Abstract
The identification of bioactive molecules that have potential to interrupt carcinogenesis continues to garner research interest. In particular, molecules that have dietary origin are most attractive because of their safety, cost-effectiveness and feasibility of oral administration. Nutraceuticals have played an important role in the overall well-being of humans for many years, with or without rigorous evidence backing their health claims. Traditional medicine systems around the world have utilized plants for millennia that have medicinal properties, providing an opportunity for modern day researchers to assess their efficacies against ailments such as cancer. Withania somnifera (WS) is a plant that has been used in Ayurveda (an ancient form of medicine in Asia) and in the recent past, has been demonstrated to have anti-tumorigenic properties in experimental models. While scientific research performed on WS has exploded in the past decade, much regarding the mode of action and molecular targets involved remains unknown. In this review, we discuss the traditional uses of the plant, the experimental evidence supporting its chemopreventive potential as well as roadblocks that need to be overcome in order for WS to be evaluated as a chemopreventive agent in humans.
Keywords: Cancer, Chemoprevention, Nutraceuticals, Withania somnifera, Withaferin A, Ayurveda
1. Chemoprevention
In spite of major breakthroughs made in the field of drug discovery, researchers struggle to manipulate the complex biology of cancer with the current therapeutics available. While discovering novel therapeutics and providing adequate care to patients with cancer is an important aspect of uprooting the burden completely, prevention is an equally important consideration that has largely been ignored [1]. In appreciation of the idea that the carcinogenic process is so prolonged, and therefore can be targeted for various interventions, the notion of chemoprevention was brought forward by Michael Sporn in 1976 [2]. To that end, chemoprevention was defined as the use of natural, synthetic, biologic or chemical agents to reverse, suppress, or prevent the process of carcinogenesis. Coupled with the idea that prevention of cancer can be more beneficial than treatment, both at the population level and more recently with personalized or precision prevention, chemoprevention has become an area for active research. Encouragingly, in human trials, tamoxifen and aromatase inhibitors have been shown to be effective to prevent breast cancer [3] and retinol has been effectively used to prevent a certain subset of skin cancers [4]. Even though opportunities exist to maximize chemoprevention, it is exciting to acknowledge that a robust pipeline of promising natural agents is currently under clinical investigations for cancer chemoprevention. Sulforaphane derived from cruciferous vegetables such as broccoli for prostate cancer prevention and turmeric constituent curcumin for colorectal cancer prevention are a few examples of trials that are currently ongoing. Food-based chemoprevention is particularly useful due to the fact that it can be a relatively inexpensive strategy by which longevity and quality of life could be improved around the globe. Therefore, identifying novel opportunities for food-based chemoprevention may be extremely fruitful in battling the burden of cancer. Traditional medicine systems around the world have used indigenous plants against various ailments for centuries. Researchers are just beginning to unravel the medicinal benefits and the modes of action of bioactive components of such plants. In fact, some of these agents have made it into the Western markets as dietary supplements or nutraceuticals and are insinuated as having beneficial uses against a variety of conditions, including cancer.
2. Withania somnifera (WS): an introduction
2.1. Uses of WS in Ayurveda/ traditional medicine in South Asia
WS (Ashwagandha; Indian winter cherry, Indian ginseng) is a medicinal plant that has been utilized in traditional medicine in many parts of South Asia for millennia. It belongs to the diverse Solanaceae family of flowering plants. Withania species show a particularly wide distribution throughout drier climates of the world. Although there are 23 known Withania species, only W. somnifera and W. coagulans (Rishyagandha) are believed to have medicinal benefits [5]. While they have several similarities, the WS plant is much more branched and has larger leaves compared to W. coagulans. WS is more commonly used in traditional medicine but some specific preparations also utilize W. coagulans. A few studies have identified that W. coagulans may also have important implications as a therapeutic to type II diabetes [6,7].
WS roots (Figure 1) are used in over 200 formulations in Ayurveda, Siddha and Unani medicine. Ashwagandha churna, powdered root of the WS plant is frequently used to treat a variety of ailments [8]. Further, it is also used with other ingredients. WS is used as the major component in Saraswati churna, which is a herbal powder mixture utilized to treat neurological conditions. Ashwagandhadhi lehyam is another preparation that includes WS, primarily utilized as a rejuvenation supplement, a treatment for male impotence and as an energy enhancer [9]. While these uses may seem highly divergent, it is likely that specific proportions and interactions with the other ingredients used in the preparations could result in highly differential outcomes. Interestingly, only the root of the plant is used for traditional medicine preparations. The use of WS in Ayurvedic concoctions has recently been evaluated by alternative medicine researchers where it has been shown that utilizing standardization, phytochemical screenings and testing for pathogen/heavy metal contamination can significantly improve the actions of Ashwagandadhi lehyam [9].
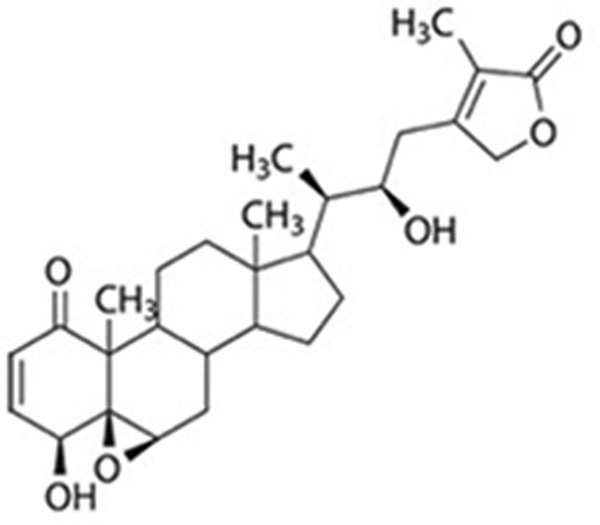
Figure 1.
A) Roots of WS. B) Structure of WA
2.2. WS a modern-day nutraceutical
WS is currently sold in the US market as a herbal supplement in the form of dried powder capsules and as alcoholic extracts. According to the Dietary Health Education Act (DSHEA) of 1994, dietary supplements (a vitamin, a mineral, a herb, an amino acid, a concentrate etc.) was defined as a type of food intended to supplement the diet, but cannot be represented as conventional foods [10]. Ashwagandha supplements are mostly recommended for energy enhancement and to improve human exercise performance [11]. It is also used against a variety of other conditions- arthritis, anxiety, insomnia and bronchitis. The exact mechanism of action for mediating a seemingly unrelated list of ailments has not been proposed to date, perhaps due to the fact that Ashwagandha in general terms promotes homeostatic conditions allowing for optimal physiological well-being. This idea is endorsed by Ayurvedic practitioners where Ashwagandha is recognized as an adaptogen further suggesting that it may or may not have direct effects against disease but rather have implications in reinstating homeostasis and physiological stability. However, precise scientific studies that evaluate these phenomena are currently lacking. As a result of the exemption herbal supplements obtain from being tested in clinical trials before market release, there are limited reports on potency, efficacy and side effects of the specific preparations of Ashwagandha that are available in the US market. While most of the modern uses of Ashwagandha stem from its place in traditional medicine, the lack of accompanying research evidence makes it challenging to determine its full potential as a nutraceutical. In an evaluation of several different herbs used to improve athletic performance, it was identified that Ashwagandha root contained a high concentration of starch which likely affected its positive impact on exercise performance via carbohydrate supplementation [11]. Therefore, in the absence of careful quality control, it becomes tricky to determine the relevant biological effects of such nutraceuticals.
