inhibition of phagocytosis by the macrophage.19,20 CD47 expression does not decrease during storage of RBCs under standard Dutch blood banking conditions (de Korte and van Bruggen, unpublished). However, it is not known how CD47 expression is maintained after transfusion. We did not find a change in CD47 expression after transfusion of BioRBCs cells as compared to untransfused and unlabeled cells. Expression did also not differ between 2D and 35D stored cells.
A general difficulty with investigations on exposure of these markers on labelled cells is that RBCs with increased levels may already have been cleared from the circulation before the first collection of blood samples. A limitation in the determination of clearance of any population study of labeled RBCs, including the BioRBC technique, is that clearance during the mixing time cannot be determined; to unambiguously avoid any mixing artifact, we elected to sample blood no sooner than 10 minutes after infusion. However, we infer that the immediate loss of BioRBCs after infusion is negligible for the following reasons; First: earlier studies reported little or no loss of labeled RBCs between 5 and 20 minutes after infusion; Second, circulating red cell volume calculated with data from BioRBCs agreed with the red cell volume calculated from minor antigen mismatch and Third: another study compared BioRBCs with 51Cr and also did not detect any immediate loss of BioRBCs.30 Although these observations do not directly prove that no labeled cells are cleared from the circulation, they do suggest that there is negligible loss. Moreover, in the current study, we found 1) that clearance over time was low; 2) that expression of clearance markers on RBC samples direct from the storage bag was unchanged in either group; and 3) that levels of hemolysis parameters did not increase. On balance, we infer that it is unlikely that we have missed a substantial number of cells expressing high clearance signals that were cleared from the circulation, either immediately after infusion or later during the experiment.
There are several factors that might explain the discrepancy between our results and those of previously published patient studies. It could be argued that the LPS did not produce adequate inflammation, but at a dose of 2 ng/kg all volunteers fulfilled the SIRS criteria (Critical Care Medicine, article in press, DOI 10.1097/CCM.0000000000001614). Another factor that may be responsible for the differences between our study and those previously published is that we used autologous transfusion products to investigate the isolated effect of storage time on RBC clearance. This partly limits the comparability to clinical practice but on the other hand allowed us to investigate the effect of storage time without confounding by allogeneic mismatch. To our opinion, this is a major strength of our study design. A third factor that might have influenced our results is that we investigated the direct effect of inflammation during endotoxemia and that we cannot rule out that a longer period of inflammation is required to induce increased clearance of transfused RBCs. However, a study in very low birth weight critically ill premature infants (gestational age 26-30 weeks) did not show any clearance of biotin labelled stored allogeneic RBCs 24 hours after transfusion.31
The two studies that detected increased RBC clearance in clinical patients made use of a minor-antigen mismatch technique.9,10 This technique does not require any additional labeling of the RBCs while biotin labeling involves several incubation and washing steps. This raised the question whether the labeling procedure influences RBC clearance. However, a study comparing post-transfusion recovery of biotin labeled RBCs to minor-antigen mismatch in infants that received both autologous and allogeneic RBCs found no differences between the two different methods and also found little difference between autologous and allogeneic RBCs.32 A study in endotoxin primed volunteers using allogeneic blood products might shed some light on these conundrums. However, ethical suitability of exposing volunteers to allogeneic blood products is unclear.
Conclusion
Clearance of autologous RBCs in humans is not dependent on storage time in the presence of inflammation. Future studies should focus on the effect of allogeneic properties of transfusion products on RBC clearance.
Acknowledgments
We wish to acknowledge the contribution of Robert L. Schmidt, B.S., in sharing his laboratory expertise in the transfer of knowledge required for establishing the RBC biotinylation method in our laboratory.
The sponsors of this work had no role in this work including study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the manuscript for publication. The sponsors of this work included The United States Public Health Service National Institutes of Health Grant P01 HL046925; The National Center for Research Resources, a part of the National Institutes of Health (NIH), Grant Numbers UL1RR024979, UL1TR000039, and 1S10 RR027219. APV has received a personal grant by the Netherlands Organization for Health Research and Development (ZonMW), NWO-VENI grant 2013 (project number 016.146.082).