2.3. Potential to target cancer
Mounting evidence from cell culture and animal studies suggest that WS possesses anti-tumorigenic properties. In 1967, it was first demonstrated experimentally that the root extract resulted in lowered cancer incidence in vivo [12]. Ever since, research interest in WS as an anti-tumorigenic agent has grown. This is apparent from the increase in the number of publications citing Withaferin A (WA; a withanolide from the WS plant) over the past decade from less than 5 in 2002 [13] to more than 50 in 2015. Researchers are just starting to scratch the surface of molecular pathways modulated by WS and its withanolides in order to counter the carcinogenic process. Not only has WS and its withanolides been shown to have therapeutic potential against cancer, some of them have also been shown to possess cancer preventive properties [14,15] [16]. These studies are discussed in detail in later sections. The cancer fighting properties have been seen not only with root extracts, but also with leaf extracts which is a relatively underused part of the Ashwagandha plant [17]. In addition to directly protecting against carcinogenesis, WS and especially WA has been shown to be hepatoprotective [18,19]. From the perspective of Ayurvedic medicine, there are several important implications of Ashwagandha for the treatment and prevention of cancer. As mentioned previously, the role of Ashwagandha in regeneration and rejuvenation can potentially be pivotal to improve longevity and quality of human life. Thus, this idea of overall health promotion may lead towards prevention of chronic disease like cancer. However, the dosage of Ashwagandha administered as treatment for cancer is presumably quite different to what is given as a general supplement that promotes good health. Careful research needs to be conducted to determine these parameters so that the factors pertaining to the use of Ashwagandha as a chemopreventive agent can be accurately established.
3. Bioactivity of Withania somnifera: Withaferin A and other withanolides
3.1. Extraction and isolation
Multiple methods are utilized to extract Ashwagandha from whole roots or leaves of the plant. Conventional methods usually involve extensive drying followed by grinding into a fine powder. Next, aqueous or organic solvent-based extraction procedures are performed where research suggests several ways in which extraction yields could be improved [20]. For example, microwave-assisted extraction can be optimized by modifying extraction time, temperature and solvent ratio. It has been identified that the major compounds isolated through alcoholic extraction of WS are alkaloids and withanolides [21]. Ultimately the best determinant of the success of the extraction or isolation methodology is how well the extract itself performs against a given disease process. A study showed that water extraction is just as viable as organic solvent extraction of Ashwagandha in affecting cancer cell progression [22]. More sophisticated methods such as high performance liquid chromatography (HPLC) coupled with mass spectrometric quantification have allowed more extensive and consistent isolation of bioactives from Ashwagandha [23]. On the other hand, non-extraction based isolation methods are also used, albeit infrequently, especially within the realm of Ayurveda where whole plant parts are dried and used directly as a powder. While this method may preserve the integrity of the plant parts, using whole plant products increases chances of contamination with pathogens and heavy metals and may also reduce the potency due to the presence of chemicals other than the bioactive components in the plant. Conversely, elements of the plant matrix could enhance the bioactivity of WA. Nonetheless, given the high variability of withanolide concentrations in different plant parts and the existence of chemotypes of WS [24], standardization techniques need to be incorporated into these isolation practices. Furthermore, it is very important that preparations are made in accordance with guidelines published by the World Health Organization to minimize pathogens, aflatoxins, pesticide residues and heavy metals [23].
3.2. Pharmacology
Characterization studies of WS have identified that the bioactive compounds present in the root, leaf and stem extract includes alkaloids and steroidal lactones. The bioactive compounds of WS have been further identified as withanolides, a type of steroidal lactone. So far, 12 alkaloids, 35 withanolides and several other sitoindosides (a withanolide containing a glucose molecule at carbon 27) have been identified [25,26] suggesting the diverse chemical makeup of the plant. Studies have shown that there is differential distribution of withanolides in different parts of the WS plant where WA is most abundant in the leaves as opposed to 12-deoxywithastramonolide and Withanolide A which is more profuse in the plant root [27]. In an in vitro model system that closely mimicked cellular absorption using Madin-Darby canine kidney cells, WA had much lower absorption compared to other withanolides [28]. WA has also been demonstrated to have higher bioavailability compared to Withanolide A when WS root extract was administered to Swiss Albino female mice orally [23]. The half-life of withanolides was evaluated in the same study where t1/2 of WA was shown to be approximately 60 minutes whereas Withanolide A had a shorter t1/2 of 45 minutes [23]. Given this rapid half-life, it may be worth considering twice daily (BID) or three times daily (TID) of WS in dosing regimens. While withanolides as a whole possess several properties that could potentially be utilized against a variety of diseases, the majority of research work that has been conducted on withanolides involves WA. This in part, is due to the notion that WA is the most potent withanolide identified thus far from the Ashwagandha plant and was one of the first withanolides to ever be isolated [29,30,31]. The structure of WA is shown in Figure 1.
The pathways for the metabolism and biotransformation of the withanolides of WS are poorly understood. In vitro microbial transformation of WA to 14 alpha-hydroxywithaferin A has been shown [32]. Given the structure of WA, it is likely that it undergoes hydrolysis (by epoxide hydrolase) and other reduction/oxidation reactions followed by conjugation to glutathione, glucuronides or sulfates. However, experimental evidence to support this claim is limited and is therefore an area that needs to be considered especially when studying the pharmacokinetics of withanolides.
Reports of major side effects of Ashwagandha are relatively scarce making it an attractive agent for cancer chemoprevention in humans. To assess acute toxicity, Wistar rats were administered a very large dose of 2000 mg/kg WS root extract for 14 days where no mortality or signs of toxicity were observed [33]. However, in another study where Sprague-Dawley rats were fed WS (dose not noted) for 14 days changes in liver and kidney histopathology was observed [34]. Understandably, purified withanolides have been associated with some minor side effects, likely due to the fact that biological effects are enhanced with a purified compound as compared to a crude plant extract. Administering 16 mg/kg WA intraperitoneally for 30 days to C57BL/6J mice resulted in loss of body weight and changes in serum enzymes [35]. Some sedation, ptosis and ataxia were observed in Sprague-Dawley rats 15–20 minutes of administering a herbal concoction that contained WS at a large dose of 1–2 g/kg body weight [36]. From a structural standpoint, it has been hypothesized that observed cytotoxicity of WA against cancer cells is attributable to its epoxide group [30]. Further research is required to determine if the aforementioned toxic side effects can be alleviated by using structural analogs that have the epoxide group or any other potentially important chemical group modified. These studies suggest that an in vivo safe dosage range is available for WS but need to be established in pre-clinical studies using appropriate models.
3.3. Structures and mechanisms of action
Novel withanolides are still being identified by researchers [37,38]. As mentioned previously, extensive work has been performed with WA where several of its structural properties have been identified. The cysteine-reactive nature of the α,β- unsaturated carbonyl group of WA is well-established [39]. WA has further been shown to directly bind to key cysteine residues of proteins such as Vimentin [40], GFAP [41], IKKβ [42] and β-Tubulin [43]. WA has also been shown to modulate important cellular signaling processes such as autophagy [44], proteasomal degradation [45,46] and the heat shock response [47]. Whether modulation of these processes originates from direct binding has not been elucidated. A study that evaluated the heat shock inducing activity of WA and several structural analogs showed that undesired cytotoxicity from WA could be minimized while enhancing cytoprotective activity by modifying WA structurally [48]. This study also suggested that there are key chemical moieties of the WA molecule that might be responsible for specific biological activities.
4. Cancer pathways modulated by Withania somnifera and its withanolides
4.1. Cell survival/ apoptosis
Most discussions on anti-tumorigenic properties of WS pertain to its ability to activate apoptotic pathways in cancer cells. Even within the realm of cancer chemoprevention, cell survival and the activation of pro-apoptotic pathways holds important implications where successful reversal of the carcinogenesis process essentially requires the early clearance or destruction of impaired cells. Several currently known chemopreventive agents such as the isothiocyanate, sulforaphane [49] and the triterpenoid, CDDO-Im [50] exhibit this property. A plethora of in vitro evidence exists about the induction of apoptosis by WS [51], WA [52,53] as well as other withanolides [54]. Some of the earliest hints of tumor suppression by WS came from a study that evaluated the potential of leaf extract to inhibit tumor formation in nude mice subcutaneously injected with fibrosarcoma HT1080 cells [55]. It was observed that treating mice with the leaf extract (0.3 mL of 24 µg/mL extract in cell growth medium, s.c.) resulted in reduced tumor size and was in part mediated via upregulation of p53. Interestingly, the authors of the paper used NMR to identify the component responsible for this action to be withanone. Induction of apoptosis by WA has been noted in some in vivo models where treatment with 4 mg/kg WA, i.p. 5 times for 2 weeks markedly reduced MDA-MB-231 tumor weights in nude mice as well as increased apoptosis compared to tumors in control mice [56].
While the exact mechanisms for induction of apoptosis by WS and its withanolides are yet to be established, data from several publications suggest that enhanced expression of pro-apoptotic genes as well as the suppression of proliferative pathways are possible targets. In a study conducted on a xenograft mouse model of cervical cancer, it was shown that 8 mg/kg WA, i.p. treatment for 6 weeks resulted in 70% reduction in tumor volume compared to controls as well as heightened expression of p53 and lowered expression of pro-caspase 3/ Bcl2 [57]. The ability of WA to downregulate oncogenic proteins that have anti-apoptotic function such as Bcl2 has been reported by others as well [58,59]. Whether this phenomena occurs in vivo within the tumor micro-environment to the extent that WA can selectively slow the growth of tumor cells via the aforementioned mechanisms while stabilizing the apoptotic function of normal cells has not been clearly determined. Ultimately, to utilize WS as a chemopreventive agent, the pharmacological conditions under which normal cells will survive while pre-cancerous/ cancerous cells will undergo death need to be assessed. Selective killing of cancer cells by WA is an idea that has been put forward by many. By comparing cell lines that are cancerous and non-cancerous, WA has been shown to be cytotoxic to only cancerous cell lines [60]. A point to note is that, these cell lines have inherent differences that can result in differential drug uptake, retention and toxicity. Therefore, mechanistic explorations of how tumor cells vs. non-tumor cells respond to WS and its withanolides require further investigation.
4.2. Angiogenesis
It is widely accepted that angiogenesis is a vital process exploited by tumors to facilitate their own growth. In addition to tumor masses, early stage carcinogenic events may also utilize angiogenesis suggesting that it could be attenuated in a cancer preventive context. Angiogenesis has been categorized as a marker of cancer progression given the differences that occur in new blood vessel formation during early and late stages of carcinogenesis [61]. The role of WS and its withanolides on angiogenesis has been studied. The first report related to anti-angiogenic effect was published in 2004, where WA was shown to be a potent inhibitor of angiogenesis both in vitro and in vivo [62]. In another study, WS was shown to inhibit angiogenesis in a VEGF-induced neovascularization model in vivo [63]. An in silico study along with molecular docking analyses corroborated the mechanism of this finding by showing that WA may directly bind to VEGF and thereby hamper angiogenesis [64]. Further in vitro and in vivo experimentation is required to validate the physiological relevance of this finding.
4.3. Stress response
In recent years, the role of stress response pathways in cancer chemoprevention has been closely evaluated [65]. WS and some of its withanolides have been shown to be mediators of the heat shock response. The heat shock response is essential to cellular homeostasis given its function in facilitating the degradation of misfolded proteins. Transcriptional regulation of multiple classes of genes by Heat shock transcription factor 1 (HSF1) is considered to be an important regulatory step of this mechanism. WA has been shown to bind HSP90 to inhibit its chaperone activity through an ATP-dependent mechanism in pancreatic cancer cells [47].This has been proposed to be one of the mechanisms by which WA exerts its anti-tumorigenic activity. A multiple compound screening study that utilized heat shock response induction as an endpoint identified WA as one of the potent mediators of the heat shock response wherein 1–4 µM WA was shown to be thiol-reactive and also shown to induce protein expression of HSP72 and 27 [39]. In a subsequent analysis, Wijeratne et al. [48] demonstrated that modulation of heat shock inducing activity of WA is feasible by structural modifications. It is important to point out that the effect of leaf or root extracts of WS on heat shock response has not been determined.
In addition to the heat shock response, several other stress response pathways have also been shown to be affected by WS and some of its withanolides. Several reports note that WA is a strong inducer of oxidative stress, mediated primarily via the generation of reactive oxygen species [66,67]. Interestingly, a report by Kaur et al. [68] suggested that WS extract did not provide any protection against oxidative damage caused by high glucose and hydrogen peroxide in human cancer cells, possibly suggesting that the pro-oxidant characteristics of WA would not render useful in protecting against oxidative damage. The exact percentages of withanolides in this leaf extract were not revealed, making it difficult to understand the exact mechanism underlying the observation. Furthermore, whether oxidative stress induction by WA is a very early molecular event that facilitates downstream cytoprotective pathways in order to ultimately guard cells and organisms is also currently unknown. WS and its withanolides have also been shown to up regulate the expression of several phase II enzymes [69,70] suggesting that other cytoprotective pathways, such as Nrf2 directly or indirectly, may be mediated by the action of withanolides.
4.4. Inflammation and immune regulation
Researchers are on the brink of identifying the pivotal roles played by inflammation and immune function in cancer. Reducing chronic inflammation to prevent certain types of cancers (e.g., hepatitis virus-induced inflammation and liver cancer) as well as utilizing immunotherapy as a successful treatment strategy for cancer are two key widely sought after areas of current cancer research. It is indeed desirable that some future chemopreventive drugs possess anti-inflammatory properties and also exhibit the ability to induce a robust immune response against early stage malignancies. Whether certain compounds that activate the immune system could potentially be utilized to prevent cancer has not been studied in detail, perhaps due to the fact that hyperactivation of the immune system could lead to several undesired challenges. Nevertheless, controlled activation of the immune system by WS is well-documented. In fact, two human studies with WS have looked at immunological end points [71,72]. These studies suggest that the mechanism of action is driven by lymphocyte and NK cell activation. Anti-inflammatory properties of WA are attributable to directly targeting cysteine 179 of IKK-β leading to the inhibition of NF-kB activity [42]. WA has also shown COX-2 inhibitory activity in some experimental models [73]. The anti-inflammatory and immune effects of WS and withanolides warrants further investigation, especially given the role of Ashwagandha as an adaptogen in traditional medicine.
5. Cancer chemoprevention with Withania somnifera/ withanolides: Pre-clinical studies
Several in vivo studies strongly suggest the chemopreventive potential of WS and its withanolides. While many of these studies have been conducted with WA, with the appropriate extrapolation experiments, the findings can be extended to WS plant extracts as well providing a rationale to use WS in human chemoprevention studies. A summary of these studies is presented in Table 1.
Table 1.
Chemopreventive activity of WA in animal models
| Organ Site |
Species | Carcinogen/ Genetic Modification |
Withaferin A Dose, route and frequency |
Efficacy against carcinogenesis |
Reference |
|---|---|---|---|---|---|
| Head & Neck |
Hamster ♂ Golden Syrian |
DMBA | 20 mg/kg, p.o. 3X/wk for 14 wk |
tumor incidence in DMBA alone=100% (10/10); DMBA+WA= 0% (0/10) |
[15] |
| Head & Neck |
Hamster ♂ Golden Syrian |
DMBA | 20 mg/kg, p.o. 3X/wk for 14 wk |
tumor incidence in DMBA alone=100% (6/6); DMBA+WA= 0% (0/6) |
[75] |
| Head & Neck |
Hamster ♂ Golden Syrian |
DMBA | 20 mg/kg, p.o. 3X/wk for 14 wk; WA administered on alternating days from DMBA at 8:00, 12:00 or 24:00 |
WA at 8:00 or 12:00 completely prevented tumors; 50% reduction in incidence with WA at 24:00 |
[74] |
| Mammary gland |
Mice ♀ | MMTV neu | 100 µg WA, i.p., 3X/wk for 28 wk |
Reduction in macroscopic tumor size and pulmonary metastasis |
[14] |
| Skin | Mice ♀ |
DBA/2; (DMBA+ TPA) |
20 µg WA, topical application 1X/day, 5X/wk for 14 wk |
100% protection against tumor formation |
[16] |
Some of the earliest work that established the chemopreventive potential of WA was performed on a DMBA-induced oral cancer model in Golden Syrian Hamsters. Oral administration of 20 mg/kg WA for 14 weeks completely prevented oral tumor formation in these animals [15]. In a follow-up study, Manoharan et al. [74] showed that this chemopreventive capacity was dependent on a circadian pattern where hamsters dosed with WA at 8 AM and 12 PM showed 100% protection from oral tumor formation while those treated at 12 AM showed 50% incidence in oral tumors [74]. Furthermore, this observation was in synchrony with diurnal changes in lipid peroxidation and antioxidant enzyme activity. Panjamurthy et al. [75] also demonstrated that there was marked reduction of p53 and Bcl2 protein expression in the animals treated with WA and DMBA compared to animals treated with DMBA alone.