Footnotes
Authorship: A.L.P, D.M.M., R.v.B., D.d.K., J.A.W., and A.P.J.V. designed the investigation; A.L.P., and A.P.J. performed the research; B.B., R.v.B. analyzed the samples; A.L.P. analyzed the data; N.P. cosupervised the conduct of the study; A.P.J.V. supervised the conduct of the study; and A.L.P., A.P.J.V. and R.v.B. wrote the paper. All authors read and corrected the paper.
Conflicts of Interest: The authors declare that they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION.
References
- 1.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–74. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 2.Tinmouth A, Fergusson D, Yee IC, Hebert PC, Investigators A, Canadian Critical Care Trials G Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–27. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 3.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 4.Villanueva C, Colomo A, Bosch A, Concepcion M, Hernandez-Gea V, Aracil C, Graupera I, Poca M, Alvarez-Urturi C, Gordillo J, Guarner-Argente C, Santalo M, Muniz E, Guarner C. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 5.Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ. 2015;350:h1354. doi: 10.1136/bmj.h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014;107:1–9. doi: 10.1111/vox.12130. [DOI] [PubMed] [Google Scholar]
- 7.Lelubre C, Vincent JL. Relationship between red cell storage duration and outcomes in adults receiving red cell transfusions: a systematic review. Crit Care. 2013;17:R66. doi: 10.1186/cc12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander PE, Barty R, Fei Y, Vandvik PO, Pai M, Siemieniuk RA, Heddle NM, Blumberg N, McLeod SL, Liu J, Eikelboom JW, Guyatt GH. Transfusion of fresher versus older red blood cells in hospitalized patients: a systematic review and meta-analysis. Blood. 2015 doi: 10.1182/blood-2015-09-670950. [DOI] [PubMed] [Google Scholar]
- 9.Zeiler T, Müller JT, Kretschmer V. Flow-cytometric determination of survival time and 24-hour recovery of transfused red blood cells. Transfusion Medicine and Hemotherapy. 2003;30:14–9. [Google Scholar]
- 10.Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, de Grip WJ, Bos HJ, Bosman GJ. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48:1478–85. doi: 10.1111/j.1537-2995.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 11.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–60. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 12.Luten M, Roerdinkholder-Stoelwinder B, Bost HJ, Bosman GJ. Survival of the fittest?--survival of stored red blood cells after transfusion. Cell Mol Biol (Noisy-le-grand) 2004;50:197–203. [PubMed] [Google Scholar]
- 13.Berezina TL, Zaets SB, Morgan C, Spillert CR, Kamiyama M, Spolarics Z, Deitch EA, Machiedo GW. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102:6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 14.Relevy H, Koshkaryev A, Manny N, Yedgar S, Barshtein G. Blood banking-induced alteration of red blood cell flow properties. Transfusion. 2008;48:136–46. doi: 10.1111/j.1537-2995.2007.01491.x. [DOI] [PubMed] [Google Scholar]
- 15.Simchon S, Jan KM, Chien S. Influence of reduced red cell deformability on regional blood flow. Am J Physiol. 1987;253:H898–903. doi: 10.1152/ajpheart.1987.253.4.H898. [DOI] [PubMed] [Google Scholar]
- 16.Lipowsky HH, Cram LE, Justice W, Eppihimer MJ. Effect of erythrocyte deformability on in vivo red cell transit time and hematocrit and their correlation with in vitro filterability. Microvasc Res. 1993;46:43–64. doi: 10.1006/mvre.1993.1034. [DOI] [PubMed] [Google Scholar]
- 17.Chin-Yee IH, Gray-Statchuk L, Milkovich S, Ellis CG. Transfusion of stored red blood cells adhere in the rat microvasculature. Transfusion. 2009;49:2304–10. doi: 10.1111/j.1537-2995.2009.02315.x. [DOI] [PubMed] [Google Scholar]