In a study conducted with MMTV-neu mice that are predisposed to developing mammary carcinogenesis, it was shown that there was a 33% reduction in tumor formation in mice that were on a diet containing 750 mg WS root extract /kg of diet for 10 months [76]. This study is in fact complimentary to a more detailed study that was carried out previously using WA in the same mouse model where it was shown that 100 µg/mouse WA (i.p., 3 times/week for 28 weeks) resulted in lowered macroscopic tumor weights and reduced lung metastasis compared to control mice [14]. WA-treated mice had reduced expression of glycolysis and TCA cycle-related proteins, suggesting alterations in intermediary metabolism. A follow-up study that used tumor samples from this model [14] showed that WA inhibited self-renewal of breast cancer stem cells [77]. This observation was coupled with lower ALDH1 activity, endorsing the idea that WA was not only able to directly inhibit the cancer process by enhancing apoptosis but was also able to hamper stem cell machinery during carcinogenesis. Extensive mechanistic details of these observations were provided by Nagalingam et al., [78] where in a mammary cancer xenograft model, it was shown that WA treatment resulted in retarded tumor growth; reduction in cell proliferation marker Ki-67, survivin, and XIAP, as well as higher numbers of TUNEL-positive apoptotic cells [78]. Higher protein expression of pERK, pRSK, CHOP and DR-5 was also observed in the WA-treated group compared to control. Interestingly, the reduction of cancer incidence by WA was not observed in a follow-up group that had shRNA knocked-down DR-5 implying the indispensible role of the DR-5 pathway in prevention of mammary carcinogenesis by WA.
In a recent study that assessed the efficacy of WA in preventing skin carcinogenesis, 100% protection against tumor formation was observed [16]. Carcinogenesis prone DBA/2 female mice were subject to tumor initiation by DMBA application for 2 weeks. Subsequently, tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA) along with 20 µg WA was applied topically on the same area of mouse skin once per day, five times per week, for 14 weeks. In the TPA+WA group, WA was applied 30 minutes prior to TPA treatment. In addition to marked protection against tumorigenesis, WA also blocked carcinogen-induced up regulation of acetyl-CoA carboxylase (ACC1).
In addition to direct models of cancer prevention, the effect of WA on mouse xenografts has been assessed. Some of these studies are summarized in Table 2. Treatment of 4 or 8 mg/kg WA (i.p., daily for 28 days) resulted in inhibition of PC-3 tumor growth and inhibition of proteasomal chymotrypsin-like activity in male nude mice [45]. Implanting a patch that delivered a total dose of 4 mg/kg WA resulted in 60% inhibition of A549 lung cancer xenograft growth compared to sham control [79]. The fact that many of the pre-clinical chemoprevention studies have been carried out with WA but not with WS presents the challenge of not knowing how WS may perform as a chemopreventive agent altogether. Utilizing carcinogenesis models that are most relevant to humans and parallel testing of WS extracts and purified withanolides can further strengthen the argument of chemopreventive prospective of WS.
Table 2.
Anti-tumor activity of WA in cancer xenograft models
| Organ Site |
Species | Carcinogen/ Genetic Modification |
Withaferin A Dose, route and frequency |
Efficacy against cancer xenograft |
Reference |
|---|---|---|---|---|---|
| Prostate | Mouse ♂ | PC-3 tumor cell xenograft |
4 or 8 mg/kg, i.p. daily for 28 days |
70% Inhibition of tumor volume in WA-treated animals |
[45] |
| Breast | Mice ♀ | MDA-MB- 231 tumor cell xenograft |
4 mg/kg, i.p. 5X/wk for 2 wk |
Reduced tumor weight by WA |
[56] |
| Medullary thyroid |
Mice ♂ | DRO81–1 tumor cell xenograft |
8 mg/kg, i.p. daily for 6 weeks; WA treatment started 2 weeks post tumor injection |
50% reduction in tumor volume by WA |
[88] |
| Cervical | Mice ♀ |
CaSki tumor cell xenograft |
8 mg/kg WA, i.p., q.o.d. for 6 wk |
70% reduction in tumor volume |
[57] |
| Lung | Mice ♀ |
A549 tumor cell xenograft |
Total dose= 4 mg/kg WA, i.p. or implant |
60% lower tumor volume by WA |
[79] |
| Colon | Mice ♀ | HCT116 cell tumor xenograft |
2 mg/kg WA, i.p. 3X/wk, 32 days |
30% reduction in tumor volume by WA |
[89] |
6. Extending pre-clinical efforts to human chemoprevention trials using WS
6.1. Past human clinical trials using WS
So far, no clinical trials in human populations have been carried out with WS or WA with cancer or cancer biomarkers as end points. However, WS has been tested in a few clinical trials against other disorders and conditions. While many of these studies suffer from major drawbacks, including but not limited to small sample sizes, use of mixtures of compounds and utilizing only older individuals as study participants, there are some important lessons learned for future clinical and translational work involving WS and WA.
A few studies have assessed the effects of WS on immunologic endpoints such as lymphocyte activation and NK cell activity. In a smaller study carried out by Mikolai et al., [71] 5 healthy study participants were administered 6 ml of WRE with cow’s milk, twice daily for 5 days [71]. Significant increases in expression of CD4+ and CD3+ T cells as well as CD56+ NK cells were observed after 96 hours. Bhat et al. [72] performed a study with a larger number of participants, but also used a concoction of several different herbs. Volunteers consumed three cups of either regular tea or natural care tea that included 4 herbs including WS. The results showed that natural care tea consumption enhanced NK cell activity. Second-generation antipsychotic drugs are associated with higher incidence of metabolic syndrome. Clinically diagnosed schizophrenia patients who had received antipsychotic medications for 6 months or more received either a capsule with 400 mg of WS extract (n=15), three times daily, for 1 month [80]. Results after one month showed significant reduction in serum triglycerides and fasting blood glucose levels in the WS extract- treated group compared to the placebo.
Biswal et al. [81] estimated the potential of WS to reduce chemotherapy-induced fatigue and quality of life in a prospective, open-label, non-randomized comparative clinical trial. Patients in the control arm experienced significantly higher estimated marginal means of fatigue scores compared with the treatment arm that received 2 g of WS root extract every 8 hours throughout the course of chemotherapy. Additionally, a survival analysis showed that patients in the WS treatment group had a better 24-month survival rate of 76% as compared to the control, which was 56%. Although this finding was not statistically significant, it could be attributed to the high heterogeneity in breast cancer types. Also, the possible drug-drug interaction between chemotherapy agents and WS was not evaluated, making it particularly difficult to determine whether the observed effects are direct or not. In an interesting translational study, the role of WA in cancer epigenetics was evaluated. First it was shown that DNA methyltransferases (DNMT) are over expressed in human invasive ductal tissue isolated from cancer patients [82]. The researchers subsequently showed that in MCF7 and MDA-MB-231 breast cancer cells WA treatment suppressed transcription of DNMT. These results indicated that WA is a modifier of the epigenetic response, a finding that warrants further exploration.
6.2. Utilizing WS as a nutraceutical to prevent cancer: Challenges and lessons for the future
Promoting WS towards chemoprevention in humans requires overcoming several barriers. These are summarized in Table 3. From an agricultural perspective, bulk manufacturing of WS is associated with several concerns. Firstly, the plant thrives under specific environmental conditions and requires time and effort to grow conventionally. However, new technologies such as in vitro hairy root cultures of Ashwagandha that may assist in this matter have emerged [83]. Secondly, research has also identified that there is large variability in amount of alkaloids and withanolides produced by each plant, and in different parts of the plant which can directly interfere with production of preparation with uniform bioactivity [24,84]. Thus, strict quantification and standardization methods should be utilized to pre-determine concentrations of alkaloids and withanolides in each preparation. Thirdly, even though the WS plant is resistant to pest attacks, some mite and insect infestations have been noticed. Ensuring that fertilizers and pesticides are not overused, especially given the fact that plant roots are used, is vital. In addition, utilizing novel methods to produce WS and its withanolides can help meet the increasing global demand in a sustainable way and will facilitate determination of its chemopreventive potential.
Table 3.
Proposed actions for developing WS as a chemopreventive agent in human populations
| Phase | Action items |
|---|---|
Agricultural
|
|
Quality control
|
|
Pre-clinical
|
|
Clinical
|
|
Design of chemoprevention trials should incorporate insights from the traditional uses of WS as well. Its common use as an adaptogen that promotes homeostasis and as an energy enhancer may suggest potentially useful modes of action of the drug. Research work cited here are dedicated to understand these biological pathways modulated by WS and its withanolides. Pre-clinical studies, performed either in cell culture or in animal models that mimic appropriately relevant conditions of populations, need to be utilized for this purpose. Evaluating the chemopreventive efficacy of not only WA but also extracts of WS in a broader range of animal carcinogenesis models would bolster the potential role of WS to prevent cancer. Data gathered from such studies would be beneficial in 1) understanding the pharmacodynamics and pharmacokinetics of Ashwagandha 2) modifying structural moieties of withanolides to assess the role of chemical structures in the mechanism of action 3) identifying molecular events involved in WS-mediated effects to specifically target signaling pathways that are validated for cancer chemoprevention 4) determining whether co-administration with other agents (compounds used in traditional medicine to complement Ashwagandha or agents that are currently used in Western cancer chemoprevention trials) could potentially render higher benefits for cancer prevention.
Inasmuch as there many naturally-occurring dietary agents currently under investigation for their potential to prevent or treat cancer, it becomes important to prioritize these compounds based on efficacy and safety. Although many of these agents may target different biological pathways and may have inherently different pharmacological profiles, they need to be compared in common models, especially in vivo ones. This has not been done to date. Nonetheless, the evidence that WS can be beneficial against a wide array of diseases highlighted in the previous section serves as a testimony to the need develop WS as a chemopreventive agent against cancer in humans. Invariably, considerations for dosage, time course as well as other pharmacological parameters of the agent need to be accounted for. Especially for chemopreventive studies, a non-toxic dose of WS will need to be administered over a prolonged period of time to determine whether it can prevent, block or reverse aspects of the carcinogenic process. Therefore, determining a safe dosage window, developing a precise administration regimen as well as understanding the bioavailability in preclinical studies is a prerequisite of promoting WS for clinical work as a chemopreventive agent. Being able to track the response to a chemopreventive agent in a non-invasive manner to the study participants is pivotal, particularly in long-term trials. Determining if WS metabolites can be detected in human blood, urine or saliva would be a useful tool to have [85]. Clinical development pathways with foods like sulforaphane-rich broccoli provide reasonable starting points for novel plant-derived agents such as WS [86]. With plant extracts, it is imperative that careful characterization of components in the extract is performed prior to starting the intervention regimen. Given the prolific evidence that WA performs well in preventing DMBA-induced oral carcinogenesis, it maybe a worthwhile consideration to first test WS in head and neck cancer prevention human trials. The fact that head and neck cancers are highly prevalent in South Asian nations, including India [87], where coincidentally WS is abundantly used in traditional medicine, presents an important and appropriate target population to fulfill a public health need.
With a rapidly expanding elderly population and relatively underdeveloped cancer research, treatment and care programs, less developed nations are likely to be the hardest hit by the cancer tsunami in the next few decades. Therefore, prevention of cancer, specifically by means of food or other dietary agents is likely the most cost-effective and sustainable method of dealing with this epidemic. The identification and characterization of dietary agents with chemopreventive potential are pivotal steps in this process. Traditional medicine systems such as Ayurveda have deep roots in many of these underdeveloped communities and present a great opportunity for battling the global burden of cancer from the stand point of primary prevention. Seeking out and researching plant-based agents that have a long standing history in traditional medicine can possibly be more effective than developing cancer treatment drugs from scratch, in terms of cost, convenience of administration and cultural acceptability. Given the central role of WS in Ayurveda and its promising actions in the realm of modern cancer research, it has potential to move forward as a cancer chemopreventive nutraceutical.
Acknowledgments
This study was funded by NIH, grant number CA197222
Abbreviations
- ALDH1
Aldehyde dehydrogenase
- BCL2
B-cell lymphoma 2
- CDDO-Im
1-(2-Cyano-3,12,28-trioxooleana-1,9(11)-dien-28-yl)-1H–imidazole
- CHOP
C/EBP homologous protein
- COX
Cyclooxygenase
- DMBA
dimethylbenz[a]anthracene
- DR-5
Death receptor 5
- ERK
Extracellular signal regulated kinase
- GFAP
Glial fibrillary acidic protein
- HSF1
Heat shock transcription factor 1
- HSP
Heat shock protein
- IKK
I kappa B kinase
- MMTV
Murine mammary tumor virus
- NFκB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NK
Natural killer
- NMR
Nuclear magnetic resonance
- NRF2
Nuclear factor (erythroid-derived 2)-like 2
- RSK
ribosomal s6 kinase
- TPA
12-O-tetradecanoylphorbol-13-acetate
- VEGF
vascular endothelial growth factor
- WA
Withaferin A
- WRE
Withania somnifera root extract
- WS
Withania somnifera
- XIAP
X-linked inhibitor of apoptosis protein
Footnotes
The authors have declared no conflict of interest
Reference List
- 1.Geneau R, Stuckler D, Stachenko S, McKee M, Ebrahim S, Basu S, Chockalingham A, Mwatsama M, Jamal R, Alwan A, Beaglehole R. Raising the priority of preventing chronic diseases a political process. Lancet. 2010;376:1689–1698. doi: 10.1016/S0140-6736(10)61414-6. [DOI] [PubMed] [Google Scholar]
- 2.Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35:1332–1338. [PubMed] [Google Scholar]
- 3.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG, Wolmark N. Tamoxifen for the prevention of breast cancer current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 4.Moon TE, Levine N, Cartmel B, Bangert JL, Rodney S, Dong Q, Peng YM, Alberts DS. Effect of retinol in preventing squamous cell skin cancer in moderate-risk subjects a randomized, double-blind, controlled trial. Southwest Skin Cancer Prevention Study Group. Cancer Epidemiol Biomarkers Prev. 1997;6:949–956. [PubMed] [Google Scholar]
- 5.Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Palazon J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14:2373–2393. doi: 10.3390/molecules14072373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ojha S, Alkaabi J, Amir N, Sheikh A, Agil A, Fahim MA, Adem A. Withania coagulans fruit extract reduces oxidative stress and inflammation in kidneys of streptozotocin-induced diabetic rats. Oxid Med Cell Longev. 2014;2014:201436. doi: 10.1155/2014/201436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Upadhyay BN, Gupta V. A clinical study on the effect of Rishyagandha (Withania coagulans) in the management of Prameha (Type II Diabetes Mellitus) Ayu. 2011;32:507–511. doi: 10.4103/0974-8520.96124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baliga MS, Meera S, Vaishnav LK, Rao S, Palatty PL. Rasayana drugs from the Ayurvedic system of medicine as possible radioprotective agents in cancer treatment. Integr Cancer Ther. 2013;12:455–463. doi: 10.1177/1534735413490233. [DOI] [PubMed] [Google Scholar]