- 18.Kempe DS, Akel A, Lang PA, Hermle T, Biswas R, Muresanu J, Friedrich B, Dreischer P, Wolz C, Schumacher U, Peschel A, Gotz F, Doring G, Wieder T, Gulbins E, Lang F. Suicidal erythrocyte death in sepsis. J Mol Med. 2007;85:273–81. doi: 10.1007/s00109-006-0123-8. [DOI] [PubMed] [Google Scholar]
- 19.Sparrow RL, Healey G, Patton KA, Veale MF. Red blood cell age determines the impact of storage and leukocyte burden on cell adhesion molecules, glycophorin A and the release of annexin V. Transfus Apher Sci. 2006;34:15–23. doi: 10.1016/j.transci.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–4. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 21.Dasgupta SK, Abdel-Monem H, Guchhait P, Nagata S, Thiagarajan P. Role of lactadherin in the clearance of phosphatidylserine-expressing red blood cells. Transfusion. 2008;48:2370–6. doi: 10.1111/j.1537-2995.2008.01841.x. [DOI] [PubMed] [Google Scholar]
- 22.Peters AL, van Bruggen R, de Korte D, Jonkers RE, Bonta PI, R L, Zeerleder SS, Van der Poll T, Juffermans NP, Vlaar APJ. S81-040B: Transfusion of 35 days stored autologous red blood cells in endotoxin primed human volunteers does not result in transfusion related acute lung injury AABB Annual Meeting. Anaheim. 2015 [Google Scholar]
- 23.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. Measurement of circulating red cell volume using biotin-labeled red cells: validation against 51Cr-labeled red cells. Transfusion. 1999;39:149–55. doi: 10.1046/j.1537-2995.1999.39299154728.x. [DOI] [PubMed] [Google Scholar]
- 24.Mock DM, Matthews NI, Zhu S, Burmeister LF, Zimmerman MB, Strauss RG, Schmidt RL, Nalbant D, Cress GA, Widness JA. Red blood cell (RBC) volume can be independently determined in vivo in humans using RBCs labeled at different densities of biotin. Transfusion. 2011;51:148–57. doi: 10.1111/j.1537-2995.2010.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 26.Mock DM, Matthews NI, Zhu S, Strauss RG, Schmidt RL, Nalbant D, Cress GA, Widness JA. Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities. Transfusion. 2011;51:1047–57. doi: 10.1111/j.1537-2995.2010.02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boas FE, Forman L, Beutler E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci U S A. 1998;95:3077–81. doi: 10.1073/pnas.95.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart A, Urbaniak S, Turner M, Bessos H. The application of a new quantitative assay for the monitoring of integrin-associated protein CD47 on red blood cells during storage and comparison with the expression of CD47 and phosphatidylserine with flow cytometry. Transfusion. 2005;45:1496–503. doi: 10.1111/j.1537-2995.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 29.Dinkla S, Peppelman M, Van Der Raadt J, Atsma F, Novotny VM, Van Kraaij MG, Joosten I, Bosman GJ. Phosphatidylserine exposure on stored red blood cells as a parameter for donor-dependent variation in product quality. Blood Transfus. 2014;12:204–9. doi: 10.2450/2013.0106-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mock DM, Widness JA, Veng-Pedersen P, Strauss RG, Cancelas JA, Cohen RM, Lindsell CJ, Franco RS. Measurement of Posttransfusion Red Cell Survival With the Biotin Label. Transfus Med Rev. 2014;28:114–25. doi: 10.1016/j.tmrv.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nalbant D, Bhandary P, Matthews NI, Schmidt RL, Bogusiewicz A, Cress GA, Zimmerman MB, Strauss RG, Mock DM, Widness JA. Comparison of multiple red cell volume methods performed concurrently in premature infants following allogeneic transfusion. Pediatr Res. 2013;74:592–600. doi: 10.1038/pr.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Widness JA, Kuruvilla DJ, Mock DM, Matthews NI, Nalbant D, Cress GA, Schmidt RL, Strauss RG, Zimmerman MB, Veng-Pedersen P. Autologous infant and allogeneic adult red cells demonstrate similar concurrent post-transfusion survival in very low birth weight neonates. J Pediatr. 2015;167:1001–6. doi: 10.1016/j.jpeds.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]