- 9.Rasheed A, Satyanarayana KV, Gulabi PS, Rao MS. Chemical and pharmacological standardization of Ashwagandhadi lehyam an ayurvedic formulation. J Complement Integr Med. 2013;10 doi: 10.1515/jcim-2012-0026. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Rahman A, Anyangwe N, Carlacci L, Casper S, Danam RP, Enongene E, Erives G, Fabricant D, Gudi R, Hilmas CJ, Hines F, Howard P, Levy D, Lin Y, Moore RJ, Pfeiler E, Thurmond TS, Turujman S, Walker NJ. The safety and regulation of natural products used as foods and food ingredients. Toxicol Sci. 2011;123:333–348. doi: 10.1093/toxsci/kfr198. [DOI] [PubMed] [Google Scholar]
- 11.Bucci LR. Selected herbals and human exercise performance. Am J Clin Nutr. 2000;72:624S–636S. doi: 10.1093/ajcn/72.2.624S. [DOI] [PubMed] [Google Scholar]
- 12.Shohat B, Gitter S, Abraham A, Lavie D. Antitumor activity of withaferin A (NSC-101088) Cancer Chemother Rep. 1967;51:271–276. [PubMed] [Google Scholar]
- 13.Vanden Berghe W, Sabbe L, Kaileh M, Haegeman G, Heyninck K. Molecular insight in the multifunctional activities of Withaferin A. Biochem Pharmacol. 2012;84:1282–1291. doi: 10.1016/j.bcp.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 14.Hahm ER, Lee J, Kim SH, Sehrawat A, Arlotti JA, Shiva SS, Bhargava R, Singh SV. Metabolic alterations in mammary cancer prevention by withaferin A in a clinically relevant mouse model. J Natl Cancer Inst. 2013;105:1111–1122. doi: 10.1093/jnci/djt153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manoharan S, Panjamurthy K, Menon VP, Balakrishnan S, Alias LM. Protective effect of Withaferin-A on tumour formation in 7,12-dimethylbenz[a]anthracene induced oral carcinogenesis in hamsters. Indian J Exp Biol. 2009;47:16–23. [PubMed] [Google Scholar]
- 16.Li W, Zhang C, Du H, Huang V, Sun B, Harris JP, Richardson Q, Shen X, Jin R, Li G, Kevil CG, Gu X, Shi R, Zhao Y. Withaferin A suppresses the up-regulation of acetyl-coA carboxylase 1 and skin tumor formation in a skin carcinogenesis mouse model. Mol Carcinog. 2015 doi: 10.1002/mc.22423. [DOI] [PubMed] [Google Scholar]
- 17.Yadav B, Bajaj A, Saxena M, Saxena AK. In Vitro Anticancer Activity of the Root, Stem and Leaves of Withania Somnifera against Various Human Cancer Cell Lines. Indian J Pharm Sci. 2010;72:659–663. doi: 10.4103/0250-474X.78543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jadeja RN, Urrunaga NH, Dash S, Khurana S, Saxena NK. Withaferin-A Reduces Acetaminophen-Induced Liver Injury in Mice. Biochem Pharmacol. 2015;97:122–132. doi: 10.1016/j.bcp.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sudhir S, Budhiraja RD. Comparison of the protective effect of Withaferin-’A’ and hydrocortisone against CCL4 induced hepatotoxicity in rats. Indian J Physiol Pharmacol. 1992;36:127–129. [PubMed] [Google Scholar]
- 20.Mirzajani F, Ghassempour A, Jalali-Heravi M, Mirjalili MH. Optimisation of a microwave-assisted method for extracting withaferin A from Withania somnifera Dunal. using central composite design. Phytochem Anal. 2010;21:544–549. doi: 10.1002/pca.1230. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni SK, Singh K, Bishnoi M. Comparative behavioural profile of newer antianxiety drugs on different mazes. Indian J Exp Biol. 2008;46:633–638. [PubMed] [Google Scholar]
- 22.Wadhwa R, Singh R, Gao R, Shah N, Widodo N, Nakamoto T, Ishida Y, Terao K, Kaul SC. Water extract of Ashwagandha leaves has anticancer activity identification of an active component and its mechanism of action. PLoS One. 2013;8:e77189. doi: 10.1371/journal.pone.0077189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patil D, Gautam M, Mishra S, Karupothula S, Gairola S, Jadhav S, Pawar S, Patwardhan B. Determination of withaferin A withanolide A in mice plasma using high-performance liquid chromatography-tandem mass spectrometry application to pharmacokinetics after oral administration of Withania somnifera aqueous extract. J Pharm Biomed Anal. 2013;80:203–212. doi: 10.1016/j.jpba.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Kushwaha S, Roy S, Maity R, Mallick A, Soni VK, Singh PK, Chaurasiya ND, Sangwan RS, Misra-Bhattacharya S, Mandal C. Chemotypical variations in Withania somnifera lead to differentially modulated immune response in BALB/c mice. Vaccine. 2012;30:1083–1093. doi: 10.1016/j.vaccine.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 25.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha):a review. Altern Med Rev. 2000;5:334–346. [PubMed] [Google Scholar]
- 26.Vaishnavi K, Saxena N, Shah N, Singh R, Manjunath K, Uthayakumar M, Kanaujia SP, Kaul SC, Sekar K, Wadhwa R. Differential activities of the two closely related withanolides, Withaferin A and Withanone bioinformatics and experimental evidences. PLoS One. 2012;7:e44419. doi: 10.1371/journal.pone.0044419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gajbhiye NA, Makasana J, Kumar S. Accumulation of Three Important Bioactive Compounds in Different Plant Parts of Withania somnifera and its Determination by the LC-ESI-MS-MS (MRM) Method. J Chromatogr Sci. 2015 doi: 10.1093/chromsci/bmv088. [DOI] [PubMed] [Google Scholar]
- 28.Devkar ST, Kandhare AD, Sloley BD, Jagtap SD, Lin J, Tam YK, Katyare SS, Bodhankar SL, Hegde MV. Evaluation of the bioavailability of major withanolides of using an absorption model system. J Adv Pharm Technol Res. 2015;6:159–164. doi: 10.4103/2231-4040.165023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z, Garcia A, Xu S, Powell DR, Vertino PM, Singh S, Marcus AI. Withania somnifera root extract inhibits mammary cancer metastasis and epithelial to mesenchymal transition. PLoS One. 2013;8:e75069. doi: 10.1371/journal.pone.0075069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi P, Misra L, Siddique AA, Srivastava M, Kumar S, Darokar MP. Epoxide group relationship with cytotoxicity in withanolide derivatives from Withania somnifera. Steroids. 2014;79:19–27. doi: 10.1016/j.steroids.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Antony ML, Lee J, Hahm ER, Kim SH, Marcus AI, Kumari V, Ji X, Yang Z, Vowell CL, Wipf P, Uechi GT, Yates NA, Romero G, Sarkar SN, Singh SV. Growth arrest by the antitumor steroidal lactone withaferin A in human breast cancer cells is associated with down-regulation and covalent binding at cysteine 303 of beta-tubulin. J Biol Chem. 2014;289:1852–1865. doi: 10.1074/jbc.M113.496844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosazza JP, Nicholas AW, Gustafson ME. Microbial transformations of natural antitumor agents. 7. 14-alpha-Hydroxylation of withaferin-A by Cunninghamella elegans (NRRL 1393) Steroids. 1978;31:671–679. doi: 10.1016/s0039-128x(78)80007-5. [DOI] [PubMed] [Google Scholar]
- 33.Prabu PC, Panchapakesan S, Raj CD. Acute and sub-acute oral toxicity assessment of the hydroalcoholic extract of Withania somnifera roots in Wistar rats. Phytother Res. 2013;27:1169–1178. doi: 10.1002/ptr.4854. [DOI] [PubMed] [Google Scholar]
- 34.Arseculeratne SN, Gunatilaka AA, Panabokke RG. Studies of medicinal plants of Sri Lanka. Part 14 Toxicity of some traditional medicinal herbs. J Ethnopharmacol. 1985;13:323–335. doi: 10.1016/0378-8741(85)90078-9. [DOI] [PubMed] [Google Scholar]
- 35.Uma DP, Kamath R. Radiosensitizing effect of withaferin A combined with hyperthermia on mouse fibrosarcoma and melanoma. J Radiat Res. 2003;44:1–6. doi: 10.1269/jrr.44.1. [DOI] [PubMed] [Google Scholar]
- 36.Dey D, Chaskar S, Athavale N, Chitre D. Acute and chronic toxicity, cytochrome p450 enzyme inhibition, and HERG channel blockade studies with a polyherbal, ayurvedic formulation for inflammation. Biomed Res Int. 2015;2015:971982. doi: 10.1155/2015/971982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pramanick S, Roy A, Ghosh S, Majumder HK, Mukhopadhyay S. Withanolide Z, a new chlorinated withanolide from Withania somnifera. Planta Med. 2008;74:1745–1748. doi: 10.1055/s-2008-1081357. [DOI] [PubMed] [Google Scholar]
- 38.Siddique AA, Joshi P, Misra L, Sangwan NS, Darokar MP. 5,6-de-epoxy-5-en-7-one-17-hydroxy withaferin A, a new cytotoxic steroid from Withania somnifera L. Dunal leaves. Nat Prod Res. 2014;28:392–398. doi: 10.1080/14786419.2013.871545. [DOI] [PubMed] [Google Scholar]
- 39.Santagata S, Xu YM, Wijeratne EM, Kontnik R, Rooney C, Perley CC, Kwon H, Clardy J, Kesari S, Whitesell L, Lindquist S, Gunatilaka AA. Using the heat-shock response to discover anticancer compounds that target protein homeostasis. ACS Chem Biol. 2012;7:340–349. doi: 10.1021/cb200353m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bargagna-Mohan P, Hamza A, Kim YE, Khuan Abby HY, Mor-Vaknin N, Wendschlag N, Liu J, Evans RM, Markovitz DM, Zhan CG, Kim KB, Mohan R. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem Biol. 2007;14:623–634. doi: 10.1016/j.chembiol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bargagna-Mohan P, Paranthan RR, Hamza A, Dimova N, Trucchi B, Srinivasan C, Elliott GI, Zhan CG, Lau DL, Zhu H, Kasahara K, Inagaki M, Cambi F, Mohan R. Withaferin A targets intermediate filaments glial fibrillary acidic protein and vimentin in a model of retinal gliosis. J Biol Chem. 2010;285:7657–7669. doi: 10.1074/jbc.M109.093765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heyninck K, Lahtela-Kakkonen M, Van dV, Haegeman G, Vanden Berghe W. Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKbeta. Biochem Pharmacol. 2014;91:501–509. doi: 10.1016/j.bcp.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Antony ML, Lee J, Hahm ER, Kim SH, Marcus AI, Kumari V, Ji X, Yang Z, Vowell CL, Wipf P, Uechi GT, Yates NA, Romero G, Sarkar SN, Singh SV. Growth arrest by the antitumor steroidal lactone withaferin A in human breast cancer cells is associated with down-regulation and covalent binding at cysteine 303 of beta-tubulin. J Biol Chem. 2014;289:1852–1865. doi: 10.1074/jbc.M113.496844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hahm ER, Singh SV. Autophagy fails to alter withaferin A-mediated lethality in human breast cancer cells. Curr Cancer Drug Targets. 2013;13:640–650. doi: 10.2174/15680096113139990039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang H, Shi G, Dou QP. The tumor proteasome is a primary target for the natural anticancer compound Withaferin A isolated from “Indian winter cherry”. Mol Pharmacol. 2007;71:426–437. doi: 10.1124/mol.106.030015. [DOI] [PubMed] [Google Scholar]
- 46.Yang H, Wang Y, Cheryan VT, Wu W, Cui CQ, Polin LA, Pass HI, Dou QP, Rishi AK, Wali A. Withaferin A inhibits the proteasome activity in mesothelioma in vitro and in vivo. PLoS One. 2012;7:e41214. doi: 10.1371/journal.pone.0041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y, Hamza A, Zhang T, Gu M, Zou P, Newman B, Li Y, Gunatilaka AA, Zhan CG, Sun D. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem Pharmacol. 2010;79:542–551. doi: 10.1016/j.bcp.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wijeratne EM, Xu YM, Scherz-Shouval R, Marron MT, Rocha DD, Liu MX, Costa-Lotufo LV, Santagata S, Lindquist S, Whitesell L, Gunatilaka AA. Structure-activity relationships for withanolides as inducers of the cellular heat-shock response. J Med Chem. 2014;57:2851–2863. doi: 10.1021/jm401279n. [DOI] [PubMed] [Google Scholar]
- 49.Sharma C, Sadrieh L, Priyani A, Ahmed M, Hassan AH, Hussain A. Anti-carcinogenic effects of sulforaphane in association with its apoptosis-inducing and anti-inflammatory properties in human cervical cancer cells. Cancer Epidemiol. 2011;35:272–278. doi: 10.1016/j.canep.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Kim EH, Deng CX, Sporn MB, Liby KT. CDDO-imidazolide induces DNA damage, G2/M arrest and apoptosis in BRCA1-mutated breast cancer cells. Cancer Prev Res (Phila) 2011;4:425–434. doi: 10.1158/1940-6207.CAPR-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malik F, Kumar A, Bhushan S, Mondhe DM, Pal HC, Sharma R, Khajuria A, Singh S, Singh G, Saxena AK, Suri KA, Qazi GN, Singh J. Immune modulation apoptosis induction Two sides of antitumoural activity of a standardised herbal formulation of Withania somnifera. Eur J Cancer. 2009;45:1494–1509. doi: 10.1016/j.ejca.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 52.Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007;67:246–253. doi: 10.1158/0008-5472.CAN-06-2430. [DOI] [PubMed] [Google Scholar]
- 53.Das T, Roy KS, Chakrabarti T, Mukhopadhyay S, Roychoudhury S. Withaferin A modulates the Spindle assembly checkpoint by degradation of Mad2-Cdc20 complex in colorectal cancer cell lines. Biochem Pharmacol. 2014;91:31–39. doi: 10.1016/j.bcp.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 54.Mondal S, Mandal C, Sangwan R, Chandra S, Mandal C. Withanolide D induces apoptosis in leukemia by targeting the activation of neutral sphingomyelinase-ceramide cascade mediated by synergistic activation of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase. Mol Cancer. 2010;9:239. doi: 10.1186/1476-4598-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Widodo N, Kaur K, Shrestha BG, Takagi Y, Ishii T, Wadhwa R, Kaul SC. Selective killing of cancer cells by leaf extract of Ashwagandha identification of a tumor-inhibitory factor and the first molecular insights to its effect. Clin Cancer Res. 2007;13:2298–2306. doi: 10.1158/1078-0432.CCR-06-0948. [DOI] [PubMed] [Google Scholar]
- 56.Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68:7661–7669. doi: 10.1158/0008-5472.CAN-08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis. 2011;32:1697–1705. doi: 10.1093/carcin/bgr192. [DOI] [PubMed] [Google Scholar]
- 58.Rah B, Ur RR, Nayak D, Yousuf SK, Mukherjee D, Kumar LD, Goswami A. PAWR-mediated suppression of BCL2 promotes switching of 3-azido withaferin A (3-AWA)-induced autophagy to apoptosis in prostate cancer cells. Autophagy. 2015;11:314–331. doi: 10.1080/15548627.2015.1017182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayola E, Gallerne C, Esposti DD, Martel C, Pervaiz S, Larue L, Debuire B, Lemoine A, Brenner C, Lemaire C. Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis. 2011;16:1014–1027. doi: 10.1007/s10495-011-0625-x. [DOI] [PubMed] [Google Scholar]
- 60.Nishikawa Y, Okuzaki D, Fukushima K, Mukai S, Ohno S, Ozaki Y, Yabuta N, Nojima H. Withaferin A Induces Cell Death Selectively in Androgen-Independent Prostate Cancer Cells but Not in Normal Fibroblast Cells. PLoS One. 2015;10:e0134137. doi: 10.1371/journal.pone.0134137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma RA, Harris AL, Dalgleish AG, Steward WP, O’Byrne KJ. Angiogenesis as a biomarker and target in cancer chemoprevention. Lancet Oncol. 2001;2:726–732. doi: 10.1016/S1470-2045(01)00586-1. [DOI] [PubMed] [Google Scholar]
- 62.Mohan R, Hammers HJ, Bargagna-Mohan P, Zhan XH, Herbstritt CJ, Ruiz A, Zhang L, Hanson AD, Conner BP, Rougas J, Pribluda VS. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7:115–122. doi: 10.1007/s10456-004-1026-3. [DOI] [PubMed] [Google Scholar]
- 63.Mathur R, Gupta SK, Singh N, Mathur S, Kochupillai V, Velpandian T. Evaluation of the effect of Withania somnifera root extracts on cell cycle and angiogenesis. J Ethnopharmacol. 2006;105:336–341. doi: 10.1016/j.jep.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 64.Saha S, Islam MK, Shilpi JA, Hasan S. Inhibition of VEGF a novel mechanism to control angiogenesis by Withania somnifera’s key metabolite Withaferin A. In Silico Pharmacol. 2013;1:11. doi: 10.1186/2193-9616-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, Bates TE, Giuffrida Stella AM, Schapira T, Dinkova Kostova AT, Rizzarelli E. Cellular stress response a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33:2444–2471. doi: 10.1007/s11064-008-9775-9. [DOI] [PubMed] [Google Scholar]
- 66.Grogan PT, Sleder KD, Samadi AK, Zhang H, Timmermann BN, Cohen MS. Cytotoxicity of withaferin A in glioblastomas involves induction of an oxidative stress-mediated heat shock response while altering Akt/mTOR and MAPK signaling pathways. Invest New Drugs. 2013;31:545–557. doi: 10.1007/s10637-012-9888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hahm ER, Moura MB, Kelley EE, Van HB, Shiva S, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One. 2011;6:e23354. doi: 10.1371/journal.pone.0023354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaur K, Rani G, Widodo N, Nagpal A, Taira K, Kaul SC, Wadhwa R. Evaluation of the anti-proliferative and anti-oxidative activities of leaf extract from in vivo and in vitro raised Ashwagandha. Food Chem Toxicol. 2004;42:2015–2020. doi: 10.1016/j.fct.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 69.Kostecka A, Sznarkowska A, Meller K, Acedo P, Shi Y, Mohammad Sakil HA, Kawiak A, Lion M, Krolicka A, Wilhelm M, Inga A, Zawacka-Pankau J. JNK-NQO1 axis drives TAp73-mediated tumor suppression upon oxidative and proteasomal stress. Cell Death Dis. 2014;5:e1484. doi: 10.1038/cddis.2014.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reuland DJ, Khademi S, Castle CJ, Irwin DC, McCord JM, Miller BF, Hamilton KL. Upregulation of phase II enzymes through phytochemical activation of Nrf2 protects cardiomyocytes against oxidant stress. Free Radic Biol Med. 2013;56:102–111. doi: 10.1016/j.freeradbiomed.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 71.Mikolai J, Erlandsen A, Murison A, Brown KA, Gregory WL, Raman-Caplan P, Zwickey HL. In vivo effects of Ashwagandha (Withania somnifera) extract on the activation of lymphocytes. J Altern Complement Med. 2009;15:423–430. doi: 10.1089/acm.2008.0215. [DOI] [PubMed] [Google Scholar]
- 72.Bhat J, Damle A, Vaishnav PP, Albers R, Joshi M, Banerjee G. In vivo enhancement of natural killer cell activity through tea fortified with Ayurvedic herbs. Phytother Res. 2010;24:129–135. doi: 10.1002/ptr.2889. [DOI] [PubMed] [Google Scholar]
- 73.Min KJ, Choi K, Kwon TK. Withaferin A down-regulates lipopolysaccharide-induced cyclooxygenase-2 expression and PGE2 production through the inhibition of STAT1/3 activation in microglial cells. Int Immunopharmacol. 2011;11:1137–1142. doi: 10.1016/j.intimp.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 74.Manoharan S, Panjamurthy K, Balakrishnan S, Vasudevan K, Vellaichamy L. Circadian time-dependent chemopreventive potential of withaferin-A in 7,12-dimethylbenz[a]anthracene-induced oral carcinogenesis. Pharmacol Rep. 2009;61:719–726. doi: 10.1016/s1734-1140(09)70125-2. [DOI] [PubMed] [Google Scholar]
- 75.Panjamurthy K, Manoharan S, Nirmal MR, Vellaichamy L. Protective role of Withaferin-A on immunoexpression of p53 and bcl-2 in 7,12-dimethylbenz(a)anthracene-induced experimental oral carcinogenesis. Invest New Drugs. 2009;27:447–452. doi: 10.1007/s10637-008-9199-z. [DOI] [PubMed] [Google Scholar]
- 76.Khazal KF, Hill DL, Grubbs CJ. Effect of Withania somnifera root extract on spontaneous estrogen receptor-negative mammary cancer in MMTV/Neu mice. Anticancer Res. 2014;34:6327–6332. [PMC free article] [PubMed] [Google Scholar]
- 77.Kim SH, Singh SV. Mammary cancer chemoprevention by withaferin A is accompanied by in vivo suppression of self-renewal of cancer stem cells. Cancer Prev Res (Phila) 2014;7:738–747. doi: 10.1158/1940-6207.CAPR-13-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagalingam A, Kuppusamy P, Singh SV, Sharma D, Saxena NK. Mechanistic elucidation of the antitumor properties of withaferin a in breast cancer. Cancer Res. 2014;74:2617–2629. doi: 10.1158/0008-5472.CAN-13-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gupta RC, Bansal SS, Aqil F, Jeyabalan J, Cao P, Kausar H, Russell GK, Munagala R, Ravoori S, Vadhanam MV. Controlled-release systemic delivery - a new concept in cancer chemoprevention. Carcinogenesis. 2012;33:1608–1615. doi: 10.1093/carcin/bgs209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agnihotri AP, Sontakke SD, Thawani VR, Saoji A, Goswami VS. Effects of Withania somnifera in patients of schizophrenia a randomized, double blind, placebo controlled pilot trial study. Indian J Pharmacol. 2013;45:417–418. doi: 10.4103/0253-7613.115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Biswal BM, Sulaiman SA, Ismail HC, Zakaria H, Musa KI. Effect of Withania somnifera (Ashwagandha) on the development of chemotherapy-induced fatigue and quality of life in breast cancer patients. Integr Cancer Ther. 2013;12:312–322. doi: 10.1177/1534735412464551. [DOI] [PubMed] [Google Scholar]
- 82.Mirza S, Sharma G, Parshad R, Gupta SD, Pandya P, Ralhan R. Expression of DNA methyltransferases in breast cancer patients and to analyze the effect of natural compounds on DNA methyltransferases and associated proteins. J Breast Cancer. 2013;16:23–31. doi: 10.4048/jbc.2013.16.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sivanandhan G, Arunachalam C, Selvaraj N, Sulaiman AA, Lim YP, Ganapathi A. Expression of important pathway genes involved in withanolides biosynthesis in hairy root culture of Withania somnifera upon treatment with Gracilaria edulis and Sargassum wightii. Plant Physiol Biochem. 2015;91:61–64. doi: 10.1016/j.plaphy.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 84.Kaul MK, Kumar A, Ahuja A, Mir BA, Suri KA, Qazi GN. Production dynamics of Withaferin A in Withania somnifera (L.) Dunal complex. Nat Prod Res. 2009;23:1304–1311. doi: 10.1080/14786410802547440. [DOI] [PubMed] [Google Scholar]
- 85.Patial P, Gota V. Rapid and sensitive method for determination of withaferin-A in human plasma by HPLC. Bioanalysis. 2011;3:285–289. doi: 10.4155/bio.10.207. [DOI] [PubMed] [Google Scholar]
- 86.Kensler TW, Egner PA, Agyeman AS, Visvanathan K, Groopman JD, Chen JG, Chen TY, Fahey JW, Talalay P. Keap1-nrf2 signaling a target for cancer prevention by sulforaphane. Top Curr Chem. 2013;329:163–177. doi: 10.1007/128_2012_339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rajaraman P, Anderson BO, Basu P, Belinson JL, Cruz AD, Dhillon PK, Gupta P, Jawahar TS, Joshi N, Kailash U, Kapambwe S, Katoch VM, Krishnan S, Panda D, Sankaranarayanan R, Selvam JM, Shah KV, Shastri S, Shridhar K, Siddiqi M, Sivaram S, Seth T, Srivastava A, Trimble E, Mehrotra R. Recommendations for screening and early detection of common cancers in India. Lancet Oncol. 2015;16:e352–e361. doi: 10.1016/S1470-2045(15)00078-9. [DOI] [PubMed] [Google Scholar]
- 88.Samadi AK, Mukerji R, Shah A, Timmermann BN, Cohen MS. A novel RET inhibitor with potent efficacy against medullary thyroid cancer in vivo. Surgery. 2010;148:1228–1236. doi: 10.1016/j.surg.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi BY, Kim BW. Withaferin-A Inhibits Colon Cancer Cell Growth by Blocking STAT3 Transcriptional Activity. J Cancer Prev. 2015;20:185–192. doi: 10.15430/JCP.2015.20.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]